Abstract
Iodine deficiency and excess are both associated with adverse health consequences, with fetuses, children and pregnant women being most vulnerable to the devastating effects of severe deficiency. It is often assumed that the iodine status of a population if displaced or in a remote or emergency situation is low. However, there is little evidence available to support this assumption, especially among long‐term food‐aid‐dependent pregnant women. An effectiveness trial of a prenatal multiple‐micronutrient supplement that contained 150 µg day−1 iodine was conducted in two refugee camps in the North Eastern Province of Kenya in 2002. Urinary iodine concentration (UIC) was measured in a subsample of pregnant women attending antenatal care in Dagahaley (control camp) (n = 74) and Ifo (intervention camp) (n = 63). There was no significant difference in median UIC between the two camps (P = 0.118). The combined median UIC was 730 µg L−1 (interquartile range, 780) (5.77 µmol L−1) and exceeded the upper safe limit of 500 µg L−1 (3.95 µmol L−1) for pregnant women (P < 0.001), indicating excessive iodine intake. About 20% of the study subjects had ‘more than adequate’ urinary iodine, while over 71% had excessive UIC. Salt iodine content varied between 5.1 and 80.1 ppm in the five market salt samples analysed. In conclusion, excessive iodine intake was evident in the Dadaab refugee camps. Further research needs to be conducted to investigate the source of excess iodine, to determine the measures needed to address excessive iodine intake and to reconsider the World Health Organization/World Food Programme/United Nations Children's Fund guidance on supplementation of vulnerable groups in emergencies.
Keywords: iodine, maternal nutrition, refugees, food aid
Introduction
Iodine is essential for the synthesis of thyroid hormones that regulate metabolic activities and brain development during fetal and post‐natal life (Delange 2000; Andersson et al. 2005). Deficiency of this element leads to a range of disorders commonly referred to as iodine deficiency disorders (IDD), which includes irreversible mental retardation. Universal salt iodization has been recommended as the most cost‐effective, safe and sustainable strategy for iodine supplementation (WHO/UNICEF/ICCIDD 2007). However, balancing iodine intake and the risk of health problems is delicate, with both extreme high and low levels of intake having adverse health consequences. This results in a narrow optimal range of iodine nutrition (Laurberg et al. 2001; Zhao 2001; Delange 2002). Excessive iodine intake has been associated with goitre and with both hypo‐ and hyperthyroidism (Thomopoulos 2005; Teng et al. 2006). The most severe consequences are iodine‐induced hyperthyroidism, which may lead to death due to cardiac problems in vulnerable individuals (Dunn et al. 1998). While the public health benefits of iodine fortification exceed the health risks of iodine excess, the need for careful attention to minimize the risk of excessive iodine intake is clear.
In Africa, good progress has been made towards the elimination of IDD through increased household utilization of iodized salts (ICCIDD 2003). About 70% of the households in developing countries are covered with iodized salt, which has led to a significant reduction in IDD (WHO/UNICEF/ICCIDD 2007). Kenya is considered to have attained 91% coverage of household iodized salt use. Despite this, pockets of iodine deficiency still exist, and the median urinary iodine concentration (UIC) has been estimated to be around 118 µg L−1 (0.93 µmol L−1), with about 36% of the population being iodine deficient (UIC < 100 µg L−1) (0.79 µmol L−1) (WHO 2004). The iodine status of the population in Somalia, where the refugees included in this study originated, is unknown. However, countries in conflict situations typically have less than 20% coverage, as salt iodization and distribution is often hindered by conflict (WHO 2004).
Despite data indicating residual iodine deficiency, there is also evidence of emerging excessive intake in some African countries. Surveys have shown excessive UIC in Liberia and Uganda, and ‘more than adequate’ UIC in Rwanda, Democratic Republic of Congo, Mali, Niger, Benin, Botswana and Zimbabwe (WHO 2004). Studies done in six African refugee camps in East, North and Southern Africa have also shown excessive median UIC among adolescent populations in five out of the six camps, where concentrations ranged from 570 to 1170 µg L−1 (4.50 to 9.24 µmol L−1) (Seal et al. 2006). Although the iodine status of adolescents does not necessarily mirror the status of pregnant women (Stilwell et al. 2008), it does provide an indication of widespread excessive iodine intake in African refugees. No adverse health consequences associated with excessive iodine intake have so far been reported in these refugee populations; however, underdiagnosis due to lack of knowledge and an ill‐equipped health infrastructure remains a possibility.
Pregnant and lactating women, and children are considered the most vulnerable to iodine deficiency. In 2007, the World Health Organization (WHO), the World Food Programme (WFP) and the United Nations Children's Fund (UNICEF) issued two joint statements, calling for optimal iodine nutrition and micronutrient supplementation of these groups in emergencies (WHO/UNICEF 2007; WHO/WFP/UNICEF 2007). The daily iodine supplementation recommended for pregnant women was 250 µg, and the joint statement states that it is to be given in all emergency situations, independently of whether fortified salt is available. Many refugee camps, including Dadaab in Northeast Kenya, fit within the definition of ‘emergency’ adopted by the joint statement. Their populations could therefore be considered as candidates for additional iodine supplementation if the recommendations of the joint statement were implemented.
The refugees in Dadaab camps depend on international organizations for food and non‐food items. Food aid items included maize, beans, oil and salt. The exact iodine content of these foods is not known, but a typical daily ration of 2100 kcal is expected to provide 150–200 µg iodine. This is consistent with daily requirements for the general population but below the daily requirement of 250 µg for pregnant and lactating women (WHO/UNICEF/ICCIDD 2007). A community intervention trial was conducted in 2001–2002 to evaluate the effectiveness of a multiple‐micronutrient supplement in improving the nutritional status of pregnant women. To assess the adequacy of iodine intake in women receiving and not receiving the supplement, we measured the UIC of a subsample attending antenatal care clinics.
Key messages
-
•
Iodine is an essential nutrient for fetal development and maternal health, but excessive intakes may be harmful.
-
•
Current policy guidance assumes that populations affected by nutritional emergencies require blanket supplementation with iodine.
-
•
The finding of excessive intakes in pregnant Somali refugees who were dependent on international food aid calls for a reassessment of the policy on iodine supplementation during emergencies.
Materials and methods
Study site
The Dadaab refugee camps are in the North Eastern Province of Kenya and border Somalia's lower Juba Region in the East, and Kenya's Wajir and Garissa Districts in the north and west, respectively. They were established in early 1991 after the collapse of the Somali government and mainly host refugees from Somalia, with some smaller groups from Ethiopia and Sudan. The camps are located 18 km apart and cover an area of about 50 km2, with a semiarid climate, sparse vegetation and little surface water. At the time of the study (June 2002), the total population of the three camps was estimated to be approximately 130 000, with 37 000 in Dagahaley, 48 000 in Hagardera and 45 000 in Ifo (SCN 2003). About 90% of the refugees depended on relief food aid and non‐food items provided by international organizations. Two of the three refugee camps in Dadaab, Ifo and Dagahaley were selected for the study based on their similar health and demographic profiles, and logistic feasibility.
Sampling method
The iodine study reported in this paper was part of a larger intervention trial conducted between 2001 and 2002 among pregnant women. This intervention compared the effectiveness of a multiple‐micronutrient supplement, containing 15 vitamins and minerals (including iodine), with the standard supplements of iron, folate and vitamin C, which were provided by the health service provider, Medecins Sans Frontieres‐Belgium. Pregnant women in the Ifo refugee camp (intervention camp) who attended the antenatal care clinics were provided with the trial supplement tablet that contained 150 µg of iodine per day, while in Dagahaley (control camp), no supplemental iodine was provided. Women enrolled in the antenatal care programme between 16 and 24 weeks of pregnancy and would therefore have been receiving supplements for an average of 2 months prior to participation in this study. The sampling method was based on simple random sampling of pregnant women attending the antenatal clinics. WHO recommends the collection of 30 urine samples from each group to assess iodine nutritional status (WHO/UNICEF/ICCIDD 2007), but generally, the larger the number of samples, the better the precision achieved (Andersen et al. 2008). A total of 137 urine samples were collected, with 74 and 63 samples from Dagahaley and Ifo, respectively. Gestational age was estimated using fundal height.
Ethical approval
The study was approved by the Refugee Health and Nutrition Committee under the auspices of the Kenyan Ministry of Health and supported by the Research Ethics Committee of the Institute of Child Health, University College London. Signed consent was obtained from all participants who accepted to take part in the study. It was emphasized that participants could withdraw from the study at anytime they wanted without affecting their rights to health care. A series of meetings with camp elders, leaders and stakeholders were held prior to the start of the study to explain the procedures and to seek acceptance for the study. All the study records were kept in locked cabinets at the study main office in Dadaab.
Collection of urine
Urine samples were collected from randomly selected pregnant women attending the antenatal clinics in the camps. Women were provided with a 100‐mL urine container to collect urine samples. Urine samples were collected before midday and then transferred into labelled screw‐capped 10‐mL collection tubes (Urine‐Monovette, Sarstedt Ltd, Nümbrecht, Germany) at the health post. Following transfer to the laboratory, they were frozen at −10°C and shipped in dry ice to London. Urine samples from four volunteer staff were also collected for analysis, and each of these samples was divided into three aliquots with unique sample numbers to permit assessment of laboratory analytical performance. All urine samples were analysed in Belgium at the Department of Clinical Chemistry, Saint‐Pierre Hospital, Brussels.
Laboratory analysis
Urine samples were analysed using a Technicon Autoanalyser II (Technicon Corporation). Analysis involved digestion of the urine samples with strong acid in the presence of ceric ammonium sulfate. Reduction of the yellow ceric ions by iodide leads to the production of colourless cerous ions. The decrease in absorbance was measured at 410 nm (Belling 1982; Dunn et al. 1993). Urine samples containing interfering substances were oxidized by alkaline ashing after adding 1 mL of 1 M potassium hydroxide to 3 mL aliquots of urine. Samples with a high iodine concentration were diluted and reanalysed (Belling 1982).
Within‐subject precision was measured by the inclusion of four volunteer quality‐control urine samples (three aliquots of each) and to which the analyst was not aware. The acceptable within‐subject coefficient of variation (CV %) was set at 10%. To check the accuracy of the analysis, a subsample of the urine samples was reanalysed using an inductively coupled plasma–mass spectrometry method.
Analysis of salt
Salt iodine content was determined through a titration method (Mannar 1995). Iodine was liberated by the addition of sulfuric acids and titrated with sodium thiosulphate using starch as an indicator. All salt samples were analysed in duplicate or triplicate, and those containing large crystals were ground with a pestle and mortar prior to titration. The within‐sample acceptable CV % was set at 10%. The within‐sample CV % achieved ranged from 0.2% to 5.5%.
Data analysis
Data entry and analysis was done using SPSS version 11 (SPSS Inc., Chicago, IL), Excel 2003 (Microsoft Corporation, Redmond, WA) and Epi Info 6.04d (Centers for Disease Control and Prevention, Atlanta, GA). Classification of severity was based on the WHO urinary iodine cut‐offs for pregnant women: iodine deficiency, <150 µg L−1 (1.19 µmol L−1); sufficient, 150–249 µg L−1 (1.19–1.97 µmol L−1); more than adequate, 250–499 µg L−1 (1.98–3.94 µmol L−1); excessive, >500 µg L−1 (3.95 µmol L−1) (WHO/UNICEF/ICCIDD 2007).
A Mann–Whitey test was used to test for differences between the median UIC in the two camps. A sign test was used to determine if the median UIC exceeded the recommended upper safe limit of 500 µg L−1 (3.95 µmol L−1). The level of significance was taken as P < 0.05 (Kirkwood 1988). A box plot was used to compare the UIC of the two camps and explore the distribution of the UIC.
Results
Demographic characteristics of the two refugee camps are given in Table 1. Both groups had similar characteristics, except the middle upper arm circumference, which differed significantly between the two camps (P = 0.001). The mean gestational age for the combined camps was 28.8 weeks and ranged from 16 to 40 weeks. There was no significant relationship found between UIC and any demographic variables.
Table 1.
Demographic characteristics of study participants*
| Characteristics | Dagahaley (n = 73) | Ifo (n = 62) |
|---|---|---|
| Age | 24.2 ± 6.7 | 26.1 ± 5.9 |
| Parity | 3.3 ± 2.8 | 3.5 ± 2.6 |
| Gestational age (weeks) † | 29.1 ± 6.7 | 28.4 ± 7.0 |
| BMI (kg m−2) | 22.4 ± 2.9 | 21.3 ± 2.4 |
| MUAC (cm) ‡ | 25.0 ± 3.0 | 23.5 ± 2.0 |
| HB (g dL−1) § | 10.4 ± 1.9 | 10.0 ± 1.6 |
BMI, body mass index; HB, haemoglobin; MUAC, middle upper arm circumference. Data are shown as means ± SD.
† Estimated by fundal height.
Means differ significantly (P = 0.001).
n = 61 in Ifo.
Figure 1 shows the distribution of the UIC of the two study camps. Pregnant women from Ifo camp had a median UIC of 845 µg L−1 (IQR 960) (6.68 µmol L−1), while the Dagahaley group had a median of 660 µg L−1 (IQR 718) (5.21 µmol L−1). However, there was no significant difference in median UIC between the two camps (P = 0.118). The UIC of the combined groups had a median of 730 µg L−1 (IQR 780) (5.77 µmol L−1), which exceeded the WHO upper safe limit of 500 µg L−1 (3.95 µmol L−1) (P < 0.001). Grouping the data into the various WHO cut‐off categories showed that 20.4% of the study population had UIC considered ‘more than adequate’, while almost three quarters of the study subjects (71.5%) had excessive UIC that exceeded the upper safe limit of 500 µg L−1 (3.95 µmol L−1).
Figure 1.
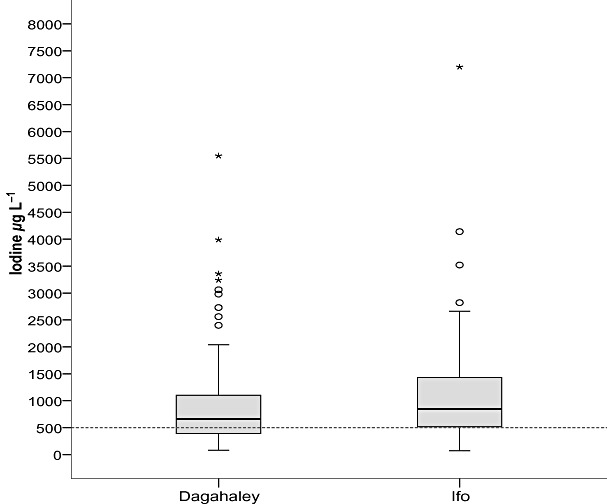
Box plot of urinary iodine concentration from pregnant women in Dagahaley and Ifo refugee camps. The World Health Organization recommended safe upper limit of 500 µg L−1 is shown with a dashed line. The International System of Units (SI) concentration conversion factor applied was 1 µg L−1 = 0.0079 µmol L−1 iodine. Outliers more than 1.5 interquartile ranges (IQRs) from the end of the box are denoted by circles, and outliers more than 3 IQRs from the end of the box are denoted by an asterisk (Pallant 2005).
The iodine concentrations of five salt samples, sampled from the three camp markets (Dagahaley, Ifo and Hagardera), were measured by titration and ranged between 5.9 and 78.5 ppm, with a mean of 50.7 ppm. Four samples exceeded the WHO‐recommended iodine concentration of 20–40 ppm, but all were less than 100 ppm, the mandatory upper salt iodine concentration in Kenya.
Discussion
This is the first study to investigate the iodine status of pregnant women in long‐term food‐aid‐dependent refugee camps. Previous studies in African refugee camps have investigated the iodine status of adolescents (Seal et al. 2006), but this does not necessarily reflect the iodine status of pregnant mothers (Stilwell et al. 2008). The current study showed no significant difference in UIC associated with the iodine supplementation of pregnant women. However, excessive iodine intake was demonstrated within this population with extremely high UIC in some individual samples.
The CV % within the quality‐control urine samples was less than 10% and ranged from 2.7% to 7.8%, indicating good laboratory precision. Collection techniques for urine samples in Kenya were done under strictly controlled conditions to avoid evaporation and contamination that could affect the iodine concentration. Validation of the colorimetric analytical technique performed using a reference inductively coupled plasma–mass spectrometry gave comparable results (regression equation: y = 0.895x + 6.43; R 2 = 0.998) (Seal et al. 2006) and confirmed the high level of iodine excretion.
Iodine urinary excretion, via the kidneys, is considered to be the best indicator of iodine intake. However, careful interpretation of pregnant women's UICs are important as ioduria during pregnancy is expected to increase due to elevated glomerula filtration. This physiological phenomenon forms the basis of the higher upper safe limit of 500 µg L−1 (3.95 µmol L−1) recommended for this population group and used in this study, compared with the upper limit of 300 µg L−1 (2.37 µmol L−1) used for other groups (WHO/UNICEF/ICCIDD 2007).
In this study of micronutrient supplementation in antenatal care, pregnant women in the Ifo camp received 150 µg per day more iodine than those in Dagahaley, the control camp. The median UIC was 185 µg L−1 (1.46 µmol L−1) higher in Ifo, which is similar to the daily supplemental dose. However, the difference in the medians was not significant. This may be related to the very high background concentrations of consumed iodine as well as the relatively small sample size. Over 90% and 93% of the study subjects in the intervention and control camps, respectively, had a UIC that was ‘more than adequate’ or excessive.
Although our finding of excessive iodine intake in African refugees is surprising, it is by no means unprecedented. Studies performed by WHO between 1993 and 2003 in 34 African countries have shown at least nine countries with a median UIC more than adequate, with excessive UICs in Uganda and Liberia (WHO 2004). This contrasts with the situation in Kenya where the median UIC was estimated to be 118 µg L−1 (0.93 µmol L−1) and where 36% of the population were iodine deficient (urinary iodine <100 µg L−1) (0.79 µmol L−1) (ICCIDD 2003). There is no published data on iodine status in Somalia, where the refugees originated. The question raised by this study is whether the high UIC is only a problem in refugee settings within Kenya, due to specific sources of iodine, or whether it is a wider problem that affects other regions of the country. A more systematic and representative survey both within the country and in the refugee camps is necessary to determine this.
One obvious source of dietary iodine in this population is the iodized salt found in the general food aid ration. It was assumed that the salt distributed in the refugee camps is obtained from manufacturers in Kenya. Random salt samples collected from the markets in the refugee camps showed that iodized and non‐iodized salts were available. The salt iodine content was well above the WHO recommended level of 20–40 ppm in iodized salt samples. This finding is also in agreement with previous studies in Kenya where an iodine content of about 100 ppm was reported (ICCIDD 2003). However, it is considered unlikely that salt iodine alone can account for this high UIC, and other possible sources could also coexist. Other possible sources include the drinking water and other food items supplied to the refugees. Further work is required to establish which of these sources are responsible.
Our data show that iodine intake can be excessive in populations regarded as remote, displaced and in an emergency setting. The need for initial urinary iodine assessment will be important in the future before supplementation of such populations. Where such assessments are not possible due to lack of resources or capacity, a review of the most recently available data from comparable populations should be carried out. Regular monitoring of salt iodization programmes should also be ensured. Iodine excess can be harmful, particularly in populations that may be initially deficient, and the most severe consequences of iodine‐induced hyperthyroidism have been well documented in Africa (Todd et al. 1995; Bourdoux et al. 1996). With rudimentary health care services and poor economic conditions in remote areas, the adverse effects of iodine excess could be severe and damaging.
In conclusion, this study shows that excessive iodine nutrition can occur in long‐term refugee contexts in the absence of any supplemental iodine. However, blanket additional supplementation of such populations is currently recommended by the WHO/WFP/UNICEF joint statement, which may lead to an elevated risk of adverse effects (WHO/WFP/UNICEF 2007). This study highlights the need for careful, evidence‐based decision making prior to any supplementation interventions. Routine assessment of iodine status should be considered before implementing any additional supplementation programs in ‘specific situation’ areas. Urgent priority should be given to investigate the sources of iodine excess identified in this study, which may originate from food aid or other sources. As an interim measure, governments should be encouraged to adapt and enforce their legislation to ensure adherence to the safe limits recommended by WHO for salt iodization (WHO/UNICEF/ICCIDD 1996; WHO 2008).
Source of funding
This work was funded by United Nations High Commissioner for Refugees, Geneva via a grant from the United Nations Fund for International Partnership.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors are grateful to the staff of the United Nations High Commissioner for Refugees Branch and Sub‐Offices. We thank MSF‐B for their organization, logistic and administrative support. We also thank the local and international staff from collaborating non‐governmental organizations for their support during this study. We also want to thank the refugee women who participated in the study; without the support of the refugee communities, this work would not have been possible.
References
- Andersen S., Karmisholt J., Pedersen K.M. & Laurberg P. (2008) Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. The British Journal of Nutrition 99, 813–818. [DOI] [PubMed] [Google Scholar]
- Andersson M., Takkouche B., Egli I., Allen H.E. & de Benoist B. (2005) Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bulletin of the World Health Organization 83, 518–525. [PMC free article] [PubMed] [Google Scholar]
- Belling G.B. (1982) Determination of Iodine. CSIRO Division of Human Nutrition: Adelaide. [Google Scholar]
- Bourdoux P., Ermans A.M., Mukalay W.A., Filetti S. & Vigneri R. (1996) Iodine induced thyrotoxicosis in Kiwu Zaire. Lancet 347, 552–553. [DOI] [PubMed] [Google Scholar]
- Delange F. (2000) The role of iodine in brain development. The Proceedings of the Nutrition Society 59, 75–79. [DOI] [PubMed] [Google Scholar]
- Delange F. (2002) Iodine deficiency in Europe and its consequences: an update. European Journal of Nuclear Medicine and Molecular Imaging 29, 404–416. [DOI] [PubMed] [Google Scholar]
- Dunn J.T., Crutchfield H.E., Gutekunst R. & Dunn A.D. (1993) Methods for Measuring Iodine in Urine. ICCIDD: The Netherlands. [DOI] [PubMed] [Google Scholar]
- Dunn J.T., Semigran M.J. & Delange F. (1998) The prevention and management of iodine‐induced hyperthyroidism and its cardiac features. Thyroid 8, 101–106. [DOI] [PubMed] [Google Scholar]
- ICCIDD (2003) Iodine Nutrition in Africa. International Council for the Control of Iodine Deficiency Disorders; IDD Newsletter 19, pp. 1–6. [Google Scholar]
- Kirkwood B.R. (1988) Essentials of Medical Statistics. Blackwell Publishing Ltd: Massachusetts. [Google Scholar]
- Laurberg P., Bulow P.I., Knudsen N., Ovesen L. & Andersen S. (2001) Environmental iodine intake affects the type of non‐malignant thyroid disease. Thyroid 11, 457–469. [DOI] [PubMed] [Google Scholar]
- Mannar V. (1995) Salt Iodization for the Elimination of Iodine Deficiency. International Council for Control of Iodine Deficiency Disorders: The Netherlands. [Google Scholar]
- Pallant J. (2005) SPSS Survival Manual 2nd Edition: A Step by Step Guide to Data Analysis Using SPSS Version 12. Open University Press: Maidenhead. [Google Scholar]
- SCN (2003) Report on the Nutritional Situation of Refugee and Displaced Populations. United Nations Sub‐Committee on Nutrition, World Health Organization: Geneva. [Google Scholar]
- Seal A.J., Creeke P.I., Gnat D., Abdalla F. & Mirghani Z. (2006) Excess dietary iodine intake in long‐term African refugees. Public Health Nutrition 9, 35–39. [DOI] [PubMed] [Google Scholar]
- Stilwell G., Reynolds P.J., Parameswaran V., Blizzard L., Greenaway T.M. & Burgess J.R. (2008) The influence of gestational stage on urinary iodine excretion in pregnancy. The Journal of Clinical Endocrinology and Metabolism 93, 1737–1742. [DOI] [PubMed] [Google Scholar]
- Teng W., Shan Z., Teng X., Guan H., Li Y., Teng D. et al (2006) Effects of iodine intake on thyroid diseases in China. The New England Journal of Medicine 354, 2783–2793. [DOI] [PubMed] [Google Scholar]
- Thomopoulos P. (2005) Iodine excess and thyroid dysfunction. La Revue du Praticien 55, 180–182. [PubMed] [Google Scholar]
- Todd C.H., Allain T., Gomo Z.A.R., Hasier J.A., Ndiweni M. & Oken E. (1995) Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet 346, 1563–1564. [DOI] [PubMed] [Google Scholar]
- WHO (2004) Iodine Status Worldwide: WHO Global Database on Iodine Deficiency. World Health Organisation: Geneva. [Google Scholar]
- WHO (2008) Salt as a Vehicle for Fortification. Report of a WHO Expert Consultation. World Health Organisation: Geneva. [Google Scholar]
- WHO/UNICEF (2007) Joint Statement by the World Health Organization and the United Nations Children's Fund: Reaching Optimal Iodine Nutrition in Pregnant and Lactating Women and Young Children. World Health Organization: Geneva. [Google Scholar]
- WHO/UNICEF/ICCIDD (1996) Recommended Iodine Levels in Salt and Guidelines for Monitoring Their Adequacy and Effectiveness. WHO/NUT 96.13. World Health Organization: Geneva. [Google Scholar]
- WHO/UNICEF/ICCIDD (2007) Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd edn. World Health Organization: Geneva. [Google Scholar]
- WHO/WFP/UNICEF (2007) Joint Statement by the World Health Organization, the World Food Programme and the United Nations Children's Fund: Preventing and Controlling Micronutrient Deficiencies in Populations Affected by an Emergency. World Health Organization: Geneva. [Google Scholar]
- Zhao J. (2001) Iodine Deficiency and Iodine Excess in Jiangsu Province, China. Thesis Wageningen University: Wageningen. [Google Scholar]


