Abstract
Good clinical practice recommends folic acid supplementation 1 month prior to pregnancy and during the first trimester to prevent congenital malformations. However, high rates of fetal growth and development in later pregnancy may increase the demand for folate. Folate and vitamins B12 and B6 are required for DNA synthesis and cell growth, and are involved in homocysteine metabolism. The primary aim of this study was to determine if maternal folate, vitamin B12, vitamin B6 and homocysteine concentrations at 18–20 weeks gestation are associated with subsequent adverse pregnancy outcomes, including pre‐eclampsia and intrauterine growth restriction (IUGR). The secondary aim was to investigate maternal B vitamin concentrations with DNA damage markers in maternal lymphocytes. A prospective observational study was conducted at the Women's and Children's Hospital, Adelaide, South Australia. One hundred and thirty‐seven subjects were identified prior to 20 weeks gestation as at high or low risk for subsequent adverse pregnancy outcome by senior obstetricians. Clinical status, dietary information, circulating micronutrients and genome damage biomarkers were assessed at 18–20 weeks gestation. Women who developed IUGR had reduced red blood cell (RBC) folate (P < 0.001) and increased plasma homocysteine concentrations (P < 0.001) compared with controls. Maternal DNA damage, represented by micronucleus frequency and nucleoplasmic bridges in lymphocytes, was positively correlated with homocysteine (r = 0.179, P = 0.038 and r = 0.171, P = 0.047, respectively). Multivariate regression analysis revealed RBC folate was a strong predictor of IUGR (P = 0.006). This study suggests that low maternal RBC folate and high homocysteine values in mid pregnancy are associated with subsequent reduced fetal growth.
Keywords: folate, B vitamins, homocysteine, pregnancy and nutrition, pregnancy outcome, low birth weight
Introduction
It is widely recognised that periconceptional folic acid (FA) supplementation reduces the risk of neural tube defects and other congenital malformations. Observational studies have suggested that reduced circulating folate in pregnancy is associated with increased risk for preterm birth, placental abruption, intrauterine growth restriction (IUGR) and pre‐eclampsia (Hibbard 1964; Ray & Laskin 1999; Lindblad et al. 2005; Czeizel et al. 2010), although clinical trials with FA have demonstrated varied effects (Scholl & Johnson 2000; Chiaffarino et al. 2010). Current good clinical practice in Australia recommends FA supplementation from at least 1 month prior to pregnancy and during the first trimester of pregnancy. Given that folate demand is increased in pregnancy (McPartlin et al. 1993), it may perhaps be of benefit to supplement throughout pregnancy. A recent publication has stated that further research to measure the effect of folate intake during pregnancy on reducing low birth weight and adverse pregnancy outcomes is an ‘urgent priority’ (Czeizel et al. 2010).
FA is an oxidised synthetic form of the vitamin, which does not exist in nature, being only found in fortified foods, supplements and pharmaceuticals. FA and dietary folate lack the ability to act as a substrate until they have been absorbed from the gastrointestinal tract and hepatically converted to the metabolically active 5‐methyltetrahydrofolate (5‐methyl‐THF) (Pietrzik et al. 2010). Active folates transfer carbon units, with vitamins B12 and B6 acting as cofactors for enzyme reactions in one‐carbon metabolism. One‐carbon metabolism is required for the synthesis of amino acids such as methionine and glycine, purines and pyrimidines, and the methylation of a large number of nucleic acids, proteins and lipids (Cravo et al. 1994; Fenech 2001).
When circulating concentrations of folate are high, homeostatic mechanisms prevent the accumulation of excessive folate in the tissues. Oral doses of FA in excess of 200–400 µg have been reported to lead to the direct appearance of unmetabolised FA in systemic circulation (Lucock et al. 1989; Kelly et al. 1997). This indicates a possible saturation point and arguably is indicative that large doses of FA may be pharmacological, rather than physiological
An inadequate supply of 5‐methyl‐THF, together with genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and other enzymes which slow the activity required for the conversion of homocysteine (Hcy) to methionine, results in increased Hcy concentrations. Hyperhomocysteinaemia is associated with reduced methylation, endothelial dysfunction and increased DNA damage, and has been linked to a number of serious diseases, including pregnancy complications (Dekker et al. 1995; Fenech et al. 1998; Vollset et al. 2000; Guerra‐Shinohara et al. 2002; Smith 2008). Growing evidence suggests that excessive Hcy may exert pathological effects through oxidative damage and apoptosis (Roberts & Redman 1993; Kark et al. 1999; Forges et al. 2007). It is plausible that these metabolic events contribute to placental vascular dysfunction and to the maternal endothelial dysfunction that has been associated with adverse pregnancy outcomes (Hague 2003).
Red blood cell (RBC), serum folate and plasma total Hcy concentrations are used for assessing folate status in humans. Serum folate concentrations change immediately after ingestion of FA and are therefore commonly measured in the fasting state for bioavailability studies, whereas RBC folate concentrations change slowly, providing a better indication of long‐term folate status (Pietrzik et al. 2010).
The hypothesis for this study was that low B vitamin status and increased plasma Hcy concentrations in pregnant women at 18–20 weeks gestation are associated with subsequent adverse pregnancy outcomes as well as with increased markers of maternal DNA damage.
Key messages
-
•
This study indicates that low maternal red blood cell and serum folate and high plasma homocysteine concentrations at 18–20 weeks gestation in human pregnancy are associated with the subsequent development of intrauterine growth restriction.
-
•
In addition, maternal homocysteine correlates with markers of maternal DNA damage in mid pregnancy.
Materials and methods
Study design
A prospective cohort study was conducted at the Women's and Children's Hospital (WCH), Adelaide, South Australia with approval from the Children's Youth and Women's Health Service Research Ethics Committee. Women were enrolled after obtaining informed consent. Fasting blood samples and questionnaires, including demographic, dietary and clinical data, were collected at 18–20 weeks gestation. Vitamin supplement information including brand, type, dose and daily intake was recorded. Pregnancy outcome data were collected after delivery by examination of the full clinical records, and confirmed after review by senior clinical medical staff.
Power analysis
Statsoft STATISTICA 10 software (StatSoft, Tulsa, OK, USA) was used to perform the power and sample size calculation for a case control study with continuous variables. Based on previous literature, the standard deviation (SD) used for plasma Hcy concentration at 18–20 weeks gestation was 1.0 µmol L−1 (Dodds et al. 2008). Using an α of 0.05 with 80% power, the sample size to determine a difference of 1.0 µmol L−1 plasma Hcy between normal and adverse pregnancy outcomes was calculated to be 17. To the best of our knowledge there are no published studies quoting the mean ± SD for RBC folate at 18–20 weeks gestation. Pilot data from our groups indicate a SD of 235 nmol L−1; using an α of 0.05 with 80% power the sample size to determine a difference of 200 nmol L−1 between normal and adverse pregnancy outcomes was calculated to be 23.
Patient selection criteria, classification and clinical diagnosis
Women were recruited to the study both from the high‐risk pregnancy clinic and from the routine antenatal clinics at the WCH. The inclusion criterion was a viable singleton pregnancy between 6 and 20 weeks gestation. The exclusion criteria included any condition requiring termination of pregnancy, a major fetal anomaly or fetal demise, multifetal pregnancy, any disorder requiring systemic steroids and pre‐existing maternal renal disease (serum creatinine >100 µmol L−1).
Women were classified as at ‘high risk’ of developing an adverse pregnancy outcome based on obstetric risk factors, including a history of one or more of pre‐eclampsia/eclampsia, early‐onset IUGR (<34 weeks gestation and birthweight <10th centile), placental abruption, preterm birth <34 weeks gestation, recurrent pregnancy loss (three or more miscarriages) and previous fetal demise. Women were classified as at ‘low risk’ of developing an adverse pregnancy outcome if they had no known pre‐existing medical (including chronic hypertension and diabetes mellitus) or obstetric disorders, and had had a previous normal pregnancy (birth >37 weeks gestation, customised birthweight >10th centile, with no gestational hypertension).
Pre‐eclampsia and gestational hypertension were defined according to the criteria of the Australasian Society for the Study of Hypertension in Pregnancy (Brown et al. 2000). Customised centiles were used to adjust birthweight for maternal height, weight, parity, ethnic group, fetal sex and gestational age at birth (http://www.gestation.net). IUGR was defined as a serial tapering of growth in abdominal circumference and of estimated fetal weight below the 10th centile of population‐based growth charts and the Australasian Society for Ultrasound in Medicine biometric charts with serial ultrasound scans by an experienced sonographer.
Micronutrient and vitamin quantification
Following venepuncture and collection of fasting samples, the blood was kept on ice until separation of plasma, serum and cells within 60 min in the laboratory. Total L‐Hcy in plasma was quantified using the AxSYM® homocysteine assay (Abbott, Wiesbaden, Germany) and expressed as Hcy µmol L−1. Serum and RBC folate were quantified using the ARCHITECT® folate assay (Abbott Laboratories, Abbott Park, IL, USA), expressed as nmol L−1. Vitamin B12 in serum was quantified using the ARCHITECT® B12 assay (Abbott Laboratories), expressed as pmol L−1. Vitamin B6 was tested using red cell aspartate amino‐transferase activation by pyridoxal phosphate (Mount et al. 1987): pyridoxal phosphate activation (PPA) activity greater than 63% was taken as representative of Vitamin B6 deficiency (Mount et al. 1987). Micronutrient quantification was performed by the Division of Clinical Biochemistry, SA Pathology, South Australia. The folate and B vitamin supplement intake was calculated based on the daily dose consumed obtained from the dietary questionnaire data. Participants were asked the type, brand, dosage and number of times per day they consumed the supplement. The dietary questionnaire for epidemiological studies, formerly known as the food frequency questionnaire produced by the Victorian Cancer Council, a validated questionnaire (Hodge et al. 2000), was administered to determine dietary intake including foods fortified with FA (with particular reference to breakfast cereals and other such foods) as well as dietary fibre. This study was conducted before the mandatory FA fortification of flour from 18 September 2009 in Australia.
DNA damage markers
DNA damage in lymphocytes was measured using the cytokinesis‐block micronucleus cytome assay (Fenech 2007). In this assay, chromosome damage is measured in cells that complete nuclear division ex vivo following mitogenic stimulation and are recognised by their binucleated appearance after cytokinesis block using cytochalasin‐B. The two DNA damage biomarkers measured in binucleated cells are (1) micronuclei (MN) which originate from acentric chromosome fragments or whole chromosome loss events during mitosis due to malsegregation of chromosomes and (2) nucleoplasmic bridges (NPBs) joining the nuclei in binucleated cells which originate from dicentric chromosomes formed as a result of either misrepair of DNA strand breaks or telomere end fusions to excess telomere shortening or telomere dysfunction.
Statistics
The associations between the continuous variables were determined by Pearson's bivariate correlation analyses. The pregnancy classification groups were compared using t‐tests, analysis of variance (ANOVA) and chi‐squared analysis. Backward logistic regression analysis was used to identify predictors of IUGR. The analysis was performed using software package PASW version 17.0 (SPSS, Chicago, IL, USA). Effects with P < 0.05 were considered statistically significant.
Results
Out of 143 eligible pregnant women recruited to the study before 20 weeks gestation, six were not included because of pregnancy loss between the time of recruitment and the planned assessment at 20 weeks gestation (n = 2), or personal choice (n = 1), or with incomplete data (n = 3). The final study cohort consisted of 46 ‘low risk’ subjects and 91 subjects classified as ‘high risk’ for a subsequent adverse pregnancy outcome. From the total study cohort of 137 subjects, 63 went on to have a normal, uncomplicated pregnancy and 74 had subsequent adverse pregnancy outcomes: 15 pre‐eclampsia, 21 IUGR and 38 other adverse outcomes (i.e. those other than pre‐eclampsia and IUGR, such as gestational hypertension and gestational diabetes). The breakdown of the study groups is represented in Fig. 1.
Figure 1.
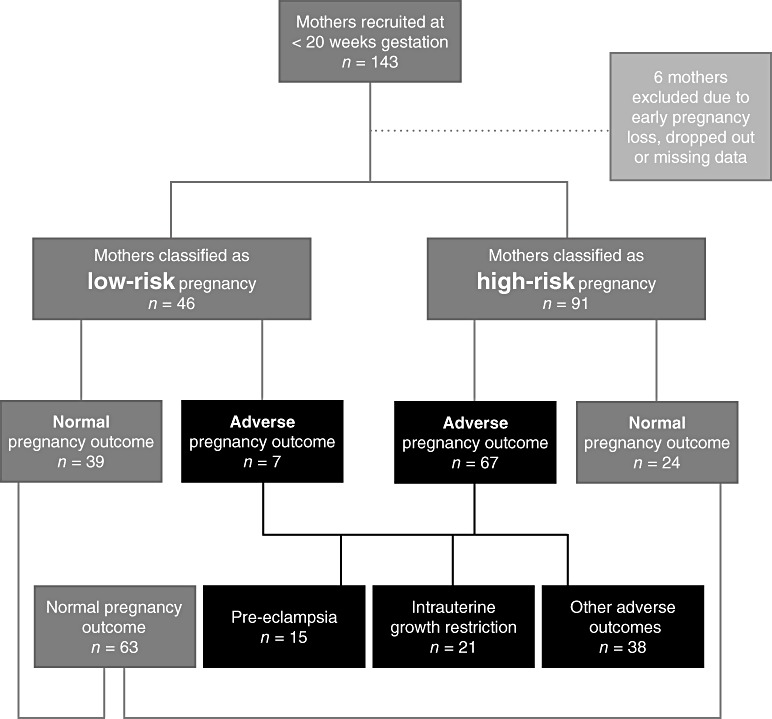
Pregnancy classification groups at 18–20 weeks gestation and subsequent outcomes. Low risk, healthy women with low risk of developing pregnancy complications; High risk, women with high risk of developing pregnancy complications; Normal, pregnancies with clinically normal outcomes; Adverse, pregnancies with adverse outcome; Other adverse outcomes, adverse outcome other than pre‐eclampsia or intrauterine growth restriction, e.g. gestational hypertension.
Subject characteristics
The mean age of the subjects in the study was 33 years, with the ‘high risk’ subjects being significantly older than the ‘low risk’ subjects. ‘High risk’ subjects had a higher body mass index (BMI) and were more likely to smoke cigarettes than ‘low risk’ subjects. Table 1 provides baseline data at 18–20 weeks gestation. Dietary folate/FA intake was correlated with circulating RBC folate concentration r = 0.288, P = 0.039. No associations were detected between plasma Hcy and dietary B vitamin intake. Increased fibre intake was negatively correlated with plasma Hcy (r = −0.189, P = 0.047).
Table 1.
Maternal characteristics, supplement use and circulating B vitamin concentrations according to pregnancy risk classification at 18–20 weeks gestation
| Demographics | Total cohort | Pregnancy groups | P value | |
|---|---|---|---|---|
| Low risk | High risk | |||
| n | 137 | 46 | 91 | |
| Age (years) | 33.0 (31.8–34.2) | 31.1 (29.5–32.4) | 34.0 (32.4–35.6) | 0.020 |
| BMI (kg) | 28.5 (27.5–29.7) | 26.5 (25.4–27.8) | 29.5 (28.1–31.1) | 0.010 |
| Smokers | 21 (15.3%) | 3 (6.5%) | 18 (19.8%) | 0.042 |
| Vitamin supplements | ||||
| Folic acid | 110 (80.3%) | 33 (71.7%) | 77 (84.6%) | 0.074 |
| Vitamin B12 | 65 (47.4%) | 21 (45.7%) | 44 (48.4%) | 0.748 |
| Vitamin B6 | 65 (47.4%) | 21 (45.7%) | 44 (48.4%) | 0.748 |
| Dietary intake | ||||
| Folate (µg/day) | 283 (254–311) | 308 (268–327) | 261 (256–297) | 0.526 |
| Blood micronutrients | ||||
| Hcy µmol/L | 4.6 (4.4–4.9) | 4.3 (4.0–4.5) | 4.7 (4.4–5.1) | 0.095 |
| RBC folate nmol/L | 652 (613–692) | 652 (587–718) | 652 (602–702) | 0.994 |
| Serum folate nmol/L | 26.5 (24.9–28.2) | 25.4 (22.9–27.8) | 27.1 (24.9–29.3) | 0.369 |
| Serum B12 pmol/L | 239 (215–265) | 231 (194–269) | 244 (212–276) | 0.555 |
| RBC B6%* | 41.9 (39.1–44.7) | 48.5 (45.5–51.6) | 38.6 (34.8–42.4) | 0.001 |
Values are mean (95% confidence interval) or numbers (percentage) calculated using t‐tests and chi‐squared analyses. BMI, body mass index; RBC, red blood cell. *Based on pyridoxal phosphate activation activity that is inversely related to vitamin B6 concentration. Bold indicates P values that are significant (P < 0.050).
B vitamin supplementation in women with low‐risk and high‐risk pregnancies
From the total study cohort, 27 (19.7%) subjects reported no vitamin supplement intake. The most common supplements used during the study were Elevit (Bayer Australia Ltd., NSW, Australia), with a standard daily dose of one tablet (FA: 800 µg, vitamin B12: 4 µg, vitamin B6: 2600 µg), and Blackmores Pregnancy and Breastfeeding (Blackmores, NSW, Australia), with a standard dose of two tablets per day (FA: 250 µg, vitamin B12: 1.5 µg, vitamin B6: 750 µg per tablet). A number of ‘high risk’ women were also being supplemented with FA 5 mg tablets (Megafol, various manufacturers, Australia) (Table 2).
Table 2.
B vitamin supplement dosage (µg) consumed daily
| Supplement | RDI | Pregnancy groups | ||
|---|---|---|---|---|
| Low risk | High risk | P value | ||
| Folic acid | 600 µg | 668 (389–947) | 2116 (1615–2617) | <0.001 |
| Vitamin B12 | 2.6 µg | 9.1 (0.0–21.7) | 34.3 (22.6–46.1) | 0.012 |
| Vitamin B6 | 1900 µg | 1031 (653–1426) | 1301 (898–1703) | 0.422 |
Values are mean (95% confidence interval) calculated using independent sample t‐tests. RDI, recommended dietary intake for pregnant women in Australia (NHMRC 2006). Bold indicates P values that are significant (P < 0.050).
B vitamin supplement intake and blood micronutrient levels
The mean age of subjects who supplemented with FA was 33.8 years compared with 29.9 years of those who did not supplement (P = 0.009). Older subjects had increased RBC folate, serum folate, and vitamins B12 and B6 (P < 0.001). Plasma Hcy was negatively associated with age (P = 0.028). Increased BMI was associated with higher Hcy (P = 0.014). RBC folate, serum folate, and vitamins B12 and B6 were all positively correlated with each other (P < 0.001), while Hcy was negatively correlated with RBC and serum folate (both P < 0.001) and vitamin B12 (P = 0.008), data not shown. FA supplementation was associated with increased blood micronutrient concentrations (Table 3).
Table 3.
Blood micronutrients in those who did and did not supplement with folic acid
| Circulating micronutrients | Daily folic acid intake | P value | ||
|---|---|---|---|---|
| No folic acid | 250–800 µg | >1000 µg | ||
| n | 27 | 67 | 41 | |
| Hcy µmol/L | 5.0 (4.5–5.4) | 4.7 (4.3–5.1) | 4.3 (3.8–4.7) | 0.177 |
| RBC folate nmol/L | 523 (423–622) | 613 (559–668) b | 800 (745–844) a | <0.001 |
| Serum folate nmol/L | 19.5 (15.7–23.3) | 25.9 (23.8–27.6) bc | 32.5 (30.0–35.5) a | <0.001 |
| Serum B12 pmol/L | 173 (134–212) | 215 (199–258) b | 315 (265–353) a | <0.001 |
| RBC B6%* | 51.5 (46.7–56.3) | 46.9 (44.3–49.7) b | 27.8 (23.2–33.4) a | <0.001 |
All data are represented as mean (95% confidence interval) calculated using one‐way analysis of variance. RBC, red blood cell. *Based on pyridoxal phosphate activation activity that is inversely related to vitamin B6 concentration. a P < 0.001 compared with no folic acid consumption; b P < 0.001 compared with folic acid consumption >1000 µg; c P < 0.005 compared with no folic acid consumption. Bold indicates P values that are significant (P < 0.050).
Smoking, micronutrients and DNA damage
Serum and RBC folate were decreased and plasma Hcy increased in those who smoked (Table 4). Cigarette smokers were younger and delivered smaller babies compared with those who did not smoke. The mean DNA damage rate, represented by MN frequency, was 19.6 MN in non‐smokers compared with 26.3 MN in smokers (P < 0.001). Plasma Hcy was correlated with the DNA damage biomarkers, MN: r = 0.179, P = 0.038 and NPB: r = 0.171, P = 0.047, in maternal lymphocytes. Maternal circulating RBC folate and plasma Hcy at 18–20 weeks gestation were respectively positively and negatively correlated with subsequent customised birthweight centiles of the offspring (RBC folate, r = 0.310, P = 0.015; Hcy, r = −0.273, P = 0.044).
Table 4.
Demographics, supplement intake and blood micronutrients in smokers
| Characteristics | Smoking status | P value | |
|---|---|---|---|
| Non‐smoker | Smoker | ||
| n | 116 | 21 | |
| Maternal age | 34 (32–35) | 30 (27–33) | 0.028 |
| Maternal BMI | 28 (27–29) | 31 (27–34) | 0.115 |
| Birthweight g | 3290 (3155–3433) | 2889 (2530–3248) | 0.039 |
| Growth centile | 52 (47–57) | 35 (18–52) | 0.029 |
| Gestational age | 267 (264–272) | 260 (253–267) | 0.087 |
| B Vitamin supplements | |||
| Folic acid | 93 (80.2%) | 17 (81.0%) | 1.00 |
| Vitamin B12 | 59 (54.6%) | 6 (28.6%) | 0.034 |
| Vitamin B6 | 58 (53.7%) | 7 (33.3%) | 0.100 |
| Blood micronutrients | |||
| Hcy µmol/L | 4.4 (4.1–4.6) | 6.0 ± (5.0–6.9) | <0.001 |
| RBC folate nmol/L | 687 (647–727) | 463 (354–571) | <0.001 |
| Serum folate nmol/L | 27.3 (25.5–29.0) | 22.6 (17.9–27.3) | 0.035 |
| Serum B12 pmol/L | 244 (217–271) | 209 (144–273) | 0.317 |
| RBC B6%* | 41.5 (38.4–44.6) | 44.5 (38.1–50.9) | 0.448 |
Values are mean (95% confidence interval) or numbers (percentage) calculated using t‐tests and chi‐squared analyses. BMI, body mass index; RBC, red blood cell. *Based on pyridoxal phosphate activation activity that is inversely related to vitamin B6 concentration. Bold indicates P values that are significant (P < 0.050).
Pregnancy outcomes
Subjects who subsequently developed pre‐eclampsia tended to be older and more obese (BMI > 30 kg/m2) than those who did not (Table 5). Those who subsequently developed both pre‐eclampsia and IUGR were more likely to smoke (P = 0.004) and were more likely to deliver preterm, compared with those having normal pregnancy outcomes (P < 0.001). Hcy was higher among all subjects with adverse pregnancy outcome groups, particularly in those who developed IUGR. Serum and RBC folate as well as B12 were lower in cases of IUGR (Table 5). When analysed separately, the ‘high risk’ subjects, whose subsequent pregnancy outcomes were normal, demonstrated higher RBC folate (mean: 714.7 nmol L−1 SD 209.5) and lower Hcy (mean: 4.0 µmol L−1 SD 0.9) when compared with all other pregnancy groups using ANOVA (data not shown).
Table 5.
Pregnancy outcome data
| Characteristics | Pregnancy outcome | P value | |||
|---|---|---|---|---|---|
| Normal | Pre‐eclampsia | IUGR | Other* | ||
| n | 63 | 15 | 21 | 38 | |
| Maternal age | 33 (32–35) | 35 (31–39) | 31 (27–34) | 33 (31–36) | 0.312 |
| BMI (kg) | 27 (26–28) | 32 (27–37) | 28 (25–31) | 29 (27–32) | 0.100 |
| Smokers | 5 (7.2%) | 5 (33.3%) c | 7 (33.3%) c | 4 (10.5%) | 0.003 |
| Birthweight | 3680 (3552–3755) | 2523 (2050–2996) a | 2518 (2092–2945) a | 3158 (2931–3385) b | <0.001 |
| Growth centile † | 60 (53–66) | 23 (9–37) a | 22 (8–36) a | 57 (48–67) | <0.001 |
| Gestational age | 277 (275–278) | 251 (235–266) a | 254 (238–268) a | 262 (255–268) b | <0.001 |
| Blood micronutrients | |||||
| Hcy µmol/L | 4.2 (3.9–4.4) | 4.8 (3.9–5.8) | 5.3 (4.6–6.2) a | 4.8 (4.3–5.4) | 0.007 |
| RBC folate nmol/L | 690 (640–740) | 664 (521–807) | 481 (379–582) a | 680 (598–762) | 0.003 |
| Serum folate nmol/L | 26.6 (25–29) | 29.6 (24–35) | 21.1 (17–26) b | 28 (25–32) | 0.024 |
| Serum B12 pmol/L | 243 (209–277) | 319 (200–438) | 205 (182–262) | 222 (182–262) | 0.109 |
| RBC B6% ‡ | 41.9 (38–46) | 41.5 (29–54) | 50.0 (45–55) | 38.0 (33–44) | 0.062 |
Values are mean (95% confidence interval) or numbers (percentage) calculated using analysis of variance and chi‐squared analyses. BMI, body mass index; IUGR, intrauterine growth restriction; RBC, red blood cell; *Other: All other adverse pregnancy outcomes not including pre‐eclampsia or IUGR. †Birthweight centiles indicates birthweight according to gestation, maternal height, weight, ethnicity, parity and sex of the baby. ‡Based on pyridoxal phosphate activation activity that is inversely related to vitamin B6 concentration. a P < 0.001 compared with normal pregnancy outcome; b P < 0.01 compared with normal pregnancy outcome; c P < 0.05 compared with normal pregnancy outcome. Bold indicates P values that are significant (P < 0.050).
Predictive potential
To analyse the potential of RBC folate and plasma Hcy concentration at 18–20 weeks gestation to be predictors for increased risk of IUGR, a backward stepwise logistic regression was performed taking into account confounding factors. The saturated model included RBC folate, Hcy, age, smoking, BMI and DNA damage markers (MN and NPBs). The parsimonious model correctly predicts 93.4% normal pregnancy outcomes and 52.4% of IUGR cases (Table 6).
Table 6.
Eighteen to twenty weeks gestation predictors of IUGR
| Putative risk factor | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Parsimonious model |
|---|---|---|---|
| RBC folate nmol/L | 0.995 (0.993–0.997) | 0.996 (0.993–1.0) | 0.996 (0.993–0.999) |
| Hcy µmol/L | 2.208 (1.332–3.659) | 1.556 (0.798–3.033) | 1.693 (0.918–3.122) |
| Serum B12 pmol/L | 0.998 (0.994–1.002) | 1.001 (0.996–1.006) | |
| Maternal age | 0.958 (0.843–1.089) | 0.958 (0.843–1.089) | |
| Maternal BMI | 1.026 (0.937–1.122) | 1.016 (0.903–1.144) | |
| Smoking | 6.400 (1.770–23.136) | 1.309 (0.254–6.759) | |
| MN frequency | 1.071 (1.014–1.131) | 1.101 (1.013–1.197) | 1.086 (1.015–1.163) |
| NPB frequency | 0.998 (0.894–1.113) | 0.946 (0.779–1.150) |
All data are represented as mean (95% CI) adjusted using multivariate regression analysis. BMI, body mass index; CI, confidence interval; MN, micronuclei; NPB, nucleoplasmic bridges; OR, odds ratio; RBC, red blood cell.
Discussion
IUGR and pre‐eclampsia are each part of a spectrum of placental vascular disorders affecting the fetus and the mother, respectively, and may occur separately or together. While the clinical syndromes occur late in pregnancy, the origin of the disorders has been attributed, at least in part, to abnormal placentation in early pregnancy (Khong et al. 1986). This study demonstrates that reduced maternal serum and RBC folate and increased plasma Hcy in mid pregnancy are associated with the subsequent development of IUGR. Maternal folate and plasma Hcy were not increased at 18–20 weeks gestation in those who developed pre‐eclampsia. These results may be explained by the fact that those with pre‐eclampsia were older and consuming high‐dose vitamin supplements, reducing their Hcy concentration. Age is a known risk factor for adverse pregnancy outcomes, perhaps suggesting that age has a larger effect than Hcy on the development of pre‐eclampsia. It may also support the hypothesis that Hcy is a bystander rather a cause of the vascular dysfunction of pre‐eclampsia. In randomised trials of folate supplementation in subjects with cardiovascular disease outside of pregnancy, Hcy was reduced, but outcome was no different (Marcus et al. 2007).
Our results are of particular interest because, despite high‐dose supplementation with FA in women with ‘high risk’ pregnancies, RBC folate was similar and, while plasma Hcy was lower, it was not different from that in ‘low risk’ subjects (P = 0.095). However, as expected when the total cohort was analysed together, there was a significant increase in serum and RBC folate with increased FA consumption (P < 0.001). This may perhaps indicate that high‐risk patients need higher dose folate supplements to bring them to concentrations that the general, healthy population readily achieves. The ‘high risk’ subjects were heavier at the first trimester booking visit (mean BMI 29.5 kg/m2, i.e. bordering on obesity), and were also more likely to smoke compared with the ‘low risk’ cohort, and these factors were associated with reduced RBC folate and high circulating Hcy. In addition, a higher BMI was associated with increased Hcy in the total cohort. The relationship between increased BMI and/or fat mass with Hcy has been reported in previous studies (Chan et al. 2002; Guzelmeric et al. 2007; Sanlier & Yabanci 2007). The interactions between Hcy and lipid metabolism may be clinically important, but is not well understood. It has been suggested that hypomethylation associated with hyper‐Hcy is responsible for lipid accumulation in tissues (Obeid & Herrmann 2009). However, further studies are required to understand the metabolic relationship. It is likely that lifestyle factors such as poor diet and lack of exercise are the common link between Hcy and BMI.
Increased Hcy was associated with increased maternal DNA damage, represented by MN and NPBs, which is consistent with in vitro data showing that MN and NPB are increased with folate‐deficiency‐induced high Hcy (Kimura et al. 2004). Our group has previously shown that maternal DNA damage in mid pregnancy is increased in women with subsequent pre‐eclampsia and IUGR (Furness et al. 2010), suggesting that Hcy may be a causal factor in DNA damage. How this relates to the subsequent development of placental vascular dysfunction remains an unanswered question. Studies in non‐pregnant populations have shown that it is necessary to supplement with higher B vitamin doses than the recommended daily intake (RDI) to minimise DNA damage (Fenech 2001).
Subjects who smoked during pregnancy had a 33% lower RBC folate and 27% higher plasma Hcy compared with non‐smokers, despite having similar FA consumption. The adverse effect of smoking on folate concentrations in the pregnant population and in normal adults is well documented (Mannino et al. 2003; Delpisheh et al. 2006). Folate status may be influenced by smoking through a number of possible mechanisms, as intermediates in one‐carbon metabolism are sensitive to the redox balance within the cell and may alter the ability of the cell to metabolise and store folate (Mannino et al. 2003). Therefore, adjusted nutrient requirement values should be considered for pregnant smokers. Smoking is a well‐recognised risk factor for IUGR (Kho et al. 2009), and as might be expected, our data show that smokers delivered babies 400 g lighter and 1 week earlier at 37 weeks gestation, compared with those who did not smoke. Unlike previous studies, however, smoking did not have a protective effect for pre‐eclampsia. This may be explained by the fact that many of the subjects in this cohort had suffered previous obstetric complications putting them at increased risk for pre‐eclampsia, compared with the general population.
Although FA supplementation is regarded as safe, it has been hypothesised that high‐dose FA could lead to the presence of unmetabolised FA that may be detrimental (Wright et al. 2007). Over the past decade, little research has been published explaining the absorption and saturation levels of folate. There are two studies (Lucock et al. 1989; Kelly et al. 1997) that claim that the absorption and biotransformation process of folate is readily saturated at doses of less than 400 µg/day. However, in the present study, higher doses of FA were associated with a marginal, but significant increase in RBC folate.
Unmetabolised FA may also promote growth of tumours, e.g. colorectal adenomas (Cole et al. 2007), while there are also recent data showing an impact of FA in the reduction of cytotoxicity of natural killer (NK) cells (Troen et al. 2006). The latter finding may be important in the context of pre‐eclampsia, given recent findings of impairment of NK cell activity in the aetiology of abnormal placentation (Redman & Sargent 2010). It will therefore be important to develop a better understanding of the mechanism of accumulation of unmetabolised FA in humans as well as of its consequences (Bailey & Ayling 2009).
The strengths of the present study include the way in which birth outcome data were collected. These outcomes were closely monitored and classified by an expert sonographer and an obstetrician using strict criteria, as opposed to relying on hospital discharge summary diagnoses. The nutritional questionnaires and vitamin supplement data were applied and recorded by one observer, who interviewed every woman involved in the study. Weaknesses of the study include lack of data on socio‐economic status and inability to obtain Hcy measurements on all women before starting vitamin supplementation. Many women who had experienced a previous adverse pregnancy outcome had been advised to begin 5 mg FA because of detection of increased plasma Hcy and/or MTHFR polymorphisms before entering the study. Furthermore, a larger cohort with more cases and controls is needed to validate these findings.
Our study suggests that low maternal B vitamins and increased Hcy in mid pregnancy are associated with the subsequent development of IUGR, but not pre‐eclampsia. Of the 91 high‐risk women recruited, those who did achieve the highest RBC folate and lowest Hcy did not develop an adverse pregnancy outcome. FA supplementation >1000 µg/day resulted in the highest RBC folate and lowest Hcy, and, therefore women with previous obstetric complications and polymorphisms in genes that slow folate metabolism may need to supplement at higher doses than the RDI to achieve adequate micronutrient levels. A randomised controlled trial to determine whether high‐dose FA is more efficacious in preventing recurrent adverse pregnancy outcomes than the standard recommended dose is required, at the same time monitoring the safety of such supplementation on longer term fetal outcomes.
Source of funding
Funding support from Channel 7 Children's Research Foundation and the Cooperative Research Centre for Diagnostics.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributions
DF, project design, patient recruiting, data collection, analysis and manuscript writing; GD, project design, patient recruiting, data collection and analysis; MF, project design, analysis and manuscript writing; YK, project design, manuscript editing; CR, data analysis and manuscript writing; BH, project design, patient recruiting, data collection and manuscript editing.
Acknowledgements
We thank the South Australian Women's and Children's Hospital, especially Denise Healy and Michelle Cox, for assistance with recruiting and patient care.
References
- Bailey S.W. & Ayling J.E. (2009) The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proceedings of the National Academy of Sciences of the United States of America 106, 15424–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.A., Hague W.M., Higgins J., Lowe S., McCowan L., Oats J. et al (2000) The detection, investigation and management of hypertension in pregnancy: full consensus statement. The Australian & New Zealand Journal of Obstetrics & Gynaecology 40, 139–155. [DOI] [PubMed] [Google Scholar]
- Chan S.J., Chang C.N., Hsu J.C., Lee Y.S. & Shen C.H. (2002) Homocysteine, vitamin B(6), and lipid in cardiovascular disease. Nutrition 18, 595–598. [DOI] [PubMed] [Google Scholar]
- Chiaffarino F., Ascone G.B., Bortolus R., Mastroia‐Covo P., Ricci E., Cipriani S. et al (2010) Effects of folic acid supplementation on pregnancy outcomes: a review of randomized clinical trials. Minerva Ginecologica 62, 293–301. [PubMed] [Google Scholar]
- Cole B.F., Baron J.A., Sandler R.S., Haile R.W., Ahnen D.J., Bresalier R.S. et al (2007) Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA: The Journal of the American Medical Association 297, 2351–2359. [DOI] [PubMed] [Google Scholar]
- Cravo M., Fidalgo P., Pereira A.D., Gouveia‐Oliveira A., Chaves P., Selhub J. et al (1994) DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. European Journal of Cancer Prevention 3, 473–479. [DOI] [PubMed] [Google Scholar]
- Czeizel A.E., Puho E.H., Langmar Z., Acs N. & Banhidy F. (2010) Possible association of folic acid supplementation during pregnancy with reduction of preterm birth: a population‐based study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 148, 135–140. [DOI] [PubMed] [Google Scholar]
- Dekker G.A., de Vries J.I., Doelitzsch P.M., Huijgens P.C., von Blomberg B.M., Jakobs C. et al (1995) Underlying disorders associated with severe early‐onset preeclampsia. American Journal of Obstetrics and Gynecology 173, 1042–1048. [DOI] [PubMed] [Google Scholar]
- Delpisheh A., Kelly Y., Rizwan S. & Brabin B.J. (2006) Socio‐economic status, smoking during pregnancy and birth outcomes: an analysis of cross‐sectional community studies in Liverpool (1993–2001). Journal of Child Health Care 10, 140–148. [DOI] [PubMed] [Google Scholar]
- Dodds L., Fell D.B., Dooley K.C., Armson B.A., Allen A.C., Nassar B.A. et al (2008) Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clinical Chemistry 54, 326–334. [DOI] [PubMed] [Google Scholar]
- Fenech M. (2001) The role of folic acid and Vitamin B12 in genomic stability of human cells. Mutation Research 475, 57–67. [DOI] [PubMed] [Google Scholar]
- Fenech M. (2007) Cytokinesis‐block micronucleus cytome assay. Nature Protocols 2, 1084–1104. [DOI] [PubMed] [Google Scholar]
- Fenech M., Aitken C. & Rinaldi J. (1998) Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogenesis 19, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Forges T., Monnier‐Barbarino P., Alberto J.M., Gueant‐Rodriguez R.M., Daval J.L. & Gueant J.L. (2007) Impact of folate and homocysteine metabolism on human reproductive health. Human Reproduction Update 13, 225–238. [DOI] [PubMed] [Google Scholar]
- Furness D.L., Dekker G.A., Hague W.M., Khong T.Y. & Fenech M.F. (2010) Increased lymphocyte micronucleus frequency in early pregnancy is associated prospectively with pre‐eclampsia and/or intrauterine growth restriction. Mutagenesis 25, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra‐Shinohara E.M., Paiva A.A., Rondo P.H., Yamasaki K., Terzi C.A. & D'Almeida V. (2002) Relationship between total homocysteine and folate levels in pregnant women and their newborn babies according to maternal serum levels of vitamin B12. BJOG: An International Journal of Obstetrics and Gynaecology 109, 784–791. [DOI] [PubMed] [Google Scholar]
- Guzelmeric K., Alkan N., Pirimoglu M., Unal O. & Turan C. (2007) Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecological Endocrinology 23, 505–510. [DOI] [PubMed] [Google Scholar]
- Hague W.M. (2003) Homocysteine and pregnancy. Best Practice & Research. Clinical Obstetrics & Gynaecology 17, 459–469. [DOI] [PubMed] [Google Scholar]
- Hibbard E.D. (1964) The Figlu‐excretion test and defective folic‐acid metabolism in pregnancy. Lancet 2, 1146–1149. [DOI] [PubMed] [Google Scholar]
- Hodge A., Patterson A.J., Brown W.J., Ireland P. & Giles G. (2000) The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle‐aged women in a study of iron supplementation. Australian and New Zealand Journal of Public Health 24, 576–583. [DOI] [PubMed] [Google Scholar]
- Kark J.D., Selhub J., Adler B., Gofin J., Abramson J.H., Friedman G. et al (1999) Nonfasting plasma total homocysteine level and mortality in middle‐aged and elderly men and women in Jerusalem. Annals of Internal Medicine 131, 321–330. [DOI] [PubMed] [Google Scholar]
- Kelly P., McPartlin J., Goggins M., Weir D.G. & Scott J.M. (1997) Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. The American Journal of Clinical Nutrition 65, 1790–1795. [DOI] [PubMed] [Google Scholar]
- Kho E.M., North R.A., Chan E., Stone P.R., Dekker G.A. & McCowan L.M. (2009) Changes in Doppler flow velocity waveforms and fetal size at 20 weeks gestation among cigarette smokers. BJOG: An International Journal of Obstetrics and Gynaecology 116, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Khong T.Y., De Wolf F., Robertson W.B. & Brosens I. (1986) Inadequate maternal vascular response to placentation in pregnancies complicated by pre‐eclampsia and by small‐for‐gestational age infants. British Journal of Obstetrics and Gynaecology 93, 1049–1059. [DOI] [PubMed] [Google Scholar]
- Kimura M., Umegaki K., Higuchi M., Thomas P. & Fenech M. (2004) Methylenetetrahydrofolate reductase C677T polymorphism, folic acid and riboflavin are important determinants of genome stability in cultured human lymphocytes. The Journal of Nutrition 134, 48–56. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Zaman S., Malik A., Martin H., Ekstrom A.M., Amu S. et al (2005) Folate, vitamin B12, and homocysteine levels in South Asian women with growth‐retarded fetuses. Acta Obstetricia et Gynecologica Scandinavica 84, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Lucock M., Wild J., Smithells R. & Hartley R. (1989) Biotransformation of pteroylmonoglutamic acid during absorption: implications of Michaelis‐Menten kinetics. European Journal of Clinical Nutrition 43, 631–635. [PubMed] [Google Scholar]
- Mannino D.M., Mulinare J., Ford E.S. & Schwartz J. (2003) Tobacco smoke exposure and decreased serum and red blood cell folate levels: data from the Third National Health and Nutrition Examination Survey. Nicotine & Tobacco Research 5, 357–362. [DOI] [PubMed] [Google Scholar]
- Marcus J., Sarnak M.J. & Menon V. (2007) Homocysteine lowering and cardiovascular disease risk: lost in translation. The Canadian Journal of Cardiology 23, 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartlin J., Halligan A., Scott J.M., Darling M. & Weir D.G. (1993) Accelerated folate breakdown in pregnancy. Lancet 341, 148–149. [DOI] [PubMed] [Google Scholar]
- Mount J.N., Heduan E., Herd C., Jupp R., Kearney E. & Marsh A. (1987) Adaptation of coenzyme stimulation assays for the nutritional assessment of vitamins B1, B2 and B6 using the Cobas Bio centrifugal analyser. Annals of Clinical Biochemistry 24, 41–46. [DOI] [PubMed] [Google Scholar]
- NHMRC (2006) Nutrient reference values for Australia and New Zealand including recommended dietary intakes. Available at: http://nhmrc.publications@nhmrc.gov.au
- Obeid R. & Herrmann W. (2009) Homocysteine and lipids: S‐adenosyl methionine as a key intermediate. FEBS Letters 583, 1215–1225. [DOI] [PubMed] [Google Scholar]
- Pietrzik K., Bailey L. & Shane B. (2010) Folic acid and L‐5‐methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clinical Pharmacokinetics 49, 535–548. [DOI] [PubMed] [Google Scholar]
- Ray J.G. & Laskin C.A. (1999) Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre‐eclampsia and spontaneous pregnancy loss: a systematic review. Placenta 20, 519–529. [DOI] [PubMed] [Google Scholar]
- Redman C.W. & Sargent I.L. (2010) Immunology of pre‐eclampsia. American Journal of Reproductive Immunology 63, 534–543. [DOI] [PubMed] [Google Scholar]
- Roberts J.M. & Redman C.W. (1993) Pre‐eclampsia: more than pregnancy‐induced hypertension. Lancet 341, 1447–1451. [DOI] [PubMed] [Google Scholar]
- Sanlier N. & Yabanci N. (2007) Relationship between body mass index, lipids and homocysteine levels in university students. Journal of the Pakistan Medical Association 57, 491–495. [PubMed] [Google Scholar]
- Scholl T.O. & Johnson W.G. (2000) Folic acid: influence on the outcome of pregnancy. The American Journal of Clinical Nutrition 71, 1295S–1303S. [DOI] [PubMed] [Google Scholar]
- Smith A.D. (2008) The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food and Nutrition Bulletin 29, S143–S172. [DOI] [PubMed] [Google Scholar]
- Troen A.M., Mitchell B., Sorensen B., Wener M.H., Johnston A., Wood B. et al (2006) Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. The Journal of Nutrition 136, 189–194. [DOI] [PubMed] [Google Scholar]
- Vollset S.E., Refsum H., Irgens L.M., Emblem B.M., Tverdal A., Gjessing H.K. et al (2000) Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. The American Journal of Clinical Nutrition 71, 962–968. [DOI] [PubMed] [Google Scholar]
- Wright A.J., Dainty J.R. & Finglas P.M. (2007) Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. The British Journal of Nutrition 98, 667–675. [DOI] [PubMed] [Google Scholar]


