Abstract
We tested the acceptability of three new lipid‐based nutrient supplements (LNSs) in two independent phases among 18 8–12‐month‐old healthy rural Malawians and their caregivers. In phase 1, acceptability was assessed by offering three new LNSs in random order, and an LNS already determined to be acceptable, Nutributter®, each added to 30 g of warm maize porridge over three consecutive days. In phase 2, infants from each village were provided one of the new supplements for a 2‐week home‐use trial. Outcome measures included the amount consumed, time completion of the dose and the maternal rating of likeability on a 5‐point scale. The supplements were rated acceptable if consumption was over 50% of the offered dose in phase 1. The mean (95% confidence interval) proportion of the LNS test meals consumed under direct observation was 88% (82–94%) for LNS‐10gM, 90% (84–95%) for LNS‐20gM, 87% (79–95%) for LNS‐20gNoM, and 86% (83–90%) for Nutributter. The median (25th and 75th centile) time (minutes) for completing the offered test meal was 4 (2, 7) for LNS‐10gM, 5 (3, 6) for LNS‐20gM, 4 (3, 8) for LNS‐20gNoM and 4 (2, 6) for Nutributter. During both phases, almost all caregivers rated all study foods very likeable for themselves and their children, with mean scores slightly lower among the caregivers than among the infants. In the home‐use phase, the test foods were almost exclusively used by the study participants with minimal sharing with siblings and other household members. Some infants were reported to prefer the new investigational products over traditional complementary food. Considering that the novel LNS was largely acceptable. Efficacy trials are now needed to assess their impact on child growth and development.
Keywords: fortified spread, infants and young children, acceptability trial, supplementary feeding, undernutrition
Introduction
In Malawi, like in most low‐income countries, childhood malnutrition is very common (National Statistical Office and ORC Macro 2001). The peak incidence is between 6 and 18 months of age, and stunting with moderate underweight is the most common presentation (National Statistical Office and ORC Macro 2001; Maleta et al. 2003). Apart from causing acute morbidity and adverse long‐term sequelae, malnutrition is estimated to contribute to approximately one‐third of the worldwide deaths and disability‐adjusted life years in under 5‐year‐old children (Black et al. 2008).
Lipid‐based nutrient supplements (LNSs), typically made from vegetable oil, peanut paste, milk powder, sugar, and a mixture of vitamins and minerals, have been used very successfully to treat severe malnutrition (Collins & Sadler 2002; Diop et al. 2003; Manary et al. 2004; Sandige et al. 2004) and may also offer an effective and convenient food‐based means for growth promotion and malnutrition prevention (Adu‐Afarwuah et al. 2007; Lin et al. 2008; Phuka et al. 2008). The cost of such supplements has, however, been reasonably high ($0.09 to $0.30 per daily ration), which might limit their longer‐term use among the most vulnerable segments of the population. More concentrated products that do not contain milk powder or imported rapeseed (canola) oil would be cheaper and could be locally produced from mostly domestic ingredients.
To assess lower‐cost food‐based interventions to prevent malnutrition, we have developed three new investigational LNS products. All of these have a higher concentration of oil and essential fatty acids (EFAs) and less sugar than the previous LNS versions. To facilitate future local production, soybean oil is used instead of rapeseed (canola) oil, the former being readily available in Africa but comparable with the latter in its EFA contents. One of the novel products does not contain milk powder, which has accounted for approximately 40% of the ingredient costs for the earlier LNS products. Lastly, one of the new products is more concentrated in micronutrients than the previous versions because its daily ration of 10 g contains the same amount of micronutrients as in those provided at 20 g daily.
The changes are expected to reduce the costs of the LNS, and are hypothesized to retain or improve its positive impact on growth and development. Changing the composition might, however, also affect the acceptability of the LNS among the intended target group, i.e. growing infants. Before beginning clinical efficacy trials, we therefore conducted acceptability tests of these new LNS products among healthy Malawian infants and their caregivers. The acceptability tests were conducted in two phases: phase 1 was aimed at accurately measuring the consumption of each of the novel LNS product, while phase 2 was aimed at collecting experiences of longer‐term use of the novel LNS products. Results from these studies are reported in this publication.
Key messages
-
•
All three new LNS products were highly acceptable to infants and their caregivers and were comparable in acceptability to Nutributter.
-
•
Changing the oil and milk content of the LNS and doubling the concentration of vitamins and minerals in the 10 g daily dose formula did not affect acceptability; thus, there is potential to reduce the cost of LNS formulations without decreasing acceptability.
Methods
General set‐up
The study consisted of two phases. In phase 1, eligible participants consumed three directly observed test meals of investigational LNS in a random order on three consecutive days, followed on a fourth day by a similar meal containing Nutributter® (Nutriset S.A.S, Malaunay, France), a commercially available LNS known to be acceptable to infants (Adu‐Afarwuah et al. 2007). The primary outcome was the proportion of the test dose consumed in a 15‐min period; secondary outcomes comprised median time to completion of the test meal, maternal estimate of the supplement's palatability to the child (on a hedonic 5‐point scale) and the caregiver's own score of the supplements (with the same hedonic scale). In phase 2, a different group of participants were allocated to receive one of the three investigational LNS products for a 2‐week supplementation period, followed by individual structured interviews and focus group discussions. This phase was used to produce descriptive information on LNS consumption patterns among the study participants.
Study area and timing
The study was conducted in Mangochi district of Malawi, south‐eastern Africa. In this area, exclusive breastfeeding for a full 6 months is almost non‐existent, and infant diet is typically complemented with thin maize porridge (phala, 10% dry weight of maize flour) beginning at 2–6 months of age. Underweight and stunting are very common; in a recent prospective cohort study of 813 newborns, underweight (weight‐for‐age z‐score ≤2) and stunting (height‐for‐age z‐score ≤2) prevalence was 10% and 50% by 6 months of age and 40% and almost 80% by 18 months of age, respectively (Maleta et al. 2003). There is one rainy season between December and March during which the staple food maize is grown. Data collection for the trial was done during the harvest season, i.e. in April–May 2009.
Participants, enrolment and group allocation
Inclusion criteria for both trial phases included age at least 8 but less than 12 months, current breastfeeding and consumption of complementary porridges, residence in the study area throughout the follow‐up period and signed informed consent from at least one caregiver. Exclusion criteria were moderate or severe wasting, presence of oedema, congenital malformations, history of peanut allergy or any severe allergic reaction, severe illness warranting hospitalization, concurrent participation in another clinical trial or any symptoms of food intolerance within 30 min after the ingestion of a 6‐g test dose of Nutributter (given to all potential participants to exclude the possibility of peanut allergy).
For enrolment, trained health surveillance assistants from a local health centre contacted families who were known to live in one purposively selected village in the study area and to have a baby of the correct age. Caregivers, whose infant's age was appropriate and verified from an under‐five clinic card, were invited for further screening to the health centre. At the enrolment session, caregivers were given detailed information on the trial contents, and the infants and children were fully assessed for eligibility. Those meeting eligibility criteria and whose caregivers signed a consent form were given a trial identification number and randomized into phase 1 of the study. For phase 2, six villages were randomly selected in the catchment area, and two villages were assigned to each of the three investigational products. Caregivers of age‐eligible infants and children were invited to the nearest health centre where, upon confirmation of all eligibility criteria, the infants and children were included in the study. The caregivers were the primary respondents.
For phase 1, a statistician not involved in the implementation of the study pre‐made a randomization list that indicated the order of the daily test foods for each participant, and ensured that each investigational product was preceded and followed in the consumption order equally often by all other investigational products. The identification numbers and the corresponding sequence of various test meals were sealed in individual opaque envelopes. When a participant was enrolled, the caregiver chose one of the envelopes, and its content determined the choice of test meals for each day. In phase 2, group allocation was systematic and based on the village of residence of the participant.
Nutrient supplements used in the study
The supplements included three newly developed investigational LNSs and one earlier developed product (Nutributter) as a positive control. Of the investigational products, LNS‐20gM differed from Nutributter mainly in its EFA content, whereas LNS‐20gNoM contained less protein because it contained no milk powder, and LNS‐10gM was more concentrated in micronutrients (Table 1). For each supplement, the intended daily ration contained approximately 100% of the recommended daily allowance of several micronutrients. All supplements were produced by Nutriset S.A.S. LNS‐10gM, LNS‐20gM and LNS‐20gNoM were made of vegetable oil, dried skimmed milk powder (except LNS20gNoM), peanut paste, sugar, maltodextrines, and mineral and vitamin mix. Nutributter was formulated using the same ingredients plus whey.
Table 1.
Nutrient content of the three investigational LNS‐products and Nutributter
| LNS‐10gM | LNS‐20gM | LNS‐20gNoM | Nutributter | |
|---|---|---|---|---|
| Daily ration (g) | 10.0 | 20.0 | 20.0 | 20.0 |
| Total energy (kcal) | 56.0 | 118.0 | 118.0 | 108.0 |
| Protein (g) | 1.3 | 2.6 | 0.9 | 2.6 |
| Fat (g) | 4.8 | 9.6 | 9.6 | 7 |
| Linoleic acid (g) | 2.2 | 4.5 | 4.5 | 1.29 |
| α‐Linolenic acid (g) | 0.29 | 0.58 | 0.58 | 0.29 |
| Vitamin A (µg RE) | 400.0 | 400.0 | 400.0 | 400.0 |
| Vitamin C (mg) | 30.0 | 30.0 | 30.0 | 30.0 |
| Vitamin B1(mg) | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin B2 (mg) | 0.4 | 0.4 | 0.4 | 0.4 |
| Niacin (mg) | 4.0 | 4.0 | 4.0 | 4.0 |
| Folic acid (µg) | 80.0 | 80.0 | 80.0 | 80.0 |
| Pantothenic acid (mg) | 1.8 | 1.8 | 1.8 | 1.8 |
| Vitamin B6 (mg) | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin B12 (µg) | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin D (IU) | 200.0 | 200 | 200 | – |
| Vitamin E (mg) | 6.0 | 6.0 | 6.0 | – |
| Vitamin K (µg) | 30.0 | 30.0 | 30.0 | – |
| Iron (mg) | 6.0 | 6.0 | 6.0 | 9.0 |
| Zinc (mg) | 8.0 | 8.0 | 8.0 | 5.0 |
| Copper (mg) | 0.34 | 0.34 | 0.34 | 0.2 |
| Calcium (mg) | 280.0 | 280.0 | 280.0 | 100.0 |
| Phosphorus (mg) | 190.0 | 190.0 | 190.0 | 82.0 |
| Potassium (mg) | 200.0 | 200.0 | 200.0 | 152.0 |
| Magnesium (mg) | 40.0 | 40.0 | 40.0 | 16.0 |
| Selenium (µg) | 20.0 | 20.0 | 20.0 | 10.0 |
| Iodine (µg) | 90.0 | 90.0 | 90.0 | 90.0 |
| Manganese (mg) | 1.2 | 1.2 | 1.2 | 0.08 |
| Phytate (mg) | 11.7 | 23.3 | 23.3 | 31.4 |
LNS, lipid‐based nutrient supplement; IU, international unit; RE, retinol equivalents.
The products were packed in identical 140‐g opaque containers, each marked with one of eight different letter codes (A, B, C, D, E, F, G, H) corresponding to the different LNS composition (two different letter codes per product), in order to keep the research assistants masked to product formulation.
For consumption, all LNS products were to be mixed in complementary porridge made from maize flour produced locally from milling whole grain maize. In phase 2, the flour was packed in opaque plastic bags, each containing 500 g of the maize flour.
Directly observed test meals
All test meals were consumed at the study office, where the caregivers reported on five consecutive mornings at approximately 8 am. The first day involved a run‐in procedure, during which the children ate a test meal (consisting of a randomly selected investigational product), and data were collected but were not included in the final analysis. The actual data collection happened on the following 4 days, with the three investigational products being offered in random order and Nutributter (positive control) on the last day. Because it was known that Nutributter was liked by children, and it is considerably sweeter than the test LNS products, it was always offered on the last day to avoid any possible interference with consumption of or hedonic responses to the test products.
Research assistants prepared test meals from either 15 g of LNS‐20gM, 15 g of LNS‐20gNoM, 7.5 g of LNS‐10gM or 15 g of Nutributter, added to 30 g of freshly prepared, warm maize porridge. At each test meal, the mother was first asked to breastfeed the participating infant; thereafter, there was a 60‐min waiting period, during which no foods or liquids were offered to the infant. The porridge–LNS mixture was then given to the mother in a pre‐weighed bowl, and she was asked to feed the child with a standard spoon. The test feeding continued under direct observation of a research assistant until the participant completed the given portion or a maximum of 15 min had elapsed from the beginning of the meal. Food consumption was calculated by subtracting the final weight of the bowl, leftovers and utensils from their combined weight at the beginning of the meal.
At the end of each test meal, the caregiver was asked to indicate how much she thought her child liked the food. The scoring was done on a 5‐point hedonic scale, graphically illustrated in a series of human face symbols with various degrees of smile or discontent. On the fifth test day, after the children had consumed their test meal of Nutributter, 20 mothers were given five 5‐g test doses of LNS products in a random order and were asked to rate their taste with the previously mentioned 5‐point hedonic scale. The five LNS products, given without any added porridge, consisted of the same four products given to the infants and an extra one that was being developed specifically for pregnant and lactating women (data not shown).
Experience from longer‐term LNS consumption
In phase 2, caregivers were provided with a 2‐week supply of one of the three investigational LNS products. They were instructed to feed their infants twice daily with a portion that consisted of approximately two tablespoons of porridge mixed with either one teaspoon (5 g) of LNS‐10gM or two teaspoons (10 g) of LNS‐20gM or LNS‐20gNoM. After the 2‐week supplementation period, the caregivers were interviewed with structured questionnaires about the LNS consumption patterns in their household. During these individual interviews, the caregivers were also asked to rate the acceptability of the provided supplement by their infants and by themselves, using the same 5‐point hedonic scale that was used during the directly observed test meals. Because the amount consumed was to be established from phase 1, during which a scale and trained staff were available, in phase 2 of the study, the focus was on collecting self‐reported experiences at the end of the 2‐week supplementation period.
To obtain more thorough insights into the consumption patterns, we organized six focus group discussions. At each session, approximately eight mothers from one village got together with a trained facilitator to discuss maternal perceptions on the taste, likeability, acceptability and feasibility of LNS products for child nutrition. Qualitative analysis was used to highlight themes that were related to caregivers' opinions on the longer‐term use of the various investigational LNS products.
Sample size calculation
For phase 1, the purpose was to determine whether each of the three investigational products had adequate acceptability defined as a minimum of 50% mean intake from an offered dose. The test dose, on the other hand, was set to represent 150% of the intended single portion in an eventual intervention where LNS would be given in two equal daily portions. The test dose was higher than the recommended portion for home use because we anticipated that measurement precision in the homes might not be perfect, and hence the children would occasionally be given larger portions. Consumption of a minimum of 50% of two LNS portions comparable with the test meal would mean that the participant obtained from the supplement at least 75% of the recommended daily allowance for the micronutrients included in it, which, together with normal feeding, was assumed to ensure appropriate micronutrient intake.
The sample size was based on the desire to test the hypothesis that mean consumption of each type of LNS would be at least 50% of the amount offered. We assumed that the standard deviation of consumption would be 30% of the amount offered. The sample size of 18 would therefore allow us to reject the null hypothesis with 90% power if the true mean were at least 75%. For maternal rating of the products, a row balanced Latin square design of 20 mothers was included to even out order and carryover effects because they consumed the food on the same day, although only 18 mothers were required per sample size calculation. The order and carryover effect was not a concern for the children as they tested the products on different days, hence the sample size remained 18.
For phase 2, the selected sample size was based on the plan to organize a focus group discussion in all the six villages where participants were enrolled. In an earlier research, it has been shown that about eight participants per focus group interview is a socially feasible number of participants (Barbour & Kitzinger 1999 ), which would dictate a minimum of 48 participants.
Data management and analysis
Raw data were initially recorded on paper forms and their coherence was checked daily by a senior research assistant. The data were then double‐entered into a tailor‐made microsoft access 2003 (Microsoft Corporation, Redmond, WA, USA) database. The two entries were electronically compared; all extreme and otherwise susceptible values were confirmed or corrected.
Statistical analysis was completed with stata for Windows (version 11.0) (StataCorp LP, College Station, TX, USA). For the main end point (amount in grams of test food consumed during the 15‐min test feeding session), which is a continuous variable, we calculated the mean [95% confidence interval (CI)] intake for each of the test foods separately, whereas for ordinal data, we calculated the median for each group. For qualitative data, individual interviews and focus group discussion sessions were transcribed and translated and back translated from Chiyao to English, and were analysed using Atlas.ti (version 6, Scientific Software Development GmbH, Berlin, Germany) for emerging themes. All children were analysed in the group to which they were initially randomized, i.e. on an intention to treat basis.
Ethics, study registration and participant safety
The trial was performed according to International Conference on Harmonisation‐Good Clinical Practice guidelines, and it adhered to the principles of Helsinki declaration and regulatory guidelines in Malawi. Before the onset of enrolment, the trial protocol was reviewed and approved by the College of Medicine Research and Ethics Committee (University of Malawi) and the Ethical Committee of Pirkanmaa Hospital District (Finland). Key details of the protocol were published at the clinical trial registry of the National Library of Medicine, Bethesda, MD, USA (http://www.clinicaltrials.gov, trial identification is NCT00885144).
All suspected serious adverse events (SAEs) were monitored by the research team without the use of an external Data Safety Monitoring Board. SAE was defined as any untoward medical occurrence that either resulted in death or was life‐threatening or required inpatient hospitalization or prolongation of existing hospitalization, or resulted in persistent or significant disability/incapacity or other serious medical condition.
Results
For phase 1, 36 mother–infant dyads were approached, and the 32 who were eligible based on the infant's age were invited for randomization. Of those invited, 22 showed up, but only the first 18 were enrolled and randomized. For phase 2, 94 caregivers with age‐eligible children were approached, and of those, 49 who showed up at the enrolment site and accepted to participate were enrolled. None of those approached refused participation, but no reasons were solicited for those who did not show up. None of the participants showed any adverse reaction to the 6‐g test dose of Nutributter. Selected characteristics of the trial participants are shown in Table 2.
Table 2.
Background characteristics of the participants at enrolment
| Participant characteristics | Phase 1 | Phase 2 |
|---|---|---|
| Children | ||
| Number of participants | 18 | 49 |
| Number (%) of females | 7 (39%) | 15 (30%) |
| Mean (SD) age, months | 9.4 (1.1) | 9.3 (1.3) |
| Mean (SD) length‐for‐age z‐score | −1.35 (1.17) | −1.43 (1.35) |
| Mean (SD) weight‐for‐length z‐score | −0.29 (1.10) | −0.09 (1.05) |
| Caregivers' characteristics | ||
| Number of participants | 20 | 49 |
| Mean (SD) age, years | 25 (5) | 24 (5) |
| Mean (SD) number of children alive | 3.0 (1.2) | 2.9 (1.7) |
| Mean (SD) time at school, completed years | 5 (3) | 5 (3) |
| Number (%) married | 19 (95) | 36 (73) |
SD, standard deviation.
The mean (95% CI) percentage of the LNS test meals consumed by the study children under direct observation was 88% (82–94%) for LNS‐10gM, 90% (84–95%) for LNS‐20gM, 87% (79–95%) for LNS‐20gNoM and 86% (83–90%) for Nutributter. The inter‐individual variation in intakes was slightly higher for LNS‐10gM and lower for Nutributter than for the two other test products (Fig. 1). All children who consumed less than 60% of the offered test dose were reported to be either ill or irritable by their caregivers. The median (25th, 75th centile) time for completing the offered test meal was 4 (2, 7) minutes for LNS‐10gM, 5 (3, 6) minutes for LNS‐20gM, 4 (3, 8) minutes for LNS‐20gNoM and 4(2, 6) minutes for Nutributter.
Figure 1.
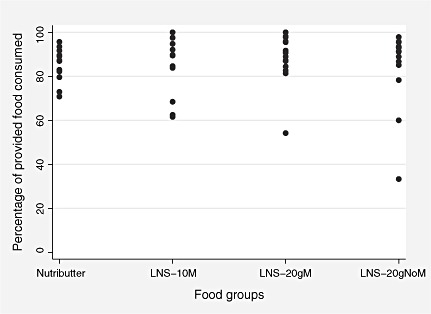
Inter‐individual variation in consumption of different test meals by the participants (each dot represents one individual). LNS, lipid‐based nutrient supplement.
When queried about the palatability of the directly observed test meals, almost all caregivers thought their children liked all study foods very much. The median hedonic score reported by caregivers was 5 in each of the LNS groups. Likewise, that reported for Nutributter was 5. The caregivers also gave a high liking score for all test foods themselves. Except for LNS‐10gM, for which the mothers reported a median hedonic score of 4, all other test foods scored 5 (2, 3).
Figure 2.
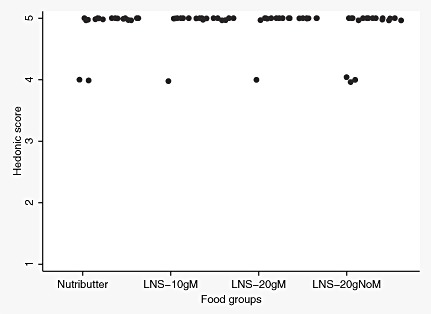
Distribution of hedonic scores reported by caregivers on child liking of the test food. LNS, lipid‐based nutrient supplement.
Figure 3.
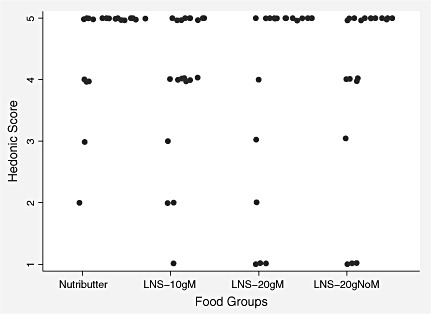
Distribution of hedonic scores reported by caregivers on their own liking of the test food. LNS, lipid‐based nutrient supplement.
In phase 2, the participants were given a 2‐week ration of one of the test products for home consumption. At the end of the intervention, the whole ration had been finished in all but one household. In an interview concerning the latter supplementation week, caregivers reported that few infants had difficulties in eating LNS, and that they usually finished the whole LNS ration offered to them. Most caregivers (39/49) reported that LNS was consumed only by the participating child; some sharing was reported to siblings (five cases), neighbour's children (one case), both sibling and neighbour's children (three cases) or adults (one case). The majority of caregivers (38/49) reported that the provided dose was enough for 2 weeks, but others said that their children liked LNS so much that they finished the supplement in a shorter period. There were no differences in the consumption patterns for the three different LNS products tested in phase 2 (Table 3).
Table 3.
Consumption patterns of the three LNS products during the second week of a 14‐day supplementation period
| Variable | LNS‐20gM | LNS‐20gNoM | LNS‐10gM |
|---|---|---|---|
| Number of participants | 16 | 16 | 17 |
| Reported no difficulties in LNS consumption (%) | 94 | 88 | 88 |
| Consumed LNS every day (%) | 94 | 94 | 94 |
| Always finished the offered LNS ration (%) | 75 | 75 | 88 |
| LNS given in two daily doses (%) | 81 | 81 | 94 |
| LNS ration mixed with complementary porridge (%) | 94 | 100 | 94 |
| Ration provided was sufficient for 2 weeks (%) | 75 | 75 | 82 |
LNS, lipid‐based nutrient supplement.
When interviewed about the palatability of the test products after the 14‐day feeding period, all the 49 caregivers reported that their infants appeared to like the test food, and the caregivers themselves also reported liking the food. After testing the food, the caregivers reported median hedonic scores of 5 for all the test foods (data not shown). No adverse events with the LNSs were reported during either phase of the study.
Focus group discussions, held with the caregivers of the participants who had been consuming the LNSs for 2 weeks, supported the questionnaire‐based data and gave a more detailed picture of feeding LNS. All of the caregivers who had children in the study took part in one focus group interview session. Caregivers indicated that the children preferred LNS mixed with porridge over the other complementary foods and that LNS meals were easy to feed. They speculated that this was because of the sugar it included (though the sugar content of the new LNS formulations was 50% lower than the amount in Nutributter). Some children started accepting LNS mixed with porridge right from the very first meal, while others required a few meals to get used to it. Although not instructed to do so, the caregivers had tasted the product their child was consuming and they typically liked it, too.
The focus group participants found LNS very acceptable also from the point of preparing the meal. An advantage that was mentioned many times was that they could store LNS at home, and thus prepare the child's meal early in the morning. Normally, they would feed plain porridge in the morning and other complementary foods only after going to the market.
Focus group participants also reported how they had to ration LNS in order to make it last for 2 weeks. Participants who received only one container of LNS (LNS‐10gM) said that rationing would have been easier with two containers (LNS‐20g). Some described how they did not manage to ration the LNS because children often wanted to consume more of it than recommended. Also, some caregivers reported that it was difficult for them to prevent older siblings from eating LNS. As a side effect, some caregivers said that the child would not accept regular porridge after having eaten porridge with LNS during the same meal even if the child still appeared hungry. Yet, participants of all focus group discussions generally felt that there were no disadvantages related to LNS.
On a more general level, caregivers associated LNS with healthy development of their children. Sometimes they even referred to LNS as medicine. However, they only discussed the physical health of the children and absence of illnesses. They did not associate LNS with the behavioural development of the children. No information on previous usage of similar products like ready‐to‐use therapeutic food was assessed or reported during the focus group discussions.
Discussion
The present trial was carried out to test the acceptability of three new LNSs among healthy infants and their caregivers in rural Malawi. In the selected sample, the LNS‐containing test meals were consumed rapidly and almost completely, and the caregivers reported that both they and their infants liked all the three investigational supplements. The opinions were similar after a 2‐week supplementation period, and individual and group interviews revealed no major problems associated with the consumption of the novel supplements. In contrast, some caregivers reported that their infants strongly preferred the investigational products over traditional complementary foods. The caregivers were also otherwise very positive about supplementing infant diets with LNS.
The study design ensured that the order of introduction was balanced between the investigational products; no refusal for participation was reported; follow‐up was complete; and all rather low intakes were explained. Hence, the sample findings are likely representative of the population from which the sample was drawn, lending support to the hypothesis that all the tested investigational LNS products would be acceptable to infants and their caregivers in the study area.
Interpretation of our phase 2 results, however, is limited to each of the pairs of villages in which each LNS type was assessed, and not all six villages as we did not balance village across all LNS types.
LNSs were initially designed for use as a rehabilitation food for severely malnourished children (Briend et al. 1999). Recently, there has been a major interest to expand their use in malnutrition prevention, as a food‐based approach to promote linear growth and general well‐being. Initial attempts in this direction have been promising, and 6–12‐month‐long complementation of the infants' diet has been associated with improved motor development and reduced incidence of severe stunting in infancy (Adu‐Afarwuah et al. 2007; Phuka et al. 2008). However, the price of the earlier used products may not be affordable by low‐income households, which might hinder scaling‐up of this approach. Hence, there is a need for cheaper alternatives.
The newly developed LNS versions were designed to reduce the manufacturing costs while maintaining or even improving the nutritional value of the products. Cost reductions are possible by a change in the oil source (LNS‐20gM), removal of milk powder from the ingredients (LNS‐20gNoM) or concentrating the product so that daily dose would be 10 g instead of 20 g (LNS‐10gM). With these changes, the estimated production costs for a daily dose for the new supplements range from 4 to 8 US cents, which might be low enough to allow widespread adoption of LNS use, should they prove biologically effective.
Two other trials have recently assessed the acceptability of novel LNS products in sub‐Saharan Africa: one in Ghana assessing LNS designed for pregnant and lactating women and their children, and the other one in Burkina Faso, testing LNS with altered zinc content but otherwise similar to the LNS‐20gM product evaluated herein (Adu‐Afarwuah et al. in press; Hess et al. in press). Both studies produced similar results to ours and concluded that modified LNS was acceptable to the intended target groups in the defined settings (Adu‐Afarwuah et al. in press; Hess et al. in press). The consistency of the results obtained from three different settings suggests that the acceptability of LNS products is rather robust despite changes in the raw ingredients, thus allowing tailoring of the product to specific target groups. Encouraged by the acceptability results, our research group is currently carrying an efficacy trial in Malawi on the growth‐promoting effect of the three LNS products tested in the present study.
Source of funding
The trial was funded by a grant from the Bill & Melinda Gates Foundation to the University of California, Davis. Per Ashorn is supported by a personal grant from the Academy of Finland (grant number 130313).
Conflicts of interest
Mamane Zeilani is employed by Nutriset S.A.S, a company that produces and sells Plumpy Nut, which is a therapeutic version of LNSs. Other authors declare no conflict of interest. The funders of the trial had no role in the implementation, analysis or reporting.
Contributions
All authors contributed in protocol drafting. JP and KM supervised data collection. JP, UA, PA and KM performed data analyses. Y‐B contributed in the statistical supervision of data analyses. JP, UA, PA, Y‐B and KM drafted the manuscript. The manuscript was edited by JP, UA, PA, MZ, Y‐B and KM. MM contributed in the reviewing of the manuscript.
Acknowledgements
We are grateful to the people in Mangochi District and our research assistants for their positive attitude, support and help in all stages of the study, and to Laszlo Csonka for designing the data collection forms and data entry programmes.
References
- Adu‐Afarwuah S., Lartey A., Brown K.H., Briend A., Zlotkin S. & Dewey K.G. (2007) Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition 86, 412–420. [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S., Lartey A., Zeilani M. & Dewey K.G. (2010) Acceptability of lipid‐based nutrient supplements (LNS) by Ghanaian infants and pregnant or lactating women. Maternal and Child Nutrition doi: 10.1111/j.1740‐8709.2010.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour R.S. & Kitzinger J. (eds) (1999) Developing Focus Group Research: Politics, Theory and Practice. Sage: London. [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Briend A., Lacsala R., Prudhon C., Mounier B., Grellety Y. & Golden M.H. (1999) Ready‐to‐use therapeutic food for treatment of marasmus. Lancet 353, 1767–1768. [DOI] [PubMed] [Google Scholar]
- Collins S. & Sadler K. (2002) Outpatient care for severely malnourished children in emergency relief programmes: a retrospective cohort study. Lancet 360, 1824–1830. [DOI] [PubMed] [Google Scholar]
- Diop E.I., Dossou N.I., Ndour M.M., Briend A. & Wade S. (2003) Comparison of the efficacy of a solid ready to use food and a liquid milk‐based diet for the rehabilitation of severely malnourished children: a randomized trial. American Journal of Clinical Nutrition 78, 302–307. [DOI] [PubMed] [Google Scholar]
- Hess S.Y., Bado L., Aaron G.J., Ouedraogo J.‐B., Zeilani M. & Brown K. (2010) Acceptability of zinc‐fortified, lipid‐based nutrient supplements (LNS) prepared for young children in Burkina Faso. Maternal and Child Nutrition doi: 10.1111/j.1740‐8709.2010.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.A., Manary M.J., Maleta K., Briend A. & Ashorn P. (2008) An energy‐dense complementary food is associated with a modest increase in weight gain when compared with a fortified porridge in Malawian children aged 6–18 months. Journal of Nutrition 138, 593–598. [DOI] [PubMed] [Google Scholar]
- Maleta K., Virtanen S., Espo M., Kulmala T. & Ashorn P. (2003) Timing of growth faltering in rural Malawi. Archives of Disease in Childhood 88, 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manary M.J., Ndekha M., Ashorn P., Maleta K. & Briend A. (2004) Home‐based therapy for severe malnutrition with ready‐to‐use food. Archives of Disease in Childhood 89, 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office (Malawi) and ORC Macro (2001) Malawi Demographic and Health Survey 2000. National Statistical Office and OCR Macro: Zomba, Malawi and Calverton, MD. [Google Scholar]
- Phuka J.C., Maleta K., Thakwalakwa C., Cheung Y.B., Briend A., Manary M.J. et al (2008) Complementary feeding with fortified spread and incidence of severe stunting in 6–18 month old rural Malawians. Archives of Pediatrics and Adolescent Medicine 162, 619–626. Erratum in: Archives of Pediatrics and Adolescent Medicine 162 (10): 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandige H., Ndekha M.J., Briend A., Ashorn P. & Manary M.J. (2004) Home‐based treatment of malnourished Malawian children with locally produced or imported ready‐to‐use food. Journal of Paediatric Gastroenterology and Nutrition 39, 141–146. [DOI] [PubMed] [Google Scholar]


