Abstract
Iron deficiency anaemia is arguably the most important public health problem in developing countries. In Sub‐Saharan Africa, iron deficiency anaemia has largely been attributed to poor infant feeding practices where complementary foods low in iron bioavailability are offered to at‐risk infants. Home fortification of complementary foods using micronutrient Sprinkles has been shown to reduce iron deficiency anaemia in many resource‐poor settings. In this study, the benefit of using the micronutrient Sprinkles as a home fortificant for some Kenyan complementary foods was assessed using an in vitro Caco‐2 cell model. In each case when micronutrient Sprinkles were added to the complementary food, the amount of Caco‐2 cell ferritin formation increased. For example, the addition of Sprinkles to corn porridge increased ferritin formation 5‐fold from 5.8 to 31.8 ng mg−1. Therefore, as indicated by the results, micronutrient Sprinkles would be a suitable form of home fortification for Kenyan complementary foods. Their use should be encouraged so as to improve infant feeding practices in Kenya.
Keywords: sprinkles, Kenyan complementary foods, iron bioavailability, iron, infant feeding, in vitro digestion/Caco‐2 model
Introduction
Micronutrient deficiencies and their consequences among infants, the causes of which are multifactorial, remain a major concern in many parts of the world. In this regard, iron deficiency and iron deficiency anaemia are arguably the most important public health problems in developing countries (Brabin et al. 2001; Underwood 2001). In Sub‐Saharan Africa, iron deficiency anaemia has largely been attributed to low iron bioavailability from non‐diverse home‐prepared complementary foods, coupled with less use or the absence of commercially available fortified foods, and little or no consumption of iron‐rich foods such as meats (Tatala et al. 1998; Yip & Ramakrishnan 2002; Davidsson 2003). Thus, over the past decade, novel food‐based approaches aimed at improving iron bioavailability from complementary foods have been designed and explored in various developing country settings with good results (Ruel & Levin 2002).
As discussed by Yip & Ramakrishnan (2002), the challenge of combating iron deficiency in developing countries is largely restricted by limited resources, where many of these countries and the populations affected are poor. Also the conditions under which anaemia occurs are diverse; therefore, a multifaceted approach that minimizes cost while improving accessibility by the poor is required to combat iron deficiency in these populations. With respect to iron, fortification of complementary foods at the household level has been shown to reduce iron deficiency anaemia in resource‐poor settings (Gibson & Hotz 2000; Davidsson 2003). An example of this is the use of micronutrient Sprinkles for home fortification of complementary foods. In some documented studies carried out in Bangladesh, Cambodia, Ghana and Haiti, a micronutrient sprinkle that contains iron and other key micronutrients added to complementary foods prepared at home from locally available staples and foods was shown to improve haemoglobin levels and reduce anaemia in previously anaemic infants (2001, 2003a, 2005; Hyder et al. 2007; Christofides et al. 2006; Giovannini et al. 2006; Menon et al. 2007). Thus, given proper monitoring and education of caregivers, home fortification of complementary foods can be a cost‐effective and feasible strategy for addressing childhood anaemia at the most critical time – when complementary feeding commences.
The in vitro iron bioavailability system/Caco‐2 cell model that mirrors the gastric and intestinal digestion of humans provides an attractive, rapid and low‐cost option for initial screening of iron bioavailability. It has been shown to be very useful in addressing iron bioavailability issues as it provides qualitatively similar results to human studies (Yun et al. 2004; Pynaert et al. 2006). It is also a quick tool for screening multiple foods or/and food combinations. Given the cost and logistic constraints of running efficacy trials over relatively large geographical areas or in areas where a variety of home‐prepared complementary foods are available, it is beneficial to utilize this screening tool to carry out preliminary studies and determine if the use of micronutrient Sprinkles on specific complementary foods shows promise in improving iron bioavailability. The purpose of this study was to assess the benefit of using the micronutrient Sprinkles as a home fortificant for some Kenyan complementary foods.
Materials and methods
Samples
Five complementary food samples were analysed in this study. They included a Ghanaian non‐fortified fermented maize porridge known locally in Ghana as koko, three Kenyan non‐fortified plant‐based complementary foods including a corn porridge made from corn meal, a home recipe porridge prepared from (corn + sorghum + millet + cassava) in the ration of (6 : 1.5 : 1.5 : 1) and a non‐fortified Kenyan commercial plant‐based recipe porridge. The other complementary food used in the experiment was Cerelac, a fortified complementary food manufactured by Nestlé. Cerelac is one of the most popular commercially available fortified baby foods in Africa and has previously been used in a number of experiments as a reference standard for various nutrients (Onofiok & Nnanyelugo 1998; Mosha et al. 2000; Okorie & Nwanekezi 2002). It was used in this experiment as a reference standard for amount of bioavailable iron.
All complementary food ingredients were purchased from Kenya while the micronutrient Sprinkles – Babyfer – were generously provided by the Sprinkles Global Health Initiative, Toronto, Ontario, Canada.
Sample preparation
For the Kenyan porridges, 20 g of the porridge flour was boiled in 200 mL of water for 20 min. For the koko, 2.5 cups of corn meal (1 cup = 160 g of corn meal) were mixed in 2 cups (470 mL) of water then fermented in a covered bowl at room temperature for 3 days prior to cooking. The Cerelac sample was prepared by mixing 25 g of powder in 160 mL of cow milk. For each complementary food, a 1 mL volume was sampled and assessed for amount of bioavailable iron. For home fortification, one sachet of the micronutrient Sprinkles was added to 1 cup of gruel (235 mL) and after thorough mixing, 1 mL of the fortified product was sampled and analysed for amount of bioavailable iron.
Chemicals, enzymes and hormones
Unless otherwise stated, all chemicals, enzymes and hormones were purchased from Sigma Chemicals (St. Louis, MO).
Cell culture
Caco‐2 cells were obtained from the American Type Culture Collection (Rockville, MD) at passage 17 and used in experiments at passages 25–33. Cells were seeded at a density of 50 000 cells cm−2 in collagen‐treated six‐well plates (Costar, Cambridge, MA). The cells were grown in Dulbecco's Modified Eagle Medium (GIBCO, Grand Island, NY) with 10% v/v foetal calf serum (GIBCO), 25 mmol L−1 HEPES and 1% antibiotic anti‐mycotic solution (GIBCO). The cells were maintained at 37°C in an incubator with a 5% CO2/95% air atmosphere at constant humidity, and the medium was changed every 2 days. The cells were used in the iron uptake experiments at 13 days post seeding.
In vitro digestion and harvesting of Caco‐2 cell monolayers for cell ferritin formation
The preparation of the digestion solutions – pepsin, pancreatin and bile extract, the in vitro digestion and harvesting of the cell monolayers were performed as previously published (Glahn et al. 1998). Exactly 1.0 mL of liquid samples was used for each sample digestion. In this model system, cell ferritin formation is used as the measure of cell Fe uptake.
Analyses
Caco‐2 cell protein was measured on samples that had been solubilized in 0.5 mol L−1 NaOH, using a semimicro adaptation of the Bio‐Rad DC protein assay kit (Bio‐Rad Laboratories, Hercules, CA). A one‐stage, two‐site immunoradiometric assay was used to measure Caco‐2 cell ferritin content (FER‐Iron II Ferritin Assay, RAMCO Laboratories, Houston, TX). A 10 µL sample of the Caco‐2 cell monolayer harvested in 2 mL of water was used for each ferritin measurement expressed per unit cell protein (ng ferritin per mg cell protein). All glassware used in the sample preparation and analyses was acid‐washed.
Statistics
Statistical analyses of the data were performed using the software package GraphPad Prism v4 (GraphPad Software, San Diego, CA) and JMP v6.0 (SAS Institute, Cary NC). Means were considered to be significantly different if P values were ≤0.05.
Results and discussion
As earlier mentioned, Cerelac, a fortified complementary food produced by Nestlé Foods, is one of the most popular commercially available fortified baby foods in Kenya and was used also in this experiment as a reference standard. In addition to its main ingredients – wheat gluten and corn flour – Cerelac is fortified with several nutrients including iron in the form of ferrous fumarate (see Table 1). As indicated on the label, one serving of Cerelac contains about 3.9 mg of iron which translates to approximately 26% of the RDA for infants of 6–7 months of age. Thus, fed twice a day, Cerelac would provide about one half of the daily iron requirements for infants. In this context and as seen on Fig. 1, the mean comparison with Cerelac shows with convincing significance that the amount of bioavailable iron in Cerelac was statistically significantly higher (P < 0.001) compared with the other foods. The ferritin formation from Cerelac was at least fourfold that from koko (a non‐fortified complementary food with a higher response in the Caco‐2 cell model compared with the other porridges) which speaks to the iron inadequacy of the non‐fortified complementary foods.
Table 1.
Micronutrients contained in per serving* of Cerelac and Sprinkles
| Micronutrient | Cerelac | Sprinkles (mg) |
|---|---|---|
| Iron | 3.9 mg | 12.5 |
| Zinc | 3.1 mg | 5 |
| Vitamin A | 860 IU/260 µg | 0.3 |
| Folic acid | 0.029 mg | 0.16 |
| Vitamin C | 22 mg | 30 |
Cerelac – 25 g of powder + 160 mL of milk; micronutrient Sprinkles – 1 sachet (1000 mg) per cup of complementary food.
Figure 1.
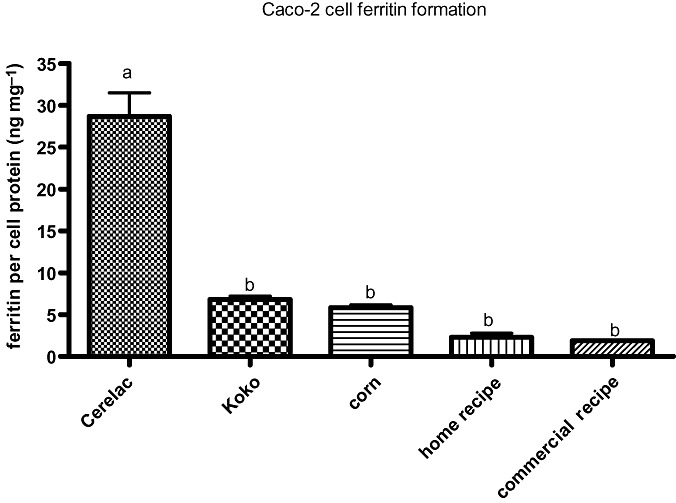
Amount of bioavailable iron in complementary foods assessed using the Caco‐2 cell model. Ferritin formation in the cells is an index of iron bioavailability. One millilitre of each porridge was analysed in the model and mean comparison with Cerelac as control was done using Dunnett's method. Bar values (mean ± SEM, n = 6) with different letters are statistically different (P < 0.05).
After fortification using micronutrient Sprinkles, an analysis of variance showed no statistically significant difference between amounts of bioavailable iron from Cerelac, koko and corn porridge. These results can be explained by the fact that per serving of the Sprinkles adds more iron and more ascorbic acid to koko and corn porridge. Both of these additives should increase cell iron uptake. The same results, however, are not seen with the Sprinkles‐fortified home recipe and the Sprinkles‐fortified Kenyan commercial recipe porridges. As shown on Fig. 2, the amount of bioavailable iron in Cerelac was still statistically significantly different from that in the Sprinkles‐fortified home recipe and the Sprinkles‐fortified Kenyan commercial recipe porridges (P < 0.001). The low in vitro iron availability of these porridges could be explained by the fact that, unlike koko and corn porridge, they contain ingredients such as sorghum and finger millet that are known to be high in polyphenols, which may have reduced iron uptake by the cells (Glahn et al. 2002; Dykes & Rooney 2006; Hu et al. 2006).
Figure 2.
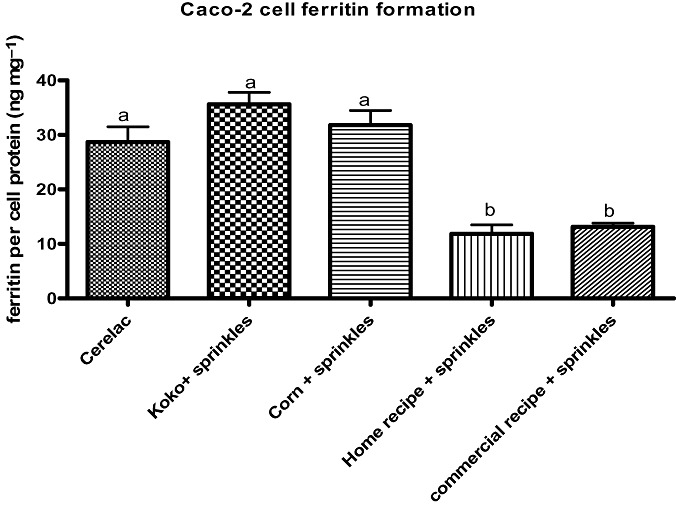
Amount of bioavailable iron in Cerelac and porridges fortified using micronutrient Sprinkles assessed using the Caco‐2 cell model. Ferritin formation in the cells is an index of iron bioavailability. One millilitre of each porridge was analysed in the model and mean comparison for all pairs was done using the Turkey–Kramer HSD method. Bar values (mean ± SEM, n = 6) with different letters are statistically different (P < 0.05). HSD, honestly significant difference.
Overall, however, as seen on Fig. 3, in each case when Sprinkles were added to the complementary food, the amount of Caco‐2 cell ferritin formation increased at least fivefold, indicating that the addition of micronutrient Sprinkles provided significantly more bioavailable iron to the complementary foods (P < 0.001). Therefore, although the Sprinkles‐fortified home recipe and the Sprinkles‐fortified Kenyan commercial recipe porridges are not equal to Cerelac in terms of amount of bioavailable iron, use of micronutrient Sprinkles with these porridges may confer some nutritional benefits to infants and should be encouraged.
Figure 3.
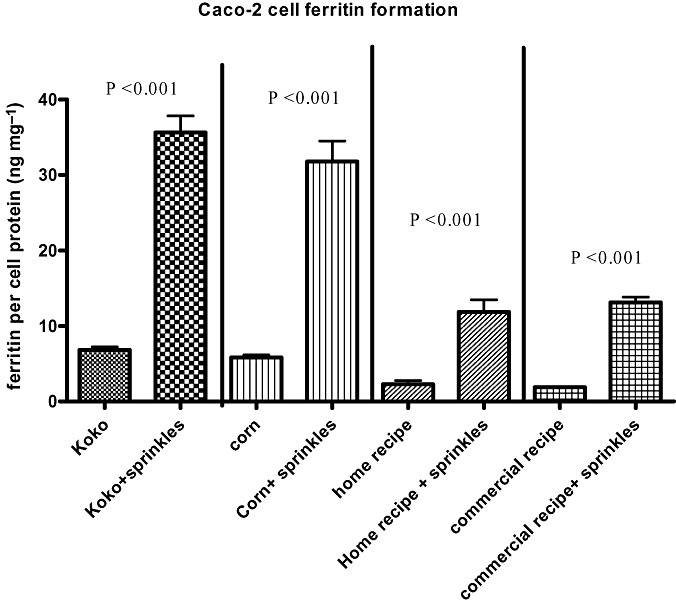
Pair‐wise comparison of samples with/without Sprinkles. Amount of bioavailable iron in porridges before and after fortification using micronutrient Sprinkles assessed using the Caco‐2 cell model. Ferritin formation in the cells is an index of iron bioavailability. Bar values (mean ± SEM, n = 6) and (P < 0.05) are statistically different.
Furthermore, as seen on 2, 3, both the Sprinkles‐fortified home recipe and the Sprinkles‐fortified Kenyan commercial recipe porridges had similar increases in ferritin formation. This is important to highlighting because it shows that non‐fortified commercial recipes are not necessarily superior to home recipes. Therefore, mothers who lack access to commercial recipes or those in resource‐poor settings who cannot afford to purchase non‐fortified commercial recipes should be helped to recognize that commercial recipes are not automatically a better option over home recipes.
Because it is neither possible nor ethical in many circumstances to randomize infants into feeding practices, studies involving micronutrient Sprinkles have been designed as efficacy studies. In those studies, there has not been a simultaneous comparison of iron bioavailability between a control group receiving complementary foods without Sprinkles and one receiving complementary foods with Sprinkles. Such a direct comparison could give an absorption ratio. Thus, at present a direct comparison of absorption ratios in infants is not possible. However, as shown by the results and especially Fig. 3, the Caco‐2 cell model enables the direct comparison of two sets of samples (with and without Sprinkles). Using the detected relative amounts of available iron from each sample, this screening tool can help us to draw conclusions on what differences or increases in vitro could result in physiological benefits in vivo. For example, unfortified koko is used as the primary complementary food in Ghana and has been implicated in the high prevalence of child malnutrition (Lartey et al. 1999). However, in subsequent studies where micronutrient Sprinkles have been added to koko, infants were able to recover from iron deficiency anaemia (Zlotkin et al. 2003b; Christofides et al. 2006). As seen in our experiment, when the Sprinkles were added to koko, the change in the amount of bioavailable iron was about 30 ferritin units. This observation implies that a change of this magnitude in the in vitro model would have a physiological impact in anaemic infants.
Conclusion
The amount of bioavailable iron in Kenyan plant‐based home‐prepared complementary foods is low. However, the use of micronutrient Sprinkles will improve the amounts of bioavailable iron from these foods and particularly in corn porridge. Therefore, the use of micronutrient Sprinkles as a home fortificant may be one solution to iron‐deficient complementary diets for those populations especially in the rural areas that lack access to fortified baby food products and those that grow, mill and consume their own grain. Also as indicated by the results, the Caco‐2 cell model would be a valuable screening tool for research on iron bioavailability of foods fortified using micronutrient Sprinkles if a suitable reference food, such as koko that has been previously used in a clinical study, can be incorporated as an internal control because it adds insight and clarity to the data generated.
Key messages
-
•
Some plant‐based Kenyan complementary foods provide very little amounts of bioavailable iron, thus iron fortification of cereal and porridge flours should be encouraged.
-
•
Micronutrient Sprinkles add more bioavailable iron to some Kenyan complementary foods and should therefore be considered for home fortification of baby foods especially in rural Kenya.
-
•
The in vitro Caco‐2 cell iron model would be a valuable tool for initial screening of iron bioavailability from foods fortified using micronutrient Sprinkles.
Conflicts of interest
None declared.
References
- Brabin B.J., Premji Z. & Verhoeff F. (2001) An analysis of anemia and child mortality. Journal of Nutrition 131, 636S–648S. [DOI] [PubMed] [Google Scholar]
- Christofides A., Asante K.P., Schauer C., Sharieff W., Owusu‐Agyei S. & Zlotkin S. (2006) Multi‐micronutrient Sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial. Maternal & Child Nutrition 2, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson L. (2003) Approaches to improve iron bioavailability from complementary foods. Journal of Nutrition 133, 1560S–1562S. [DOI] [PubMed] [Google Scholar]
- Dykes L. & Rooney L.W. (2006) Sorghum and millet phenols and antioxidants. Journal of Cereal Science 44, 236–251. [Google Scholar]
- Gibson R.S. & Hotz C. (2000) The adequacy of micronutrients in complementary foods. Pediatrics 106, 1298–1299. [PubMed] [Google Scholar]
- Giovannini M., Sala D., Usuelli M., Livio L., Francescato G., Braga M. et al (2006) Double‐blind, placebo‐controlled trial comparing effects of supplementation with two different combinations of micronutrients delivered as sprinkles on growth, anemia, and iron deficiency in Cambodian infants. Journal of Pediatric Gastroenterology and Nutrition 42, 306–312. [DOI] [PubMed] [Google Scholar]
- Glahn R.P., Lee O.A., Yeung A., Goldman M.I. & Miller D.D. (1998) Caco‐2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco‐2 cell culture model. Journal of Nutrition 128, 1555–1561. [DOI] [PubMed] [Google Scholar]
- Glahn R.P., Wortley G.M., South P.K. & Miller D.D. (2002) Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: studies using an in vitro digestion/Caco‐2 cell model. Journal of Agriculture and Food Chemistry 50, 390–395. [DOI] [PubMed] [Google Scholar]
- Hu Y., Cheng Z., Heller L., Glahn R.P. & Welch R.M. (2006) Kaempferol and quercitrin effect on iron bioavailability in white and colored bean seeds (phaseolus vulgaris l.) using an in vitro digestion/human Caco‐2 cell model. The FASEB Journal 20, A197. [DOI] [PubMed] [Google Scholar]
- Hyder S.M.Z., Haseen F., Rahman M., Tondeur M.C. & Zlotkin S. (2007) Effect of daily versus once‐weekly home fortification with micronutrient Sprinkles on hemoglobin and iron status among young children in rural Bangladesh Food and Nutrition Bulletin 28, 156–164. [DOI] [PubMed] [Google Scholar]
- Lartey A., Manu A., Brown K.H., Peerson J.M. & Dewey K.G. (1999) A randomized, community‐based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. American Journal of Clinical Nutrition 70, 391–404. [DOI] [PubMed] [Google Scholar]
- Menon P., Ruel M.T., Loechl C.U., Arimond M., Habicht J.P., Pelto G. et al (2007) Micronutrient sprinkles reduce anemia among 9‐ to 24‐mo‐old children when delivered through an integrated health and nutrition program in rural Haiti. Journal of Nutrition 137, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Mosha T.C.E., Laswai H.S. & Tetens I. (2000) Nutritional composition and micronutrient status of home made and commercial weaning foods consumed in Tanzania. Plant Foods for Human Nutrition (Formerly Qualitas Plantarum) 55, 185–205. [DOI] [PubMed] [Google Scholar]
- Okorie S.U. & Nwanekezi E.C. (2002) Effects of processing methods on the quality of maize‐groundnut infant weaning food. Global Journal of Pure and Applied Sciences 8, 209–214. [Google Scholar]
- Onofiok N.O. & Nnanyelugo D.O. (1998) Weaning foods in West Africa: nutritional problems and possible solutions. Food and Nutrition Bulletin 19, 26–33. [Google Scholar]
- Pynaert I., Armah C., Fairweather‐Tait S., Kolsteren P., Van Camp J. & De Henauw S. (2006) Iron solubility compared with in vitro digestion‐Caco‐2 cell culture method for the assessment of iron bioavailability in a processed and unprocessed complementary food for Tanzanian infants (6–12 months). British Journal of Nutrition 95, 721–726. [DOI] [PubMed] [Google Scholar]
- Ruel M.T. & Levin C.E. (2002) Assessing the potential for food‐based strategies to reduce vitamin a and iron deficiencies: a review of recent evidence. Discussion Paper 92. International Food Policy Research Institute (IFPRI): Washington DC.
- Tatala S., Svanberg U. & Mduma B. (1998) Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi District of Tanzania. American Journal of Clinical Nutrition 68, 171–178. [DOI] [PubMed] [Google Scholar]
- Underwood B. (2001) Nutritional anemias worldwide: a historical overview In: Nutritional Anemias (ed. Ramakrishnan U.), pp 1–6. CRC Press: Boca Raton, FL. [Google Scholar]
- Yip R. & Ramakrishnan U. (2002) Experiences and challenges in developing countries. Journal of Nutrition 132, 827S–830. [DOI] [PubMed] [Google Scholar]
- Yun S., Habicht J.P., Miller D.D. & Glahn R.P. (2004) An in vitro digestion/Caco‐2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. Journal of Nutrition 134, 2717–2721. [DOI] [PubMed] [Google Scholar]
- Zlotkin S., Arthur P., Antwi K.Y. & Yeung G. (2001) Treatment of anemia with microencapsulated ferrous fumarate plus ascorbic acid supplied as Sprinkles to complementary (weaning) foods. American Journal of Clinical Nutrition 74, 791–795. [DOI] [PubMed] [Google Scholar]
- Zlotkin S., Antwi K.Y., Schauer C. & Yeung G. (2003a) Use of microencapsulated iron (II) fumarate Sprinkles to prevent recurrence of anaemia in infants and young children at high risk. Bulletin of the of the World Health Organization 81, 108–115. [PMC free article] [PubMed] [Google Scholar]
- Zlotkin S., Arthur P., Schauer C., Antwi K.Y., Yeung G. & Piekarz A. (2003b) Home‐fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. Journal of Nutrition 133, 1075–1080. [DOI] [PubMed] [Google Scholar]
- Zlotkin S., Schauer C., Christofides A., Sharieff W., Tondeur M.C. & Hyder Z.S.M. (2005) Micronutrient sprinkles to control childhood anaemia. PLoS Medicine 2, e1. doi:10.1371/journal.pmed. 0020001 [DOI] [PMC free article] [PubMed] [Google Scholar]


