Abstract
In two recent publications in Molecular Cell, Boulias et al. (2019) and Sendinc et al. (2019) use complementary approaches to map m6Am modification sites transcriptome-wide and demonstrate that m6Am can repress translation while increasing the stability of a subset of low-abundance transcripts.
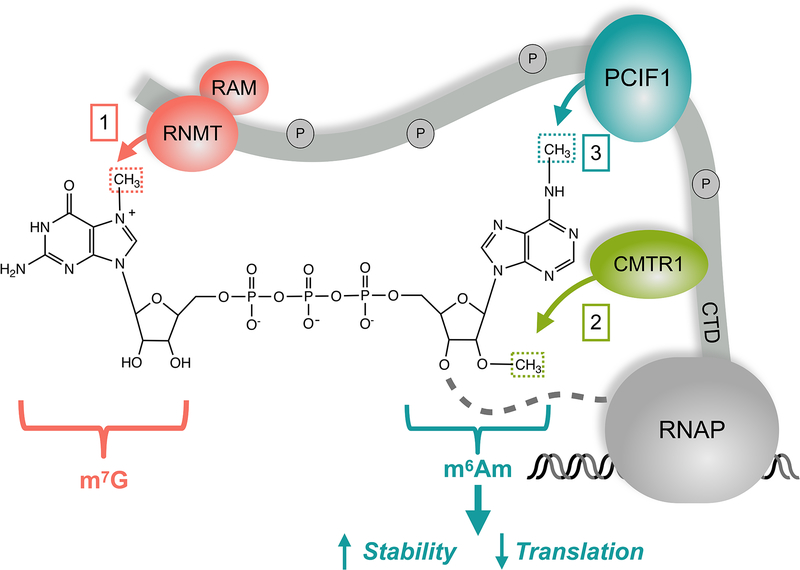
Cellular RNAs can be decorated with over 150 distinct post-transcriptional modifications (Frye et al., 2018). The variety of possible chemical modifications provide opportunities to regulate RNA function. Although we have known about the existence of modifications on abundant RNAs for a long time, recent advances in detecting and mapping the chemical modification sites in the transcriptome have expanded our understanding of how they affect mRNA function. One of the most common and well-characterized modifications in mRNAs is adenine methylation on nitrogen-6 (N6-methyladenosine or m6A). When adenosine is the first nucleotide after the 5’ cap structure (7-methylguanosine or m7G), it can be methylated at both 2’-O and N6 positions, yielding N6, 2′-O-dimethyladenosine (m6Am) (Fig 1). Recent work from multiple groups including the two articles in this issue of Molecular Cell identified PCIF1 as the enzyme responsible for N6 methylation required to create m6Am marks. The two papers use complementary methods to reveal the location of m6Am sites in the transcriptome, and suggest that m6Am can affect gene expression on multiple levels (Boulias et al., 2019; Sendinc et al., 2019).
Figure 1.
Capped mRNAs are methylated by multiple enzymes recruited by the phosphorylated CTD of RNA polymerase II (RNAP). First, RNMT-RAM methylate the cap to create m7G. Second, the 2’-O of the first transcribed nucleotide is methylated by CMTR1. If this nucleotide is an adenosine, it can be further methylated by PCIF1 at the N6 position to create an m6Am modification. The resultant m7Gppp-m6Am modification can suppress translation efficiency and stabilize certain mRNAs.
PCIF1 (Phosphorylated CTD Interacting Factor 1) associates with the C-terminal domain of RNA polymerase II via a WW domain, which would allow PCIF1 to greet the nascent RNA chain as it exits the tunnel after synthesis (Fan et al., 2003). Both Sendic et al. and Boulias et al. show that PCIF1 knockout (KO) in human cell lines leads to a complete loss of m6Am modification. Thus, PCIF1 is likely to be the only enzyme capable of writing m6Am marks in mammals, in agreement with previous findings (Akichika et al., 2019; Sun et al., 2019). Quantitative studies using recombinant proteins show that PCIF1 substrates require an adenine base adjacent to the cap structure, as well as a prior 2’O-Me on the ribose ring (Akichika et al., 2019; Boulias et al., 2019; Sendinc et al., 2019; Sun et al., 2019). Moreover, although PCIF1 can act on the m7Gppp-Am dinucleotides (Sendinc et al., 2019), additional nucleotides 3’ to the modified adenosine enhance PCIF1 activity, and beyond 6 nucleotides may be superfluous (Akichika et al., 2019). A crystal structure of PCIF1 bound with visible m7G cap helps explain the biochemical findings on the specificity of PCIF1 (Akichika et al., 2019). Localization of PCIF1 near the polymerase and its substrate requirements suggest that m6Am may be added co-transcriptionally, although more studies are needed to test this hypothesis. Biochemical and structural approaches help define the intrinsic specificity of PCIF1 in isolation, but further investigation of in vivo activity and specificity of PCIF1 will shed light on how m6Am writing may be regulated in cells.
To investigate the role of m6Am in gene expression, the two groups adopt distinct approaches to identify the modification sites in the transcriptome. Sendinc et al. developed “m6Am-Exo-Seq”, which utilizes a 5’ exonuclease to enrich for the capped RNA fragments, thereby reducing the noise caused by internal m6A, which can be captured by the same antibody. Subsequent decapping then helps with enhancing the ability to detect m6Am with antibodies. Boulias et al. applied miCLIP, a method that can identify m6A sites with high resolution (Linder et al., 2015), to PCIF1 KO cells, to distinguish m6Am from m6A. The two complementary sequencing methods provide higher confidence transcriptome-wide maps of m6Am and thus m6A as well, an improvement over the previous studies (Mauer et al., 2017). The updated maps should prompt the RNA modification field to revisit the findings based on incomplete and inaccurate maps of m6A and m6Am, in order to distinguish the effects of the two modifications, as both can impact expression of the mRNA.
PCIF1-mediated m6Am modification tends to occur on highly abundant transcripts. However, the direct effect of m6Am on mRNA levels is not as dramatic. For highly-expressed mRNAs, Boulias et al. observe little change in RNA stability, measured by SLAM-Seq, upon knocking out PCIF1. However, less abundant mRNAs can become stabilized in the presence of PCIF1 (Boulias et al., 2019). Sendinc et al. report that PCIF1 KO causes certain transcripts to increase or decrease, and that most of these changes are due to changes in transcription, rather than RNA degradation. Altered transcription does not correlate with m6Am modification, suggesting that PCIF1 KO does not affect RNA synthesis directly. Overall, PCIF1 may not have as much of an effect on global RNA stability as previously thought (Mauer et al., 2017; Wei et al., 2018), but more research is needed to understand how m6Am might stabilize a subset of mRNAs in different contexts.
The 5’ end of mRNAs, including the cap structure, plays an important role in eukaryotic translation. Ribosome profiling experiments by Akichika et al. and Boulias et al. do not show a substantial change in the distribution of ribosomes on mRNAs upon knocking out PCIF1 in HEK293T cells (Akichika et al., 2019; Boulias et al., 2019). In contrast, Sendinc et al. propose that m6Am downregulates translation. In melanoma cells, they use reporter assays to determine that the transcripts containing m7Gppp-m6Am are translated poorly in comparison to those with m7Gppp-Am. The repressive role of m6Am in translation is also confirmed by measuring protein levels using quantitative proteomics. Furthermore, in vitro translation assay results corroborate the cell-based studies, showing that cap-dependent translation specifically is repressed by the m6Am modification (Sendinc et al., 2019). Although the effects on translation from different experiments might seem incongruent, m6Am may not change the distribution of ribosomes while nevertheless reducing translation efficiency. Moreover, the effect of m6Am on translation may also depend on cell type and growth conditions. Finally, it is important to note that PCIF1 KO models were used to determine m6Am effects on both RNA stability and translation, rather than using the point mutation that deactivates the catalytic activity of PCIF1. Additional work will be required to distinguish the effects of m6Am modification from the non-catalytic effects of PCIF1.
New findings of PCIF1 effects on gene expression raise questions regarding the role of m6Am-mediated gene regulation in biology. PCIF1 KO does not regularly affect cell growth but does increase sensitivity to oxidative stress (Akichika et al., 2019). Interestingly, Boulias et al. identify m6Am at a translation start site of ATF4 known to switch under stress. What is missing, however, is the mechanism through which m6Am affects translation. Future work on how m6Am affects specific steps in translation and interactions between mRNA and translation factors, such as the cap binding complex including eIF4E (Akichika et al., 2019), may lead to further insights on the function of m6Am. Moreover, understanding how stress or cell signaling can regulate PCIF1 activity or specificity may also reveal how m6Am is utilized to regulate gene expression.
References
- Akichika S, Hirano S, Shichino Y, Suzuki T, Nishimasu H, Ishitani R, Sugita A, Hirose Y, Iwasaki S, Nureki O, et al. (2019). Science 363. [DOI] [PubMed] [Google Scholar]
- Boulias K, Toczydlowska-Socha D, Hawley BR, Liberman-Isakov N, Takashima K, Zaccara S, Guez T, Vasseur J-J, Debar F, Aravind L, et al. (2019). Molecular Cell 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Sakuraba K, Komuro A, Kato S, Harada F, and Hirose Y (2003). Biochem Biophys Res Commun 301, 378–385. [DOI] [PubMed] [Google Scholar]
- Frye M, Harada BT, Behm M, and He C (2018). Science 361, 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, and Jaffrey SR (2015). Nat Methods 12, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, et al. (2017). Nature 541, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendinc E, Valle-Garcia D, Dhall A, Chen H, Henriques T, Navarrete-Perea J, Sheng W, Gygi SP, Adelman K, and Shi Y (2019). Molecular Cell 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhang M, Li K, Bai D, and Yi C (2019). Cell Res 29, 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, Shi H, Cui X, Su R, Klungland A, et al. (2018). Mol Cell 71, 973–985 e975. [DOI] [PMC free article] [PubMed] [Google Scholar]