Abstract
Nail toxicities, such as paronychia and pyogenic granuloma-like lesions, are well-recognized side effects of epidermal growth factor receptor inhibitor (EGFR-I) therapy that can significantly impair patient’s quality of life and compliance to anticancer treatment. Numerous therapeutic options are available, with variable rates of success. Recently, topical β-blockers have emerged as a novel, non-invasive treatment strategy. We tested the effectiveness of topical timolol 0.5% gel, twice daily, under occlusion for 30 days, on paronychia and periungual pyogenic granuloma-like lesions in 9 patients being treated with EGFR-I. We also reviewed the available literature on this topic, which is the use of topical β-blockers in the management of EGFR-I-induced nail toxicities. We assessed 25 lesions consistent with the diagnosis of EGFR-I-induced pyogenic granuloma-like lesions and paronychia (21 diagnosed as pyogenic granuloma-like, and four as paronychia). Thirteen of the 25 lesions achieved complete resolution, 9/25 reached at least improvement, and only 3/25 did not respond to the intervention. As for the review, four papers met the scope of our research. The results confirmed at least partial benefit in the majority of treated patients. Among current strategies, high-potency topical corticosteroids are a well-known treatment option especially for paronychia, targeting the inflammatory component of such lesions; nevertheless, the management of pyogenic granuloma-like lesion is often more complex and the success rate is variable. Nail plate avulsion and phenol chemical matricectomy are not highly effective and display some degree of invasiveness. Topical β-blockers seem to be promising alternatives, especially in fragile cancer patients who may be unsuitable candidates for an invasive procedure.
Keywords: epidermal growth factor, paronychia adverse event, pyogenic granuloma, topical beta-blockers
Introduction
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein belonging to the ErbB family of tyrosine kinase receptors, which is primarily expressed on keratinocytes of the basal and supra-basal layers of the epidermis, and on the outer sheath of the hair follicles. EGFR plays a critical role in the normal development and function of the skin and its appendages; its activation mediates cell survival, proliferation, angiogenesis and tumour invasiveness (growth and progression).1 Blockage of EGFR-driven pathways leads to keratinocytes growth arrest, deranged and premature differentiation, and increased rate of apoptosis, ultimately resulting in decreased epidermal thickness and reduced integrity of the epidermal barrier.2
EGFR inhibitors (EGFR-I) are a class of targeted therapies approved for the treatment of several types of solid organ tumours such as non-small cell lung cancer, colorectal, breast, head and neck and pancreatic cancer.3–7 There are two types of EGFR-I: (1) monoclonal antibodies such as cetuximab and panitumumab, which bind the EGFR extracellular domain, and (2) tyrosine kinase inhibitors such as erlotinib, gefitinib, lapatinib, afatinib and osimertinib that target the intracellular tyrosine kinase domain of the receptor.
Given the EGFR specificity, these drugs display a class-specific spectrum of adverse reactions, which parallel the EGFR distribution across the body. Cutaneous toxicities are frequent, affecting 45–100% of patients treated with EGFR-I.8,9 The most common dermatologic adverse reactions are papulopustular rash, xerosis, itching, hair and nail changes and fissures of the heels and fingertips,10,11 which significantly affect patient’s quality of life (QoL) representing both a physical and a psychological burden. Dose reduction or treatment discontinuation may be warranted for the most severe cases, thus compromising patients’ clinical endpoint.
Paronychia and pyogenic granuloma-like lesions are periungual lesions observed in 10–15% of cancer patients treated with EGFR-I, both with monoclonal antibodies and tyrosine kinase inhibitors.12 These lesions typically manifest as late onset adverse events, at least 4–8 weeks after initiation of anticancer therapy, a finding that is consistent with the slow kinetics of nail growth. Multiple nails of both hands and feet may be affected at the same time, the great toe being the most common one to be involved. Early manifestations are erythema, oedema, swelling of the lateral nail folds and tenderness. Periungual lateral friable granulation tissue resembling a pyogenic granuloma may develop in a subset of patients.13 EGFR-I-induced epidermal thinning and altered barrier function are believed to be the initiating events. Increased skin fragility coupled with local trauma, or penetration of nail plate fragments piercing the paronychium subsequently drive a foreign body-like reaction, with local release of inflammatory mediators and recruitment of inflammatory cells.14 Bacterial or fungal infections are a common secondary process; the most frequently isolated pathogens are coagulase-negative Staphylococci.15 Despite being non-life-threatening conditions, paronychia and pyogenic granuloma-like lesions are highly disabling for the patients, resulting in painful functional limitation of daily activities. Current strategies for prevention and management of side effects are based on physicians’ experience as randomized controlled trials and established guidelines are lacking. Oral tetracyclines, topical corticosteroids and antibiotics, topical adapalene, cryotherapy with liquid nitrogen, topical silver nitrate and phenol chemical matricectomy represent the current therapeutic approaches to treat periungual pyogenic granuloma and paronychia.16 Recently, topical β-blockers have emerged as a novel, non-invasive treatment for EGFR-I-induced nail toxicity.17
We evaluated the efficacy and tolerability of topical timolol in the management of EGFR-I-induced paronychia and pyogenic granuloma-like lesions in a series of adult patients and reviewed the available literature on the use of β-blockers to treat such cutaneous lesions.
Materials and methods
Case series
Pyogenic granuloma-like lesions and paronychia on the fingers of nine patients under treatment with EGFR-I were diagnosed and managed from 1 February 2018 to 1 May 2019 in the setting of a combined Dermatology/Oncology outpatient clinic. All lesions were treated with timolol 0.5% gel applied twice daily, under occlusion, until complete response (CR) or up to a maximum of 4 weeks, on the basis of earlier studies.17–20 Treatment efficacy was assessed at week 4 by clinical examination and photographic documentation. CR was defined as disappearance of the lesion, and absence of pain and bleeding; partial response (PR) as improvement in any of the three categories: lesion, pain or bleeding); and no response (NR). The patients were consecutive to our outpatient clinic but selected for the type of skin pathology we investigated (i.e. we included and analyzed only patients affected by paronychia and pyogenic granuloma-like lesions). Signed consent was obtained from all the patients.
Literature review
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed throughout the literature search process to structure the framework for the review. The literature review on treatment on this topic was performed using the following search items “paronychia” or “pyogenic granuloma” and “β-blocker” in the following databases: PubMed, Embase and Cochrane. Eligibility criteria were English language, availability of full text, and publications from January 2009 to June 2019.
Results
Case series
Our patients included six women and three men (age range: 58–83 years, mean: 70.5 years), who were under treatment with cetuximab (n=4) for colorectal cancer, or afatinib (n=3) or gefitinib (2) for non-small cell lung cancer. The mean antineoplastic treatment duration was 2.1 months (range: 1–4 months) (Table 1). An overall number of 25 pyogenic granuloma-like lesions, affecting all nine patients, were diagnosed. Anatomical location included the fingernails in 14 lesions (10 localized on the lateral nail fold and four on the proximal nail fold) and toenails in seven lesions (six localized on the lateral nail fold and one on the proximal nail fold). In addition, four paronychia lesions located on the fingernails were observed in two of the nine patients. The mean time from initiation of anticancer therapy to treatment of skin lesions was 2 months (range 1–6 months). After 4 weeks of timolol 0.5% treatment applied twice daily, complete response (CR) and partial response (PR) was observed in 13/25 (52%) lesions, which included eight pyogenic granuloma-like lesions on the hands, one pyogenic granuloma-like on the feet and four paronychia of the fingernails (Figures 1 and 2). PR was achieved in 9/25 (36%) lesions, including six pyogenic granuloma-like lesions on the hands and three pyogenic granuloma-like lesions on the feet. No response (NR) was found in three pyogenic granuloma-like lesions located on the feet, all three occurring on the same patient, probably due to poor patient compliance (Table 1). No local or systemic side effects were detected during treatment. After a mean follow-up period of 7.1 months (range 6–10 months), no relapse of successfully treated lesions was detected.
Table 1.
Patient characteristics and response to treatment.
| Patient N./Sex/Age | Tumour type and stage | Antineoplastic treatment | Antineoplastic treatment duration before lesions (months) | No. of lesions (PG and paronychia) | Localization of the lesions (PG and paronychia) | Other skin disease | Clinical response | Follow up (months) |
|---|---|---|---|---|---|---|---|---|
| 1/F/58 | CRC stage IV | Cetuximab | 2 | 3 PG | Left foot (I & II toes) Right foot (I toe) |
Acneiform rash CTCAE grade 2 | No response | 6 |
| 2/M/70 | CRC stage IV | Cetuximab | 1 | 1 PG | Left hand (thumb) | Acneiform rash CTCAE grade 2 | PR | 8 |
| 3/F/83 | NSCLC stage IIIB | Afatinib | 4 | 1 PG 2 Paronychia |
Right hand (IV finger) Left hand (III & IV fingers) |
NA | PR 2 CR |
10 |
| 4/M/64 | CRC stage IV | Cetuximab | 2 | 4 PG | Right hand (IV finger) Left hand (III finger) Left foot (I & II toes) |
Acneiform rash CTCAE grade 2 | CR PR 1CR, 1 PR |
6 |
| 5/F/79 | NSCLC stage IV | Gefitinib | 3 | 5 PG | Right hand (II, III, & IV fingers) Left hand (IV & V fingers) |
Acneiform rash CTCAE grade 1 | 3 CR 1 CR, 1 PR |
|
| 6/F/76 | NSCLC stage IV | Gefitinib | 2 | 3 PG | Right hand (III finger) Left hand (III finger) Right foot (II toe) |
Acneiform rash CTCAE grade 2 | CR PR PR |
6 |
| 7/F/71 | NSCLC stage IV | Afatinib | 2 | 2 PG | Left hand (I & III finger) | Acneiform rash CTCAE grade 1 | 2 CR | 6 |
| 8/F/68 | NSCLC stage IV | Afatinib | 1 | 2 Paronychia 1 PG |
Right hand (II & III fingers) Left hand (IV finger) |
Acneiform rash CTCAE grade 1 | 2 CR PR |
6 |
| 9/M/66 | CRC stage IV | Cetuximab | 2 | 1 PG | Right foot (I toe) | Acneiform rash CTCAE grade 2 | PR | 9 |
Abbreviations: CRC, colorectal cancer; CTCAE, common terminology criteria for adverse events; NA, not applicable; NSCLC, non-small cell lung cancer; PG, pyogenic granuloma; CR, complete response; PR, partial response.
Figure 1.
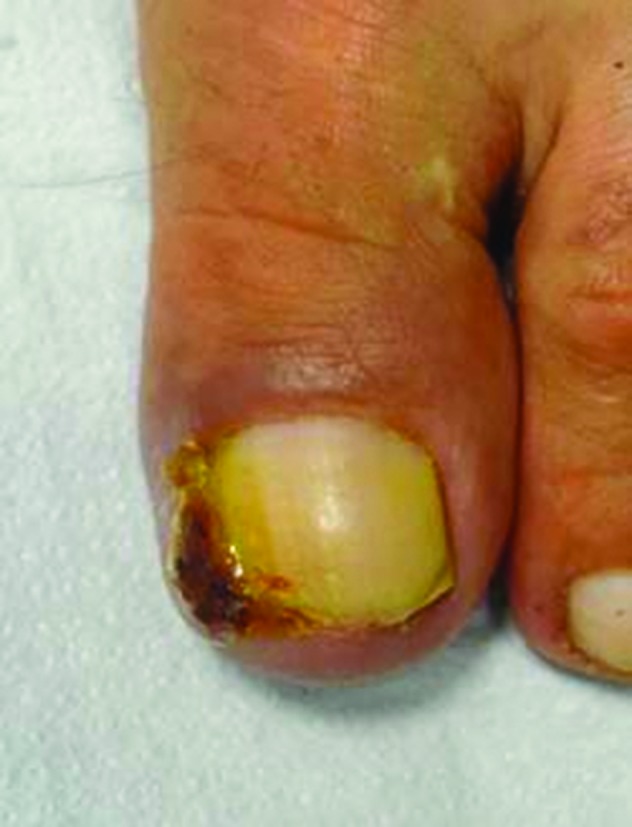
Pyogenic granuloma of the lateral fold of the first toe (left foot).
Figure 2.
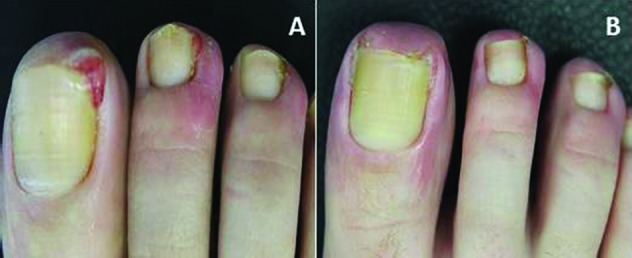
Pyogenic granuloma of the first toe (A) before and (B) after treatment with timolol 0.5% gel.
Literature review
The literature review yielded a total of 43 papers, 27 of which were on PubMed, 15 on Embase and the remaining one on the Cochrane database (Figure 3). Three studies were excluded because they were duplicates. A total of 40 articles underwent evaluation for inclusion into our study. On the basis of the eligibility criteria, two articles were removed because they were not in the English language, and one was discarded because it was not published within the last 10 years. Only four papers met the criteria of our review,17–20 that is the evaluation of the use of topical β-blockers in the management of paronychia and pyogenic granuloma-like lesions occurring in the setting of anti-EGFR cancer therapy (Table 2). The other studies were excluded either because the treatment for EGFR-I induced nail toxicity and was not a topical β-blocker, or because the β-blockers were used for treatment of paronychia and pyogenic granuloma-like lesions which were not EGFR-I related.
Figure 3.
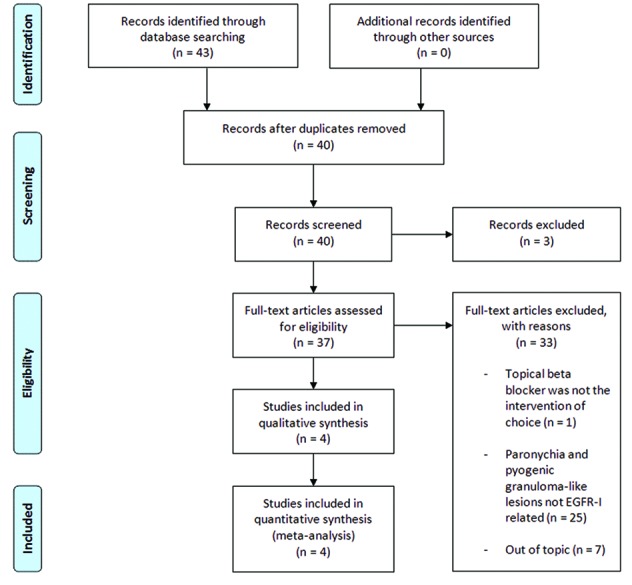
Flow diagram based on PRISMA 2009.
Table 2.
Summary of articles included in our review.
| Title | Type of topical treatment | Patients (n) | Response | Follow up |
|---|---|---|---|---|
| Piraccini et al.17 | Propanolol 1% cream, once daily, under occlusion for a maximum of 45 days | 10 | CR: 3/10 PR: NA NR: 7/10 |
– |
| Cubiró et al.18 | Timolol 0.5% gel, twice daily, under occlusion, for 1 month | 10 | CR: 9/10 PR: 1/10 NR: NA |
8 months |
| Sibaud et al.19 | Timolol 0.5% gel, twice daily, under occlusion for 1 month | 13 | CR: 2/13 PR: 6/13 NR: 5/13 |
– |
| Yen et al.20 | Betaxolol 0.25% eye drops, once daily, under occlusion for 1 month | 1 | CR: 1/1 PR: NA NR: NA |
– |
CR, complete response; NA, not applicable; NR, no response; PR, partial response.
Among the publications included in our review, Piraccini and colleagues17 examined the efficacy of topical propranolol 1% cream applied under occlusion overnight for 30–45 days on periungual and subungual granulomas of the hands and feet in 10 patients. Three of 10 patients were under targeted therapy (1 on trastuzumab, 1 on capecitabine and lapatinib, and 1 on erlotinib), whereas the remaining 7 patients were not on an anticancer regimen, and therefore their pyogenic granuloma-like lesions had rather a traumatic cause. CR of the pyogenic granuloma-like lesions localized on the fingernails was achieved; however, similar satisfactory results could not be obtained for granulomas of the toenails, which were unchanged or worsened after therapy. No local or systemic side effects were reported.
Two studies on topical timolol 0.5% gel have been published. Cubirò and colleagues18 assessed the efficacy and tolerability of topical timolol 0.5% gel, applied under occlusion twice daily for four weeks, on paronychia and pyogenic granuloma-like lesions of the hands and feet in 10 patients, nine of whom treated with EGFR-I, (cetuximab [n=4], panitumumab [n=4] and erlotinib [n=1]); the remaining patient was not treated with EGFR-I and therefore was not considered for our review. Five patients presented with paronychia, either alone or associated with pyogenic granuloma-like lesion, whereas four patients presented only with pyogenic granuloma-like lesions, localized on the toenail or on fingernails or both. Overall, 27 lesions were considered in such case series: 12 lesions were diagnosed as paronychia, seven of which were localized on the fingernails and five on the toenails; whereas 15 were pyogenic granuloma-like lesions, 10 affecting the fingernails and five the toenails.18 CR, defined as disappearance of the lesion, absence of pain and/or bleeding was achieved after 4 weeks of treatment in 25 lesions of eight patients, including 15 pyogenic granuloma-like lesions and 10 paronychia lesions. Partial remission was observed in one patient with two paronychia on the toenails, defined as the improvement in one of the three categories. No local or systemic adverse events were reported at the 1-month assessment and throughout the 8 months of follow-up. A retrospective, single-center study was recently published by Sibaud and colleagues19 who evaluated the efficacy of topical timolol 0.5% gel applied twice daily under occlusion in 13 patients treated with EGFR-I (four patients with lapatinib, four patients with afatinib, three patients with cetuximab and two patients with erlotinib). At 1-month evaluation, 2/13 (15%) patients achieved CR, defined as clearance of lesion, absence of pain and/or bleeding; 6/13 (46%) had a partial response with improvement in at least one of the three parameters; 5/13 (39%) did not have any clinical benefit. No local or systemic side effects were reported. No differences in the clinical outcome had been observed between fingernail and toenail pyogenic granuloma-like lesions.
Successful treatment of relapsing afatinib-induced paronychia and pyogenic granuloma-like lesion of the left thumb has been reported with topical betaxolol 0.25% eye drops under occlusion used once daily for four weeks.20 β-blockers were chosen as a third-line option, after failure of topical bethametasone and gentamycin, and an attempt of cauterization with 10% aqueous silver nitrate solution.
The results reported so far are rather heterogeneous. Cubirò and colleagues18 showed excellent clinical results, i.e., CR in eight of nine patients, applying timolol 0.5% gel, twice daily, for treatment of pyogenic granuloma-like lesions located on the fingernails and toenails. Satisfactory clinical results were also obtained in another case series including three patients affected by EGFR-I-induced nail toxicities, with clearance of the fingernail pyogenic granuloma-like lesions by applying propanolol 1% cream, once daily. In contrast, no benefit was seen for toenail lesions.17 It is conceivable that the lack of response might be caused by the cream vehicle, which was not adequate to penetrate into the thicker skin of the feet, or that the drug concentration was not appropriate. Sibaud and colleagues19 demonstrated a partial response in nearly two thirds of cancer patients (8/13) at 1-month evaluation. Our case series included nine patients with paronychia and pyogenic granuloma-like lesions of the fingernails and/or toenails which were treated with topical timolol 0.5% gel, twice daily, under occlusion, for 4 weeks. Overall, our results are in line with earlier case reports indicating that topical timolol is a valid therapeutic approach in pyogenic granuloma-like lesions and paronychia in patients treated with EGFR-I. We found that the majority of the lesions managed with topical β-blockers achieved at least a significant improvement, and only three toenail pyogenic granuloma-like lesions, all affecting the same patient, did not have any clinical response. In this specific case, we believe that patient’s low compliance might be a key factor affecting the clinical outcome. In our patients, no relapses were identified after a mean follow-up period of 6 months. No systemic side effects have been registered in our case series or in any of the four studies reported earlier in the literature.17–20
Conclusion
Cutaneous adverse events occurring in the oncologic treatment course can be severe and can significantly impair patient’s quality of life. Compliance to anticancer therapies can be negatively affected, and dose reduction or treatment discontinuation are needed for the most severe cases, thus compromising patient’s clinical outcome.11 Paronychia and pyogenic granuloma-like lesions due to EGFR-I therapies are relatively common dermatologic adverse events, and represent a physical and a psychological burden for cancer patient. Patients should be instructed to avoid pressure and friction on the fingertips and on the nailfolds to limit the development of the lesions. Among current strategies, nail plate avulsion and phenol chemical matricectomy are not highly effective and display some degree of invasiveness.9,12 Indeed, a fragile oncologic patient may often be an unsuitable candidate for such treatment options. High-potency topical corticosteroids are a well-known traditional first-line approach for paronychia and pyogenic granuloma-like lesions.9 Corticosteroids primarily target the inflammatory component of such lesions; therefore, they are mostly effective for paronychia not complicated by the simultaneous occurrence of pyogenic granuloma-like lesions where highly vascularized and granulation tissues are prominent. Treatment with corticosteroids may not be sufficient for severe pyogenic granuloma-like lesions, and a combination therapy or a switch to other strategies specifically aiming at removing excessive granulation tissue should be preferred. β-blockers are emerging as a non-invasive treatment for the management of paronychia and pyogenic granuloma-like lesions occurring in the setting of anti-EGFR cancer therapy.17–20 Reports of satisfactory treatment of paediatric cutaneous and mucosal pyogenic granuloma prompted for the novel application of these drugs also in the oncologic setting.21 Vascular endothelial cells have been found to display β-adrenergic receptors; their antagonism would lead to vasoconstriction, endothelial cell apoptosis, modulation of proangiogenic factors, and regression of the vascular lesion.22 Based on this assumption, improvement of EGFR-I-induced pyogenic granuloma-like lesions would be expected.
The optimal topical β-blockers treatment duration for pyogenic granuloma-like lesions and paronychia is not known as data provided in the literature are scarce: small patient sample size, lack of a protocol or standardization of the procedure. Indeed, the differences on clinical outcome that we found in the scientific articles analysed should be due to the differences in the type of β-blocker prescribed, administration schedule and duration of the treatment. Moreover, the proportion and rate of recurrence requires longer follow-up than those described so far. Cancer patients are a high-need group and β-blockers showed a high degree of patient satisfaction and excellent cosmetic results.
Acknowledgments
None.
Footnotes
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/10/dic.212613-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Sollena P, Mannino M, Tassone F, Calegari MA, D’Argento E, Peris K. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 13 September 2019
References
- 1.Normanno N, de Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21(14):2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MH, Johnson JR, Chen YF, et al. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist. 2005;10(7):461–466. doi: 10.1634/theoncologist.10-7-461. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303–306. doi: 10.1634/theoncologist.8-4-303. [DOI] [PubMed] [Google Scholar]
- 5.Ryan Q, Ibrahim A, Cohen MH, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER2. Oncologist. 2008;13:1114–1119. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- 6.Giusti RM, Shastri KA, Cohen MH, et al. FDA drug approval summary: panitumumab (Vectibix) Oncologist. 2007;12(5):577–583. doi: 10.1634/theoncologist.12-5-577. [DOI] [PubMed] [Google Scholar]
- 7.Greig SL. Osimertinib: First Global Approval. Drugs. 2016;76(2):263–273. doi: 10.1007/s40265-015-0533-4. [DOI] [PubMed] [Google Scholar]
- 8.Balagula Y, Garbe C, Myskowski P, et al. Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol. 2011;50(2):129–146. doi: 10.1111/j.1365-4632.2010.04791.x. [DOI] [PubMed] [Google Scholar]
- 9.Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19(8):1079–1095. doi: 10.1007/s00520-011-1197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 11.Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116(16):3916–3923. doi: 10.1002/cncr.25090. [DOI] [PubMed] [Google Scholar]
- 12.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(9):1425–1433. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 13.Ho P, Lin I, Yang X, et al. Using a novel scoring system for paronychia related to oncologic treatments (SPOT) for assessing paronychia severity and its correlation with pain index and quality of life. J Eur Acad Dermatol Venereol. 2019;33(1):204–212. doi: 10.1111/jdv.15121. [DOI] [PubMed] [Google Scholar]
- 14.Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56(2):317–326. doi: 10.1016/j.jaad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Eames T, Grabein B, Kroth J, et al. Microbiological analysis of epidermal growth factor receptor inhibitor therapy-associated paronychia. J Eur Acad Dermatol Venereol. 2010;24(8):958–960. doi: 10.1111/j.1468-3083.2009.03516.x. [DOI] [PubMed] [Google Scholar]
- 16.Robert C, Sibaud V, Mateus C, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16(4):e181–e189. doi: 10.1016/S1470-2045(14)71133-7. [DOI] [PubMed] [Google Scholar]
- 17.Piraccini BM, Alessandrini A, Dika E, et al. Topical propranolol 1% cream for pyogenic granulomas of the nail: open-label study in 10 patients. J Eur Acad Dermatol Venereol. 2016;30(5):901–902. doi: 10.1111/jdv.13071. [DOI] [PubMed] [Google Scholar]
- 18.Cubiró X, Planas-Ciudad S, Garcia-Muret MP, et al. Topical timolol for paronychia and pseudopyogenic granuloma in patients treated with epidermal growth factor receptor inhibitors and capecitabine. JAMA Dermatol. 2018;154(1):99–100. doi: 10.1001/jamadermatol.2017.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibaud V, Casassa E, D’Andrea M. Are topical beta-blockers really effective “in real life” for targeted therapy-induced paronychia. Support Care Cancer. 2019;27(7):2341–2343. doi: 10.1007/s00520-019-04690-8. [DOI] [PubMed] [Google Scholar]
- 20.Yen CF, Hsu CK, Lu CW. Topical betaxolol for treating relapsing paronychia with pyogenic granuloma-like lesions induced by epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2018;78(6):e143–e144. doi: 10.1016/j.jaad.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Wine Lee L, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonists. Pediatr Dermatol. 2014;31(2):203–207. doi: 10.1111/pde.12217. [DOI] [PubMed] [Google Scholar]
- 22.Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25(11):1446–1451. doi: 10.1038/modpathol.2012. [DOI] [PubMed] [Google Scholar]

