Abstract
Determining early‐life risk factors for obesity in later life is essential in order to effectively target preventative interventions to reduce obesity. The aim of this systematic review was to investigate current evidence to determine whether the timing of introducing solid foods is associated with obesity in infancy and childhood. Relevant randomized and observational studies from developed countries were identified by searching the following six bio‐medical databases (Medline, Embase, British Nursing Index, CINAHL, Maternity and Infant Care, and PsycINFO) and hand‐searching reference lists. Studies of pre‐term or low birthweight infants were excluded. Twenty‐four studies met the inclusion criteria for the systematic review. Data from over 34 000 participants were available for interpretative analysis. No clear association between the age of introduction of solid foods and obesity was found. It is likely that a whole family approach to obesity prevention will be most effective and health professionals should continue to promote healthy infant feeding in line with national recommendations.
Keywords: solid foods, obesity, body mass index, childhood, systematic review
Introduction
Obesity is currently a leading global public health concern. Raised body mass index (BMI) is a major risk factor for cardiovascular disease, diabetes, musculoskeletal disorders and some cancers. Cardiovascular disease has been rated as the world's top cause of death (17 million people per year) by the World Health Organization (WHO), which also projects that deaths from diabetes will increase by more than 50% worldwide by 2015 (WHO 2006a). UK National Health Service (NHS) costs attributable to obesity in 2007 were estimated at £2.3 billion, with projections suggesting that this amount will rise to £7.1 billion/year in 2050 (Foresight 2007). Wider total costs of overweight and obesity to the UK economy in 2007 were estimated at £15.8 billion, predicted to increase to £49.9 billion in 2050. Employment, welfare and social care costs were included in this model (Foresight 2007). A cross‐government strategy to support people to maintain a healthy weight and lower levels of obesity in the UK population was published following these estimates (Department of Health and the Department for Children, Schools and Families 2008).
The latest figures from the National Child Measurement programme in England show that 24% of boys and 21.5% of girls aged 4–5 years are overweight or obese. At age 10–11 years, 34.5% of boys and 30.7% of girls are overweight or obese (NHS Information Centre, Lifestyle Statistics 2009). It is estimated that if nothing is done to curb these increasing levels, 55% of boys and 70% of girls could be overweight or obese by 2050 (Foresight 2007). A better understanding of early predictors of obesity is essential in order to target public health interventions effectively. In recent years, much research has concentrated on the preventative effects of breastfeeding (von Kries et al. 1999; Dewey 2003; Arnez et al. 2004) and interventions in school‐aged children (Summerbell et al. 2005) for reducing obesity. However, another potentially influential stage in childhood development has received less attention – the timing of introducing solid foods.
Recommendations about the introduction of solid foods
During the first 2 years of life, children are particularly vulnerable and at this time, adequate nutrition is essential to optimize health and physical and mental development. Poor nutrition can lead to significant mortality and morbidity; for example, malnourished infants and children are more vulnerable to other diseases such as diarrhoea and malaria (PAHO 2003). Poor nutrition in infancy and early childhood can also have longer‐term effects on adolescent and adult intellectual performance and general health, which may ultimately affect their life chances and ability to work (PAHO 2003). Inappropriate feeding practices, such as reduced breastfeeding, are a major cause of poor nutrition early in a child's life. The time when solid foods are introduced is recognized as a time of particular vulnerability (WHO 2005).
The optimum age at which to introduce solid foods to an infant's diet is a much‐debated, contentious issue. In 2001, the WHO issued a global recommendation that mothers should exclusively breastfeed for the first 6 months of life, with the introduction of solid foods commencing at 6 months (WHO 2001); this is supported by a systematic review conducted by Kramer & Kakuma (2002) that concluded that breastfeeding for 6 months conferred benefits to both mother and infant; particularly reducing infant morbidity and mortality by decreased incidence of gastrointestinal infections. Despite this recommendation, the age at which solid foods are introduced to infants' diets varies worldwide – even within European countries, there are wide ranging differences. It is common practice in some countries, such as the UK, for solid foods to be introduced early (e.g. before 4 months), and in other countries it is not uncommon for the introduction of solid foods to be delayed for more than 6 months (ESPGHAN Committee on Nutrition 2008). In the UK, the Scientific Advisory Committee on Nutrition endorsed the WHO resolution, and the Department of Health (DH) Infant Feeding Survey Recommendations stated ‘exclusive breastfeeding is recommended for the first 26 weeks of an infant's life’ (DH 1994); however, the seventh National Infant Feeding Survey showed that half of mothers had introduced solid foods by 4 months and only 2% met the WHO recommendation and delayed the introduction of solid foods until 6 months (Bolling et al. 2007). More recently, there has been a move towards ‘baby‐led weaning’ practices in which infants are not fed solid foods until around 6 months when they show physical signs that they are ready to feed themselves. The Start4Life campaign, a NHS initiative in the UK, now recommends an approach to the introduction of solid foods in line with that of ‘baby‐led weaning’ practices (DH 2009).
Defining childhood obesity
There is currently no universally accepted way of defining childhood obesity. A number of different classification systems have been used by different countries, making international comparisons difficult. In the UK, the 1990 BMI percentile charts which are age and sex specific, are commonly used to define overweight and obesity at a population level using the 85th and 95th percentiles, and at an individual level in clinical practice using the 91st and 98th percentile (Cole et al. 1995). These were developed from a British reference population consisting of 15 636 males and 14 899 females aged 0–23 years (NEPHO 2009). In addition, the International Obesity Task Force (IOTF) has developed a classification system based upon an international population including UK, Brazil, Hong Kong, Netherlands, Singapore and the USA. The IOTF definition uses age‐ and sex‐specific cut off points which are extrapolated from the adult BMI points of 25 kg m2 for overweight and 30 kg m2 for obesity (Cole et al. 2000). In response to the global rise in obesity rates, the WHO have developed and released new international growth standards based on pooled data from the USA National Centre for Health Statistics 1977 (de Onis et al. 2007) and WHO Multi‐centre Growth Reference Study from Brazil, Ghana, Norway, India, Oman and USA (de Onis et al. 2007). The charts are designed so that the centile curves align with the new WHO child growth standards at 5 years and the adult BMI cut offs of 25 kg m2 and 30 kg m2 at age 19 (NEPHO 2009). It is hoped that the WHO growth charts will provide a single international standard for childhood obesity.
In 2009, The Royal College of Paediatrics and Child Health (RCPCH), WHO and Department of Health (DH) launched the UK‐WHO 0–4 years growth charts for breastfed babies. The revised charts combined the UK 1990 and WHO data. The previous charts were devised based mainly on data from studies that included infants who were at least partially formula fed and therefore did not represent the growth patterns of exclusively breastfed infants (RCPCH 2009). As a result, breastfed infants had often appeared to be underweight when measured against the charts. This may have led to parents stopping breastfeeding or supplementing their child's diet with formula milk causing overfeeding. Evidence suggests that this practice of mixed feeding can increase the risk of overweight in childhood (Hammer et al. 1999; von Kries et al. 1999).
Existing evidence
The relationship between breastfeeding and obesity has been investigated and it is now generally agreed that breastfeeding may reduce the risk of obesity in later life (Dewey 2003; Arnez et al. 2004; Horta et al. 2007). However, the association between the age at which solids are introduced and the risk of obesity in later life is still not clear. Lanigan et al. (2001) conducted a systematic review evaluating evidence published between 1982 and 1998 investigating growth, nutritional status and morbidity outcomes in relation to the age at which solid foods were introduced. They concluded that at the time, there was a lack of clear evidence to support or refute a change to the WHO infant recommendations to exclusively breastfeed for the first 6 months of life (Lanigan et al. 2001). One review has specifically investigated the protective effect of introducing solid foods after 4 months of age on obesity in adulthood (Sass 2006). The study concluded that the introduction of solid foods before 4 months was not associated with an increased risk of obesity in adulthood; however, the review was not systematic and the conclusions were based upon the outcome of only five studies (Sass 2006). Therefore, the aim of the present study is to address these gaps by undertaking a systematic review to investigate whether there is an association between the timing of introducing solid foods, and obesity in infancy and childhood.
Key messages
-
•
This review did not find an association between the timing of introducing solid foods and obesity in infancy and childhood.
-
•
Health care professionals should continue to encourage and promote healthy infant feeding practices in line with national recommendations.
-
•
A whole family approach to obesity prevention is more likely to be effective than working to change a single factor such as the timing of the introduction of solid foods.
Materials and methods
Search strategy
The Cochrane Library and the Database of Reviews of Effectiveness were searched, as well as other online databases: Medline, Embase, CINAHL, PsycINFO, British Nursing Index, and Archives and Maternity and Infant Care. The search strategy was generated directly from the review question, which was broken down into key facets using the Population‐Intervention‐Outcomes‐Design (P‐I‐O‐D) model, as shown in Fig. 1. For each of the facets identified using the P‐I‐O‐D model, possible synonyms, spelling variants and subject headings were identified. A preliminary search was conducted using the search terms to ensure the sensitivity and specificity of the search strategy. An initial search was conducted in 2007 and was updated in December 2008 and July 2010. Limits were applied for English language text and studies on humans. The search did not include grey literature. No date restrictions were applied for the initial search; however, date restrictions were applied to the updated search (Table 1). Hand‐searching of reference lists of retrieved articles was also conducted.
Figure 1.
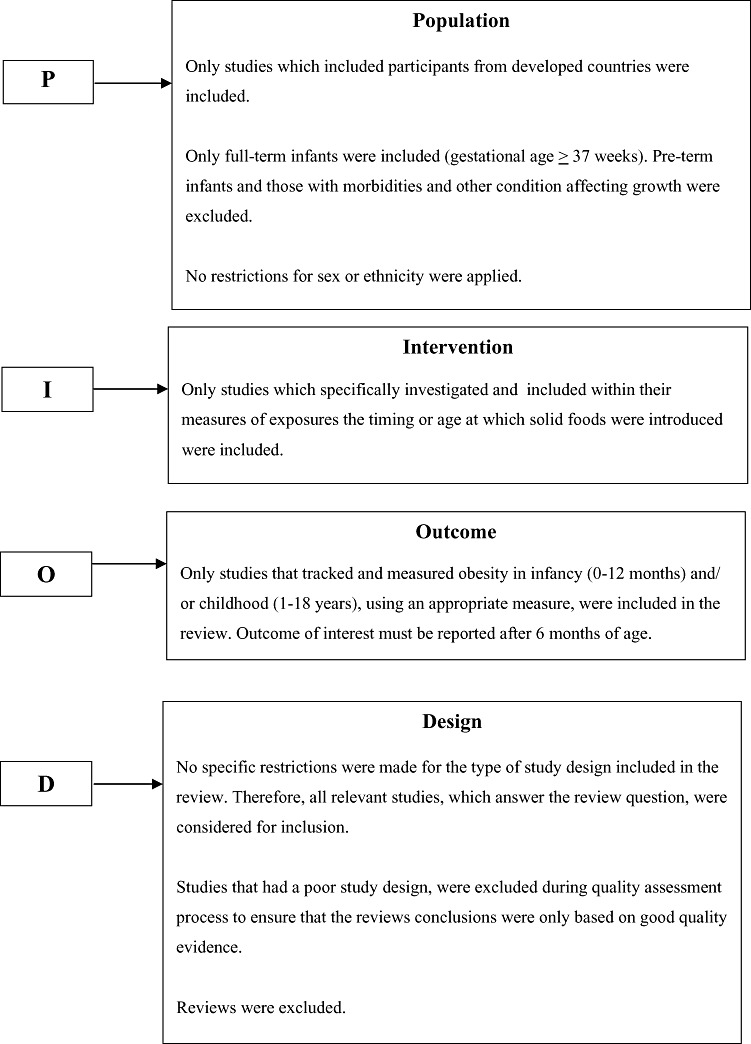
Population‐Intervention‐Outcomes‐Design Model.
Table 1.
Biomedical databases searched
| Biomedical Database | Description | Initial search – Provider and dates searched | First updated search – Provider and dates searched | Second updated search – Provider and dates searched |
|---|---|---|---|---|
| Medline | Medical information covering all aspects of medicine. It is the bibliographic database of the USA'S national Library for Medicine, holding records of over 3500 journals | Dialog 1966–2007 | Ovid 1980–Dec 2008 | Ovid Dec 2008–July 2010 |
| Embase | Covers all aspects of biomedicine, drugs and pharmacology with a European, rather than an American emphasis. It also covers public, occupational and environmental health. | Dialog 1974–2007 | Ovid 1980–Dec 2008 | Ovid Dec 2008–July 2010 |
| CINAHL (Cumulative Index to Nursing and Allied Health Literature) | Provides access to virtually all English Language nursing journals, as well as journals from 17 allied health disciplines. | Dialog 1983–2007 | EBSCO 1983–Dec 2008 | EBSCO Dec 2008–July 2010 |
| PsycINFO | Produced by the American Psychological Association and covers psychology and psychological aspects of medicine, psychiatry, nursing pharmacology, physiology, linguistics, anthropometry, business and law. | Dialog 1806–2007 | Ovid 1987–Dec 2008 | Ovid Dec 2008–July 2010 |
| British Nursing Index (BNI) and Archive | Provides citations from the UK's most popular nursing, midwifery, and community healthcare material. | Dialog 1994–2007 | Ovid 1994–Dec 2008 | Ovid Dec 2008–July 2010 |
| Maternity and Infant Care | Not searched | 1971–Dec 2008 | Dec 2008–July 2010 |
Study inclusion and exclusion criteria
Papers from citations identified through the search strategy were retrieved for pre‐screening when titles and abstracts met the pre‐defined inclusion criteria. Randomized, observational and case‐control studies were included and reviews were excluded; studies included in reviews were assessed. Only studies of participants living in developed countries were included to ensure that the review was applicable to these populations. Countries were defined as developed according to the World Development Report (World Bank 2001). No restrictions for sex or ethnicity were implemented. Studies of pre‐term infants (born at <37 weeks gestational age) and infants with conditions that can influence growth were excluded. Studies which specifically investigated or included within their measures of exposures the effects of the ‘timing’ or ‘age’ at which solid foods were introduced and that measured and reported obesity in infancy (up to 12 months) and/or childhood (1–18 years), using an appropriate measure were included in the review. Appropriate outcome measures included height, weight, BMI and measures of adiposity, such as skin fold and circumference measures. Only studies published in English were included due to lack of resources and expertise available for translation.
Assessment of study eligibility
The title and abstract of papers identified by electronic searches were independently assessed by two researchers against the review inclusion and exclusion criteria. Those identified for possible inclusion in the review, including those identified through manual searching of reference lists, were then retrieved for further quality assessment before being included in the review. Most studies could be excluded by reviewing the title and abstract. Studies that could not be clearly excluded were retrieved for full evaluation (Fig. 2).
Figure 2.
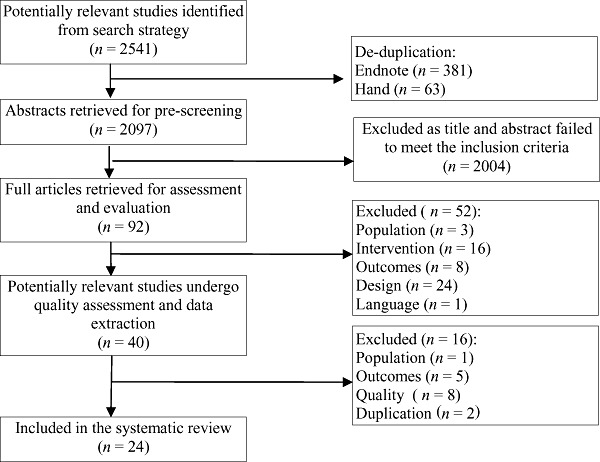
Identification and assessment of studies for inclusion in the systematic review.
Quality assessment
The aims of the quality assessment process were to check whether the study addressed the review question, and to rate the methodological quality of the study to ensure that the findings were valid and not subject to bias or confounding factors. A critical appraisal proforma was developed using the existing guidance from Critical Appraisal Skills Programme (CASP 2006), Scottish Intercollegiate Guidelines Network (SIGN), School of Health and Related Research (Scharr), Centre for Reviews and Dissemination (CRD) and the work of Oxman and Guyatt (1993). The studies were rated based upon the SIGN grading recommendations in evidence‐based guidelines (SIGN 2001). Giving each study included in the review a quality rating helped to eliminate poor quality studies and assisted with more effective data handling when interpreting the results. The study design, methods, participants, setting and any other key measures and variables were assessed using the critical appraisal proforma. As well as assessing study methodology for bias, the tool also helped to reduce the likelihood of type I or type II errors occurring during the critical appraisal process.
Data extraction
Study eligibility was re‐verified during the data extraction process. A proforma was devised based upon the data extraction sheet produced by CRD (2001). Use of the proforma ensured that data were extracted systematically and that comprehensive summary tables were completed (CRD 2001). The form included general information about the study, such as the title, author(s) and journal publication information. In order to verify the eligibility of the study in answering the review question, specific information regarding the study population, exposures, outcomes and the study design were also included on the proforma. Once the study eligibility was established, further information was extracted, which directly related to the review question. This included information on the study methodology, such as the quality, sample selection, blinding, population characteristics, setting in which the study was conducted, exposures and baseline measurements. Information relating to the outcome measures and findings was also extracted such as length of follow‐up, completeness of follow‐up, missing data, statistical analysis conducted, and presentation of results and the size of the effect of the results.
Pre‐screening, quality assessment and data extraction were carried out by two independent reviewers. Any differences were then discussed and assessed by a third reviewer if differences were not resolved.
Synthesis of data
A narrative approach was used for data synthesis. Meta‐analysis was not feasible due to the heterogeneous nature of the studies in terms of both outcomes and the timing of the intervention of interest – introduction of solid food. For example, a range of outcome measures were used [weight, length, BMI, ponderal index, skin‐fold measures, fat mass, lean mass and dual X‐ray absorptiometry (DXA) scanning] and these were measured and reported at different ages, also the introduction of solid food was not categorized consistently across studies.
Results
Results of the search
A total of 2541 titles and abstracts were identified by the search strategy, and were imported into Endnote reference managing software. Endnote identified 381 duplicates and a further 63 duplicates were identified by hand. After de‐duplication, a total of 2097 papers remained for pre‐screening. Pre‐screening of abstract and title identified 92 papers for full retrieval and further assessment. Of the 92 papers retrieved, 52 were initially excluded from the review, 24 papers also excluded due to their study design, a further 16 papers did not measure the intervention of interest (timing of introduction to solid foods), eight papers did not report on the outcome of interest; one was not available in English language text and three papers were excluded as studies were conducted in developing countries.
A further 16 papers were excluded after quality assessment. Eight papers (Lauver et al. 1981; Yeung et al. 1981; Quandt 1984; Wolman 1984; Patterson et al. 1986; Agras et al. 1990; Carruth et al. 2000; Boyington & Johnson 2004) were excluded due to poor quality. Five papers (Gillman et al. 2001; Ong et al. 2006; De la Hunty 2009; Van Dijk & Innis 2009; Worobey et al. 2009) were excluded as our outcome of interest was collected but not fully reported and we did not have the capacity within this review to contact authors. One paper (Khadivzadeh & Parsai 2004) was excluded as it studied participants from a developing country. Two further papers (Kramer et al. 1986; Van't Hof 2000) were duplicates.
Following critical appraisal of study design, methodology and possible sources of bias, studies considered to provide good quality evidence were included in the review (see Table 2). To ensure the findings of the review were based upon the best available evidence those that rated low on the quality assessment proforma were excluded (Appendix 1).
Table 2.
Summary of data extracted
| Age when outcomes were measured | First author and Date | Design Number started/reported | Age at which solids introduced (how collected) | Outcome measured (calculated) | Method of milk feeding How BF was taken into account | Study quality grade | Outcome |
|---|---|---|---|---|---|---|---|
| 3 months 6 months 1 year | Mehta 1998 | RCT US 165/147 | 3–4 months vs. 6 months (randomized) | Weight, length, fat mass and lean mass | Infants all fed formula from 3 months may have breastfed before BF not analysed | ++ | No differences in anthropometric measurements or body composition were found between study groups at 3, 6 or 12 months |
| 1 year | Baker 2004 | Cohort Danish National Birth cohort 5330/3768 | <16 weeks vs. ≥16 weeks (Maternal reporting) | Weight and length | >95% any breastfeeding at 1 month, 11% at 12 months 20% never fed formula Analysed by breastfeeding duration | + | Infants fed solid foods earlier gained significantly more weight from birth to one year (P < 0.0001) |
| 8 weeks 13 weeks 26 weeks 1 year | Forsyth 1993 | Cohort Dundee Study 671/548 | ≤12 weeks vs. >12 weeks (Standard questionnaire completed by HV) | Weight | 14% exclusively breastfed, 47% breastfed (not excl) 39% formula fed Regression analysis included breastfeeding | ++ | The early introduction of solids was independently associated with increased weight at 8 (P = 0.003), 13 (P = 0.006) and 26 weeks (P = 0.009) of age but not at 1 year (P = 0.3) |
| 12 weeks 9 months | Morgan 2004 | Data from two RCTs 940/680 | ≤12 weeks vs. >12 weeks (Maternal reporting) | Weight, length, triceps skin folds, subscapular skin folds | Predominantly breastfed for at least 6 weeks Analysis of covariance adjusted for type of milk feeding | + | Infants given solid foods later gained a small amount more weight and length between 12 weeks and 9 months but this did reach significance. |
| 1 year | Haschke 2000 | Cohort Euro‐growth study 504/355 | <4–5 months vs. ≥4–5 months | Weight and length (BMI z‐scores) | Only breastfed infants | ++ | Fully breastfed infants fed solid foods before 4–5 months were longer and lighter than fully breastfed infants fed solid foods after 4–5 months – Mean z‐scores for BMI were lower (P < 0.05) |
| 6 months 1 year | Kramer 1985a | Cohort Canada 462/358 | 2 months, 3 months, 6 months (Maternal reporting) | Weight, length, right triceps, subscapular and suprailiac skin folds | 58% breastfed at birth, 19% at 4 months, 10% at 6 months Regression analysis included duration of breastfeeding | + | Later age at introduction of solid foods was a significant determinant for lower weight (r 2 = 0.296, P < 0.0001) and for less total skin‐fold thickness (r 2 = 0.038, P = 0.002) at 12 months but not for BMI. Results stated to be similar but to explain less of the variance at 6 months. |
| 1 year | Lande 2005 | Cohort Norway 3000/1441 | ≤4 months vs. >4 months (Parental semi‐quantitative food frequency questionnaire) | Weight and length (BMI) | 42% exclusively breastfed at 4 months Regression analysis included exclusive breastfeeding and breastfeeding duration | ++ | Time of introduction of solid foods was found to be significantly associated with BMI at 1 year in the univariate analyses (P ≤ 0.02), but not in the multiple linear regression analysis. |
| 6 months | Baird 2008 | Cohort Southampton Women's Study 1841/1740 | <5 months vs. ≥5 months (maternal report) | Weight, length and skin‐fold thicknesses | Milk feeding 0–6 months in 6 categories. 21% formula fed only, 7.5% exclusively breastfed Multivariable linear regression analysis examined the independent influences of milk feeding | ++ | At 6 months infants introduced to solid foods earlier were heavier and longer than other infants independent of 0–6 month milk feeding and maternal factors. No association between timing of introduction of solid foods and skin‐fold thickness at 6 months. |
| 10 to 22 months Mean 14 m (SD + 2.38) | Sloan 2008 | Cohort Northern Ireland 1218/210 | <4 months vs. >4 months (Retrospectively) | Weight (converted to z‐scores after adjustment for gender and exact age when weight measured) | 68% initiated breastfeeding, mean duration of breastfeeding 20 weeks Breastfeeding controlled for in linear modelling | + | Infants given solid foods earlier had higher z‐scores at 14 months (P = 0.004) When duration of breastfeeding was controlled for, there was still a significant effect of early introduction of solid food on 7‐month weight z‐scores (P = 0.046) and 14‐month weight z‐scores (P = 0.035), and weight gain between 8 weeks and 14 months (P = 0.029). |
| 18 months | Heinig 1993 | Cohort Davis Area Research on Lactation, Infant Nutrition and Growth study 144/80 | 16–25 weeks vs. >26 weeks N.B No solids before 16 weeks (4 day weighed food diary) | Weight and length monthly to 18 months(z‐scores for weight‐for‐age, length‐for‐age and weight‐for‐length) | Analysis conducted separately for breastfed and formula fed infants | + | Timing of introduction of solid foods was not found to be related to weight or to length at any month to 18 months in either breastfed or formula fed infants |
| Morgan 2004 | Data from 2 RCTs 940/680 | <12 weeks vs. >12 weeks (Maternal reporting) | Weight, length, triceps skin folds, sub scapular skin folds | Predominantly breastfed for at least 6 weeks Analysis of covariance adjusted for type of milk feeding | + | No significant differences were found for any of the measures at 18 months | |
| 2 years | Kramer 1985a,b | Cohort Canada 462/347 | 2 months, 4 months, 6 months (Maternal reporting) | Weight, length, right triceps skin fold, subscapular skin fold, suprailiac skin fold | 58% breastfed at birth, 19% at 4 months, 10% at 6 months Regression analysis included duration of breastfeeding | + | Age at which solids were introduced was not a significant determinant of weight, total skin‐fold thickness or BMI at 2 years |
| Forsyth 1993 | Cohort Dundee study UK 671/392 | <12 weeks vs. >12 weeks (Standard questionnaire completed by HV) | Weight | 14% exclusively breastfed, 47% breastfed (not excl) 39% formula fed Regression analysis included breastfeeding | ++ | No significant difference in weight between the groups was found at 2 years (P = 0.7) | |
| Haschke 2000 | Cohort Euro‐growth study 504/351 | <4–5 months vs. >4–5 months (Only breastfed infants who received solids early and late – not formula fed) | Length and weight (BMI z‐scores) | Only breastfed infants | ++ | No significant differences were found at 2 years between fully breastfed infants fed solid foods before and after 4–5 months | |
| 3 years | Cohort Euro‐growth study 504/246 | No significant differences found at 3 years between fully breastfed infants fed solid foods before and after 4–5 months | |||||
| Hawkins 2009 | Millennium Cohort Study UK 14 630/13 188 | <17.4 weeks (4 months) vs. ≥17.4 weeks (4 months) (maternal report) | Height and weight Overweight and obesity defined by International Obesity Task Force cut‐offs for BMI | Never breastfed n = 4203 Breastfed for <4 months n = 5515 Breastfed for ≥4 months n = 3454 Breastfeeding duration examined as one of many risk factors in regression analysis | ++ | At age 3, 18% were overweight and an additional 5% were obese. In the fully adjusted model, introduction of solid foods before 4 months was associated with childhood overweight at age 3 years (adjusted OR 1.12 95% CI 1.02 to 1.23) | |
| Griffiths 2009 | Millennium Cohort Study UK 12 268/10 533 Term, singleton, white infants only | <17.4 weeks (4 months) vs. ≥17.4 weeks (4 months) (maternal report) | Conditional weight gain z‐scores from birth to 3 years | Never breastfed n = 3651 Ever breastfed n = 6882 Breastfed <4 months n = 4251 Breastfeeding initiation and duration were tested by linear regression | ++ | Conditional weight gain was associated with age at introduction of solid foods with and without adjustment for maternal social class, pre‐pregnancy BMI, parity, smoking during pregnancy and duration of breastfeeding. However, this association was no longer significant after adjustment for the child's height z‐score at 3 years (2.01, 95% CI.04 to 0.03). | |
| 4 years | Kuperberg 2006 | Cohort First Nations Canada 102/71 | <3 months vs. >3 months (Parental reporting) | Weight and length (BMI >85th percentile at 48 months classified as overweight) | At 3 m, 27% exclusive bf, 33.3% bf not exclusive and 39.7% (n = 25/63) had stopped breastfeeding Breastfeeding not reported in relation to introduction of solid foods | + | No differences were found in the proportions of children overweight at 4 years according to age at introduction of solid foods |
| Zive 1992 | Cohort SCAN Project US 351/331 | Mean range 4–5 months (Retrospective infant feeding questionnaire) | Height, weight, triceps skin fold, subscapular skin fold (BMI) | High breastfeeding initiation Infant feeding variables were included in regression analysis | + | No associations were found between the age at which solids were introduced or any of the other infant feeding variables and measures of adiposity at 4 years | |
| Robinson 2009 | Cohort Southampton Women's Study UK 782/569 | Up to 3 months Up to 4 months Up to 5 months >5 months | Height and weight Body composition by DXA | 12.5% never breastfed 20% breastfed 4–6 13.3% BF >12 months Infant feeding variables were included in regression analysis | ++ | The weak inverse association found between fat mass at 4 years and age at introduction of solid foods (P 0.034) was not seen after adjustment for confounding factors | |
| 5 years | Poskitt and Cole 1978 | Cohort UK 300/203 | 1, 2, 3, 4, 8, 13, 14–25 weeks Mean 6.4 weeks (Maternal reporting) | Weight and height Eid Index | Very low rates of breastfeeding, 28% breastfed (not excl) 6.3% received any breast milk after 12 weeks. No analysis by method of milk feeding | + | Children who were overweight at age 5 years had not been given solid foods earlier than normal weight 5‐year‐olds |
| Burdette 2006 | Cohort US 372/313 | <4 months vs. >4 months (Maternal recall) | Height, weight Adiposity, by DXA scan (BMI > 85th centile defined as overweight) | 30% exclusively bf 4 months, 35% never breastfed Examined the relationship between breastfeeding and introduction of solid foods in multivariate regression analysis | ++ | Timing of the introduction of solid foods was not associated with adiposity at age 5 years | |
| Griffiths 2010 | Millennium Cohort Study UK 12 989/11 653 | <17.4 weeks (4 months) vs. ≥17.4 weeks (4 months) (maternal report) | 3–5‐year weight gain z‐scores Weight, height Overweight and obesity defined by International Obesity Task Force cut‐offs for BMI | Described in Hawkins et al. 2009 Breastfeeding duration was included as one of many risk factors in logistic regression analysis | ++ | Neither timing of introduction of solid foods nor breastfeeding duration were factors in the fully adjusted explanatory model for rapid weight gain between 3 and 5 years | |
| Brophy 2009 | Millennium Cohort Study UK 17 561/13 745 | <3 months vs. ≥3 months (maternal report) | Height and weight Overweight and obesity defined by International Obesity Task Force cut‐offs for BMI | Described in Griffiths et al. 2009 Breastfeeding is not included in the analysis | ++ | Feeding solid foods before 3 months was associated with higher levels of obesity at age 5 (OR 1.2 95% CI 1.02–1.5). This effect was greater in higher income and White/European families than in Asian and African families | |
| 7 years | Reilly 2005 | Cohort ALSPAC UK 8234/7758 | 1 months, 1–2 months, 2–3 months, 3–4 months, 4–6 months, not yet introduced (Child feeding questionnaire at 6 m) | Height and weight (Obesity defined as BMI ≥95th centile relative to reference data for the UK population in 1990) | Exclusive and non‐exclusive breastfeeding and never breastfed categories used. Breastfeeding was included in regression analysis | ++ | Timing of introduction of solid foods was not significantly related to the risk of obesity at age 7 years after controlling for any breastfeeding and duration of breastfeeding |
| Wilson 1998 | Cohort Dundee Study 674/405 | <15 weeks vs. >15 weeks (Home visit, recorded by HV) | Height, weight and skin‐fold thickness at four sites (% body fat calculated from total skin‐fold thickness) | Exclusive breastfeeding for 15 weeks, exclusive formula feeding and mixed. Mean duration of breastfeeding 9.5 weeks. Breastfeeding was included in regression analysis | ++ | Early introduction of solids significantly associated with increased weight (P < 0.025) and increased % body fat (P < 0.01) | |
| 1–14 years (and older time points to 42 years, not reported here) | Schack‐Neilson 2010 | Copenhagen Perinatal Cohort Subsample n = 5068 Highest follow‐up for BMI was 1675 at 1 year Lowest was 367 at 7 years | <3.5 months vs. ≥3.5 months (maternal recall at 1 year interview by physician) | Height and weight | 25% did not breastfeed beyond 2 weeks 9% did not receive any breast milk Duration of any breastfeeding used in regression analysis | + | Late introduction of solid foods (≥3.5 months) was associated with a lower BMI only at age 42 years. Earlier in life (including at 1, 3, 6, 7, 8, 9, 10, 11, 12, 13 and 14 years) no effect of age at introduction of solid foods was seen |
| 12–18 years | Kramer 1981 | Case control Canada 639/517 cases 533/390 controls | Not reported Maternal recall at 12–18 years | Height, weight and skin‐fold thickness at two sites (Classified obese if % median weight for height and age >120% and either skin fold >95th percentile, or both >90th percentile) | Considered to be breastfeeding if given no more than one formula feed a day. Duration of breastfeeding used in regression analysis | + | Age at introduction of solid foods was found not to be associated with obesity after controlling for any breastfeeding and duration of breastfeeding |
BMI, body mass index; CI, confidence interval; DXA, dual x‐ray absorptiometry; OR, odds ratio; RCT, randomized controlled trial. +If some of criteria from check list fulfilled; where criteria not fulfilled or were not adequately described, the conclusions of the study were thought unlikely to alter; ++If all or most of criteria from check list fulfilled; where criteria not fulfilled or were not adequately described, the conclusions of the study were thought unlikely to alter.
Description of included studies
In total, 24 studies met the inclusion criteria for the systematic review, and data from over 34 000 participants were available for interpretive analysis. These included a randomized controlled trial (Mehta et al. 1998) a re‐analysis of data from two randomized controlled trials (Morgan et al. 2004), cohort studies (Poskitt & Cole 1978; 1985a, 1985b; Zive et al. 1992; Forsyth et al. 1993; Heinig et al. 1993; Wilson et al. 1998; Haschke & van't Hof 2000; Baker et al. 2004; Lande et al. 2005; Reilly et al. 2005; Burdette et al. 2006; Kuperberg & Evers 2006; Baird et al. 2008; Sloan et al. 2008; Brophy et al. 2009; Robinson et al. 2009; Griffiths et al. 2009, 2010; Hawkins et al. 2009; Schack‐Nielson et al. 2010) and a case‐control study (Kramer 1981). It is important to note that some of these studies analysed data from the same cohorts (e.g. the UK Millennium Cohort Study). The studies were categorized according to the length of follow‐up (Table 2).
All included studies investigated the age at which solid foods were introduced and a measure of obesity in infancy or in childhood (1–18 years). The cut‐off ages used to define ‘early’ or ‘late’ introduction of solid foods in these studies varied, including 2 months (Forsyth et al. 1993), 3 months (Lande et al. 2005), 4 months (Wilson et al. 1998; Baker et al. 2004; Burdette et al. 2006) and 6 months (Kramer et al. 1985a; Heinig et al. 1993), with some studies not categorizing the timing of the introduction of solid foods.
All the studies included in the review measured weight and height (or length), with some calculating BMI and z‐scores. Other outcome measures also used to determine growth and body composition included DXA scanning (Burdette et al. 2006), skin‐fold measures (Kramer et al. 1986; Zive et al. 1992), circumference measures and ponderal index (Lande et al. 2005) and Eid index (Poskitt & Cole 1978). Some studies clearly described the quality control measures used such as calibration of measuring equipment, training of staff collecting measurements and dress of participants for each measurement. In some of the studies, however, it is not clear whether the same researcher(s) carried out the measurements at each follow‐up, which could lead to systematic errors and bias.
The majority of studies included in the review used regression analysis to measure the effect of the confounding variables identified and measured, and presented adjusted effects of the timing of introduction of solid foods on measures of obesity in infancy and/or childhood. Regression analysis is one of the most commonly used and reliable methods to deal with confounders in cohort studies, providing all the potential confounding variables have been measured (Normand et al. 2005). The advantage of regression analysis is that it uses the data from all the participants included in the study (Normand et al. 2005). Confounders varied considerably across studies; maternal socio‐economic status, maternal weight or BMI, and method of milk feeding were commonly used in adjusted analyses. The strong relationship between milk feeding and consumption of solid foods has frequently been identified (e.g. Griffiths et al. 2009; Robinson et al. 2009), but is not reported consistently in the studies included in this review (see Table 2).
Age at which solid foods are introduced and obesity in infancy (≤12 months)
Eight studies assessed the effects of the age at which solids were introduced on weight in infancy; seven of these also reported length, with or without other anthropometric measurements (Table 2). Outcomes were measured at 12 months in six studies. At 12 months, three studies (Forsyth et al. 1993; Mehta et al. 1998; Lande et al. 2005) found no association between the age at which solid foods were introduced and anthropometric measurements. Two studies found that earlier introduction of solid foods was associated with higher weight at 12 months; however, Kramer et al. (1985a) noted that BMI did not differ, and Baker et al. (2004) did not report length or BMI by age at introduction of solid foods. The sixth study included only fully breastfed infants (Haschke & van't Hof 2000), and found that those fed solids earlier were longer and lighter and had lower mean z‐scores for BMI at 12 months. A similarly mixed picture was found when outcomes were measured in infants less than 12 months of age (Table 2). Of five studies, three reported an association between early introduction of solid foods and increased weight (Kramer et al. 1985a; Forsyth et al. 1993; Baird et al. 2008), one reported a small increase in weight and length but this was not statistically significant (Morgan et al. 2004) and one reported no difference between groups (Mehta et al. 1998). However, none of these studies related their measures to obesity.
Mehta et al. (1998) randomized 165 white infants to have solids introduced either at 3–4 months or at 6 months in a controlled trial (18 were withdrawn before 12 months for failure to attend a visit). They found no differences between groups in weight, length, fat mass or lean mass at 3, 6 or 12 months. Morgan et al. (2004) used data from five randomized controlled trials (in which they had investigated other questions) to examine the influence of age at introduction of solid food on growth and health outcomes. Two of these trials had included term infants with birthweights appropriate for gestational age, and data presented by Morgan et al. (2004) from these are reported here. In one study, infant feeding data had been collected prospectively at 12 weeks; in the other, mothers had been asked at recruitment, 9 months after the birth, to recall the age at which their infants had first received solid foods. Infants who first received solid foods aged ≤12 weeks were heavier at 12 weeks than those who first received solid foods aged >12 weeks and gained a small amount more between 12 weeks and 9 months, but this did not reach statistical significance. Baker et al. (2004) followed 3768 predominantly breastfed infants in a Danish cohort study and found that infants introduced to solid foods before 16 weeks had significantly greater weight gain from birth to 12 months compared with infants introduced to solid foods at or after 16 weeks (P = 0.0001). The introduction of solid foods before 16 weeks was associated with 224.2 g of additional weight from birth to 12 months. The age at which solid foods were introduced was found to interact significantly with the duration of breastfeeding in that infants who were exclusively breastfed for longer were fed solids slightly later (Baker et al. 2004). Kramer et al. (1985a) followed 358 Canadian infants until 12 months of age, and in a regression analysis found that the age at which solids were introduced was a significant predictor of weight (r 2 = 0.296, P < 0.0001) and total skin‐fold thickness (r 2 = 0.038, P = 0.002) at 12 months (significance levels at 6 months not reported; stated to be similar to, but to explain less of the variance than at 12 months). Although they observed the same positive association between breast feeding and the delayed introduction of solid foods as Baker et al. (2004), they state that this only explained a small percentage of variance in weight or adiposity as it is outweighed by other factors.
Forsyth et al. (1993) conducted a cohort study. They followed 548 Scottish (Dundee) infants to 12 months of age and found no association between the age at which solid foods were introduced and weight at 12 months (P = 0.3), but they did find an association between early introduction of solid foods and increased weight at 8 (P = 0.003), 13 (P = 0.006) and 26 (P = 0.009) weeks of age. Baird et al. (2008) analysed findings from 1740 infants born to participants in the Southampton Women's survey. They found that 6‐month‐old infants who had been fed solid foods before 5 months were heavier and longer than those fed solid foods at or after 5 months, but timing of introduction of solid foods was not significantly associated with skin‐fold thickness. Haschke & van't Hof (2000) conducted a longitudinal study involving 355 infants who were categorized into two groups. All the infants were fully breastfed; one group received solid foods before 4 to 5 months, and the other group received solid foods after 4 to 5 months. They found that infants fed solid foods early were leaner and had lower BMI z‐scores between 1 and 12 months of age than those who were fed solids later. Lande et al. (2005) followed 1441 Norwegian infants for 12 months and found that in a univariate analysis, the introduction of solid foods was significantly associated with BMI at 12 months (P ≤ 0.02, direction of association not stated in the paper), but in the multiple linear regression analysis no significant association was found.
Age at which solid foods were introduced and obesity in childhood (>1 to 18 years)
Nineteen studies reported the age at which solid foods were introduced in relation to measures of obesity in childhood. All reported weight; seventeen also reported height or length and six of these reported measures of adiposity such as skin‐fold thickness (Table 2). Fourteen of the nineteen studies found no association between the age at which solid foods were introduced and study measures in childhood (>1–18 years). In four studies, earlier introduction of solid foods was associated with being heavier in childhood (at 14 months in Sloan et al. 2008, at 3 years Hawkins et al. 2009, at 5 years Brophy et al. 2009 and at 7 years in Wilson et al. 1998). In contrast, Haschke & van't Hof (2000) in their study of breastfed infants, found a positive correlation between early solid foods and length gain between 1 and 24 months of age, but the age of introduction of solid foods was not associated with change in BMI at age 2 or 3 years.
Sloan et al. (2008) followed 210 participants up to a mean age of 14 months [range 10 to 22 months standard deviation (SD) ± 2.38; z‐scores were adjusted for age at which the child was actually weighed) and categorized early introduction of solids as <4 months and late introduction of solids as ≥4 months. They found that infants aged <4 months at introduction of solids had higher 14 month weight z‐scores (P = 0.004) and a faster rate of weight gain between 8 weeks and 14 months (P = 0.003). After controlling for duration of any breastfeeding (mean 20 weeks, SD ± 18.41), significant effects of early introduction of solids were still found on z‐scores at 14 months (P = 0.035) and rate of weight gain between 8 weeks and 14 months (P = 0.029). Although they found that infants introduced to solids before 4 months had higher BMI z‐scores and a faster rate of weight gain, it is not clear from the study whether they were more likely to be obese as obesity is not defined.
Two studies found no significant associations between the age at which solid foods were introduced, and weight or length at 18 months of age. Heinig et al. (1993) followed 80 participants for 18 months and categorized early introduction of solid foods as 16–25 weeks and late introduction of solid foods as >26 weeks of age. They found no significant associations between weight and length at 18 months and timing of introduction of solid foods. Morgan et al. (2004) used data from two randomized controlled trials of term infants and found no significant differences between infants who first received solid foods aged ≤12 weeks and those who first received solid foods aged later in any of the growth or health measures at 18 months. This suggests that earlier introduction of solid foods was not associated with a greater risk of these infants becoming overweight at 18 months of age.
Three studies reported outcomes at 2 years of age (all of these also reported outcomes at 12 months and one at 3 years, see Table 2). Two studies reported weight, length and BMI (1985a, 1985b), or BMI z‐score (Haschke & van't Hof 2000), and one (Forsyth et al. 1993) reported weight only. No significant differences between early and late introduction of solid foods groups were found at 2 years in these three studies. Kramer et al. (1985a) continued their study to measure outcomes on 347 children at 2 years (Kramer et al. 1985b). They found that the introduction of solid foods at 2, 4 or 6 months was not a significant determinant of weight, skin‐fold thickness or BMI at two years. In their cohort of 671 participants, Forsyth et al. (1993) followed 392 children until 2 years of age. They defined early introduction of solid foods as ≤12 weeks and late introduction of solid foods as >12 weeks. They concluded that although infants who were introduced to solid foods at or before 12 weeks were heavier at ages 4, 8, 13 and 26 weeks than those who first received solid foods after 12 weeks, at 2 years of age there were no significant differences between the groups. Haschke & van't Hof (2000) conducted a longitudinal study and measured outcomes at 12 months (reported above) and at two and 3 years of age for fully breastfed infants. They found the age of introduction of solid food was no longer positively correlated to change in BMI between 1 and 24 months, or 1 and 36 months of age.
Two further studies reported outcomes at 3 years of age; both were analyses of the Millennium Cohort Study (Griffiths et al. 2009; Hawkins et al. 2009). Hawkins et al. (2009) used overweight and obesity as outcomes (defined using the IOTF cut‐offs for BMI). They analysed a range of potential individual, family, community and area risk factors for obesity and found that children were more likely to be overweight if they had been introduced to solid foods before 4 months of age. They initially conducted unadjusted logistic regression analyses and included a range of factors (significant at the P ≤ 0.05 level) in a stepped approach to adjusted models; overweight at age 3 years was directly associated with the introduction of solid foods before 4 months in these adjusted models, whereas breastfeeding was found to be a protective factor. Griffiths et al. (2009) used conditional weight gain z‐scores from birth to 3 years (adjusted for birthweight) as the main outcome measure and examined introduction of solid food before 4 months as one of three main exposure variables (the others were breastfeeding initiation and duration). They found conditional weight gain to be associated with the introduction of solid foods after adjustment for a range of confounders including maternal social class, pre‐pregnancy BMI and duration of breastfeeding, but this association was no longer significant after adjustment for height z‐score at 3 years.
Three studies found no significant associations between the age at which solid foods were introduced and measures of obesity at 4 years. Kuperberg & Evers (2006) enrolled a cohort of 102 children from a First Nations community in Canada and measured 71 children at 4 years. Early introduction of solid foods was defined as <3 months, late introduction of solid foods as >3 months, and children were classified as overweight if their BMI was ≥85th centile. Overall, 27.8% of children were found to be overweight at 4 years, but no association between being overweight at 4 years and age at introduction of solids was found. Zive et al. (1992) surveyed 351 Anglo‐American and Mexican‐American low socio‐economic status families with a 4‐year‐old child. They found no significant relationships between infant feeding variables including age at introduction of solid foods and either BMI or sum of skin‐fold thicknesses at age 4 years. However, these findings do need to be interpreted with some caution as the age at which solid foods were introduced is not categorized as ‘late’ or ‘early’ and infant feeding data were collected retrospectively. In a more recent study of 536 children, Robinson et al. (2009) used DXA to assess body composition. They found a weak association between increased fat mass and later introduction of solid foods (P = 0.034), but this was not seen after adjustment for confounding factors (maternal age, BMI, height, education, social class, smoking in late pregnancy and birth weight).
Three studies found no significant associations between the age at which solid foods were introduced and measures of obesity at 5 years, and one study found introducing solid foods before 3 months was associated with higher levels of obesity at 5 years. Burdette et al. (2006) defined early introduction of solid foods as <4 months, late introduction of solid foods as ≥4 months and followed 313 participants to 5 years. They found no statistically significant difference in adiposity (fat mass) at age 5 years, according to age at introduction of solid foods (4.49 ± 0.12 vs. 4.63 ± 0.12 kg, P = 0.42). However, one limitation of this study is that the measure of exposure relied on parental recall at 3 years, which could compromise validity. Poskitt & Cole (1978) followed 203 infants until 5 years. The age at which solid foods were introduced was measured but not categorized as early or late, and adiposity was calculated and defined using the Eid index. They concluded that children who were overweight at age 5 years had not been given solid food earlier than normal weight 5‐year‐olds. Griffiths et al. (2010) analysed data from 11 653 children participating in the Millennium Cohort Study. Using 3 to 5 years of weight gain z‐scores as an outcome measure (and classifying the top quarter as gaining weight rapidly), they examined risk factors for rapid weight gain between 3 and 5 years – neither timing of introduction of solid foods nor breastfeeding duration were factors in the fully adjusted model. In contrast to these studies, Brophy et al. (2009) also analysed data from the Millennium Cohort Study and used obesity as an outcome measure, found feeding solid foods before 3 months was associated with higher levels of obesity at 5 years of age. They examined a range of risk factors for obesity including; activity, family behaviours, socio‐economic factors, birth weight and infant eating habit but interestingly did not examine breastfeeding.
Two cohort studies reported age at which solid foods were introduced and measures of obesity at 7 years. Reilly et al. (2005) categorized age at introduction of solid foods using a child feeding questionnaire at 6 months as less than 1 month, 1–2 months, 2–3 months, 3–4 months, 4–6 months and not yet introduced. Height and weight of 7758 participants were measured at 7 years and BMI was calculated from these. Obesity was defined as BMI ≥95th centile relative to reference data for the UK population in 1990. Using multivariable analysis for the prevalence of obesity they concluded that the timing of introducing solid foods was not significantly related to risk of obesity at age 7 years. However, Wilson et al. (1998), in their Dundee cohort of 674 infants that compared early (<15 weeks) with late introduction of solid foods (≥15 weeks) and found significantly increased weight (412 children, P < 0.025) and percentage body fat (405 children, P < 0.01) with early introduction of solid foods at mean age 7.2 years (range 6.9 to 10.0 years).
Age at which solid foods were introduced in infancy and obesity in adolescence (12–18 years)
Two studies examined the relationship between the timing of introduction of solid foods and being obese or overweight at age 12–18, and neither found an association. Schack‐Nielson et al. (2010) explored the effect of both breastfeeding and introduction of solid foods on BMI throughout childhood (measured at 1 and 3 years and then yearly from 6–14 years) and into adulthood (age 42 years). They did not find an association between introduction of solid foods (defined as spoon‐feeding) and BMI at any point in childhood. Kramer (1981) conducted a case‐control study and found not breastfeeding to be a significant predictor of obesity, and that longer duration of breastfeeding was associated with significant extra protection. However, later introduction of solid foods was not found to be associated with relative weight (percent median weight for height and age) at age 12–18 years (r = −0.064 P > 0.05). Limitations of this study are that it relied on parental recall when the children were aged 12–18 years to determine the age at which solid foods were introduced, which could affect the validity of the findings, and the authors failed to define what was meant by delayed introduction of solid foods.
Discussion
This review used rigorous systematic methods to ensure the selection of the best quality evidence currently available and has not found a clear association between timing of introduction of solid food and risk of overweight and obesity in infancy and childhood. The majority of studies reported no difference in measures of obesity for infants introduced to solid foods at different times. In children over 1 year, two studies reported increased measures of obesity when solid foods were introduced earlier (Wilson et al. 1998; Sloan et al. 2008) although it is notable that these studies only use weight as outcome measures and these were not related to height/length. Two other studies, both using data from the Millennium Cohort Study found associations between timing of introduction of solid foods and child overweight at age 3 (Hawkins et al. 2009) and age 5 (Brophy et al. 2009) although this latter study did not include breastfeeding in the analysis. Findings of studies measuring outcomes in children at or less than 12 months of age were also not consistent, but again, in the majority of studies, there was no clear association with time of introduction of solid foods and measures of obesity. At 12 months two studies found infants fed solids earlier were heavier, another study reported a similar finding at 6 months but did not find an association between early introduction of solid foods and skin‐fold thickness. One study reported that infants fed solid foods earlier were lighter and longer. Thus it is apparent that findings varied across different ages of infants and different outcome measures – none of the studies of infants clearly defined overweight and obesity.
Perhaps unsurprisingly, given the difficulty of studying an intervention such as timing of introduction of solid food, few randomized controlled trials were identified. The majority of studies included in the review were cohort studies – inevitably susceptible to selection bias and confounding. All but three of the cohort studies included in this review (Poskitt & Cole 1978; Morgan et al. 2004; Sloan et al. 2008) used regression analysis to assess the extent of the effect of confounding variables such as: maternal BMI, education, socio‐economic status and smoking during pregnancy, birth weight and method of milk feeding. Potentially important confounders were not always identified and measured. One of the most important confounders is likely to be the method of milk feeding. A complex relationship exists between milk feeding and the introduction of solid foods; for example, some studies have found that infants breastfed for longer are introduced to solid foods later than formula‐fed infants (Kramer 1981; Forsyth et al. 1993; Baker et al. 2004; Burdette et al. 2006). The information available about the method of milk feeding in each study is presented in Table 2. Most studies considered the effect of breastfeeding as a variable in regression analysis but this was not the case for all studies (Poskitt & Cole 1978; Kuperberg & Evers 2006; Brophy et al. 2009). However, examination of this does not provide any further explanations for the inconsistent findings.
Unpublished papers are not included in this review as searching was restricted to electronic databases and hand searching of reference lists. This may mean the review is subject to publication bias; however, the findings described above in which the majority of studies reported no difference in measures of obesity suggest this unlikely to have been a major problem in this review. Another potential limitation of this study is that it did not include studies from developing countries. Obesity is a global concern and is increasingly becoming an important issue in developing countries (WHO 2006a). WHO have published growth standards for infants and children up to five that indicate optimal growth for children; produced by studying populations in both developed and developing countries and patterns of growth were similar across these populations (WHO 2006b). Thus studies conducted in developing countries should be included in future reviews of infant feeding.
Studies included in this review used different outcome measures to determine overweight and obesity, which makes drawing comparisons between the included studies challenging. Outcomes measures included: weight, length, BMI, ponderal index, skin‐fold measures, fat mass, lean mass and DXA scanning (Table 2). BMI was the most commonly used measure and although it is a useful indicator, there are limitations; it is not a direct measure of body fat as ‘bone, muscle and organs will be reflected in BMI’ (Dai et al. 2002 p.728), and it does not determine fat mass or its distribution (Dai et al. 2002; Hall & Cole 2006). In addition to BMI as an outcome measure, some of the studies included in the review did measure skin‐fold thickness to give an indication of fat mass and distribution. Studies that reported pre‐determined relevant measures of obesity were included in this review but it is notable that few of the older studies stated definitions of overweight and obesity, although several studies conducted more recently used definitions defined by the IOTF.
The included studies vary in size; the majority are relatively small and could therefore lack sufficient power to detect a meaningful association between the effects of infant feeding practices and obesity in later childhood. Seven studies have large sample sizes (Baker et al. 2004; Lande et al. 2005; Reilly et al. 2005; Baird et al. 2008; Hawkins et al. 2009; 2009, 2010). Infant feeding methods were sometimes collected retrospectively relying on mothers to recall events (e.g. Kramer 1981; Zive et al. 1992 and Burdette et al. 2006) affecting the internal validity of the findings.
It is important to note that the current increase in child overweight and obesity has occurred at a time when later introduction of solid foods has been recommended (PAHO 2003; WHO 2006a), and although not all parents follow these recommendations (Bolling et al. 2007; ESPGHAN Committee on Nutrition 2008; Schiess et al. 2010), the general trend has been towards later introduction of solid food. In relation to rapidly rising rates of childhood overweight and obesity, this suggests there are many other more important determining factors, such as maternal BMI and socioeconomic status and method of milk feeding (Whitaker 2004; Foresight 2007; Butte 2009). A strong association between maternal BMI and increased risk of obesity in childhood was found in some of the studies included in this review (Zive et al. 1992; Baker et al. 2004; Reilly et al. 2005), and is reported in a Department of Health report which showed that children who had one or more obese parent were at higher risk of being obese themselves (Dhriti et al. 2006). Rapid growth at different stages in infancy and childhood is also associated with a higher risk of obesity in childhood (Baird et al. 2005). When this whole complex situation is considered, it leads to the conclusion that a whole family approach to the prevention of overweight and obesity in childhood is necessary, and concentrating on a range of modifiable factors is likely be more effective than concentrating on any single factor in isolation.
Conclusion
This review did not find a clear association between the timing of introducing solid foods and obesity in infancy and childhood. It is likely that genetic and environmental factors, or conditions that cause rapid growth, have more impact on childhood obesity. This leads to the conclusion that a whole family approach to obesity prevention is likely to be more effective than working to change a single factor such as the timing of the introduction of solid foods. Health care professionals should continue to encourage healthy eating practices in line with national recommendations.
Source of funding
None.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank Janette Colclough, Library Services at The University of York for her support with the search and Professor Josephine Green for reviewing and commenting on a draft of the paper.
Appendix 1. Papers excluded from the review
| Authors and citation | Title | Reason for exclusion | |
|---|---|---|---|
| 1 | Centre for Reviews and Dissemination. Effective Health Care. 2002;7(6):1–12 | The prevention and treatment of childhood obesity | Design – review |
| 2 | Agras WS, Kraemer HC et al. Journal of Pediatrics. 1990;116(5):805–9 | Influence of early feeding style on adiposity at 6 years of age | Quality – low quality assessment score |
| 3 | Baranowski T, Bryan GT, Harrison JA, Rassin DK, Greaves KA, Baranowski JH. Journal of Clinical Epidemiology. 1992;45(5):513–8 | Height, infant feeding practices and cardiovascular functioning among 3 or 4 year old children in three ethnic groups | Intervention – not timing of introducing solid foods |
| 4 | Baughcum AE, Powers SW, Johnson SB, Chamberlin LA, Deeks CM, Jain A et al. Journal of Developmental and Behavioural Practices. 2001;22(6):391–408 | Maternal feeding practices and beliefs and their relationship to overweight in early childhood | Intervention – not timing of introducing solid foods |
| 5 | Boyington JA, Johnson AA. Journal of the Medical Association. 2004;96(3):351–362 | Maternal perception of body size as a determinant of infant adiposity in African‐American community | Quality – low quality assessment score |
| 6 | Butte NF. The Journal of Nutrition. 2009;139:412S–16S | Impact of infant feeding practices on childhood obesity | Design – review |
| 7 | Carruth BR, Skinner JD et al. Journal of the American College of Nutrition. 2000;19(3):405–12 | Addition of supplementary foods and infant growth (2–24 months) | Quality – low quality assessment score |
| 8 | Castiglia P. Journal of Pediatric Health Care. 1987 Jul–Aug;1(4):218–20 | Obesity in infants and toddlers | Design – not a study |
| 9 | Costom BH, Shore D. Clinical Pediatrics. 1983;22(2):105–11 | Effect of a comprehensive nutritional program on the growth and ponderosity of infants | Outcome – length and weight measured but in group as a whole not related to timing of introduction of solid foods |
| 10 | Darnton‐Hill I, Nishida C, James W. Public Health Nutrition. 2004;7(1A):101–21 | A life course approach to diet, nutrition and the prevention of chronic diseases . . . Diet, Nutrition and the Prevention of Chronic Diseases: scientific background papers of the Joint WHO/FAO Expert Consultation (Geneva, 28 January‐1 February 2002) | Design – not a study |
| 11 | De la Hunty A. British Nutrition Foundation Nutrition Bulletin. 2009;34:403–6 | The EU Childhood Obesity Project | Outcome – outcomes of interest not fully reported |
| 12 | Dewey KG. Pediatrics. 2000;106(5):1281 | Complementary feeding and infant growth and body composition | Design – Review |
| 13 | Ferris AG, Laus MJ et al. American Journal of Clinical Nutrition. 1980;33(12):2635–42 | The effects of diet on weight gain in infancy | Population – only includes female participants |
| 14 | Fewtrell MS, Lucas A, Morgan JB. Archives of Disease in Childhood and Fetal and Neonatal Edition. 2003;88(4) | Factors associated with introduction of solid food in full term and pre term infants | Design – Review |
| 15 | Greenhalgh T, Buchan I, Heller R, Clayton P, Bundred P, Cole T, et al. BMJ. 2005 Aug 20–27;331(7514):453–4 | Early life risk factors for obesity in childhood . . . Reilly JJ, Armstrong J, Dorosty AR et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005;330;1357. (11 June) | Design – letter about study screened and included in the review |
| 16 | Gillman MW, Rifas‐Shiman SL et al. Journal of the American Medical Association. 2001;285(19):2461–7 | Risk of overweight among adolescents who were breastfed as infants | Outcome – outcomes of interest not fully reported |
| 17 | Goodell LS, Wakefield DB. Journal Community Health. 2009;34:370–5 | Rapid weight gain during the first year of life predicts obesity in 2–3 year olds from a low‐income, minority population | Intervention – not timing of introducing solid foods |
| 18 | Gunnarsdottir I, Thorsdottir I. International Journal of Obesity. 2003;27(12):1523–7 | Relationship between growth and feeding in infancy and body mass index at the age of 6 years | Intervention – not timing of introducing solid foods |
| 19 | Gunnarsdottir I, Schack‐Nielsen L, Michaelsen KF et al. Public Health Nutrition. 2010;13(2):201–7 | Infant weight gain, duration of exclusive breast‐feeding and childhood BMI – two similar follow‐up cohorts | Intervention – not timing of introducing solid foods |
| 20 | Gunther AL, Remer T, Kroke A et al. American Journal of Clinical Nutrition. 2007 December;86(6):1765–72. | Early protein intake and later obesity risk: which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age? | Intervention – not timing of introducing solid foods |
| 21 | Hamosh M. Journal of Pediatric Gastroenterology & Nutrition. 1988;7(1):10–6 | Does infant nutrition affect adiposity and cholesterol levels in the adult? | Design – review |
| Population – adults | |||
| 22 | Harder T, Schellong K, Plagemann A, Owen C, Whincup P, Cook D, et al. Pediatrics. 2006/03/;117(3):987–8 | Differences between meta‐analyses on breastfeeding and obesity support causality of the association . . . Owen CG, Martin RM, Whincup PH et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of the published evidence. Pediatrics 2005;115:1367–1377 | Design – letter |
| 23 | Harvey et al. British Journal of Nutrition. 2009;102(6): 915–20. | Breast‐feeding and adherence to infant feeding guidelines do not influence bone mass at age 4 years | Intervention – not timing of introducing solid foods |
| 24 | Hetzner Philipsen NM, Razza RA, Malone LM, Brooks‐Gunn J. Maternal Child Health Journal. 2009;13:795–805 | Associations Among Feeding Behaviors During Infancy and Child Illness at Two years | Outcome – outcomes of interest not reported |
| 25 | Kathy C. Journal of Family Health Care. 2010;20(1):20–3 | Complementary feeding for infants 6 to 12 months | Design – commentary |
| 26 | Khadivzadeh T, Parasai S. Eastern Mediterranean Health Journal. 2004;10(3):289–94 | Effect of exclusive breastfeeding and complementary feeding on infant growth and morbidity | Population – not from an industrialized country (Iran) |
| 27 | Kleinman RE. Pediatrics. 2000;106(5II):1287–8 | Complementary feeding and later health | Design – review |
| 28 | Koletzko B. Scandinavian Journal of Food and Nutrition. 2006;50(1):30–1 | Infant feeding and later obesity risk: What is the relationship? | Intervention – not timing of introducing solid foods |
| 29 | Koletzko B, von Kries RD, Monasterolo RC et al. The American Journal of Clinical Nutrition. 2009; 89(suppl):1502S–8S | Can infant feeding choices modulate later obesity risk? | Intervention – not timing of introducing solid foods |
| 30 | Kramer MS, Barr RG et al. Canadian Journal of Public Health. 1986;77(Suppl 1):98–103 | Determinants of weight and adiposity in early childhood | Duplicate – duplicate of Kramer (1985b) vol 107 |
| 31 | Kramer M, Guo T, Platt R, Sevkovskaya Z, Dzikovich I, Collet J, et al. American Journal of Clinical Nutrition. 2003/08/;78(2):291–5 | Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding | Intervention – not timing of solid foods |
| 32 | Kramer M, Guo T, Platt R, Vanilovich I, Sevkovskaya Z, Dzikovich I et al. Feeding effects on growth during infancy. Journal of Pediatrics. 2004/11/;145(5):600–5 | Feeding effects on growth during infancy | Outcome – not reported by timing of introduction of solid food |
| 33 | Kramer MS. The American Journal of Clinical Nutrition. 2010;91:500–1 | Breastfeeding, complementary (solid) foods, and long‐term risk of obesity | Design – editorial |
| 34 | Labbok M. SCN [Sub‐Committee on Nutrition] News. 2004(29):23–6 | Infant feeding and obesity | Design – review |
| 35 | Lamb et al. Annals of Nutrition and Metabolism. 2010;56(1):16–22 | Early‐life predictors of higher body mass index in healthy children | Intervention – not timing of introducing solid foods |
| 36 | Lauver MA, Hizon L et al. Journal of the Kansas Medical Society. 1981;82(9):403–6 | Infant feeding practices: the effect on six month weight | Outcome – no outcomes reported after 6 months of age |
| 37 | MacDonald A. J R Soc Promot Health. 2003;123(3):169–74 | Is breast best? Is early solid feeding harmful? | Design – review |
| 38 | McIntosh J. Child: Care, Health & Development. 1986;12(4):215–33 | Introduction of solid food practices in a sample of working class primiparae | Outcome – no review outcomes measured |
| 39 | Michaelson KF, Larnkjaer A et al. Current Opinion in Clinical Nutrition & Metabolic Care. 2010;13(3): 277–83 | Science base of complementary feeding practice in infancy | Design – review |
| 40 | Neutzling et al. International Journal of Pediatric Obesity. 2009;4(3):143–9 | Infant feeding and obesity at 11 years: prospective birth cohort study | Population – not from an industrialized country |
| 41 | O'Callaghan MJ, Williams GM, Andersen MJ, Bor W, Najman JM. Journal of Paediatrics and Child Health. 1997;33(4):311–6 | Prediction of obesity in children at 5 years: A cohort study | Intervention – not timing of introducing solid foods |
| 42 | Oddy WH, Kendall GE et al. Journal of Pediatrics. 2010;156(4):568–74 | The long‐term effects of breastfeeding on child and adolescent mental health: a pregnancy cohort study followed for 14 years | Outcome – outcome of interest not reported. |
| 43 | Olson CM, Strawderman MS, Dennison BA. Maternal and Child Health Journal. 2009;13:839–46 | Maternal Weight Gain During Pregnancy and Child Weight at Age 3 years | Intervention – not timing of introducing solid foods |
| 44 | Ong K, Emmett P et al. Pediatrics. 2006;117(3):e503–8 | Dietray energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index | Outcome – outcome of interest not fully reported |
| 45 | Parsons TJ, Power C, Logan S, Summerbell CD. Journal of Obesity. 1999;23(Suppl 8):S1–107 | Childhood predictors of adult obesity | Design – review |
| Population – adults | |||
| 46 | Patterson RE, Typpo JTL et al. Journal of the American Dietetic Association. 1986;86(10):1376–81 | Factors related to obesity in preschool children | Quality – low quality assessment score |
| 47 | Pinot de Moira A, Power C, Leah L. American Journal of Epidemiology. 2010;171:1289–98 | Changing Influences on Childhood Obesity: A Study of 2 Generations of the 1958 British Birth Cohort | Intervention – not timing of introducing solid food |
| 48 | Pridham K, Brown R, Clark R, Sondel S, Green C. Res Nurs Health. 2002/10/;25(5):394–410 | Infant and care giving factors affecting weight‐for‐age and motor development of full‐term and premature infants at 1 year post‐term | Intervention – measures nutritional content of infants diet rather than timing of introducing solid foods |
| 49 | Quandt SA. Journal of the American Dietetic Association. 1984;84(1):47–51 | The effect of beikost on the diet of breastfed infants | Population – no outcomes reported after 6 months of age |
| 50 | Reilly J, Wilson M, Summerbell C. Archives Disease in Childhood. 2002 Jun;86(6):392–5 | Obesity: diagnosis, prevention, and treatment: evidence based answers to common questions | Design – evidence appraisal |
| 51 | Rowland MG. American Journal of Clinical Nutrition. 1985;41(2 Suppl):459–63 | The ‘why’ and ‘when’ of introducing food to infants: growth in young breast‐fed infants and some nutritional implications | Population – Country not industrialized (the Gambia) |
| 52 | Sass P. Evidence Based Practice. 2006;9(7):2 | Evidence in Nutrition. Does early introduction of solid food (before 4 months of age) lead to an increased risk of adult obesity? | Design – review |
| Population – adults | |||
| 53 | Schiess S, Grote V et al. Journal of Pediatric Gastroenterology & Nutrition. 2010;50(1):92–8 | Introduction of complementary feeding in 5 European countries | Outcome – outcomes of interest not reported |
| 54 | Shepherd A. Br J Healthcare Assistants. 2008;2(3):132–8 | Paediatrics: nutrition for babies and young children | Design – not a study |
| 55 | Simon et al. Revista de Saude Publica 2009;43(1):60–9 | Breastfeeding, complementary feeding, overweight and obesity in pre‐school children. | Language – not English |
| 56 | Snethen JA, Hewitt JB, Goretzke M. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2007;36(5):501 | Childhood obesity: the infancy connection | Design – review |
| 57 | Stuebe AM, Schwarz EB. Journal of Perinatology. 2010;30(3):155–62 | The risks and benefits of infant feeding practices for women and their children | Design – review |
| 58 | Taitz LS, Lukmanji Z. Human Biology. 1981;53(3):313–20 | Alterations in feeding patterns and rates of weight gain in South Yorkshire infants, 1971–1977 | Intervention – not timing of introduction of solid foods. Age at introduction of solid food is measured but not treated as an independent variable |
| 59 | Tavaras EM, Gillman MW et al. Pediatrics. 2010;125(4):686–95 | Racial/ethnic differences in early‐life risk factors for childhood obesity | Timing of introduction of solid foods not linked to obesity |
| 60 | Twells L, Newhook LA. Canadian Journal of Public Health. 2010. | Can exclusive breastfeeding reduce the likelihood of childhood obesity in some regions of Canada? | Intervention – not timing of introduction of solid foods |
| 61 | Van Dijk CE, Innis SM. Pediatrics. 2009;123;102–8 | Growth‐Curve Standards and the Assessment of Early Excess Weight Gain in infancy | Outcome – outcome of interest not fully reported |
| 62 | Van't Hof MA. Pediatrics. 2000;106(5II):1281–2 | The influence of breastfeeding and complementary foods on growth until 3 years of age in the Euro‐Growth Study | Duplicate –Haschke & van't Hof (2000) |
| 63 | Wishon P, Kinnick V. MCN. 1986;11(2):118–21 | Helping infants overcome the problem of obesity | Design – review |
| 64 | Wolman PG. Journal of the American Dietetic Association. 1984;84(4):436–8 | Feeding practices in infancy and prevalence of obesity in pre‐school children | Quality – low‐quality assessment score |
| 65 | Worobey J, Lopez MI, Hoffman DJ. Journal of Nutrition Education and Behavior. 2009;3:169–75 | Maternal Behavior and Infant Weight Gain in the First year | Outcome – outcome of interest not fully reported |
| 66 | Yeung DL, Pennell MD et al. Journal of the American Dietetic Association. 1981;79(5):531–5 | Infant fatness and feeding practices: a longitudinal assessment | Quality – low‐quality assessment score |
| 67 | Yew KS, Webber B, Hodges J. Clinical Inquiries. 2009; 58:219 | Are there any known health risks to early introduction of solids to an infant's diet? | Design – evidence‐based report not related to obesity |
| 68 | Ziol‐guest KM, Hernandez DC. Journal of the American Dietetic Association 2010;110(5):702–9 | First‐ and second‐trimester WIC participation is associated with lower rates of breastfeeding and early introduction of cow's milk during infancy | Outcome – outcome of interest not reported |
References
- Agras W.S., Kraemer H.C., Berkowitz R.I. & Hammer L.D. (1990) Influence of early feeding style on adiposity at 6 years of age. Journal of Pediatrics 116, 805–809. [DOI] [PubMed] [Google Scholar]
- Arnez R., Ruckerl R., Koletzko B. & Von Kries R. (2004) Breast‐feeding and childhood obesity‐a systematic review. International Journal of Obesity 28, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Baird J., Fisher D., Lucas P., Kleijnen J., Roberts H. & Law C. (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. British Medical Journal 331, 929. doi:10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J., Poole J., Robinson S., Marriott L., Godfrey K., Cooper C. et al (2008) Milk feeding and dietary patterns predict weight and fat gains in infancy. Paediatric and Perinatal Epidemiology 22, 575–586. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Michaelsen K.F., Rasmussen K.M. & Sorensen T.I.A. (2004) Maternal pre‐pregnant body mass index, duration of breastfeeding, timing of complementary food introduction are associated with infant weight. American Journal of Clinical Nutrition 80, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Bolling K., Grant C., Hamlyn B. & Thornton A. (2007) Infant Feeding Survey 2005. The Information Centre: Leeds. [Google Scholar]
- Boyington J.A. & Johnson A.A. (2004) Maternal perception of body size as a determinant of infant adiposity in an African‐American community. Journal of the National Medical Association 96, 351–362. [PMC free article] [PubMed] [Google Scholar]
- Brophy S., Cooksey R., Gravenor M.B., Mistry R., Thomas N., Lyons R.A. & Williams R. (2009) Risk factors for childhood obesity at age 5: anaylsis of the millennium cohort study. BMC Public Health 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette H.L., Whitaker R.C., Hall W.C. & Daniels S.R. (2006) Breastfeeding, introduction of complementary foods, and adiposity at age 5 y of age. American Journal of Clinical Nutrition 83, 550–558. [DOI] [PubMed] [Google Scholar]
- Butte N.F. (2009) Impact of infanct feeding practices on childhood obesity. The Journal of Nutrition 139, 412S. [DOI] [PubMed] [Google Scholar]
- Carruth B.R., Skinner J.D., Houck K.S. & Moran J.D. (2000) Addition of supplementary foods and infant growth (2 to 24 months). Journal of the American College of Nutrition 19, 405–412. [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination (CRD) (2001) Undertaking Systematic Reviews of Research on Effectiveness. Report 4, 2nd edn, The University of York: York. [Google Scholar]
- Cole T.J., Freeman J.V. & Preece M.A. (1995) Body mass index reference curves for the UK, 1990. Archives of disease in childhood 73, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T.J., Bellizzi M.C., Flegal K.M. & Dietz W.H. (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal 320, 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme (CASP) (2006) Appraisal Tools. Public Health Resource Unit. [On‐line] Available at: http://www.sph.nhs.uk/what-we-do/public-healthworkforce/resources/critical-appraisals-skills-programme[Accessed 21 October 2010].
- Dai S., Labarthe D.R., Grunbaum J.A., Harrist R.B. & Mueller W.H. (2002) Longitudinal analysis of changes in indices of obesity from age 8 years to age 18 years. Project Heartbeat! American Journal of Epidemiology 156, 720–729. [DOI] [PubMed] [Google Scholar]
- De La Hunty A. (2009) The EU childhood obesity project. British nutrition foundation. Nutrition Bulletin 34, 403–406. [Google Scholar]
- De Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C. & Siekmann J. (2007) Development of a WHO growth reference for school‐aged children and adolescents. Bulletin of the World Health Organization 85, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health (DH) (1994) Introduction of Solid Food and the Introduction of Solid Food Diet Report of the Introduction of Solid Food Working Group on the Introduction of Solid Food Diet of the Committee on Medical Aspects of Food Policy. Report on Health and Social Subjects No 45 . HMSO: London. [Google Scholar]
- Department of Health (DH) (2009) Start for Life. Department of Health. [On‐line] Available at: http://www.nhs.uk/start4life/pages/welcome-to-start4life.aspx[Accessed 19 August 2010].
- Department of Health and the Department for Children, Schools and Families (2008) Healthy Weight Healthy Lives: A Cross Government Strategy for England. Department of Health: London. [Google Scholar]
- Dewey K.G. (2003) Is breastfeeding protective against child obesity? International Journal of Lactation 19, 9–18. [DOI] [PubMed] [Google Scholar]
- Dhriti J., Moody A., Stamatakis E. & Wardle H. (2006) Obesity Among Children Under 11. National Centre for Social Research: London. [Google Scholar]
- ESPGHAN Committee on Nutrition , Agostoni C., Decsi T., Fewtrell M., Goulet O., Kolacek S. et al (2008) Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 46, 99–110. [DOI] [PubMed] [Google Scholar]
- Foresight (2007) Tackling Obesities: Future Choices. Government Office for Science: London. [Google Scholar]
- Forsyth J.S., Ogston S.A., Clark A., Florey C.D. & Howie P.W. (1993) Relation between early introduction of solid food to infants and their weight and illnesses during the first two years of life. British Medical Journal 306, 1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman M.W., Rifas‐Shiman S.L., Camargo C.A., Berkley C.S., Frazier A.L., Rockett H.R.H., Field A.E. & Colditz G.A. (2001) Risk of overweight among adolescents who were breastfed as infants. Journal of the American Medical Association 285 , 2461–2467. [DOI] [PubMed] [Google Scholar]
- Griffiths L.J., Smeeth L., Sherburne Hawkins S., Cole T.J. & Dezateux C. (2009) Effects of infant feeding practice on weight gain from birth to 3 years. Archives of Disease in Childhood 94, 577–582. [DOI] [PubMed] [Google Scholar]
- Griffiths L.J., Hawkins S.S., Cole T.J., Dezateux and the Milleneum Cohort Study Child Health Group (2010) Risk factors for rapid weight gain in preschool children: findings from a UK‐wide prospective study. International Journal of Obesity 34, 624–632. [DOI] [PubMed] [Google Scholar]
- Hall D.M.B. & Cole T.J. (2006) What use is the BMI? Archives of Disease in Childhood 91, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer L.D., Bryson S. & Agras W.S. (1999) Development of feeding practices during the first 5 years of life. Archives of Pediatrics & Adolescent Medicine 153, 189–194. [DOI] [PubMed] [Google Scholar]
- Haschke F. & Van't Hof M.A. (2000) Euro‐Growth Study G. Euro‐Growth references for breast‐fed boys and girls: influence of breast‐feeding and solids on growth until 36 months of age. Journal of Pediatric Gastroenterology and Nutrition 31, S60–S71. [DOI] [PubMed] [Google Scholar]
- Hawkins S.S., Cole T.J. & Law C. (2009) An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. Journal of Epidemiology and Community Health 63, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig M.J., Nommsen L.A., Peerson J.M., Lonnerdal B. & Dewey K.G. (1993) Intake and growth of breast‐fed and formula‐fed infants in relation to the timing of introduction of complementary foods: the DARLING study. Davis area research on lactation, infant nutrition and growth. Acta Paediatrica 82, 999–1006. [DOI] [PubMed] [Google Scholar]
- Horta B.L., Bahl R., Martines J.C. & Victora C.G. (2007) Evidence on the Long‐Term Effects of Breastfeeding: Systematic Reviews and Meta‐Analyses. World Health Organization: Geneva. [Google Scholar]
- Khadivzadeh T. & Parsai S. (2004) Effect of exclusive breastfeeding and complementary feeding on infant growth and morbidity. Eastern Mediterranean Health Journal 10, 289–294. [PubMed] [Google Scholar]
- Kramer M.S. (1981) Do breast‐feeding and delayed introduction of solid foods protect against subsequent obesity? Journal of Pediatrics 98, 883–887. [DOI] [PubMed] [Google Scholar]
- Kramer M.S. & Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews 2002, Issue 1. Art No:CD003517, DOI:10.1002/14651858.CD003517. [DOI] [PubMed]
- Kramer M.S., Barr R.G., Leduc D.G., Boisjoly C., McVey‐White L. & Pless I.B. (1985a) Determinants of weight and adiposity in the first year of life. Journal of Pediatrics 106, 10–14. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Barr R.G., Leduc D.G., Boisjoly C. & Pless I.B. (1985b) Infant determinants of childhood weight and adiposity. Journal of Pediatrics 107, 104–107. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Barr R.G. & Pless I.B. (1986) Determinants of weight and adiposity in early childhood. Canadian Journal of Public Health 77 (1), 98–103. [PubMed] [Google Scholar]
- Kuperberg K. & Evers S. (2006) Feeding patterns and weight among First Nations children. Canadian Journal of Dietetic Practice & Research 67, 79–84. [DOI] [PubMed] [Google Scholar]
- Lande B., Andersen L.F., Henriksen T., Baerug A., Johansson L., Trygg K.U. et al (2005) Relations between high ponderal index at birth, feeding practices and body mass index in infancy. European Journal of Clinical Nutrition 59, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Lanigan J.A., Bishop J.A., Kimber A.C. & Morgan J. (2001) Systematic review concerning the age of introduction of complementary foods to the healthy full‐term infant. European Journal of Clinical Nutrition 55, 309–320. [DOI] [PubMed] [Google Scholar]
- Lauver M.A., Hizon L., Bulla A., Connell C. & Wagoner B. (1981) Infant feeding practices: the effect on six month weight. Journal of the Kansas Medical Society 82, 403–406. [PubMed] [Google Scholar]
- Mehta K.C., Specker B.L., Bartholmey S., Giddens J. & Ho M.L. (1998) Trial on timing of introduction to solids and food type on infant growth. Pediatrics 102, 569–573. [DOI] [PubMed] [Google Scholar]
- Morgan J.B., Lucas A. & Fewtrell M.S. (2004) Does weaning influence growth and health up to 18 months? Archives of Disease in Childhood 89, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Information Centre, Lifestyle Statistics (2009) National Child Measurement Programme: England, 2008/09 school year. The Health and Social Care Information Centre. [On‐line] Available at: http://www.shef.ac.uk/scharr/sections/ir/links[Accessed 15 December 2009].
- Normand S.‐L.T., Sykora K., Li P., Mamdani M., Rochon A. & Anderson G.M. (2005) Readers guide to critical appraisal of cohort studies: 3 analytical strategies to reduce confounding. British Medical Journal 330, 1021–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North East Public Health Observatory (NEPHO) (2009) BMI and Children. NEPHO. [On‐line] Available at: http://www.nepho.org.uk/obesity/child_and_maternal/bmi_children[Accessed 30 June 2009].
- Ong K., Emmett P., Noble S., Ness A. & Dunger D. (2006) Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics 117, e503–e508. [DOI] [PubMed] [Google Scholar]
- Oxman A.D. & Guyatt G.H. (1993) The science of reviewing research. Annals of the New York Acadamy of Sciences 703, 125–133. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organisation (PAHO) (2003) Guiding Principles for Complementary Feeding of the Breastfed Child. World Health Organization: Geneva. [Google Scholar]
- Patterson R.E., Typpo J.T., Typpo M.H. & Krause G.F. (1986) Factors related to obesity in preschool children. Journal of the American Dietetic Association 86, 1376–1381. [PubMed] [Google Scholar]
- Poskitt E.M. & Cole T.J. (1978) Nature, nurture, and childhood overweight. British Medical Journal 1, 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt S.A. (1984) The effect of beikost on the diet of breast‐fed infants. Journal of the American Dietetic Association 84, 47–51. [PubMed] [Google Scholar]
- Reilly J.J., Armstrong J., Dorosty A.R., Emmett P.M., Ness A., Rodgers I. et al (2005) Early life risk factors for obesity in childhood: cohort study. British Medical Journal 330, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.M., Marriott L.D., Crozier S.R., Harvey N.C. & Gale C.R. (2009) Variations in infant feeding practice are associated with body composition in childhood: A prospective cohort study. The Journal of Clinical Endocrinology and Metabolism 94, 2799–2805. [DOI] [PubMed] [Google Scholar]
- Royal College of Paediatrics and Child Health (RCPCH) (2009) UK‐WHO Growth Charts: Early Years . RCPCH. [On‐line] Available at: http://www.rcpch.ac.uk/Research/UK-WHO-Growth-Charts. [Accessed 21 January 2010].
- Sass P. (2006) Does early introduction of solid food (before 4 months of age) lead to an increased risk of adult obesity? Evidence Based Practice 9, 9. [Google Scholar]
- Schack‐Nielsen L., Sørensen T.I.A., Mortensen E.L. & Michaelsen K.F. (2010) Late introduction of complementary feeding, rather than duration of breastfeeding, may protect against adult overweight. The American Journal of Clinical Nnutrition 91, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess S., Grote V., Scaglioni S., Luque V., Martin F. et al (2010) Introduction of complementary feeding in 5 European countries. Journal of Pediatric Gastroenterology & Nutrition 50, 92–98. [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN) (2001) Critical Appraisal . SIGN. [On‐line] Available at: http://www.sign.ac.uk/methodology/checklists.html[Accessed 21 October 2010].
- Sloan S., Gildea A., Stewart M., Sneddon H. & Iwaniec D. (2008) Early weaning is related to weight and rate of weight gain in infancy. Child: Care, Health and Development 34, 59–64. [DOI] [PubMed] [Google Scholar]
- Summerbell C.D., Waters E., Edmunds L., Kelly S.A.M., Brown T. & Campbell K.J. (2005) Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews 3, CD001871. [DOI] [PubMed] [Google Scholar]
- Van Dijk C.E. & Innis S.M. (2009) Growth‐curve standards and the assessment of early excess weight gain in infancy. Pediatrics 123, 102–108. [DOI] [PubMed] [Google Scholar]
- Van't Hof M.A. (2000) The influence of breastfeeding and complementary foods on growth until three years of age in the Euro‐Growth Study. Pediatrics 106, 1281–1282. [PubMed] [Google Scholar]
- Von Kries R., Koletzko B., Saurwald T., Von Mutius E., Barnert D., Grunert V. et al (1999) Breastfeeding and obesity: a cross‐sectional study. British Medical Journal 319, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R.C. (2004) Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114, e29–e36. [DOI] [PubMed] [Google Scholar]
- Wilson A.C., Forsyth J.S., Greene S.A., Irvine L., Hau C. & Howie P.W. (1998) Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. British Medical Journal 316, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman P.G. (1984) Feeding practices in infancy and prevalence of obesity in preschool children. Journal of the American Dietetic Association 84, 436–438. [PubMed] [Google Scholar]
- World Bank (2001) World Development Report. Oxford University Press: Oxford. [Google Scholar]
- World Health Organisation (WHO) (2001) Recommendation on the optimal duration of exclusive breastfeeding‐guidance on infant feeding. WHO. [On‐line] Available at: http://www.who.int/healthtopics/breastfeeding/en/htm[Accessed 5 November 2008].
- World Health Organization (WHO) (2005) Guiding Principles for Feeding Non‐breastfed Children 6–24 months of Age. World Health Organization: Geneva. [Google Scholar]
- World Health Organisation (WHO) (2006a) Obesity and Overweight. What are overweight and obesity? Fact Sheet No 311. WHO. [On‐line] Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html[Accessed 20 January 2010].
- World Health Organisation (WHO) (2006b) Child Growth Standards. World Health Organisation: Geneva. [Google Scholar]
- Worobey J., Lopez M.I. & Hoffman D.J. (2009) Maternal behavior and infant weight gain in the first year. Journal of Nutrition Education and Behavior 41, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung D.L., Pennell M.D., Leung M. & Hall J. (1981) Infant fatness and feeding practices: a longitudinal assessment. Journal of the American Dietetic Association 79, 531–535. [PubMed] [Google Scholar]
- Zive M.M., McKay H., Frank‐Spohrer G.C., Broyles S.L., Nelson J.A. & Nader P.R. (1992) Infant‐feeding practices and adiposity in 4‐y‐old Anglo‐ and Mexican‐ Americans. American Journal of Clinical Nutrition 55, 1104–1108. [DOI] [PubMed] [Google Scholar]


