Abstract
Background
Aedes aegypti is a vector mosquito of major public health importance, transmitting arthropod-borne viruses (arboviruses) such as chikungunya, dengue, yellow fever and Zika viruses. Wild mosquito populations are persistently infected at high prevalence with insect-specific viruses that do not replicate in vertebrate hosts. In experimental settings, acute infections with insect-specific viruses have been shown to modulate arbovirus infection and transmission in Ae. aegypti and other vector mosquitoes. However, the impact of persistent insect-specific virus infections, which arboviruses encounter more commonly in nature, has not been investigated extensively. Cell lines are useful models for studying virus-host interactions, however the available Ae. aegypti cell lines are poorly defined and heterogenous cultures.
Methodology/Principle findings
We generated single cell-derived clonal cell lines from the commonly used Ae. aegypti cell line Aag2. Two of the fourteen Aag2-derived clonal cell lines generated harboured markedly and consistently reduced levels of the insect-specific bunyavirus Phasi Charoen-like virus (PCLV) known to persistently infect Aag2 cells. In contrast to studies with acute insect-specific virus infections in cell culture and in vivo, we found that pre-existing persistent PCLV infection had no major impact on the replication of the flaviviruses dengue virus and Zika virus, the alphavirus Sindbis virus, or the rhabdovirus vesicular stomatitis virus. We also performed a detailed characterisation of the morphology, transfection efficiency and immune status of our Aag2-derived clonal cell lines, and have made a clone that we term Aag2-AF5 available to the research community as a well-defined cell culture model for arbovirus-vector interaction studies.
Conclusions/Significance
Our findings highlight the need for further in vivo studies that more closely recapitulate natural arbovirus transmission settings in which arboviruses encounter mosquitoes harbouring persistent rather than acute insect-specific virus infections. Furthermore, we provide the well-characterised Aag2-derived clonal cell line as a valuable resource to the arbovirus research community.
Author summary
Mosquito-borne viruses usually only infect humans through the bite of a mosquito that carries the virus. Viruses transmitted by the ‘yellow fever mosquito’ Aedes aegypti, including dengue virus, Zika virus, yellow fever virus and chikungunya virus, are causing an ever-increasing number of human disease cases globally. Mosquito-borne viruses have to infect and replicate inside the mosquito before they are transmitted to humans, and the presence of other infectious agents can change the efficiency of virus transmission. Mosquitoes are known to be infected with ‘insect-specific viruses’ that only infect mosquitoes and cannot cause human disease. We have shown here that in laboratory cell cultures derived from the Aedes aegypti mosquito, pre-existing infection with an insect-specific virus called Phasi Charoen-like virus does not affect the infection and growth of the mosquito-borne viruses dengue virus, Zika virus, Sindbis virus or vesicular stomatitis virus. Our research provides important new insights into whether and how insect-specific viruses may affect mosquito-borne virus replication. Ultimately, this information could inform ongoing research into whether insect-specific viruses could be used to prevent the transmission of mosquito-borne viruses to reduce global disease burdens.
Introduction
Arthropod-borne viruses (arboviruses) are a major public health concern worldwide, with many considered emerging or re-emerging pathogens [1]. Significant taxons to which arboviruses belong include the positive-sense single-stranded RNA (+ssRNA) families Flaviviridae (genus Flavivirus) and Togaviridae (genus Alphavirus), and the negative-sense single-stranded RNA (-ssRNA) order Bunyavirales and family Rhabdoviridae (genus Vesiculovirus). Many arboviral taxons also include related insect-specific viruses that can infect vector insects but not vertebrate hosts [2,3]. Arboviruses transmitted by the vector mosquito Aedes aegypti are of particular concern to human health, as this mosquito species thrives in urban environments and is highly anthropophilic, feeding primarily on humans [4]. Ae. aegypti is the primary vector for the emerging and re-emerging flaviviruses dengue virus (DENV), yellow fever virus (YFV) and Zika virus (ZIKV), and the alphavirus chikungunya virus (CHIKV) [5].
Vector competence is the intrinsic ability of an arthropod to be infected with and transmit vector-borne pathogens [6]. Vector competence varies between individuals and populations based on many factors, including the combination of pathogen and vector genotype, co-infection status of the vector with other microbes, and other environmental factors [4,7–9]. There is widespread interest in understanding the underlying mechanisms influencing vector competence to gain a better understanding of how arboviruses are transmitted and emerge on a global and local scale, especially because this knowledge could aid the development of mosquitoes unable to transmit arboviruses of human public health concern. For example, mosquitoes harbouring the obligate intracellular bacteria Wolbachia spp. are less able to transmit DENV and other arboviruses [10–13] and are being released in endemic settings to test their impact on human disease burdens [14]. Similarly, insect-specific viruses have also been proposed as potential biocontrol agents to reduce arbovirus transmission [2,15].
Insect-specific viruses are highly prevalent in wild mosquito populations [16–28], with a number of studies investigating whether insect-specific viruses influence vector competence [reviewed in 15]. There is no consensus on how insect-specific viruses affect arbovirus replication in tissue culture or in vivo, with the experimental outcome varying depending on the combination of arbovirus, insect-specific virus and mosquito species (and potentially the specific mosquito line or cell line used), as well as other variations in the experimental set up [15]. Thus, previous studies have found insect-specific viruses to either increase [29,30], decrease [19,31–41] or have no effect [31,35,40,42,43] on the replication of various arboviruses across different mosquito species and cell lines. The majority of these studies were performed in the context of acute insect-specific virus infection, which may not accurately recapitulate the effects of the persistent insect-specific virus infections more commonly encountered in nature.
Since any persistent infection must invariably originate from an acute infection, and because many viruses remain present for the full duration of the mosquito’s remaining life following the initial infection, it is worth defining our interpretation of the term ‘persistence’ in the context of insect-specific viruses. In vivo, we would consider an insect-specific virus to be persistent if it were maternally inherited and already present in the embryo, implying some level of stable maintenance within the population. In cell culture, we would consider an insect-specific virus to be persistent only if it were already present at the start of any superinfection experiment in cells seeded from a cell line stock in which the virus had been stably maintained over multiple passages. Since viral levels might slowly drop to clearance over time, a useful indicator that persistence has been achieved might be the point at which viral infection is maintained at similar levels over multiple generations in vivo (% population infected) or cell passages (% cells infected, or viral RNA and/or titres). Where insect-specific virus loads have been shown to vary over time in cell culture [44], the point at which viral levels begin to increase after an initial decrease could be defined as the establishment of persistence.
To our knowledge, there are no in vivo studies on the impact of persistent insect-specific virus infection on arbovirus replication in Aedes spp. Of the in vitro studies that tested arbovirus replication in Aedes spp.-derived cell lines in which persistent insect-specific virus infection was maintained over multiple cell passages, Burivong et al. found that DENV replication was reduced in the presence of persistent but not acute co-infection with Aedes albopictus densovirus (AalDV; family Parvoviridae) in the Ae. albopictus cell line C6/36 [40]. This highlights the differential effects persistent and acute insect-specific virus infections can have on arbovirus replication. Meanwhile, Fujita et al. showed that persistent infection with Shinobi tetravirus (SHTV; family Permutotetraviridae) and Menghai rhabdovirus (MERV; family Rhabdoviridae) alone and in combination reduce ZIKV replication in C6/36 cells, with the two viruses combined also suppressing the replication of the flaviviruses DENV and Japanese encephalitis virus (JEV) [39]. Both studies may not fully recapitulate the conditions encountered in nature, since C6/36 cells are immunocompromised [45], and the mosquito immune system is known to pose a barrier to arboviral infection, within-vector dissemination and transmission [46–50], and is a known contributor to the observed variability in vector competence [46,49]. Notably, Parry and Asgari observed only a modest reduction in DENV replication in the Ae. aegypti Aa20 cell line in the presence of Aedes anphevirus (AeAV; order Mononegavirales) [19]. Therefore, further studies into whether and how persistent infection with insect-specific viruses might modulate arbovirus replication in vivo or in immunocompetent Aedes spp. cell lines are needed.
The Ae. aegypti-derived cell line Aag2 is one of the most commonly used cell lines for studies into virus-vector interactions in tissue culture. One of the benefits of Aag2 cells is that they are immunocompetent [51,52]. A major antiviral immune response in mosquitoes is the RNA interference (RNAi) pathway, in which viral double-stranded RNAs (dsRNAs) are processed by Dicer-2 into small interfering RNAs (siRNAs) that are loaded into the RNA-induced silencing complex (RISC) to target and degrade viral RNAs and thus reduce viral replication and spread [53]. In addition, innate immune pathways such as the Janus kinase-signal transducer and activator of transcription (Jak-STAT) pathway and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-regulated Toll and immunodeficiency (IMD) pathways regulate the expression of antimicrobial peptides that are induced upon microbial stimulation [53].
The Aag2 cell line was originally generated in the 1960’s by Peleg from whole homogenised embryos, and has been referred to as ‘Aag2’ since the 1990’s when Lan and Fallon adapted the culture for growth in E-5 medium [54]. Cells within the culture exhibit differing morphologies (Fig 1A), and it has been suggested that the varying morphologies of mosquito cells in culture may be indicative of the presence of a diversity of embryonic and differentiated cell types [55,56]. Furthermore, Aag2 cells are known to be persistently infected with a number of insect-specific viruses. Cell fusing agent virus (CFAV; family Flaviviridae, genus Flavivirus) was the first insect-specific virus discovered and has long been known to persistently infect Aag2 cells and other Ae. aegypti cell lines [3,57,58]. In addition, we previously discovered Aag2 cells to be persistently infected with the insect-specific virus Phasi Charoen-like virus (PCLV; order Bunyavirales, family Phenuiviridae, genus Phasivirus) [59]. CFAV and PCLV both circulate in Ae. aegypti in the wild [16,17,25], and may have entered the cell line during its establishment or later on from an infected laboratory mosquito colony or environmental sample. While some research groups have found their Aag2 cell lines to also be persistently infected with the insect-specific viruses AeAV [19] or Culex Y virus [44], this is not the case for our Aag2 cells [59].
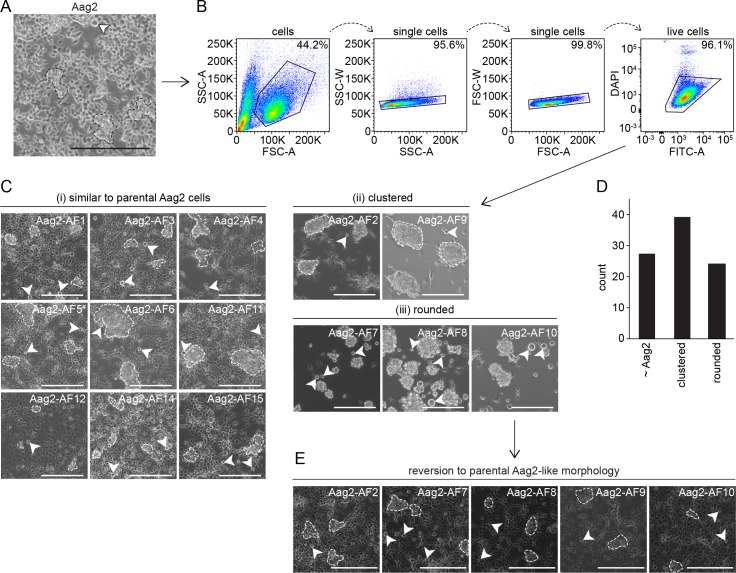
Fig 1. Generation of clonal Aag2-derived cell lines originating from single cells.
(A) Brightfield microscopy image of heterogeneous Aag2 cell population consisting of multicellular ‘clusters’ (examples indicated by hashed lines throughout) and large rounded floating cells (arrows) interspersed across a loose monolayer. (B) FACS gating strategy illustrating selection of live single cells from DAPI-stained Aag2 cell suspension. (C) Resultant Aag2-derived clonal cell line morphologies following limited expansion; (i) similar appearance to parental Aag2 cells, (ii) highly clustered cells with no monolayer formation (some rounded floating cells present), (iii) only large rounded floating cells observable (individual cells and large multi-cell floating aggregates). Only those fourteen clones selected for further study are shown. Images were taken immediately following three-week expansion from single cells into confluent 24-well plate culture. # The Aag2-AF5 cell line was selected for CRISPR gene editing [60,61] (see main text). (D) Total number of clonal cell lines of each morphology generated. (E) Reversion of ‘clustered’ and ‘rounded’ clonal cell lines back to parental Aag2-like morphology following extended culture. Scale bar is 200 μm.
Here, we single-cell sorted Aag2 cells to generate clonal Aag2-derived cell lines so as to provide a better-defined homogeneous Aag2-derived cell line for the research community, and as a starting point for our own CRISPR experiments [60,61]. Although we initially selected clonal cell lines exhibiting different morphologies corresponding to those observed within the original Aag2 cell line (herein referred to as the ‘parental’ Aag2 cell line), these morphologies were not stable and all cell lines reverted to the parental Aag2 cell morphology. This suggests that the various cell morphologies observed in Aag2 cell cultures do not represent fundamentally different cell types. Furthermore, two of the clones selected for further characterisation were found to be ‘cured’ of PCLV, or at least harboured markedly and consistently reduced levels of PCLV. We used these clones to test the effect persistent (rather than acute) insect-specific virus infection has on superinfection with DENV, ZIKV, Sindbis virus (SINV) and vesicular stomatitis virus (VSV), and observed no notable reproducible impact on the replication of these arboviruses. Finally, we characterised a clone we termed ‘Aag2-AF5’ in further detail, and have provided this to the research community as a more well-defined version of the parental Aag2 cell line via the European Collection of Authenticated Cell Cultures (ECACC) (phe-culturecollections.org.uk). Our findings provide important insights into the impact that insect-specific viruses have on mosquito vector competence for arboviruses. Furthermore, our single cell-derived clone Aag2-AF5 represents a much needed standardised and well-defined Ae. aegypti cell line that will benefit the vector research community.
Methods
Cells
Aag2 cells were a kind gift from Raul Andino (University of California, San Francisco, CA USA), and were maintained in Leibovitz’s L-15 medium supplemented with 2 mM glutamine (Sigma-Aldrich, St. Louis, MO USA), 0.1 mM non-essential amino acids (Sigma-Aldrich), 10% (v/v) tryptose phosphate broth (Sigma-Aldrich), 100 U/ml penicillin, 100 μg/ml streptomycin and 10% (v/v) foetal bovine serum (FBS) at 28°C in a humidified atmosphere without CO2. In our hands, the source of FBS is critically important for culturing Aag2 cells and derived clones, with ThermoFisher Scientific (Waltham, MA USA) product number 16000044 being optimal. C6/36 cells were a kind gift from Jorge Muñoz-Jordán (Centers for Disease Control and Prevention, San Juan, Puerto Rico), and were maintained in Roswell Park Memorial Institute (RPMI) medium supplemented with 0.15% (w/v) sodium bicarbonate (Sigma-Aldrich), 0.1 mM non-essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate and 10% (v/v) FBS at 33°C in a humidified atmosphere with 5% CO2. The species identity of all mosquito cell lines entering our lab was verified by sequencing the cytochrome oxidase I or II genes using established PCR protocols [25]. We also tested for RNAi deficiency, which is a distinguishing feature of some mosquito cell lines [45,55,56].
Baby hamster kidney (BHK) cells were a kind gift from Sujan Shresta (La Jolla Institute for Allergy and Immunology, La Jolla, CA USA), and were maintained in minimal essential medium (α-MEM) GlutaMAX supplemented with 10% (v/v) FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM HEPES at 37°C in a humidified atmosphere with 5% CO2. Unless stated, reagents were from ThermoFisher Scientific. Madin Darby Canine Kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA USA).
Viruses
DENV serotype 2 (DENV-2) strain 16681 [62] was a kind gift from Richard Kinney (Arbovirus Disease Branch, Centers for Disease Control and Prevention, Fort Collins, CO USA). ZIKV strain MR766 [63] was obtained from ATCC. Green fluorescent protein (GFP)-expressing SINV, based on clone dsTE12Q [64,65], was a kind gift from Christopher Basler (Georgia State University, Atlanta, GA USA). VSV was the Indiana strain and expresses GFP [66], and was a kind gift from Adolfo Garcia-Sastre (Icahn School of Medicine at Mount Sinai, New York, NY USA). GFP-expressing Newcastle disease virus (NDV), based on clone Hitchner B1 [67], was a kind gift from Christopher Basler. DENV-2 and ZIKV were routinely grown on C6/36 cells at 33°C, and SINV and VSV were routinely grown on BHK cells at 37°C, in cell culture medium supplemented with 2% (v/v) FBS. Briefly, for ZIKV, SINV and VSV, confluent cell monolayers were infected at multiplicity of infection (MOI) 0.05 one day post-seeding. Culture supernatant was harvested seven (ZIKV) or two (SINV, VSV) days post-infection and clarified by centrifugation before storage, titration and use in experiments. For DENV-2, C6/36 cells were seeded at 1 x 106 cells per 75 cm2 culture flask and infected one day later at MOI 0.5; virus was harvested seven days post-infection as described above.
For one-step growth curves, Aag2 cells were seeded at 5 x 105 cells/well in 12-well plates (DENV-2), 3 x 105 cells/well in 24-well plates (SINV, VSV) or 1 x 105 cells/well in 96-well plates (ZIKV) and infected the next day by replacing culture medium with inoculum in phosphate-buffered saline (PBS). Inoculum was removed after 1 h and cells were washed once in PBS before adding fresh culture medium. To compare virus replication in Aag2-derived clonal cell lines, cells were seeded at 1 x 105 cells/well in 96-well plates and infections performed as above. All viruses were titrated on confluent BHK cells one day post-seeding in culture medium containing 2% (v/v) FBS and 0.5% (w/v) methyl cellulose (Sigma Aldrich). For DENV-2 titrations, cells were moved to 33°C during and following infection. Titrations were fixed six (DENV-2) or three (ZIKV, SINV, VSV) days post-infection in 1% crystal violet solution in 20% ethanol following removal of the methyl cellulose overlay.
Bacteria
Escherichia coli DH5α (ThermoFisher Scientific) were cultured overnight at 37°C with shaking in Luria Bertani (LB) medium (Sigma Aldrich) without antibiotics, and titrated on LB agar in 6-well plates overnight at 37°C. Listeria monocytogenes cultures were a kind gift from Adolfo Garcia-Sastre and Staphylococcus aureus cultures were a kind gift from Flora Samaroo (Icahn School of Medicine at Mount Sinai); both were cultured and titrated in brain heart infusion broth/agar (Sigma Aldrich) as for E. coli. Bacteria were pelleted, washed once in PBS and resuspended in PBS before heat-inactivation at 60°C for 3 h (E. coli, L. monocytogenes) or at 75°C for 6 h (S. aureus).
Plasmids
The GFP expression vector pIEx-EGFP [68] was a kind gift from Doug Brackney (The Connecticut Agricultural Experiment Station, New Haven, CT USA). The constitutive firefly luciferase expression plasmid pKM19 was generated by amplifying the firefly luciferase gene from pLUC-MCS (Agilent Technologies, Santa Clara, CA USA) and cloning it into pIEx-EGFP after the enhanced GFP (EGFP) sequence was removed by digestion with XhoI and NcoI, using In-Fusion cloning (Takara Biosciences, Mountain View, CA USA). The constitutive Renilla luciferase expression plasmid pKM50 was generated by amplifying the Ae. aegypti ubiquitin UbL40 promoter from pSLfa-UbL40-EGFP [69] (a kind gift from Raul Andino) and cloned by In-Fusion into pRL-TK-Renilla (Promega, Madison, WI USA) after the TK promoter was removed by digestion with BglII and BstBI. pKM19 and pKM50 have been made available via Addgene (addgene.org, Watertown, MA USA) with reference numbers 123655 and 123656 respectively.
Single cell sorting of Aag2 cells
Prior to cell sorting, Aag2 cells were grown in Leibovitz’s L-15 medium with supplements as described above, including 20% (v/v) FBS. Adherent cells were trypsinised, pelleted by centrifugation, washed once in PBS, pelleted again by centrifugation and resuspended at 3–5 x 106 cells/ml in sterile PBS containing 1.25 μg/ml 4',6-diamidino-2-phenylindole dihydrochloride (DAPI; ThermoFisher Scientific) and stored on ice until required. Immediately prior to sorting, cells were passed through a 35 μm filter. Single cells were sorted on a FACSAria II (BD Biosciences, San Jose, CA USA) using a 100 μm nozzle and a sheath pressure of 35 psi into individual wells of a 96-well plate each containing 200 μl Leibovitz’s L-15 medium with supplements as above including 20% (v/v) FBS. Cells were gated to select DAPIlow (live) single cell clones (Fig 1B). Fast-growing clonal cell lines confluent after three weeks of growth were expanded and confirmed to be mycoplasma-negative using the Myco-Alert PLUS kit (Lonza, Basel, Switzerland) prior to freezing for long-term storage in liquid nitrogen. Clone Aag2-AF5 has been made available via ECACC (phe-culturecollections.org.uk; Public Health England, London, UK).
Microscopy
Images were captured using an EVOS XL Core (Fig 1) or EVOS FL (Fig 5) Cell Imaging System (ThermoFisher Scientific). To measure transfection efficiency, images were captured 48 h after transient transfection with 300 ng pIEx-EGFP per well of a 12-well plate, each containing 1 x 106 cells, at the time of cell seeding using TransIT-insect transfection reagent (Cambridge Biosciences, Cambridge, UK) as per manufacturer’s instructions. Transfection efficiency was calculated manually using Fiji (ImageJ) software (National Institutes of Health, Bethesda, MA USA) [70]. At least 900 individual cells were counted across three separate fields of view at 40X magnification for each experiment.
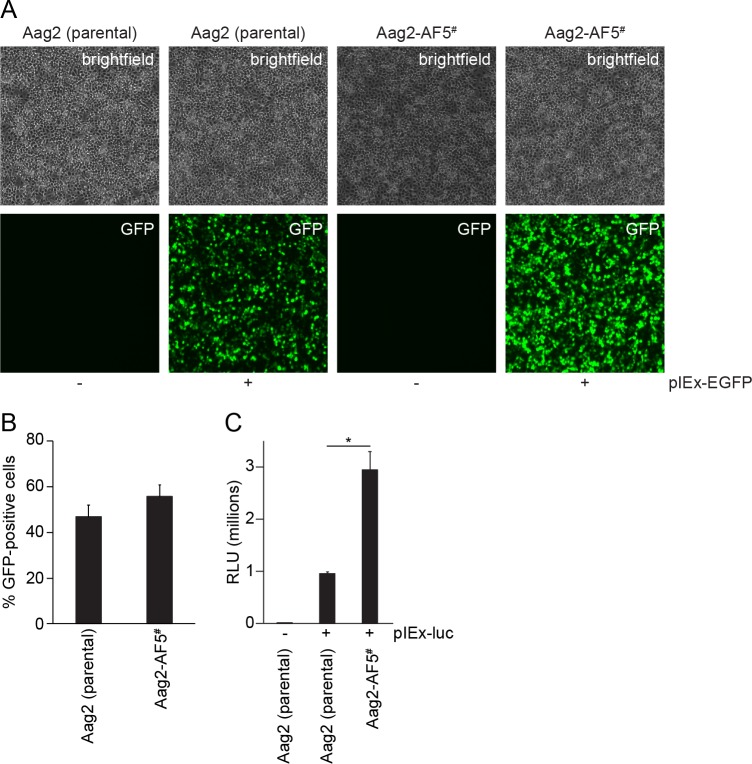
Fig 5. Transfection efficiency of clone Aag2-AF5 relative to parental Aag2 cells.
(A) Cells were imaged at 10X magnification 48 h after transient transfection with a constitutively active GFP expression vector (pIEx-EGFP). (B) Quantification of transfection efficiency; differences are non-significant. (C) Cells were transiently transfected with a constitutively active firefly luciferase reporter plasmid (pIEx-luc) and luciferase activity was measured four days later. * P < 0.05 (two-tailed Student’s t test). RLU, relative light units. All error bars represent standard deviation. # Aag2-AF5 cell line used for CRISPR gene editing [60,61].
PCR and RT-PCR
To analyse the genomic integration of PCLV, total DNA or total RNA was extracted from 1–3 x 106 cells using the Quick-DNA or Quick-RNA Miniprep Kits (Zymo Research, Irvine, CA USA) respectively, as per manufacturer’s instructions. RNA samples were spiked with NDV prior to isolation. Nucleic acids were treated with DNase for 40 min at 37°C using the DNA-free DNA Removal Kit (ThermoFisher Scientific) or with RNase A (Sigma-Aldrich) for 1 h at 37°C as per manufacturers’ instructions. Nucleases were removed by re-purifying the nucleic acids as described above. cDNA was generated from RNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA USA) with random hexamers as per manufacturer’s instructions. PCR amplification was performed using the HOT FIREPol EvaGreen qPCR Supermix Plus (no ROX) (Solis BioDyne, Tartu, Estonia) at 95°C for 10 min followed by 26 cycles of 95°C for 15 s and 60°C for 30 s, as per manufacturer’s instructions. Primers (Sigma-Aldrich) were as follows; Rps7, prKM27F CCACGATCCCGCACTCTGA, prKM27R TACGCTTGCCGACGACTTCA; NDV, forward GACAATGCTTGATGGTGAAC, reverse CAATGCTGAGAGACAATAGGTC; PCLV L, prKM110F CACTGCTACACCGCCTAGAG, prKM110R TGACCTGTTGGCCTGTTGTT, prKM111F GCACCTTTAACAGGAGATGCAA, prKM111R ACTACGCCACAATGCGATGA, prKM112F GACTCCCCGATTGAGTAAAGAAC, prKM112R TCCAAGGAATCACTTTCTGATGC, prKM113F GTCGATTTCGAAGAAGTAGGTGC, prKM113R TCTATCGGTGATGTGCGTTCC, prKM231F AGGAGGCACAAATCAAGGTAGT, prKM231R GCGAGCTCACTTTGATGAATGG, prKM232F AGCCAGAGAAAGCAAACCAGA, prKM232R TCCATGTCATCAGTGTTGGTGT; PCLV M, prKM233F AGGCATGAAGACCTGGACTC, prKM233R GCATGCATCTGCTCTATGGG, prKM234F TTGCAGAGGAAGATCTCTGAGG, prKM234R TTCGCTTATCAGCCTGCAGTT, prKM235F GCCTGTCCCATCTGCGAAT, prKM235R AACCTGTGACTCGTGTGCAA, prKM236F AGCTGTTCTGGTAATGTTGTGGA, prKM236R TCTTCCAAGCAGGTTGGTTTG; PCLV S, prKM237F AGCAATAGATACGACTGCTAGTGA, prKM237R GCATTCATCTCCATACGCACA, prKM238F GCGTCATTCGTTTCGAGCAT, prKM238R TCAGCAGACGGAAATCGTTGT.
To test Aag2-derived clonal cell lines for the presence of insect-specific viruses, RNA was extracted from 1–3 x 106 cells using the Quick-RNA Miniprep Kit (Zymo Research) as per manufacturer’s instructions. cDNA was synthesised using the Maxima H Minus First Strand cDNA Synthesis Kit (ThermoFisher Scientific) with random hexamers or gene-specific primers. PCR amplification was performed using the AccuPrime Taq High Fidelity DNA Polymerase (ThermoFisher Scientific) at 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s and 68°C for 1 min, followed by a final extension at 68°C for 5 min, as per manufacturer’s instructions. Primers were as follows; Rps7, prKM259F TGCTTTCGAGGGACAAATCGG, prKM259R AATTCGAACGTAACGTCACGTCC; CFAV, prKM258F TCATCTTATGTTGCACATGGACGC, prKM258R CACCCTCCGGAAATCCGATTG; PCLV L, prKM254F CATCAATTGATGAAGCCAGAGAAAG, prKM254R GTCTTTATGTTTTCTGTACAGCCATAAT; PCLV M, prKM256F AATGCAAACTGTTCTTGCAGATTCTG, prKM256R GTAGCTTAAAATCTGCGTCGTTAGT; PCLV S, prKM257F ATTATAAATATTCAAACACCCCAGTTATAAG, prKM257R TTCTGATCATTTAACATTCTCAGAGCTA.
RT-qPCR
To measure PCLV and CFAV RNA levels, RNA was extracted from 1 x 106 cells using 1 ml TRIzol reagent (ThermoFisher Scientific) and treated with DNase for 40 min at 37°C using the DNA-free DNA Removal Kit, as per manufacturers’ instructions. To measure immune gene induction, cells were seeded at 1 x 105 cells/well in 96-well plates and stimulated one day later by replacing the culture medium with culture medium containing 1,000 colony-forming units (CFU)/cell heat-inactivated bacteria. RNA was isolated 24 h later using the Quick-RNA Miniprep Kit (Zymo Research) as per manufacturer’s instructions. RNA was reverse transcribed using the iScript cDNA Synthesis Kit with random hexamers as per manufacturer’s instructions. PCR amplification was performed using the HOT FIREPol EvaGreen qPCR Supermix Plus (no ROX) at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 30 s, as per manufacturer’s instructions. Primers were as follows; PCLV L, prKM110F/R (see above); CFAV, prKM34F CGGGTTCTATGGCTTTGGGT, prKM34R ACCACCTGCTACTGTTGAGC; Rps7 (Genbank accession number XM_001660119), prKM27F CCACGATCCCGCACTCTGA, prKM27R TACGCTTGCCGACGACTTCA; Defensin D (XM_001657239), prKM14F TGCACCGGGGCCATTAC, prKM14R CAGGTGGCCCGTTTCAGG; Cecropin B (XM_001648640), prKM17F GAAGCTGGTCGGCTGAAGAA, prKM17R CAACGGGTAGTCCCTTCTGG; Cecropin D (XM_001649131), prKM16F AGCTGTTCGCAATTGTGCTGT, prKM16R TACAACAACCGGGAGAGCCTT. PCLV L segment RNA levels were measured by RT-qPCR in clones Aag2-AF10 and Aag2-AF12 in at least four independent experiments (N = 4, n = 3; Fig 2D), plus at four separate passages for three independent cultures of each cell line (N = 3, n = 1; Fig 2Ei).
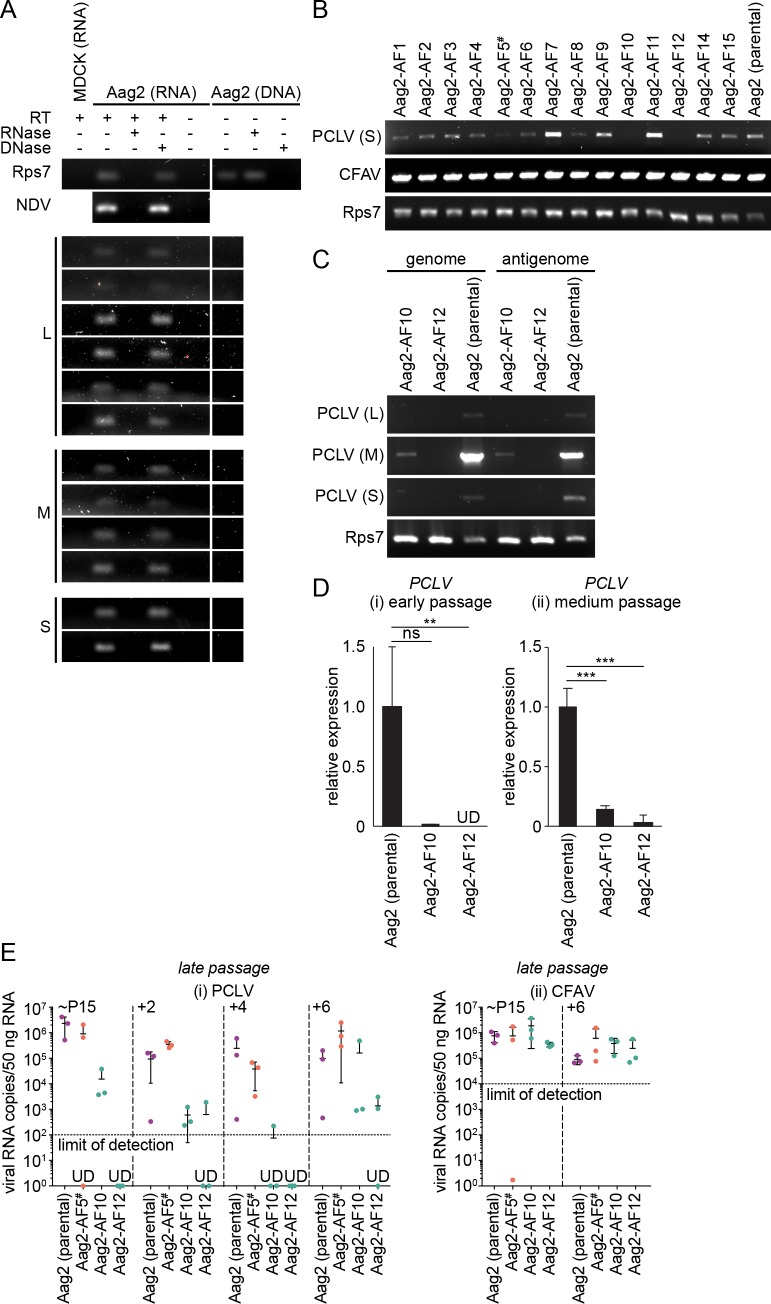
Fig 2. Aag2-AF10 and Aag2-AF12 cell lines harbour barely detectable levels of phasi charoen-like virus.
(A) Short PCR amplicons spanning the three PCLV genome segments (L, M, S) amplified from RNA or genomic DNA isolated from parental Aag2 cells, either with or without a reverse transcription step (RT). Purified nucleic acids were treated with RNase or DNase prior to PCR. MDCK cell RNA, the cellular Rps7 gene/mRNA and the RNA virus NDV, which was spiked into cells immediately prior to RNA extraction, serve as controls. (B) Detection of the PCLV S segment and CFAV by RT-PCR in Aag2-derived clonal cell lines. Cellular Rps7 mRNA serves as a loading control. (C) Detection of the PCLV L, M and S genome (-ssRNA) and antigenome (+ssRNA) segments in select Aag2-derived clonal cell lines by sense-specific RT-PCR. Rps7 mRNA serves as a loading control. (D) PCLV L segment RT-qPCR ΔΔCt (normalised to Rps7 mRNA) for select Aag2-derived clonal cell lines expressed relative to parental Aag2 cell line at (i) early passages (Aag2-AF10, passage 2; Aag2-AF12, passage 3) and (ii) later (‘medium’) passages (Aag2-AF10, passage 8; Aag2-AF12, passage 12). (E) RT-qPCR quantification of viral RNA copies for PCLV L segment (i) and CFAV (ii) at late passages in Aag2 (parental), Aag2-AF5, Aag2-AF10 and Aag2-AF10 cells. Starting passages (“~P15”) were passage 15 (parental Aag2), 12 (Aag2-AF5), 14 (Aag2-AF10) and 13 (Aag2-AF12). Data points represent three independently passaged lines. Error bars represent standard deviation. ** P < 0.01; *** P < 0.001; ns, not significant (one-tailed Student’s t test). UD, undetected. # Aag2-AF5 cell line used for CRISPR gene editing [60,61].
For absolute quantification of PCLV and CFAV viral RNA, RNA standards for RT-qPCR were prepared. Amplicons of ~1,000 bp were amplified from RNA extracted from parental Aag2 cells as described above; annealing temperatures were 59°C (PCLV) and 55°C (CFAV). Primers were as follows; PCLV L segment, prKM133F CCGCTTGAATTCCCACAGCC, prKM133R TAATACGACTCACTATAGGGAGGCAAGCATGGCAATGAGAAG; CFAV, prKM40F TCGATTGCTAATACGACTCACTATAGGGTGAAGCTCATTGCTAACAAC, prKM40R CGCTTATTGCATAATTCGAG. The forward (CFAV) or reverse (PCLV) primer contains a T7 promoter sequence used to generate ssRNA positive-sense (CFAV) or negative-sense (PCLV) RNA from the gel purified cDNA template by in vitro transcription using the MEGAscript T7 High Yield Transcription Kit (ThermoFisher Scientific). Transcripts were DNase treated (see above) and gel purified before quantifying the RNA concentration on a Nanodrop spectrophotometer (ThermoFisher Scientific).
RNAi assay
Cells were seeded at 1 x 105 cells/well in 96-well plates and concurrently transiently transfected with 10 ng/well pKM50 (Renilla), 50 ng/well pKM19 (firefly luciferase) and 1 nM dsRNA directed against EGFP or firefly luciferase using TransIT-insect transfection reagent as per manufacturer’s instructions. Cells were harvested and luciferase activity measured two days post-transfection. Transfections for measuring transfection efficiency by firefly luciferase expression were set up in the same way, without the addition of dsRNA.
dsRNAs were generated by PCR amplification from plasmid templates pIEx-EGFP (EGFP dsRNA; primers prKM57F TAATACGACTCACTATAGGGCGTAAACGGCCACAAGTTCA, prKM57R TAATACGACTCACTATAGGGGGCGGACTTGAAGAAGTCGT) or pKM19 (firefly luciferase dsRNA; primers prKM168F TAATACGACTCACTATAGGGCAATCCGGAAGCGACCAACG, prKM168R TAATACGACTCACTATAGGGTTCCGCCCTTCTTGGCCTTT). Primers contain a 5’ T7 polymerase promoter used for in vitro transcription from gel purified amplicons using the MEGAshortscript in vitro transcription kit (ThermoFisher Scientific). dsRNA was gel purified prior to use.
Bioinformatic analysis of PCLV insertions in Aag2 genome
Our previously published full-length PCLV genome sequences from Aag2 cells (Genbank accession numbers KU936055, KU936056 and KU936057) [59] were searched against the Aag2 cell reference genome [71] using the BLAST function at vectorbase.org [72].
Statistics
Heteroscedastic Student’s t test (assuming unequal variance) was performed in Microsoft Excel (Microsoft Corporation, Redmund, WA USA).
Images
Graphs were plotted in Microsoft Excel. FACS images were generated in FlowJo (FlowJo LLC, Ashland, OR USA). Figures were prepared in Adobe Illustrator (Adobe Systems, San Jose, CA USA). Images were cropped, annotated and modified to optimise brightness and contrast only.
Results
Establishment of clonal cell lines derived from Aag2 cells
The Ae. aegypti Aag2 cell line forms a discontinuous monolayer of cells interspersed with three-dimensional cell clusters attached to the substrate and large rounded cells floating in isolation through the culture medium (Fig 1A). It is in principle possible that these morphological differences reflect underlying functional differences [55,56]. We therefore derived clonal cell lines from Aag2 cells to provide a more homogeneous and better-defined experimental background, for example for the generation of our previously reported CRISPR-edited cell lines [60,61]. Parental Aag2 cells were individually sorted into three 96-well plates using flow cytometry, with a stringent double gating scheme for single cells (Fig 1B). Of the 288 single cells plated, 90 clones expanded into multi-cell cultures in 24-well plates within three weeks. Fifteen of these clones were selected for further study and assigned reference numbers preceded by the prefix ‘AF’ (Aag2-AF1, Aag2-AF2 etc.). Clone Aag2-AF13 succumbed to fungal infection and is not discussed further. The set of clones was selected to be representative of the different morphologies observed across the population of clonal cell lines generated. Some clones resembled the parental Aag2 cell line (Fig 1Ci) while others did not form monolayers and instead either grew in large clusters attached to the substrate (Fig 1Cii) or grew floating individually and in aggregates in the culture medium (Fig 1Ciii). These clustered and floating morphologies are also observed in parental Aag2 cells (Fig 1A). Across all single-cell clones generated, these three morphologies (‘parental Aag2-like’, ‘clustered’, ‘rounded’) were represented at similar levels, with slightly more ‘clustered’ cell lines observed (Fig 1D). However, all of the clonal cell lines reverted back to the parental Aag2 morphology over time (Fig 1E), with this parental Aag2 morphology being stably maintained over many passages. This suggests that the different cell morphologies observed in the parental Aag2 cell line are not indicative of the presence of different cell types within the heterogeneous Aag2 cell culture.
We observed no gross differences in the growth kinetics of any of the Aag2-derived single-cell clones compared to the parental Aag2 cell line during routine culture. Before proceeding, we also confirmed that all clonal cell lines tested negative for mycoplasma. Note that we have used clone Aag2-AF5 as a well-defined single cell-derived starting point for the generation of Aag2 mutants using CRISPR [60,61].
Parental Aag2 cells do not contain PCLV-Derived DNA sequences integrated into their genome
In mosquitoes and their derived cell lines, fragments of RNA virus genomes, including insect-specific virus genomes, can be reverse transcribed into DNA by endogenous reverse transcriptases [73,74], following which these fragments can become integrated into the cellular genome [75–78]. DNA sequences derived from members of the Flaviviridae, Rhabdoviridae and other viral families are known to be integrated within the Aag2 genome [56,76]. We were ultimately interested in studying the potential impact of persistent PCLV infection on acute superinfection with arboviruses in our Aag2-derived clonal cell lines, and therefore first investigated whether sequences derived from PCLV specifically are also integrated into the parental Aag2 cell genome. We performed a BLASTn search against the Aag2 reference genome [71] using our previously published full-length genome sequences for the PCLV known to infect the parental Aag2 cell line [59]. We did not identify any statistically significant (E-value <10−5) PCLV-derived sequences from any of the three viral genome segments (L, M, S) in the Aag2 reference genome sequence.
To rule out the possibility that fragments derived from persistent PCLV infection are integrated into the specific version of the parental Aag2 cell line growing in our lab, we designed primers to amplify short (50-150-nt) fragments covering each genome segment in 1,000-nt intervals. As a positive control we used RNA purified from parental Aag2 cells that had been subjected to a reverse transcription reaction (Fig 2A). To control for template that was only present in RNA form, we spiked the samples with the RNA virus Newcastle disease virus (NDV). To confirm that the RNA samples were not contaminated with residual DNA, we treated RNA with RNase or DNase prior to performing RT-PCR. Here, the mosquito genomic ribosomal subunit 7 (Rps7) served as a control, as this sequence is present in both RNA and DNA forms within the cell. For each PCLV-specific primer pair, and the NDV and Rps7 controls, RNase treatment eliminated the PCR signal, while DNase treatment did not (Fig 2A). This confirms the purity of the RNA samples. No PCLV signal was detected when PCR was performed on RNA that had not been subjected to reverse transcription, or on RNA isolated from the mammalian Madin-Darby canine kidney (MDCK) cell line, which should not contain genomic integrations of insect-specific virus sequences (Fig 2A). We then repeated the experiment using genomic DNA isolated from parental Aag2 cells, and detected no evidence of DNA sequences derived from PCLV (Fig 2A). Importantly, Rps7 was amplified when genomic DNA was treated with RNase, but not DNase, confirming the purity of the DNA samples (Fig 2A). Although we cannot exclude the possibility that the primers we designed missed smaller fragments of integrated PCLV sequence, our data suggest that PCLV fragments are not integrated into the genome of parental Aag2 cells, which is in agreement with data from other research groups [58].
Identification of Aag2-derived clonal cell lines harbouring consistently low levels of persistent insect-specific bunyavirus infection
We next tested whether all of our Aag2-derived clonal cell lines still contained both of the insect-specific viruses known to persistently infect parental Aag2 cells. We detected CFAV RNA by RT-PCR in parental Aag2 cells and in all of our clonal cell lines, with cellular Rps7 RNA serving as a template control (Fig 2B). In contrast, clones Aag2-AF10 and Aag2-AF12 did not contain detectable levels of the PCLV S segment in this assay, while PCLV RNA was clearly detectable to varying degrees in parental Aag2 cells and in the other clones (Fig 2B). To verify this result, we performed strand-specific RT-PCR to amplify genome and antigenome sequences from each of the three PCLV genome segments, with Rps7 serving as a template control. In this experiment we did detect PCLV M segment RNA in the Aag2-AF10 clone (Fig 2C). Although any amplification of the L and S segments were below the limit of detection, the presence of both genome and antigenome sequences for the M segment indicates that the virus must be replicating its RNA and therefore the L (RdRp) and S (nucleocapsid) segments, which are both required for genome replication, must also be present. No PCLV RNA was detected in the Aag2-AF12 clone in this assay.
Finally, we measured PCLV L segment RNA by RT-qPCR and detected low levels of PCLV RNA in the Aag2-AF10 clone, with PCLV RNA consistently maintained at lower levels relative to the parental Aag2 cell line over multiple cell passages (Fig 2D and 2E). We did not detect PCLV RNA at early passages in the Aag2-AF12 clone, though very low levels of PCLV were detected at later passages (Fig 2D and 2E). Reduced levels of PCLV RNA were maintained in clones Aag2-AF10 and Aag2-AF12 over multiple independent passages (Fig 2Ei). PCLV RNA levels in clones Aag2-AF10 and Aag2-AF12 were generally maintained at levels at least 2–3 logs lower than in the parental cell line, with PCLV RNA mostly undetectable in clone Aag2-AF12. In contrast, the levels of CFAV RNA were comparable in clones Aag2-AF5, Aag2-AF10, Aag2-AF12 and the parental cell line (Fig 2Eii). Overall, our data indicate that clones Aag2-AF10 and Aag2-AF12 harbour markedly reduced levels of PCLV infection that are maintained at consistently low levels over multiple cell passages.
All Aag2-Derived clonal cell lines have a functional RNAi pathway
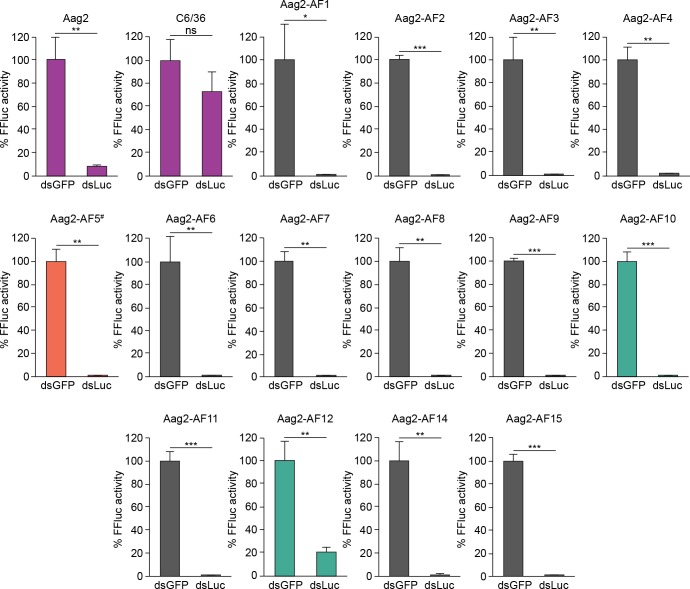
Derived cell lines can have markedly different characteristics compared to their parental cell lines, and several mosquito cell lines in particular are known to be immunodeficient compared to the cell lines they were derived from [45,55,56]. Before proceeding, we therefore tested our Aag2-derived clonal cell lines for RNAi functionality, since this immune pathway is defective in several mosquito cell lines, such as C6/36 and C7/10 [45,55,56]. In parental Aag2 cells, transient co-transfection of a constitutively active firefly luciferase reporter plasmid with a dsRNA directed against firefly luciferase significantly reduced luciferase expression compared to a non-specific dsRNA directed against GFP, confirming that RNAi is active in the parental Aag2 cell line (Fig 3). In contrast, the luciferase dsRNA did not significantly reduce reporter activity in RNAi-defective C6/36 cells (Fig 3). The luciferase-specific dsRNA significantly reduced firefly luciferase activity in all of the Aag2-derived clonal cell lines, confirming that all of the clones have a functional RNAi pathway (Fig 3). Furthermore, the clonal cell line Aag2-AF5 was previously shown to have a functional RNAi pathway as measured by the production of 21-nt siRNAs during viral infection [60,61].
Fig 3. All Aag2-derived clonal cell lines have a functional RNAi pathway.
C6/36 cells, the parental Aag2 cell line and its derived clonal cell lines were transiently transfected with plasmids constitutively expressing firefly luciferase and Renilla luciferase (transfection control) in the presence of dsRNA directed against GFP (dsGFP) or firefly luciferase (dsLuc). Mean Renilla-normalised firefly luciferase (FFluc) expression is expressed relative to the dsGFP negative control. * P < 0.05; ** P < 0.01; *** P < 0.001; ns, not significant (one-tailed Student’s t test). Error bars represent standard deviation. # Aag2-AF5 cell line used for CRISPR gene editing [60,61] is highlighted in orange. PCLV-low clones Aag2-AF10 and Aag2-AF12 are highlighted in green; parental Aag2 cells and C6/36 cells (negative control) are shown in purple.
Pre-existing persistent infection With PCLV Does Not modulate acute superinfection with flaviviruses
The isolation of the clonal cell lines Aag2-AF10 and Aag2-AF12 provided an opportunity to test whether a pre-existing persistent infection with an insect-specific virus (in this case PCLV) modulates the replication of arboviruses in cell culture by comparing these PCLV-low clones to clones harbouring higher levels of PCLV. We started by testing the flavivirus DENV serotype 2 (DENV-2), which replicated with a peak in titres six days post-infection in a one-step growth curve at high multiplicity of infection (MOI 2) in the parental Aag2 cell line (Fig 4A). DENV-2 replicated with similar kinetics (1, 2, 3 days post-infection) and to a similar level three days post-infection in clones Aag2-AF10 and Aag2-AF12 relative to the parental Aag2 cell line at MOI 2 (Fig 4B). This indicates that the markedly suppressed PCLV infection in clones Aag2-AF10 and Aag2-AF12 had very little impact on DENV-2 replication. Although DENV-2 exhibited somewhat different growth kinetics across the Aag2-derived clonal cell lines, titres three days post-infection did not deviate from the parental Aag2 cell line by more than one log, and were close to within half a log of the parental Aag2 cell line for all clones (Fig 4B).
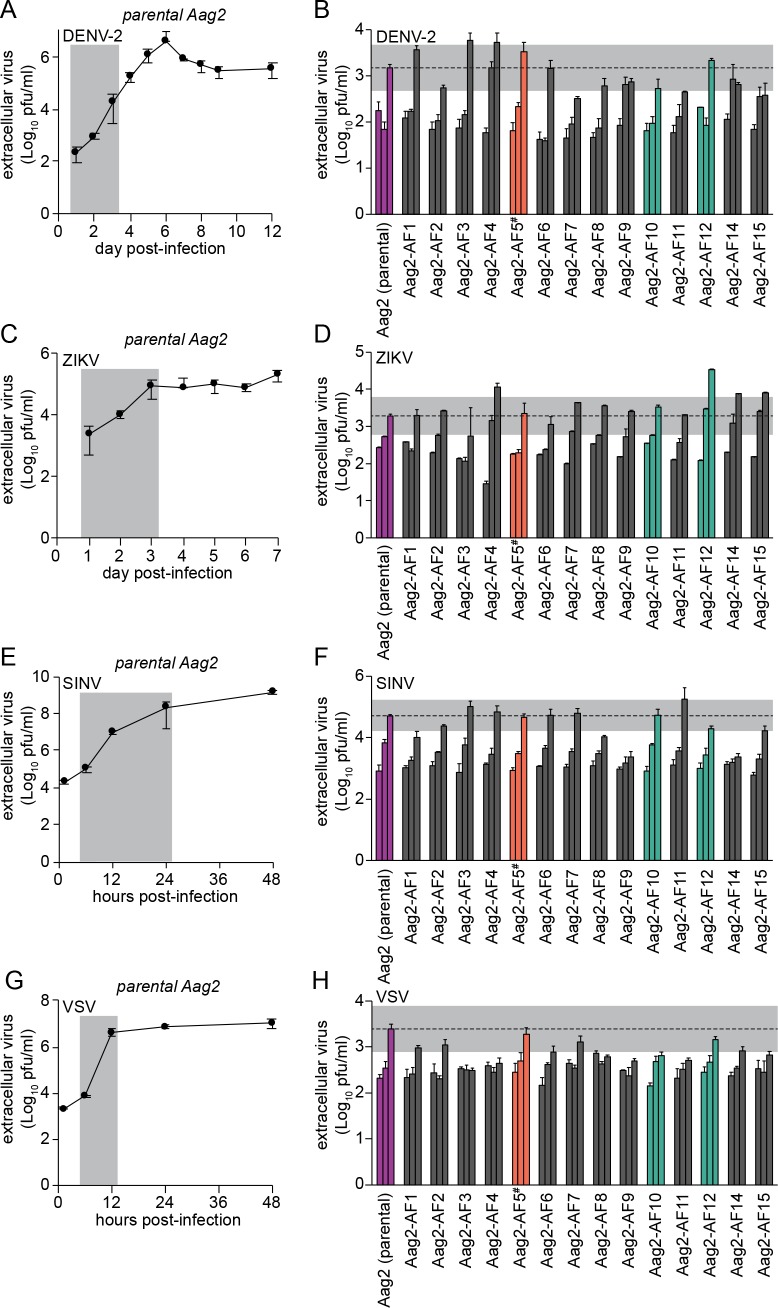
Fig 4. Susceptibility of Aag2-derived clonal cell lines to infection with arboviruses.
(A, C, E, G) Single-step growth kinetics of DENV-2 (A), ZIKV (C), SINV (E) and VSV (G) in the parental Aag2 cell line (MOI 2). Grey shading highlights time points tested in Aag2-derived clonal cell lines. (B, D, F, H) Replication of DENV-2 (B) and ZIKV (D) at 1, 2 and 3 days post-infection, replication of SINV (F) at 6, 12 and 24 hours post-infection (hpi), and replication of VSV (H) at 6, 9 and 12 hpi in the parental Aag2 cell line and its derived clonal cell lines (all MOI 2). Grey shading indicates 0.5 Log10 above and 0.5 Log10 below peak extracellular titres detected in parental Aag2 cell line. Error bars represent standard deviation. # Aag2-AF5 cell line used for CRISPR gene editing [60,61] is highlighted in orange. PCLV-low clones Aag2-AF10 and Aag2-AF12 are highlighted in green; parental Aag2 cells and C6/36 cells (negative control) are shown in purple.
We next tested the flavivirus ZIKV, which replicated with a peak in titres three days post-infection in the parental Aag2 cell line at MOI 2 (Fig 4C). While ZIKV replicated with faster kinetics (1, 2, 3 days post-infection) and to a more than one-log higher titre at its peak in clone Aag2-AF12, the growth kinetics and peak titres were similar to the parental Aag2 cell line in clone Aag2-AF10 (Fig 4D). Therefore, although clone Aag2-AF12 appears to be more permissive to ZIKV replication, this is not linked to PCLV levels, which are also reduced in clone Aag2-AF10. Again, ZIKV replication kinetics varied somewhat across the other clones, but fell close to within half a log from the parental Aag2 cell line at their peak.
Replication kinetics of both DENV-2 and ZIKV in clone Aag2-AF5 were comparable to the parental Aag2 cell line (Fig 4B and 4D).
Pre-existing persistent infection with PCLV does not modulate acute superinfection with the alphavirus sindbis virus
Alphaviruses, like flaviviruses, are +ssRNA viruses, and we next tested replication of the model alphavirus SINV in our Aag2-derived clonal cell lines. SINV replication peaked 48 hpi in parental Aag2 cells infected at MOI 2 (Fig 4E). SINV replication kinetics (6, 12, 24 hpi) and peak viral titres were similar in clones Aag2-AF10 and Aag2-AF12 compared to parental Aag2 cells (Fig 4F), indicating that persistent PCLV infection does not markedly alter SINV replication. Again, variability in the replication kinetics and peak titres of SINV were observed across the other Aag2-derived clonal cell lines. SINV replication kinetics and peak titres in clone Aag2-AF5 were comparable to the parental Aag2 cell line.
Pre-existing persistent infection with PCLV does not modulate acute superinfection with the rhabdovirus vesicular stomatitis virus
As a contrast to the +ssRNA arboviruses tested, we next tested the -ssRNA rhabdovirus VSV. In a one-step growth curve (MOI 2), VSV replication peaked 12 hpi in parental Aag2 cells (Fig 4G). As for the other viruses tested, peak titres of VSV in clones Aag2-AF10 and Aag2-AF12 were within one log compared to the parental Aag2 cell line at 12 hpi, with some variability in replication kinetics (6, 9, 12 hpi) observed across all Aag2-derived single-cell clones (Fig 4H). VSV replicated similarly in clone Aag2-AF5 and the parental Aag2 cell line.
Overall, we therefore conclude that pre-existing persistent infection with PCLV does not notably alter the replication of a diverse range of +ssRNA and -ssRNA arboviruses, since replication kinetics and peak titres of DENV-2, ZIKV, SINV and VSV were not markedly different from the parental Aag2 cell line in clones Aag2-AF10 and Aag2-AF12, which harbour drastically reduced levels of persistent PCLV infection.
Transfection efficiency of clone Aag2-AF5
Next, we further characterised clone Aag2-AF5 with the goal of providing a better-defined Aag2-derived cell line for the research community. Clone Aag2-AF5 was selected because, of all the isolated clones, arboviral infectivity in Aag2-AF5 cells was most similar to the parental Aag2 cells (Fig 4). Furthermore, this clone formed a more uniform monolayer and was more resilient and easier to handle in culture than parental Aag2 cells.
First, we tested the transfection efficiency of this clone by transient transfection with a constitutive GFP expression plasmid (Fig 5A). A similar proportion of Aag2-AF5 cells (56%) were detectably GFP-positive compared to the parental Aag2 cell line (47%) (Fig 5B); differences were not statistically significant. However, the GFP signal was brighter in Aag2-AF5 cells (Fig 5A). This higher level of transgene expression in Aag2-AF5 cells was confirmed by transient transfection with a constitutively active firefly luciferase reporter plasmid (Fig 5C). Therefore, while the overall proportion of cells transiently expressing a transgene is comparable for clone Aag2-AF5 and the parental Aag2 cell line, individual Aag2-AF5 cells express transgenes to higher levels, making this clone well-suited for molecular experiments including gene editing using CRISPR [60,61].
Antimicrobial peptide induction in clone Aag2-AF5
Clone Aag2-AF5 was already confirmed to have an active antiviral RNAi pathway (Fig 3) [60,61], and we next tested whether this clone was also competent for antimicrobial peptide induction via inducible innate immune signalling pathways. When stimulated with heat-inactivated Gram-negative (Escherichia coli) or Gram-positive (Listeria monocytogenes or Staphylococcus aureus) bacteria, which are well-defined stimuli of inducible innate immune signalling pathways [53], upregulation of the antimicrobial peptides defensin D (DefD), cecropin B (CecB) and cecropin D (CecD) was detected in both parental Aag2 cells and clone Aag2-AF5 with all stimuli (Fig 6). There was however some variability in the relative levels of antimicrobial peptide induction in clone Aag2-AF5 compared to the parental cell line for different gene/stimulus combinations. Thus, all tested antimicrobial peptide genes were less inducible in clone Aag2-AF5 with E. coli stimulation (Fig 6A), and CecD was also less inducible in clone Aag2-AF5 for all stimuli tested (Fig 6Aiii, 6Biii and 6Ciii), though some of these differences were not significant. In contrast, DefD and CecB were more inducible in clone Aag2-AF5 during stimulation with Gram-positive bacteria compared to the parental Aag2 cell line (Fig 6Bi, 6Bii, 6Ci and 6Cii), with some of these differences again being non-significant. Therefore, while there may be subtle differences in the immune sensitivity of clone Aag2-AF5 in terms of antimicrobial peptide production compared to parental Aag2 cells, there is no gross defect in inducible innate immune pathways in response to bacterial stimulation.
Fig 6. Antimicrobial peptide induction in clone Aag2-AF5 compared to parental Aag2 cells.
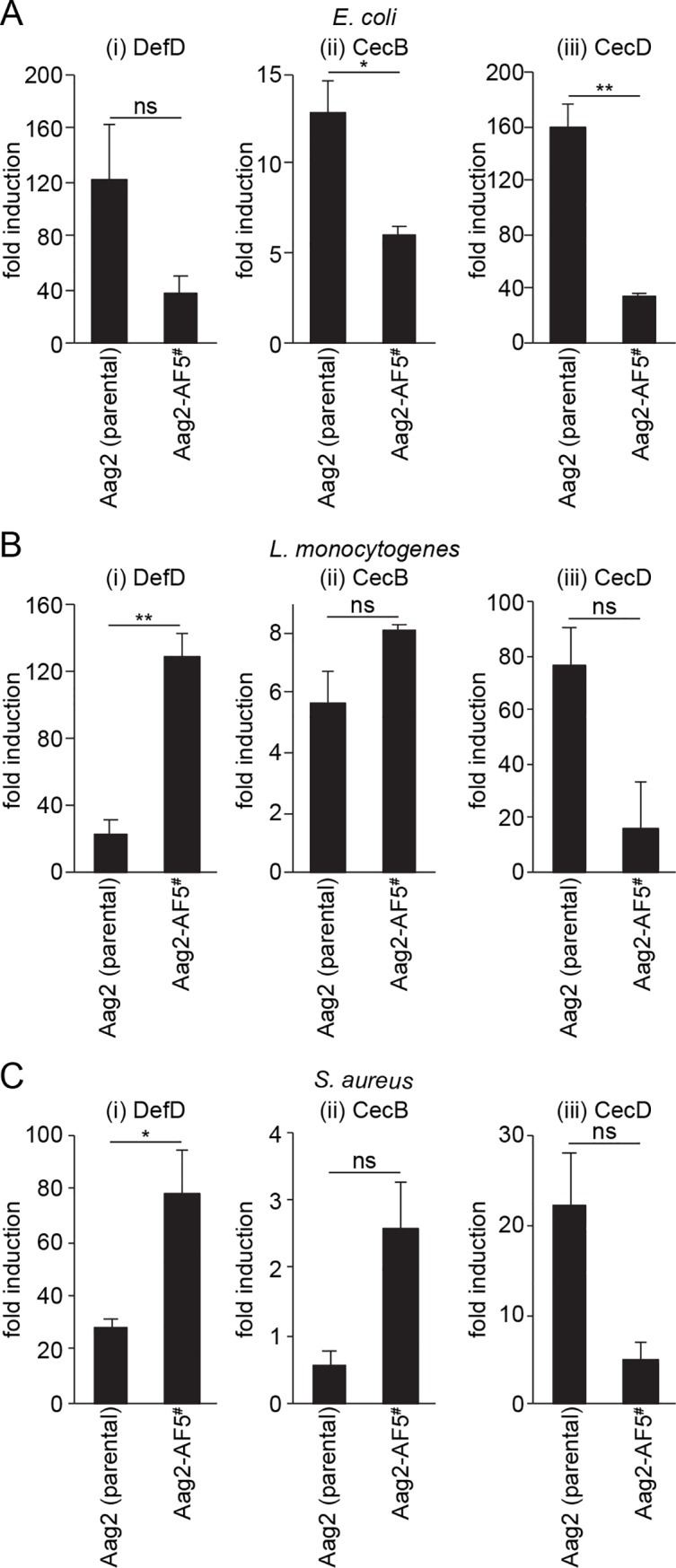
Cells were stimulated with heat-inactivated E. coli (A), L. monocytogenes (B) or S. aureus (C) for 24 h and induction of DefD (i), CecB (ii) or CecD (iii) was measured by RT-qPCR. Gene induction is relative to the respective unstimulated cell line. * P < 0.05; ** P < 0.01; ns, not significant (two-tailed Student’s t test). Error bars represent standard error of the mean. # Aag2-AF5 cell line used for CRISPR gene editing [60,61].
Discussion
In this study, we generated clonal cell lines from the widely used Aag2 cell line. While the different morphologies within the parental Aag2 cell line could have been indicative of the presence of different embryo-derived cell types, this appears not to be the case as all of the clonal cell lines reverted back to the parental Aag2 cell morphology, with cell clusters and floating cells above the monolayer. In our hands, the Aag2 morphology is highly susceptible to culture conditions, and therefore the different cell morphologies may instead reflect cellular responses to growth phase, cell density or nutrient status.
Across all of the clonal Aag2-derived cell lines, there was minor variability in the replication kinetics and peak titres for all of the viruses tested. These effects were not consistent across viruses or cell lines and did not overall correlate consistently with the presence or level of insect-specific viruses in the culture. Neither are these effects likely to be linked to variability in immune responses because all clonal cell lines had a functional RNAi pathway, considered to be the major antiviral immune pathway in insects [51,52]. However, we did not extensively test inducible immune pathways such as Toll, IMD or Jak-STAT signalling in all clones. The clonal cell lines may exhibit variability in the expression of pro- or anti-viral factors, and could therefore be useful for identifying viral restriction factors or host proteins required for viral replication in mosquito cells.
Clone Aag2-AF5 as a defined cell line for the arbovirus research community
There has been a drive to improve data reproducibility, with the standardisation of experimental methods and tools representing one important means of achieving this end [79]. We believe that our Aag2-derived clone Aag2-AF5 represents a useful standardised Ae. aegypti cell line for the arbovirus research community, and have made the cell line available via ECACC (phe-culturecollections.org.uk). The parental Aag2 cell line has previously been shown to be a valuable tool for studying mosquito immune responses to arbovirus infection [51,52], and we confirmed that Aag2-AF5 cells are also competent for RNAi and antimicrobial peptide induction. Furthermore, the viruses tested all replicated with similar kinetics and to similar peak titres in Aag2-AF5 cells compared to the parental Aag2 cell line, and this clone was therefore chosen to allow comparison to experiments performed in parental Aag2 cells. Morphologically, Aag2-AF5 cells are similar to the parental Aag2 cell line, and easier to work with in culture. Both CFAV and PCLV remain present in Aag2-AF5 cells. Aag2-AF5 cells are readily transfected and express exogenous proteins to high levels. We have also previously shown that Aag2-AF5 cells are easily gene edited using CRISPR [60], and Aag2-AF5 cells provide a more homogeneous background for gene editing experiments compared to the parental Aag2 cell line.
We have shared Aag2-AF5 cells widely within the research community, and their availability via ECACC should further increase their utility in standardising cell culture experiments to provide more reproducible data on arbovirus-vector interactions in Ae. aegypti cells.
Impact of persistent insect-specific virus infection on arbovirus replication
Two of the clonal Aag2-derived cell lines (Aag2-AF10 and Aag2-AF12) exhibited markedly reduced levels of persistent PCLV infection. PCLV levels remained consistently low over multiple passages, and in the case of Aag2-AF12 cells were so low as to be only intermittently detectable. In the wild, insect-specific viruses primarily cause persistent infection of mosquitoes, and comparison of the PCLV-low clones Aag2-AF10 and Aag2-AF12 to parental Aag2 cells allowed us to test the impact of persistent insect-specific virus infection on arbovirus replication, in comparison to previous studies that tested the impact of acute insect-specific virus infection on arbovirus replication [31,34–37,41–43]. We observed no consistent impact of PCLV on the replication of representative flaviviruses (DENV-2, ZIKV), alphaviruses (SINV) or rhabdoviruses (VSV), representing both +ssRNA and -ssRNA arboviruses. Göertz et al. recently generated Aag2-derived cell lines that lack CFAV but are still persistently infected with PCLV [80], and these would be an interesting tool to test whether the persistent infection of Aag2 cells with CFAV has a more notable effect on arbovirus replication.
To our knowledge, only one previous study tested the impact of persistent insect-specific virus (AeAV) infection on arbovirus (DENV) replication in immunocompetent Aedes spp. cell cultures [19], with at most a minor reduction in DENV replication in the presence of AeAV. Our data agree with this study in that no major impact of persistent insect-specific virus infection was observed on arbovirus replication, with our study extending this observation to a broader set of arboviral families. A study by Kuwata et al. also found that persistent insect-specific virus (CxFV) infection did not reduce, and in fact increased, arbovirus (DENV, JEV) replication in a Culex tritaeniorhynchus cell line [29]. To our knowledge there are no studies testing the impact of persistent insect-specific virus infection on arbovirus replication in vivo in Aedes spp., however our cell line data are also in agreement with an in vivo study in Culex spp. that found no impact of persistent insect-specific virus (CxFV) infection on arbovirus (West Nile virus, WNV) transmission [32].
The lack of impact of persistent insect-specific virus infection on arbovirus replication in Aedes spp. cell culture is noteworthy because a number of studies have reported reduced replication of arboviruses in the presence of acute insect-specific virus infection in cell lines [31,34–37] and in the presence of acute insect-specific virus infection in vivo [31,35–37,41] in both Aedes spp. and Culex spp. Arboviruses have been shown to be affected differently when entering cells harbouring acute versus persistent insect-specific virus infection [40], which likely expose arb oviruses to markedly different cellular environments. For instance, immune responses likely differ under acute versus persistent infection scenarios, and persistent infection with at least one insect-specific virus (Culex Y virus) has been shown to modulate RNAi responses [81]. Therefore, studies linking acute or persistent insect-specific virus infection to reduced arbovirus replication in the immunocompromised C6/36 cell line [32,33,39,40] may also not fully reflect the effects of persistent insect-specific virus infection in natural settings.
Our findings and those of others [19,32] suggest that the persistent insect-specific virus infections encountered by arboviruses in nature and in mosquitoes hypothetically infected for environmental release may not reduce, and may in fact enhance [29], arbovirus transmission. However, further studies are required to reconcile the contradictory observations made by different research groups, which may be influenced by insect-specific virus infection status (acute versus persistent), as well as the tripartite combination of arbovirus, insect-specific virus and mosquito species. Representative in vivo studies in particular are much needed, since cell culture experiments do not fully recapitulate all facets of the arbovirus infection process in mosquitoes.
We therefore provide new insights that may have important implications for the use of insect-specific viruses as biocontrol agents to reduce the transmission of arboviruses. Furthermore, clone Aag2-AF5 represents a valuable new clonal and better-defined cell line to provide a more standardised system for studying arbovirus-vector interactions in cell culture.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by Wellcome Trust (wellcome.ac.uk) grant 096062 and grant MR/R010315/1 cofunded by the Medical Research Council (MRC; mrc.ukri.org) and the Department for International Development (DFID; https://www.gov.uk/government/organisations/department-for-international-development) to KM, MRC grant G0801973 to AD, Department of Defence (DOD)/Defense Advanced Research Projects Agency (DARPA) (defense.gov/darpa.mil) grant HR0011-11-C-0094 (PROPHECY) and National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID) (niaid.nih.gov) grants R01AI073450, R21AI116022 and U19AI118610 to AF-S, and a University of Surrey (surrey.ac.uk) Doctoral College studentship to LEW. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu Rev Med. 2017;69: 395–408. 10.1146/annurev-med-050715-105122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses. 2015;7: 4911–4928. 10.3390/v7092851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook S, Moureau G, Kitchen A, Gould EA, de Lamballerie X, Holmes EC, et al. Molecular evolution of the insect-specific flaviviruses. J Gen Virol. 2012;93: 223–234. 10.1099/vir.0.036525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuno G, Chang G-JJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18: 608–637. 10.1128/CMR.18.4.608-637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver SC, Barrett ADT. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Micro. 2004;2: 789–801. 10.1038/nrmicro1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer LD. Complexity of virus-vector interactions. Curr Opin Virol. 2016;21: 81–86. 10.1016/j.coviro.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: A review. Infect Genet Evol. 2018;67: 191–209. 10.1016/j.meegid.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beerntsen BT, James AA, Christensen BM. Genetics of Mosquito Vector Competence. Microbiol Mol Biol Rev. 2000;64: 115–137. 10.1128/mmbr.64.1.115-137.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer MC, Liang D, Tatomer DC, Gold B, March ZM, Cherry S, et al. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes Dev. 2015;29: 2168–2182. 10.1101/gad.270421.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476: 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 11.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139: 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 12.Blagrove MSC, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci. 2012;109: 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010;6: e1000833 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson NM. Challenges and opportunities in controlling mosquito-borne infections. Nature. 2018;559: 490–497. 10.1038/s41586-018-0318-5 [DOI] [PubMed] [Google Scholar]
- 15.Öhlund P, Lundén H, Blomström A-L. Insect-specific virus evolution and potential effects on vector competence. Virus Genes. 2019;64: 1–11. 10.1007/s11262-018-01629-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakrzewski M, Rasic G, Darbro J, Krause L, Poo YS, Filipovic I, et al. Mapping the virome in wild-caught Aedes aegypti from Cairns and Bangkok. Sci Rep. 2018;8: 4690 10.1038/s41598-018-22945-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Huang S, Jin T, Lin P, Huang Y, Wu C, et al. Discovery and high prevalence of Phasi Charoen-like virus in field-captured Aedes aegypti in South China. Virology. 2018;523: 35–40. 10.1016/j.virol.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 18.Yamao T, Eshita Y, Kihara Y, Satho T, Kuroda M, Sekizuka T, et al. Novel virus discovery in field-collected mosquito larvae using an improved system for rapid determination of viral RNA sequences (RDV ver4.0). Arch Virol. 2009;154: 153–158. 10.1007/s00705-008-0285-5 [DOI] [PubMed] [Google Scholar]
- 19.Parry R, Asgari S. Aedes anphevirus (AeAV): an insect-specific virus distributed worldwide in Aedes aegypti mosquitoes that has complex interplays with Wolbachia and dengue virus infection in cells. J Virol. 2018; 92:e00224–18. 10.1128/JVI.00224-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasilakis N, Guzman H, Firth C, Forrester NL, Widen SG, Wood TG, et al. Mesoniviruses are mosquito-specific viruses with extensive geographic distribution and host range. Virol J. 2014;11: 97 10.1186/1743-422X-11-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huhtamo E, Putkuri N, Kurkela S, Manni T, Vaheri A, Vapalahti O, et al. Characterization of a novel flavivirus from mosquitoes in northern europe that is related to mosquito-borne flaviviruses of the tropics. J Virol. 2009;83: 9532–9540. 10.1128/JVI.00529-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangdee K, Pattanakitsakul S-N. Comparison of Mosquito Densoviruses: Two Clades of Viruses Isolated from Indigenous Mosquitoes. Southeast Asian J Trop Med Public Health. 2013;44: 586–593. [PubMed] [Google Scholar]
- 23.Junglen S, Kopp A, Kurth A, Pauli G, Ellerbrok H, Leendertz FH. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from uranotaenia mosquitoes from a tropical rain forest. J Virol. 2009;83: 4462–4468. 10.1128/JVI.00014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasar F, Palacios G, Gorchakov RV, Guzman H, Da Rosa APT, Savji N, et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci. 2012;109: 14622–14627. 10.1073/pnas.1204787109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook S, Bennett SN, Holmes EC, De Chesse R, Moureau G, de Lamballerie X. Isolation of a new strain of the flavivirus cell fusing agent virus in a natural mosquito population from Puerto Rico. J Gen Virol. 2006;87: 735–748. 10.1099/vir.0.81475-0 [DOI] [PubMed] [Google Scholar]
- 26.Cook S, Moureau G, Harbach RE, Mukwaya L, Goodger K, Ssenfuka F, et al. Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J Gen Virol. 2009;90: 2669–2678. 10.1099/vir.0.014183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman CM, Cerutti F, Anderson TK, Hamer GL, Walker ED, Kitron UD, et al. Culex Flavivirus and West Nile Virus Mosquito Coinfection and Positive Ecological Association in Chicago, United States. Vector-Borne Zoonotic Dis. 2011;11: 1099–1105. 10.1089/vbz.2010.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calzolari M, Ze-Ze L, Ruzek D, Vazquez A, Jeffries C, Defilippo F, et al. Detection of mosquito-only flaviviruses in Europe. J Gen Virol. 2012;93: 1215–1225. 10.1099/vir.0.040485-0 [DOI] [PubMed] [Google Scholar]
- 29.Kuwata R, Isawa H, Hoshino K, Sasaki T, Kobayashi M, Maeda K, et al. Analysis of Mosquito-Borne Flavivirus Superinfection in Culex tritaeniorhynchus (Diptera: Culicidae) Cells Persistently Infected with Culex Flavivirus (Flaviviridae). J Med Entomol. 2015;52: 222–229. 10.1093/jme/tju059 [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Asad S, Khromykh AA, Asgari S. Cell fusing agent virus and dengue virus mutually interact in Aedes aegypti cell lines. Sci Rep. 2017;7: 6935 10.1038/s41598-017-07279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romo H, Kenney JL, Blitvich BJ, Brault AC. Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg Microbes Infect. 2018;7: 181 10.1038/s41426-018-0180-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427: 90–97. 10.1016/j.virol.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobson-Peters J, Yam AWY, Lu JWF, Setoh YX, May FJ, Kurucz N, et al. A New Insect-Specific Flavivirus from Northern Australia Suppresses Replication of West Nile Virus and Murray Valley Encephalitis Virus in Co-infected Mosquito Cells. Wang T, editor. PLoS One. 2013;8: e56534 10.1371/journal.pone.0056534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenney JL, Solberg OD, Langevin SA, Brault AC. Characterization of a novel insect-specific flavivirus from Brazil: potential for inhibition of infection of arthropod cells with medically important flaviviruses. J Gen Virol. 2014;95: 2796–2808. 10.1099/vir.0.068031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goenaga S, Kenney JL, Duggal NK, Delorey M, Ebel GD, Zhang B, et al. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses. 2015;7: 5801–5812. 10.3390/v7112911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasar F, Erasmus JH, Haddow AD, Tesh RB, Weaver SC. Eilat virus induces both homologous and heterologous interference. Virology. 2015;484: 51–58. 10.1016/j.virol.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall-Mendelin S, McLean BJ, Bielefeldt-Ohmann H, Hobson-Peters J, Hall RA, van den Hurk AF. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit Vectors. 2016;9: 414 10.1186/s13071-016-1683-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz MJ, Frydman HM, Connor JH. Dual Insect specific virus infection limits Arbovirus replication in Aedes mosquito cells. Virology. 2018;518: 406–413. 10.1016/j.virol.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita R, Kato F, Kobayashi D, Murota K, Takasaki T, Tajima S, et al. Persistent viruses in mosquito cultured cell line suppress multiplication of flaviviruses. Heliyon. 2018;4: e00736 10.1016/j.heliyon.2018.e00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burivong P, Pattanakitsakul S-N, Thongrungkiat S, Malasit P, Flegel TW. Markedly reduced severity of Dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV). Virology. 2004;329: 261–269. 10.1016/j.virol.2004.08.032 [DOI] [PubMed] [Google Scholar]
- 41.Baidaliuk A, Miot EF, Lequime S, Moltini-Conclois I, Delaigue F, Dabo S, et al. Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo. J Virol. 2019;93: e00705–19. 10.1128/JVI.00705-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent RJ, Crabtree MB, Miller BR. Transmission of West Nile Virus by Culex quinquefasciatus Say Infected with Culex Flavivirus Izabal. PLoS Negl Trop Dis. 2010;4: e671 10.1371/journal.pntd.0000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talavera S, Birnberg L, Nuñez AI, Muñoz-Muñoz F, Vázquez A, Busquets N. Culex flavivirus infection in a Culex pipiens mosquito colony and its effects on vector competence for Rift Valley fever phlebovirus. Parasit Vectors. 2018;11: 405 10.1186/s13071-018-2985-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franzke K, Leggewie M, Sreenu VB, Jansen S, Heitmann A, Welch SR, et al. Detection, infection dynamics and small RNA response against Culex Y virus in mosquito-derived cells. J Gen Virol. 2018;99: 1739–1745. 10.1099/jgv.0.001173 [DOI] [PubMed] [Google Scholar]
- 45.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, et al. C6/36 Aedes albopictus Cells Have a Dysfunctional Antiviral RNA Interference Response. PLoS Negl Trop Dis. 2010;4: e856 10.1371/journal.pntd.0000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambrechts L, Quillery E, Noel V, Richardson JH, Jarman RG, Scott TW, et al. Specificity of resistance to dengue virus isolates is associated with genotypes of the mosquito antiviral gene Dicer-2. Proc R Soc B. 2012;280: 20122437–20122437. 10.1101/gad.1482006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4: e1000098 10.1371/journal.ppat.1000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106: 17841–17846. 10.1073/pnas.0905006106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sim S, Jupatanakul N, Ramirez JL, Kang S, Romero-Vivas CM, Mohammed H, et al. Transcriptomic Profiling of Diverse Aedes aegypti Strains Reveals Increased Basal-level Immune Activation in Dengue Virus-refractory Populations and Identifies Novel Virus-vector Molecular Interactions. PLoS Negl Trop Dis. 2013;7: e2295 10.1371/journal.pntd.0002295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jupatanakul N, Sim S, Angleró-Rodríguez YI, Souza-Neto J, Das S, Poti KE, et al. Engineered Aedes aegypti JAK/STAT Pathway-Mediated Immunity to Dengue Virus. PLoS Negl Trop Dis. 2017;11: e0005187 10.1371/journal.pntd.0005187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barletta A, Silva MCLN, Sorgine M. Validation of Aedes aegypti Aag-2 cells as a model for insect immune studies. Parasit Vectors. 2012;5: 148 10.1186/1756-3305-5-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang R, Zhu Y, Pang X, Xiao X, Zhang R, Cheng G. Regulation of Antimicrobial Peptides in Aedes aegypti Aag2 Cells. Front Cell Infect Microbiol. 2017;7: 22 10.3389/fcimb.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merkling SH, van Rij RP. Beyond RNAi: Antiviral defense strategies in Drosophila and mosquito. J Insect Physiol. 2013;59: 159–170. 10.1016/j.jinsphys.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 54.Lan Q, Fallon M. Small heat shock proteins distinguish between two mosquito species and confirm identity of their cell lines. Am J Trop Med Hyg. 1990;43: 669–676. 10.4269/ajtmh.1990.43.669 [DOI] [PubMed] [Google Scholar]
- 55.Walker T, Jeffries CL, Mansfield KL, Johnson N. Mosquito cell lines: history, isolation, availability and application to assess the threat of arboviral transmission in the United Kingdom. Parasit Vectors. 2014;7: 382 10.1186/1756-3305-7-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN, Myles KM. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 2012;8: e1002470 10.1371/journal.ppat.1002470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stollar V, Thomas VL. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology. 1975;64: 367–377. 10.1016/0042-6822(75)90113-0 [DOI] [PubMed] [Google Scholar]
- 58.Weger-Lucarelli J, Rückert C, Grubaugh ND, Misencik MJ, Armstrong PM, Stenglein MD, et al. Adventitious viruses persistently infect three commonly used mosquito cell lines. Virology. 2018;521: 175–180. 10.1016/j.virol.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maringer K, Yousuf A, Heesom KJ, Fan J, Lee D, Fernandez-Sesma A, et al. Proteomics informed by transcriptomics for characterising active transposable elements and genome annotation in Aedes aegypti. BMC Genomics. 2017;18: 1–18. 10.1186/s12864-016-3406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varjak M, Maringer K, Watson M, Sreenu VB, Fredericks AC, Pondeville E, et al. Aedes aegypti Piwi4 Is a Noncanonical PIWI Protein Involved in Antiviral Responses. mSphere. 2017;2: e00144–17. 10.1128/mSphere.00144-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varjak M, Donald CL, Mottram TJ, Sreenu VB, Merits A, Maringer K, et al. Characterization of the Zika virus induced small RNA response in Aedes aegypti cells. PLoS Negl Trop Dis. 2017;11: e0006010 10.1371/journal.pntd.0006010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halstead SB, Simasthien P. Observations related to the pathogenesis of dengue hemorrhagic fever. II. Antigenic and biologic properties of dengue viruses and their association with disease response in the host. Yale J Biol Med. 1970;42: 276–292. [PMC free article] [PubMed] [Google Scholar]
- 63.Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46: 509–520. 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- 64.Levine B, Goldman JE, Jiang HH, Griffin DE, Hardwick JM. Bc1-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93: 4810–4815. 10.1073/pnas.93.10.4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hardwick M, Levine B. Sindbis virus vector system for functional analysis of apoptosis regulators. Methods Enzymol. 2013;322: 1–17. [DOI] [PubMed] [Google Scholar]
- 66.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4: 263–275. 10.1016/S1535-6108(03)00241-1 [DOI] [PubMed] [Google Scholar]
- 67.Park MS, Shaw ML, Munoz-Jordan J, Cros JF, Nakaya T, Bouvier N, et al. Newcastle Disease Virus (NDV)-Based Assay Demonstrates Interferon-Antagonist Activity for the NDV V Protein and the Nipah Virus V, W, and C Proteins. J Virol. 2003;77: 1501–1511. 10.1128/JVI.77.2.1501-1511.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott JC, Brackney DE, Campbell CL, Bondu-Hawkins V, Hjelle B, Ebel GD, et al. Comparison of Dengue Virus Type 2-Specific Small RNAs from RNA Interference-Competent and–Incompetent Mosquito Cells. PLoS Negl Trop Dis. 2010;4: e848 10.1371/journal.pntd.0000848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson MAE, Gross TL, Myles KM, Adelman ZN. Validation of novel promoter sequences derived from two endogenous ubiquitin genes in transgenic Aedes aegypti. Insect Mol Biol. 2010;19: 441–449. 10.1111/j.1365-2583.2010.01005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9: 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitfield ZJ, Dolan PT, Kunitomi M, Tassetto M, Seetin MG, Oh S, et al. The Diversity, Structure, and Function of Heritable Adaptive Immunity Sequences in the Aedes aegypti Genome. Curr Biol. 2017;27: 3511–3519.e7. 10.1016/j.cub.2017.09.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giraldo-Calderón GI, Emrich SJ, Maccallum RM, Maslen G, Dialynas E, Topalis P, et al. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015;43: D707–13. 10.1093/nar/gku1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goic B, Vodovar N, Mondotte JA, Monot C, Frangeul L, Blanc H, et al. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat Immunol. 2013;14: 396–403. 10.1038/ni.2542 [DOI] [PubMed] [Google Scholar]
- 74.Goic B, Stapleford KA, Frangeul L, Doucet AJ, Gausson V, Blanc H, et al. Virus-derived DNA drives mosquito vector tolerance to arboviral infection. Nat Commun. 2016;7: 12410 10.1038/ncomms12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horst Ter AM, Nigg JC, Dekker FM, Falk BW. Endogenous viral elements are widespread in arthropod genomes and commonly give rise to piRNAs. J Virol. 2019; 93(6): e02124–18. 10.1128/JVI.02124-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palatini U, Miesen P, Carballar-Lejarazú R, Ometto L, Rizzo E, Tu Z, et al. Comparative genomics shows that viral integrations are abundant and express piRNAs in the arboviral vectors Aedes aegypti and Aedes albopictus. BMC Genomics. 2017;18: 512 10.1186/s12864-017-3903-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki Y, Frangeul L, Dickson LB, Blanc H, Verdier Y, Vinh J, et al. Uncovering the repertoire of endogenous flaviviral elements in Aedes mosquito genomes. J Virol. 2017; 91(15). e00571–17. 10.1128/JVI.00571-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crochu S, Cook S, Attoui H, Charrel RN, De Chesse R, Belhouchet M, et al. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol. 2004;85: 1971–1980. 10.1099/vir.0.79850-0 [DOI] [PubMed] [Google Scholar]
- 79.Mullane K, Williams M. Enhancing reproducibility: Failures from Reproducibility Initiatives underline core challenges. Biochem Pharmacol. 2017;138: 7–18. 10.1016/j.bcp.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 80.Göertz GP, Miesen P, Overheul GJ, van Rij RP, van Oers MM, Pijlman GP. Mosquito Small RNA Responses to West Nile and Insect-Specific Virus Infections in Aedes and Culex Mosquito Cells. Viruses. 2019;11: 271 10.3390/v11030271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Cleef KWR, van Mierlo JT, Miesen P, Overheul GJ, Fros JJ, Schuster S, et al. Mosquito and Drosophila entomobirnaviruses suppress dsRNA- and siRNA-induced RNAi. Nucleic Acids Res. 2014;42: 8732–8744. 10.1093/nar/gku528 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.