Abstract
A range of compounds with negative nutritional impact – ‘anti‐nutrients’ – are found in most plant foods. The contents of anti‐nutrients in processed foods depend on the ingredients and processing. Anti‐nutrients in complementary foods for children can have a negative impact on nutritional status. The aim of this study was to screen complementary foods from developing countries for the anti‐nutritional compounds, phytate, polyphenols, inhibitors of trypsin and chymotrypsin, and lectins. Commercial products based on whole grain cereals were included as a ‘worst‐case’ scenario for anti‐nutrient exposure in Europe. Contents of minerals (iron, zinc and calcium), in which absorption or utilisation is affected by anti‐nutrients, were analysed. Thirty‐six products representing foods used in food aid programmes, local blended foods, fortified instant porridges and ‘baby foods’ were analysed. The content of minerals indicated that the fortification of a number of products did not meet the declared levels of iron, zinc and calcium. The phytate content ranged from 68 to 1536 mg/100 g, confirming a persistent problem of high levels of phytate in processed cereal‐ and legume‐based products. The phytate : Fe molar ratio exceeded the recommended level of <1.0 in 32 of the 36 products. The total polyphenols varied from 1.3 to 9.3 mg gentisic acid equivalents g−1. Screening low‐molecular weight soluble polyphenols may be more relevant in complementary foods than total polyphenolic compounds. Trypsin and chymotrypsin inhibitors and lectins were found in residual amounts in most products, indicating efficient degradation by heat processing. However, young infants and malnourished children may have reduced pancreatic function, and upper limits for residual trypsin inhibitors are needed.
Keywords: minerals, phytate, polyphenols, trypsin inhibitor, lectins
Introduction
Anti‐nutritional compounds exist as a natural component of cereals, legumes and other plant foods. Owing to the relatively high nutrient requirements of children from 6 to 24 months of life, anti‐nutrients in complementary foods can negatively affect the nutritional status of these children. However, complementary foods are seldom analysed for the contents of anti‐nutrients. Among the anti‐nutritional compounds of greatest importance to be mapped in complementary foods are phytate (or phytic acid1 ), polyphenols, inhibitors of pancreatic enzymes and lectins.
Phytate [inositol hexaphosphate (IP6)] is widely present in plant‐based foods. IP6 chelates with divalent metal ions and hereby interferes with the absorption or utilisation of important minerals, especially iron, zinc and calcium. It is well documented that even small amounts of phytate in food will significantly reduce iron absorption (Davidsson et al. 1997; Hurrell 2004). Phytate contents in cereals and legumes can be reduced either by mechanical removal of the phytate‐containing parts of the seeds (e.g. the germ in maize or the hull in beans) or by enzymatic degradation by phytases during food processing and digestion. Inositol pentaphosphate (IP5) also binds with minerals, although more weakly than phytate. Inositol phosphates with smaller numbers of phosphate groups – IP4 and lower inositol phosphates – have little or insignificant anti‐nutritional impact on mineral absorption (Brune et al. 1992). Phosphorus bound in inositol phosphates is almost unavailable for absorption.
An early study showed that solubility of proteins in peanut was inhibited by phytate (Fontaine et al. 1946). Later animal studies have confirmed the tendency of negative impact of phytate on protein utilisation (Rham & Jost 1979; Boling‐Frankenbach et al. 2001; Pontoppidan et al. 2007), although this was not confirmed in human studies.
Recent analyses of both indigenous and processed complementary foods in developing countries found that phytate concentrations were highest in complementary foods based on unrefined cereals and legumes (∼600 mg/100 g dry weight), followed by refined cereals (∼100 mg/100 g dry weight) and then starchy roots and tubers (<20 mg/100 g dry weight) (Gibson et al. 2010). Phytate content is evaluated as molar ratios to iron, zinc and calcium. To efficiently minimise mineral absorption inhibition, the molar ratios for phytate : Fe should be less than 1.0 (Hurrell 2004), phytate : Zn molar ratio less than 15 [World Health Organization (WHO) 2004] and phytate : Ca molar ratio less than 0.17 (Umeta et al. 2005).
Polyphenols are a large and diverse group of compounds, including tannins and isoflavons. Tannins are found in plant foods and strongly inhibit iron absorption by forming insoluble chelates. Variable proportions of the polyphenolic compounds are low‐molecular weight (LMW) compounds (molar weight below ∼3000 g mol−1), e.g. isoflavones such as genistein and daidzein (Manach et al. 2004). The potential hormonal effects of isoflavons from soya have been investigated in soy‐based formula for infants. There are, however, no firm evidence for the negative health effects of using soy‐based formula, although potential long‐term effects are unknown and should be investigated (Agostoni et al. 2006; Bhatia & Greer 2008). One study found a higher proportion of female infants with increased breast‐bud tissue in a group fed soy‐based formula compared with a group receiving cow milk‐based formula (Zung et al. 2008). The LMW polyphenols make up a heterogeneous fraction of the total pool of primarily insoluble high‐molecular weight polyphenolic compounds in plant foods. It is analytically challenging to determine and quantify the LMW compounds (Ajila et al. 2011). However, identifying and quantifying the presence of LMW polyphenolic compounds with potential harmful effects, such as isoflavons, may be more relevant from a nutritional and health perspective, compared with the determination of the total pool of polyphenolic compounds.
The proteases trypsin and chymotrypsin are produced from pancreatic proenzymes. They are needed to break down protein into amino acids that can be absorbed by the gut. Trypsin and chymotrypsin inhibitors are widely present in cereals and legumes (Lajolo & Genovese 2002). These inhibitors reduce the intestinal ability to digest protein and, thus, decrease the availability of protein needed for optimal growth and health. Protease inhibitors can be destroyed by aqueous heat treatment. The efficiency of this process is closely dependent on temperature and duration of processing. In soya bean samples, it was found that trypsin and chymotrypsin inhibitor activity was abolished by 10 min of aqueous heat treatment at 100°C. Treated at 90°C, chymotrypsin inhibition was eliminated after 20 min, while trypsin inhibition remained detectable after 40‐min treatment (Armour et al. 1998).
Lectins are carbohydrate‐binding proteins that can cause intestinal epithelial injury and diarrhoea. The main source of lectins is beans (Champ 2008). Lectins are known for their specific binding to cellular and intracellular membrane‐associated carbohydrates (glycoprotein and glycolipids). They also bind to carbohydrates in the ingested food and limit or change their potential hydrolysis and absorption from the gut. Thus, they reduce the amount of energy that can be utilised for growth and maintenance (Pusztai et al. 1990; Machado et al. 2008). They can also increase proliferation of pathogenic bacteria in the gut (Pusztai et al. 1995) and increase intestinal permeability (Banwell et al. 1983). Similar to the proteases inhibitors, lectins are destroyed by aqueous heat treatment. A study showed that aqueous treatment at 100°C eliminates lectin activity after 10 min, while temperatures ≤90°C left traces of lectins after 40 min (Armour et al. 1998).
Anti‐nutritional compounds may also have potential health benefits, which go beyond the potential adverse nutritional effects. For example, lectin might be used for obesity treatment and trypsin inhibitor may have anti‐carcinogenic effects. However, in complementary food for infants and young children, the immediate anti‐nutritional properties impacting growth and nutrient utilisation may counteract the potential long‐term health benefits, and systematic elimination of anti‐nutrients in complementary foods has been advocated (Champ 2008). Diets in developing countries are largely plant‐based, and although they contain some nutrients important for growth, the presence of anti‐nutrients makes it challenging to achieve sufficient amounts of bioavailable nutrients from these diets to sustain healthy growth. Animal source foods contain no anti‐nutritional compounds, and even small amounts of animal source foods are nutritionally beneficial in children vulnerable to malnutrition due to a predominantly plant‐based non‐diverse diet (Marquis et al. 1997; Gibson et al. 1998; Hotz & Gibson 2007; Neumann et al. 2007; Michaelsen et al. 2009).
An informal consultation on moderate malnutrition among children under 5 years of age was held jointly by WHO, United Nations Children's Fund (UNICEF), World Food Program (WFP) and The United Nations Refugee Agency in October 2008 (WHO 2008). The consultation recommended that because of the likely negative impact of anti‐nutritional factors and fibre on malnutrition, upper limits need to be established. It was also recommended that a separate expert group should be established, also in collaboration with the Codex Alimentarius, to examine different endogenous food components that have potential negative effects and develop upper limits for these anti‐nutrients and toxins. To be able to set upper limits, it is necessary to first know these existing levels in foods used for complementary feeding and treatment of malnourished children and whether they differ from diets consumed in countries where children have an adequate growth.
In addition, samples of industrially processed foods for the European market were included as a first screening of levels of anti‐nutrients. Products for complementary feeding available in the European market are extremely diverse. The variation in commercial products between countries as well as within countries is high, and in addition, there are cultural differences in composition and use of home‐made complementary food. On a population basis, there are no general public health concerns for child nutrition and health in Europe, and thereby there are no indications of general public health concerns of the nutritional adequacy of complementary foods. For the purpose of this study, a number of products were selected for having components of unrefined cereals, categorised as ‘whole grain’ products. As most commercial complementary food products available are based on refined cereals, the selected whole grain products represent a ‘worst‐case’ scenario for European children for being exposed to anti‐nutrients in complementary feeding in cases when caregivers systematically prefer whole grain products. Caregiver preference for whole grain complementary food products may also be reflected in a family diet with the same preferences.
The directive issued by the European Commission in 2006 on processed cereal‐based foods and baby foods for infants and young children (European Communities 2006) states that ‘there is a great variety of the products in question reflecting the widely varied diet of infants being weaned and young children owing to social and cultural circumstances existing in the Community’. This directive establishes guidelines for contents of macro‐ and micronutrients, and upper levels for residuals of pesticides, but not on anti‐nutrient compounds. Screening for anti‐nutrients in European whole grain products can help identify if there is a problem of anti‐nutrients in these ‘worst‐case scenario’ foods.
Our overall goal in this study was to compare the levels of anti‐nutrients in complementary foods and investigate whether there is a gradation of anti‐nutrients among local complementary foods, low‐cost centrally processed complementary foods and complementary food supplements, and foods sold in rich countries. This broad goal covers a wide variation in complementary foods from different continents and within different cultures and socio‐economic conditions. The present study presents a first step to achieve the overall goal by investigating the variation in core anti‐nutrients in foods for infants and young children, including a specific focus on complementary foods used in programmes for either preventing or treating malnutrition among children under 2 years of age in resource‐poor settings.
Key messages
This screening confirms that there is a persistent problem of high phytate levels in cereal‐ and legume‐based complementary foods, with the exception of foods based on white rice. There is a need for guidelines for manufacturers to efficiently reduce phytate in processed foods.
Upper limits for residual contents of trypsin and chymotrypsin inhibitors are needed for products fed to malnourished infants and young children.
Establishing upper limits for polyphenolic compounds needs to be carried out separately for polyphenolic subclasses and not for the total polyphenolic pool.
Commercial whole grain products representing a ‘worst‐case’ scenario for anti‐nutrient exposure in Europe showed high levels of phytate and, in some cases, other anti‐nutrients. Whole grain products should be limited in, or eliminated from, the complementary feeding of infants and young children.
Materials and methods
Selection and sampling of products
To screen for the levels of anti‐nutrients in complementary foods, foods from Asia, Africa and Europe were sampled, and the levels of anti‐nutritional compounds (phytate, total polyphenol, trypsin and chymotrypsin inhibitors and lectins) were determined. A total of 52 samples representing 36 different products were identified and shipped to Copenhagen through contacts to manufacturers, organisations or individuals. The selection of products was based on covering a wide range of primarily industrially processed foods used for complementary feeding, either as a part of a daily diet or as therapeutic treatment of malnourished children. Fortified blended foods were selected for being used in food aid programmes supported by international or local agencies or organisations. Local blended foods were included as examples to identify differences from industrially processed foods. The therapeutic foods selected for sampling were lipid‐based pastes defined as ready‐to‐use therapeutic foods (RUTFs) and ready‐to‐use supplementary foods (RUSFs). RUTF and RUSF are similar products by having high energy density around 5 kcal g−1 and by being prepared and packed for direct consumption. RUTF products are for treatment of severely malnourished children and RUSF products are modified RUTF aimed for supplementary feeding of moderate malnourished children (de Pee & Bloem 2009). Industrially processed complementary food products – instant porridges and semi‐solid ready‐to‐eat products (‘baby food’) – for a European market were bought in supermarkets in Denmark and in the United Kingdom. These products represent an upper level of anti‐nutrients from unrefined cereals in foods marketed for children aged 6–12 months in Denmark and 4–12 months in the United Kingdom. The selected foods represented different plant ingredients (wheat, maize, rice, amaranth grain, sorghum, chickpea, soya, peanut and oats).
Fortified blended foods used in food aid programmes, local blended foods and RUTFs and RUSFs were sampled in replicates (duplicate or triplicate) when samples from separate production batches were available. Industrially processed European instant porridges and baby foods were sampled in single samples as production batches were not identified. The identification of the products and manufacturers was blinded in this comparative presentation of the results. Information about the products – composition, fortificants and processing – was obtained from the manufacturers, from open‐sources and from labelled declarations.
Analytical methods
Drying and homogenising
Samples were freeze‐dried for the determination of moisture content. If needed, dried samples were homogenised in an iron‐free titanium grinder.
Minerals
Iron, zinc and calcium were analysed by atomic absorption spectrometry (Spektr‐AA 200; Varian, Zug, Switzerland), following acidic destruction in DigiTUBEs (SCP Science, Quebec, Canada), in a DigiPREP MS Digestion System (SCP Science). The material ‘Typical diet’ 1548a (National Institute of Standards and Technology, Gaithersburg, MD, USA) was used as a reference material.
Phytate/phytic acid
Phytic acid and other inositol phosphates were analysed on a high‐performance liquid chromatography system, equipped with a guard column (Dionex CarboFac PA‐100, 4 × 50 mm) and a high‐performance ion chromatography CarboPac PA‐100 (4 × 250 mm) analytical column (Dionex Corp., Sunnyvale, CA, USA). Triplicate 40 mg of freeze‐dried samples were extracted in 1.6 mL of 0.5 M HCl. Lipid‐based products were treated with ultrasound to ensure complete disintegration of the sample and extraction. Furthermore, 100 μL was loaded with an autoinjector (SIL‐10Ai). The eluents (0.8 mL min−1) were mixed with 0.1% ferric nitrate (Sigma‐Aldrich, Brøndby, Denmark) in a 2% solution of perchloric acid in a post‐column reactor. The combined flow rate was 1.2 mL min−1. Reported results are means of three replicate extractions and analysis of each sample. The details of the method were described elsewhere (Bohn et al. 2008).
Extraction for polyphenols, trypsin and chymotrypsin inhibitors, and lectin/glycoprotein
Sample (0.5 g freeze‐dried) was extracted in buffer (0.1 M methanol acetate, pH 4.9) with an Ultra Turrax homogeniser (IKA‐Werke GmbH & Co. KG, Staufen, Germany) for 2 min and centrifuged at 4000 g. The supernatant was transferred to a collection vial; 5 mL buffer was added to the remaining pellet and the extraction and centrifugation step described earlier was repeated. Combined supernatants were stored at 5°C overnight. The combined supernatant was centrifuged and pellet was discarded (Arentoft et al. 1991; Soerensen et al. 1999; Elnif et al. 1988). The clear supernatant was used for the following analyses.
Polyphenols
Total polyphenolic compounds were determined with standard colorimetric method using Folin–Ciocalteu reagents (Singleton et al. 1999). The content was measured against a standard of gentisic acid [2,5‐dihydroxybenzoic (DHB)], a derivate of benzoic acid with two hydroxyl groups and a common polyphenolic compound widely distributed in plants. The weight equivalents of the polyphenolic compounds are calculated from the molar weight of gentisic acid, 154 g mol−1. Reported results are the mean of two replicate analyses of each food sample.
Two food samples, representing two common types of food aid product – a fortified blended flour (product 3) and a peanut‐based paste (product 25) – were selected for analysis for LMW compounds on a three‐column hyphenated chromatography system. Column A separated cationic LMW compounds, column B separated anionic compounds and column C separated neutral compounds. Fingerprint‐type analysis – micellar electrokinetic capillary chromatography (MECC) with photodiode‐array detection (DAD) – was used to estimate the LMW compounds in the samples (Bjerg et al. 1988; Soerensen et al. 1999).
Trypsin and chymotrypsin inhibitors
The activities of the enzyme inhibitors for trypsin and chymotrypsin were determined at 50% inhibition of the enzymes using the linear area for enzyme and inhibitor activities and at least two series of inhibitor activities, which allows the calculation of the amounts resulting in 50% inhibition (Arentoft et al. 1991). From this, the inhibitor activities were calculated as inhibitor units (IU) per gram of sample. Enzyme unit (U) was defined as 1 U = amount of enzyme activities required for catalytic transformation of 1 μmol of substrate into product per minute under defined conditions (μmol min−1). So, 1 IU was defined as the amount of inhibitor required for inhibition of 1 U at defined conditions.
All measurements were performed in microtitre wells with the following procedure: 120 μL of sample (extract) was placed in well A1; 60 μL of Tris buffer was placed in well B1 to H1, with twofold dilution from A1 to H1 (120 μL of Tris buffer in A1 to H1); and 200 μL of Tris buffer was placed in A11 to H11 for blinds. Furthermore, 180 μL of Tris buffer was placed in A12 to H12 for controls for chymotrypsin activity. The enzymes – porcine trypsin, 0.054 mg mL−1 or bovine pancreas chymotrypsin, 7.1 mg mL−1 – dissolved in 0.001 M HCl were added from A1 to H1 and A12 to H12, respectively, followed by kinetic reading for 5 min. Then, 100 μL of the substrate – either benzoyl‐dl‐arginine‐p‐nitroanilide, 0.24 mg mL−1 or glupheba 0.38 mg mL−1, for trypsin and chymotrypsin, respectively – was added to all wells, followed by kinetic reading for 5 min. The reported results are means of two replicate analyses of each sample (Soerensen et al. 1999).
Lectin
Lectins were quantified as glycoprotein isolated by affinity chromatography using concanavalin A (ConA) columns (Soerensen et al. 1999). Glass wool was placed as a stopper in a 1‐mL pipette. In addition, 0.5 mL of Con Sepharose was added to the pipette and washed with 2 mL of buffer (0.02 M Tris–HCL + 0.5 M NaCl, pH 7.4). Then, 2 mL of the extract was added to the pipette and washed with 5 × 1 mL buffer. Proteins were eluted with 3 × 1 mL methyl‐α‐d‐mannopyranoside. After 5 mL wash with buffer, the concentration of the eluted proteins on a 1 mL fraction was measured by UV at 280 nm (scanned from 200 to 400 nm).
Results
Product characteristics
The analysed products are described in Table 1. The products are described by food type and regional origin. The declared cereal‐ and legume‐based ingredients potentially contributing anti‐nutrients are listed. The products are grouped in the following food types:
Fortified blended food: blended flours (cereals, beans or both) with vitamin and mineral premix produced either for local use or for a global food aid market. The products were based on blended cereal and legume flours, pre‐cooked by extrusion or similar industrial processing. The dry pre‐cooked flours were sampled and analysed. The products were prepared for additional heating (boiling for 5–10 min in water) before serving (n = 10 products).
Local blended food: blended non‐fortified foods based on traditional recipes and local foods produced by local small‐ and medium‐sized enterprises. The products were based on blended cereal and legume flours. The dry flours were analysed. These products were prepared to be mixed with or boiled in water before serving (n = 5 products).
Lipid‐based paste products: RUTF and RUSF (n = 12 products).
Fortified instant porridge (‘processed cereal‐based foods’): products for European market, bought in supermarkets in Denmark and in the United Kingdom. Only products declared as ‘whole grain’ were selected. The products were pre‐cooked by industrial processing and ready for serving after adding warm or boiling water (n = 6 products).
‘Semi‐solid’ products for European market (‘baby foods’ ready‐to‐eat), bought in supermarkets in Denmark and in the United Kingdom, selected for declared whole grain contents. Two semi‐solid products (‘baby foods’) and one ‘biscuit’ to be prepared as semi‐solid meal by crunching it in milk at the time of serving (n = 3 products).
Table 1.
Product description. Manufacturers of trade names blinded. Sorted by food groups
| Product ID[Link] | Use for | Produced in | No. of replicate samples of same product | Primary content | Other ingredients (except soya) | Contain soya + or − | Mineral and vitamin premix added + or − |
|---|---|---|---|---|---|---|---|
| Fortified blended food | |||||||
| 1 | Local commercial CF | Asia | 2 | Wheat | Sugar | + | + |
| 2 | Food aid product | United States | 1 | Maize (70%) | Oil | + appro. 20% defatted flour | + |
| 3 | Food aid product | Europe for global market | 2 | Maize (78%) | + appro. 20% flour | + | |
| 4a[Link] | Food aid product for CF | Europe for global market | 1 | Maize (58%) | Milk (8%), sugar, oil | + appro. 20% dehulled | + |
| 4b[Link] | Food aid product for CF | Europe for global market | 2 | Maize (58%) | Milk (8%), sugar, oil | + appro. 20% dehulled | + |
| 5 | Food aid product for CF | Africa | 1 | Maize (58%) | Milk (8%), sugar, oil | + appro. 20% dehulled | + |
| 6 | Local commercial CF | Asia | 1 | Wheat | Sugar, oil | + | + |
| 7 | Local commercial CF | Asia | 1 | Wheat | Sugar, oil | + | + |
| 17 | Food aid product | Asia | 2 | Wheat | Maize | + | + |
| 18 | Local commercial CF | Asia | 1 | Soya | Whole wheat | + 29% isolates and 12% dried soya milk | + |
| 19 | Local commercial CF | Asia | 1 | Soya | − | + 95% dried soya milk | + |
| Local blended food | |||||||
| 8 | Local CF for hospitals | Asia | 2 | Maize, wheat | Chickpea | + | − |
| 9 | Local CF for hospitals | Asia | 2 | Wheat | Rice | + | − |
| 10 | Local CF for hospitals | Asia | 2 | Maize (33%) | Wheat (33%) | + 33% | − |
| 20 | Traditional CF | Africa | 1 | Amaranth grain (80%) | Maize (20%), | − | − |
| 21 | Traditional CF | Asia | 1 | White rice (85%) | Fish (10%), oil, sugar | − | − |
| Lipid‐based paste | |||||||
| 22 | RUTF | Asia | 1 | Peanut | Skimmed milk powder, sugar, oil | + lipid | + |
| 23 | LNS | Europe for global market | 3 | Peanut | Whey, sugar, oil | − | + |
| 24 | RUTF | Asia | 1 | Peanut | Milk powder, sugar, oil | + protein | + |
| 25 | RUTF | Europe for global market | 3 | Peanut | Skimmed milk powder, sugar | − | + |
| Lipid‐based paste (continued) | |||||||
| 26 | RUTF | Africa | 1 | Peanut | Skimmed milk powder, sugar, oil | − | + |
| 27 | RUSF | Africa | 1 | Peanut | Whey, sugar, oil | + | + |
| 28 | RUSF | Asia | 1 | Peanut | Sugar, oil | + low trypsin | + |
| 29 | RUTF/RUSF | Asia | 1 | Peanut | Sugar, oil | + defatted | + |
| 30 | RUSF | Asia | 1 | Peanut | Rice, sugar, oil | + defatted | + |
| 31 | RUSF | Europe for global market | 3 | Peanut | Whey, sugar, oil | + | + |
| 32a[Link] | RUSF | Africa | 1 | Maize | Sorghum (5%), milk powder, oil | + | + |
| 32b[Link] | RUSF | Africa | 1 | Maize | Sorghum (5%), milk powder, oil | + | + |
| 33 | RUTF | Africa | 2 | Peanut | Milk powder, sugar, oil | − | + |
| Fortified instant porridge | |||||||
| 11 | Industrial product – CF (from age 8 months) | Europe for European market | 1 | Rice | Oat, wholemeal wheat | − | + |
| 12 | Industrial product – CF (from age 6 months) | Europe for European market | 1 | Wholemeal wheat | Refined wheat, oat | − | + |
| 13 | Industrial product – CF (from age 12 months) | Europe for European market | 1 | Wholemeal wheat, | Refined wheat, oat, rye | − | + |
| 14 | Industrial product – CF (from age 8 months) | Europe for European market | 1 | Wholemeal wheat | Refined wheat, oat, fruit | − | + |
| 15 | Industrial product – CF (from age 6 months) | Europe for European market | 1 | Wheat | Oat, fruit | − | + |
| 16 | Industrial product – CF (from age 4 months) | Europe for European market | 1 | Rice | Unrefined maize, vegetables, cheese | − | + |
| ‘Semi‐solid’ food for European market | |||||||
| 34 | Industrial product – composite meal for CF (from age 6 months) | Europe for European market | 1 | Wholemeal wheat | Wholemeal oat, rice, fruit | − | + |
| 35 | Industrial product – composite meal for CF (no declaration of age) | Europe for European market | 1 | Durum whole wheat | Whole grain pasta (durum) | − | − |
| 36 | Industrial product – ‘biscuit’ for CF (from age 6 months when crunched in milk) | Europe for European market | 1 | Wheat | Wheat flour, added ‘dietary fibre’ (inulin) | − | + |
CF, complementary food; LNS, lipid‐based nutritional supplement; RUSF, ready‐to‐use supplemental food; RUTF, ready‐to‐use therapeutic food. *Products 4 and 32 were sampled in replicates with different premix compositions. Micronutrient compositions are reported separately.
Mineral contents
The declared nutrient composition and the analysed contents of iron, zinc and calcium on weight basis are shown in Table 2. Minerals were analysed on dried samples and expressed per 100 g of food by adjusting for the measured moisture contents in the samples. The fortified products had higher levels of iron, zinc and calcium than the samples of local blended non‐fortified foods. For example, the mean contents of iron, zinc and calcium of 13 samples of fortified blended food representing eight different products were 12.32 mg Fe/100 g (range: 9.5–19.9), 12.7 mg Zn/100 g (range: 2.4–23.4) and 408 mg Ca/100 g (range: 73–654). Two products (products 18 and 19) are not included in this mean as these products are designed as a supplement to a habitual meal. The mean of eight samples of local blended foods representing five different products were 5.4 mg Fe/100 g (range: 3.0–10.5), 3.4 mg Zn/100 g (range: 1.8–5.2) and 111.5 mg Ca/100 g (range: 29–410). Of the 12 lipid‐based paste products, the analysed iron contents in 8 products (products 22, 23, 24, 28, 29, 30, 31 and 32a) were less than 80%. The analysed contents of zinc and calcium were less than 80% in seven and six products, respectively.
Table 2.
Declared contents of energy, protein, fat and minerals, and analysed mineral contents sorted by food groups. Product ID description is shown in Table 1
| Product ID | Declared nutrient composition per 100 g food | Nutrient density per 1000 kcal | Analysed minerals per 100 g food | Minerals. analysed as % of declared | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | Protein (g) | Fat (g) | Iron (mg Fe) | Zinc (mg Zn) | Calcium (mg) | Iron (mg Fe) | Zinc (mg Zn) | Calcium (mg) | Iron (mg Fe) | Fe (SD) | Zinc (mg Zn) | Zn (SD) | Calcium (Mg Ca) | Ca (SD) | Iron | Fe (SD) | Zinc | Zn (SD) | Calcium | Ca (SD) | |
| Fortified blended foods | |||||||||||||||||||||
| 1 | ND | ND | ND | 15.00 | 8.40 | 200 | ND | ND | ND | 19.90 | 0.06 | 17.95 | 7.69 | 311.92 | 8.46 | 132.67 | 0.37 | 213.72 | 91.54 | 155.96 | 4.23 |
| 2 | 376.00 | 17.20 | 6.90 | 17.49 | 5.00 | 831 | 46.52 | 13.30 | 2210.11 | 9.50 | 5.45 | 133.67 | 54.30 | 108.93 | 16.09 | ||||||
| 3 | 410.00 | 16.00 | 9.00 | 6.50 | 5.00 | 130 | 15.85 | 12.20 | 317.07 | 9.59 | 0.14 | 7.74 | 0.52 | 177.77 | 6.49 | 147.60 | 2.20 | 154.79 | 10.31 | 136.75 | 4.99 |
| 4a | 410.00 | 16.00 | 9.00 | 6.50 | 5.00 | 600 | 15.85 | 12.20 | 1463.41 | 10.43 | 0.79 | 8.48 | 0.56 | 654.13 | 160.42 | 12.10 | 169.54 | 11.28 | 109.2 | ||
| 4b | 410.00 | 16.00 | 9.00 | 6.50 | 5.00 | 130 | 15.85 | 12.20 | 317.10 | 9.97 | 0.02 | 8.15 | 0.07 | 255.54 | 3.29 | 153.44 | 0.36 | 163.05 | 1.32 | 196.57 | 2.53 |
| 5 | 410.00 | 16.00 | 9.00 | 6.50 | 5.00 | 130 | 15.85 | 12.20 | 317.07 | 10.01 | 8.08 | 329.53 | 153.99 | 161.52 | 253.48 | ||||||
| 6 | 460.00 | 16.00 | ND | ND | ND | ND | ND | ND | ND | 9.91 | 2.37 | 300.09 | ND | ND | ND | ND | ND | ND | |||
| 7 | 460.00 | 16.00 | ND | ND | ND | ND | ND | ND | ND | 10.76 | 2.59 | 73.25 | ND | ND | ND | ND | ND | ND | |||
| 17 | 380.00 | 16.00 | 6.00 | 6.50 | 5.00 | 175 | 17.10 | 13.20 | 460.50 | 14.90 | 0.80 | 5.65 | 1.73 | 174.78 | 0.88 | 229.26 | 12.38 | 113.06 | 34.51 | 99.87 | 0.50 |
| 18 | 367.50 | 37.17 | ND | 62.50 | 41.67 | 2272 | 170.07 | 113.38 | 6182.31 | 41.07 | 40.60 | 1.336.02 | 65.71 | 97.43 | 58.80 | ||||||
| 19 | 372.86 | 25.00 | 11.70 | 41.70 | 41.70 | 1750 | 111.84 | 111.84 | 4693.49 | 37.85 | 41.55 | 1.463.90 | 90.77 | 99.64 | 83.65 | ||||||
| Local blended foods | |||||||||||||||||||||
| 8 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.51 | 0.03 | 3.06 | 0.16 | 40.42 | 0.94 | ND | ND | ND | ND | ND | ND |
| 9 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.50 | 0.71 | 1.83 | 0.01 | 30.32 | 0.62 | ND | ND | ND | ND | ND | ND |
| 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 6.78 | 0.73 | 4.26 | 0.02 | 73.66 | 5.82 | ND | ND | ND | ND | ND | ND |
| 20 | 436.00 | 17.50 | 7.50 | ND | ND | ND | ND | ND | ND | 10.55 | 3.84 | 196.24 | ND | ND | ND | ND | ND | ND | |||
| 21 | 389.00 | 15.20 | 3.20 | ND | ND | ND | ND | ND | ND | 5.70 | 5.21 | 410.18 | ND | ND | ND | ND | ND | ND | |||
| Lipid‐based paste | |||||||||||||||||||||
| 22 | 528.50 | 18.00 | 30.50 | 12.00 | 5.00 | 300 | 22.71 | 9.46 | 567.64 | 2.00 | 2.65 | 315.49 | 16.66 | 52.97 | 105.16 | ||||||
| 23 | 540.00 | 12.80 | 35.40 | 45.00 | 20.00 | 500 | 83.33 | 37.04 | 925.93 | 34.72 | 4.77 | 20.75 | 1.39 | 408.44 | 33.42 | 77.16 | 10.61 | 103.77 | 6.93 | 81.69 | 6.68 |
| 24 | 514.30 | 18.00 | 28.60 | 12.00 | 5.00 | 300 | 23.33 | 9.72 | 583.32 | 6.19 | 2.56 | 174.07 | 51.57 | 51.19 | 58.02 | ||||||
| 25 | 543.48 | 13.59 | 35.76 | 11.52 | 14.02 | 300 | 21.20 | 25.80 | 552.00 | 10.41 | 0.83 | 11.34 | 0.36 | 287.26 | 13.86 | 90.37 | 7.22 | 80.86 | 2.56 | 95.75 | 4.62 |
| 26 | 543.48 | 13.59 | 35.76 | 11.52 | 14.02 | 300 | 21.20 | 25.80 | 552.00 | 11.23 | 13.25 | 317.39 | 97.46 | 94.45 | 105.80 | ||||||
| 27 | 543.48 | 13.59 | 35.76 | 11.52 | 14.02 | 300 | 21.20 | 25.80 | 552.00 | 13.52 | 12.10 | 418.05 | 117.38 | 86.26 | 139.35 | ||||||
| 28 | 501.00 | 18.60 | 26.50 | 12.00 | 5.00 | 300 | 23.95 | 9.98 | 598 | 7.72 | 2.58 | 107 | 64.30 | 51.57 | 35.75 | ||||||
| 29 | 501.00 | 19.40 | 26.50 | 12.00 | 5.00 | 300 | 23.95 | 9.98 | 598 | 5.58 | 2.57 | 103 | 46.52 | 51.44 | 34.51 | ||||||
| 30 | 496.00 | 17.90 | 22.00 | 12.00 | 5.00 | 300 | 24.19 | 10.08 | 604 | 6.72 | 2.46 | 105 | 56.03 | 49.28 | 35.32 | ||||||
| 31 | 543.48 | 13.59 | 35.76 | 11.52 | 14.02 | 300 | 21.20 | 25.80 | 552 | 9.15 | 0.77 | 10.66 | 1.20 | 202 | 39.28 | 79.44 | 6.67 | 76.02 | 8.57 | 67.64 | 13.09 |
| 32a | 520.00 | 9.00 | 54.00 | 36.50 | 32.00 | 1980 | 70.19 | 61.54 | 3807 | 17.10 | 14.17 | 889 | 46.86 | 44.39 | 44.94 | ||||||
| 32b | 520.00 | 9.00 | 54.00 | 4.50 | 7.00 | 300 | 8.65 | 13.46 | 576 | 9.34 | 10.56 | 325 | 207.61 | 150.90 | 108.55 | ||||||
| 33 | 520.00 | 14.00 | 54.00 | 12.20 | 12.50 | 300 | 23.46 | 24.04 | 576 | 11.18 | 2.28 | 12.26 | 0.49 | 261 | 29.51 | 91.60 | 18.67 | 98.11 | 3.92 | 87.10 | 9.89 |
| Fortified instant porridges | |||||||||||||||||||||
| 11 | 410.00 | 15.00 | 10.00 | 9.50 | 3.00 | 600 | 23.17 | 7.32 | 1463 | 9.48 | 4.17 | 590 | 99.84 | 139.15 | 98.47 | ||||||
| 12 | 423.81 | 16.00 | 13.00 | 10.00 | ND | 600 | 23.60 | ND | 1415 | 9.44 | 3.55 | 673 | 94.36 | 112.17 | |||||||
| 13 | 428.57 | 15.00 | 17.00 | 8.50 | 4.00 | 600 | 19.83 | 9.33 | 1400 | 9.56 | 4.14 | 586 | 112.44 | 103.54 | 97.83 | ||||||
| 14 | 440.48 | 14.00 | 19.00 | 8.50 | 2.00 | 600 | 19.30 | 4.54 | 1362 | 8.11 | 4.53 | 564 | 95.40 | 226.36 | 94.02 | ||||||
| 15 | 443.57 | 12.50 | 15.30 | 7.50 | ND | 331 | 16.91 | ND | 746 | 8.95 | 2.21 | 312 | 119.34 | 94.37 | |||||||
| 16 | 407.62 | 15.30 | 6.90 | 7.00 | 4.00 | 470 | 17.17 | 9.81 | 1153 | 7.13 | 5.76 | 486 | 101.85 | 144.10 | 103.44 | ||||||
| ‘Semi‐solid’ foods (Europe) | |||||||||||||||||||||
| 34 | 150.00 | 4.00 | 2.00 | ND | ND | ND | ND | ND | ND | 0.33 | 0.19 | 7 | ND | ND | ND | ND | ND | ND | |||
| 35 | 130.61 | 7.62 | 1.36 | ND | ND | ND | ND | ND | ND | 0.74 | 0.72 | 17 | ND | ND | ND | ND | ND | ND | |||
| 36 | 407.62 | 10.00 | 9.70 | 6.00 | 4.00 | 317 | 14.72 | 9.81 | 777 | 5.82 | 4.90 | 270 | 97.00 | 122.59 | 85.41 | ||||||
ND, no data available; SD, standard deviation when replicating samples of products. *Products 4 and 32 were sampled in replicates with different premix compositions. Micronutrient composition is reported separately.
For most products, the iron compounds used for fortification were not specified. In five products, the fortified iron was specified as a combination of iron salt (ferrous fumarate) and NaFeEDTA iron. In products 3, 4, 5 and 17, NaFeEDTA iron contributed 2.5 mg Fe of a total of 6.5 mg fortified iron, and in product 18, half of the fortified iron was NaFeEDTA iron.
Phytate and lower inositol phytates
The analysed contents for inositol phosphates are shown in Table 3. The contents are given in nmol g−1 and for IP6, also in mg/100 g (molar weight 660). IP6 content ranged from 68 to 1536 mg/100 g on dry weight basis (Table 3). IP4 and IP5 were found in two configurations, IP4a and b, and IP5a and b, respectively. The a and b forms are the results of degradation by different phytases originating from different plants or from microbial activity. In nine products, no IP5 or IP4 were detected. Expressed on weight basis, maximum total IP5 was 373 mg/100 g on dry weight basis. For the 11 fortified blended food products, the phytate content averaged 840 mg/100 g, ranging by a factor of 10 from 142 to 1450 mg/100 g. The phytate content in the peanut‐based pastes ranged from 73 to 1055 mg/100 g, averaging 514 mg/100 g. Of the 12 lipid‐based pastes, 10 products had peanut as the main ingredient, either as the only plant food or in combination with soya. A single peanut‐based product also contained rice (product 30). Phytate contents in the analysed nine whole grain products from a European market (instant porridges and semi‐solid foods) averaged 734 mg/100 g, ranging from 211 to 1248.
Table 3.
Molar contents of inositol hexaphosphate (IP6 – phytate) and inositols IP4‐IP5. IP4 and IP5 were detected in two different configurations (a and b). IP6 shown as mg/100 g (molar weight 660 g mol−1). Listed by food groups. Product ID description is shown in Table 1
| Product ID | Composition | Inositol phosphates | ||||||
|---|---|---|---|---|---|---|---|---|
| Primary content | Phytate sources | IP4a (nmol g−1) | IP4b (nmol g−1) | IP5a (nmol g−1) | IP5b (nmol g−1) | IP6 (nmol g−1) | IP6 (mg/100 g) | |
| Fortified blended foods | ||||||||
| 1 | Wheat | Wheat, soya | 0 | 346 | 867 | 0 | 4 661 | 308 |
| 2 | Maize | Maize, defatted soya | 0 | 0 | 402 | 0 | 18 898 | 1247 |
| 3 | Maize | Maize, soya | 0 | 1701 | 0 | 2166 | 16 291 | 1076 |
| 4a | Maize | Maize, dehulled soya | 0 | 0 | 0 | 0 | 15 301 | 1001 |
| 4b | Maize | Maize, dehulled soya | 0 | 0 | 0 | 1426 | 15 093 | 996 |
| 5 | Maize | Maize, dehulled soya | 0 | 115 | 0 | 0 | 9 895 | 653 |
| 6 | Wheat | Wheat, soya | 0 | 0 | 0 | 1471 | 21 976 | 1450 |
| 7 | Wheat | Wheat, soya | 0 | 0 | 0 | 1343 | 17 798 | 1175 |
| 17 | Wheat | Wheat, maize, soya | 0 | 281 | 418 | 0 | 10 574 | 698 |
| 18 | Soya | Whole wheat, soya isolate | 0 | 585 | 1015 | 0 | 7 441 | 491 |
| 19 | Soya | Soya‐milk powder‐ | 0 | 110 | 0 | 0 | 2 154 | 142 |
| Local blended foods | ||||||||
| 8 | Maize, wheat | Wheat, maize, chickpea, soya | 0 | 654 | 964 | 0 | 5 142 | 339 |
| 9 | Wheat | Wheat, rice, soya | 0 | 498 | 994 | 0 | 13 538 | 894 |
| 10 | Maize, wheat, soya (1:1:1) | Yellow maize, wheat, soya | 0 | 261 | 693 | 0 | 8 018 | 529 |
| 20 | Amaranth grain | Amaranth grain, maize | 0 | 0 | 0 | 0 | 25 600 | 1536 |
| 21 | Rice | Rice | 0 | 0 | 0 | 0 | 449 | 68 |
| Lipid‐based paste | ||||||||
| 22 | Peanut | Peanut, soya lipid | 0 | 303 | 642 | 0 | 4 513 | 298 |
| 23 | Peanut | Peanut | 0 | 252 | 266 | 0 | 10 819 | 714 |
| 24 | Peanut | Peanut, soya protein | 0 | 647 | 959 | 0 | 6 912 | 456 |
| 25 | Peanut | Peanut | 0 | 0 | 401 | 0 | 15 979 | 1055 |
| 26 | Peanut | Peanut | 0 | 200 | 160 | 0 | 5 625 | 371 |
| 27 | Peanut | Peanut, soya | 0 | 0 | 0 | 0 | 1 106 | 730 |
| 28 | Peanut | Peanut, soya | 0 | 0 | 0 | 353 | 3 975 | 262 |
| 29 | Peanut | Peanut, defatted soya | 0 | 0 | 0 | 566 | 8 132 | 537 |
| 30 | Peanut | Peanut, rice, defatted soya | 0 | 500 | 0 | 759 | 5 348 | 353 |
| 31 | Peanut | Peanut, soya | 236 | 1533 | 1891 | 0 | 11 540 | 762 |
| 32a | Maize | Maize, sorghum, soya | 72 | 0 | 0 | 0 | 3 121 | 206 |
| 32b | Maize | Maize, sorghum, soya | 0 | 0 | 0 | 0 | 5 046 | 337 |
| 33 | Peanut | Peanut | 0 | 0 | 357 | 0 | 11 865 | 783 |
| Fortified instant porridges | ||||||||
| 11 | Rice | Rice, oat, wholemeal wheat | 0 | 0 | 673 | 0 | 14 332 | 946 |
| 12 | Wheat | Wholemeal wheat, wheat, oat | 0 | 0 | 0 | 0 | 18 907 | 1248 |
| 13 | Wheat | Wholemeal wheat, wheat, oat, rye | 0 | 496 | 880 | 213 | 3 857 | 255 |
| 14 | Wheat | Wholemeal wheat, wheat, oat | 0 | 128 | 604 | 0 | 3 202 | 211 |
| 15 | Wheat | Wheat, oat | 0 | 696 | 1124 | 0 | 7 892 | 521 |
| 16 | Rice | Rice, maize | 0 | 0 | 0 | 0 | 11 724 | 774 |
| ‘Semi‐solid’ food (Europe) | ||||||||
| 34 | Wheat | Wholemeal wheat, oat, rice | 0 | 0 | 0 | 0 | 18 182 | 1200 |
| 35 | Durum whole wheat | Whole grain pasta (durum) | 735 | 1255 | 0 | 1194 | 5 175 | 342 |
| 36 | Wheat | Wheat flour, ‘dietary fibre’ (inulin) | 0 | 0 | 0 | 0 | 16 841 | 1112 |
The molar ratios between IP6 and iron, zinc and calcium are shown in Fig. 1. Only 4 of the 36 products had phytate : Fe ratio below the recommended cut‐off value of <1.0, while 25 of the 36 products were below the recommended cut‐off value for phytate : Zn of maximum 15. The products below phytate : Fe ratio were two fortified blended flours based on soya (products 18 and 19), one rice‐based local blended food and one lipid‐based peanut paste. Fifteen products were below the recommended cut‐off value of 0.17 for phytate : Ca. The products exceeding the cut‐off value for Zn were equally distributed in the food groups (Fig. 1), while the products exceeding the cut‐off value for calcium were among the fortified blended foods (6 of 11) and local blended foods (4 of 5) and the semi‐solid foods (3 of 3). For the lipid‐based pastes, 4 of 12 products exceeded the phytate : Ca molar ratio cut‐off value, and 3 were bordering the cut‐off value, while all fortified instant porridges were below the cut‐off value for phytate : Ca ratio.
Figure 1.
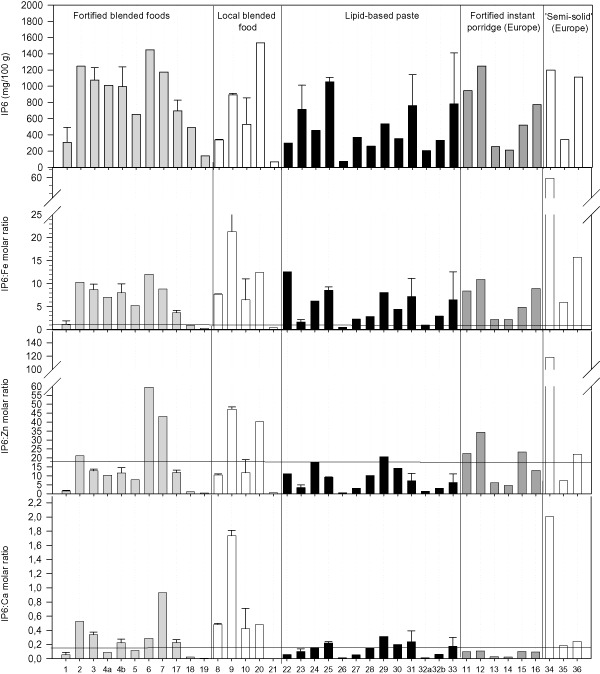
Phytate [inositol hexaphosphate (IP6)] contents and phytate : mineral molar ratios by product. Error bars indicate standard deviation between replicate samples of products. Product description is shown in Table 1. Products 4 and 32 were sampled in replicates with different premix compositions. Horizontal lines indicate recommended maximum molar ratios for optimal mineral bioavailability. Phytate : iron ratio =1; phytate : Zn ration =15 and phytate : Ca ratio = 0.17.
Iron is the most critical mineral to meet the maximum molar ratio to phytate <1.0 to ensure acceptable absorption. Four products met the cut‐off value, and these products also met the cut‐off values for zinc and calcium. The four products meeting all the recommended phytate : mineral molar ratios were products 18 and 19, both fortified blended foods based on processed soya, product 21, a local rice‐based blended food with a very low phytate content, and product 26, a lipid‐based peanut paste.
In Fig. 2, the contents of phytate (IP6), polyphenols/tannins, trypsin and chymotrypsin inhibitors, and lectins in the screened products are shown by ‘daily portion’ in order to compare across products with different energy density, as well as products that are produced as a daily food supplement to be added to a habitual porridge (products 18, 19, 23 and 32).
Figure 2.
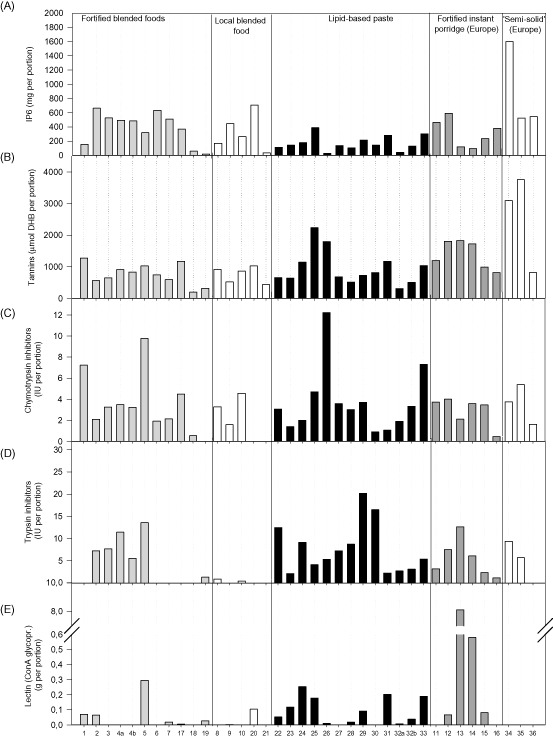
Anti‐nutrient by‐products by exposure from a daily portion. A daily portion is calculated as 200 kcal, except for products 18 and 19, which are supplements to be added to a porridge in a daily ration of 12 g (44 kcal) and products 23 and 32a, which are supplement to be used in a 20 g daily ration. Product description is shown in Table 1. Products 4 and 32 were sampled in replicates with different premix compositions. (A) Inositol hexaphosphate (IP6); (B) total polyphenols [tannins, Folin–Ciocalteu, standard: gentisic acid (2,5‐dihydroxybenzoic – DHB)]; and (C,D) chymotrypsin and trypsin inhibitors: IU g−1 = inhibitor units (IU) per gram of samples. Lectin: concanavalin A reactive glycoprotein.
Polyphenols
The contents of total polyphenolic compounds – expressed in gentisic acid (DHB) equivalents – are shown in Table 4. Folin–Ciocalteu reactive compounds were detected in all samples, with a mean of 22.0 μmol DBH equivalents g−1 (standard deviation: 14.1; range: 8.5–60.7). Expressed as gentisic acid weight equivalents, the measured contents ranged from 1.3 to 9.3 mg DHB g−1. The lowest content was found in product 21, a rice‐based local blended food, and the highest content in product 25, a lipid‐based paste.
Table 4.
Anti‐nutrients: lectin (convacavalin A reactive glycoprotein), polyphenols and enzyme inhibitors. Product description in Table 1
| Product ID | Primary content | Anti‐nutrient sources | Total polyphenols | Trypsin inhibitors | Chymotrypsin inhibitors | Lectin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| μmol DHB g−1 (mg DHB g−1) | SD (μmol g−1) | IU g−1 | SD | IU g−1 | SD | ConA reactive glycoprotein (mg g−1) | SD | |||
| Fortified blended foods | ||||||||||
| 1 | Wheat | Wheat, soya | 25.50 (3.93) | 0.67 | 0.00 | 0.00 | 0.14 | 0.04 | 1.40 | 0.22 |
| 2 | Maize | Maize, defatted soya | 10.59 (1.63) | 0.14 | 0.04 | 1.21 | ||||
| 3 | Maize | Maize, soya | 13.32 (2.05) | 1.50 | 0.16 | 0.07 | 0.07 | 0.00 | 0.00 | 0.00 |
| 4 | Maize | Maize, dehulled soya | 17.61 (2.71) | 1.00 | 0.15 | 0.07 | 0.07 | 0.01 | 0.00 | 0.00 |
| 5 | Maize | Maize, dehulled soya | 21.10 (3.35) | 0.28 | 0.20 | 6.05 | ||||
| 6 | Wheat | Wheat, soya | 17.11 (2.63) | 0.00 | 0.04 | 0.00 | ||||
| 7 | Wheat | Wheat, soya | 13.64 (2.10) | 0.00 | 0.05 | 0.42 | ||||
| 17 | Wheat | Wheat, maize, soya | 22.22 (3.42) | 4.32 | 0.00 | 0.00 | 0.09 | 0.02 | 0.09 | 0.13 |
| 18 | Soya | Whole wheat, soya isolate | 16.87 (2.60) | 0.00 | 0.05 | 0.00 | ||||
| 19 | Soya | Soya milk powder | 26.30 (4.05) | 0.11 | 0.00 | 2.28 | ||||
| Local blended foods | ||||||||||
| 8 | Maize, wheat | Wheat, maize, chickpea, soya | 18.25 (2.81) | 0.32 | 0.02 | 0.01 | 0.07 | 0.01 | 0.00 | 0.00 |
| 9 | Wheat | Wheat, rice, soya | 10.36 (1.60) | 0.65 | 0.00 | 0.00 | 0.03 | 0.01 | 0.05 | 0.07 |
| 10 | Maize, wheat, soya (1:1:1) | Yellow maize, wheat, soya | 17.24 (2.65) | 1.13 | 0.01 | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 |
| 20 | Amaranth grain | Amaranth grain, maize | 22.39 (3.45) | 0.00 | 0.00 | 2.91 | ||||
| 21 | Rice | Rice | 8.53 (1.31) | 0.00 | 0.00 | 0.50 | ||||
| Lipid‐based paste | ||||||||||
| 22 | Peanut | Peanut, soya lipid | 17.29 (2.66) | 0.33 | 0.08 | 1.38 | ||||
| 23 | Peanut | Peanut | 32.09 (4.94) | 4.77 | 0.10 | 0.05 | 0.07 | 0.06 | 5.91 | 3.98 |
| 24 | Peanut | Peanut, soya protein | 29.41 (4.53) | 0.23 | 0.05 | 6.51 | ||||
| 25 | Peanut | Peanut | 60.72 (9.35) | 33.47 | 0.11 | 0.00 | 0.13 | 0.11 | 4.83 | 3.20 |
| 26 | Peanut | Peanut | 48.73 (7.50) | 0.15 | 0.33 | 0.25 | ||||
| 27 | Peanut | Peanut, soya | 18.60 (2.86) | 0.20 | 0.10 | 0.00 | ||||
| Lipid‐based pastes (continued) | ||||||||||
| 32 | Maize | Maize, sorghum, soya | 14.29 (2.20) | 1.73 | 0.11 | 0.04 | 0.09 | 0.01 | 0.66 | 0.44 |
| 33 | Peanut | Peanut | 26.91 (4.14) | 8.51 | 0.14 | 0.03 | 0.19 | 0.01 | 4.91 | 1.76 |
| 28 | Peanut | Peanut, soya protein | 12.96 (2.00) | 0.22 | 0.08 | 0.44 | ||||
| 29 | Peanut | Peanut, defatted soya | 18.13 (2.79) | 0.51 | 0.09 | 2.28 | ||||
| 30 | Peanut | Peanut, rice, defatted soya | 20.15 (3.10) | 0.41 | 0.02 | 0.00 | ||||
| 31 | Peanut | Peanut, soya | 31.84 (4.90) | 3.55 | 0.06 | 0.04 | 0.03 | 0.05 | 5.49 | 1.85 |
| Fortified instant porridges | ||||||||||
| 11 | Rice | Rice, oat, wholemeal wheat | 24.55 (3.78) | 0.06 | 0.08 | 0.00 | ||||
| 12 | Wheat | Wholemeal wheat, wheat, oat | 38.19 (5.88) | 0.16 | 0.08 | 1.41 | ||||
| 13 | Wheat | Wholemeal wheat, wheat, oat, rye | 39.19 (6.04) | 0.27 | 0.05 | 55.00 1) | ||||
| 14 | Wheat | Wholemeal wheat, wheat, oat | 37.99 (5.85) | 0.13 | 0.08 | 12.79 | ||||
| 15 | Wheat | Wheat, oat | 21.96 (3.38) | 0.05 | 0.08 | 1.80 | ||||
| 16 | Rice | Rice, maize | 16.65 (2.56) | 0.02 | 0.01 | 0.00 | ||||
| ‘Semi‐solid’ foods (Europe) | ||||||||||
| 34 | Wheat | Wholemeal wheat, wholemeal oat, rice | 23.20 (3.57) | 0.07 | 0.03 | 0.00 | ||||
| 35 | Durum whole wheat | Whole grain pasta (durum) | 24.56 (3.78) | 0.04 | 0.04 | 0.00 | ||||
| 36 | Wheat | Wheat flour, ‘dietary fibre’ (inulin) | 16.67 (2.57) | 0.00 | 0.03 | 0.00 | ||||
DHB, dihydroxybenzoic acid (gentisic acid) equivalents; SD, standard deviation.
The DAD spectra for MECC fingerprint analyses after the ionic separation and of the crude extract of two samples are shown in Fig. 3. MECC indicates that this relatively simple technique could be a useful tool for screening for the small pool of LMW absorbable polyphenols that may have direct health impact, rather than quantifying the total pool of dominantly high‐molecular weight and insoluble polyphenols. The MECC spectrum for the peaks provides initial indications of the presence of phyto‐oestrogenic isoflavons, which is shown to be present in the soya‐based product (product 3) and not in the peanut‐based product (product 25). The method could potentially be developed as a relatively simple screening method for relevant polyphenolic compounds. This would contribute to set upper limits and develop guidelines for manufactures for how to limit these compounds.
Figure 3.
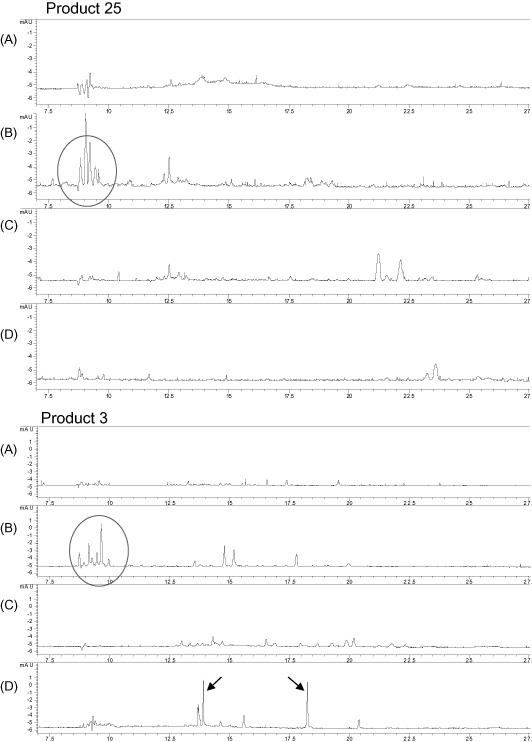
Example of low‐weight molecular separation of two samples, one peanut‐based lipid paste (product 25) and one maize‐ and soya‐based fortified blended food (product 3). Separation was conducted by the three‐column ionic separation technique, followed by simple fingerprint‐type analysis using micellar electrokinetic capillary chromatography. (A) Unseparated crude extract (diluted ten times); (B) cationic fraction; (C) anionic fraction; and (D) neutral unloaded fraction. Peaks marked in fraction B indicate non‐harmful peptides. Peaks marked in fraction D in the soya‐based sample indicate detectable levels of the isoflavones daidzein and genistein. The compounds are not quantified.
Trypsin and chymotrypsin inhibitors
The levels of trypsin and chymotrypsin inhibitor activities are shown in Table 4. The maximum contents were 0.51 and 0.20 IU g−1 for trypsin and chymotrypsin inhibitors, respectively. By comparison, typical contents in raw soya are 30–50 and 15–25 IU g−1, respectively (Champ 2008).
There were no clear systematic differences in contents between products with and without soya. The mean trypsin inhibition in samples without soya was 0.10 IU g−1, and samples with soya averaged 0.11 IU g−1. The two highest contents of trypsin inhibitor, 0.41 and 0.51 U g−1, were measured in lipid‐based paste products produced in Asia (products 29 and 30).
Lectins
The contents of ConA reactive glycoprotein are shown in Table 4 and expressed as exposure from a daily ratio in Fig. 2. ConA affinity assesses the contents of glycoproteins containing N‐acetyl‐α‐ or β‐galactopyranosyl residues and is applied as a simple and relevant alternative to measuring the anti‐nutritional effect of lectins based on haemagglutinating activity in erythrocytes from humans or other mammals (Bures et al. 1973). Haemagglutinating activity in corn–soya blend assessed with rabbit erythrocytes was shown to be efficiently reduced by heat treatment (Machado et al. 2008). The content of ConA reactive glycoproteins is below 10 mg g−1 in all products, except in two products, both industrially processed instant porridges for European market. The two products were from the same producer and were based on wholemeal wheat and oat. One product has higher level of ConA reactive glycoprotein, 55 mg g−1, which could indicate that heat treatment during processing has been insufficient. This product was pre‐cooked but needs further preparation by boiling before consumption, which could contribute to reduce the level of ConA reactive glycoprotein before serving. However, attention is needed to the finding of lectins above residual levels in products marketed for complementary feeding of young children.
For other products, the ConA reactive content was variable and there are no indications of correlation with food type or contents. The content averaged 1.8 mg g−1, leaving out the two products with contents >10 mg g−1.
Discussion
The representativeness of the selected food products
The fortified blended flours and lipid‐based pastes are industrially processed foods specifically developed and manufactured for food aid and rehabilitation programmes. These products are available from a relatively small number of manufacturers, selling either to a regional market in Asia or Africa, or to a global market for food aid programmes, e.g. through WFP or UNICEF. The fortified blended foods and lipid‐based pastes selected and analysed in this study represent a significant proportion of these types of food products distributed in aid in programmes for severely or moderately malnourished children.
In contrast, local and indigenous foods are extremely heterogeneous, and a systematic coverage of global variation in composition and processing of local complementary foods would demand higher numbers of samples. A recent review of 26 indigenous and 27 local commercially processed plant‐based complementary foods from low‐income countries complements this study with regard to phytate and mineral contents (Gibson et al. 2010). Also, the sampled products of instant porridges and semi‐solid ready‐to‐eat products (‘baby food’) for a European market represent a very heterogeneous food market. These products were selected for whole grain contents representing a ‘worst‐case’ scenario for exposure to anti‐nutrient compounds, as outlined earlier. In this study, these products represent an upper level of anti‐nutrients from unrefined cereals in foods marketed for children aged 6–12 months in Denmark and 4–12 months in the United Kingdom.
Declared and measured mineral contents
The analysed contents of iron, zinc and calcium indicated that the actual fortification of a number of products was below the declared contents of these minerals. The fortified blended foods generally met the declared fortification better than the lipid‐based paste products. The reason for the difference between the two types of foods is not clear; the declarations are not updated or are there technological issues related to fortified lipid‐based pastes. The unfortified local blended foods had – as expected – generally lower contents of iron, zinc and calcium, confirming the notion that meeting the requirements of these critical minerals with local foods is challenging. A recent screening of iron, zinc and calcium contents in 57 fortified complementary foods from Africa and Asia found that less than 5% of the products had fortification levels, which, in daily servings of complementary feeding, could meet the WHO recommendations for intake of iron, zinc and calcium for children (Gibbs et al. 2011). This indicates that closer monitoring of actual fortification levels is needed to ensure that fortified products meet the declared contents.
Of the five products fortified with NaFeEDTA iron, four were fortified blended foods with high phytate : Fe molar ratios between 3.7 and 8.7 (products 3, 4, 5 and 17). NaFeEDTA iron is documented to be more bioavailable from phytate‐rich meals than electrolytic iron (Andang'o et al. 2007) and than iron salts (Hurrell et al. 2010), and the use of NaFeEDTA iron could partially compensate for the high phytate ratios in these products. Product 18 was also fortified partially with NaFeEDTA iron. This product had low phytate : Fe ratio relative to the recommended cut‐off value, and as NaFeEDTA iron has improved bioavailability specifically from phytate‐rich foods (Hurrell et al. 2010), the use of NaFeEDTA iron may not improve bioavailability in this specific product. NaFeEDTA will help increase iron bioavailability from local foods high in phytate (such as maize or wheat). NaFeEDTA iron is a more expensive compound compared with iron salts, and the feasibility as fortification needs to be assessed by product. The level of NaFeEDTA fortification in specific products needs to assure that the total EDTA intake will not exceed the recommended Acceptable Daily Intake of maximum 1.9 mg EDTA kg−1 body weight (Hurrell et al. 2010). Mass use of NaFeEDTA fortification will require an assessment of the total intake from the diet, not from single foods.
The phytate level in the analysed products varied with the ingredients in the foods. Highest and lowest phytate contents were found in local blended food, the highest in a food based on amaranth grain (product 20), and the lowest in a food based on white rice (product 21) – in line with that, white rice generally has low phytate content compared with other cereals and legumes (Chan et al. 2007). Also, Asian recipes for complementary foods based on rice as the only cereal and no legumes were found to have low phytate contents, with a maximum of 35 mg IP6 + IP5/100 g food (Gibson et al. 2010).
The phytate content in the fortified blended foods ranged from 142 to more than 1000 mg/100 g dry food, with the highest content found in the maize‐ and soya‐based products, and the lowest contents in product 18, a product based on soya isolate. The mean of 840 mg IP6/100 g dry food in the fortified blended foods was slightly higher than the mean contents in foods based on unrefined cereals and legumes in the review by Gibson et al. (2010).
The small sample size does not allow general conclusions but indicate that continuous screening of local and indigenous foods to identify local food composition and processing, which can reduce phytate, can contribute to improve nutritional quality of complementary foods.
The high variation in phytate in the peanut‐based pastes indicated that these products may be reviewed for ‘best practices’ to identify if the variation is due to variation in the raw peanut batches or in the processing procedures. Products 25 and 26 are two similar products, both RUTFs based on peanut with no other sources of phytate, and these products had phytate contents of 1055 and 371 mg/100 g, respectively. This may help in developing improved guidelines for manufacturers for reduction of phytate in the lipid‐based pastes.
This screening confirms a persistent picture of high phytate levels in processed cereal‐ and legume‐based products used for complementary feeding in developing countries. The lipid‐based products primarily based on peanut also had high phytate contents, exceeding the recommended molar ratio for specifically iron and, to some extent, zinc and calcium. The whole grain product samples from European supermarkets and selected for this screening as examples of the ‘worst‐case’ exposure of anti‐nutrients had high contents of phytate and tended to have phytate : mineral molar ratios unsuitable for infants and young children. The products represent a fraction of the highly variable commercial market of complementary foods available for European children, but call for the development of guidelines for the age at which whole grain products could be introduced in the complementary diet of children. One of the semi‐solid products produced for the European market (product 34) had moderate phytate content but very low contents of minerals (0.3 mg Fe/100 g, 0.2 mg Zn/100 g and 7 mg Ca/100 g) and, consequently, high molar ratios. The phytate : Zn ratio exceeded 100 due to the very low content of Zn. This specific semi‐solid whole grain complementary food product was not fortified and the mineral content was not declared. The findings of commercial product not meeting the general standards for accepted good nutritional quality for food for the age group it is marketed for need attention.
Polyphenols
When evaluating the nutritional significance of the polyphenolic compounds in foods, it is well recognised that polyphenols constitute a highly complex biochemical group with highly different nutritional properties (Bravo 1998), and the evaluation of the nutritional significance is therefore complex. Comparison between studies is constrained by the use of different analytical methods and measurement against different polyphenolic compounds as standard (Manach et al. 2004; Ajila et al. 2011; Champ 2008).
A main nutritional concern of polyphenolic compounds in this context of complementary foods is the chelating properties with minerals, especially iron, lowering the bioavailability. Early studies showed that intake of tea together with a meal inhibited non‐heme iron absorption and the inhibiting effect was identified to be caused by tannins, a group of large polyphenolic compounds with hydroxyl groups that bind with minerals in insoluble complexes (Disler et al. 1975; Hallberg & Rossander 1982). Derivates of hydroxybenzoic acids, which have up to three hydroxyl groups (gallic acid, a trihydroxybenzoic acid and gentistic acid, a dihydroxybenzoic acid), have similar iron‐binding properties as tannic acid and are therefore used as standards for assessing polyphenolic contents in foods of interest for evaluating the iron‐inhibiting properties (Brune et al. 1989). The polyphenolic contents of foods with negative impact on iron absorption were reported in tannic acid equivalents by Hallberg & Hulthen (2000), which also gave an algorithm for quantifying the impact of polyphenols on iron absorption from a meal. This algorithm is given in tannic acid equivalents. However, tannic acid is a large composite polyphenolic compound with dihydroxy‐ and trihydroxybenzoate groups, and a conversion to other standards is not specified. Hallberg & Hulthen (2000) reported no measurable tannin content in peanuts and cereals, except for whole wheat.
In a study using the Folin–Ciocalteu reagent method, cereals and legumes were screened for total polyphenols using gallic acid as a standard. The total polyphenolic content in a yellow soya bean and whole wheat grain were 18.7 and 16.2 mg gallic acid equivalents, respectively (Djordjevic et al. 2011), corresponding to 110 and 95 μmol gallic acid g−1 (170 g mol−1 for gallic acid). Compared with the maximum content in this study of 60 μmol gentistic acid g−1 dried food sample, and also that 17 of the 36 products have contents below 20 μmol DHB equivalents g−1, the levels were relatively low compared with unrefined cereals and legumes.
The product with the highest polyphenolic content (60 μmol DHB equivalents g−1) was a peanut‐based paste (product 25). Other peanut‐based products ranged from 14 to 48 μmol DHB equivalents g−1. A study on the effect of processing on the content of polyphenols in peanuts found a total polyphenolic content in boiled and roasted peanuts in the range of 20–38 gallic acid equivalents g−1 using Folin–Ciocalteu reagent method (Chukwumah et al. 2007). The results are not directly comparable to this study due to the different standards, but indicate that the level of hydrobenzoic acids is in a similar range as in the peanut pastes analysed in this study.
One study has specifically investigated the impact of phytate and polyphenols on iron absorption in in vitro and in vivo models from maize and bean varieties (Beiseigel et al. 2007), a promising contribution to quantifying the impact of polyphenols on iron absorption. However, the authors concluded that the applied method for extracting and measuring iron‐binding polyphenols – galloyl groups by the method developed by Brune et al. (1991) – was inadequate to measure the iron‐binding polyphenols in beans, and the values for measured polyphenols in the investigated maize and beans were not reported. That study on the iron absorption measured in vitro and in vivo was also used for validation of an algorithm for calculating iron absorption from food composition, developed from in vitro studies where synthetic tannins were added to test meals in controlled doses (Yun et al. 2004). It was concluded that the impact of polyphenolic compounds on iron absorption predicted by the algorithm developed from the in vitro tannin studies was not valid for beans and maize, and it was recommended that polyphenolic compounds needed to be separated in subclasses in food analysis in order to understand the impact on iron absorption.
In addition to the complexity of separating the polyphenolic compounds, a specific limitation in using the Folin–Ciocalteu method for quantifying the polyphenolic compounds in composite foods is that the Folin–Ciocalteu reagent also reacts with amino acids and peptides, a well‐recognised interference of the method (Soerensen et al. 1999; Chukwumah et al. 2007). The indication of high contents of polyphenolic compounds in, e.g. RUTFs, based on the Folin–Ciocalteu method is therefore not conclusive and may, in part, be due to the analytical interference of peptides and amino acids.
Another aspect of the anti‐nutritional properties of polyphenols is the potential hormonal effects of LMW isoflavons. As outlined earlier, if present, these compounds make up a small fraction of the total polyphenols in beans and legumes and are analytically challenging to identify and quantify. We made a separation of LMW polyphenolic compounds in two samples to explore the potential for a relatively simple separation (DAD) and characterisation (MECC) technique.
With this method, the interference with peptides is eliminated as the peptides are separated in the cationic fraction (column B), and the presence of the isoflavones daidzein and genistein in product 3, the product with soya, was detectable.
Establishing the upper limits for polyphenolic compounds needs to be performed for specific subclasses with anti‐nutritional properties, and not for the total polyphenolic pool.
Trypsin and chymotrypsin inhibitors
The levels of trypsin and chymotrypsin inhibitor in the analysed products were generally low, corresponding to only a fraction of the level in raw soya, indicating that the heat processing has efficiently degraded these anti‐nutritional compounds. So, the contents are not of immediate concern. However, attention should be given to possible critical residual levels of specifically trypsin inhibition in single products or production batches, particularly for the lipid‐based paste products, as these products are for direct consumption for feeding malnourished children. The fortified blended foods are processed for further cooking, e.g. 5–10 min of boiling, which will contribute to further degradation of residuals of the inhibitors.
The pancreatic enzyme inhibitors bind strongly to their corresponding enzymes in a 1:1 ratio (Arentoft et al. 1993; Soerensen et al. 1999). The levels of pancreatic enzymes in newborn or very young mammals are low and increase with age (Arentoft et al. 1993; Elnif et al. 1988; Hedemann et al. 2011). In piglets, the pancreatic activity, including trypsin and chymotrypsin activity, tends to drop during the critical weaning period (Thymann et al. 2007). This indicates risks of immature or temporary reduced pancreatic functions in young individuals.
In humans, a study investigated pancreatic excretion in infants and found a median trypsin excretion output in 16 children, average age of 3 months and with healthy pancreatic function, to be 45 IU in 15 min (range: 14–100); in a group of 14 children averaging 5 months of age, the median trypsin excretion output was 170 IU in 15 min (range: 70–240) (Carroccio et al. 1997). The same pattern of increased excretion from 3 to 5 months of age was found for chymotrypsin. The study confirmed the general pattern that pancreatic excretion increases with age. Therefore, special attention to potential harmful levels of enzyme inhibition must be focused on foods given to the very young children with immature pancreatic excretions of trypsin and chymotrypsin.
A study on pancreatic function of malnourished children in West Africa found that in 15 ‘protein–calorie’ malnourished children, average age of 1.6 years, the mean trypsin excretion was 14.1 IU in 15 min, which is significantly lower than that in a control group in France (Sauniere & Sarles 1988).
These measured excretion outputs over 15 min were applied to evaluate the potential harm of the highest content of trypsin inhibition measured in the products. The highest content was 0.51 IU g−1 in a lipid‐based peanut product produced in Asia. If a child consumes 50 g of this product, corresponding to about 260 kcal, it will ingest 25 IU of trypsin inhibitor. This trypsin inhibition capacity of a consumed portion would not be of concern in a healthy 1‐year‐old French control child reported in the West Africa study, as they averaged an excretion of >100 IU in 15 min. However, a trypsin inhibition capacity of 25 IU in a meal may potentially be harmful to the protein digestion in young and malnourished children with reduced pancreatic function as the ingested amount of inhibiting compounds can block a significant proportion and, in worse case, the total amounts of excreted trypsin in a 15‐min period.
The highest measured content of chymotrypsin inhibitor was 0.33 IU g−1 in a lipid‐based paste produced in Africa. In the study of pancreatic excretion, the median excretion in young infants (3 months of age) was 120 U in 15 min (range: 40–300), and the median excretion output in the older group of 5‐month‐old infants was 460 U in 15 min (range: 240–680). However, in the referred West Africa study, the group of 15 malnourished children from Abidjan had severely reduced chymotrypsin excretion – mean of 26 IU in 15 min – compared with the French control group, which exceeded 800 IU in chymotrypsin excretion output. A 50‐g portion of the product with the highest chymotrypsin inhibition would contribute 16 IU of inhibitor. It indicates that the risk of reaching potential critical levels of chymotrypsin inhibition in these products is less pronounced than the risk of potential critical trypsin inhibition, but there is a need for attention of residual chymotrypsin inhibition in products for young infants and malnourished children.
Overall, the contents of trypsin and chymotrypsin inhibitors in the analysed products are not of immediate concern, indicating that the food processing efficiently degrades these harmful anti‐nutrients. However, the measured residual trypsin inhibitor level of up to 0.51 IU g−1 needs further attention to establish if this level is a potential risk. The products are designed and distributed for the prevention and treatment of malnutrition in young children, who may have a reduced pancreatic function. There is a need for further documentation of the variation in residual contents of trypsin inhibitors in particular in products that may be consumed by malnourished children, and for investigating the need for specifying upper limits.
Compared with the published values for trypsin excretion in young children, this study indicated that the upper limit of residual trypsin inhibition in processed products delivered for consumption without further heating should be lower than 0.5 IU g−1, which was found to be the highest value in the analysed products. The exact upper limit needs to be based on screening of variations between production batches of each product in order to establish a realistic estimate of the total exposure to different groups of consumers, relative to the risks of reduced pancreatic excretion.
As discussed earlier, there are no alarming findings of high exposure, but attention is needed particularly to the exposure from lipid‐based pastes to be consumed by young and malnourished children without further heating.
Lectin
The content of ConA reactive glycoproteins is below 10 mg g−1 in all products, except in two products, both industrially processed instant porridges for the European market. The two products were from the same producer and were based on wholemeal wheat and oat. The relatively high level of ConA reactive glycoprotein could indicate insufficient heat treatment during processing. Attention is needed to finding lectins above the residual levels in products marketed for young children.
Overall, the contents of lectin in the products are residuals following the heat processing, and it has been suggested that residual levels of lectins have no general human health implications (Lajolo & Genovese 2002). There are no upper levels set for these residuals in processed foods, as proper heat processing easily eliminate these compounds. However, the presence of residual contents of lectins in products distributed as ready‐to‐eat foods for malnurished children needs further evaluation for potential harm. These products will not be heated before consumption, a processing which could contribute to eliminate the residuals.
Food components and processing
Of the total 52 samples, representing the 36 products, 27 samples contained soya and 25 samples did not contain soya. The information about the exact soya fraction was not available for all products. The soya contents were either whole soya, dehulled soya, defatted soya or soya isolates. One product was declared to have 95% ‘soya milk powder’ (product 19), while others were cereal‐soy blends in different compositions.
Raw soya beans contain all the anti‐nutrients of concern and the quality of the final product depends on adequate processing. The general effect of the processing techniques of soya beans on the nutrient composition is generally well described, but the effect on the profile in a final blended food may need to be investigated further. For example, products 3 and 4 are both fortified blended foods based on maize and soya in the ratio of 58–78% maize and 20% soya. In product 3, the soya component is whole soya, while product 4 contains dehulled soya. The phytate contents in the two products are almost the same, indicating that little or no nutritional benefit in terms of phytate reduction is achieved by dehulling the soya, either because the phytate in the soya bean is located in other compartments, e.g. in the cotyledon, or because the unrefined maize is the dominant source in the blended foods.
Product 19 is a pure soya product, declared to be made from soya milk powder. This product has low contents of the analysed anti‐nutrient, indicating that intensive processing can eliminate the anti‐nutrients from soya. The product is declared as a supplement to be added to a habitual meal in a daily portion of 12 g.
The products sampled from the European market selected for having a component of whole grain, primarily wheat, as well as the semi‐solid baby foods, did have variable anti‐nutritional profiles. These products are available in a highly diverse commercial market and in cultures where the majority of children will get a highly varied diet combining family food and a selection of commercial products. It is therefore unlikely that single commercial product will contribute a large proportion of a child's diet. However, the marketing of whole grain products for complementary feeding not meeting basic nutritional recommendations for the age group calls for recommendations for which age whole grain products should be introduced to.
The screening included one product based on white rice as the only plant ingredient (product 21). This product had low or no contents of the measured anti‐nutrients, indicating that rice is a recommended food in complementary foods in order to limit anti‐nutrients. In the referred review of phytate in complementary foods (Gibson et al. 2010), foods with rice and no legumes had low phytate contents, equal to foods based on starchy roots and tubers.
For the peanut‐based lipid pastes, the variation in anti‐nutrients, particularly phytate, was high, also between similar products. There is no obvious explanation for the difference, and further attention should focus on identifying the phytate source and possibly differences in processing.
The highest level of residual trypsin and chymotrypsin inhibitors was found in peanut‐based products, and it is recommended that variation between products and production batches is further investigated in order to establish more documentation on risk exposure.
The study was limited by the number of samples being analysed. However, it provides a first step for identifying specific needs for improving nutritional quality of complementary foods and food aid and products for infants and young children, through setting upper limits for specific anti‐nutrients, and improved guidelines for manufactures.
Conclusion
The identified needs are as follows:
There is a need for closer monitoring of declared and actual levels of mineral fortification of processed foods to ensure that the nutritional quality is correctly declared.
This screening confirmed a consistent high level of phytate in most complementary foods and food aid products for children. There is a need for setting upper limits and for development of guidelines to manufacturers on how to efficiently reduce phytate in processed foods, e.g. by improved mechanical refinement, or through the use of industrially processed microbial phytases. The high molar ratios between phytate and especially iron is a combined result of high phytate and, in some products, low iron contents. Fewer products exceeded the molar ratios for phytate : Zn and phytate : Ca than for iron, but the overall picture is that the level of phytate in complementary food is high, and reduction will improve the nutritional quality of the products, and likely contribute to improved child nutrition. Some almost identical products had highly different phytate contents, especially for the peanut‐based pastes, and tracing ‘best practices’ for the processing of the product with low phytate may help identify practical solutions to phytate reduction in commercial productions.
The screening showed that the levels of anti‐nutrients in complementary foods were variable with the ingredients and process used. High contents of anti‐nutrients are found across products based on wheat, maize and other cereals, and products with and without soya. Intensive processing can reduce the anti‐nutrients in, e.g. soya, as products based on processed soya had a highly acceptable nutritional profile. However, there is a need for closer monitoring of the total anti‐nutrient contents of composite foods, as processing of one food component to reduce anti‐nutrients, e.g. dehulling soya, does not necessarily significantly reduce the total anti‐nutrient contents in a blended food. This was seen in the comparison of products 3 and 4. In these products, a 20% soya component was dehulled in product 4, but the phytate content is on the same level as in product 3, which contained unrefined soya.
The contents of trypsin and chymotrypsin inhibitors are not of immediate concern, but there is a need for setting upper levels for residuals of these inhibitors in products specifically manufactured and distributed for young infants and malnourished children at risk of reduced pancreatic excretion. Also, the levels of lectins were not of immediate concern, but possible negative impact of residuals of lectins in the same products needs further investigation.
Source of funding
The study was funded by the Global Alliance for Improving Nutrition (GAIN), Geneva, Switzerland (contract 109300).
Conflicts of interest
All authors of this paper have declared to have no conflicts of interest in the present study.
Contributions
Søren Kjærsgaard Rasmussen (SKR) was in charge of the phytic acid analyses. Jens Christian Sørensen (JCS) and Hilmer Sørensen (HS) were in charge of the analyses of polyphenols, trysin and chymotrypsin inhibitors and lectin/glycoproteins. Nanna Roos (NR) was in charge of sampling, sample preparation and mineral analyses, and for coordination of the analysis. Sandra L. Huffman (SLH) and André Briand (AB) developed the initial idea to the study. Zhenyu Yang (ZY) supported the study design, sampling and data compiling. NR compiled the data and drafted the manuscript. All authors contributed to the final edition of the manuscript.
Acknowledgement
Manufactures and organizations providing food samples for the present study are acknowledged for their kind collaboration. Information on the contents of minerals and anti‐nutrients in specific products is communicated directly to the manufactures.
Footnotes
Phytate is the insoluble complex formed between phytic acid and a mineral. In food chemistry referring to anti‐nutritional effects, phytate and phytic acid are interchangeable terminologies.
References
- Agostoni C., Axelsson I., Goulet O., Koletzko B., Michaelsen K.F., Puntis J. et al (2006) Soy protein infant formulae and follow‐on formulae: a commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 42, 352–361. [DOI] [PubMed] [Google Scholar]
- Ajila C.M., Brar S.K., Verma M., Tyagi R.D., Godbout S. & Valero J.R. (2011) Extraction and analysis of polyphenols: recent trends. Critical Reviews in Biotechnology 31, 227–249. [DOI] [PubMed] [Google Scholar]
- Andang'o P.E., Osendarp S.J., Ayah R., West C.E., Mwaniki D.L., De Wolf C.A. et al (2007) Efficacy of iron‐fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet 369, 1799–1806. [DOI] [PubMed] [Google Scholar]
- Arentoft A.M., Mortensen K. & Sørensen H. (1991) Pea proteinase inhibitor activity. Aspects of Applied Biology 27, 211–215. [Google Scholar]
- Arentoft A.M., Frokiaer H., Michaelsen S., Soerensen H. & Soerensen S. (1993) High‐performance capillary electrophoresis for the determination of trypsin and chymotrypsin inhibitors and their association with trypsin, chymotrypsin and monoclonal‐antibodies. Journal of Chromatography. A 652, 189–198. [DOI] [PubMed] [Google Scholar]
- Armour J.C., Perera R.L.C., Buchan W.C. & Grant G. (1998) Protease inhibitors and lectins in soya beans and effects of aqueous heat‐treatment. Journal of the Science of Food and Agriculture 78, 225–231. [Google Scholar]
- Banwell J.G., Boldt D.H., Meyers J. & Weber F.L. Jr (1983) Phytohemagglutinin derived from red kidney bean (Phaseolus vulgaris): a cause for intestinal malabsorption associated with bacterial overgrowth in the rat. Gastroenterology 84, 506–515. [PubMed] [Google Scholar]
- Beiseigel J.M., Hunt J.R., Glahn R.P., Welch R.M., Menkir A. & Maziya‐Dixon B.B. (2007) Iron bioavailability from maize and beans: a comparison of human measurements with Caco‐2 cell and algorithm predictions. The American Journal of Clinical Nutrition 86, 388–396. [DOI] [PubMed] [Google Scholar]
- Bhatia J. & Greer F. (2008) Use of soy protein‐based formulas in infant feeding. Pediatrics 121, 1062–1068. [DOI] [PubMed] [Google Scholar]
- Bjerg B., Ebmeyer E., Eggum B.O., Larsen T., Robbelen G. & Soerensen H. (1988) The nutritive‐value of 10 inbred lines of faba beans (Vicia faba L.) in relation to their content of antinutritional constituents and protein‐quality. Plant Breeding 101, 277–291. [Google Scholar]
- Bohn L., Meyer A.S. & Rasmussen S.K. (2008) Phytate: impact on environment and human nutrition. A challenge for molecular breeding. Journal of Zhejiang University. Science B 9, 165–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling‐Frankenbach S.D., Peter C.M., Douglas M.W., Snow J.L., Parsons C.M. & Baker D.H. (2001) Efficacy of phytase for increasing protein efficiency ratio values of feed ingredients. Poultry Science 80, 1578–1584. [DOI] [PubMed] [Google Scholar]
- Bravo L. (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews 56, 317–333. [DOI] [PubMed] [Google Scholar]
- Brune M., Rossander L. & Hallberg L. (1989) Iron‐absorption and phenolic‐compounds – importance of different phenolic structures. European Journal of Clinical Nutrition 43, 547–558. [PubMed] [Google Scholar]
- Brune M., Hallberg L. & Skanberg A.B. (1991) Determination of iron‐binding phenolic groups in foods. Journal of Food Science 56, 128–131. [Google Scholar]
- Brune M., Rossanderhulten L., Hallberg L., Gleerup A. & Sandberg A.S. (1992) Iron‐absorption from bread in humans – inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. The Journal of Nutrition 122, 442–449. [DOI] [PubMed] [Google Scholar]
- Bures L., Entliche G., Ticha M. & Kocourek J. (1973) Studies on phytohemagglutinins. Hemagglutinins of pea and lentil – advantage of their application in comparison to concanavalin A in some agglutination reactions. Experientia 29, 1546–1547. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Cavataio F., Montalto G., Soresi M., Lorello D., Notarbartolo A. et al (1997) Evaluation of pancreatic function development after hydrolyzed protein‐based and soy‐based formulas in unweaned infants. Scandinavian Journal of Gastroenterology 32, 273–277. [DOI] [PubMed] [Google Scholar]
- Champ M. (2008) Non‐nutrient bioactive substaces of pulses. The British Journal of Nutrition 88 (Suppl. 3), S307–S319. [DOI] [PubMed] [Google Scholar]
- Chan S.S.L., Ferguson E.L., Bailey K., Fahmida U., Harper T.B. & Gibson R.S. (2007) The concentrations of iron, calcium, zinc and phytate in cereals and legumes habitually consumed by infants living in East Lombok, Indonesia. Journal of Food Composition and Analysis 20, 609–617. [Google Scholar]
- Chukwumah Y., Walker L., Vogler B. & Verghese M. (2007) Changes in the phytochemical composition and profile of raw, boiled, and roasted peanuts. Journal of Agricultural and Food Chemistry 55, 9266–9273. [DOI] [PubMed] [Google Scholar]
- Davidsson L., Galan P., Cherouvrier F., Kastenmayer P., Juillerat M.A., Hercberg S. et al (1997) Bioavailability in infants of iron from infant cereals: effect of dephytinization. The American Journal of Clinical Nutrition 65, 916–920. [DOI] [PubMed] [Google Scholar]
- de Pee S. & Bloem M.W. (2009) Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6‐ to 23‐month‐old children and for treating moderate malnutrition among 6‐to 59‐month‐old children. Food and Nutrition Bulletin 30, S434–S463. [DOI] [PubMed] [Google Scholar]
- Disler P.B., Lynch S.R., Charlton R.W., Torrance J.D., Bothwell T.H., Walker R.B. et al (1975) Effect of tea on iron‐absorption. Gut 16, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic T.M., Siler‐Marinkovic S.S. & Dimitrijevic‐Brankovic S.I. (2011) Antioxidant activity and total phenolic content in some cereals and legumes. International Journal of Food Properties 14, 175–184. [Google Scholar]
- Elnif J., Hansen N.E., Mortensen K. & Sørensen H. (1988) Properties of Mink Trypsinogen/trypsin and Chymotrypsinogen/Chymotrypsin Compared with Corresponding Properties of these Enzymes from Other Animals . In Biology, Pathology and Genetics of Fur Bearing Animals. 4th International Scientific Congress in Fur Animal Production, Toronto & Wisconsin: pp 308–319.
- European Communities (2006) Commision Directive 2006/125/EC of 5 December 2006 on Processed Cereal‐Based Foods and Baby Foods for Infants and Young Children . Official Journal of the European Union, The Commisson of the European Communities, Brussel, Belgium.
- Fontaine T.D., Pons W.A. & Irving G.W. (1946) Protein‐phytic acid relationship in peanuts and cottonseed. The Journal of Biological Chemistry 164, 487–507. [PubMed] [Google Scholar]
- Gibbs M., Bailey K.B., Lander R.D., Fahmida U., Perlas L., Hess S.Y. et al (2011) The adequacy of micronutrient concentrations in manufactured complementary foods from low‐income countries. Journal of Food Composition and Analysis 24, 418–426. [Google Scholar]
- Gibson R.S., Ferguson E.L. & Lehrfeld J. (1998) Complementary foods for infant feeding in developing countries: their nutrient adequacy and improvement. European Journal of Clinical Nutrition 52, 764–770. [DOI] [PubMed] [Google Scholar]
- Gibson R.S., Bailey K.B., Gibbs M. & Ferguson E.L. (2010) A review of phytate, iron, zinc, and calcium concentrations in plant‐based complementary foods used in low‐income countries and implications for bioavailability. Food and Nutrition Bulletin 31, S134–S146. [DOI] [PubMed] [Google Scholar]
- Hallberg L. & Hulthen L. (2000) Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. The American Journal of Clinical Nutrition 71, 1147–1160. [DOI] [PubMed] [Google Scholar]
- Hallberg L. & Rossander L. (1982) Effect of different drinks on the absorption of non‐heme iron from composite meals. Human Nutrition. Applied Nutrition 36, 116–123. [PubMed] [Google Scholar]
- Hedemann M.S., Clausen T.N. & Jensen S.K. (2011) Changes in digestive enzyme activity, intestine morphology, mucin characteristics and tocopherol status in mink kits (Mustela neovision) during the weaning period. Animal 5, 394–402. [DOI] [PubMed] [Google Scholar]
- Hotz C. & Gibson R.S. (2007) Traditional food‐processing and preparation practices to enhance the bioavailability of micronutrients in plant‐based diets. The Journal of Nutrition 137, 1097–1100. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F. (2004) Phytic acid degradation as a means of improving iron absorption. International Journal for Vitamin and Nutrition Research 74, 445–452. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F., de Ranum P., Pee S., Biebinger R., Hulthen L., Johnson Q. et al (2010) Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food and Nutrition Bulletin 31, S7–S21. [DOI] [PubMed] [Google Scholar]
- Lajolo F.M. & Genovese M.I. (2002) Nutritional significance of lectins and enzyme inhibitors from legumes. Journal of Agricultural and Food Chemistry 50, 6592–6598. [DOI] [PubMed] [Google Scholar]
- Machado F.P.P., Queiroz J.H., Oliveira M.G.A., Piovesan N.D., Peluzio M.C.G., Costa N.M.B. et al (2008) Effects of heating on protein quality of soybean flour devoid of Kunitz inhibitor and lectin. Food Chemistry 107, 649–655. [Google Scholar]
- Manach C., Scalbert A., Morand C., Remesy C. & Jimenez L. (2004) Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition 79, 727–747. [DOI] [PubMed] [Google Scholar]
- Marquis G.S., Habicht J.P., Lanata C.F., Black R.E. & Rasmussen K.M. (1997) Breast milk or animal‐product foods improve linear growth of Peruvian toddlers consuming marginal diets. The American Journal of Clinical Nutrition 66, 1102–1109. [DOI] [PubMed] [Google Scholar]
- Michaelsen K.F., Hoppe C., Roos N., Kaestel P., Stougaard M., Lauritzen L. et al (2009) Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food and Nutrition Bulletin 30, S343–S404. [DOI] [PubMed] [Google Scholar]
- Neumann C.G., Murphy S.P., Gewa C., Grillenberger M. & Bwibo N.O. (2007) Meat supplementation improves growth, cognitive, and behavioral outcomes in Kenyan children. The Journal of Nutrition 137, 1119–1123. [DOI] [PubMed] [Google Scholar]
- Pontoppidan K., Pettersson D. & Sandberg A.S. (2007) Interaction of phytate with protein and minerals in a soybean‐maize meal blend depends on pH and calcium addition. Journal of the Science of Food and Agriculture 87, 1886–1892. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Ewen S.W., Grant G., van Peumans W.J., Damme E.J., Rubio L. et al (1990) Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion 46 (Suppl. 2), 308–316. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Ewen S.W., Grant G., van Peumans W.J., Damme E.J., Coates M.E. et al (1995) Lectins and also bacteria modify the glycosylation of gut surface receptors in the rat. Glycoconjugate Journal 12, 22–35. [DOI] [PubMed] [Google Scholar]
- Rham O.D. & Jost T. (1979) Phytate‐protein interactions in soybean extracts and low‐phytate soy protein products. Journal of Food Science 44, 596–600. [Google Scholar]
- Sauniere J.F. & Sarles H. (1988) Exocrine pancreatic function and protein‐calorie malnutrition in Dakar and Abidjan (West Africa) – silent pancreatic insufficiency. The American Journal of Clinical Nutrition 48, 1233–1238. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R. & Lamuela‐Raventos R.M. (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin‐Ciocalteu reagent. Oxidants and Antioxidants, PtA Book Series: Methods in Enzymology 299, 152–178. [Google Scholar]
- Soerensen H., Soerensen S., Bjergegaard C. & Michaelsen S. (1999) Chromatography and Capillary Electrophoresis in Food Analysis. The Royal Society of Chemistry: Cambridge, UK. [Google Scholar]
- Thymann T., Soerensen K.U., Hedemann M.S., Elnif J., Jensen B.B., Banga‐Mboko H. et al (2007) Antimicrobial treatment reduces intestinal microflora and improves protein digestive capacity without changes in villous structure in weanling pigs. The British Journal of Nutrition 97, 1128–1137. [DOI] [PubMed] [Google Scholar]
- Umeta M., West C.E. & Fufa H. (2005) Content of zinc, iron, calcium and their absorption inhibitors in foods commonly consumed in Ethiopia. Journal of Food Composition and Analysis 18, 803–817. [Google Scholar]
- World Health Organization (WHO) (2004) Human Vitamin and Mineral Requirements . Report of a joint FAO/WHO consultation, Bangkok, Thailand. Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO), Rome.
- WHO (2008) Proceedings of the WHO, UNICEF, WFP and UNHCR Consultation on the dietary management of moderate malnutrition in under‐5 children. Compiled by: Shoham J & Duffield A. Available at: http://www.who.int/nutrition/publications/moderate_malnutrition/MM_meeting_report.pdf
- Yun S., Habicht J.P., Miller D.D. & Glahn R.P. (2004) An in vitro digestion/Caco‐2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. The Journal of Nutrition 134, 2717–2721. [DOI] [PubMed] [Google Scholar]
- Zung A., Glaser T., Kerem Z. & Zadik Z. (2008) Breast development in the first 2 years of life: an association with soy‐based infant formulas. Journal of Pediatric Gastroenterology and Nutrition 46, 191–195. [DOI] [PubMed] [Google Scholar]


