Abstract
With increasing interest in the potential effects of n‐6 and n‐3 fatty acids in early life, there is a need for data on the dietary intake of polyunsaturated fatty acids (PUFA) in low‐income countries. This review compiles information on the content in breast milk and in foods that are important in the diets of low‐income countries from the few studies available. We also estimate the availability of fat and fatty acids in 13 low‐income and middle‐income countries based on national food balance sheets from the United Nations' Food and Agriculture Organization Statistical Database (FOASTAT). Breast milk docosahexaenoic acid content is very low in populations living mainly on a plant‐based diet, but higher in fish‐eating countries. Per capita supply of fat and n‐3 fatty acids increases markedly with increasing gross domestic product (GDP). In most of the 13 countries, 70–80% of the supply of PUFA comes from cereals and vegetable oils, some of which have very low α‐linolenic acid (ALA) content. The total n‐3 fatty acid supply is below or close to the lower end of the recommended intake range [0.4%E (percentage of energy supply)] for infants and young children, and below the minimum recommended level (0.5%E) for pregnant and lactating women in the nine countries with the lowest GDP. Fish is important as a source of long‐chain n‐3 fatty acids, but intake is low in many countries. The supply of n‐3 fatty acids can be increased by using vegetable oils with higher ALA content (e.g. soybean or rapeseed oil) and by increasing fish production (e.g. through fish farming).
Keywords: food balance sheets, breast milk, fish, vegetable oil, cereals, PUFA
Introduction
It is only recently that there has been an increasing scientific interest in the potential beneficial effects of an optimal fatty acid composition of the diet in low‐income countries. This interest is focusing on intake of essential fatty acids in early life, i.e. during pregnancy and the first post‐natal years, when fatty acid composition potentially has important effects on growth, development and long‐term health. There has been a long‐standing interest in the intake of total fat, which has an important role in the diet of infants and young children because it increases energy density, energy intake and thereby growth in populations with a very low fat intake. Several publications, including some from low‐income countries, are available on this topic (Michaelsen & Jorgensen 1995; Prentice & Paul 2000; Uauy et al. 2000). However, information about the fatty acid composition of diets in low‐income countries is scarce. One of the reasons is that many food composition databases do not include data on the fatty acid composition of relevant foods. Data on n‐3 fatty acids are of special interest and it is often these data that are lacking.
The aim of this paper is to review the available data on dietary intake of fatty acids in low‐income countries, with special focus on pregnancy and the first years of life. The review includes data on the fatty acid content of relevant foods, the amount of long‐chain polyunsaturated fatty acids (LCPUFA) in breast milk, fatty acid intake during the complementary feeding period, and data on fatty acid availability in selected low‐income and middle‐income countries based on Food and Agriculture Organization (FAO) food balance data. In the calculation of intakes and availability, we have mainly used the US Department of Agriculture (USDA 2009) and the Australian NUTTAB (FSANZ 2006) databases, which we found had the most complete information on fatty acid composition. Even when fatty acid composition data are available, there is still some uncertainty about the data because the fatty acid composition of certain foods can vary considerably depending on the ecological setting, season, and how animals and fish have been fed.
Key messages
-
•
In countries with low economic status, the availability of fat and n‐3 fatty acids in the food supply is low, often below the minimum recommended intake for vulnerable groups.
-
•
In populations living on a predominantly plant‐based diet, vegetable oils and cereals are important sources of PUFA.
-
•
For children under 2 years of age, the key sources of LCPUFA, especially n‐3 fatty acids, are breast milk and fish. Therefore, continued breastfeeding up to at least 2 years and increased intake of fish (e.g. small indigenous farmed fish) should be promoted.
-
•
Some vegetable oils (e.g. soy and rapeseed oil) have high contents of n‐3 fatty acids while other oils have very low n‐3 fatty acid levels. It should therefore be mandatory to declare the type of oils used in declarations of ingredients for foods.
-
•
There is a need for better data on PUFA intake in vulnerable groups in low‐income countries.
Fat and fatty acid recommendations
FAO and World Health Organization (WHO) arranged a joint expert consultation on fats and fatty acids in human nutrition in Geneva in November 2008. An interim summary of conclusions and dietary recommendations from the meeting is available at the WHO web site (FAO/WHO 2008).
The recommendations for infants and young children from 6 to 24 months of age and for pregnant and lactating women are given in Table 1. For infants and young children, the committee found that the evidence behind the recommendations for total fat and n‐6 fatty acids was ‘convincing’, while the evidence for the recommendations for total polyunsaturated fatty acids (PUFA) and n‐3 fatty acids was thinner and was therefore judged as ‘probable’. Concerning the ratio between n‐6 and n‐3 fatty acids, or linoleic acid (LA, 18:2n‐6) to α‐linolenic acid (ALA, 18:3n‐3) acid, the report concluded that there was no compelling scientific rationale for recommending a specific ratio. However, in the global CODEX standards for infant formula, it is recommended that the LA/ALA ratio be between 5 and 15 (CODEX 1981; Koletzko et al. 2005).
Table 1.
Recommended dietary intakes for total fat and essential fatty acids for infants and young children (6–24 months) and pregnant and lactating women (FAO/WHO 2008)
| Infants and young children (6–24 months) | Pregnancy and lactation* | |
|---|---|---|
| Fat in food supply | Gradual reduction to 35%E, depending on physical activity | 20–35%E |
| Polyunsaturated fatty acid (PUFA) | <15%E (U‐AMDR) | 6–11%E (AMDR) |
| n‐6 PUFA(18:2 undifferentiated) | 3.0–4.5%E (AI)† | 2–3%E (AI)† |
| n‐3 PUFA (18:3 undifferentiated) | 0.4–0.6%E (AI)‡ | 0.5–2%E (AMDR)§ |
Recommended intake for healthy adults;
†recommended intake for LA only;
†recommended intake for ALA only;
recommended intake for ALA + n‐3LCPUFA. AMDR, acceptable macronutrient distribution range; U‐AMDR, upper value for AMDR; AI, adequate intake (range); LCPUFA, long‐chain polyunsaturated fatty acids.
For moderately malnourished children below 5 years of age, Golden has suggested that the diet should contain at least 4.5%E (percentage of energy supply) of n‐6 fatty acids (5 g/1000 kcal), and at least 0.5%E of n‐3 fatty acids (0.85 g/1000 kcal) (Golden 2009). For ready‐to‐use therapeutic foods, WHO and World Food Programme (WFP) have recommended that 3–10%E should come from n‐6 fatty acids and 0.3–2.5%E from n‐3 fatty acids and that the fat content should be 45–60%E (FAO/WHO 2008; UNICEF 2010). None of these recommendations has any guidelines on the intake of LCPUFA vs. the shorter‐chain PUFA. However, conversion of the n‐3 precursor (ALA) to the longer‐chain n‐3 fatty acids [e.g. docosahexaenoic acid (DHA, 22:6n‐3)], which exert many of the essential functions of n‐3 fatty acids within the body, is generally low (Lauritzen et al. 2001). Therefore, consuming preformed LCPUFA is a more efficient way to fulfil the needs for n‐3 PUFA.
LCPUFA content in breast milk
In a comprehensive review by Brenna et al. of the DHA and arachidonic acid (AA, 20:4n‐6) content in human milk, many of the studies were from low‐ and middle‐income countries (Brenna et al. 2007). The values from these studies are ranked in Table 2 according to the DHA level in the breast milk. The range and median values for these countries are not very different from the values in studies from high‐income countries. In the review, the mean value worldwide was 0.32% of fatty acids, exactly the same as the median of the studies included in Table 2. For comparison, the Adequate Intake in the FAO/WHO recommendations from 2008 was between 0.2 and 0.4% of fatty acids (FAO/WHO 2008). The lowest value in Table 2, 0.06%, is from a study in Pakistan including eight mothers of malnourished children. Other values in Table 2 are also below the lower reference limit of 0.2%. These were from rural South Africa and two sites in China, but similar values are observed in many studies in high‐income countries, e.g. those from Canada, United States, France and the Netherlands (Brenna et al. 2007). In a study from Tanzania, the breast milk DHA content from two different populations was compared (Kuipers et al. 2005). In a group of mothers living close to a lake and eating local fish fried in sunflower oil, the DHA value was 0.75%; whereas in a group of mothers who had fish about every second week and meat one time weekly, the DHA value was 0.13%. The AA content of breast milk was less variable, with most values from the low‐ and middle‐income countries lying within ±1SD of the mean for all countries, which was 0.47%. The milk from mothers of malnourished children from Pakistan (Kuipers et al. 2005), which had the lowest DHA content, also had the lowest AA value among all the studies included in the review.
Table 2.
Breast milk docosahexaenoic acid (DHA) and arachidonic acid (AA) values (percentage of total fatty acids) found in studies from low‐income and middle‐income countries ranked according to DHA level (modified from the review by Brenna et a l. 2007)
| Country | DHA | AA | Reference |
|---|---|---|---|
| Pakistan | 0.06 | 0.24 | Smit et al. (2000) |
| Rural South Africa | 0.10 | 1.00 | van der Westhuyzen et al. (1988) |
| Enshi, China | 0.15 | 0.35 | Dodge et al. (1999) |
| China | 0.18 | 0.51 | Xiang et al. (2005) |
| Nigeria, Niger | 0.20 | 0.51 | Vanderjagt et al. (2000) |
| Nigeria, Niger | 0.20 | 0.52 | Knox et al. (2000) |
| Urban South Africa | 0.20 | 0.60 | van der Westhuyzen et al. (1988) |
| Belize | 0.21 | 0.44 | van Beusekom et al. (1990) |
| Xichang, China | 0.22 | 0.52 | Dodge et al. (1999) |
| Mexico | 0.26 | 0.42 | Yuhas et al. (2006) |
| Tanzania | 0.27 | 0.60 | Muskiet et al. (1987) |
| Beijing, China | 0.28 | 0.63 | Dodge et al. (1999) |
| Nigeria | 0.32 | 0.58 | Okolo et al. (2000) |
| Panama | 0.32 | 0.52 | Rueda et al. (1998) |
| Nigeria | 0.33 | 0.44 | Okolo et al. (2000) |
| Nigeria | 0.34 | 0.56 | Ogunleye et al. (1991) |
| China | 0.35 | 0.49 | Yuhas et al. (2006) |
| Dominican Republic | 0.40 | 0.50 | van Beusekom et al. (1993) |
| Suriname | 0.41 | 0.58 | Muskiet et al. (1987) |
| Cuba | 0.43 | 0.67 | Krasevec et al. (2002) |
| Curao | 0.43 | 0.71 | Muskiet et al. (1987) |
| Saint Lucia | 0.53 | 0.58 | Boersma et al. (1991) |
| Congo | 0.55 | 0.44 | Rocquelin et al. (1998) |
| Philippines | 0.74 | 0.39 | Yuhas et al. (2006) |
| Dominican Republic | 0.91 | 0.33 | van Beusekom et al. (1990) |
In summary, the breast milk samples from populations in low‐ and middle‐income countries, which typically rely primarily on a plant‐based diet, do not in general have DHA and AA levels that are considerably lower than the levels in high‐income countries, where the average intake of animal source foods is considerably higher. However, the data suggest that the consumption of marine foods is the most important determinant of breast milk DHA levels.
Fatty acid intake during the complementary feeding period
The complementary feeding period is critical regarding intake of PUFA and especially n‐3 fatty acids. The brain is still growing at a rapid rate and a low intake of n‐3 fatty acids may have a negative effect on mental and motor development. In populations with a predominantly plant‐based diet, infants and young children will typically have an intake of n‐3 fatty acids far below the recommended intake if breastfeeding is stopped early, or if the DHA content of the breast milk is low because of lack of animal source foods, especially fish, in the mother's diet.
One of the most comprehensive descriptions of fatty acid intake during the first years of life in a low‐income population is reported in a review by Prentice & Paul (2000), which provides data from the Gambia. The results are shown in the three graphs in Fig. 1. Intakes of total fat, n‐6 fatty acids and n‐3 fatty acids are given as daily intake in g/day. During the first 17 months, total fat intake is constant, but as the child grows, energy intake increases and breast milk is displaced by complementary foods lower in fat, which is reflected in a gradual decrease in percentage of energy from fat in the diet from about 50%E to 25%E. At 2 and 3 years of age, fat intake is much lower, about 15%E. The figures illustrate the important role of breast milk fatty acids during the first year of life in this population. Furthermore, the data show that during the second and third years, the most important sources of PUFA in this population are cereals and peanuts.
Figure 1.
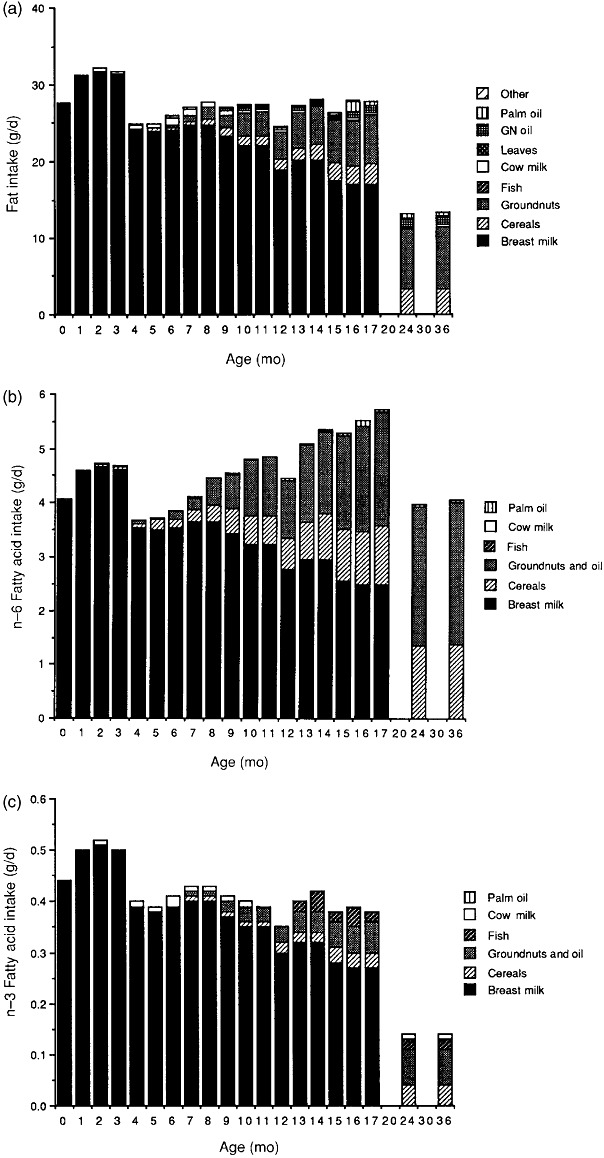
The contribution of different foods to the intake in Gambian children to (a) fat intake, (b): n‐6 fatty acid intake and (c) n‐3 fatty acid intake (from Prentice & Paul 2000, reprinted with permission from American Journal of Clinical Nutrition). GN oil, Ground nut oil.
The fatty acid content of five complementary foods available in Nigeria has been analysed and compared with the content calculated by using available food tables (Fernandez et al. 2002). The main ingredients were corn, soybean, rice, millet and peanuts. An interesting finding was that the analysed fatty acid content differed considerably from the content calculated from the food tables. The fat content of the complementary foods was generally low, with values ranging from about 17%E to 26%E, and one even as low as ∼6%E. The analysed content of ALA and LA was only about 40–60% of the calculated content. The authors have no explanation for the large difference, but suggest that it could be because of differing soils, climates, agricultural practices or the different varieties of plants used to prepare the complementary foods and those used for the USDA database, or it could be a result of differing analytical methods. Not surprisingly, the three complementary foods with soy had LA/ALA ratios within the recommended range (8, 10 and 11), while the two mainly rice‐based foods had ratios of 29 and 81.
Fatty acid content in relevant foods
Food composition tables are generally incomplete in providing values for fatty acid content and composition of foods relevant to diets in low‐income countries, which is a major constraint for reliable quantification of fatty acid intake. National‐ and culture‐specific food composition tables are essential for this purpose. Among national food composition tables, the Australian NUTTAB published by Food Standard Australia New Zealand (FSANZ 2006) has specifically been expanded to include fatty acid composition. The USDA National Nutrient Database for Standard Reference, Release 22 (USDA 2009) provides detailed information on fatty acid composition for some foods – especially fish products – and cumulated values for n‐6 and n‐3 fatty acids for most food items.
Table 3 shows the fat content and the LA and ALA contents and ratios in some important staple foods and examples of legumes. The major staple foods – rice, wheat and maize – all have low total fat content, negligible LA content and only traces of ALA. Refining rice, wheat and maize lowers the fat content, consequently further reducing the amounts of n‐6 and n‐3 PUFA per weight unit. Thus, whole grain cereals are a better source of essential fatty acids than refined cereals, but whole grain cereals are generally not recommended for infants and young children because of the higher content of anti‐nutrients (e.g. phytate and polyphenols) (American Academy of Pediatrics 1998). Because cereal grains are consumed in large amounts in many low‐income countries, they are an important source of fatty acids despite their very low essential fatty acid content.
Table 3.
Fatty acid composition in selected plant foods (g/100 g)
| Fatty acid | |||||
|---|---|---|---|---|---|
| Total fat | Total PUFA | LA | ALA | LA : ALA | |
| Staple food (raw) | |||||
| Rice, white flour | 1.40 | 0.50 | 0.46 | 0.02 | 23:1 |
| Rice, brown | 2.40 | 0.80 | 0.78 | 0.03 | 26:1 |
| Wheat, refined flour | 1.20 | 0.50 | 0.50 | 0.03 | 17:1 |
| Wheat, bran | 4.60 | 2.30 | 2.15 | 0.16 | 13:1 |
| Maize, whole flour* | 3.86 | 1.76 | 1.71 | 0.05 | 34:1 |
| Maize, degermed flour* | 1.75 | 0.63 | 0.62 | 0.02 | 40:1 |
| Sorghum* | 3.30 | 1.37 | 1.31 | 0.07 | 20:1 |
| Millet* | 4.22 | 2.13 | 2.02 | 0.12 | 17:1 |
| Teff* | 2.38 | 1.07 | 0.94 | 0.14 | 7:1 |
| Wild rice (Zizania sp.)* | 1.08 | 0.68 | 0.38 | 0.30 | 1:1 |
| Legumes (dried, raw) | |||||
| Soya bean | 20.2 | 12.7 | 10.99 | 1.56 | 7:1 |
| Lima bean | 1.70 | 0.80 | 0.55 | 0.24 | 2:1 |
| Haricot bean | 2.20 | 1.30 | 0.50 | 0.82 | 0.6:1 |
| Red kidney bean | 1.80 | 1.00 | 0.40 | 0.63 | 0.6:1 |
Source: FSANZ, except values marked ‘*’ which are from USDA.
PUFA, polyunsaturated fatty acids; LA, linoleic acid; ALA, α‐linolenic acid; FSANZ, Food Standard Australia New Zealand; USDA, US Department of Agriculture.
Teff is a staple grain food from Ethiopia that has a favourable fatty acid composition compared with other staple foods. In addition, the Zizania species (‘wild rice’), which is a different genus than rice (Oryza), has relatively high ALA content, and a favourable LA : ALA ratio. These examples serve to draw attention to the fact that indigenous cereal foods might be important – and possibly overlooked – sources of fatty acids in some diets.
Legumes represent a diverse food group covering many species and varieties. The fat and fatty acid composition of selected legumes are shown in Table 3. Soya bean is a good source of total fat and PUFA with an LA : ALA ratio around 7. Soya bean products may be an important source of LA and ALA in vulnerable population groups without access to animal source foods. Other legumes generally have much lower total fat content, but some varieties – such as red kidney beans and lima beans – are good sources of PUFA and especially ALA. However, legumes have high contents of major anti‐nutrients such as polyphenols (Petry et al. 2010), phytate (Morris & Hill 1996; American Academy of Pediatrics 1998) and certain oligosaccharides (Suarez et al. 1999), and must be used with caution in foods for infants and young children.
Vegetable oil (Table 4) is one of the most important sources of PUFA in many low‐income countries, where the intake of PUFA from animal foods, including fish, is often very low. There are many low‐ and middle‐income countries where more than 50% of the PUFA intake comes from vegetable oil (Table 7). However, several of the most commonly used oils have very low levels of PUFA (e.g. palm oil) and often very low levels of ALA (e.g. sunflower oil, sesame oil, and palm oil). The highest levels of PUFA, with the most balanced ratio between LA and ALA, are found in soybean oil and canola, or rapeseed oil.
Table 4.
Fatty acid composition of plant oil (g/100 g)
| Fatty acid | |||||
|---|---|---|---|---|---|
| Total fat | Total PUFA | LA | ALA | LA : ALA | |
| Vegetable oil | |||||
| Sunflower oil | 100.0 | 59.8 | 59.4 | 0.3 | 205:1 |
| Sesame oil | 100.0 | 41.2 | 40.7 | 0.5 | 85:1 |
| Palm oil | 100.0 | 9.3 | 9.0 | 0.2 | 48:1 |
| Olive oil | 100.0 | 9.5 | 8.8 | 0.7 | 13:1 |
| Soybean oil | 100.0 | 62.3 | 55.1 | 7.2 | 8:1 |
| Canola oil | 100.0 | 28.5 | 18.4 | 10.0 | 1.8:1 |
Source: Food Standard Australia New Zealand.
Sorted by decreasing LA : ALA ratio. LA, linoleic acid; ALA, α‐linolenic acid; PUFA, polyunsaturated fatty acid.
Table 7.
Total fat supply, supply of PUFA, n‐6 and n‐3 fatty acids (%E) and n‐6 : n‐3 PUFA ratio in 13 countries ranked according to GDP (lowest to highest)*
| Country | GDP per capita (US$) ‡ | Fat supply (% E) | PUFA (% E) | N‐6 fatty acids (% E) | N‐3 fatty acids † (% E) | Ratio n‐6 : n‐3 |
|---|---|---|---|---|---|---|
| Malawi | 288 | 16.4 | 5.8 | 5.4 | 0.3 | 19 |
| Ethiopia | 317 | 16.3 | 4.0 | 3.7 | 0.3 | 14 |
| Bangladesh | 497 | 12.1 | 3.3 | 2.8 | 0.5 | 6 |
| Burkina Faso | 522 | 27.3 | 7.9 | 7.5 | 0.3 | 22 |
| Ghana | 713 | 19.9 | 4.3 | 3.8 | 0.4 | 10 |
| India | 1 017 | 21.6 | 4.5 | 4.0 | 0.5 | 9 |
| Vietnam | 1 051 | 18.6 | 3.3 | 2.9 | 0.3 | 9 |
| Bolivia | 1 720 | 35.8 | 5.8 | 5.2 | 0.5 | 11 |
| Indonesia | 2 246 | 25.3 | 4.0 | 3.5 | 0.4 | 8 |
| Guatemala | 2 848 | 28.5 | 8.2 | 7.4 | 0.7 | 10 |
| China | 3 267 | 24.2 | 5.6 | 4.8 | 0.6 | 8 |
| South Africa | 4 678 | 32.4 | 8.2 | 7.5 | 0.6 | 12 |
| Mexico | 10 232 | 35.8 | 7.3 | 6.5 | 0.7 | 9 |
*Calculations based on FAO Food Balance Sheets. FAOSTAT (2010); † includes total n‐3 fatty acids for fish products; ‡ World Bank 2008. PUFA, polyunsaturated fatty acid; GDP, gross domestic product; FAO, Food and Agriculture Organization.
The market price and the availability of oils are the primary determinants of which oils are used. The tendency to use the cheapest oil is supported by the tradition to not declare which oils are used in many products – the ingredients list says simply ‘vegetable oil’. However, even taking into consideration the availability and price, it is possible in many countries to choose vegetable oils with a high content of ALA. According to a review by Wolmarans (2009), the three oils produced in the highest amounts in developing countries are palm oil, soybean oil and rapeseed/mustard oil, which constituted 37, 23 and 8% of the total vegetable oil production, respectively. Soybean oil had the highest increase in production, increasing 74% from 1995 to 2001. The price for vegetable oils in low‐income countries varies considerably according to local production and availability. However, when comparing prices for the most relevant oils according to the WFP commodity price lists [September 2010 (free on board prices out of Antwerp)], there is not much difference among the most common oils. The prices for soybean oil (US$/MT 1190) and canola/rapeseed oil (US$/MT 1145), both with a high ALA content, were actually lower than for sunflower oil (US$/MT 1320). Palm oil was not much cheaper, around US$/MT 1000 (out of Malaysia or Indonesia). Thus, soybean oil or rapeseed oil seems to be realistic choices in many settings in low‐income countries.
Animal‐source foods, including meat, poultry, egg, milk and fish, are important sources of n‐6 and n‐3 fatty acids. Examples of the fat content and fatty acid composition of meat, poultry, egg and full fat milk are shown in Table 5. Beef is a relatively good source of n‐3 PUFA compared with pork and lamb. Poultry and eggs generally have less favourable n‐6 : n‐3 PUFA ratios compared with meat. The exact content of PUFAs will however depend on the feed of the animals. Chicken and eggs are often more accessible than meat in poor households in low‐income countries. The variation in the fatty acid composition of the flesh and eggs from different chicken breeds is unknown. It would be useful to obtain information about the fatty acid composition of flesh and eggs from scavenging and semi‐scavenging ‘backyard’ or ‘village’ chickens in populations where these foods are known to be used for complementary feeding. In a study in Greece, eggs from ‘free range’ hens in a village had an n‐6/n‐3 PUFA ratio of 1.8, much lower than the value of 19.8 for eggs from US supermarkets (Simopoulos & Sidossis 2000).
Table 5.
Fatty acid composition in animal source foods (g/100 g)
| Total fat | Total PUFA | LA | ALA | AA | EPA | DHA | n‐6 PUFA | n‐3 PUFA | n‐6:n‐3 PUFA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Meat (raw) | ||||||||||
| Minced pork (9.4% fat) | 9.4 | 1.2 | 0.98 | 0.09 | 0.06 | 0.00 | 0.03 | 1.04 | 0.12 | 9:1 |
| Minced beef (10.8% fat) | 10.8 | 1.2 | 0.54 | 0.13 | 0.23 | 0.09 | 0.02 | 0.77 | 0.39 | 2:1 |
| Minced lamb (6.9% fat) | 6.9 | 0.5 | 0.22 | 0.11 | 0.03 | 0.02 | 0.01 | 0.25 | 0.17 | 1:1 |
| Poultry (raw) | ||||||||||
| Duck, lean and skin | 36.9 | 4.5 | 4.09 | 0.24 | 0.13 | 0.00 | <0.01 | 4.22 | 0.24 | 17:1 |
| Quail, flesh and skin | 11.0 | 2.6 | 2.24 | 0.17 | 0.10 | 0.00 | 0.03 | 2.34 | 0.20 | 12:1 |
| Turkey breast | 8.5 | 2.3 | 2.05 | 0.16 | 0.05 | 0.00 | 0.01 | 2.10 | 0.17 | 12:1 |
| Chicken breast | 9.4 | 1.3 | 1.13 | 0.09 | 0.04 | 0.00 | 0.01 | 1.17 | 0.11 | 11:1 |
| Eggs and milk | ||||||||||
| Chicken egg, hard boiled | 8.6 | 1 | 0.72 | 0.02 | 0.19 | 0.00 | 0.07 | 0.91 | 0.10 | 9:1 |
| Duck egg, hard boiled | 13.2 | 0.9 | 0.54 | 0.10 | 0.31 | 0.00 | <0.01 | 0.85 | 0.10 | 9:1 |
| Milk (full fat) | 4.0 | 0.1 | 0.08 | 0.03 | 0.00 | 0.00 | 0.00 | 0.08 | 0.04 | 2:1 |
Source: Food Standard Australia New Zealand.
PUFA, polyunsaturated fatty acids; LA, linoleic acid; ALA, α‐linolenic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Fish and seafood is the only food group that is a significant source of n‐3 LCPUFA [e.g. eicosapentaenoic acid (20:5n‐3, EPA) and DHA]. The fatty acid composition varies significantly between fish species. In Table 6, the relative fatty acid composition is shown for species grouped as cold‐water marine species, cold‐water freshwater species and warm‐water freshwater species. The composition is shown as relative values, i.e. as percentage of total fatty acids. The general trend is that marine species are the best sources of n‐3 LCPUFA. Freshwater species have relatively less n‐3 LCPUFA, and among freshwater species, those living in a warm environment tend to have relatively less n‐3 LCPUFA compared with the cold‐water species. The freshwater species tend to have a higher content of ALA, but because of the low conversion to LCPUFA in humans, this is less biologically active. Frog, cricket and spider are shown as examples of other potential PUFA sources in traditional diets, with a relatively high content of n‐3 fatty acids, although again a large proportion of this tends to be ALA.
Table 6.
Composition of selected fatty acids in fish and other animals (percentage of total fatty acid)
| LA | ALA | AA | EPA | DHA | Total n‐6 PUFA | Total n‐3 PUFA | n‐6:n‐3 PUFA | |
|---|---|---|---|---|---|---|---|---|
| Marine fish | ||||||||
| Atlantic herring (Clupea herengus)* | 0.7 | 0.3 | 0.4 | 7.4 | 3.9 | 1.1 | 11.6 | 1:10 |
| Cod (Gadus morhua) † | 1.1 | 0.2 | 4.8 | 14.0 | 26.3 | 5.9 | 40.6 | 1:6 |
| Thai sardine (Sardinella gibbosa)* | 1.2 | 0.5 | 2.7 | 6.1 | 9.7 | 3.9 | 16.3 | 1:4 |
| Cod liver oil | 1.8 | 0.7 | 1.7 | 8.9 | 9.3 | 3.5 | 18.9 | 1:5 |
| Cold‐water freshwater fish | ||||||||
| Rainbow trout (Oncorhynchus mykiss)* | 4.6 | 5.2 | 2.2 | 5 | 19 | 6.8 | 29.2 | 1:4 |
| Perch (Perca fluviatilis)* | 1.5 | 0.5 | 9.1 | 8.8 | 26.5 | 10.6 | 35.8 | 1:3 |
| Roach (Rutilus rutilus)* | 4.5 | 2.8 | 5.5 | 10.7 | 14.9 | 10 | 28.4 | 1:3 |
| Atlantic salmon (Salmo salar)* | 2.7 | 4.6 | 4.2 | 5.1 | 17.6 | 6.9 | 27.3 | 1:4 |
| Warm‐water freshwater fish | ||||||||
| Snakehead (Channa striatus)* | 8.2 | 0.5 | 2.2 | 0.3 | 1.5 | 10.4 | 2.3 | 5:1 |
| Common carp (Cyprinus carpio) * | 7.9 | 2.9 | 8.6 | 8.8 | 6.5 | 16.5 | 18.2 | 1:1 |
| Nile tilapia (Oreochromis niloticus)* | 9.0 | 0.8 | 1.5 | 0.8 | 9 | 10.5 | 10.6 | 1:1 |
| Eel (Monopterus albus) ‡ | 5.6 | 0.9 | 0.7 | 2.7 | 0.2 | 6.3 | 6.1 | 1:1 |
| Catfish (Clarias macrocephalus)* | 6.6 | 2.7 | 13.5 | 3.2 | 6.7 | 20.1 | 12.6 | 2:1 |
| Climbing perch (Anabas testudineus)* | 5.4 | 1.3 | 12.4 | 1.1 | 9.2 | 17.8 | 11.6 | 2:1 |
| Other animals | ||||||||
| Field cricket (Teleogryllus testaceus) § | 26.6 | 9.0 | 0 | 0 | 0 | 26.6 | 9 | 3:1 |
| Chinese edible frog (Haplobatrachus rugulosus) § | 9.2 | 2.3 | 10.8 | 1.7 | 3.5 | 20 | 7.5 | 3:1 |
| Cambodian spider (Haplopelmaalbostriatum) ¶ | 7.6 | 3.6 | 11.2 | 1.6 | 1.7 | 18.8 | 6.9 | 3:1 |
Sources: *Cited from Karapanagiotidis et al. (2010); †US Department of Agriculture. USDA National Nutrient database; ‡ Rahman et al. (1995); § Nurhasan et al. (2010); ¶Own data (Roos N, unpublished). Total n‐6 PUFA is the sum of LA and AA and total n‐3 PUFA is the sum of ALA, EPA and DHA. PUFA, polyunsaturated fatty acids; LA, linoleic acid; ALA, α‐linolenic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Different populations may have different intakes of n‐3 fatty acids and especially n‐3 LCPUFA based on the types of fish species that are available for consumption. In Fig. 2, the total available fish supply for selected countries is extracted from the United Nations Food and Agriculture Organization Statistical Database (FAOSTAT 2010), showing the contribution of freshwater fish to the total supply. The source of fish supply varies with the ecological conditions, with countries like Ghana having a high supply of marine fish compared with Bangladesh, where the supply is dominated by freshwater fish. It is notable that India, despite having a long coastline, has a low total fish supply per capita and a limited marine fish supply.
Figure 2.
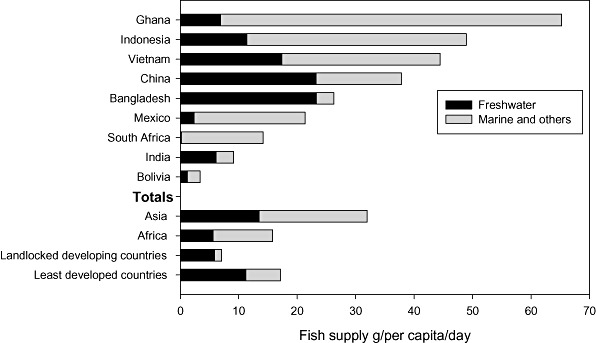
Total fish supply for selected countries extracted from FAOSTAT (2010), showing the supply of freshwater fish to the total supply. g/cap/day = grams per capita per day.
The supply of fish for food to population groups in low‐income countries vulnerable to malnutrition will typically reflect the local ecology and natural resources for fisheries. Resource‐poor coastal populations might have access to marine fish with low market value, while populations in landlocked countries may have access to freshwater fisheries if supported by the local hydrology. While trade in fishery commodities is increasing, including between developing countries (Delgado et al. 2003), fish for food is becoming increasingly detached from local production. The poorest segments of the population will typically not have access to the traded fish commodities, such as tuna and other high‐value marine species, but rely on low‐value local or regional supplies with no international commercial value.
Fish stocks are under great pressure everywhere in the world. Over the past several decades, aquaculture has developed and expanded to compensate for the declining supply from fisheries. Farmed fish are fed differently from wild fish and this has raised the question of whether farmed fish have the same level and composition of fatty acids as wild fish. A recent meta‐analysis of 39 studies on fatty acid composition of farmed and wild fish concluded that for marine fish, there is virtually no difference in the content of n‐3 LCPUFA (EPA + DHA) or the n‐6 : n‐3 PUFA ratio. The meta‐analysis also found that farmed freshwater fish had slightly lower n‐3 PUFA content compared with wild fish of the same species (Sales 2010). However, for some poor populations, aquaculture might be the only future stable source of fish. In the development of aquaculture, the species selected to be farmed is most often not the same species as the wild fish supply that the farmed fish will replace. The selection of fish species to be farmed should take into consideration the nutritional value of the farmed fish, including both fatty acid composition and micronutrient content, which can be high in small farmed fish.
Fatty acid availability: country profiles
The availability of total fat and fatty acids was estimated for 13 different countries using the food supply data (g/per capita/day) from the FAO Food Balance Sheets (FBS) FAOSTAT for 2007 (FAOSTAT 2010). The fatty acid composition of the food items reported by FAO was calculated using the USDA National Nutrient Database (release 22) (USDA 2009).Values provided for 18:2 undifferentiated fatty acids and 18:3 undifferentiated fatty acids were used for the estimation of n‐6 and n‐3 PUFA content, respectively. However, the total n‐3 PUFA content for fish and fish products was obtained by summing the 18:3 undifferentiated fatty acids with DHA and EPA. Food items in the FBS categories for sugars, vegetables, fruits and miscellaneous foods were excluded from the calculations. For items in the FBS for which the fatty acid content was available for different types of the same food (e.g. different types of freshwater fish or different types of wheat), an average value was calculated. When the supply of individual food items within the FBS category was not specified (e.g. tree nuts, offals), an average fatty acid content of the foods available in the USDA National Nutrient Database pertaining to that category was used. Because the specific types of food consumed within a category could vary among countries, but data on supply of different foods or species within a food category were not available for all countries, an ‘average’ scenario using all foods available in the nutrient composition database with data on fatty acid content was created. This approach was used only for tree nuts and fish, categories that would have the most impact on total fatty acid intake, especially n‐3 fatty acids. The ‘average scenario’ included the same specific foods for all countries, assuming that differences in the types of food available in each country within these two FBS categories would not have a major impact on the total fatty acid content of the food supply. To test this assumption, two more extreme scenarios of total fatty acid availability were constructed for each country; one in which the fatty acid content of foods in a given category was based on the food item in that category that had the lowest n‐3 fatty acid content, and one in which the fatty acid content of foods in a given category was based on the food item in that category with the highest n‐3 fatty acid content. For example, the ‘low content’ scenario for the tree nuts category was based on the amount of n‐3 and n‐6 fatty acids reported for almonds, which have the lowest n‐3 fatty acid content (18:3 undifferentiated = 0.006 g/100 g) of all the tree nuts available in the USDA database. The ‘high content’ scenario used the fatty acid composition of pecans (18:3 undifferentiated = 0.986 g/100 g) for the calculations, and the ‘average scenario’ included the averaged fatty acid composition of macadamia nuts, almonds, pine nuts, pecans, hazelnuts, cashew nuts and pistachios (walnuts were not included because of their very high fatty acid content and general lack of availability in low‐income countries). When comparing the high and low scenarios, differences in total fatty acid availability were not very large (data not shown) because of the small contribution of these food groups to the total food supply in low‐income countries. Therefore, the fatty acid content obtained from the ‘average’ scenario was used to calculate the total fatty acid content of the food supply in each country. Fatty acid availability from each of the food categories available in the FBS was also calculated as the percentage of the total energy (kcal/capita/day) reported by FAO.
The results are shown in 7, 8 and 3, 4, 5, 6. It should be emphasized that these data reflect the availability of fat and fatty acids in the food supply, not necessarily the dietary intake of these constituents by the target groups relevant to this paper (infants and young children, and pregnant or lactating women). Because the results are expressed as percentage of energy from the available foods (not as absolute g/day), it is assumed that dietary intake patterns would be similar to these estimates for the general population in each country. However, there is likely to be a wide range of fat or fatty acid intakes within each country, probably related to socio‐economic status. Moreover, it is possible that young children and pregnant or lactating women have less access to foods that are rich in these constituents than other members of the household. Thus, these estimates are probably the ‘best case’ scenario for fatty acid intakes by low‐income women and for intakes from complementary foods by infants and young children. For the latter subgroup, fat intake from breast milk represents a major source of fat and essential fatty acids, so total intake may not be low even if the complementary foods are deficient in these constituents. However, if the mothers themselves have low intakes of certain fatty acids, this will be reflected in the composition of their breast milk, and thus their infants may have inadequate total fatty acid intake.
Table 8.
Sources of PUFA (percentage of total PUFA) from selected food groups in 13 countries ranked according to GDP (lowest to highest)
| Country | Cereals | Starchy roots and pulses | Tree nuts and oil crops | Vegetable oils | Animal source foods* | Fish and seafood |
|---|---|---|---|---|---|---|
| Malawi | 57.5 | 4.7 | 15.0 | 18.9 | 2.8 | 1.2 |
| Ethiopia | 69.9 | 6.5 | 6.3 | 10.1 | 7.2 | 0.1 |
| Bangladesh | 17.2 | 2.1 | 5.0 | 66.4 | 3.6 | 5.8 |
| Burkina Faso | 47.3 | 2.7 | 20.7 | 23.9 | 5.1 | 0.3 |
| Ghana | 25.2 | 3.9 | 17.4 | 41.3 | 4.1 | 8.2 |
| India | 21.6 | 3.7 | 8.3 | 58.9 | 6.3 | 1.3 |
| Vietnam | 16.8 | 0.8 | 31.8 | 18.7 | 26.5 | 5.4 |
| Bolivia | 26.4 | 1.2 | 22.0 | 22.2 | 27.9 | 0.3 |
| Indonesia | 24.2 | 0.8 | 21.5 | 38.5 | 8.4 | 6.7 |
| Guatemala | 29.5 | 1.1 | 8.5 | 50.3 | 10.2 | 0.4 |
| China | 12.2 | 0.5 | 9.7 | 52.7 | 21.4 | 3.5 |
| South Africa | 28.0 | 0.3 | 2.1 | 57.0 | 11.7 | 0.8 |
| Mexico | 31.1 | 0.9 | 5.8 | 40.8 | 20.4 | 1.1 |
Excludes fish and seafood products. PUFA, polyunsaturated fatty acid; GDP, gross domestic product.
Figure 3.
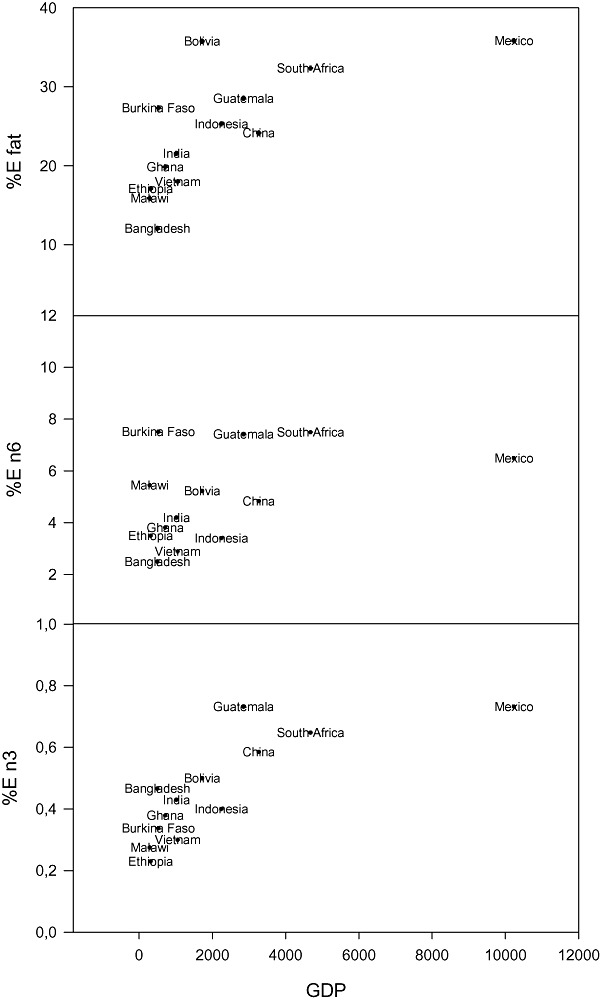
The average country supply of total fat, n‐6 and n‐3 fatty acids (weight %) relative to the gross domestic product (GDP) in US$ for each country. Source for supply data: FAOSTAT (2010). Source for GDP data: World Bank (2008).
Figure 4.
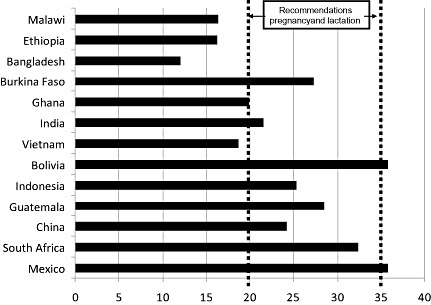
Total fat supply [percentage of energy supply (%E)] in 13 countries ranked according to increasing gross domestic product. The recommended range in fat intakes by pregnant and lactating women is shown. The recommendation for the 6–24 months age group is a gradual reduction to 35%E (FAO/WHO 2008).
Figure 5.
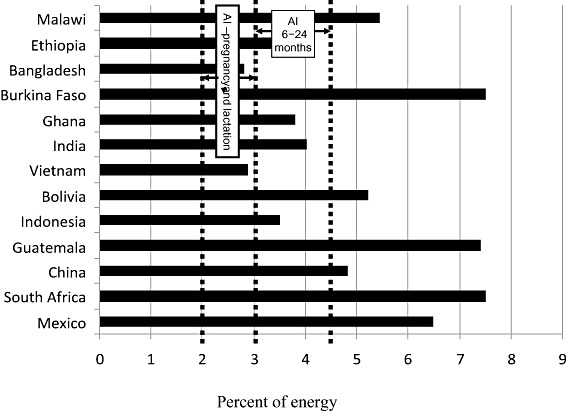
The n‐6 fatty acid supply [percentage of energy supply (%E)] in 13 countries ranked according to increasing gross domestic product. The Adequate Intake (AI) ranges for pregnant and lactating women and for the 6–24 months age group are shown (FAO/WHO 2008).
Figure 6.
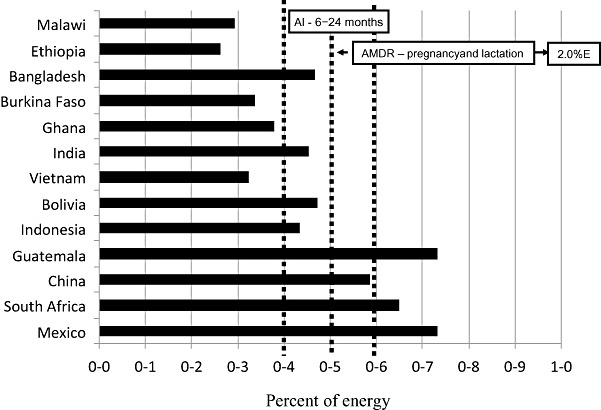
The n‐3 fatty acid supply [percentage of energy supply (%E)] in 13 countries ranked according to gross domestic product. The AMDR for pregnant and lactating women and the AI for the 6–24 months age group are shown (FAO/WHO 2008). AMDR, acceptable macronutrient distribution range; AI, adequate intake.
Table 7 gives an overview of the dietary fat supply and the supply of PUFA, n‐6 and n‐3 fatty acids as %E in the selected 13 countries. Total fat availability ranges from 12.1 to 35.8%E; and PUFA availability ranges from 3.3 to 8.2%E. The supply of n‐6 and n‐3 fatty acids is discussed in the following sections. It is of interest that the ratio of n‐6 to n‐3 fatty acids for most of the countries is within the range that is considered satisfactory, when the total fat supply to the population is taken into account. Most countries have a value between 5 and 15, which is considered optimal for infants (Koletzko et al. 2005). However, in two countries (Burkina Faso and Malawi) the values are quite high, slightly above 22 and 19, respectively. This could potentially compromise the individual's ability to desaturate and elongate the n‐3 fatty acids to DHA, leading to lower delivery of LCPUFA to the fetus or via breast milk.
In Fig. 3, the supply of total fat, n‐3 fatty acids and n‐6 fatty acids for the 13 countries included in the analysis is plotted against the GDP of the country. There is a strong positive association between total fat supply and GDP (linear regression r = 0.6, P = 0.01). The positive association between n‐3 fatty acid availability and GDP is even stronger (r = 0.8, P = 0.02). Interestingly, Bolivia and Bangladesh have almost the same n‐3 PUFA supply despite a very large difference in total fat supply. The explanation is that fish availability in Bangladesh is much higher than in Bolivia. The association between GDP and n‐6 PUFA supply is weaker and non‐significant (r = 0.5, P = 0.12).
Figure 4 indicates that total fat availability from foods is below the recommended minimum of 35%E for children 6–24 months of age in all of the countries except Bolivia and Mexico, and it is very low (<20%E) in Bangladesh, Ethiopia, Ghana, Malawi and Vietnam. This illustrates the importance of continued breastfeeding as a key source of dietary fat in these populations. Figure 4 also illustrates that total fat availability is below the recommended range of 20–35%E for pregnant and lactating women in Bangladesh, Ethiopia, Ghana, Malawi and Vietnam.
The n‐6 fatty acid supply is below the lower end of the recommended range (<3%E) for infants and young children in Bangladesh and Vietnam, whereas it appears to be adequate (>2%E) for pregnant and lactating women in all countries (Fig. 5).
The n‐3 fatty acid supply is below or very close to the lower end of the recommended range (0.4%E) for infants and young children in all countries except China, Guatemala, Mexico and South Africa (Fig. 6). Similarly, n‐3 fatty acid supply is below the minimum recommended level (0.5%E) for pregnant and lactating women in all but those four countries. This suggests that the DHA content of breast milk in many of those countries may be relatively low, and thus that the total n‐3 fatty acid intake by breastfed children may not be adequate. However, we did not find any large studies from low‐income countries with simultaneous measurements of intake and breast milk fatty acid composition to support this. The countries with the lowest percentage of energy from n‐3 fatty acids are Burkina Faso, Ethiopia, Ghana, Malawi and Vietnam.
For many of the low‐ and middle‐income countries included in this analysis, the main sources of PUFA are cereals and vegetable oils (Table 8). For most of the countries, these two food groups contribute 70–80% of the PUFA supply. In the two countries with the lowest GDP, where fat supply is also very low (∼16%E), more than half of the PUFA comes from cereals. The main source of PUFA in most of the other countries included in the analysis is vegetable oil, and the proportion of PUFA from oil is especially high in the countries with a high GDP, as well as in India and Bangladesh. This highlights the importance of the fatty acid composition of vegetable oils available to low‐ and middle‐income countries, as mentioned previously. The other food groups that contribute considerably to the PUFA supply in some countries are tree nuts/oil crops (which includes soybeans) and animal source foods. Tree nuts/oil crops provide 20–30% of the PUFA in four countries: Burkina Faso, Vietnam, Bolivia and Indonesia. In Vietnam, Bolivia, China and Mexico, about 20–30% of the PUFA supply comes from meat and other animal source foods. Fish and seafood provide only a small proportion of the PUFA supply, and in some countries less than 1% (Ethiopia, Burkina Faso, Bolivia, Guatemala and South Africa). The four countries with the highest supply of PUFA from fish and seafood (5–8%) are Bangladesh, Ghana, Vietnam and Indonesia.
Conclusions
There are only very limited data available on fatty acid intake in low‐income countries. This is not only because of a general lack of good data on dietary intake for infants and young children and pregnant and lactating women, but also because food composition tables have incomplete data on fatty acid content, and many local foods are not included in the food tables.
Breast milk is the most important source of PUFA during the first 2 years of life, but the breast milk DHA content is very dependent on the mother's diet and can be very low in populations living on a primarily plant‐based diet with no or limited fish intake. The food balance data described herein show that n‐3 fatty acid supply in most of the countries is below the minimum recommended level (0.5%E) for pregnant and lactating women. However, even small amounts of fish and seafood can increase the DHA content of breast milk considerably. In general, the levels of DHA in breast milk in low‐income countries are not very different from those in high‐income countries despite the difference in animal source food intake, but it should be noted that breast milk DHA levels in many high‐income countries are suboptimal.
During the complementary feeding period, breast milk is still a key source of PUFA because complementary foods in low‐income countries are typically cereal‐based with low fat content, and therefore contribute very little PUFA. The food balance data show that n‐3 fatty acid supply (not including breast milk) in most of the countries is below or very close to the lower end of the recommended range of intake (0.4%E) for infants and young children. An addition of fish, seafood and vegetable oils with high ALA content can improve the PUFA intake considerably during this period.
In the 13 countries included in the food balance calculations, there was a strong positive association between the economic status of the country (GDP) and the supply of total fat and n‐3 fatty acids. Most of the fat and PUFA intake comes from vegetable oils and cereals among families living on a primarily plant‐based diet. Fat content of cereals is low and mainly located in the outer layers of the kernels, so whole grains are a better source of fatty acids than refined cereals. A very important source of PUFA in low‐income countries is vegetable oils, but they differ considerably in PUFA content and especially in the LA/ALA ratio. Soybean oil and canola/rapeseed oil have the highest ALA content. In declarations of ingredients for foods, it should be mandatory to declare the type of vegetable oil because the type of oil has such an important effect on PUFA intake.
Fish and seafood are the most important sources of n‐3 LCPUFA, with marine fish generally a better source than freshwater fish. However, the availability of marine fish is limited, as many species are overfished. It has therefore been suggested that fishing resources should be protected by implementing the Code of Conduct for Responsible Fisheries. A potential way forward is sustainable fish farming. For poor rural populations in particular, farming of small fish in rice fields and lakes could provide an important extra supply of n‐3 fatty acids. Other potentially sustainable and cheap sources of LCPUFA are indigenous foods (e.g. amphibia, worms and insects) which are underutilized in many populations.
This review underlines the need for more data on PUFA intake in vulnerable groups in low‐income countries, which is only possible if the food databases are updated with detailed data on n‐6 and n‐3 fatty acids of foods eaten in low‐income countries. Improved knowledge of PUFA intake combined with data on fatty acid status is necessary to better understand the adequacy of PUFA and especially n‐3 fatty acid intakes in low‐income countries.
References
- American Academy of Pediatrics (1998) American Academy of Pediatrics. Committee on Nutrition. Soy protein‐based formulas: recommendations for use in infant feeding. Pediatrics 101, 148–153. [PubMed] [Google Scholar]
- van Beusekom C., Martini I.A., Rutgers H.M., Boersma E.R. & Muskiet F.A. (1990) A carbohydrate‐rich diet not only leads to incorporation of medium‐chain fatty acids (6:0–14:0) in milk triglycerides but also in each milk‐phospholipid subclass. American Journal of Clinical Nutrition 52, 326–334. [DOI] [PubMed] [Google Scholar]
- van Beusekom C.M., Nijeboer H.J., van der Veere C.N., Luteyn A.J., Offringa P.J., Muskiet F.A. et al (1993) Indicators of long chain polyunsaturated fatty acid status of exclusively breastfed infants at delivery and after 20–22 days. Early Human Development 32, 207–218. [DOI] [PubMed] [Google Scholar]
- Boersma E.R., Offringa P.J., Muskiet F.A., Chase W.M. & Simmons I.J. (1991) Vitamin E, lipid fractions, and fatty acid composition of colostrum, transitional milk, and mature milk: an international comparative study. American Journal of Clinical Nutrition 53, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Brenna J.T., Varamini B., Jensen R.G., Diersen‐Schade D.A., Boettcher J.A. & Arterburn L.M. (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. American Journal of Clinical Nutrition 85, 1457–1464. [DOI] [PubMed] [Google Scholar]
- CODEX (1981) Codex Stan 72, FAO/WHO Food Standards, Standards for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. http://www.codexalimentarius.net/web/standard_list.do?lang=en
- Delgado C., Wada N., Rosegrant M., Meijer S. & Ahmed M. (2003) Fish to 2020 Supply and Demand in Changing Global Market. International Food Policy Research Institute: Washington, D.C. and WorldFish Center, Penang, Malaysia. [Google Scholar]
- Dodge M.L., Wander R.C., Xia Y., Butler J.A. & Whanger P.D. (1999) Glutathione peroxidase activity modulates fatty acid profiles of plasma and breast milk in Chinese women. Journal of Trace Elements in Medicine and Biology 12, 221–230. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2008) Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatty Acids. From the Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition, WHO, Geneva. http://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf
- FAOSTAT (2010) United Nations Food and Agriculture Organization (FAO)'s Statistical Database. http://faostat.fao.org/
- Fernandez D.R., Vanderjagt D.J., Williams M., Huang Y.S., Chuang L.T. & Millson M. (2002) Fatty acid, amino acid, and trace mineral analyses of five weaning foods from Jos, Nigeria. Plant Food for Human Nutrition 57, 257–274. [DOI] [PubMed] [Google Scholar]
- Food Standards Australia New Zealand (FSANZ) (2006) Food Composition Table NUTTAB 2006 On‐line Version.. http://www.foodstandards.gov.au/consumerinformation/nuttab2006/onlineversionintroduction/onlineversion.cfm
- Golden M.H. (2009) Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Bulletin 30, S267–S342. [DOI] [PubMed] [Google Scholar]
- Karapanagiotidis I.T., Yakupitiyage A., Little D.C., Bell M.V. & Mente E. (2010) The nutritional value of lipids in various tropical aquatic animals from rice‐fish farming systems in northeast Thailand. Journal of Food Composition and Analysis 23, 1–8. [Google Scholar]
- Knox E., Vanderjagt D.J., Shatima D., Huang Y.S., Chuang L.T. & Glew R.H. (2000) Nutritional status and intermediate chain‐length fatty acids influence the conservation of essential fatty acids in the milk of northern Nigerian women. Prostaglandins, Leukotrienes, and Essential Fatty Acids 63, 195–202. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Baker S., Cleghorn G., Neto U.F., Gopalan S., Hernell O. et al (2005) Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. Journal of Pediatric Gastroenterology and Nutrition 41, 584–599. [DOI] [PubMed] [Google Scholar]
- Krasevec J.M., Jones P.J., Cabrera‐Hernandez A., Mayer D.L. & Connor W.E. (2002) Maternal and infant essential fatty acid status in Havana, Cuba. American Journal of Clinical Nutrition 76, 834–844. [DOI] [PubMed] [Google Scholar]
- Kuipers R.S., Fokkema M.R., Smit E.N., van der Meulen J., Boersma E.R. & Muskiet F.A. (2005) High contents of both docosahexaenoic and arachidonic acids in milk of women consuming fish from lake Kitangiri (Tanzania): targets for infant formulae close to our ancient diet? Prostaglandins, Leukotrienes, and Essential Fatty Acids 72, 279–288. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Hansen H.S., Jørgensen M.H. & Michaelsen K.F. (2001) The essentiality of long chain n‐3 fatty acids in relation to development and function of the brain and retina. Progress in Lipid Research 40, 1–94. [DOI] [PubMed] [Google Scholar]
- Michaelsen K.F. & Jorgensen M.H. (1995) Dietary fat content and energy density during infancy and childhood; the effect on energy intake and growth. European Journal of Clinical Nutrition 49, 467–483. [PubMed] [Google Scholar]
- Morris E.R. & Hill D. (1996) Inositol phosphate content of selected dry beans, peas, and lentils, raw and cooked. Journal of Food Composition and Analysis 9, 2–12. [Google Scholar]
- Muskiet F.A., Hutter N.H., Martini I.A., Jonxis J.H., Offringa P.J. & Boersma E.R. (1987) Comparison of the fatty acid composition of human milk from mothers in Tanzania, Curacao and Surinam. Human Nutrition – Clinical Nutrition 41, 149–159. [PubMed] [Google Scholar]
- Nurhasan M., Maehre H.K., Malde M.K., Stormo S.K., Halwart M., James D. et al (2010) Nutritional composition of aquatic species in Laotian rice field ecosystems. Journal of Food Composition and Analysis 23, 205–213. [Google Scholar]
- Ogunleye A., Fakoya A.T., Niizeki S., Tojo H., Sasajima I., Kobayashi M. et al (1991) Fatty acid composition of breast milk from Nigerian and Japanese women. Journal of Nutritional Science and Vitaminology 37, 435–442. [DOI] [PubMed] [Google Scholar]
- Okolo S.N., VanderJagt T.J., Vu T., VanderJagt T.A., Vanderjagt D.J., Okonji M. et al (2000) The fatty acid composition of human milk in northern Nigeria. Journal of Human Lactation 16, 28–35. [DOI] [PubMed] [Google Scholar]
- Petry N., Egli I., Zeder C., Walczyk T. & Hurrell R. (2010) Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. The Journal of Nutrition 140, 1977–1982. [DOI] [PubMed] [Google Scholar]
- Prentice A.M. & Paul A.A. (2000) Fat and energy needs of children in developing countries. American Journal of Clinical Nutrition 72, 1253S–1265S. [DOI] [PubMed] [Google Scholar]
- Rahman S.A., Huah T.S., Nassan O. & Daud N.M. (1995) Fatty acid composition of some Malaysian freshwater fish. Food Chemistry 54, 45–49. [Google Scholar]
- Rocquelin G., Tapsoba S., Mbemba F., Gallon G. & Picq C. (1998) Lipid content and fatty acid composition in foods commonly consumed by nursing Congolese women: incidences on their essential fatty acid intakes and breast milk fatty acids. International Journal of Food Sciences & Nutrition 49, 343–352. [DOI] [PubMed] [Google Scholar]
- Rueda R., Ramirez M., Garcia‐Salmeron J.L., Maldonado J. & Gil A. (1998) Gestational age and origin of human milk influence total lipid and fatty acid contents. Annals of Nutrition and Metabolism 42, 12–22. [DOI] [PubMed] [Google Scholar]
- Sales J. (2010) Quantification of the differences in flesh fatty acid components between farmed and wild fish. Journal of Aquatic Food Product Technology 19, 298–309. [Google Scholar]
- Simopoulos A.P. & Sidossis L.S. (2000) What is so special about the traditional diet of Greece. The scientific evidence. World Review of Nutrition and Dietetics 87, 24–42. [DOI] [PubMed] [Google Scholar]
- Smit E.N., Oelen E.A., Seerat E., Muskiet F.A. & Boersma E.R. (2000) Breast milk docosahexaenoic acid (DHA) correlates with DHA status of malnourished infants. Archives of Disease in Childhood 82, 493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez F.L., Springfield J., Furne J.K., Lohrmann T.T., Kerr P.S. & Levitt M.D. (1999) Gas production in human ingesting a soybean flour derived from beans naturally low in oligosaccharides. American Journal of Clinical Nutrition 69, 135–139. [DOI] [PubMed] [Google Scholar]
- Uauy R., Mize C.E. & Castillo‐Duran C. (2000) Fat intake during childhood: metabolic responses and effects on growth. American Journal of Clinical Nutrition 72, 1354S–1360S. [DOI] [PubMed] [Google Scholar]
- United Nations Children's Fund (UNICEF) (2010) Community‐based Management of Severe Acute Malnu trition. http://www.unicef.org/publications/files/Community_Based_Management_of_Sever_Acute__Malnutirtion.pdf
- U. S. Department of Agriculture , Agricultural Reserch Service . (2010) USDA National Nutrient Database for Standard Reference, Release 23. Nutrient Data Laboratory Home Page, http://www.ars.usda.gov/ba/bhnrc.ndl
- Vanderjagt D.J., Arndt C.D., Okolo S.N., Huang Y.S., Chuang L.T. & Glew R.H. (2000) Fatty acid composition of the milk lipids of Fulani women and the serum phospholipids of their exclusively breast‐fed infants. Early Human Development 60, 73–87. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen J., Chetty N. & Atkinson P.M. (1988) Fatty acid composition of human milk from South African black mothers consuming a traditional maize diet. European Journal of Clinical Nutrition 42, 213–220. [PubMed] [Google Scholar]
- Wolmarans P. (2009) Background paper on global trends in food production, intake and composition. Annals of Nutrition and Metabolism 55, 244–272. [DOI] [PubMed] [Google Scholar]
- World Bank . (2008) GDP per capita 2008. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 30 November 2010.
- Xiang M., Harbige L.S. & Zetterstrom R. (2005) Long‐chain polyunsaturated fatty acids in Chinese and Swedish mothers: diet, breast milk and infant growth. Acta Paediatrica 94, 1543–1549. [DOI] [PubMed] [Google Scholar]
- Yuhas R., Pramuk K. & Lien E.L. (2006) Human milk fatty acid composition from nine countries varies most in DHA. Lipids 41, 851–858. [DOI] [PubMed] [Google Scholar]


