Abstract
This investigation describes the pattern of changes in mid‐upper arm circumference (MUAC), triceps, biceps and subscapular skinfold thicknesses during the course of pregnancy, and its relationship with maternal and newborn outcomes. A prospective cohort of 1066 pregnant women were selected in seven different urban regions in Argentina. Measurements of MUAC were carried out at 16, 28 and 36 gestational weeks. In a subsample of 488 women, triceps, biceps and subscapular skinfold thicknesses were measured. Mean total increase in subscapular, tricipital and bicipital skinfolds from 16 to 36 weeks of gestation were 4.5, 3.6 and 2.6 mm, respectively. MUAC showed a mean increase of 1.7 cm in the same period. Overweight or obese women at the start of pregnancy had lower increases in all measurements compared with women with normal or low body mass index. Maternal anthropometry was related to birthweight; women who gave birth to infants of less than 3000 g had lower average values in all measurements than those who had normal birthweight infants. LMS curves for MUAC and skinfolds by gestational age are presented, which can be used as a reference to assess maternal nutrition status during pregnancy. MUAC, tricipital and subscapular skinfold for gestational age curves are proposed for monitoring maternal nutritional status during pregnancy. MUAC cut‐off points of 24.5, 25.5 and 26.5 cm for 16, 28 and 36 weeks of gestation, respectively, are also proposed as a proxy to detect low birthweight.
Keywords: maternal anthropometry assessment, MUAC, skinfold thicknesses
Introduction
Anthropometric assessment has many advantages for nutritional evaluation: it is relatively simple, non‐invasive and causes minimal discomfort to the patient. During pregnancy, maternal anthropometry assessment helps to identify women at risk of malnutrition and adverse pregnancy outcomes. Prepregnancy body mass index (BMI) and gestational weight gain are the most used anthropometric indicators because they are closely related to neonatal birthweight, and many charts have been generated for prenatal use in order to identify low or excessive weight gain. Nevertheless, in developing countries, prepregnancy weight may be frequently unknown and thus total weight gain cannot be calculated. In these cases, mid‐upper arm circumference (MUAC) is recognized as an effective tool for screening purposes because of its strong correlation with body weight, but knowledge about its changes during the course of pregnancy is still limited (James et al. 1994; Pelletier et al. 1995; Kelly et al. 1996).
Skinfold thicknesses reflect subcutaneous fat stores that are used to meet the energy needs of the fetus and the mother during pregnancy and lactation. Changes in these measurements could also be used to assess maternal nutritional status, but as in the case of MUAC, few studies have evaluated its variation at different gestational ages (Licitra et al. 1998; Zekan et al. 1998; Araújo et al. 2009).
In late pregnancy, abnormal body weight gain can be related to clinical oedema; in such cases, both MUAC and skinfold measurements are alternative parameters to be used for nutritional assessment as they are not so influenced by leg oedema (Davison 1997; Reynolds 2003).
In this study, we describe the pattern of changes in MUAC, triceps, biceps and subscapular skinfold thicknesses during the course of pregnancy, and its relationship with maternal weight gain and newborn outcomes.
Key messages
-
•
MUAC cut‐off points of 24.5 cm at less than 16 weeks of gestation, 25.5 cm at week 28 and 26.5 cm at week 36 can be used as a proxy to detect both low maternal BMI and low birthweight when maternal weight gain cannot be calculated.
-
•
Average MUAC of mothers of normal‐birthweight infants was almost 2 cm higher during the whole course of pregnancy than that of mothers of low‐birthweight newborns.
-
•
MUAC increase was lower in overweight and obese women, compared with normal‐weight or underweight mothers.
-
•
Subscapular and tricipital skinfolds could also be used as a proxy to detect low‐birthweight newborns and can be applied as complementary measurements to assess maternal nutritional status.
Population and methods
Subjects
Data were collected as part of a larger project designed to evaluate maternal weight gain during the course of pregnancy (Calvo et al. 2009). A prospective cohort of 1066 pregnant women was selected from antenatal clinics in seven different urban regions in Argentina with the aim of taking geographical variability into account, but without probabilistic sampling procedures. Data were collected from May 2005 to December 2006.
The study protocol was conducted pursuant to local ethical standards according to the Helsinki Declaration and was approved by the National Commission ‘Salud Investiga’ from the Ministry of Health. All the subjects involved signed an informed consent form.
Eligibility criteria included women aged 19–49 years with singleton pregnancies and a gestational age of less than 12 weeks at entry (or less than 16 weeks if prepregnancy weight was remembered), without clinical symptoms of any concomitant pathology at entry, parity 0–5, non‐smokers or smoking less than five cigarettes per day and non‐alcohol consumers or drinking less than 20 g of ethanol per day.
Study design
At the first visit, a questionnaire on social characteristics and a medical history were completed, and weight and height were measured according to standardized techniques; prepregnancy BMI (kg m2) was calculated and classified according to the Institute of Medicine (1990). Gestational age was determined by dating the last menstrual period at the time of registration and was corrected by first trimester ultrasonographic examinations if the difference exceeded 5 days.
Serial measurements of MUAC were carried out with a flexible steel tape (Rosscraft Innovations Inc., Surray, Canada) at less than 16, 28 ± 2 and 36 ± 2 gestational weeks. In a subsample of 488 women, triceps, biceps and subscapular skinfold thicknesseses were measured by trained nutritionists using a Lange skinfold caliper according to standardized methods. The average of three measurements was recorded at each site.
Before data collection, a standardization procedure was carried out to homogenize anthropometric results among investigators; intra‐ and inter‐observer errors for skinfolds and circumference were calculated. Only three nutritionists with the highest precision were selected to perform skinfold measurements in a subsample of pregnant women. Neonatal sex, weight and height were obtained from hospital records.
Statistical analysis
Summary statistics (means and 95% confidence intervals) were calculated using Epi‐Info software, version 3.2 [Center for Disease Control and Prevention (CDC), Atlanta, GA, USA]. Quantitative data were analysed by contrasting means using t‐test or analysis of variance, and qualitative data were analysed by using the chi‐square test. A significance level of P < 0.05 was used in all tests. Centile curves of MUAC and skinfolds by gestational age were developed for those pregnant women who delivered neonates with birthweights between 2500 and 4000 g. The LMS method, which summarizes the distribution by three curves representing the median (M), coefficient of variation (S), and skewness expressed as a Box–Cox power transform (L) (Cole 1990), was used to fit the smoothed curves (lmsChartMaker, The Institute of Child Health, London, UK).
Changes in MUAC and skinfolds were analysed as a function of initial maternal BMI and categories of birthweight.
The receiver operating characteristic (ROC) curves were applied to obtain cut‐off points of MUAC at different gestational ages, based on the current data set plus data on BMI of the same women (Calvo et al. 2009). We intended to find the best cut‐off point of MUAC in the different trimesters to predict low maternal BMI and/or risk of low birthweight [Epidat 3.1 (2006), Xunta de Galicia – PAHO/WHO, Washington DC, USA].
Results
Maternal and newborn characteristics for the whole population and the subsample with skinfolds measurements are summarized in Table 1. There were no differences in age, parity, educational level and prepregnancy weight between women in both samples. Although women from the subsample with skinfold measurements were taller, there were no differences in prepregnancy BMI classification with the whole population. There were minor differences in birthweight (58 g) and length (1.6 cm), but these are not conditions associated with sampling procedures, could not be prevented and are not likely to affect the interpretation of data.
Table 1.
Sample characteristics
| Maternal characteristics | MUAC sample (n = 1066) | Skinfolds sample (n = 488) | P‐value |
|---|---|---|---|
| Age [years, mean (SD)] | 27.0 (5.8) | 27.6 (6.0) | 0.060 |
| Parity [mean (SD)] | 1.04 (1.2) | 1.06 (1.2) | 0.760 |
| Completed secondary school [n (%)] | 795 (74.9) | 385 (79.2) | 0.065 |
| Prepregnancy weight [kg, mean (SD)] | 60.1 (12.5) | 60.1 (12.0) | 1 |
| Height [cm, mean (SD)] | 159.6 (6.7) | 160.6 (7.0) | 0.004 |
| Prepregnancy BMI [kg m2, mean (SD)] | 23.3 (4.3) | 23.1 (4.1) | 0.270 |
| Prepregnancy BMI classification [n (%)] | 0.540 | ||
| Underweight (BMI < 19.8) | 160 (15.0) | 84 (17.2) | |
| Normal weight (BMI 19.8–25.9) | 682 (64.0) | 314 (64.3) | |
| Overweight (BMI 26–29) | 120 (11.3) | 49 (10.0) | |
| Obese (BMI > 29) | 104 (9.8) | 41 (8.4) | |
| Total weight gain [mean (SD)] | 11.9 (4.4) | 11.7 (3.9) |
| Newborn characteristics | |||
|---|---|---|---|
| Mean birthweight (g) | 3239.0 ± 492.4 | 3180.8 ± 474.9 | 0.030 |
| Mean birth length (cm) | 47.4 ± 3.8 | 45.8 ± 4.4 | 0.000 |
| Birthweight classification [n (%)] | 0.073 | ||
| Low birthweight (<2500 g) | 55 (5.16) | 27 (5.53) | |
| Insufficient birthweight (2500–2999 g) | 224 (21.01) | 131 (26.84) | |
| Normal birthweight (3000–4000 g) | 736 (69.04) | 307 (62.91) | |
| High birthweight (>4000 g) | 51 (4.78) | 23 (4.71) | |
| Prematurity (less than 37 weeks) | 61 (5.7) | 23 (4.7) | |
| Infant gender, male (%) | 48.8 | 47.5 | 0.630 |
MUAC, mid‐upper arm circumference; SD, standard deviation; BMI, body mass index.
According to the selection criteria, only 10.65% of women smoked (less than five cigarettes per day), and 1.97% developed diabetes and 2.62% developed pre‐eclampsia during the follow‐up.
Means and 95% confidence intervals for MUAC and skinfold measurements at 16, 28 and 36 weeks of gestation are shown in Table 2. The total number of subjects in the MUAC sample was 1066, 910 and 905 at weeks 16, 28 and 36, respectively. The subsample for skinfold measurements included 488 women at week 16; 431 women at week 28; and 415 at week 36.
Table 2.
MUAC and skinfolds at 16, 28 and 36 weeks of gestation according to neonatal birthweight (means and 95% confidence intervals)
| Gestational age <16 weeks | Gestational age 28 weeks | Gestational age 36 weeks | |
|---|---|---|---|
| MUAC (cm) | 25.7 (25.5–26) | 26.9 (26.6–27.2) | 27.5 (27.2–27.8) |
| Neonatal weight <3000 g (n = 279) | 24.4 (23.9–24.9)* | 25.5 (25.0–26.1)* | 26.4 (25.7–27.0)* |
| Neonatal weight >3000 g (n = 787) | 26.2 (25.9–26.5)* | 27.4 (27.1–27.7)* | 28.0 (27.6–28.3)* |
| Bicipital skinfold (mm) | 10.1 (9.7–10.6) | 11.7 (11.3–12.2) | 12.8 (12.4–13.3) |
| Neonatal weight <3000 g (n = 158) | 8.3 (7.7–8.9)* | 10.1 (9.4–10.7)* | 11.3 (10.6–11.9)* |
| Neonatal weight >3000 g (n = 330) | 11.0 (10.5–11.5)* | 12.5 (11.9–13.1)* | 13.5 (12.9–14.1)* |
| Tricipital skinfold (mm) | 19.2 (18.5–19.8) | 21.5 (20.8–22.1) | 23.0 (22.3–23.7) |
| Neonatal weight <3000 g (n = 158) | 16.1 (15.0–17.3)* | 18.6 (17.5–19.8)* | 19.9 (18.8–20.9)* |
| Neonatal weight >3000 g (n = 330) | 20.6 (19.8–21.4)* | 22.8 (22.0–23.6)* | 24.4 (23.6–25.1)* |
| Subscapular skinfold (mm) | 19.4 (18.8–20.1) | 22.4 (21.7–23.1) | 24.2 (23.5–24.9) |
| Neonatal weight <3000 g (n = 158) | 16.9 (15.8–17.9)* | 19.9 (18.8–21.0)* | 21.9 (20.9–22.9)* |
| Neonatal weight >3000 g (n = 330) | 20.7 (19.9–21.5)* | 23.6 (22.7–24.4)* | 25.2 (24.3–26.1)* |
MUAC, mid‐upper arm circumference; *P‐value less than 0.000 (t‐test between neonatal weight <3000 g and ≥3000 g).
Maternal anthropometry was related to birthweight; women who gave birth to infants of less than 3000 g presented lower average values for all measurements at 16, 28 and 36 weeks of gestation than mothers of normal birthweight infants. Total change in MUAC and skinfolds is shown in Table 3. The largest absolute gain in skinfold thickness from the first trimester (<16 weeks) to week 36 of gestation was in the subscapular area, with a mean increase of 4.5 mm; tricipital and bicipital skinfolds increased 3.6 mm and 2.6 mm, respectively.
Table 3.
Increase in MUAC and skinfold thickness by maternal prepregnancy BMI classification (mean, 95% confidence interval)
| Prepregnancy weight classification* (BMI kg m2) | Increase in MUAC † (cm) | Increase in bicipital skinfold † (mm) | Increase in tricipital skinfold † (mm) | Increase in subscapular skinfold † (mm) |
|---|---|---|---|---|
| Total sample | 1.7 (1.5–1.8) | 2.6 (2.3–2.8) | 3.6 (3.2–4.0) | 4.5 (4.1–4.9) |
| Underweight | 2.4 (2.1–2.7) | 3.1 (2.7–3.5) | 5.0 (4.3–5.7) | 6.4 (5.6–7.2) |
| Normal weight | 1.9 (1.7–2.1) | 2.7 (2.4–3.0) | 3.8 (3.3–4.3) | 4.7 (4.3–5.2) |
| Overweight | 0.9 (0.4–1.3) | 2.0 (1.0–3.1) | 1.8 (0.4–3.1) | 2.2 (0.8–3.6) |
| Obese | 0.6 (0.05–1.1) | 0.6 (−1.1–2.3) | 0.4 (−1.5–2.2) | 0.6 (−1.0–2.3) |
MUAC, mid‐upper arm circumference; BMI, body mass index; *Underweight: BMI < 19.8, normal weight: BMI 19.8–25.9, overweight: BMI 26–29, Obese: BMI > 29. †P‐value less than 0.000 (analysis of variance between categories of prepregnancy weight classification).
MUAC showed a total average increase of 1.7 cm: the largest increase of 1.1 cm (95% confidence interval: 1.0–1.2 cm) was observed from the first trimester to 28 weeks, while the variation from week 28 to the end of pregnancy was only 0.6 cm (95% confidence interval: 0.5–0.7 cm).
MUAC and maternal weight were significantly associated as could be expected; the correlation coefficient was strongest in the first trimester (r = 0.735, P < 0.001), with a decrease throughout the course of pregnancy (r = 0.718 at 28 weeks and r = 0.638 at 36 weeks, P < 0.001). The association between increase in MUAC and total maternal weight gain was weaker (r = 0.165, P < 0.001).
In a previous publication, we generated and proposed the use of maternal BMI curves by gestational age to evaluate nutritional status during pregnancy (Calvo et al. 2009). According to this study, maternal BMI by gestational age lower than −1 standard deviation (SD) was related to low‐birthweight infants.
MUAC cut‐off points of 24.5 cm at gestational age of less than 16 weeks, 25.5 cm at 28 weeks and 26.5 cm at 36 weeks of gestation were selected after applying ROC. Sensitivity of these values in relation to a maternal BMI < −1 SD was 88.0%, 81.3% and 85.4%, respectively. Specificity was higher for the cut‐off at the beginning of pregnancy (71.3%) and decreased at the second and third trimesters (69.8% and 63.5%, respectively). These cut‐off points were also a proxy to predict neonatal birthweight lower than 3000 g with sensitivity in the range of 48%–56%. Areas under the ROC curves varied from 60% to 62% for birthweight lowe than 3000 g and from 82% to 87% for prediction of a maternal BMI < −1 SD at different gestational ages.
LMS curves of MUAC, tricipital and subscapular skinfolds for gestational age were calculated using measurements throughout pregnancy of only those women who delivered newborns with birthweights between 2500 and 4000 g (1, 2, 3). LMS curves were based on the serial measurements of 948 women for MUAC, having a total of 3412 individual measurements and 436 women for skinfolds, with 1266 individual points. The programme considers each individual point as cross‐sectional, and even if points are clustered around 4‐week intervals, there is some spread over the entire period. Specifications of the model that provided the best fit to generate the curves were: no age power transformation; degree of freedom (d.f.) (M) = 7; d.f. (S) = 3, and d.f. (L) = 3.
Figure 1.
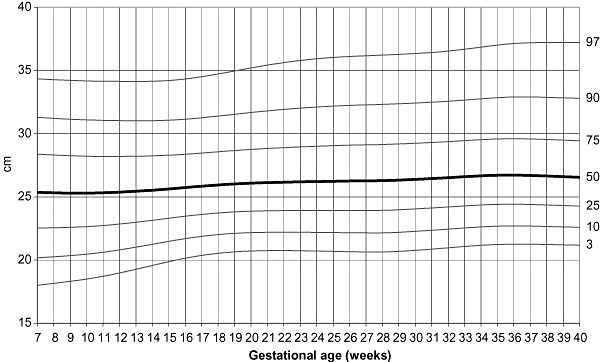
Mid‐upper arm circumference centiles according to gestational age.
Figure 2.
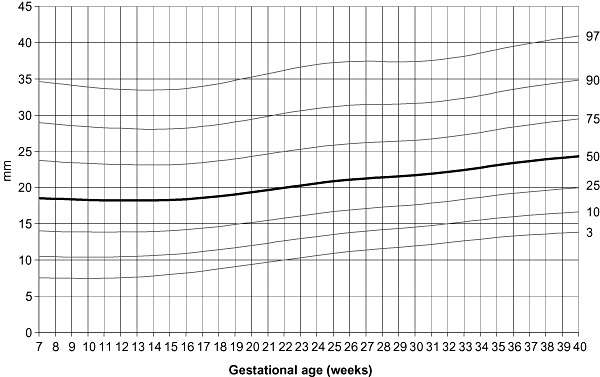
Subscapular skinfold centiles according to gestational age.
Figure 3.
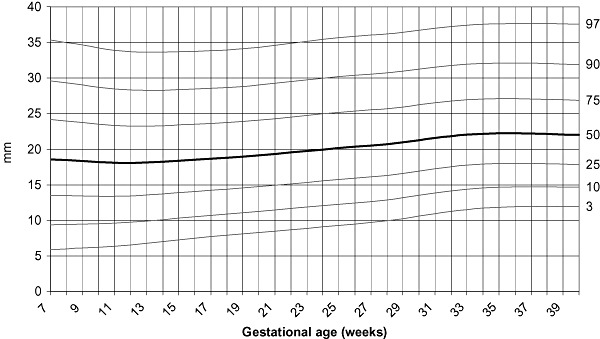
Tricipital skinfold centiles according to gestational age.
Discussion
In developing countries, pregnant women usually initiate their prenatal care after the first months of pregnancy and thus prepregnancy weight can be unknown; in such cases, total weight gain is difficult to determine. Therefore, the value of MUAC has been evaluated in many studies as an alternative or complementary measurement during prenatal care (Pelletier et al. 1995; Ogbonna et al. 2007; Thame et al. 2007). Khadivzadeh studied 2000 healthy women at reproductive age in Iran and found a strong correlation of MUAC with maternal weight and BMI (Khadivzadeh 2002). Olukoya et al.studied the correlation between MUAC and women's weight in Nigeria; they found that a value of MUAC lower than 23 cm had a sensitivity of 85.7% and a positive predictive value of 54.5% for a first trimester weight under 45 kg (Olukoya 1990; Olukoya & Giwa‐Osagie 1991).
Although a positive relationship between MUAC and birthweight has also been reported in many studies (Pelletier et al. 1995; Ricalde et al. 1998; Janjua et al. 2008), there is no consensus about the cut‐off point that can be associated with an increased risk of low or insufficient birthweight, prematurity or inadequate maternal weight gain. In Mozambican pregnant women, MUAC below 25 cm has been proposed as a warning of malnutrition and below 23 cm as a strong indicator of malnutrition. Researchers suggest that, particularly during early pregnancy, MUAC can be a better predictor of prematurity than weight or BMI (Liljestrand & Bergström 1991). Ojha & Malla (2007) found that low birthweight was twice more common in Nepalese pregnant women with MUAC lower than 22 cm, and the same figure has been proposed to identify pregnant women in South Africa who can be at risk of adverse pregnancy outcomes (Kruger 2005).
In our research, we suggest MUAC cut‐off points from 24.5 cm at the first weeks of pregnancy to 26.5 cm at the end of gestation as a proxy to identify both maternal BMI lower than −1 SD and neonatal weight lower than 3000 g. These values were rounded to the next 0.5 cm of the selected point in the ROC curves in order to be easily remembered. Although these figures are higher than those proposed by other researchers based on populations from Africa or Asia, our cut‐off points could be more appropriate to identify low maternal BMI and newborns with insufficient weight in Latin American pregnant women. We have selected a neonatal birthweight lower than 3000 g as an outcome because many studies indicate that birthweights lower than 3000 g are a possible risk factor for developing both undernutrition in late childhood and cardiovascular diseases in adult life (Eriksson et al. 2004; Varela‐Silva et al. 2009). Moreover, our study included only 55 women whose newborns weighed less than 2500 g, making our comparisons less reliable.
Changes in MUAC during pregnancy have been less investigated. In the present study, we observed a mean increase of 1.7 cm in MUAC from <16 to 36 weeks of gestation, this value being higher than the 0.8 cm reported by Mahaba et al. (2001) in pregnant women from Egypt. Other researchers have not found variations in MUAC during gestation and even suggest that MUAC is independent of gestational age (Piperata et al. 2002). Moreover, Krasovec & Anderson (1991) have stated that in developing countries, where pregnancy weight gain is scarce, a consistent decrease in MUAC can be observed, with 70% of women experiencing a loss in MUAC over pregnancy.
In the study by Piperata et al. (2002), which evaluated the anthropometric characteristics of pregnant women in Colombia, mothers of normal‐birthweight newborns (3000 g or more) had a higher MUAC than mothers of low‐birthweight newborns. In our study, average MUAC of mothers of normal‐birthweight infants was almost 2 cm higher during the whole course of pregnancy than that of mothers of low‐birthweight newborns.
There is no published information related to MUAC changes during pregnancy and its relationship with prepregnancy weight or BMI. In our study, the MUAC increase was lower in overweight and obese women, compared with normal‐weight or underweight mothers. In a previous publication (Calvo et al. 2009), we observed that there were no differences in weight gain among women who enter pregnancy with low weight, normal weight or overweight; only those women with a prepregnancy BMI in the range of obesity showed a significantly lower weight gain. A similar pattern of change in MUAC measurements was observed in this study: women who presented overweight or obesity at the start of pregnancy (BMI > 26 kg m2) had lower increases in MUAC compared with women with normal or low BMI.
In our study, we classified women according to early pregnancy BMI (instead of any of the measurements under study), and repeated measurements were made on the whole population. However, in the interpretation of changes in MUAC or skinfolds throughout pregnancy, the phenomenon of regression towards the mean cannot be ruled out.
LMS values from 7 to 40 weeks of gestation for MUAC are presented and can be used as a guide to monitor maternal nutritional status. Nevertheless, although the statistical model applied allows the generation of the proposed smooth curves, only three measurements have been made in each pregnant women, and therefore, more extensive surveys are required before generalization for clinical purposes. Taking into account these limitations, we also propose to use cut‐off points of 24.5 cm at less than 16 weeks of gestation, 25.5 cm at week 28 and 26.5 cm at week 36 as predictive figures to detect both low maternal BMI and low birthweight, pointing out the usefulness of MUAC as an alternative measure when maternal weight gain cannot be calculated. As could be expected, we found that MUAC had a greater association with maternal attained BMI than with neonatal low birthweight, with a sensitivity higher than 85% for maternal BMI below −1 SD and in the range of 48%–56% for insufficient birthweight. Although maternal weight gain and neonatal weight are influenced by many other determinants, the strong relationship between MUAC and maternal weight gain has already been established (Krasovec & Anderson 1991). Therefore, MUAC is still a valuable anthropometric tool for nutritional evaluation, particularly for screening purposes in areas where adequate scales are not available. It is customary in the literature to use measurements in each trimester for evaluating pregnant women nutritional gains; a more detailed schedule could be more sensitive, but changes in MUAC are not of great magnitude to overcome measurement errors in repeated measurements.
Skinfolds are good indirect indicators of subcutaneous body fat, and their measurement can be used to describe the patterns of fat change during pregnancy (Huston Presley et al. 2000). In our study, bicipital, tricipital and subscapular skinfolds were evaluated. A mean increase of 3.6 mm was observed in tricipital skinfold from early pregnancy to 36 weeks of gestation; this figure is higher than the values reported by other researchers who observed changes in the range of 1.1 mm–1.9 mm (Paxton et al. 1998; Mahaba et al. 2001; Sidebottom et al. 2001). Differences could be explained by socio‐economic and demographic characteristics of the populations, and because measurements were performed at different gestational ages.
However, average subscapular skinfold increase in our research (4.5 mm) was similar to that found by Sidebottom et al. (2001) (4.2 mm) and smaller than the 5.9 mm observed by Forsum et al. (1989). In all three studies, the largest increase was observed in subscapular skinfold. These findings support the idea that during pregnancy, central skinfold thicknesses increase more than those at peripheral sites and also that the pattern of changes shows a peak increase at the end of the third trimester.
In this study, all measurements (at 16, 28 and 36 weeks of gestational age) of bicipital, tricipital and subscapular skinfolds from mothers who delivered infants with a birthweight below 3000 g had a lower average than measurements from mothers of normal‐weight newborns; similar findings have been reported by Piperata et al. (2002).
Soltani & Fraser (2000) have postulated that skinfolds increase according to women prepregnancy body weight. In their study, at 6 months post‐partum, obese women had a higher increase in fat mass compared with normal‐weight women. Although they could not find a significant difference in maternal fat mass during pregnancy, probably because of the small sample size, their findings suggest a different pattern of skinfold variation for overweight and obese women compared with normal‐weight women. In our survey, we have observed that in normal‐weight women, the increase in subscapular skinfold from 16 to 36 weeks of gestation was around 2.5 mm higher than in obese women. These findings are again related to our population having a lower than average weight gain in the obese group, with a mean increase of 10.2 kg in contrast with 12.2 kg in normal‐weight women (Calvo et al. 2009).
As opposed to MUAC, in the literature there are no skinfold thickness cut‐off points to be applied during prenatal control. Our data suggest that subscapular and tricipital skinfolds could be used as a proxy to detect low‐birthweight newborns and can be applied as complementary measurements to evaluate maternal nutritional status. As there are no published data from Latin American countries about MUAC or skinfold changes during the course of pregnancy, the LMS values from 7 to 40 weeks of gestation for MUAC, tricipital and subscapular skinfolds, as well as the MUAC cut‐off points obtained from a healthy cohort of pregnant women, are proposed to be used in the region as complementary measurements during prenatal control.
Over the last few years, there has been a notable shift in the demographic and epidemiologic profiles of childbearing women in developed countries. Overweight and obesity prior to pregnancy, and excess gestational weight gain are common nutritional problems. Prepregnancy weight and weight gain during the course of pregnancy are the most reliable anthropometric indicators to monitor nutritional status. In such cases, MUAC can add little information and could be used as a complementary measure.
Skinfold measurements are difficult to standardize and lack the precision required to estimate changes in fat mass accurately. Nevertheless, in this context of preventing post‐partum weight retention, skinfold measurements become an appropriate complementary tool for nutritional assessment besides weight gain. In addition, low skinfold increase during the course of pregnancy could be a proxy of insufficient birthweight.
In developing countries where weight gain monitoring is not feasible because of limitations in facilities, staff and/or coverage of prenatal care, MUAC becomes an alternative tool for anthropometric evaluation. Alternatively, MUAC can be used as a first screen in order to refer women to facilities for a more complete assessment of nutritional risk. As different patterns of MUAC change have been described in diverse settings, MUAC is not recommended for monitoring, but it is a useful tool for screening.
Source of funding
The National Commission ‘Salud Investiga’, Ministry of Health, financially supported this study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors wish to acknowledge Ester Barrientos, Silvina Belingueres, Silvia Garciarena, Carina González, Letizia Hossly and Lucía Quintana for their contribution in data gathering.
References
- Araújo C.L., Hallal P.C., Nader G.A., Neutzling M.B., Defátima Vieira M., Menezes A.M. et al. (2009) Effect of birth size and proportionality on BMI and skinfold thickness in early adolescence: prospective birth cohort study. European Journal of Clinical Nutrition 63, 627–633. [DOI] [PubMed] [Google Scholar]
- Calvo E.B., López L.B., Balmaceda Y., Poy M.S., González C., Quintana L. et al. (2009) Reference charts for weight gain and body mass index during pregnancy obtained from a healthy cohort. Journal of Maternal-Fetal & Neonatal Medicine 22, 36–42. [DOI] [PubMed] [Google Scholar]
- Cole T.J. (1990) The LMS method for constructing normalized growth standards. European Journal of Clinical Nutrition 44, 45–60. [PubMed] [Google Scholar]
- Davison J.M. (1997) Edema in pregnancy. Kidney International Supplement 59, S90–S96. [PubMed] [Google Scholar]
- Eriksson M., Wallander M.A., Krakau I., Wedel H. & Svärdsudd K. (2004) Birth weight and cardiovascular risk factors in a cohort followed until 80 years of age: the study of men born in 1913. Journal of Internal Medicine 255, 236–246. [DOI] [PubMed] [Google Scholar]
- Forsum E., Sadurskis A. & Wager J. (1989) Estimation of body fat in healthy Swedish women during pregnancy and lactation. The American Journal of Clinical Nutrition 50, 465–473. [DOI] [PubMed] [Google Scholar]
- Huston Presley L., Wong W.W., Roman N.M., Amini S.B. & Catalano P.M. (2000) Anthropometric estimation of maternal body composition in late gestation. Obstetrics and Gynecology 96, 33–37. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Subcommittee on Nutritional Status and Weight Gain During Pregnancy (1990) Nutrition During Pregnancy. Part I: Weight Gain. Food and Nutrition Board, National Academy of Sciences, National Academy Press: Washington, DC. [Google Scholar]
- James W.P., Mascie‐Taylor G.C., Norgan N.G., Bistrian B.R., Shetty P.S. & Ferro‐Luzzi A. (1994) The value of arm circumference measurements in assessing chronic energy deficiency in Third World adults. European Journal of Clinical Nutrition 48, 883–894. [PubMed] [Google Scholar]
- Janjua N.Z., Delzell E., Larson R.R., Meleth S., Kristensen S., Kabagambe E. et al. (2008) Determinants of low birth weight in urban Pakistan. Public Health Nutrition 11, 1–10. [DOI] [PubMed] [Google Scholar]
- Kelly A., Kevany J., de Onis M. & Shah P. (1996) A WHO collaborative study of maternal anthropometry and pregnancy outcomes. International Journal of Gynaecology and Obstetrics 53, 219–233. [DOI] [PubMed] [Google Scholar]
- Khadivzadeh T. (2002) Mid upper arm and calf circumferences as indicators of nutritional status in women of reproductive age. Eastern Mediterranean Health Journal 8, 612–618. [PubMed] [Google Scholar]
- Krasovec K. & Anderson M. (1991) Maternal Nutrition and Pregnancy Outcomes. Anthropometric Assessment. Pan American Health Organization and World Health Organization: Washington, DC. Scientific Publication No. 529. [Google Scholar]
- Kruger H.S. (2005) Maternal anthropometry and pregnancy outcomes: a proposal for the monitoring of pregnancy weight gain in outpatient clinics in South Africa. Curationis 28, 40–49. [DOI] [PubMed] [Google Scholar]
- Licitra L., Pregazzi R., Troiano L. & Guaschino S. (1998) Anthropometric skin‐fold and impedance measurement assessment of the maternal nutritional status at the end of pregnancy. Comments deduced from a study of 54 cases. Minerva Ginecologica 50, 379–382. [PubMed] [Google Scholar]
- Liljestrand J. & Bergström S. (1991) Antenatal nutritional assessment: the value of upper arm circumference. Gynecologic and Obstetric Investigation 32, 81–83. [DOI] [PubMed] [Google Scholar]
- Mahaba H.M., Ismail N.A., El Teheiwy M.M., El‐Goewily M.M. & Ramadan M.S. (2001) Development of weight gain charts for healthy Egyptian pregnant women. Journal of the Egyptian Public Health Association 76, 369–391. [PubMed] [Google Scholar]
- Ogbonna C., Woelk G.B., Ning Y., Mudzamiri S., Mahomed K. & Williams M.A. (2007) Maternal mid‐arm circumference and other anthropometric measures of adiposity in relation to infant birth size among Zimbabwean women. Acta Obstetricia et Gynecologica Scandinavica 86, 26–32. [DOI] [PubMed] [Google Scholar]
- Ojha N. & Malla D.S. (2007) Low birth weight at term: relationship with maternal anthropometry. Journal of the Nepal Medical Association 46, 52–56. [PubMed] [Google Scholar]
- Olukoya A.A. (1990) Identification of underweight women by measurement of the arm circumference. International Journal of Gynaecology and Obstetrics 31, 231–235. [DOI] [PubMed] [Google Scholar]
- Olukoya A.A. & Giwa‐Osagie O.F. (1991) Maternal weight and weight gain during pregnancy–can the arm circumference be used as surrogate? African Journal of Medicine and Medical Sciences 20, 155–162. [PubMed] [Google Scholar]
- Paxton A., Lederman S.A., Heymsfield S.B., Wang J., Thornton J.C. & Pierson R.N. Jr (1998) Anthropometric equations for studying body fat in pregnant women. The American Journal of Clinical Nutrition 67, 104–110. [DOI] [PubMed] [Google Scholar]
- Pelletier D., Arimond M., Johnson F.C., Liang E., Low J., Mvula P. et al. (1995) Maternal anthropometry predictors of intrauterine growth retardation and prematurity in the Malawi maternal and child nutrition study. Bulletin of the World Health Organization 73 (Suppl.), 80–81. [PMC free article] [PubMed] [Google Scholar]
- Piperata B.A., Dufour D.L., Reina J.C. & Spurr G.B. (2002) Anthropometric characteristics of pregnant women in Cali, Colombia and relationship to birth weight. The American Journal of Human Biology 14, 29–38. [DOI] [PubMed] [Google Scholar]
- Reynolds D. (2003) Severe gestational edema. Journal of Midwifery & Women's Health 48, 146–148. [DOI] [PubMed] [Google Scholar]
- Ricalde A.E., Velásquez‐Meléndez G., Tanaka A.C. & de Siqueira A.A. (1998) Mid‐upper arm circumference in pregnant women and its relation to birth weight. Review of Saude Publica 32, 112–117. [DOI] [PubMed] [Google Scholar]
- Sidebottom A.C., Brown J.E. & Jacobs D.R. Jr (2001) Pregnancy‐related changes in body fat. European Journal of Obstetrics, Gynecology, and Reproductive Biology 94, 216–223. [DOI] [PubMed] [Google Scholar]
- Soltani H. & Fraser R.B. (2000) A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. British Journal of Nutrition 84, 95–101. Erratum in: British Journal of Nutrition 84(6), 947. [DOI] [PubMed] [Google Scholar]
- Thame M., Trotman H., Osmond C., Fletcher H. & Antoine M. (2007) Body composition in pregnancies of adolescents and mature women and the relationship to birth anthropometry. European Journal of Clinical Nutrition 61, 47–53. [DOI] [PubMed] [Google Scholar]
- Varela‐Silva M.I., Azcorra H., Dickinson F., Bogin B. & Frisancho A.R. (2009) Influence of maternal stature, pregnancy age, and infant birth weight on growth during childhood in Yucatan, Mexico: a test of the intergenerational effects hypothesis. American Journal of Human Biology 21, 657–663. [DOI] [PubMed] [Google Scholar]
- Zekan J., Buković D., Djelmis J., Ivanisević M. & Kopljar M. (1998) Assessment of the nutritional status in Croatian pregnant women by measuring skinfolds. Collegium Antropologicum 22, 637–649. [PubMed] [Google Scholar]


