Abstract
The present review of determinants of infant fatty acid status was undertaken as part of a conference on ‘Fatty acid status in early life in low‐income countries: determinants and consequences’. Emphasis is placed on the essential fatty acids, and particularly the physiologically important long chain polyunsaturated fatty acids (LCPUFAs) of 20 and 22 carbons. We are unaware of any studies of determinants of infant fatty acid status in populations with a cultural dietary pattern with low amounts of linoleic acid (LA, 18:2n‐6) and α‐linolenic acid (ALA,18:3n‐3). Many reports suggest that there may be adverse health effects related to the increased proportion of LA in relation to ALA, which have occurred worldwide due to the increased availability of vegetable oils high in LA. The issue of dietary n‐6 to n‐3 balance may apply to infant fatty acid status both during fetal and post‐natal life; however, this review focuses on the n‐3 and n‐6 LCPUFA, in particular, docosahexaenoic acid (DHA, 22:6n‐3) and arachidonic acid (AA, 20:4n‐6), which are the predominant n‐3 and n‐6 LCPUFA found in cell membranes. The evidence that these fatty acids are preferentially transferred from maternal to fetal circulation across the placenta, and the sources and mechanisms for this transfer, are reviewed. We also address the sources of DHA and AA for the newborn including human milk DHA and AA and the factors that influence maternal DHA status and consequently the amount of DHA available for transfer to the fetus and infant via human milk.
Keywords: long‐chain polyunsaturated fatty acids, docosahexaenoic acid, fatty acid transfer, placenta, breast milk
Introduction
The dietary essential fatty acids (EFAs), linoleic acid (LA, 18:2n‐6) and α–linolenic acid (ALA, 18:3n‐3), are important for infant growth and development. Fat‐free diets fed to infants reduced the skin water barrier and thus increased energy requirement and reduced growth (Hansen 1986). The precursor EFA and their long chain metabolites [long chain polyunsaturated fatty acids (LCPUFAs)] also play important roles in reproduction. There is currently no evidence that the absolute amounts of EFA provided by any cultural dietary pattern are inadequate to meet the needs for growth of the placenta, the fetus or the infant. Higher LA intake has been associated with smaller birth size (Badart‐Smook et al. 1997), but tissue levels of LA and arachidonic acid (AA, 20:4n‐6), a known growth mediator, are inversely related. Higher AA status has been associated with both size at birth (Koletzko & Braun 1991) and first year growth achievement in preterm infants (Carlson et al. 1993a). In contrast to the paucity of data related to the 18‐carbon EFA, considerable evidence has accumulated over the past 25 years to suggest that infant and child development (Carlson et al. 1993b; O'Connor et al. 2001; Clandinin et al. 2005; Henriksen et al. 2008) and growth (Clandinin et al. 2005) can be enhanced by including docosahexaenoic acid (DHA, 22:6n‐3) and AA in the diet of preterm infants. A growing body of literature, beyond the scope of this article, provides a number of different mechanisms for the role of DHA in brain development and function (for reviews, see McNamara & Carlson 2006 and Innis 2008).
The last intrauterine trimester and the first year of life are important for the accumulation of DHA in the brain. The large number of observational studies that link higher habitual maternal DHA intake to some aspect of higher or more mature infant or child development are considered relevant in relation to the functional importance of LCPUFA transfer (Cheruku et al. 2002; Colombo et al. 2004; Bakker et al. 2007; Hibbeln et al. 2007; Jacobson et al. 2008; Kannass et al. 2009; Mendez et al. 2009) as well as postnatal randomized studies of supplemented term and preterm infants (Makrides et al. 2009 and Birch et al. 2010a are recent examples). Virtually all of the studies of development have been conducted in Europe and North America where most women have good access to animal foods that are sources of protein, micronutrients and n‐3 and n‐6 LCPUFA for the growing placenta and conceptus. Despite that, because there are few concentrated sources of DHA in foods, DHA intake in the developed world is highly variable; and women in the United States in particular have been observed to have relatively low intakes of DHA (Makrides et al. 2009).
It is reasonable to ask about the determinants and consequences of fatty acids status in developing countries, where women may have limited or no access to animal foods. Protein and micronutrients are required for placental function, healthy pregnancy and optimal fetal outcome. They are also required for n‐3 and n‐6 LCPUFA synthesis from the 18‐carbon precursor EFA, and for the fatty acid binding proteins (FABP) and fatty acid transporters (FAT) that likely ensure transfer from mother to offspring as well as the fetal and neonate uptake of available n‐3 and n‐6 LCPUFA. Animal models link poor protein, energy restriction and iron status to altered LCPUFA status (2002, 2003; LeBlanc et al. 2009).
Most of the reported studies of development have focused on brain development, because DHA and AA are the major polyunsaturated fatty acids (PUFAs) in the neuron‐rich grey matter of the brain. LCPUFA status, particularly DHA status, is linked to the immune system (Pastor et al. 2006; Field et al. 2008; Hamazaki et al. 2008; Furuhjelm et al. 2009; Thienprasert et al. 2009; Minns et al. 2010; Birch et al. 2010b) and the developing autonomic nervous system (Gustafson et al. 2008; Lauritzen et al. 2008). These systems as well as brain development could have long‐term effects on the health and chronic illness rates in the developing world and should be kept in mind when considering the potential physiological consequences of limited fetal and infant LCPUFA status.
Several major factors have the potential to influence maternal transfer of precursor EFA and n‐3 and n‐6 LCPUFA to the fetus and infant. These include placental function, maternal LCPUFA status (adipose storage, intake and circulating levels of fatty acids, particularly DHA), and FATs and FABPs that are likely involved in the apparent selectivity of DHA for mobilization, placental transfer, uptake by the fetus and further uptake into infant tissues (Larque et al. 2006; Ehehalt et al. 2008). After a brief explanation of biomarkers used to determine LCPUFA status, we focus on differences in maternal LCPUFA status and the relationship to that of the fetus and infant. Subsequent sections address other factors that influence LCPUFA status during pregnancy and lactation.
Key messages
-
•
LCPUFA transfer from mother to her fetus/newborn during pregnancy and lactation is mainly dependent upon maternal status, but genetic influences are also now recognized.
-
•
Transfer is highly variable because there are large differences worldwide in dietary intake of LCPUFA, particularly DHA.
-
•
Most studies of maternal and infant LCPUFA have been conducted in Europe and North America.
-
•
The evidence for effects of all forms of nutrient inadequacy is generally absent; however, animal models provide some evidence for adverse effects of malnutrition on LCPUFA status.
-
•
Studies in the developing world are few and needed; it is reasonable to assume that LCPUFA status is influenced by poor nutritional status.
Biomarkers of LCPUFA status
All EFAs are derived from the diet; and the levels of LA and DHA in various blood lipid pools and tissues have been shown, in particular, to reflect dietary intake and are therefore used as biomarkers of intake. Many studies have furthermore employed such markers in plasma, red blood cell (RBC) or adipose tissue to estimate functional status in relation to health in the absence of available tissue from more specific organs of interest. The PUFA content in RBC has been shown to reflect levels in the heart of adults (Harris et al. 2004). The relative amount of DHA in RBC has also been shown to correlate reasonably well with the DHA content of the brain of animals (Carlson et al. 1986; Ward et al. 1998) and human infants (Makrides et al. 1994) fed a consistent intake of EFA/LCPUFA. Thus, during early development (fetal and infant life), both RBC and plasma lipid DHA are assumed to reflect brain DHA accumulation. However, DHA in blood lipids will not necessarily reflect brain DHA during dynamic periods of depletion and repletion. For example, RBC, plasma and brain cortex of rhesus monkeys fed an ALA‐deficient diet were depleted of DHA; when fish oil was introduced to the diet, the DHA in their blood lipid compartments and brain recovered, but recovery was faster in blood lipids than in the brain and the two tissues accumulated different amounts of DHA (Connor et al. 1990).
Connor et al. (1990) were the first to show that the brain depleted of DHA during both prenatal and postnatal developments could achieve apparently normal DHA composition with later provision of DHA from fish oil. In a later study, rhesus monkey infants depleted only prenatally were given ALA for 15 weeks and brain cortex n‐3 LCPUFA was normalized; however, at 3 years of age, retinal DHA remained at only 84% of controls and the repleted group continued to have lower retinal function (Anderson et al. 2005). Although there are functional effects of presumed lower early brain DHA accumulation (for recent examples, see Makrides et al. 2009 and Birch et al. 2010a), no human study has been designed to evaluate recovery following n‐3 LCPUFA remediation.
Maternal DHA status
As noted previously, DHA in maternal blood lipids is an indicator of maternal DHA status as well as the immediate source of DHA for transport to the fetus and infant. Among the factors that determine maternal DHA and AA status are the tissue stores and dietary intake. Recent evidence suggests that maternal DHA status is influenced as well by single nucleotide polymorphisms (SNPs) in the fatty acid desaturases (FADS) (Schaeffer et al. 2006; Xie & Innis 2008). An elegant study done by Hornstra's group (Otto et al. 1997) documented the changes in plasma phospholipid (PL) DHA during pregnancy among women from a number of countries consuming different amounts of DHA. The study provides insight into the combined effects of pregnancy and presumed differences in tissue stores. Maternal plasma PL‐DHA concentration (mg L–1) and the relative percent of total fatty acids (wt/wt total fatty acids) throughout pregnancy were determined. Despite their variable DHA status, all groups experienced an increase in DHA concentration after a 14‐week gestation that was unrelated to DHA intake. Only women from the country having the highest DHA status at 14 weeks had a progressive increase in the DHA concentration in plasma to term. It is presumed that the increase occurred in response to the mobilization of DHA from adipose tissue stores under the physiological influences of pregnancy. Oestrogen has been shown to increase DHA synthesis (Pawlosky et al. 2003) and circulating oestrogen levels increase dramatically during pregnancy.
In other studies, this group of investigators demonstrated that relative percent of DHA (wt/wt of total fatty acids) in maternal plasma decreased later in pregnancy (Foreman van Drongelen et al. 1995; Al et al. 1997) and declined further after parturition. The decline was larger in women who breastfed and was mitigated by intake of DHA (Otto et al. 1999), suggesting that at least some of the decline could be because of less than optimal maternal DHA status and/or further depletion of DHA stores as well as reduced mobilization of DHA from tissue. Multiparous women have also been shown to have lower plasma DHA during pregnancy than primiparous women (both concentration and relative to total fatty acids) (Al et al. 1997). Consistent with these observations, pregnancy number was inversely related to the amount of DHA in maternal plasma (Al et al. 1997), which suggests a depletion of maternal DHA stores with an increase in the number of pregnancies.
LCPUFA transferred from the mother to her fetus during pregnancy and to her infant during lactation are incorporated into membranes in the growing tissues of the fetus and infant. In the central nervous system, the proportion of membrane fatty acids as DHA increases even as the size of the brain increases; and the increase in proportion of fatty acids continues for the second year of life even as brain growth begins to plateau (Martinez 1992). Brain cortical DHA is increased in infants fed human milk compared with formula without DHA (Farquharson et al. 1992; Makrides et al. 1994; Jamieson et al. 1999). If as has been presumed, this is related to differences in DHA intake, it cannot be assumed that brain DHA accumulation is optimal in all circumstances. The factors that could influence LCPUFA transfer to tissues, particularly to the developing brain, are a major point of this review.
Intrauterine fatty acid accumulation
Maternal and fetal blood and adipose LCPUFA
Between the 10th and 30th week of gestation, maternal fat mass increases by ∼7 lbs. The accumulation of fat mass reaches a plateau at approximately 30‐week gestation, and maternal plasma non‐esterified fatty acids (NEFAs) increased because of maternal lipolysis. Maternal NEFAs provide the fuel for fetal triacylglycerol (TAG) synthesis, responsible for the increase in fetal fat mass beginning at approximately 30‐weeks gestatio (Haggarty 2004). Interestingly (and of possible consequence to ultimate transfer of maternal LCPUFA to the fetus), NEFA from maternal intake and stores appear to be the major fuel for the fetus during the first two trimesters, while it is glucose that is mainly transferred across the placenta and is the major fuel in the 3rd trimester (Haggarty 2004).
In contrast to maternal adipose tissue accumulation, which plateaus at approximately 30 weeks of gestation, the concentration of maternal plasma TAG increases progressively throughout pregnancy (Haggarty 2004), and the PUFA in TAG of all lipoprotein classes is higher in the 3rd trimester than post‐partum (Herrera 2002). Compared with the fetus, the mother has a much higher absolute concentration of all plasma lipids, including DHA and AA; however, the relative proportion of DHA and AA in total fatty acids of circulating lipids (PL, TAG and cholesterol esters (CE)) is consistently higher in the neonate (Haggarty 2010): the increases can be as much as 16‐fold for DHA and 90‐fold for AA (Clandinin et al. 1981; Haggarty 2004). Crawford and coworkers (1976) defined this apparent preferential transfer of DHA and AA to the infant from the mother as ‘biomagnification’.
By combining data from a number of published sources, Haggarty (2004) created a composite illustration of the mother and fetus for the proportion of total fatty acids as DHA and AA in maternal and infant plasma, adipose tissue, and brain lipids. His composite data show that the per cent of total fatty acids as DHA and AA is greatly elevated in the fetal compared with maternal plasma lipid classes (PL, TAG, CE and NEFA), supporting selective transfer to the fetus (Haggarty 2004). In a later review, Haggarty (2010) compared the PL concentration of DHA and AA in maternal blood, intervillous spaces, microvillus and basal membrane of the syncytiotrophoblast, and fetal circulation, and concluded that the degree of enrichment at the microvillus border alone could be responsible for the biomagnifications of DHA in fetal circulation.
The DHA status of the neonate – whether measured in esterified blood lipids or in the umbilical artery wall – increases with gestational age (Foreman van Drongelen et al. 1995). In addition to the effects of pregnancy and gestation on DHA status of the fetus, maternal and newborn DHA status are highly correlated at the time of delivery (Fig. 1), evidence that higher maternal DHA status can increase the DHA status of the newborn.
Figure 1.
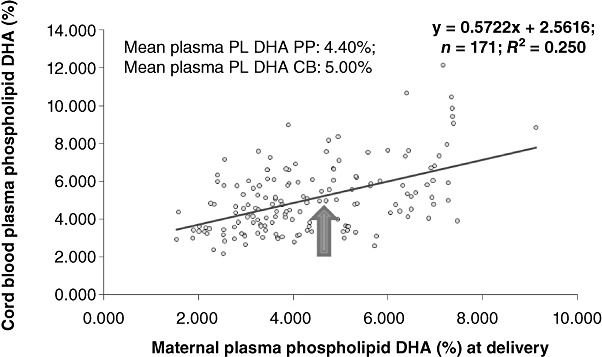
Relationship between maternal and cord blood plasma phospholipid docosahexaenoic acid (DHA; weight percent) at delivery – showing high degree of correlation but also large individual variability (Carlson, unpublished data). PL, plasma; PL PP, plasma phospholipid.
The absolute amount of DHA in fetal adipose tissue exceeds that in the brain from around the 25th week of gestation; and the last trimester increase in the quantity of DHA in fetal adipose tissue greatly exceeds the quantitative increase in brain DHA. It has been suggested that fetal accumulation of DHA in adipose tissue supports the need for fetal DHA accumulation by various organs and serves as a pool of DHA for growing organs and tissues after birth (Haggarty 2004). Farquharson et al. (1993) found an extremely low percentage of DHA in adipose tissue by 2 months of age, possible evidence that the DHA pool in adipose tissue is quite labile. The estimates made by Haggarty (2004) suggest that maximal fetal adipose DHA accumulation should occur with usual maternal DHA intakes of approximately 325 mg day−1. Recent work suggests that differences in SNPs for FADS 1/2 and phosphatidylethanolamine methyl transferase could also influence both LCPUFA‐status (Xie & Innis 2008) and DHA concentration in brain phosphatidylcholine (da Costa et al. 2010).
Intrauterine maternal to fetal fatty acid transfer
NEFAs are transported across the placenta. The NEFA concentration in the plasma of pregnant women during the last trimester is twofold higher than in non‐pregnant women (Campbell et al. 1998). Plasma lipid concentrations of the fetus and neonate, on the other hand, are low because of a limited fatty acid transfer across the human placenta (Campbell et al. 1997). Thus, at the fetal–maternal interface, there is a large concentration difference to drive the transport (Campbell et al. 1998). Placental lipid transport has specificity for DHA and AA relative to other fatty acids, but DHA is preferentially accumulated in the fetal circulation compared with other PUFA (Crabtree et al. 1998). Maternal DHA is accumulated by the fetus at a threefold higher rate than AA, but only 1.3 and 1.6 times higher rate than ALA and LA, respectively (Crabtree et al. 1998). AA crosses the placenta at higher rates but is retained in placental tissue (Crabtree et al. 1998).
Placental plasma membrane fatty acid binding protein (p‐FABPpm) has a preference for AA and DHA and is located on the maternal side of the syncytiotrophoblast, favouring the unidirectional flow of maternal LCPUFA to the syncytiotrophoblast (Campbell & Dutta‐Roy 1995; Dutta‐Roy 1997). In a human placental cell line (BeWo) that expresses p‐FABPpm (Campbell et al. 1997), DHA was preferentially incorporated into TAG whereas AA was primarily esterified in PL (Campbell et al. 1997; Crabtree et al. 1998). Differential esterification and retention by the placenta could result in preferential transport of DHA > AA, with greater AA accumulation in the placenta. The most recent support for selective transfer of DHA to the fetus comes from Gil‐Sanchez et al. (2010). These researchers used 13C‐labelled fatty acids and demonstrated elevated specific activity of DHA (but not palmitic, oleic or LA) in cord compared with maternal plasma 12 h after maternal oral application of the fatty acid.
Many reports show a relationship between maternal and newborn DHA status and it is evident that in general higher maternal DHA intake can increase infant DHA status. As shown in Fig. 1, a large amount of the variance in infant plasma PL‐DHA at birth (25%) can be explained by knowing the maternal plasma PL‐DHA. As clearly illustrated by the figure, however, the individual relationships demonstrate a considerable variability. The variability may be related to any of a number of factors including differences in placental quality, maternal to fetal transfer, fetal uptake, fetal distribution into tissues and fetal synthesis.
Fetal DHA and AA synthesis
Fetal hepatic microsomes and neonates demonstrate significant Δ6‐ and Δ5‐desaturase activities (Rodriguez et al. 1998), and stable isotope technology has provided evidence that both term and preterm infants convert LA to AA and ALA to DHA (Salem et al. 1996; Sauerwald et al. 1997; Szitanyi et al. 1999). It has later been shown that this biosynthetic capacity decreases with gestational age (Uauy et al. 2000). Carnielli et al. (2007) did the first quantitative study of DHA and AA synthesis in preterm infants, where they capitalized on the natural abundance of 13C in components of infant formula and used both time and LCPUFA intake (DHA and AA) as variables. Not only did they quantify synthesis, but they were also able to determine the proportion of synthesized DHA and AA in relation to the amount obtained from the diet, which contained 0.64 FA% DHA and 0.84 FA% AA or none of these LCPUFA. At 1 month of age (∼32 weeks gestation equivalent), infants synthesized ∼40 mg AA per kg body weight per day and approximately 13 mg DHA per kg per day or approximately 40 and 65% of the total AA and DHA (exogenous plus endogenous), respectively. By 3 months of age (∼1 month past expected term), synthesis of these same fatty acids had declined to approximately 14 and 3 mg kg−1 per day. It cannot be determined whether this was because of feedback inhibition from dietary LCPUFA or related to developmental programming, but the biosynthesis capacity of infants is significant compared with the estimated fetal accretion rate of 45 mg kg−1 per day (Lin et al. 2010). Another very recent study dosed newborn term infants with labelled ALA and labelled eicosapentaenoic acid (EPA, 20:5n‐3), and showed that although endogenously formed EPA was more efficiently converted to DHA than the externally supplied EPA, provision of EPA was almost four times as effective as ALA with respect to maintenance of DHA status (Lin et al. 2010). The investigators furthermore estimated that the infant needed 5‐mg preformed DHA per kg per day in order to maintain plasma DHA homeostasis (Lin et al. 2010).
Other factors that affect fetal DHA and AA status
Haggarty (2004) illustrated a role for longer gestation and higher maternal DHA intake in relation to the final expected accumulation of DHA in fetal adipose tissue. From this it may be inferred that infants born before term or born to women on low DHA intakes could accumulate suboptimal amounts to support later development. He notes as well the potential for post‐natal dietary intake of DHA to mitigate a deficit in DHA formed in utero. The relative adequacy of DHA accumulated by the fetus likely depends on the sum of adipose tissue accumulation in utero, the infant's capacity for biosynthesis and post‐natal DHA intake. The timing as well as the quantity of DHA accumulation appears to be important; e.g. intrauterine accumulation may be more favourable than post‐natal accumulation. Rats depleted of brain cortex DHA and repleted after weaning continued to have abnormal dopaminergic function in adulthood (Levant et al. 2004). Studies done by a different group found that this was also the case for the serotoninergic system (Kodas et al. 2004).
We also point out that estimates made by Haggarty (2004) were based on LCPUFA analysis of tissues from women consuming a self‐selected diet (and their infants). Consequently, his estimates reflect ongoing intake and the LCPUFA stores supported by that intake. It cannot be assumed that women consuming a diet (whether low in DHA or inadequate in protein or micronutrients, as might occur in women with poor financial resources) can meet the DHA needs in pregnancy. Studies are currently under way in low‐DHA consuming groups, and it will be interesting to learn what, if any, benefits will be found for infant development. At present, the observational studies (Cheruku et al. 2002; Colombo et al. 2004; Bakker et al. 2007; Hibbeln et al. 2007; Jacobson et al. 2008; Kannass et al. 2009; Mendez et al. 2009) are suggestive evidence for benefit of higher usual DHA intakes. Ramakrishnan et al. (2010) followed the pregnancies of nearly 1000 women assigned randomly to placebo or a supplement of 400 mg DHA per day beginning between the 18th and 22nd weeks of gestation. Mean baseline DHA intake in the population was very low (55 mg day−1). Neither gestational age nor infant size at birth was influenced by supplementation, although capsule compliance was excellent and the supplemented mothers and their infants had significantly higher plasma DHA at delivery.
A number of high‐risk pregnancy conditions can influence placental function and could therefore influence maternal to fetal transfer of LCPUFA. These are considered in light of the present focus on low maternal income or low intake of DHA. More studies of diabetes mellitus [types I, II or gestational diabetes mellitus (GDM)] have been reported compared with other high‐risk conditions although there do not appear to be any studies that have made a concerted effort to enroll large numbers of women with diabetes mellitus. It is difficult to generalize about the effects of diabetes mellitus on placental transfer directly or as viewed from the perspective of maternal and infant status, especially because there is no uniformity in the measures of LCPUFA status obtained among studies (a common situation during the exploratory stages of investigation of a new area). There is evidence of limited transfer to the fetus based on several studies from Crawford's group, who have reported birth fatty acid status of infants born to women with type I (Ghebremeskel et al. 2004; Min et al. 2005), type II (Min et al. 2005) and GDM (Thomas et al. 2005). In each case, the researchers reported lower plasma DHA and AA in the offspring and Ortega‐Senovilla et al. (2009) found lower AA and total n‐6 fatty acids but not lower DHA in the cord arterial blood (but not in cord venous blood, the presumed source of cord blood in studies that measure cord blood fatty acids). Some evidence exists for reduced placental LCPUFA transfer based on higher DHA and AA in the placenta of women with GDM (Bitsanis et al. 2006), consistent with two studies that found GDM to be associated with higher DHA in PL as well as TAG of maternal plasma (Wijendran et al. 1999; Thomas et al. 2004). On the other hand, Min et al. (2006) reported lower DHA and AA in the RBC‐PC of Korean and British women with GDM compared with population controls without GDM, suggesting a non‐placental effect such as differences in maternal mobilization of DHA and AA from tissue stores. A similar inability to mobilize LCPUFA could be involved in lower milk LCPUFA found in women with insulin‐dependent diabetes mellitus (Jackson et al. 1994).
Maternal smoking, obesity, pre‐eclampsia and intrauterine growth retardation (IUGR) have been mentioned in a more limited number of observational reports related to LCPUFA status [although several reports demonstrate that' a high incidence of smoking during pregnancy (Smuts et al. 2003; Magnusardottir et al. 2009) may be a confounder in pregnancy studies]. Agostoni et al. (2005) found lower DHA and AA in whole blood of 4‐day‐old infants whose mothers continued to smoke during pregnancy compared with those who did not smoke and, in the case of DHA, compared with those who stopped smoking early in the pregnancy. One study (Magnusardottir et al. 2009) implicates lower maternal DHA in RBC‐PL in smokers, but the effect was relatively small compared with the effects of DHA intake. Only one published study has compared maternal body mass index (BMI) with maternal plasma PL‐DHA: Wijendran et al. (1999) found an inverse relationship between obesity and maternal plasma PL‐DHA in a small sample. We (SE Carlson) compared maternal plasma PL‐DHA with enrollment BMI in 352 women enrolled between 12 and 20‐week gestation (see Fig. 2) and found no relationship. It would be of interest, however, to determine if maternal BMI affects newborn DHA status.
Figure 2.
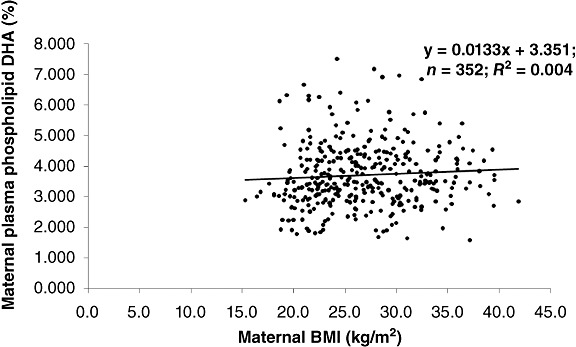
Relationship between maternal body mass index (BMI) and plasma phospholipid docosahexaenoic acid (DHA) during pregnancy (Carlson, unpublished data).
Hypertensive disorders also appear to reduce transfer of DHA and AA to the fetus (Wang et al. 1991; Velzing Aarts et al. 1999), with lower free fatty acid DHA and AA in placental tissue (Wang et al. 2005). Women from a high DHA intake group who developed pre‐eclampsia had significantly reduced DHA in the umbilical vein wall compared with women without pre‐eclampsia; however, the umbilical artery wall (coming from the fetus) had higher DHA in both groups compared with the vein wall and this did not differ between groups (Huiskes et al. 2009). These findings could be evidence of reduced maternal to fetal transfer of DHA without adverse effects on the DHA status of the fetus. Possible explanations would include some kind of compensation through fetal synthesis (the placenta does not make DHA) or accumulation of maternal DHA by the fetus prior to the development of pre‐eclampsia. IUGR infants represent a possible fruitful group to study in relation to the effects on LCPUFA transfer and newborn status. We could not find any comparisons of newborn LCPUFA status in relation to appropriate compared with low intrauterine growth. Increased placental lipolytic activity (Biale 1985) and up‐regulation of placental lipoprotein lipase (Tabano et al. 2006) have been reported in pregnancies complicated by IUGR.
Maternal to infant transfer in milk
It is well known that levels of DHA in infant blood correlate significantly with DHA in both maternal blood and milk – which again are correlated with each other (see Lauritzen et al. 2001 and Fig. 3). Curiously, no such correlation is seen for AA (Fig. 3). In 17 mothers and their term breastfed infants over the first 4 months of lactation, Jørgensen et al. (2006) found a decrease in maternal DHA status, which was not accompanied by a decrease in the status of the infant. Furthermore, the infant had a higher LCPUFA to precursor EFA ratio, which could indicate specific transfer of LCPUFA via the breast milk, but could also be explained by an extra desaturation cycle in the infant liver after ingestion of the LA and ALA from the milk (Jørgensen et al. 2006).
Figure 3.
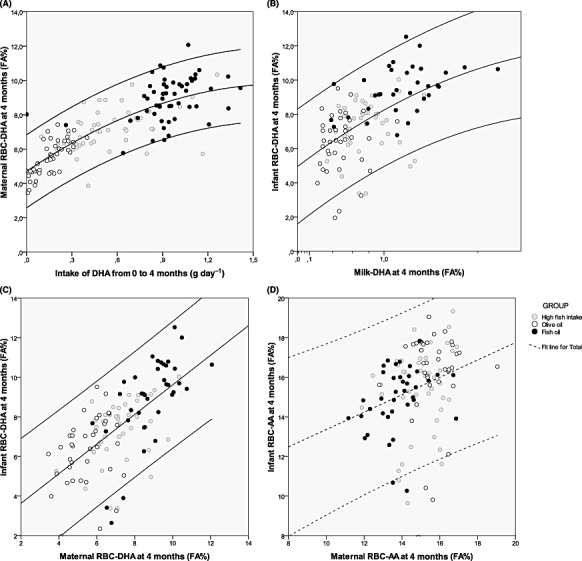
Correlations between intake and RBC status of DHA in mothers (A) and in infants (B) and between maternal and infant RBC status of DHA (C) and AA (D) 4 months after delivery. The calculated correlation coefficients for each of the curves are: (A) r = 0.806 (P < 0.001, n = 148); (B) r = 0.564 (P < 0.001, n = 115); (C) r = 0.712 (P < 0.001, n = 114); and (D) r = 0.086 (P = 0.352, n = 118). (Based on data from Lauritzen et al. 2004.)
Mammary fat excretion
Plasma TAG, predominately those from chylomicrons and very low‐density lipoprotein in fed animals, is the main source of fatty acids for milk secretion (Shennan & Peaker 2000) and diet; hepatic metabolism and adipose stores determine the fatty acid composition of the plasma TAG pool. Most of the milk lipids (about 60%) seem to be derived from maternal stores (Hachey et al. 1987). The exact adipose tissue contribution may depend on the size of the fat stores laid down during pregnancy, as the fat content of human milk has been shown to be influenced by maternal pregnancy weight gain (Michaelsen et al. 1994).
Lipoprotein lipase activity is abundant in the capillary endothelium of the mammary tissue, but the mechanism of NEFA uptake by the mammary epithelial cells has not been identified (Shennan & Peaker 2000). Several groups have isolated and characterized FAT and FABP‐analogues from mammary tissue (Barber et al. 1997). The FAT‐homologous CD36, which mediates fatty acid uptake, has been shown to be up‐regulated during lactation (Bionaz & Loor 2008). Intracellular FABP3 has also been shown to be abundant in mammary tissue and has been suggested to be one of the key components in the channelling of fatty acids towards milk fat globule synthesis in the bovine mammary gland (Bionaz & Loor 2008). As far as we know, no fatty acid specificity has been described for any of these proteins.
The lipids of mammary epithelial cells are located in small protein‐coated lipid droplets (or lipid bodies), which are secreted into the milk by exocytosis (Olofsson et al. 2009), and a unique budding process gives rise to the milk fat globules (McManaman & Neville 2003). Thus, milk fat globules have an outer shell of PL originating from the epithelial cell membrane, fractions of epithelial cell cytoplasm (and whatever substances that were dissolved herein), the original lipid droplet protein coat and a core of TAG. Milk globule formation starts in the endoplasmic reticulum and the primordial milk globule then fuse to increase the size of the globules (Olofsson et al. 2009). As lactation continues, total milk fat content increases and the ratio of PL to TAG decreases (Jensen 1999), suggesting that the milk fat globules increase in size.
Little is known about the regulation of lipid secretion by human mammary cells. As in other lipogenic tissues, mammary lipid secretion depends on the abundance of gene transcripts encoding for key lipogenic regulatory factors, e.g. sterol response element binding protein 1 (Shingfield et al. 2010) and PPARγ (Bionaz & Loor 2008). The expression of these transcription factors as well as the controlled lipogenic enzymes has been shown to vary during bovine lactation (Bionaz & Loor 2008) and to account for changes in the relative contribution of diet and stores relative to fatty acids synthesized by the mammary gland itself. The involved transcription factors have in other tissues been shown to be affected differentially by LCPUFA (Schmitz & Ecker 2008) and could thus to some extent play a role in the potential nutritional effects on milk fat composition. FADS 1/2 genotype has also been implicated in the DHA transfer from maternal plasma to breast milk: plasma but not milk DHA of women carrying the minor allele did not respond to a fish oil supplement, whereas women with the major allele (homo‐ or heterozygous) showed the expected increase in DHA in plasma and milk (Molto‐Puigmarti et al. 2010). Verification of this finding is needed as the number of subjects with the minor allele was low. FADS genotype has been shown to affect breast milk LCPUFA content, which in other studies has been interpreted as an effect on LCPUFA synthesis (Lattka et al. 2010)
The relative contribution of diet vs. stores to milk fatty acid composition is dependent upon the timing of food intake/duration of fasting. Fish or fish oil intake has been shown to have an acute effect on the content of DHA and total n‐3 fatty acids in the milk (Henderson et al. 1992; Francois et al. 1998; Lauritzen et al. 2002), which has been shown to last for 1–2 days (Francois et al. 1998). Dietary fat has been shown to have the greatest effect on milk lipids approximately 10 h after consumption (Emken et al. 1989; Francois et al. 1998), which fits with a peak in plasma TAG approximately 4 h after a meal and a mean milking frequency of around 4–5 h. At the peak of the response, the DHA content of the milk increased up to six times above the level of the unsupplemented milk (Francois et al. 1998; Lauritzen et al. 2002). Daily fish oil supplementation gradually increased morning milk DHA content, with a steady state reached after 1 week (Henderson et al. 1992).
Human milk fatty acids
A rapid post‐natal decline in infant adipose tissue DHA concentration is suggestive evidence that adipose DHA functions as a reservoir for DHA to supply the quantitatively much larger post‐natal DHA accumulation in the brain (Farquharson et al. 1993). The LCPUFAs from breast milk are a major source of infant tissue LCPUFA, but the relative contribution will depend on the milk LCPUFA content.
With dietary intake being equal, the relative LCPUFA composition of human milk declines during lactation (Luukkainen et al. 1994; Makrides et al. 1995; Jensen 1996, 1999), although the quantity per unit volume of milk may not decline (Marangoni et al. 2000). The ratio of ALA to LA in the milk correlated with that of the adipose tissue (Martin et al. 1991), although the relative content of n‐6 fatty acids in the stores was significantly higher than in the milk (1991, 1993). The fatty acid composition of breast milk closely reflects the fatty acid composition in maternal plasma lipids (Young et al. 1997; Jensen et al. 2000), but the ratio of ALA to LA of breast milk has also been shown to be higher (three to sixfold) than in plasma (Mellies et al. 1979; Marangoni et al. 2002). These observations could suggest that n‐3 fatty acids are to some degree specifically transferred to the milk. The ratio of LCPUFA to EFA (LA plus ALA), on the other hand, has been shown to be much lower (around 25%) in breast milk compared with plasma (Marangoni et al. 2002), which does not support the specific transfer of LCPUFA.
Marangoni et al. (2002) found significant correlations between plasma and milk for LA, ALA and DHA in Italian women, but a study in Indian women found no such correlations and only weak correlations between the overall content of n‐6 and n‐3 PUFA in milk and plasma (Kilari et al. 2009). Correlations for milk LCPUFA including DHA seem to be somewhat better with plasma PL (Jensen et al. 2000). LCPUFAs are specifically incorporated into the sn‐2 position of PL, the content of which decreases as milk globules increase in size during maturation of the milk. These changes could to some extent explain the decrease in LCPUFA content in the breast milk. The decline in milk LCPUFA could also be related to the decline in maternal plasma DHA to pre‐pregnancy amounts (or lower) after pregnancy or to a decline in maternal DHA status during lactation (Otto et al. 1999; Jørgensen et al. 2006).
Specific issues in low‐income countries
Based on their low fat intake, we might expect EFA levels to be low in milk and in mothers and their infants in most developing countries; however, the fatty acid composition of human milk in developing countries on the African continent has been shown to be quite similar to that of samples of European human milk (Koletzko et al. 1992). Comparisons of the worldwide variation in breast milk fatty acid composition does demonstrate a large variation in medium chain saturated fatty acids (MCFA) (e.g. Koletzko et al. 1992); and a low fat intake in developing countries has been shown to be associated with a milk MCFA > 10 FA% (Hachey et al. 1987). Low‐fat diets have been shown to result in increased mammary MCFA synthesis (Insull et al. 1959; Sanders & Reddy 1992). A study of milk fatty acid composition among tribes in Tanzania demonstrates a generally high amount of MCFA, AA and DHA, and low levels of LA (Kuipers et al. 2007). A newer review with focus on the breast milk content of DHA and AA also indicates little difference in the average content between high‐ and low‐income countries, although some values are outliers (e.g. Pakistan with very low levels of both DHA and AA) (Brenna et al. 2007). Sanders & Reddy (1992) made the observation that vegans and vegetarians have lower levels of DHA in their breast milk than omnivores. Infants of Hindu vegetarian mothers also have a lower DHA and a higher mead acid (20:3n‐9) relative to AA in cord plasma and artery wall than infants of matched omnivorous mothers (Sanders & Reddy 1992).
Conversion of ALA to DHA may be higher in a moderately undernourished population compared with a well‐nourished one because of an overall lower intake of fat and PUFA (e.g. see the paper by Gibson et al. in this issue of MCN). However, it is also possible that there will be less efficient utilization of EFA as more of the fat is used as energy at a low fat intake [e.g. EFA needs on fat‐free vs. specific EFA‐deficient diet (Cunnane & Anderson 1997)]. Several studies (e.g. Alessandri et al. 1996) have shown that the conversion rate of LA to AA and ALA to DHA is higher when the supply of LCPUFA is low, as LCPUFA inhibit the Δ6‐desaturase. De la Presa‐Owens and coworkers (1998) found a higher Δ5‐ and Δ6‐desaturase activity in liver microsomes from piglets fed formula without LCPUFA compared with a sow's milk reference. The increased activity was only evident with LA, whereas activity towards ALA was not affected. In this study, Δ6‐desaturation of ALA was decreased by addition of DHA to the formula, whereas addition of AA did not affect Δ6‐desaturation, but rather increased Δ5‐desaturation of n‐6 as well as n‐3 fatty acids. These findings are in sharp contrast to what would be expected according to the results of Emken and coworkers (1998), showing that dietary AA depressed AA as well as DHA formation in adult humans. The plasma fatty acid composition of 18‐month‐old Cambodian children has also indicated higher DHA and AA levels compared with that of Italian children (Agostoni et al. 2007), but this may be better explained by higher seafood intake in Cambodian compared with Italian children.
Beyond the first year of life, there is still a high rate of brain growth and brain DHA accretion should continue; however, the supply of n‐3 PUFA from complementary foods after weaning can be very low – this may be even more so in some developing countries depending upon the diet (see paper by Michaelsen et al. in this issue of MCN). US toddlers consumed only approximately 20 mg day−1 of DHA, and consumption of a supplement of approximately 100 mg day−1 for 2 months reduced upper respiratory infections dramatically (Minns et al. 2010). Few studies have looked at functional consequences of n‐3 LCPUFA supplementation later in childhood in developing countries. One of the few studies that did, the CHAMPION study (Muthayya et al. 2009), found no functional effects on growth or cognitive performance in 600 Indian schoolchildren after supplementation with a low dose of DHA (100 mg day−1 for 12 months). Other new studies have found indications of beneficial effects of n‐3 LCPUFA supplementation on illness and immune function in children in low‐ and high‐income countries (Pastor et al. 2006; Thienprasert et al. 2009; Minns et al. 2010).
Conclusion
Maternal DHA status is related to intrauterine and post‐natal (in human milk‐fed infants) DHA transfer. The supply of LCPUFA from the mother to the infant and the status of the mother and infant depend on the dietary intake of LCPUFA of the mother and, we now suspect from several studies of FADS alleles, the efficiency of metabolism and/or transfer. The n‐3 PUFA supplementation is more effective with n‐3 LCPUFA than ALA, but ALA supplementation could be important at low total fat intake. Some studies in the developing world indicate very good maternal LCPUFA status while others find very poor LCPUFA status. While much of the difference could be because of differences in LCPUFA intake, particularly n‐3 LCPUFA intake, there is also evidence that low (not deficient) intake of LA and ALA could contribute to higher LCPUFA status and transfer to the fetus/infant.
DHA and AA are preferentially transferred across the placenta, and in general, newborn DHA status is lower than maternal status. Nevertheless, when DHA is compared in maternal and newborn plasma phospholipids, the data suggest the existence of a factor or factors that influence maternal/placental transfer to the fetus or variable uptake by the fetus. Perhaps both kinds of influences are operative. Based on data from a recent trial (Fig. 2) that provided 600 mg day−1 DHA during the last half of pregnancy, it is clear that the women and their infants do not fall neatly into two distinct groups as randomized, indicating that other factors than intake most likely contribute to the variability.
There is currently no evidence of mechanisms of selectivity in the post‐natal supply of PUFA from the mother to the infant, although selective uptake by tissues would be anticipated based on what is known about differences in fatty acid composition among tissues regardless of dietary intake. AA levels in milk, blood and tissues are less subject to dietary modifications and thus need less attention, whereas n‐3 PUFA status may be the major problem in low‐income countries.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Agostoni C., Galli C., Riva E., Colombo C., Giovannini M. & Marangoni F. (2005) Reduced docosahexaenoic acid synthesis may contribute to growth restriction in infants born to mothers who smoke. Journal of Pediatrics 147, 854–856. [DOI] [PubMed] [Google Scholar]
- Agostoni C., Giovannini M., Sala D., Usuelli M., Livio L., Francescato G. et al (2007) Double‐blind, placebo‐controlled trial comparing effects of supplementation of two micronutrient sprinkles on fatty acid status in Cambodian infants. Journal of Pediatric Gastroenterology and Nutrition 44, 136–142. [DOI] [PubMed] [Google Scholar]
- Al M.D.M., van Houwelingen A.C. & Hornstra G. (1997) Relation between birth order and the maternal and neonatal docosahexaenoic acid status. European Journal of Clinical Nutrition 51, 548–553. [DOI] [PubMed] [Google Scholar]
- Alessandri J.M., Goustard B., Guesnet P. & Durand G. (1996) Polyunsaturated fatty acids status in blood, heart, liver, intestine, retina and brain of newborn piglets fed either sow milk or a milk replacer diet. Reproductive Nutrition and Development 36, 95–109. [DOI] [PubMed] [Google Scholar]
- Anderson G.J., Neuringer M., Lin D.S. & Connor W.E. (2005) Can prenatal n‐3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in Rhesus monkeys. Pediatric Research 58, 865–872. [DOI] [PubMed] [Google Scholar]
- Badart‐Smook A., van Houwelingen A.C., Al M.D.M., Kester A.D. & Hornstra G. (1997) Fetal growth is associated positively with maternal intake of riboflavin and negatively with maternal intake of linoleic acid. Journal of the American Dietetic Association 97, 867–870. [DOI] [PubMed] [Google Scholar]
- Bakker E.C., Hornstra G., Blanco C.E. & Vles J.S.H. (2007) Relationship between long‐chain polyunsaturated fatty acids at birth and motor function at 7 years of age. European Journal of Clinical Nutrition 63, 499–504. [DOI] [PubMed] [Google Scholar]
- Barber M.C., Clegg R.A., Travers M.T. & Vernon R.G. (1997) Lipid metabolism in the lactating mammary gland. Biochimica Biophysica Acta, Lipids and Lipid Metababolism 1347, 101–126. [DOI] [PubMed] [Google Scholar]
- Biale Y. (1985) Lipolytic‐activity in the placentas of chronically deprived fetuses. Acta Obstetrica et Gynecologica Scandinavica 64, 111–114. [DOI] [PubMed] [Google Scholar]
- Bionaz M. & Loor J.J. (2008) Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 9, 366. doi:10.1186/1471‐2164‐9‐366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch E.E., Carlson S.E., Hoffman D.R., Fitzgerald‐Gustafson K.M., Fu V.L.N., Drover J.R. et al (2010a) The DIAMOND (DHA Intake and Measurement of Neural Development) Study: a double‐masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. American Journal of Clinical Nutrition 91, 848–859. [DOI] [PubMed] [Google Scholar]
- Birch E.E., Khoury J.C., Berseth C.L., Castaneda Y.S., Couch J.M., Bean J. et al (2010b) The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. Journal of Pediatrics 156, 902–U68. [DOI] [PubMed] [Google Scholar]
- Bitsanis D., Ghebremeskel K., Moodley T., Crawford M.A. & Djahanbakhch O. (2006) Gestational diabetes mellitus enhances arachidonic and docosahexaenoic acids in placental phospholipids. Lipids 41, 341–346. [DOI] [PubMed] [Google Scholar]
- Brenna J.T., Varamini B., Jensen R.G., Diersen‐Schade D.A., Boettcher J.A. & Arterburn L.M. (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. American Journal of Clinical Nutrition 85, 1457–1464. [DOI] [PubMed] [Google Scholar]
- Burdge G.C., Dunn R.L., Wootton S.A. & Jackson A.A. (2002) Effect of reduced dietary protein intake on hepatic and plasma essential fatty acid concentrations in the adult female rat: effect of pregnancy and consequences for accumulation of arachidonic and docosahexaenoic acids in fetal liver and brain. British Journal of Nutrition 88, 379–387. [DOI] [PubMed] [Google Scholar]
- Burdge G.C., Delange E., Dubois L., Dunn R.L., Hanson M.A., Jackson A.A. et al (2003) Effect of reduced maternal protein intake in pregnancy in the rat on the fatty acid composition of brain, liver, plasma, heart and lung phospholipids of the offspring after weaning. British Journal of Nutrition 90, 345–352. [DOI] [PubMed] [Google Scholar]
- Campbell F.M. & Dutta‐Roy A.K. (1995) Plasma membrane fatty acid‐binding protein (FABPpm) is exclusively located in the maternal facing membranes of the human placenta. FEBS Letters 375, 227–230. [DOI] [PubMed] [Google Scholar]
- Campbell F.M., Clohessy A.M., Gordon M.J., Page K.R. & Dutta‐Roy A.K. (1997) Uptake of long chain fatty acids by human placental choriocarcinoma (BeWo) cells: role of plasma membrane fatty acid binding protein. Journal of Lipid Research 38, 2558–2568. [PubMed] [Google Scholar]
- Campbell F.M., Gordon M.J. & Dutta‐Roy A.K. (1998) Placental membrane fatty acid‐binding protein preferentially binds arachidonic and docosahexaenoic acids. Life Sciences 63, 235–240. [DOI] [PubMed] [Google Scholar]
- Carlson S.E., Carver J.D. & House S.G. (1986) High fat diets varying in ratios of polyunsaturated to saturated fatty acid and linoleic to linolenic acid: a comparison of rat neural and red cell membrane phospholipids. Journal of Nutrition 116, 718–725. [DOI] [PubMed] [Google Scholar]
- Carlson S.E., Werkman S.H., Peeples J.M., Cooke R.J. & Tolley E.A. (1993a) Arachidonic acid status correlates with first year growth in preterm infants. Proceedings of the National Academy of Sciences of the United States of America 90, 1073–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.E., Werkman S.H., Rhodes P.G. & Tolley E.A. (1993b) Visual acuity development in healthy preterm infants: effect of marine‐oil supplementation. American Journal of Clinical Nutrition 58, 35–42. [DOI] [PubMed] [Google Scholar]
- Carnielli V.P., Simonato M., Verlato G., Luijendijk I., De Curtis M., Sauer P.J. et al (2007) Synthesis of long‐chain polyunsaturated fatty acids in preterm newborns fed formula with long‐chain polyunsaturated fatty acids. American Journal of Clinical Nutrition 86, 1323–1330. [DOI] [PubMed] [Google Scholar]
- Cheruku S.R., Montgomery‐Downs H.E., Farkas S.L., Thoman E.B. & Lammi‐Keefe C.J. (2002) Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep‐state patterning. American Journal of Clinical Nutrition 76, 608–613. [DOI] [PubMed] [Google Scholar]
- Clandinin M.T., Chappell J.E., Heim T., Swyer P.R. & Chance G.W. (1981) Fatty acid utilization in perinatal de novo synthesis of tissues. Early Human Development 5, 355–366. [DOI] [PubMed] [Google Scholar]
- Clandinin M.T., van Aerde J.E., Merkel K.L., Harris C.L., Springer M.A., Hansen J.W. et al (2005) Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 146, 461–468. [DOI] [PubMed] [Google Scholar]
- Colombo J., Kannass K.N., Shaddy D.J., Kundurthi S., Maikranz J.M., Anderson C.J. et al (2004) Maternal DHA and the development of attention in infancy and toddlerhood. Child Development 75, 1254–1267. [DOI] [PubMed] [Google Scholar]
- Connor W.E., Neuringer M. & Lin D.S. (1990) Dietary effects on brain fatty acid composition: the reversibility of n‐3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain erythrocytes, and plasma of rhesus monkeys. Journal of Lipid Research 31, 237–247. [PubMed] [Google Scholar]
- da Costa K.A., Rai K.S., Craciunescu C.N., Parikh K., Mehedint M.G., Sanders L.M. et al (2010) Dietary docosahexaenoic acid supplementation modulates hippocampal development in the preterm mouse. Journal of Biological Chemistry 285, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J.T., Gordon M.J., Campbell F.M. & Dutta‐Roy A.K. (1998) Differential distribution and metabolism of arachidonic acid and docosahexaenoic acid by human placental choriocarcinoma (BeWo) cells. Molecular and Cellular Biochemistry 185, 191–198. [DOI] [PubMed] [Google Scholar]
- Crawford M.A., Hassam G.A., Williams G. & Whitehouse W.L. (1976) Essential fatty acids and fetal brain growth. Lancet 7957, 452–453. [DOI] [PubMed] [Google Scholar]
- Cunnane S.C. & Anderson M.J. (1997) Pure linoleate deficiency in the rat: influence on growth, accumulation of n‐6 polyunsaturates, and [1‐C‐14]linoleate oxidation. Journal of Lipid Research 38, 805–812. [PubMed] [Google Scholar]
- De la Presa‐Owens S., Innis S.M. & Rioux F.M. (1998) Addition of triglycerides with arachidonic acid or docosahexaenoic acid to infant formula has tissue‐ and lipid class‐specific effects on fatty acids and hepatic desaturase activities in formula‐fed piglets. Journal of Nutrition 128, 1376–1384. [DOI] [PubMed] [Google Scholar]
- Dutta‐Roy A.K. (1997) Transfer of long‐chain polyunsaturated fatty acids across the human placenta. Prenatal and Neonatal Medicine 2, 101–107. [Google Scholar]
- Ehehalt R., Sparla R., Kulaksiz H., Herrmann T., Fullekrug J. & Stremmel W. (2008) Uptake of long‐chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts). BMC Cell Biology 9, 45. doi:10.1186/1471‐2121‐9‐45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emken E.A., Adlof R.O., Hachey D.L., Garza C., Thomas M.R. & Brown‐Booth L. (1989) Incorporation of deuterium‐labeled fatty acids into human milk, plasma, and lipoprotein pyhospholipids and cholesteryl esters. Journal of Lipid Research 30, 395–402. [PubMed] [Google Scholar]
- Emken E.A., Adlof R.O., Duval S.M. & Nelson G.J. (1998) Effect of dietary arachidonic acid on metabolism of deuterated linoleic acid by adult male subjects. Lipids 33, 471–480. [DOI] [PubMed] [Google Scholar]
- Farquharson J., Cockburn F., Patrick W.A., Jamieson E.C. & Logan R.W. (1992) Infant cerebral cortex phospholipid fatty‐acid composition and diet. Lancet 340, 810–813. [DOI] [PubMed] [Google Scholar]
- Farquharson J., Cockburn F., Patrick W.A., Jamieson E.C. & Logan R.W. (1993) Effect of diet on infant subcutaneous tissue triglyceride fatty acids. Archives of Diseases in Childhood 69, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C.J., Van Aerdel J.E., Robinson L.E. & Clandinin M.T. (2008) Effect of providing a formula supplemented with long‐chain polyunsaturated fatty acids on immunity in full‐term neonates. British Journal of Nutrition 99, 91–99. [DOI] [PubMed] [Google Scholar]
- Foreman van Drongelen M.M., Al M.D.M., van Houwelingen A.C., Blanco C.E. & Hornstra G. (1995) Comparison between the essential fatty acid status of preterm and full‐term infants, measured in umbilical vessel walls. Early Human Development 42, 241–251. [DOI] [PubMed] [Google Scholar]
- Francois C.A., Connor S.L., Wander R.C. & Connor W.E. (1998) Acute effects of dietary fatty acids on the fatty acids of human milk. American Journal of Clinical Nutrition 67, 301–308. [DOI] [PubMed] [Google Scholar]
- Furuhjelm C., Warstedt K., Larsson J., Fredriksson M., Bottcher M.F., Falth‐Magnusson K. et al (2009) Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatrica 98, 1461–1467. [DOI] [PubMed] [Google Scholar]
- Ghebremeskel K., Thomas B., Lowy C., Min Y.J. & Crawford M.A. (2004) Type 1 diabetes compromises plasma arachidonic and docosahexaenoic acids in newborn babies. Lipids 39, 335–342. [DOI] [PubMed] [Google Scholar]
- Gil‐Sanchez A., Larque E., Demmelmair H., Acien M.I., Faber F.L., Parrilla J.J. et al (2010) Maternal‐fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. American Journal of Clinical Nutrition 92, 115–122. [DOI] [PubMed] [Google Scholar]
- Gustafson K.M., Colombo J. & Carlson S.E. (2008) Docosahexaenoic acid and cognitive function: is the link mediated by the autonomic nervous system? Prostaglandins, Leukotrienes, and Essential Fatty Acids 79, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachey D.L., Thomas M.R., Emken E.A., Garza C., Brown‐Booth L., Adlof R.O. et al (1987) Human lactation: maternal transfer of dietary triglycerides labeled with stable isotopes. Journal of Lipid Research 28, 1185–1192. [PubMed] [Google Scholar]
- Haggarty P. (2004) Effect of placental function on fatty acid requirements during pregnancy. European Journal of Clinical Nutrition 58, 1559–1570. [DOI] [PubMed] [Google Scholar]
- Haggarty P. (2010) Fatty acid supply to the human fetus. Annual Reviews of Nutrition 30, 237–255. [DOI] [PubMed] [Google Scholar]
- Hamazaki K., Syafruddin D., Tunru I.S., Azwir M.F., Asih P.B., Sawazaki S. et al (2008) The effects of docosahexaenoic acid‐rich fish oil on behavior, school attendance rate and malaria infection in school children – A double‐blind, randomized, placebo‐controlled trial in Lampung, Indonesia. Asia Pacific Journal of Clinical Nutrition 17, 258–263. [PubMed] [Google Scholar]
- Hansen H.S. (1986) The essential nature of linoleic acid in mammals. Trends in Biochemical Sciences 11, 263–265. [Google Scholar]
- Harris W.S., Sands S.A., Windsor S.L., Ali H.A., Stevens T.L., Magalski A. et al (2004) Omega‐3 fatty acids in cardiac biopsies from heart transplantation patients – Correlation with erythrocytes and response to supplementation. Circulation 110, 1645–1649. [DOI] [PubMed] [Google Scholar]
- Henderson R.A., Jensen R.G., Lammi Keefe C.J., Ferris A.M. & Dardick K.R. (1992) Effect of fish oil on the fatty acid composition of human milk and maternal and infant erythrocytes. Lipids 27, 863–869. [DOI] [PubMed] [Google Scholar]
- Henriksen C., Haugholt K., Lindgren M., Aurvag A.K., Ronnestad A., Gronn M. et al (2008) Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 121, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Herrera E. (2002) Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development – A review. Placenta 23, S9–S19. [DOI] [PubMed] [Google Scholar]
- Hibbeln J.R., Davis J.M., Steer C., Emmett P., Rogers I., Williams C. et al (2007) Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 369, 578–585. [DOI] [PubMed] [Google Scholar]
- Huiskes V.J.B., Kuipers R.S., Velzing‐Aarts F.V., Dijck‐Brouwer D.A.J., van der Meulen J. & Muskiet F.A.J. (2009) Higher de novo synthesized fatty acids and lower omega 3‐and omega 6‐long‐chain polyunsaturated fatty acids in umbilical vessels of women with preeclampsia and high fish intakes. Prostaglandins, Leukotrienes, and Essential Fatty Acids 80, 101–106. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2008) Dietary omega 3 fatty acids and the developing brain. Brain Research 1237, 35–43. [DOI] [PubMed] [Google Scholar]
- Insull W., Hirsch J., James T. & Ahrens E.H. (1959) The fatty acids of human milk. II. Alterations produced by manipulation of caloric balance and exchange of dietary fats. Journal of Clinical Investigation 38, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.B., Lammi‐Keefe C.J., Jensen R.G., Couch S.C. & Ferris A.M. (1994) Total lipid and fatty acid composition of milk from women with and without insulin‐dependent diabetes‐mellitus. American Journal of Clinical Nutrition 60, 353–361. [DOI] [PubMed] [Google Scholar]
- Jacobson J.L., Jacobson S.W., Muckle G., Kaplan‐Estrin M., Ayotte P. & Dewailly E. (2008) Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. Journal of Pediatrics 152, 356–364. [DOI] [PubMed] [Google Scholar]
- Jamieson E.C., Farquharson J., Logan R.W., Howatson A.G., Patrick W.J.A., Weaver L.T. et al (1999) Infant cerebellar gray and white matter fatty acids in relation to age and diet. Lipids 34, 1065–1071. [DOI] [PubMed] [Google Scholar]
- Jensen R.G. (1996) The lipids in human milk. Progress in Lipid Research 35, 53–92. [DOI] [PubMed] [Google Scholar]
- Jensen R.G. (1999) Lipids in human milk. Lipids 34, 1243–1271. [DOI] [PubMed] [Google Scholar]
- Jensen C.L., Maude M., Anderson R.E. & Heird W.C. (2000) Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. American Journal of Clinical Nutrition 71, 292S–299S. [DOI] [PubMed] [Google Scholar]
- Jørgensen M.H., Nielsen P.K., Michaelsen K.F., Lund P. & Lauritzen L. (2006) The relationship between long‐chain polyunsaturated fatty acids in breast‐milk, maternal and infant erythrocytes during the first four months of lactation. Maternal and Child Nutrition 2, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannass K.N., Colombo J. & Carlson S.E. (2009) Maternal DHA levels and toddler free‐play attention. Developmental Neuropsychology 34, 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilari A.S., Mehendale S.S., Dangat K.D., Yadav H.R., Kulakarni A.V., Dhobale M.V. et al (2009) Long‐chain polyunsaturated fatty acids in mothers and term babies. Journal of Perinatal Medicine 37, 513–518. [DOI] [PubMed] [Google Scholar]
- Kodas E., Galineau L., Bodard S., Vancassel S., Guilloteau D., Besnard J.C. et al (2004) Serotoninergic neurotransmission is affected by n‐3 polyunsaturated fatty acids in the rat. Journal of Neurochemistry 89, 695–702. [DOI] [PubMed] [Google Scholar]
- Koletzko B. & Braun M. (1991) Arachidonic acid and early human growth: is there a relation? Annals of Human Metabolism 35, 128–131. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Thiel I. & Abiodun P.O. (1992) The fatty acid composition of human milk in Europe and Africa. Journal of Pediatrics 120, S62–S70. [DOI] [PubMed] [Google Scholar]
- Kuipers R., Smit E.N., van der Meulen J., Dijck‐Brouwer J.D.A., Boersma R.E. & Muskiet F.A.J. (2007) Milk in the island of Chole [Tanzania] is high in lauric, myristic, arachidonic and docosahexaenoic acids, and low in linoleic acid. Reconstructed diet of infants born to our ancestors living in tropical coastal regions. Prostaglandins, Leukotrienes, and Essential Fatty Acids 76, 221–233. [DOI] [PubMed] [Google Scholar]
- Larque E., Krauss‐Etschmann S., Campoy C., Hartl D., Linde J., Klingler M. et al (2006) Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. American Journal of Clinical Nutrition 84, 853–861. [DOI] [PubMed] [Google Scholar]
- Lattka E., Illig T., Heinrich J. & Koletzko B. (2010) Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clinical Nutrition 29, 277–287. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Hansen H.S., Jørgensen M.H. & Michaelsen K.F. (2001) The essentiality of long‐chain n‐3 fatty acids in relation to development and function of the brain and retina. Progress in Lipid Research 40, 1–94. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Jørgensen M.H., Hansen H.S. & Michaelsen K.F. (2002) Fluctuations in human milk long‐chain PUFA levels in relation to dietary fish intake. Lipids 37, 237–244. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Jørgensen M.H., Mikkelsen T.B., Skovgaard M., Straarup E.M., Olsen S.F. et al (2004) Maternal fish oil supplementation in lactation: effect on visual acuity and n‐3 fatty acid content of infant erythrocytes. Lipids 39, 195–206. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Christensen J.H., Damsgaard C.T. & Michaelsen K.F. (2008) The effect of fish oil supplementation on heart rate in healthy Danish infants. Pediatric Research 64, 610–614. [DOI] [PubMed] [Google Scholar]
- LeBlanc C.P., Fiset S., Surette M.E., Turgeon O'Brien H. & Rioux F.M. (2009) Maternal iron deficiency alters essential fatty acid and eicosanoid metabolism and increases locomotion in adult guinea pig offspring. Journal of Nutrition 139, 1653–1659. [DOI] [PubMed] [Google Scholar]
- Levant B., Radel J.D. & Carlson S.E. (2004) Decreased brain docosahexaenoic acid during development alters dopamine‐related behaviors in adult rats that are differentially affected by dietary remediation. Behavioural Brain Research 152, 49–57. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Llanos A., Mena P., Uauy R., Salem N. & Pawlosky R.J. (2010) Compartmental analyses of H‐2(5)‐alpha‐linolenic acid and C‐13‐U‐eicosapentaenoic acid toward synthesis of plasma labeled 22:6‐23 in newborn term infants. American Journal of Clinical Nutrition 92, 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkainen P., Salo M.K. & Nikkari T. (1994) Changes in the fatty‐acid composition of preterm and term human‐milk from 1 week to 6 months of lactation. Journal of Pediatric Gastroenterology and Nutrition 18, 355–360. [DOI] [PubMed] [Google Scholar]
- McManaman J.L. & Neville M.C. (2003) Mammary physiology and milk secretion. Advanced Drug Delivery Reviews 55, 629–641. [DOI] [PubMed] [Google Scholar]
- McNamara R.K. & Carlson S.E. (2006) Role of omega‐3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins, Leukotrienes, and Essential Fatty Acids 75, 329–349. [DOI] [PubMed] [Google Scholar]
- Magnusardottir A.R., Steingrimsdottir L., Thorgeirsdottir H., Hauksson A. & Skuladottir G.V. (2009) Red blood cell n‐3 polyunsaturated fatty acids in first trimester of pregnancy are inversely associated with placental weight. Acta Obstetricia et Gynecologica Scandinavica 88, 91–97. [DOI] [PubMed] [Google Scholar]
- Makrides M., Neumann M.A., Byard R.W., Simmer K. & Gibson R.A. (1994) Fatty acid composition of brain, retina, and erythrocytes in breast‐ and formula‐fed infants. American Journal of Clinical Nutrition 60, 189–194. [DOI] [PubMed] [Google Scholar]
- Makrides M., Simmer K., Neumann M. & Gibson R.A. (1995) Changes in the polyunsaturated fatty acids of breast‐milk from mothers of full‐term infants over 30 wk of lactation. American Journal of Clinical Nutrition 61, 1231–1233. [DOI] [PubMed] [Google Scholar]
- Makrides M., Gibson R.A., McPhee A.J., Collins C.T., Davis P.G., Doyle L.W. et al (2009) Neurodevelopmental outcomes of preterm infants fed high‐dose docosahexaenoic acid: a randomized controlled trial. Obstetrical and Gynecological Survey 64, 297–298. [DOI] [PubMed] [Google Scholar]
- Marangoni F., Agostoni C., Lammardo A.M., Giovannini M., Galli C. & Riva E. (2000) Polyunsaturated fatty acid concentrations in human hindmilk are stable throughout 12‐months of lactation and provide a sustained intake to the infant during exclusive breastfeeding: an Italian study. British Journal of Nutrition 84, 103–109. [PubMed] [Google Scholar]
- Marangoni F., Agostoni C., Lammardo A.M., Bonvissuto M., Giovannini M., Galli C. et al (2002) Polyunsaturated fatty acids in maternal plasma and in breast milk. Prostaglandins, Leukotrienes, and Essential Fatty Acids 66, 535–540. [DOI] [PubMed] [Google Scholar]
- Martin J.C., Niyongabo T., Moreau L., Antoine J.M., Lanson M., Berger C. et al (1991) Essential fatty acid composition of human colostrum triglycerides – Its relationship with adipose tissue composition. American Journal of Clinical Nutrition 54, 829–835. [DOI] [PubMed] [Google Scholar]
- Martin J.C., Bougnoux P., Fignon A., Theret V., Antoine J.M., Lamisse F. et al (1993) Dependence of human milk essential fatty acids on adipose stores during lactation. American Journal of Clinical Nutrition 58, 653–659. [DOI] [PubMed] [Google Scholar]
- Martinez M. (1992) Tissue levels of polyunsaturated fatty acids during early human development. Journal of Pediatrics 120, S129–S138. [DOI] [PubMed] [Google Scholar]
- Mellies M.J., Ishikawa T.T., Gartside P.S., Burton K., MacGee J., Allen K. et al (1979) Effects of varying maternal dietary fatty acids in lactating women and their infants. American Journal of Clinical Nutrition 32, 299–303. [DOI] [PubMed] [Google Scholar]
- Mendez M.A., Torrent M., Julvez J., Ribas‐Fito N., Kogevinas M. & Sunyer J. (2009) Maternal fish and other seafood intakes during pregnancy and child neurodevelopment at age 4 years. Public Health Nutrition 12, 1702–1710. [DOI] [PubMed] [Google Scholar]
- Michaelsen K.F., Larsen P.S., Thomsen B.L. & Samuelson G. (1994) The Copenhagen cohort study on infant nutrition and growth – Breast‐milk intake, human milk macronutrient content and influencing factors. American Journal of Clinical Nutrition 59, 600–611. [DOI] [PubMed] [Google Scholar]
- Min Y.J., Lowy C., Ghebremeskel K., Thomas B., Offley‐Shore B. & Crawford M. (2005) Unfavorable effect of type 1 and type 2 diabetes on maternal and fetal essential fatty acid status: a potential marker of fetal insulin resistance. American Journal of Clinical Nutrition 82, 1162–1168. [DOI] [PubMed] [Google Scholar]
- Min Y.J., Nam J.H., Ghebremeskel K., Kim A. & Crawford M. (2006) A distinctive fatty acid profile in circulating lipids of Korean gestational diabetics: a pilot study. Diabetes Research and Clinical Practice 73, 178–183. [DOI] [PubMed] [Google Scholar]
- Minns L.M., Kerling E.H., Neely M.R., Sullivan D.K., Wampler J.L., Harris C.L. et al (2010) Toddler formula supplemented with docosahexaenoic acid (DHA) improves DHA status and respiratory health in a randomized, double‐blind, controlled trial of US children less than 3 years of age. Prostaglandins, Leukotrienes, and Essential Fatty Acids 82, 287–293. [DOI] [PubMed] [Google Scholar]
- Molto‐Puigmarti C., Plat J., Mensink R.P., Muller A., Jansen E., Zeegers M.P. et al (2010) FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. American Journal of Clinical Nutrition 91, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Muthayya S., Eilander A., Transler C., Thomas T., van der Knaap H.C.M., Srinivasan K. et al (2009) Effect of fortification with multiple micronutrients and n‐3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children's Health and Mental Performance Influenced by Optimal Nutrition) Study. American Journal of Clinical Nutrition 89, 1766–1775. [DOI] [PubMed] [Google Scholar]
- O'Connor D.L., Hall R., Adamkin D., Auestad N., Castillo M., Connor W.E. et al (2001) Growth and development in preterm infants fed long‐chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 108, 359–371. [DOI] [PubMed] [Google Scholar]
- Olofsson S.O., Bostrom P., Andersson L., Rutberg M., Perman J. & Boren J. (2009) Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids 1791, 448–458. [DOI] [PubMed] [Google Scholar]
- Ortega‐Senovilla H., Alvino G., Taricco E., Cetin I. & Herrera E. (2009) Gestational diabetes mellitus upsets the proportion of fatty acids in umbilical arterial but not venous plasma. Diabetes Care 32, 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S.J., van Houwelingen A.C., Antal M., Manninen A., Godfrey K., Lopez Jaramillo P. et al (1997) Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. European Journal of Clinical Nutrition 51, 232–242. [DOI] [PubMed] [Google Scholar]
- Otto S.J., van Houwelingen A.C., Badart‐Smook A. & Hornstra G. (1999) The postpartum docosahexaenoic acid status of lactating and nonlactating mothers. Lipids 34, S227. [DOI] [PubMed] [Google Scholar]
- Pastor N., Soler B., Mitmesser S.H., Ferguson P. & Lifschitz C. (2006) Infants fed docosahexaenoic acid‐ and arachidonic acid‐supplemented formula have decreased incidence of bronchiolitis/bronchitis the first year of life. Clinical Pediatrics 45, 850–855. [DOI] [PubMed] [Google Scholar]
- Pawlosky R.J., Hibbeln J.R., Lin Y.H., Goodson S., Riggs P., Sebring N. et al (2003) Effects of beef‐and fish‐based diets on the kinetics of n‐3 fatty acid metabolism in human subjects. American Journal of Clinical Nutrition 77, 565–572. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U., Stein A.D., Parra‐Cabrera S., Wang M., Imhoff‐Kunsch B., Juarez‐Marquez S. et al (2010) Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double‐blind, placebo‐controlled trial in Mexico. Food and Nutrition Bulletin 31, S108–S116. [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Sarda P., Nessmann C., Boulot P., Leger C.L. & Descomps B. (1998) Delta 6‐ and Delta 5‐desaturase activities in the human fetal liver: kinetic aspects. Journal of Lipid Research 39, 1825–1832. [PubMed] [Google Scholar]
- Salem N. Jr, Wegher B., Mena P. & Uauy R. (1996) Arachidonic and docosahexaenoic acids are biosynthesized from their 18‐carbon precursors in human infants. Proceedings of the National Academy of Sciences of the United States of America 93, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T.A.B. & Reddy S. (1992) The influence of a vegetarian diet on the fatty acid composition of human milk and the essential fatty acid status of the infant. Journal of Pediatrics 120, s71–s77. [DOI] [PubMed] [Google Scholar]
- Sauerwald T.U., Hachey D.L., Jensen C.L., Chen H., Anderson R.E. & Heird W.C. (1997) Intermediates in endogenous synthesis of C22:6 omega 3 and C20:4 omega 6 by term and preterm infants. Pediatric Research 41, 183–187. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Gohlke H., Muller M., Heid I.M., Palmer L.J., Kompauer I. et al (2006) Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Human Molecular Genetics 15, 1745–1756. [DOI] [PubMed] [Google Scholar]
- Schmitz G. & Ecker J. (2008) The opposing effects of n‐3 and n‐6 fatty acids. Progress in Lipid Research 47, 147–155. [DOI] [PubMed] [Google Scholar]
- Shennan D.B. & Peaker M. (2000) Transport of milk constituents by the mammary gland. Physiological Reviews 80, 925–951. [DOI] [PubMed] [Google Scholar]
- Shingfield K.J., Bernard L., Leroux C. & Chilliard Y. (2010) Role of trans‐fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 4, 1140–1166. [DOI] [PubMed] [Google Scholar]
- Smuts C.M., Huang M.Z., Mundy D., Plasse T., Major S. & Carlson S.E. (2003) A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstetrics and Gynecology 101, 469–479. [DOI] [PubMed] [Google Scholar]
- Szitanyi P., Koletzko B., Mydlilova A. & Demmelmair H. (1999) Metabolism of C‐13‐labeled linoleic acid in newborn infants during the first week of life. Pediatric Research 45, 669–673. [DOI] [PubMed] [Google Scholar]
- Tabano S., Alvino G., Antonazzo P., Grati F.R., Miozzo M. & Cetin I. (2006) Placental LPL gene expression is increased in severe intrauterine growth‐restricted pregnancies. Pediatric Research 59, 250–253. [DOI] [PubMed] [Google Scholar]
- Thienprasert A., Samuhaseneetoo S., Popplestone K., West A.L., Miles E.A. & Calder P.C. (2009) Fish oil n‐3 polyunsaturated fatty acids selectively affect plasma cytokines and decrease illness in Thai schoolchildren: a randomized, double‐blind, placebo‐controlled intervention trial. Journal of Pediatrics 154, 391–395. [DOI] [PubMed] [Google Scholar]
- Thomas B., Ghebremeskel K., Lowy C., Min Y. & Crawford M.A. (2004) Plasma AA and DHA levels are not compromised in newly diagnosed gestational diabetic women. European Journal of Clinical Nutrition 58, 1492–1497. [DOI] [PubMed] [Google Scholar]
- Thomas B.A., Ghebremeskel K., Lowy C., Offley‐Shore B. & Crawford M.A. (2005) Plasma fatty acids of neonates born to mothers with and without gestational diabetes. Prostaglandins, Leukotrienes, and Essential Fatty Acids 72, 335–341. [DOI] [PubMed] [Google Scholar]
- Uauy R.D., Mena P., Wegher B., Nieto S. & Salem N. Jr (2000) Long‐chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatric Research 47, 127–135. [DOI] [PubMed] [Google Scholar]
- Velzing Aarts F.V., van der Klis F.R.M., van der Dijs F.P.L. & Muskiet F.A.J. (1999) Umbilical vessels of preeclamptic women have low contents of both n‐3 and n‐6 long‐chain polyunsaturated fatty acids. American Journal of Clinical Nutrition 69, 293–298. [DOI] [PubMed] [Google Scholar]
- Wang Y.P., Kay H.H. & Killam A.P. (1991) Decreased levels of polyunsaturated fatty acids in preeclampsia. American Journal of Obstetrics and Gynecology 164, 812–818. [DOI] [PubMed] [Google Scholar]
- Wang Y.P., Walsh S.W. & Kay H.H. (2005) Placental tissue levels of nonesterified polyunsaturated fatty acids in normal and preeclamptic pregnancies. Hypertension in Pregnancy 24, 235–245. [DOI] [PubMed] [Google Scholar]
- Ward G.R., Huang Y.S., Bobik E., Xing H.C., Mutsaers L., Auestad N. et al (1998) Long‐chain polyunsaturated fatty acid levels in formulae influence deposition of docosahexaenoic acid and arachidonic acid in brain and red blood cells of artificially reared neonatal rats. Journal of Nutrition 128, 2473–2487. [DOI] [PubMed] [Google Scholar]
- Wijendran V., Bendel R.B., Couch S.C., Philipson E.H., Thomsen K., Zhang X.F. et al (1999) Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. American Journal of Clinical Nutrition 70, 53–61. [DOI] [PubMed] [Google Scholar]
- Xie L. & Innis S.M. (2008) Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n‐6) and (n‐3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast‐milk during lactation. Journal of Nutrition 138, 2222–2228. [DOI] [PubMed] [Google Scholar]
- Young C., Hikita T., Kaneko S., Shimizu Y., Hanaka S., Abe T. et al (1997) Fatty acid compositions of colostrum, cord blood, maternal blood and major infant formulas in Japan. Acta Paediatrica Japonica 39, 299–304. [DOI] [PubMed] [Google Scholar]


