Abstract
The relationship between breastfeeding, respiratory and other allergic disorders has been controversial. Our aim was to investigate the relationships between breastfeeding, respiratory outcomes, eczema and atopy at 15 months of age in a prospective birth cohort in New Zealand.
A total of 1105 children were enrolled at birth, and 1011 (91.2%) were followed up at 15 months. Logistic regression was used to model associations between breastfeeding duration and respiratory outcomes, eczema and atopy after adjusting for relevant confounding variables: ethnicity, socio‐economic status, parity, body mass index, smoking in pregnancy, gender and respiratory infections in the first 3 months of life.
Breastfeeding was associated with a significant reduction in the risk of adverse respiratory outcomes at 15 months. After adjustment for confounders, each month of exclusive breastfeeding reduced the risk of doctor‐diagnosed asthma by 20% (odds ratio 0.80, 95% confidence interval 0.71 to 0.90), wheezing by 12% (0.88, 0.82 to 0.94) and inhaler use by 14% (0.86, 0.78 to 0.93). Associations for both exclusive and additional breastfeeding durations, and respiratory outcomes remained independently significant when modelled simultaneously. Although independently associated with all respiratory outcomes, adjusting for parental history of allergic disease or maternal history of asthma did not alter our findings. Breastfeeding was not associated with eczema or atopy at 15 months.
In conclusion, there was a significant protective effect of breastfeeding on infant wheezing and other adverse respiratory outcomes that may be early indicators of asthma in New Zealand children.
Keywords: breastfeeding, asthma, wheeze, eczema, prospective birth cohort, children
Introduction
The prevalence of atopy, allergic disease and asthma has increased over the last 40 years in many industrialized nations, including New Zealand. A number of longitudinal birth cohorts such as those in Tucson, USA (Taussig et al. 1989), Melbourne (Phelan et al. 2002) and Perth (Joseph‐Bowen et al. 2004), Australia, and the German multi‐centre atopy study (MAS‐90) (Bergmann et al. 1994) have been established to explore potential risk factors for the development of atopy, allergic disease and asthma.
The relationship between early childhood nutrition, particularly breastfeeding, and subsequent respiratory and other allergic disorders has been controversial. Some studies have shown that breastfeeding is protective (Oddy et al. 1999; Wright et al. 2001; Kull et al. 2004), while others suggest that it is a risk factor for asthma in later childhood and adulthood (Sears et al. 2002), and in older children with atopy and a maternal history of asthma (Wright et al. 2001). A meta‐analysis of prospective studies by Gdalevich et al. (2001), subsequently updated by Ip et al. (2007), has suggested that breastfeeding reduces the risk of asthma. However, data from the Sears study (Sears et al. 2002), another New Zealand study, could not be included as it failed to meet the inclusion criteria as a result of the way that data about breastfeeding was collected and presented.
The aim of this study was to investigate the relationship between breastfeeding and respiratory outcomes, atopy and eczema at 15 months of age in a prospective birth cohort in New Zealand. This included the prospective collection of infant feeding histories, with data about exclusive breastfeeding, to allow a more comprehensive analysis of potential relationships between breastfeeding and respiratory outcomes in New Zealand children.
Materials and methods
Study population
The New Zealand Asthma and Allergy Cohort Study is a two‐centre prospective birth cohort that was established in 1997. Ethics committee approval for the study was obtained from the Wellington and Canterbury Regional Ethics Committees. Full details of the cohort, including assembly, demographics and methodology, have been published elsewhere (Epton et al. 2007).
Recruitment took place in Christchurch and Wellington between 1997 and 2001. Expectant mothers were recruited by midwives before birth, and consent confirmed by the study team soon afterwards. No explicit attempt was made to recruit families with a history of atopy, allergic disease or asthma. The cohort sample is largely representative of the wider New Zealand population based on national demographic statistics (Epton et al. 2007).
Breastfeeding
Detailed information about infant feeding was collected using questionnaires administered soon after birth, and at 3, 6 and 15 months. Breastfeeding was assessed in two ways: the duration of ‘exclusive’ breastfeeding (age when infant formula, food or other drinks, except water, were introduced) and the duration of ‘any’ breastfeeding (age when breastfeeding was stopped). From these, the duration of ‘additional’ breastfeeding (duration of any breastfeeding minus the duration of exclusive breastfeeding) was derived. These were modelled as continuous variables.
One hundred and seventy (16.8%) children were still being breastfed at their 15‐month assessment. The duration of any breastfeeding for these children was therefore determined from the date of this assessment.
Outcomes
Respiratory outcomes included a parental report of the following: (1) ‘doctor's diagnosis of asthma’; (2) ‘ever wheezed’; (3) ‘ever used an inhaler’; (4) ‘ever wheezed’ AND ‘doctor's diagnosis of asthma’ AND ‘ever used an inhaler’; and (5) current asthma defined as ‘doctor's diagnosis of asthma’ and [‘ever wheezed’ OR ‘used an inhaler’] by 15 months.
Eczema was defined as a parental report of eczema, and atopy was determined by the results of a skin prick test to common food and environmental allergens. Children were categorized as atopic if there was an allergen to histamine wheal ratio of greater than 0.5 following subtraction of the negative control wheal size after 15 min. A ratio measure was used in order to control for the variable response to histamine in infancy and inter‐operator and inter‐batch allergen variability (Meinert et al. 1994; Joint Task Force on Practice Parameters, representing the American Academy of Allergy Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology 1995).
Confounding variables
Potential confounding variables included sex of child, gestational age <37 weeks, study site, smoking in pregnancy, household smoking at 15 months and respiratory infections (parental report of colds, coughs, and chest and ear infections) in the first 3 months of life. These were all modelled as binary covariates. Ethnicity (Maori, Pacific, Pakeha/European and others) was modelled as a prioritized categorical variable, whereas birthweight and other anthropometric birth measures, parity, crowding (number of rooms per person), body mass index (BMI) at 15 months and the New Zealand Deprivation Index, a marker of socio‐economic status (Salmon & Crampton 2001), were modelled as continuous variables.
Those entered into the final models were: ethnicity, socio‐economic status, parity, BMI at 15 months, smoking in pregnancy, sex of child and respiratory infections in the first 3 months of life. Although relevant, birthweight and household smoking were not included in the final models because of close correlation with BMI at 15 months and smoking in pregnancy, respectively.
Parental history of allergic disease (asthma or eczema or hay fever in at least one parent) and maternal history of asthma were included as potential confounders in secondary analyses.
Statistical analysis
Unconditional binary logistic regression analyses were used to test the associations between breastfeeding and outcomes. Univariate unadjusted odds ratios and 95% confidence intervals were calculated for durations of exclusive and any breastfeeding. Multivariate models incorporating the confounding variables outlined above were used to generate adjusted odds ratios and 95% confidence intervals. In addition, both the duration of exclusive and additional breastfeeding were modelled simultaneously to determine the independent effect of these exposures on outcomes.
To investigate possible disease‐related modification of exposure and to ensure that the exposure preceded the outcome, therefore assessing the likelihood of a causal relationship between breastfeeding duration and respiratory outcomes, children with onset of wheeze during the exclusive or any breastfeeding periods were excluded from the relevant wheeze models in secondary analyses.
All analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA, Version 13, 2004). Missing data were not imputed.
Statistical power
The rationale for the cohort size was based on pilot data and research available on prevalence of atopy and asthma in New Zealand at the time (The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee 1998; Sawyer et al. 1998). Assuming that approximately 10% of the sample would be diagnosed with asthma by 15 months of age, and those exposed to more than the median duration of breastfeeding showed a >50% decline in the rates of asthma diagnosis at 15 months, then a total sample of 1000 children would have more than 90% power to detect this reduction (relative risk < 0.5) as statistically significant (two‐tailed α = 0.05).
Results
A total of 1105 children were enrolled. Of these, 1 child died, 27 parents withdrew consent and 50 children were lost to follow‐up. The parents of a further 16 children declined follow‐up at 15 months. Overall, 1011 (91.2%) children remained available for follow‐up at 15 months.
The 6‐month questionnaire was not completed for 24 children, thus restricting the exclusive breastfeeding data to 987 children. Parental history of allergic diseases required both parents to be living with the child at the time of the 3‐month questionnaire; hence, there were missing data for 111 children. Key characteristics of the cohort are listed in Table 1 and the prevalence of outcomes is given in Table 2.
Table 1.
Prevalence of selected exposure characteristics in children from the New Zealand Asthma and Allergy Birth Cohort
| Characteristics | No. (%) |
|---|---|
| Male (n = 1011) | 515 (50.9) |
| Māori (n = 1011) | 148 (14.6) |
| Gestational age <37 weeks (n = 1011) | 37 (3.7) |
| Mean (SD) gestational age (weeks; n = 1011) | 39.6 (1.47) |
| Mean (SD) NZ Index of Deprivation (n = 1011) | 4.7 (2.87) |
| Mean (SD) BMI (n = 994) | 17.5 (1.72) |
| Smoking in pregnancy (n = 1011) | 206 (20.4) |
| Respiratory infections ≤3 months (n = 1011) | 623 (61.6) |
| Smoking in household at 15 months (n = 1011) | 335 (33.1) |
| Parental history of asthma and allergic disease (n = 900) | 751 (83.4) |
| Maternal history of asthma (n = 1009) | 277 (27.5) |
| Exclusive breastfeeding | |
| Age when infant formula, food or other drinks introduced (n = 987) | |
| ≤1 week | 337 (34.1) |
| 1 week–1 month | 125 (12.7) |
| 1–2 months | 101 (10.2) |
| 2–3 months | 84 (8.5) |
| 3–4 months | 156 (15.8) |
| 4–5 months | 131 (13.3) |
| 5–6 months | 52 (5.3) |
| >6 months | 1 (0.1) |
| Any breastfeeding | |
| Age when breastfeeding stopped (n = 1011) | |
| ≤1 week | 60 (5.9) |
| 1 week–1 month | 65 (6.4) |
| 1–2 months | 54 (5.3) |
| 2–3 months | 56 (5.5) |
| 3–4 months | 33 (3.3) |
| 4–5 months | 41 (4.1) |
| 5–6 months | 72 (7.1) |
| 6–12 months | 335 (33.1) |
| >12 months | 295 (29.2) |
Data given as number (%) unless otherwise stated; NZ, New Zealand.
Table 2.
Prevalence of 15 month outcomes in children from the New Zealand Asthma and Allergy Birth Cohort
| Outcomes at 15 months | No. (%) |
|---|---|
| Doctor‐diagnosed asthma (n = 1009) | 124 (12.3) |
| Ever wheezed (n = 1011) | 397 (39.3) |
| Ever used an inhaler (n = 1010) | 222 (22.0) |
| Wheeze AND doctor‐diagnosed asthma AND inhaler (n = 1008) | 100 (9.9) |
| Current asthma (n = 1008) | 119 (11.8) |
| Ever had eczema (n = 1006) | 398 (39.6) |
| Atopy (n = 889) | 249 (28.0) |
Data given as number (%) unless otherwise stated.
There were strong associations (all, P < 0.001) between the respiratory outcomes indicating that the outcome definitions were internally consistent.
The median duration of exclusive breastfeeding was 1.4 months [interquartile range (IQR) 0–4] and of any breastfeeding was 9.0 months (IQR 4–13). The duration of exclusive breastfeeding was correlated with the duration of additional breastfeeding beyond the exclusive period (r = 0.482, P < 0.001). However, the duration of exclusive breastfeeding was found to be a stronger determinant of respiratory outcomes than the duration of any breastfeeding (Table 3).
Table 3.
Odds ratios (95% confidence intervals) for the individual effects of each month of exclusive and any breastfeeding on outcomes at 15 months
| Outcome | Exclusive breastfeeding | Any breastfeeding | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | P‐value | Adjusted* | P‐value | Unadjusted | P‐value | Adjusted* | P‐value | |
| Doctor‐diagnosed asthma | 0.79 (0.71–0.89) | <0.001 | 0.80 (0.71–0.90) | <0.001 | 0.92 (0.89–0.95) | <0.001 | 0.93 (0.89–0.97) | <0.001 |
| Ever wheezed | 0.87 (0.82–0.93) | <0.001 | 0.88 (0.82–0.94) | 0.001 | 0.95 (0.92–0.97) | <0.001 | 0.96 (0.93–0.98) | 0.001 |
| Ever used an inhaler | 0.85 (0.78–0.92) | <0.001 | 0.86 (0.78–0.93) | <0.001 | 0.91 (0.89–0.94) | <0.001 | 0.92 (0.90–0.95) | <0.001 |
| Wheeze AND doctor‐diagnosed asthma AND inhaler | 0.76 (0.67–0.86) | <0.001 | 0.76 (0.67–0.87) | <0.001 | 0.91 (0.88–0.95) | <0.001 | 0.92 (0.88–0.96) | <0.001 |
| Current asthma | 0.78 (0.70–0.88) | <0.001 | 0.79 (0.70–0.90) | <0.001 | 0.92 (0.89–0.96) | <0.001 | 0.93 (0.89–0.97) | <0.001 |
| Ever had eczema | 0.96 (0.90–1.03) | 0.231 | 0.96 (0.90–1.03) | 0.244 | 1.00 (0.98–1.03) | 0.738 | 1.00 (0.98–1.03) | 0.789 |
| Atopy | 1.03 (0.96–1.11) | 0.373 | 1.03 (0.96–1.11) | 0.397 | 1.03 (1.00–1.06) | 0.066 | 1.03 (1.00–1.06) | 0.051 |
Adjusted for ethnicity, socio‐economic status, parity, body mass index at 15 months, smoking in pregnancy, gender and respiratory infections in the first 3 months of life.
Longer durations of exclusive (all, P ≤ 0.001) and any (all, P ≤ 0.001) breastfeeding were associated with a significant reduction in the risk of adverse respiratory outcomes at 15 months (Table 3). After adjustment for confounders, each month of exclusive breastfeeding reduced the risk of doctor‐diagnosed asthma by 20% (odds ratio 0.80, 95% confidence interval 0.71–0.90), wheezing by 12% (0.88, 0.82–0.94), inhaler use by 14% (0.86, 0.78–0.93), ‘wheeze AND doctor‐diagnosed asthma AND inhaler’ by 24% (0.76, 0.67–0.87) and ‘current asthma’ by 21% (0.79, 0.70–0.90). Risks were reduced by 7% (0.93, 0.89–0.97), 4% (0.96, 0.93–0.98), 8% (0.92, 0.90–0.95), 8% (0.92, 0.88–0.96) and 7% (0.93, 0.89–0.97), respectively, for each month of any breastfeeding.
There was no significant association between breastfeeding and the risk of eczema or atopy at 15 months of age.
The results of analyses which modelled both exclusive and additional breastfeeding simultaneously, as well as other potential confounders, are given in Table 4. Associations for breastfeeding (exclusive and additional) and respiratory outcomes remained independently significant in each of the models. No significant associations were found for eczema or atopy.
Table 4.
Adjusted odds ratios (95% confidence intervals) for the independent effects of each month of exclusive and additional breastfeeding on outcomes at 15 months
| Outcome | Exclusive breastfeeding* | P‐value | Additional breastfeeding* | P‐value |
|---|---|---|---|---|
| Doctor‐diagnosed asthma | 0.81 (0.72–0.92) | <0.001 | 0.95 (0.91–0.99) | 0.021 |
| Ever wheezed | 0.88 (0.82–0.95) | <0.001 | 0.97 (0.94–1.0) | 0.038 |
| Ever used an inhaler | 0.87 (0.80–0.95) | 0.002 | 0.93 (0.90–0.97) | <0.001 |
| Wheeze AND doctor‐diagnosed asthma AND inhaler | 0.77 (0.67–0.89) | <0.001 | 0.95 (0.90–0.99) | 0.026 |
| Current asthma | 0.80 (0.71–0.91) | <0.001 | 0.95 (0.91–1.00) | 0.034 |
| Ever had eczema | 0.96 (0.90–1.03) | 0.214 | 1.02 (0.99–1.05) | 0.274 |
| Atopy | 1.03 (0.95–1.11) | 0.466 | 1.03 (1.0–1.07) | 0.076 |
Adjusted for ethnicity, socio‐economic status, parity, body mass index at 15 months, smoking in pregnancy, gender and respiratory infections in the first 3 months of life.
Figure 1 illustrates the reduction in risk of doctor‐diagnosed asthma to be expected for every 3 months of additional breastfeeding following 0–3 or greater than 3 months of exclusive breastfeeding. The figure shows that children with the lowest risk were exclusively breastfed for a period of at least 3 months. This risk was then further reduced by longer durations of additional breastfeeding.
Figure 1.
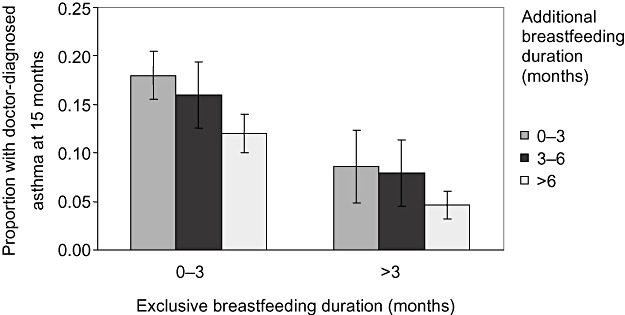
Doctor‐diagnosed asthma (95% confidence intervals) at 15 months for every 3 months of additional breastfeeding following 0–3 or greater than 3 months of exclusive breastfeeding.
Although both parental history of allergic disease and maternal history of asthma were independently associated with all respiratory outcomes in secondary analyses, neither altered the odds ratios or significance levels to any appreciable degree.
Only 29 of the 397 wheezy children had wheezed during lactation, and only six children had wheezed prior to the end of the exclusive breastfeeding period. Excluding these children from the relevant wheeze models did not change our findings. The unadjusted odds ratios were 0.87 (0.81–0.93, P < 0.001) for exclusive and 0.94 (0.92–0.97, P < 0.001) for any breastfeeding. Adjusted odds ratios were 0.88 (0.82– 0.94, P < 0.001) and 0.95 (0.93–0.98, P = 0.001), respectively.
Discussion
This study demonstrates a significant protective effect of breastfeeding on adverse respiratory outcomes at 15 months of age. Longer durations of exclusive, any and additional breastfeeding consistently reduced the risk of doctor‐diagnosed asthma, wheezing and inhaler use. There was no significant association between breastfeeding and the risk of eczema or atopy.
Strengths of the study were that data collection was prospective, and there was sufficient statistical power with a very good retention rate. Our breastfeeding information was comprehensive and the definition of exclusive breastfeeding was conservative. It is therefore likely that the protective association of breastfeeding was, if anything, underestimated. There was also a wide range of breastfeeding duration, allowing analysis of the data as a continuous variable.
Although bias regarding the frequency of infant feeding data collection is possible, this is unlikely to have impacted on the accuracy of breastfeeding data. First, data were collected on three occasions within the first 6 months of life, the likely period of exclusive breastfeeding. Second, evidence suggests that even after 3 years, 80% of women are able to recall the total duration of breastfeeding to within 1 month (Eaton‐Evans & Dugdale 1986; Li et al. 2005). As the median duration of exclusive breastfeeding was only 1.4 months, and all infant feeding data were collected within 15 months of birth, the frequency of assessment was adequate.
Our findings are consistent with those of several other prospective birth cohorts (Oddy et al. 1999; Wright et al. 2001; Kull et al. 2004). Although some of these studies have shown that breastfeeding is protective in older children (Oddy et al. 1999; Kull et al. 2004), others found that breastfeeding increased the risk of asthma in older children and adults (Sears et al. 2002), and in older children with atopy and a maternal history of asthma (Wright et al. 2001). One possible explanation for these apparently conflicting findings is the quality of breastfeeding data and whether exclusivity of breastfeeding was explored. There are issues of clarity in the description of exclusive breastfeeding used by Wright and co‐workers (Wright et al. 2001), and debate about the validity of the breastfeeding grouping in the Sears study (Sears et al. 2002). In addition, no child was likely to have been exclusively breastfed in the Sears study because it was the practice for all children to receive infant formula in hospital at that time (Sears et al. 2002).
Data from our study do not appear to support the hypothesis that the reduction in the prevalence of respiratory outcomes is due to protection provided by maternal antibodies in colostrum and breast milk against early respiratory infections, as adjustment for respiratory infections occurring in the first 3 months of life did not alter the relationship between breastfeeding and respiratory outcomes.
Other likely explanations for our findings include the exclusion of infant formula, food or other drinks containing potentially allergenic components and provision of anti‐inflammatory and nutritional or other components of human breast milk. It is also possible that breastfeeding duration, particularly the duration of exclusive breastfeeding, may be a surrogate for other important factors, such as skin‐to‐skin contact and the degree of mother–child bonding or the time at which mothers returned to work.
Although unlikely, it is possible that breastfeeding was stopped as a result of respiratory symptoms. However, this hypothesis would run contrary to public health advice about breastfeeding available to mothers at the time. Conversely, if the duration of breastfeeding was extended to mitigate respiratory symptoms, the strength of the association would have been underestimated. Our findings would indicate that the effects of breastfeeding duration were not biased by disease‐related modification of exposure.
While our research revealed a common pattern of symptoms that led to treatment being offered by a doctor, we recognize that recurrent wheeze and even doctor‐diagnosed asthma at 15 months may reflect any one of several different respiratory conditions (Sherriff et al. 2001; Martinez 2002, 2003; Kuehni 2005; 2005a, 2005b; De Sario et al. 2006). These include prior respiratory syncytial virus bronchiolitis, transient infant wheeze related to prenatal smoking exposure and asthma. Many of these factors are transient, and although a history of parental asthma, atopic eczema and recurrent wheezing strongly supports asthma diagnosis in some toddlers, it is not easy to be confident that they will have persisting childhood asthma until they reach 3–5 years of age. Despite not yet being certain whether our findings relate to asthma in later childhood, our analyses were adjusted for many of the variables relating to other infant wheezing phenotypes, in particular, smoking in pregnancy, early respiratory infections and BMI at 15 months. This issue will be further explored at 6 years of age when the cohort will undergo a detailed respiratory assessment.
In summary, these data show a very significant protective effect of breastfeeding in terms of infant wheezing and other adverse respiratory outcomes at 15 months that may be early indicators of asthma in New Zealand children.
Source of funding
This study was funded by two grants from the Health Research Council of New Zealand; a grant‐in‐aid from the Child Health Research Foundation (Christchurch) and the David and Cassie Anderson Bequest (Wellington).
Conflicts of interest
All authors declare that they have no competing interests.
Key messages
-
•
breastfeeding protects against infant wheezing and other adverse respiratory outcomes that may be early indicators of asthma;
-
•
the duration of exclusive breastfeeding was a stronger determinant of respiratory outcomes than the duration of any breastfeeding; and
-
•
breastfeeding was not associated with eczema or atopy.
Acknowledgements
The authors are very grateful to the families in Wellington and Christchurch who have generously given their time. We also acknowledge the outstanding efforts of the midwives in Wellington, Porirua and Canterbury for assistance with recruitment.
The New Zealand Asthma and Allergy Cohort Study Group comprises the following staff or past staff of the University of Otago (in alphabetical order): Professor J. Crane, Ms M. Duignan, Dr M.J. Epton, Dr D. Fishwick, Dr P. Fitzharris, Dr T. Ingham, Ms V. Irvine, Ms R. Kelly, Ms P. Lampshire, Ms J. Lane, Dr P. Leadbitter, Ms F. McCartin, Ms C. Macdonald, Ms S. McLeod, Ms A. Nicholson, Dr P. Pattemore, Mrs K. Roff, Ms G. Sawyer, Mr R. Siebers, Professor G.I. Town, Dr K. Wickens, Ms H. Wilson, Ms K. Withell.
References
- Bergmann R.L., Bergmann K.E., Lau‐Schadensdorf S., Luck W., Dannemann A., Bauer C.P. et al (1994) Atopic diseases in infancy. The German multicenter atopy study (MAS‐90). Pediatric Allergy and Immunology 5, 19–25. [DOI] [PubMed] [Google Scholar]
- De Sario M., Di Domenicantonio R., Corbo G., Forastiere F., Pistelli R. & Rusconi F. et al (2006) Characteristics of early transient, persistent, and late onset wheezers at 9 to 11 years of age. Journal of Asthma 43, 633–638. [DOI] [PubMed] [Google Scholar]
- Eaton‐Evans J. & Dugdale A.E. (1986) Recall by mothers of the birth weights and feeding of their children. Human Nutrition Applied Nutrition 40, 171–175. [PubMed] [Google Scholar]
- Epton M.J., Town G.I., Ingham T., Wickens K., Fishwick D. & Crane J. (2007) The New Zealand Asthma and Allergy Cohort Study (NZA2CS): assembly, demographics and investigations. BMC, Public Health 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalevich M., Mimouni D. & Mimouni M. (2001) Breast‐feeding and the risk of bronchial asthma in childhood: a systematic review with meta‐analysis of prospective studies. The Journal of Pediatrics 139, 261–266. [DOI] [PubMed] [Google Scholar]
- Ip S., Chung M., Raman G., Chew P., Magula N., DeVine D. et al (2007) Breastfeeding and maternal and infant health outcomes in developed countries. Evidence Report/Technology Assessment (Full Report) 153, 1–186. [PMC free article] [PubMed] [Google Scholar]
- Joint Task Force on Practice Parameters, representing the American Academy of Allergy Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology (1995) Practice parameters for the diagnosis and treatment of asthma. Journal of Allergy and Clinical Immunology 96, 707–870. [PubMed] [Google Scholar]
- Joseph‐Bowen J., De Klerk N.H., Firth M.J., Kendall G.E., Holt P.G. & Sly P.D. (2004) Lung function, bronchial responsiveness, and asthma in a community cohort of 6‐year‐old children. American Journal of Respiratory and Critical Care Medicine 169, 850–854. [DOI] [PubMed] [Google Scholar]
- Kuehni C.E. (2005) Phenotype specific treatment of obstructive airways disease in infancy and childhood: new recommendations of the Swiss Paediatric Pulmonology Group. Swiss Medical Weekly 135, 95–100. [DOI] [PubMed] [Google Scholar]
- Kull I., Almqvist C., Lilja G., Pershagen G. & Wickman M. (2004) Breast‐feeding reduces the risk of asthma during the first 4 years of life. Journal of Allergy and Clinical Immunology 114, 755–760. [DOI] [PubMed] [Google Scholar]
- Kurukulaaratchy R.J., Matthews S. & Arshad S.H. (2005a) Defining childhood atopic phenotypes to investigate the association of atopic sensitization with allergic disease. Allergy 60, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Kurukulaaratchy R.J., Waterhouse L., Matthews S.M. & Arshad S.H. (2005b) Are influences during pregnancy associated with wheezing phenotypes during the first decade of life? Acta Paediatrica 94, 553–558. [DOI] [PubMed] [Google Scholar]
- Li R., Scanlon K.S. & Serdula M.K. (2005) The validity and reliability of maternal recall of breastfeeding practice. Nutrition Reviews 63, 103–110. [DOI] [PubMed] [Google Scholar]
- Martinez F.D. (2002) Development of wheezing disorders and asthma in preschool children. Pediatrics 109, 362–367. [PubMed] [Google Scholar]
- Martinez F.D. (2003) Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatric Infectious Disease Journal 22, S76–S82. [DOI] [PubMed] [Google Scholar]
- Meinert R., Frischer T., Karmaus W. & Kuehr J. (1994) Influence of skin prick test criteria on estimation of prevalence and incidence of allergic sensitization in children. Allergy 49, 526–532. [DOI] [PubMed] [Google Scholar]
- Oddy W.H., Holt P.G., Sly P.D., Read A.W., Landau L.I., Stanley F.J. et al (1999) Association between breastfeeding and asthma in 6 year old children: findings of a prospective birth cohort study. British Medical Journal 319, 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P.D., Robertson C.F. & Olinsky A. (2002) The Melbourne Asthma Study: 1964–1999. Journal of Allergy and Clinical Immunology 109, 189–194. [DOI] [PubMed] [Google Scholar]
- Salmon C. & Crampton P. (2001) NZDep 2001 Index of deprivation. Wellington. Available at: http://wwwmoh.govt.nz/moh.nsh/Files/phi-research-report/$file/phi-research-report.pdf
- Sawyer G., Kemp T., Shaw R., Patchett K., Siebers R., Lewis S. et al (1998) Biologic pollution in infant bedding in New Zealand: high allergen exposure during a vulnerable period. Journal of Allergy and Clinical Immunology 102, 765–770. [DOI] [PubMed] [Google Scholar]
- Sears M.R., Greene J.M., Willan A.R., Taylor D.R., Flannery E.M., Cowan J.O. et al (2002) Long‐term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 360, 901–907. [DOI] [PubMed] [Google Scholar]
- Sherriff A., Peters T.J., Henderson J. & Strachan D. (2001) Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3(1/2) years. International Journal of Epidemiology 30, 1473–1484. [DOI] [PubMed] [Google Scholar]
- Taussig L.M., Wright A.L., Morgan W.J., Harrison H.R. & Ray C.G. (1989) The Tucson Children's Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. American Journal of Epidemiology 129, 1219–1231. [DOI] [PubMed] [Google Scholar]
- The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee . (1998) Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). The European Respiratory Journal 12, 315–335. [DOI] [PubMed] [Google Scholar]
- Wright A.L., Holberg C.J., Taussig L.M. & Martinez F.D. (2001) Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax 56, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]


