Abstract
Few studies appear to have investigated the prevalence of constipation for all three trimesters of the gestative period, or indeed after birth. Using a prospective 4‐ to 7‐day weighed food diary, International Physical Activity Questionnaire and 7‐day bowel habit diary, dietary factors, physical activity levels and bowel habit parameters were assessed and examined concurrently at weeks 13, 25, 35 of pregnancy and 6 weeks post‐partum. Ninety‐four primiparous pregnant women were initially recruited, and 72, 59, 62 and 55 completed the first, second, third trimester and post‐partum study stages, respectively. Key dietary factors and physical activity levels were compared between the constipated and non‐constipated groups from each of the three trimesters and after parturition. Compared with non‐constipated mothers‐to‐be, constipated participants consumed statistically significantly less water in the first trimester (P = 0.04), more food in the second trimester (P = 0.04), and less iron (P = 0.02) and food (P = 0.04) in the third trimester and after birth, respectively. No statistically significant differences were identified between light, moderate and vigorous physical activity levels when groups were compared. This study demonstrates that dietary factors may play a role in terms of preventing, or alleviating, bowel habit perturbations both throughout and after pregnancy. Further research is required to investigate the interrelationship between physical activity and constipation during and after pregnancy.
Keywords: pregnancy, water, food, constipation
Introduction
Constipation, a common disorder among Westernized communities, is especially prevalent during pregnancy, and up to 40% of women are likely to suffer symptoms at some stage of their pregnancy (Anderson 1986). With over half a million maternities taking place each year in the United Kingdom (Office for National Statistics 2004 give a figure of 596 122 for 2004), this summates to an approximate 240 000 women likely to suffer from gestational constipation. The aetiology of constipation during pregnancy is multifactorial and may be either hormonal in origin, or attributed to poor dietary habits, physical inactivity, or a combination of these factors (Wald 2003).
In terms of endocrinological factors, colonic hypomotility during pregnancy, particularly during the second and third trimesters, has been attributed to the relaxation of intestinal smooth muscle by progesterone (Wald et al. 1982; Lawson et al. 1985). It has also been suggested that increased secretions of progesterone and somatostatin during pregnancy may inhibit the release of motilin, a peptide hormone known to normally stimulate smooth muscle (Christofides et al. 1982; Jenssen et al. 1986). Relaxin, an inhibitory polypeptide released by the corpus luteum and placenta during pregnancy, also inhibits myometrial contractility and relaxes smooth muscles, including those of the gastrointestinal tract, predisposing to gestational constipation (Bonapace & Fisher 1998; Tincello et al. 2003). Total colonic perfusion studies have shown that aldosterone increases water absorption in women, between weeks 12 and 20 of pregnancy (Parry et al. 1970); a factor likely to result in ‘colonic dehydration’ and the formation of hard, small, scybalous stools that are difficult to expel (Arnaud 2003). Finally, the combined movements of the intestinal tract and uterus during late pregnancy have also been found to impede onward movement of solid feces, obtrude defecations and initiate constipation‐type symptoms (Wald 2003).
Constipation in the puerperium may be a result of anal sphincter injury (the muscle of evacuation) induced by childbirth (Shafik & El‐Sibai 2002). Previous studies have also found that forceps delivery, increased duration of second‐stage delivery and high infant birthweight may all lead to anal sphincter injury (Corman 1985; Sultan et al. 1993).
With regard to dietary factors, Burgess (1972) suggested that lack of dietary fibre, particularly Englyst fibre, also known as non‐starch polysaccharide (NSP), and water may be some of the most important factors in the aetiology of constipation during and after pregnancy. Busy post‐partum mothers may find it particularly difficult to consume a balanced diet, enriched with NSP and water when preoccupied with their young infants. Burgess suggested that an indolent bowel tends to accompany a sedentary existence, and a lack of exercise may predispose to constipation in pregnancy. Bonen et al. (1992) found that progesterone concentrations increased after pregnant subjects cycled on a bicycle ergometer for 30 min at heart rates of 130–140 beats per minute. Although it was not elucidated in this study whether the surge in progesterone levels was attributed to increased progesterone secretion rates or reductions in hepatic clearance, it is possible that aerobic exercise may slow GI transit in pregnancy through further elevation of progesterone concentrations. However, Artal & O’Toole (2003) reported that participating in light forms of physical activity during pregnancy may help to promote healthy bowel movements during this time, and Sullivan (1984) emphasized that an upright posture and ‘biochemical bouncing’ or jiggling of colonic contents can move stools into the rectum, thus stimulating bowel movements.
The available information relating to prevalence of constipation for each individual trimester of pregnancy, or after birth is fragmentary, as is its relation to dietary factors and physical activity levels during the pregnancy and post‐partum phase of the life cycle. Most of what is presently known derives from research undertaken over two decades ago (Anderson 1984, 1986, 1986; Anderson & Whichelow 1985). Therefore, the aims of the present study were twofold: (1) to re‐examine the prevalence of constipation using detailed, prospective methodologies for the three trimesters of pregnancy and post‐partum and (2) to asses the possible role of diet and physical activity in the aetiology of constipation at these stages.
Materials and methods
Subjects
Primiparous non‐smokers, aged 19–40 year (mean 33.4 year) were initially recruited from St George’s, St Thomas’ and Guys and Kings College Teaching Hospitals, London (Fig. 1). Volunteers completed a screening questionnaire to ensure all subjects were healthy and free from gastrointestinal disorders (including irritable bowel syndrome). Letters were issued to general practitioners, asking them to inform the research department if their patient had been diagnosed with any bowel or health disorders. Subjects taking medications known to influence bowel function were excluded. Data were collected over four time periods. First trimester data were collected once pregnancy had been confirmed and as soon as women were willing to participate in the study. Data were collected at the end of the second trimester (between 24 and 28 weeks), and the third part of the study was undertaken between weeks 34 and 38 of gestation. The final post‐partum section of the study was undertaken 6 weeks after birth. Ninety‐four subjects were initially recruited, and 72, 59, 62 and 55 participants completed the first, second, third and post‐partum study stages, respectively. Compliance rates were: 77% in the first trimester, 63% in the second trimester, 66% in the third trimester, and 59% after birth. Forty‐two volunteers successfully completed every study stage, an overall compliance rate of 45%. Specific inclusion criteria meant that the study population was not randomly selected and therefore not entirely representative of the UK pregnant population. Eighty‐seven per cent of volunteers were in categories 1–3 of the Office for National Statistics (2002) Socio‐economic Classification. Ethical approval was obtained from all three Hospitals and London South Bank University. Volunteers were provided with information sheets and completed a study consent form prior to study participation. Each questionnaire and diary was individually piloted by eight pregnant volunteers in their first trimester. Amendments were made accordingly before the study commenced. These included the simplification of questions and terms used in the questionnaire and food diary.
Figure 1.
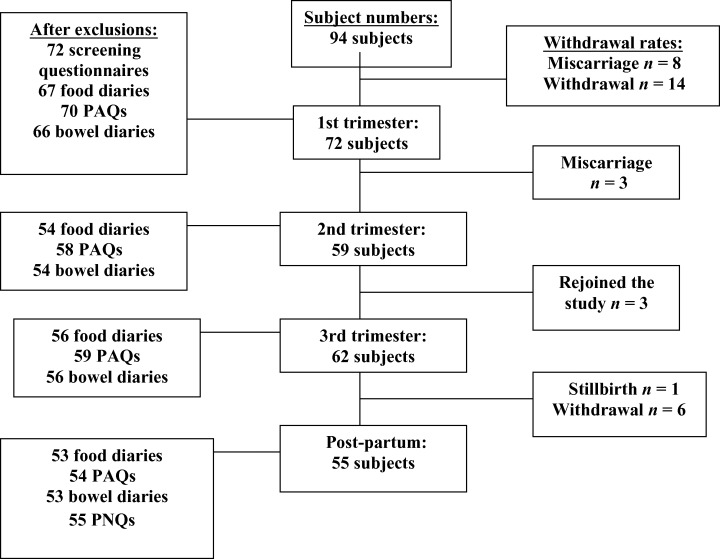
Recruitment, retention and withdrawal of participants throughout the study. PAQ, Physical Activity Questionnaire; PNQ, Post‐natal Questionnaire.
Food diaries
At the initial interview, volunteers were issued with a diet record book and electronic calibrated scales. Volunteers were asked to record and weigh food items using the cumulative weighing method, for seven consecutive days. When meals were consumed out of the home, subjects used estimated food portion sizes using household measures. These were checked and estimated according to the Ministry of Agriculture, Fisheries and Food (1994). As this study was a follow‐through study, it was decided for women experiencing difficulties with the weighing of food, 4 days would be acceptable, provided they included a weekend day. Gay (2000) found that decreasing the recording period from 7 to 4 days had a desirable impact upon response rate without influencing the precision of data. Food diaries were not used in the analysis when volunteers did not include a weekend day, food weights were not recorded, or the diary appeared to be completed retrospectively. The daily nutrient intakes of all items listed in the food diary were calculated using the computer program Dietplan 5 (Forestfield Software Ltd., West Sussex, UK). Foods consumed by subjects but not on the database were added after contacting food manufacturers for analytical data. When homemade dishes were consumed, nutrients were calculated from the food constituents. Dietary supplements were recorded in the food diaries on a daily basis, but dosages were not always recorded by subjects. Therefore, this paper focuses specifically upon dietary sources of iron.
Physical activity questionnaire
In the same week as the weighed food diary, volunteers were asked to complete a physical activity questionnaire, in order to obtain total time spent participating in vigorous, moderate and light activities (min day−1). This questionnaire had previously been used and validated by Craig et al. (2002), and had been piloted by small sample of pregnant subjects before the study commenced. Vigorous activities referred to activities that took hard physical effort, causing volunteers to breathe much harder than normal (fast bicycling, swimming and aerobics). Moderate activities referred to activities taking moderate physical effort and made volunteers breathe somewhat harder than normal (bicycling and swimming at a regular pace). Light activities were defined as minimal physical exertion (walking and meditating).
Bowel habit diary
A bowel habit diary was also completed for seven consecutive days during the same study week. To ascertain the prevalence of constipation during each trimester and after birth, it was important to compare data with diagnostic criteria. Volunteers were defined as functionally constipated when they exhibited two or more symptoms from the Rome II diagnostic criteria: straining to start for more than 25% of defecations; straining to finish for more than 25% of defecations; lumpy, hard stools; incomplete evacuation for more than 25% defecations; or less than 3 defecations per week (Table 1) (Thompson et al. 1999). Stools were defined as ‘lumpy’ if they were categorized as ‘cylindrical with deep cracks’ or ‘fragmented’ according to the Davies et al. (1986) stool form scale. Supplements, anatomical aids and lifestyle factors that may alter bowel habit were also recorded on a daily basis in the diary.
Table 1.
Rome II diagnostic criteria
| Straining to start (>25% defecations) |
| Straining to finish (>25% defecations) |
| Lumpy, hard stools |
| Incomplete evacuation (>25% defecations) |
| Defecation frequency (<3 defecations per week) |
Volunteers were defined as being functionally constipated if they experienced at least two of the following: straining to start and finish for 25% or more of defecations; lumpy, hard stools; incomplete evacuations for 25% or more of defecations; or less than 3 defecations per week.
Statistics
Statistical analyses were conducted using spss/PC version 11.5 to investigate associations between dietary factors, physical activity and prevalence of constipation. The independent samples t‐test was used to compare dietary intakes between study set points. The Mann–Whitney (two‐sample test) was used to compare physical activity parameters between non‐constipated and constipated pregnant women during each of the three trimesters and after birth. Descriptive statistics were used to calculate the mean, standard deviations and confidence interval (CI) of variables.
Results
Prevalence of constipation
Prevalence of constipation within the sample population was greatest in the first two trimesters of pregnancy, decreasing in the third trimester and after birth. As illustrated in Table 2, 35% of pregnant women were defined as functionally constipated in the first trimester (95% CI 23–47%), 39% in the second (95% CI 26–52%), 21% in the third (95% CI 10–32%) and 17% (95% CI 7–27%) at 6 weeks post‐partum. Spicy foods, baked beans and stress were reported to increase bowel habits during pregnancy (n = 6), and iron supplements were reported to cause dark stools in one subject after birth.
Table 2.
Prevalence of functional constipation throughout and after pregnancy
| Study stage (n) | Prevalence of constipation n (%) | 95% CI | 99% CI |
|---|---|---|---|
| Trimester 1 (66) | 23 (35) | 23–47 | 20–50 |
| Trimester 2 (54) | 21 (39) | 26–52 | 22–56 |
| Trimester 3 (56) | 12 (21) | 10–32 | 7–35 |
| Post‐partum (53) | 9 (17) | 7–27 | 4–30 |
Diet
In the first trimester of pregnancy, water consumption (g day−1) was statistically significantly lower in constipated mothers, when compared with non‐constipated subjects in the same trimester (P = 0.04). Food (g day−1) intake was statistically significantly higher in constipated mothers in the second trimester, P = 0.04 (Table 3), but lower in constipated subjects after birth (P = 0.04). Consumption of dietary iron was 2.9 mg per day higher in non‐constipated women in the third trimester (P = 0.02).
Table 3.
Intake of key nutrients in non‐constipated and constipated pregnant women
| Non‐constipated Mean (SD) | Constipated Mean (SD) | P‐value | |
|---|---|---|---|
| 1st trimester nutrient | n = 43 | n = 23 | |
| Food (g day−1) | 463.0 (146) | 450.0 (141) | 0.72 |
| Water (g day−1) | 2311 (654) | 1917 (728) | 0.04* |
| Energy (kcal day−1) | 1905 (465) | 1831 (528) | 0.57 |
| NSP (g day−1) | 15.2 (5.1) | 12.8 (5.3) | 0.08 |
| Iron (mg day−1) | 12.8 (4.6) | 11.9 (5.6) | 0.45 |
| 2nd trimester | n = 34 | n = 20 | |
| Food (g day−1) | 476.1 (99.2) | 606.0 (257) | 0.04* |
| Water (g day−1) | 2551 (735) | 2309 (751) | 0.34 |
| Energy (kcal day−1) | 1832 (389) | 2068 (484) | 0.07 |
| NSP (g day−1) | 14.7 (4.5) | 14.0 (4.6) | 0.63 |
| Iron (mg day−1) | 12.4 (3.4) | 12.2 (3. 7) | 0.82 |
| 3rd trimester | n = 45 | n = 11 | |
| Food (g day−1) | 487.8 (134) | 461.9 (91.8) | 0.46 |
| Water (g day−1) | 2480 (794) | 2407 (594) | 0.74 |
| Energy (kcal day−1) | 1925 (447) | 1873 (314) | 0.66 |
| NSP (g day−1) | 14.6 (5.4) | 11.6 (4.2) | 0.06 |
| Iron (mg day−1) | 13.3 (5.0) | 10.4 (3.1) | 0.02* |
| Post‐partum | n = 44 | n = 9 | |
| Food (g day−1) | 472.0 (169) | 400.6 (62.3) | 0.04* |
| Water (g day−1) | 2477 (827) | 2187 (584) | 0.23 |
| Energy (kcal day−1) | 1889 (499) | 1784 (348) | 0.46 |
| NSP (g day−1) | 13.0 (4.4) | 12.5 (6.6) | 0.83 |
| Iron (mg day−1) | 12.2 (4.3) | 13.7 (5.0) | 0.43 |
Groups were compared using the independent samples t‐test. *A statistical significance of P < 0.05. NSP, non‐starch polysaccharide.
Physical activity
Physical activity data were collated and recorded in Table 4. Study findings indicated that non‐constipated subjects participated in higher levels of vigorous, moderate and light activity in the first two trimesters of pregnancy (min day−1). In the third trimester of gestation and after birth, constipated subjects participated in higher levels of vigorous and moderate activity (min day−1); however, these findings were not statistically significant (Mann–Whitney non‐parametric test).
Table 4.
Time spent in vigorous, moderate and light activities (min day−1)
| Non‐constipated Mean (SD) | Constipated Mean (SD) | P‐value | |
|---|---|---|---|
| 1st trimester | n = 47 | n = 23 | |
| Vigorous activity | 30.8 (39) | 27.7 (37) | 0.99 |
| Moderate activity | 61.6 (62) | 53.5 (50) | 0.78 |
| Light activity | 81.8 (86) | 63.8 (68) | 0.24 |
| 2nd trimester | n = 36 | n = 22 | |
| Vigorous activity | 32.4 (47) | 20.3 (21.4) | 0.75 |
| Moderate activity | 60.8 (69) | 45.9 (28) | 0.40 |
| Light activity | 79.8 (93) | 68.3 (53) | 0.37 |
| 3rd trimester | n = 46 | n = 13 | |
| Vigorous activity | 15.8 (19) | 29.4 (41.2) | 0.76 |
| Moderate activity | 66.0 (67) | 96.7 (91) | 0.19 |
| Light activity | 48.8 (36.0) | 42.3 (45) | 0.36 |
| Post‐partum | n = 45 | n = 9 | |
| Vigorous activity | 9.5 (15) | 21.4 (44.2) | 0.54 |
| Moderate activity | 49.6 (73) | 86.7 (72) | 0.13 |
| Light activity | 70.9 (80.2) | 56.3 (30) | 0.83 |
Groups were compared using the Mann–Whitney test. P < 0.05. NSP, non‐starch polysaccharide.
Discussion
Present study findings have shown that functional constipation was greatest in the second trimester of pregnancy, with 39% of women experiencing symptoms at this study stage. The 95% CI indicated that there was some overlapping between trimesters, but overall prevalence remained to be highest in the second trimester (95% CI 26–52%) and lowest after birth (95% CI 7–27%). Occurrence of constipation in the present study was comparable with previous studies. Anderson (1984) reported that 38% and 20% of women experienced constipation in the second and third trimesters, respectively. Marshall et al. (1998) did not document the trimester for which data collection took place, but reported that 35% of women overall suffered from constipation during pregnancy. This was in accord with the mean prevalence rate (32%) of this study. As constipation was highest in early pregnancy, it seems that the endocrinological changes of pregnancy are more likely to be accountable causes of constipation, as opposed to mechanical changes of gestation, which would have been most likely to exert their influence in the third trimester. As prevalence of constipation varies not only between individuals, but also throughout and after pregnancy, different dietary requirements may be essential to alleviate such symptoms at each trimester of pregnancy and after birth. For example, high water intakes appeared to reduce symptoms of constipation in the first trimester (P = 0.04), but had limited effects in subsequent trimesters. It is therefore likely that higher water intakes may be required during these trimesters and post‐partum, particularly in the case of lactating mothers, to induce a response and prevent constipation at these stages.
This study also confirmed that water consumption was higher in non‐constipated women during all three trimesters and after birth. Through the actions of water, stools may become softer and bulkier, thus easing the defecation process and acting as a preventative measure in terms of constipation (Arnaud 2003). Parry et al. (1970) identified that colonic absorption of water was increased in early pregnancy and augmented through the secretion of aldosterone. Oestrogen and progesterone both increase renin secretion (an enzyme that converts angiotensinogen to angiotensin I), and angiotensin II formation increases (Porterfield 1997). Therefore, it can be postulated that the rise in aldosterone concentrations as pregnancy proceeds (augmented by the rise in oestrogen and progesterone concentrations), may increase water absorption further in late pregnancy, increasing dietary water requirements in the second and third trimesters. Langer et al. (1998) found that the highest activity of the rennin–angiotensin–aldosterone system was in the third trimester when levels of angiotensin I, angiotensin II and aldosterone were elevated at this study stage. Women failing to consume sufficient water may therefore experience colonic hypohydration and constipation as a result (Arnaud 2003).
With regard to food volume, the present study found that volunteers consuming a greater food volume in the second trimester experienced higher rates of constipation. It is possible that the combination of slow colonic transit in the second trimester of gestation and high food intake at this study stage resulted in ‘colonic congestion’, increased water absorption and constipation. Conversely, study findings further identified that food intake was inversely associated with symptoms of constipation after birth. As hormonal concentrations return to pre‐pregnancy levels at this phase (Christofides et al. 1982), this would account for such an association.
Dietary iron intake was statistically significantly (2.4 mg day−1) higher in non‐constipated volunteers in the third trimester. Non‐constipated subjects at this study stage may have consumed a healthier diet overall, which may have prevented constipation symptoms normally associated with iron consumption (Milman et al. 2006).
This study also confirms that although physical activity was initially higher in non‐constipated study participants in the first and second trimesters, constipated subjects undertook higher levels of vigorous and moderate activities in the third trimester and after birth. Even though not statistically significant, it is possible that women at this phase of pregnancy may have been constipated because they were participating in frequent physical activities and experiencing colonic dehydration, or attempting to alleviate constipation through the actions of physical activity. Further research using detailed prospective methodologies is required to investigate the associations between physical activity levels and constipation during and after pregnancy.
Lifestyle recommendations
In terms of lifestyle recommendations, dietary advice given to women should include an adequate water intake. Water may be provided in beverages or by consumption of foods with high moisture content. Examples of such foods include fruit and vegetables, such as melon, cucumber and celery, soups and lean meats. Nicolaïdis (2004) recommends that at least 35 mL day−1 per kg of bodyweight should be consumed by adult women and supplied through food and beverage sources.
This study has also proposed that food volume may result in colonic congestion, particularly when gastrointestinal transit is reduced in the second trimester. Therefore, it must be emphasized that a sufficient food volume must be complimented with adequate water intake throughout pregnancy, as insufficient fluid may result in intestinal obstruction (Doughty 2002).
With regard to levels of physical activity, although no statistically significant differences were found between constipated and non‐constipated mothers in the present study, the American College of Sports Medicine suggests that, in the absence of medical complications, an accumulation of 30 min of moderate exercise should occur on most days of the week during pregnancy (Artal & O’Toole 2003).
Acknowledgements
Many thanks to the dedicated volunteers who took part in this study and Reckitt Benckiser Healthcare (UK) Ltd., the sponsor of this research. There is no conflict of interest that could be perceived to bias this work.
References
- Anderson A.S. (1984) Constipation during pregnancy: incidence and methods used in its treatment in a group of Cambridgeshire women. Health Visitor 57, 363–364. [Google Scholar]
- Anderson A.S. (1986) Dietary factors in the aetiology and treatment of constipation during pregnancy. British Journal of Obstetrics and Gynaecology 93, 245–249. [DOI] [PubMed] [Google Scholar]
- Anderson A.S. & Whichelow M.J. (1985) Constipation during pregnancy: dietary fibre intake and the effect of fibre supplementation. Human Nutrition: Applied Nutrition 39A, 202–207. [PubMed] [Google Scholar]
- Arnaud M.J. (2003) Mild dehydration: a risk factor of constipation. European Journal of Clinical Nutrition 57, S88–S95. [DOI] [PubMed] [Google Scholar]
- Artal R. & O’Toole M. (2003) Guidelines of the American college of obstetricians and gynaecologists for exercise during pregnancy and the postpartum period. British Journal of Sports Medicine 37, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapace E.S. & Fisher R.S. (1998) Constipation and diarrhea in pregnancy. Gastroenterology Clinics of North America 27, 197–211. [DOI] [PubMed] [Google Scholar]
- Bonen A., Campagna P., Gilchrist L., Young D.C. & Beresford P. (1992) Substrate and endocrine responses during exercise at selected stages of pregnancy. American Physiological Society 73, 134–142. [DOI] [PubMed] [Google Scholar]
- Burgess D.E. (1972) Constipation in obstetrics In: Management of Constipation (eds. Avery Jones F. & Godding E.W.). Blackwell Scientific Publishing: Oxford. [Google Scholar]
- Christofides N.D., Ghatei M.A., Bloom S.R., Borberg C. & Gillmer M.D.G. (1982) Decreased plasma motilin concentrations in pregnancy. British Medical Journal 285, 1453–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman M.L. (1985) Anal incontinence following obstetrical injury. Diseases of the Colon and Rectum 28, 86–89. [DOI] [PubMed] [Google Scholar]
- Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E. et al. (2002) Reliability and validity study group. International physical activity questionnaire (IPAQ): 12‐country reliability and validity. Journal of Medicine, Science, Sport and Exercise 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- Davies G., Crowder M., Reid B. & Dickerson J. (1986) Bowel function measurements of individuals with different eating patterns. Gut 27, 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty D.B. (2002) When fiber is not enough: current thinking on wound management. Ostomy/Wound Management 48, 30–41. [PubMed] [Google Scholar]
- Gay C. (2000) Estimation of population distributions of habitual nutrient intake based on a short‐run weighed food diary. British Journal of Nutrition 80, 287–293. [DOI] [PubMed] [Google Scholar]
- Jenssen T.G., Holst N., Burhol P.G., Jorde R., Maltau J.M. & Vonen B. (1986) Plasma concentrations of motilin, somatostatin and pancreatic polypeptide, during and after parturition. Acta Obstetrica Gynaecologica Scandinavica 65, 153–156. [DOI] [PubMed] [Google Scholar]
- Langer B., Grima M., Coquard C., Bader A.M., Schlaeder G. & Imbs J.L. (1998) Plasma active rennin, angiotensin I, and angiotensin II during pregnancy and in preeclampsia. Obstetrics and Gynecology 91, 196–202. [DOI] [PubMed] [Google Scholar]
- Lawson M., Kern F. & Everson G.T. (1985) Gastrointestinal transit time in human pregnancy: prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology 89, 996–999. [DOI] [PubMed] [Google Scholar]
- Marshall K., Thompson K.A., Walsh D.M. & Baxter G.D. (1998) Incidence of urinary incontinence and constipation during pregnancy and postpartum: survey of current findings at the rotunda lying‐in hospital. British Journal of Obstetrics and Gynaecology 105, 400–402. [DOI] [PubMed] [Google Scholar]
- Milman N., Byg K.E., Bergholt T. & Eriksen L. (2006) Side effects of oral iron prophylaxis in pregnancy – myth or reality? Acta Haematologica 115, 53–57. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture, Fisheries and Food (1994) Food Portion Sizes, 2nd edn HMSO: London. [Google Scholar]
- Nicolaïdis S. (2004) Water: hydration and health. Danone Nutritopics 31, 1–17. [Google Scholar]
- Office for National Statistics (2002) The National Statistics Socio‐economic Classification. Office for National Statistics: London. [Google Scholar]
- Office for National Statistics (2004) Birth Statistics: Review of the Registrar General on Births and Patterns of Family Building in England and Wates, 2002. Series FMI, No. 31. Office for National Statistics: London. [Google Scholar]
- Parry E., Shields R. & Turnbull A.C. (1970) The effect of pregnancy on the colonic absorption of sodium, potassium and water. Journal of Obstetrics and Gynaecology 77, 616–619. [DOI] [PubMed] [Google Scholar]
- Porterfield S.P. (1997) Endocrinology of pregnancy In: Endocrine Physiology (ed. Porterfield S.P.), pp. 197–214. Mosby Publishing: London. [Google Scholar]
- Shafik A. & El‐Sibai O. (2002) Study of the levator ani muscle in the multipara: role of levator dysfunction in defecation disorders. Journal of Obsretrics and Gynaecology 22, 187–192. [DOI] [PubMed] [Google Scholar]
- Sullivan S.N. (1984) The effect of running on the gastrointestinal tract. Journal of Clinical Gastroenterology 6, 461–465. [DOI] [PubMed] [Google Scholar]
- Sultan A.H., Kamm M.A., Hudson C.N., Thomas J.M. & Batram C.I. (1993) Anal sphincter disruption during vaginal delivery. New England Journal of Medicine 329, 1905–1911. [DOI] [PubMed] [Google Scholar]
- Thompson W.G., Longstreth G.F., Drossman D.A., Heaton K.W., Irvine E.J. & Müller‐Lissner S.A. (1999) Functional bowel disorders and functional abdominal pain. Gut 45, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tincello D.G., Teare J. & Fraser W.D. (2003) Second trimester concentration of relaxin and pregnancy related incontinence. European Journal of Obstetrics Gynaecology and Reproductive Biology 106, 237–238. [DOI] [PubMed] [Google Scholar]
- Wald A. (2003) Constipation, diarrhea and symptomatic hemorrhoids during pregnancy. Gastroenterology Clinics of North America 32, 309–322. [DOI] [PubMed] [Google Scholar]
- Wald A., Van Thiel D., Hoechstetter L., Gavaler J.S., Egler K.M., Verm R. et al. (1982) Effect of pregnancy on gastrointestinal transit. Digestive Diseases and Sciences 27, 1015–1018. [DOI] [PubMed] [Google Scholar]


