Abstract
The aim of this paper is to investigate whether the lower rate of breastfeeding at 6 months by overweight and obese mothers is primarily due to these women giving up breastfeeding in the first week postpartum using a cross‐sectional population survey. The sample is children from the infant cohort (about 12 months of age) of Wave 1 (2004) of the Longitudinal Study of Australian Children for whom breastfeeding and maternal information were available (n = 3075). Definitions used: normal‐weight body mass index (BMI, kg/m2) 20 to <25, overweight BMI 25 to <30, obese BMI ≥30. Breastfeeding initiation was 95.1% for normal‐weight women, 92.8% for overweight women and 87.1% for obese women. At 6 months, 64% of normal‐weight women were breastfeeding, compared with 54% of overweight and 44% of obese women. On multivariate analysis, for women who initiated breastfeeding, overweight women had an odds ratio (OR) of 1.52 [95% confidence interval (CI) 1.02, 2.28] and obese women had an OR of 2.54 (95% CI 1.70, 3.79) of stopping breastfeeding by 1 week compared with normal‐weight women (adjusted for maternal age, education, smoking, level of socio‐economic disadvantage, caesarean birth, admission to special care nursery). For women who breastfed for at least 1 week, overweight women had an adjusted OR of 1.26 (1.04, 1.53) and obese women had an adjusted OR of 1.38 (1.10, 1.73) of ceasing to breastfeed before 6 months, compared with normal‐weight women. In conclusion, among overweight/obese women who initiate breastfeeding, higher rates of cessation of breastfeeding in both the immediate postpartum period and in the first 6 months contribute to the shorter duration.
Keywords: breastfeeding initiation, breastfeeding duration, obesity, determinants of breastfeeding
Introduction
In Australia, 34% of pregnant women are overweight or obese (Callaway et al. 2006) and this is an increasing problem internationally (World Health Organization 2000). As breastfeeding is important for child health (American Academy of Pediatrics Section on Breastfeeding et al. 2005), it is essential to investigate the possible relationship between maternal obesity and poorer infant feeding outcomes.
Since 1992, a number of studies have reported that women who are overweight or obese have lower rates of breastfeeding initiation and duration than other women (Rutishauser & Carlin 1992; Hilson et al. 1997; Donath & Amir 2000; Li et al. 2003; Forster et al. 2006; Baker et al. 2007). Our review also found that obese women were less likely to intend to breastfeed and/or intended to breastfeed for a shorter duration than women of normal weight (Amir & Donath 2007). Rasmussen's (2007) recent review of possible reasons for the ‘poor lactation performance’ in obese women concludes that maternal obesity may affect development of the mammary glands before, during and after pregnancy, and lead to complications of pregnancy, babies with large birthweights and large breasts which all may interfere with normal onset of lactation be associated with the decision not to breastfeed for a variety of socio‐demographic and psychosocial factors.
In her review, Rasmussen comments that many overweight/obese women give up breastfeeding in the first week postpartum (Rasmussen 2007, p. 111). The recent Danish study found 14.4% of obese class III women stopped breastfeeding in the first week, compared with 3.5% of normal‐weight women (Baker et al. 2007); the authors suggested that this was related to the lower prolactin response in overweight/obese women compared with normal‐weight women at 48 hours postpartum (Rasmussen & Kjolhede 2004) or the delayed onset of a copious milk supply (Chapman & Perez‐Escamilla 1999).
This paper uses data from a large study of Australian infants to investigate whether the lower rate of breastfeeding at 6 months by overweight and obese mothers is primarily due to these women giving up breastfeeding in the first week postpartum. We, therefore, investigate breastfeeding duration in women who are continuing to breastfeed at 1 week focusing primarily on the effect of maternal body mass index (BMI, kg/m2).
Methods
The Longitudinal Study of Australian Children (LSAC) is being implemented by a large multidisciplinary research consortium led by the Australian Institute of Family Studies (Nicholson & Sanson 2003). During 2004, over 10 000 children and their families were recruited to the study from a sample selected from the Health Insurance Commission's (HIC) Medicare database (Australian Institute of Family Studies 2004). The sample is broadly representative of all Australian children in each of two selected age cohorts (approximately 5000 in each cohort): children born between March 2003 and February 2004 (infants) and children born between March 1999 and February 2000 (children aged 4–5 years).
The main data collection for Wave 1 was a face‐to‐face interview with the parent who knew the child best, predominantly with the mother (Australian Institute of Family Studies 2004). The final response to the recruitment in the infant cohort was 57% of those families who were sent a letter by HIC.
In this paper, we included children from the infant cohort for whom breastfeeding and maternal information were available. The number of infants in the multivariate sample [i.e. data were available on breastfeeding duration to 6 months, maternal age, maternal smoking, maternal BMI (based on self‐reported height and weight at time of the interview when infants were 42 weeks old on average, range 26–79 weeks), maternal education level and level of socio‐economic disadvantage of the geographical location of the child's household (Australian Bureau of Statistics 2003)] was 3075. Definitions used: normal‐weight BMI 20 to <25, overweight BMI 25 to <30, obese BMI ≥30. Obesity has been further classified as obese class 1 (30 to <35), obese class II (35 to <40) and obese class III (≥40) based on WHO definitions (World Health Organization 2000). In this paper, we have excluded infants whose mothers had BMI <20 as we planned to compare overweight and obese women with women of normal weight. We wanted to exclude women who were underweight in case this was another confounding factor. An underweight cut‐off of 20–21 is used ‘in relation to psychological problems and psychosocial conditions related to underweight and self‐perception of body and body weight’ (Ali & Lindström 2006).
The breastfeeding data are based on the following questions: (1) breastfeeding initiation –‘was [child] ever breastfed? (including colostrum)’; and (2) breastfeeding duration –‘how old was [child] when he/she completely stopped being breastfed?’
We used multivariable logistic regression, adjusted for maternal age, education, smoking, level of socio‐economic disadvantage of the geographical location of the child's household, caesarean birth, admission to special care nursery to estimate the adjusted odds ratios (OR) of breastfeeding for at least 6 months of overweight and obese women compared with normal‐weight women (information on maternal parity was not collected in LSAC). In order to assess the relative contribution of early cessation of breastfeeding, we estimated similar adjusted OR for each of the following:
-
1
initiation of breastfeeding,
-
2
breastfeeding for at least 1 week in those who initiated, and
-
3
breastfeeding for at least 6 months in those who breastfed for at least 1 week.
In each of these analyses, an OR greater than 1 indicates that overweight and obese women are less likely to be breastfeeding than women of normal weight. A 95% confidence interval which does not include 1 indicates that this result is unlikely to have occurred by chance.
We used the suest and nlcom commands in stata 9.2 to test the hypotheses (for overweight and for obese women) that there was no difference between OR (2) and (3) listed above.
Written informed consent by a parent was obtained for each participating child, and the study was approved by the Australian Institute of Family Studies Ethics Committee.
Results
Information about the main socio‐demographic characteristics of the sample is shown in Table 1 (last column). Obese women were more likely to belong to the most disadvantaged socio‐economic group, more likely to be younger, to have less education and to smoke than women with normal BMI. The babies of obese mothers were more likely to be born by caesarean birth and to be admitted to intensive care after birth than infants of women of normal BMI (Table 1).
Table 1.
Sample characteristics by maternal BMI
| Normal BMI % (n) | Overweight % (n) | Obese % (n) | Total % (n) | |
|---|---|---|---|---|
| (n = 1567) | (n = 890) | (n = 618) | (n = 3075) | |
| Mother's age group | ||||
| 15–24 | 9.1 (142) | 10.6 (94) | 11.3 (70) | 10.0 (306) |
| 25–30 | 28.7 (449) | 29.0 (258) | 34.6 (214) | 30.0 (921) |
| 31–34 | 31.4 (492) | 33.9 (302) | 30.3 (187) | 31.9 (981) |
| 35+ | 30.9 (484) | 26.5 (236) | 23.8 (147) | 28.2 (867) |
| Education level of mother | ||||
| <Year 12 | 11.6 (182) | 14.7 (131) | 19.1 (118) | 14.0 (431) |
| Year 12 | 13.3 (208) | 14.6 (130) | 20.2 (125) | 15.1 (463) |
| Certificate | 20.0 (313) | 26.3 (234) | 30.3 (187) | 23.9 (734) |
| Diploma | 11.2 (176) | 9.8 (87) | 9.1 (56) | 10.4 (319) |
| ≥Degree | 43.3 (678) | 33.5 (298) | 20.4 (126) | 35.8 (1102) |
| Other | 0.6 (10) | 1.1 (10) | 1.0 (6) | 0.8 (26) |
| Geographic disadvantage index for location of household‐(quintiles) | ||||
| Highest disadvantage | 12.8 (200) | 13.9 (124) | 21.2 (131) | 14.8 (455) |
| 2nd | 18.7 (293) | 23.1 (206) | 24.3 (150) | 21.1 (649) |
| 3rd | 20.5 (321) | 18.8 (167) | 21.7 (134) | 20.2 (622) |
| 4th | 22.2 (348) | 23.7 (211) | 18.4 (114) | 21.9 (673) |
| Least disadvantage | 25.8 (405) | 20.4 (182) | 14.4 (89) | 22.0 (676) |
| Maternal smoking in pregnancy | 13.0 (203) | 17.3 (154) | 19.4 (120) | 15.5 (477) |
| Caesarean birth | 25.8 (405) | 35.1 (312) | 40.9 (253) | 31.5 (970) |
| Intensive care after birth | 14.4 (225) | 17.2 (153) | 20.9 (129) | 16.5 (507) |
BMI, body mass index.
Overall, 92.8% infants initiated breastfeeding. Initiation was 95.1% for normal‐weight women (n = 1569), 92.8% for overweight women (n = 890) and 87.1% for obese women (n = 619) (see Table 2). By 6 months of age, 63.7% of normal‐weight women were continuing to breastfed, compared with 53.5% of overweight women and 43.9% of obese women (Table 2).
Table 2.
Univariate results: breastfeeding initiation and duration according to maternal BMI (unweighted)
| Normal BMI (%) | Overweight (%) | Obese (%) | |
|---|---|---|---|
| Initiation | 95.1 | 92.8* | 87.1** |
| Breastfed at least 1 week | 91.7 | 87.1** | 77.7** |
| Breastfed at 6 months | 63.8 | 53.6** | 43.9** |
| Proportion of those who initiated breastfeeding, continuing to breastfeed at 1 week | 96.4 | 93.8** | 89.2** |
| Proportion of those breastfeeding at 1 week continuing to breastfeed at 6 months | 69.5 | 61.6** | 56.5** |
BMI, body mass index.
P = 0.02,
P < 0.005.
In this sample, the proportion of overweight and obese women breastfeeding was lower than the proportion of normal‐weight women at all times in the first 6 months postpartum. Stratification of BMI into five levels (Fig. 1) shows that obese class III women (BMI >40) are less likely to breastfeed than other women and the gradation between overweight, and obese classes I, II and III (n = 404, 143, 71 respectively). In this sample, less than 25% of women classified as obese class III were breastfeeding at 6 months compared with over 50% of women classified as normal.
Figure 1.
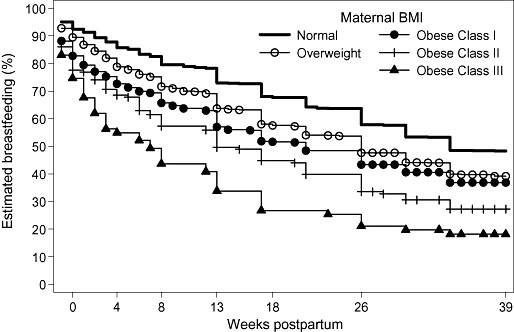
Proportion of women breastfeeding (weeks postpartum) according to maternal body mass index (BMI).
In order to exclude the effect of women who gave up breastfeeding in the first week postpartum, Fig. 2 includes only women who were breastfeeding at 1 week postpartum. Of the women breastfeeding at 1 week, the proportion of obese women breastfeeding at 6 months (56%) was 20% lower than women of normal weight (70%).
Figure 2.
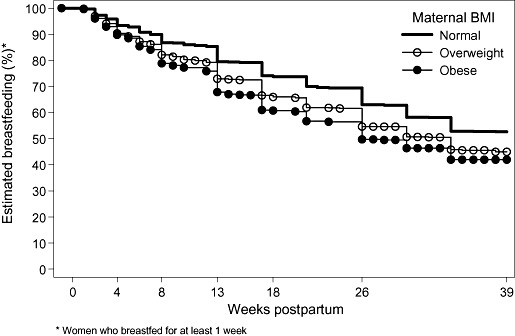
Women who breastfed for at least 1 week: proportion of women breastfeeding (weeks postpartum) according to maternal body mass index (BMI).
Table 3 presents results adjusted for maternal age, education, smoking, level of socio‐economic disadvantage of the geographical location of the child's household, caesarean birth and admission to special care nursery. Obese women had significantly higher rates of never initiating breastfeeding, and overweight and obese women had significantly higher rates of ceasing breastfeeding in the first week postpartum, and ceasing breastfeeding between 1 week and 6 months postpartum, compared with normal‐weight women. Compared with overweight women, obese women were significantly more likely to never breastfeed, or to cease breastfeeding in the first week postpartum (2, 3).
Table 3.
Multivariate analysis: non‐initiation and cessation of breastfeeding according to maternal BMI
| Overweight compared with normal maternal BMI (adjusted odds ratios, 95% CI)* | Obese compared with normal maternal BMI (adjusted odds ratios, 95% CI)* | |
|---|---|---|
| Never initiated breastfeeding | 1.30 (0.91, 1.84) | 2.10 (1.49, 2.96) |
| Ceased breastfeeding in first week (if initiated) | 1.52 (1.02, 2.28) | 2.54 (1.70, 3.79) |
| Ceased breastfeeding between 1 week and 6 months (if breastfeeding at 1 week) | 1.26 (1.04, 1.53) | 1.38 (1.10, 1.73) |
| Not breastfeeding at 6 months | 1.34 (1.12, 1.60) | 1.68 (1.37, 2.06) |
BMI, body mass index; CI, confidence interval.
Logistic regression; adjusted for maternal age, education, smoking, level of socio‐economic disadvantage of the geographical location of the child's household, caesarean birth, admission to special care nursery.
For overweight women, the adjusted OR of ceasing breastfeeding in the first week (1.52) was not significantly different from the adjusted OR of ceasing breastfeeding between 1 week and 6 months (1.26) (P = 0.45). For obese women, however, the adjusted OR of ceasing breastfeeding in the first week (2.54) was significantly higher than the adjusted OR of ceasing breastfeeding between 1 week and 6 months (1.38) (P = 0.03). In other words, for overweight women, the comparative rate of breastfeeding cessation in the first week was similar to the comparative rate of breastfeeding cessation between 1 week and 6 months; whereas, for obese women the comparative rate of breastfeeding cessation in the first week was greater than the comparative rate between 1 week and 6 months.
Discussion
Data from LSAC confirm that overweight/obese women are less likely to ever put the baby to the breast, more likely to stop breastfeeding in the first week (Rasmussen 2007) and also, given that they ever began and continued for at least 1 week, more likely to stop breastfeeding before 6 months even after adjusting for possible confounding factors. There appears to be a dose–response effect between maternal BMI and breastfeeding initiation and duration, with higher maternal BMI increasing the risk of lower rates of breastfeeding, as also seen in other studies (Hilson et al. 1997; Baker et al. 2007). This dose–response effect is particularly clear when the ‘obese’ category is further subdivided, with women in the ‘extreme obesity’ classification having much lower rates of breastfeeding than those in the ‘obese class 1’ category (Fig. 1).
Obese women and their infants are more likely to experience medical complications (Yu et al. 2006). Studies have suggested that for obese women milk may be slower to ‘come‐in’ than for other women (Dewey et al. 2003). Because these factors may lead to a greater risk of giving up breastfeeding in the first postpartum week, we examined only the women who were continuing to breastfeed at 1 week postpartum, and found that there was still a significant difference between breastfeeding duration in obese women and normal‐weight women.
Strengths of this study
This is a nationally representative sample in a population with high breastfeeding initiation, with adjustment for the common confounding factors. The sample size allows stratification into different levels of maternal obesity, which allows a clear step‐wise relationship to be seen between maternal BMI and breastfeeding duration.
Limitations of this study
In general, people underestimate their weight and overestimate their height, leading to self‐reported BMI being an underestimate of their true BMI (Hill & Roberts 1998; Schieve et al. 1999). Therefore, associations found between BMI and other variables are more likely to be underestimates than overestimates. The weight was recorded when the child was about 42 weeks old. Ideally, an estimate of maternal weight while breastfeeding would have been preferred, but other studies have used pre‐pregnancy weight (Baker et al. 2004; Hilson et al. 2004), 1 month postpartum (Rutishauser & Carlin 1992), 6 months postpartum (Forster et al. 2006) or have not been reported (Scott et al. 2006). The findings from these studies are consistent, although the timing of measurement of obesity has varied. We believe that although women's weight varies in the perinatal period, it is likely that most women would have been correctly classified.
Children of lower socio‐economic status postcodes, non‐English speaking backgrounds and lone‐parent families are known to be underrepresented in LSAC which explains why the breastfeeding rates are higher than other Australian studies (49% of infants were being breastfed in the 2001 National Health Survey) (Donath & Amir 2005). However, neither of these limitations is likely to affect the results reported here.
Overweight and obese women are also less likely to intend to breastfeed and/or to intend to breastfeed for shorter durations than women of normal weight (Barnes et al. 1997). These data do not include women's infant feeding intention, so although intention is an important determinant of breastfeeding (Donath et al. 2003), we were unable to adjust for this.
In comparison with other studies
Australian women have fairly high rates of breastfeeding initiation and duration in comparison with the USA and UK. Lower breastfeeding initiation in overweight/obese women has been found in the US, UK and Australia (1997, 2006; Donath & Amir 2000; Sebire et al. 2001; 2002, 2003; Kugyelka et al. 2004). Shorter duration of breastfeeding in overweight/obese women has been found in the US, Australia and Denmark (Rutishauser & Carlin 1992; 1997, 2006; Donath & Amir 2000; 2002, 2003; Kugyelka et al. 2004; Forster et al. 2006; Oddy et al. 2006; Scott et al. 2006).
As there are likely to be a number of factors leading to poorer lactation outcomes in overweight and obese women – from possible physiological differences, to practical difficulties with attachment, medical problems, social and psychological issues (Amir & Donath 2007) – further investigations are necessary to determine the role of these factors. As we have found that infant feeding intention is a stronger predictor of breastfeeding behaviour than other factors combined (Donath et al. 2003), future studies need to adjust for the lower breastfeeding intention of obese women. Qualitative studies are also required to explore women's views about their body size and breastfeeding attitudes and experiences.
Acknowledgements
The LSAC is funded by the Commonwealth Department of Families, Community Services and Indigenous Affairs. The authors received no funding for this analysis.
This study uses questionnaires developed for Growing up in Australia: LSAC. These questionnaires are the property of the Australian Government Department of Families, Community Services and Indigenous Affairs. LSAC is an initiative of the Australian Government Department of Families, Community Services and Indigenous Affairs (http://www.facsia.gov.au), and is being undertaken in partnership with the Australian Institute of Family Studies (http://www.aifs.gov.au), with advice being provided by a consortium of leading researchers at research institutions and universities throughout Australia.
References
- Ali S.M. & Lindström M. (2006) Socioeconomic, psychosocial, behavioural, and psychological determinants of BMI among young women: differing patterns for underweight and overweight/obesity. European Journal of Public Health 16, 324–330. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Section on Breastfeeding , Gartner L.M., Morton J., Lawrence R.A., Naylor A.J., O’Hare D. et al (2005) Breastfeeding and the use of human milk. Pediatrics 115, 496–506. [DOI] [PubMed] [Google Scholar]
- Amir L.H. & Donath S. (2007) A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Bureau of Statistics (2003) Socio‐economic Indexes for Areas, Information Paper. Census of Population and Housing, Australia 2001. Canberra: Cat 2039.0.
- Australian Institute of Family Studies (2004) Growing up in Australia: the Longitudinal Study of Australian Children, 2004 Annual Report. Available at: http://www.aifs.gov.au/growingup/pubs/ar/annualreport2004.html
- Baker J.L., Michaelsen K.F., Rasmussen K.M. & Sorensen T.I.A. (2004) Maternal pre‐pregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. American Journal of Clinical Nutrition 80, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Michaelsen K.F., Sørensen T.I.A. & Rasmussen K.M. (2007) High pre‐pregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. American Journal of Clinical Nutrition 86, 404–411. [DOI] [PubMed] [Google Scholar]
- Barnes J., Stein A., Smith T., Pollock J.I. & ALSPAC Study Team (1997) Extreme attitudes to body shape, social and psychological factors and a reluctance to breast feed. Journal of the Royal Society of Medicine 90, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway L.K., Prins J.B., Chang A.M. & McIntyre H.D. (2006) The prevalence and impact of overweight and obesity in an Australian population. Medical Journal of Australia 184, 56–59. [DOI] [PubMed] [Google Scholar]
- Chapman D.J. & Perez‐Escamilla R. (1999) Identification of risk factors for delayed onset of lactation. Journal of the American Dietetic Association 99, 450–454. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Nommsen L.A., Heinig M.J. & Cohen R.J. (2003) Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 112, 607–619. [DOI] [PubMed] [Google Scholar]
- Donath S.M. & Amir L.H. (2000) Does maternal obesity adversely affect breastfeeding initiation and duration? Journal of Paediatrics and Child Health 36, 482–486. [DOI] [PubMed] [Google Scholar]
- Donath S.M. & Amir L.H. (2005) Breastfeeding and the introduction of solids in Australian children: data from the 2001 National Health Survey. Australian and New Zealand Journal of Public Health 29, 171–175. [DOI] [PubMed] [Google Scholar]
- Donath S.M., Amir L.H. & ALSPAC Study Team (2003) Relationship between prenatal infant feeding intention and initiation and duration of breastfeeding: a cohort study. Acta Paediatrica 92, 352–356. [PubMed] [Google Scholar]
- Forster D., McLachlan H. & Lumley J. (2006) Factors associated with continuing to feed any breast milk at six months postpartum in a group of Australian women. International Breastfeeding Journal 1, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. & Roberts J. (1998) Body mass index: a comparison between self‐reported and measured height and weight. Journal of Public Health Medicine 20, 206–210. [DOI] [PubMed] [Google Scholar]
- Hilson J.A., Rasmussen K.M. & Kjolhede C.L. (1997) Maternal obesity and breast‐feeding success in a rural population of white women. American Journal of Clinical Nutrition 66, 1371–1378. [DOI] [PubMed] [Google Scholar]
- Hilson J.A., Rasmussen K.M. & Kjolhede C.L. (2004) High pre‐pregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. Journal of Human Lactation 20, 18–29. [DOI] [PubMed] [Google Scholar]
- Hilson J.A., Rasmussen K.M. & Kjolhede C.L. (2006) Excessive weight gain during pregnancy is associated with earlier termination of breast‐feeding among white women. Journal of Nutrition 136, 140–146. [DOI] [PubMed] [Google Scholar]
- Kugyelka J.G., Rasmussen K.M. & Frongillo E.A. (2004) Maternal obesity is negatively associated with breastfeeding success among Hispanic but not black women. Journal of Nutrition 134, 1746–1753. [DOI] [PubMed] [Google Scholar]
- Li R., Ogden C., Ballew C., Gillespie C. & Grummer‐Strawn L. (2002) Prevalence of exclusive breastfeeding among US infants: the third National Health and Nutrition Examination Survey (Phase II, 1991–1994). American Journal of Public Health 92, 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Jewell S. & Grummer‐Strawn L. (2003) Maternal obesity and breastfeeding practices. American Journal of Clinical Nutrition 77, 931–936. [DOI] [PubMed] [Google Scholar]
- Nicholson J.M. & Sanson A. (2003) A new longitudinal study of the health and wellbeing of Australian children: how will it help? Medical Journal of Australia 278, 282–284. [DOI] [PubMed] [Google Scholar]
- Oddy W.H., Li J., Landsborough L., Kendall G.E., Henderson S. & Downie J. (2006) The association of maternal overweight and obesity with breastfeeding duration. Journal of Pediatrics 149, 185–191. [DOI] [PubMed] [Google Scholar]
- Rasmussen K.M. (2007) Association of maternal obesity before conception with poor lactation performance. Annual Review of Nutrition 27, 103–121. [DOI] [PubMed] [Google Scholar]
- Rasmussen K.M. & Kjolhede C.L. (2004) Pre‐pregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 113, e465–e471. [DOI] [PubMed] [Google Scholar]
- Rutishauser I.H.E. & Carlin J.B. (1992) Body mass index and duration of breast feeding: a survival analysis during the first six months of life. Journal of Epidemiology and Community Health 46, 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve L.A., Perry G.S., Cogswell M.E., Scanion K.S., Rosenberg D., Carmichael S. et al (1999) Validity of self‐reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. American Journal of Epidemiology 150, 947–956. [DOI] [PubMed] [Google Scholar]
- Scott J.A., Binns C.W., Oddy W.H. & Graham K.I. (2006) Predictors of breastfeeding duration: evidence from a cohort study. Pediatrics 117, e646–655. [DOI] [PubMed] [Google Scholar]
- Sebire N.J., Jolly M., Harris J.P., Wadsworth J., Joffe M., Beard R. et al (2001) Maternal obesity and pregnancy outcome: a study of 287, 213 pregnancies in London. International Journal of Obesity and Related Metabolic Disorders 25, 1175–1182. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2000) Defining the Problem. Obesity: Preventing and Managing the Global Epidemic, Report on a WHO Consultation, Technical Report Series, No. 894. World Health Organization: Geneva. [PubMed]
- Yu C.K.H., Teoh T.G. & Robinson S. (2006) Obesity in pregnancy. BJOG: an International. Journal of Obstetrics and Gynaecology 113, 1117–1125. [DOI] [PubMed] [Google Scholar]


