Abstract
Inappropriate complementary feeding practices have led to, in part, significant disparities in growth and nutritional status between rural and urban children in China. A cluster‐randomised, controlled trial was implemented in Laishui, China to assess the effectiveness of an educational intervention on caregivers' feeding practices and children's growth. Eight townships were randomly assigned to the intervention or control. Five hundred ninety‐nine healthy infants were enrolled at 2–4 months old, and were followed up at ages 6, 9, 12, 15 and 18 months. The intervention group received information on enhanced home‐prepared recipes and food preparation and hygiene through group training, counselling and home visit. Key outcomes were children's physical growth, caregivers' knowledge and behaviours on complementary feeding, and the infant and child feeding index (ICFI). Analysis was by intention to treat. The intervention group achieved better knowledge and practices related to complementary feeding, and significantly higher ICFI scores at each follow‐up point. Children in the intervention group achieved higher z‐scores for weight‐for‐age (WAZ) and weight‐for‐height (WHZ) than the control (0.18 vs. 0.01 and 0.49 vs. 0.19, respectively) at 18 months old, and were less likely to have stunted growth (odds ratio = 0.71, 95% confidence interval: 0.53–0.94). Mixed model showed that the intervention group achieved significantly better linear growth over time, including WAZ (P = 0.016), WHZ (P = 0.030) and HAZ (P = 0.078). These results indicated that an educational intervention delivered through local health services can enhance caregivers' knowledge and practices of complementary feeding and ultimately improve children's growth.
Keywords: child growth, complementary feeding, infant feeding behaviour, nutritional interventions, randomised controlled trial
Introduction
China, as the most populated developing country in the world, has most of its population in the vast rural areas. There are great disparities in growth and nutritional status between rural and urban Chinese children. The weight and length of rural children lags behind their urban counterparts after 4 months of age (Shen et al. 1996). The prevalence of underweight and stunting among children under 5 years old in rural areas was 9.3% and 17.3%, respectively, significantly higher than those in urban areas (3.1% and 4.9%, respectively) (Li et al. 2005). Micronutrient deficiencies were also more prevalent among rural Chinese children than their urban counterparts: 21.9% of rural young children under 5 in 2005 had anaemia, significantly higher than 11.3% in urban children (Chang et al. 2007); Vitamin A deficiency was 23% in rural children, compared with 3.8% in urban areas (UNICEF 2005).
The urban–rural disparities in child growth and nutrition are accounted for by the inequitable distribution of economic and health care resources in rural areas as well as the poor complementary feeding practices (Shen et al. 1996; He & Zhai 2001). The proportion of rural infants consuming dairy and animal foods daily was less than half that of urban children (Chinese Ministry of Health 2004). The proportion of rural infants ever having consumed fruits and vegetables was 2.8 times lower than their urban counterparts, and that of ever having consumed animal foods such as egg, fish and meat was three times lower in rural infants (Fu et al. 2000).
Previous studies showed that effective educational interventions such as group training, individual counselling and interpersonal communication can change caretakers' feeding behaviours, thereby enhancing children's dietary intake and growth (Guldan et al. 2000; Salehi et al. 2004; Kilaru et al. 2005; Penny et al. 2005; Roy et al. 2005). However, there was no rigorously designed intervention study on complementary feeding practices having been conducted in China rural areas. For example, Guldan et al. implemented a community‐based intervention in Sichuan Province through growth monitoring, nutrition counselling and enhanced complementary food recipes. The intervention improved children's growth and their consumption of foods from animal sources (Guldan et al. 2000). However, the study was flawed by the lack of baseline data and randomisation. We, therefore, implemented a cluster‐randomised, controlled educational intervention trial in Laishui, a rural area in China, to promote appropriate complementary feeding practices among caregivers of young children. A total of 599 healthy infants were enrolled at age 2–4 months and randomised to receive the educational intervention or routine health care. The study was initially designed with the final data collection when the children reached 12 months of age. The initial results revealed that the intervention group achieved improvements in food diversity, meal frequency and hygiene practices, as well as better weight and length increment than did controls by 1 year of age (results were published elsewhere) (Shi et al. 2010).
Considering that inadequate nutrition during the first 2 years of life can affect child's growth and health, and that malnutrition rates increase between 6 and 18 months, the period of complementary feeding (Waterlow 1988), we extended the intervention and assessments to 18 months of age, with additional funding support and approval from the institutes and local health department, as well as consent from parents. The intervention strategies remained the same, but the educational messages were adjusted to adapt for child's developmental needs. The current paper reports the results of the study at 18 months of age.
Key messages
An educational intervention targeting improving complementary feeding practices, including the timing of introduction, food quantity and quality, and feeding behaviours, can lead to improvement in children's growth.
The intervention delivered through local health services is feasible, effective and sustainable.
ICFI can be used to evaluate the intervention effects on complementary feeding behaviours.
Materials and methods
Study setting and participants
Details of the study settings and participants were presented elsewhere (Shi et al. 2010). Briefly, the study was conducted in Laishui County, Hebei Province in northwest China with a per capita Gross Domestic Product of $500 in 2006. Eight townships were selected that has at least two primary health care providers who could provide intervention and evaluation for the study. Townships were paired for similar population, geographic type and economic condition. The paired townships were listed alphabetically in blocks of two and assigned randomly to the intervention or control.
All infants in the selected townships who were full‐term (gestational age: >37 weeks), singleton birth, without major birth defects and aged 2–4 months at the time of the baseline survey were eligible for the study. These eligibility data were obtained from the county Maternal and Child Health (MCH) hospital, which collects perinatal health care data from township hospitals on a monthly basis. Written informed consent was obtained from the children's parents. In total, 599 infants were enrolled between April and September of 2006, and followed up until they reached 18 months of age.
Procedures
The intervention was delivered by the children's primary health care providers with employment of health education and interpersonal communication strategies. Interpersonal communication strategies included group training sessions and individual counselling to provide the key messages, which were designed according to child feeding guideline [Pan American Health Organization (PAHO)/World Health Organization (WHO) 2001]. These messages were: (1) provide continue frequent, on‐demand breastfeeding until 2 years of age or beyond; (2) provide complementary foods of good quality and adequate quantity; (3) do not expose the child to family meals too early; (4) increase fluid intake, including more frequent breastfeeding, and encourage the child to eat soft foods during illness especially during diarrhoea; (5) good hygiene and proper food handling; and (6) practice responsive feeding. The group training sessions were conducted at township clinics by the township doctors who were trained by the researchers, to disseminate the key messages on child feeding to the mothers, grandmothers and fathers. After the group training sessions, individual counselling was held. In addition, the primary health care providers conducted home visits every 3 months with the purpose of identifying possible feeding problems and providing individual counselling. To identify feeding problems, the primary health care providers asked caregivers questions using a structured guideline and observed child feeding practice. Following this, the primary health care provider provided individual counselling, which was tailored based on caregivers' questions and problems that the provider identified during home visit. Educational strategies included dissemination of booklets on child feeding guidance, and demonstration of preparing enhanced recipes, which were formulated using locally available, affordable, acceptable and nutrient‐dense foods such as eggs, tomato, beans, dark green leafy vegetables, pork, beef, chicken, and bovine and chicken liver. The recipe demonstrations were conducted in the group training sessions and during home visits. Besides caregivers as the main intervention targets, other important family members such as husbands and parents‐in‐law, and community members such as village doctors and village committee leaders, were involved in the intervention. For husbands and parents‐in‐law, they were invited to participate in group training and get involved in during home visit. Village doctors were mobilised by township doctors to provide additional home visits. Village doctors and committee leaders were involved during the development of the intervention. At that stage, their opinions on local food availability, diet structure, food culture and child feeding practices were weighed in when the educational booklet and enhanced recipes were developed. During the intervention implementation phase, they were invited to the meetings with the researchers and the county MCH hospital doctors to discuss the optimal feeding practices. The control group received a standard package of child health care, which included breastfeeding counselling, but did not contain counselling on complementary feeding.
Assessments were conducted at baseline, and 6, 9, 12, 15 and 18 months of age. Each time, a 30‐min questionnaire survey was conducted with the mother of the child. The questionnaire collected information on child feeding practices, health status, caregiver's knowledge, beliefs and behaviours related to child feeding, and household socio‐demographic background. At the end of the questionnaire survey, the weight and length/height of the child were measured following standard procedures.
The primary outcome of the study was the child's physical growth measured by z‐scores for weight‐for‐age (WAZ), height‐for‐age (HAZ) and weight‐for‐height (WHZ) at age 18 months. The secondary outcomes included: (1) mothers' knowledge on child feeding, which was measured by multiple choice questions on child's age‐specific feeding practices. Responses were categorised as ‘Correct’ or ‘Wrong’ according to PAHO/WHO guideline (PAHO/WHO 2001). For example, for the question ‘Which is the best food for children aged 4–6 months?’, the correct answer was ‘breast milk’, and the other answer options such as formula milk, animal milk, fruit juice, rice porridge were wrong; and (2) the infant and child feeding index (ICFI), which was developed based on 24‐h dietary recall of all foods consumed in the previous day and food‐frequency information. It included eight age‐appropriate components such as current breastfeeding (a child who was currently breastfed at the time of the survey received 2 points, otherwise 0 point), absence of bottle‐feeding (a child who was not bottle fed received 1 points, otherwise 0 point), meal frequency (2 points were added if the age‐specific recommended meal frequency was reached, and 1 point was added if the meal frequency was less than the recommendation, but not zero. For snacks such as cookies and fruits, 1 point was given if the child consumed snacks at least twice on the day before the survey.), variety of food groups (1 point was given if the child consumed one to three food groups on the day preceding the survey out of the total of six food groups – cereals/tubers, beans, animal milk, egg, meat/fish and other foods; 2 points were given if four or more food groups were consumed), and frequency of consumption of different food groups (1 point was given if the child consumed staple foods more than once a day for infants 6–8 months of age and more than twice a day for children 9 months and older. For beans and egg/fish/meat, consumption of one to two times a week was given 1 point, and 2 points if they were consumed three times or more a week) (Ruel & Menon 2002; Zhang et al. 2009); and (3) the proportion of children receiving recommended complementary feeding practices.
The study was approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health and Peking University Health Science Center.
Statistical analysis
For budget and logistical reasons, we could only enrol four townships per treatment group. Assuming the intercluster variation to be 0.01, the standard deviation (SD) for weight to be 1 kg, average weight to be 9 kg in the control group, and 20% loss to follow‐up, in order to detect a 0.3 kg difference in weight between the intervention and control groups, with 80% power and 5% significance level, we needed to recruit 600 children at the beginning of the study. With this sample size, it allowed us to detect a difference of 0.7 cm at 18 months of age for linear growth (Salehi et al. 2004).
Descriptive statistics for continuous variables were presented as mean ± SD, while categorical variables as number (n) and proportion (%). Chi‐squared test and t‐test were used to examine the difference in subjects' characteristics and observed variables between the control and the intervention groups. Hotelling's t‐square test and t‐test were performed for data of physical growth between the control and the intervention groups. Mixed model for longitudinal data analysis was used to estimate the intervention effects over time on child growth, controlling for socioeconomic variables such as parents' age, education and employment, the child's birthweight, birth length and number of children. The interclass coefficient across townships was low at about 0.01, but considering the large cluster size, we still adjusted for clustering within township in the mixed model analysis. Statistical significance was established at the level α = 0.05, with all tests being two‐tailed. Data management and analyses were performed using SAS version 9.1, 2002 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 599 infants were recruited at baseline, 294 in the intervention and 305 in the control group (Fig. 1). The children available in the intervention and control group for the follow‐up visits at 6, 9, 12, 15 and 18 months of age were 528 (88.1%), 473 (79.0%), 490 (81.8%), 498 (83.8%) and 492 (82.8%), respectively (Fig. 1). The characteristics of children and families at baseline were comparable (Shi et al. 2010).
Figure 1.
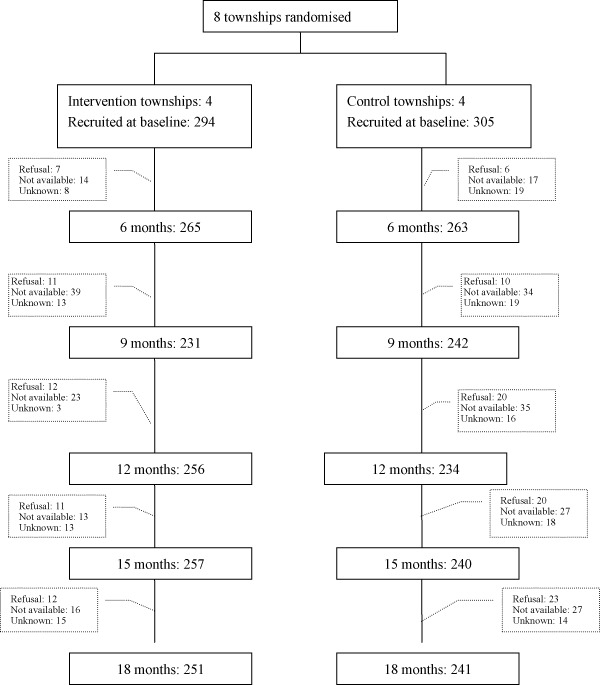
Trial profile.
Table 1 summarises mothers' knowledge of age‐specific feeding practices. At the enrolment, the proportions of mothers choosing correct answers for the questions related to child feeding were similar, except for the question on what the caregiver should do when the child refuses to eat food. At 6, 9, 12 and 18 months of age after the implementation of the intervention, more caregivers in the intervention group responded correctly to the questions on feeding practices than those in the control (statistically significant for all questions). The infants and child feeding index (ICFI) was developed. The total score of ICFI was 10. As shown in Table 2, higher scores were found at each follow‐up point in the intervention group than those in the control (statistically significant). No significant differences of current breastfeeding rate at each follow‐up point were found between the two groups (data not shown). The current breastfeeding rate remained at a high level at 6 and 9 months of age (93.6% and 93.9% in the intervention group, 93.9% and 94.6% in the control group, respectively), then decreased to about 85.0% at 12‐month old children and no more than 50% at 18 months of age (44.6% in the intervention and 48.1% in the control). However, children in the intervention group had lower rates of bottle‐feeding, higher meal frequency, greater variety of food groups and more frequent consumption of different food groups than those in the control at 9, 12, 15 and 18 months of age (statistically significant).
Table 1.
Mothers' knowledge on age‐specific feeding practices by treatment n (%)
| Knowledge on feeding practices | Intervention | Control | P |
|---|---|---|---|
| Breast milk as the best food for infants aged 4–6 months | |||
| Baseline | 261 (88.8) | 277 (90.8) | 0.245 |
| 6 months | 233 (87.9) | 231 (87.8) | 0.540 |
| 12 months | 230 (92.7) | 208 (88.9) | 0.095 |
| 18 months | 240 (95.6) | 220 (91.3) | 0.038 |
| Timing of introducing complementary food | |||
| Baseline | 217 (73.8) | 218 (71.5) | 0.292 |
| 6 months | 252 (95.1) | 240 (91.3) | 0.057 |
| 12 months | 225 (90.7) | 176 (75.2) | <0.001 |
| 18 months | 228 (90.8) | 174 (72.2) | <0.001 |
| Do not expose children less than 2 years old to table food | |||
| Baseline | 111 (37.8) | 115 (37.7) | 0.529 |
| 9 months | 126 (54.5) | 26 (10.7) | <0.001 |
| 12 months | 144 (58.1) | 62 (26.5) | <0.001 |
| 18 months | 118 (47.0) | 48 (19.9) | <0.001 |
| Caregivers wash hands before preparing food | |||
| Baseline | 197 (67.0) | 221 (72.5) | 0.086 |
| 6 months | 214 (80.8) | 200 (76.0) | 0.113 |
| 12 months | 227 (91.5) | 185 (79.1) | <0.001 |
| 18 months | 238 (94.8) | 218 (90.5) | 0.046 |
| Appropriate feeding the child during diarrhoea | |||
| Baseline | 163 (55.4) | 178 (58.4) | 0.262 |
| 6 months | 196 (74.0) | 129 (49.0) | <0.001 |
| 12 months | 213 (85.9) | 151 (64.5) | <0.001 |
| 18 months | 219 (87.3) | 167 (69.3) | <0.001 |
| Strategies when the child refuses to take foods | |||
| Baseline | 171 (58.2) | 145 (47.5) | 0.006 |
| 9 months | 181 (78.4) | 81 (33.5) | <0.001 |
| 12 months | 210 (84.7) | 86 (36.8) | <0.001 |
| 18 months | 237 (94.4) | 210 (71.0) | <0.001 |
| Appropriate use of nutrition supplement for child | |||
| Baseline | 243 (82.7) | 264 (86.6) | 0.113 |
| 9 months | 223 (96.5) | 223 (92.1) | 0.030 |
| 12 months | 240 (96.8) | 205 (87.6) | <0.001 |
| 18 months | 237 (94.4) | 171 (71.0) | <0.001 |
Table 2.
ICFI at each follow‐up point by treatment
| Group | n | ICFI scores* (mean ± standard deviation) | Unadjusted difference | P | |
|---|---|---|---|---|---|
| 6 months † | Intervention | 127 | 5.72 ± 2.17 | 1.24 (0.77, 1.71) | <0.001 |
| Control | 132 | 4.48 ± 1.61 | |||
| 9 months | Intervention | 226 | 8.09 ± 1.55 | 1.60 (1.29, 1.90) | <0.001 |
| Control | 210 | 6.50 ± 1.65 | |||
| 12 months | Intervention | 240 | 7.85 ± 1.68 | 1.54 (1.21, 1.86) | <0.001 |
| Control | 229 | 6.31 ± 1.87 | |||
| 15 months | Intervention | 252 | 8.38 ± 1.70 | 1.70 (1.41, 1.99) | <0.001 |
| Control | 229 | 6.68 ± 1.55 | |||
| 18 months | Intervention | 250 | 8.70 ± 1.61 | 1.42 (1.15, 1.70) | <0.001 |
| Control | 241 | 7.28 ± 1.49 |
ICFI, infants and child feeding index. *ICFI includes breast‐feeding, bottle‐feeding, dietary diversity, food‐frequency, variety of food groups and frequency of consumption of different food groups. The maximum score is 11. †Children less than 6 months were not included.
The two groups were comparable at baseline in terms of other feeding behaviours such as caregivers' hand‐washing practice, feeding more breast milk and easy‐to‐digest foods for children with diarrhoea, and encouraging child to eat when the child refuses. After the implementation of the intervention, more mothers in the intervention group than those in the control group reported that they washed their own hands and their children's hands before meals (80.5% vs. 67.9%; statistically significant), fed breast milk and easy‐to‐digest foods for children with diarrhoea (88.8% vs. 65.0%; statistically significant), cooked easy‐to‐digest food separately for their children with diarrhoea (67.3% vs. 32.8%, statistically significant), and encouraged children to eat when they refused food (88.0% vs. 53.9%, statistically significant).
Children's growth results as measured by WAZ, HAZ and WHZ, after adjustment for potential confounders such as parents' age, ethnicity, education, employment and the child's birthweight and length, are shown in Table 3. At baseline and the follow‐up points of 6, 9, 12 and 15 months of age, there were no significant differences in WAZ, HAZ and WHZ scores between the intervention and control groups based on univariate t‐test (not significant). By the age of 18 months, the intervention group achieved higher scores of WAZ and WHZ than the control group (0.18 ± 0.90 vs. −0.09 ± 0.93 for WAZ, and 0.49 ± 1.07 vs. 0.19 ± 0.97 for WHZ, respectively, univariate t‐test, statistically significant). At each follow‐up point, multivariate t‐square test was used, adjusted for parents' age, ethnicity, education, employment, weight and height, as well as children's birth weight and length, household size, and number of siblings. At 9 and 18 months of age, the children in the intervention group had better physical growth than those in the control (statistically significant). The rate of stunting at 18‐month old children in the intervention group was significantly lower than that in the control (3.2% vs. 7.1%, odds ratio = 0.71, 95% confidence interval: 0.53–0.94).
Table 3.
Child growth outcomes at each follow‐up point by treatment
| Group | n | WAZ | HAZ | WHZ | t square † | P | |
|---|---|---|---|---|---|---|---|
| Baseline | Intervention | 294 | 0.21 ± 1.08 | 0.05 ± 1.09 | 0.31 ± 1.05 | 1.136 | 0.769 |
| Control | 300 | 0.13 ± 1.06 | 0.03 ± 1.08 | 0.22 ± 1.19 | |||
| P * | 0.355 | 0.829 | 0.317 | ||||
| 6 months | Intervention | 258 | 0.43 ± 1.04 | 0.04 ± 1.02 | 0.63 ± 1.08 | 4.010 | 0.263 |
| Control | 254 | 0.47 ± 1.06 | 0.18 ± 1.00 | 0.60 ± 1.07 | |||
| P | 0.647 | 0.115 | 0.765 | ||||
| 9 months | Intervention | 211 | 0.47 ± 0.94 | −0.20 ± 0.97 | 0.79 ± 1.05 | 9.438 | 0.025 ‡ |
| Control | 227 | 0.40 ± 1.02 | −0.03 ± 1.19 | 0.62 ± 1.06 | |||
| P | 0.438 | 0.091 | 0.084 | ||||
| 12 months | Intervention | 256 | 0.06 ± 1.00 | −0.43 ± 0.99 | 0.34 ± 1.17 | 5.265 | 0.156 |
| Control | 234 | 0.07 ± 0.92 | −0.50 ± 1.03 | 0.41 ± 0.98 | |||
| P | 0.985 | 0.735 | 0.766 | ||||
| 15 months | Intervention | 251 | −0.07 ± 1.06 | −0.39 ± 0.99 | 0.13 ± 1.20 | 0.240 | 0.971 |
| Control | 232 | −0.08 ± 0.94 | −0.40 ± 0.94 | 0.13 ± 1.05 | |||
| P | 0.901 | 0.882 | 0.955 | ||||
| 18 months | Intervention | 248 | 0.18 ± 0.90 | −0.37 ± 0.89 | 0.49 ± 1.07 | 11.933 | 0.008 ‡ |
| Control | 228 | −0.09 ± 0.93 | −0.50 ± 1.06 | 0.19 ± 0.97 | |||
| P | 0.001 ‡ | 0.159 | 0.002 ‡ |
HAZ, height‐for‐age; WAZ, weight‐for‐age; WHZ, weight‐for‐height. Data are mean ± standard deviation. *Univariate t‐test. †Multivariate t‐square test, adjusted for household size, number of children, parents' age, ethnicity, education, employment, weight and height, as well as the child's birthweight and length.
‡ P value of multivariate t‐square test.
Further, mixed model for longitudinal data analysis was used to examine the effect of the intervention on growth over time adjusting for clustering within township and correlation within subjects among different observation points (Table 4). The control group's WAZ, HAZ and WHZ decreased over time significantly (beta = −0.094, −0.142, and −0.061, respectively, statistically significant). The intervention effect was evaluated by the interaction term of treatment and time. The interaction terms for WAZ (beta = 0.049, P = 0.016) and WHZ (beta = 0.050, P = 0.030) were positive, meaning that WAZ and WHZ improved at a faster rate over time than in the control group. For HAZ, the coefficient of the interaction term is also positive (beta = 0.037), but it is only marginally significant (P = 0.078).
Table 4.
Mixed model for longitudinal data analysis of children's growth by treatment
| Itemsa | Beta (95% confidence interval) | P |
|---|---|---|
| Weight‐for‐age | ||
| Intercept | −2.943 (−4.135, −1.751) | <0.001 ‡ |
| Intervention | −0.140 (−0.293, −0.013) | 0.071 |
| Time | −0.094 (−0.121, −0.067) | <0.001 ‡ |
| Intervention × time | 0.049 (0.010, 0.088) | 0.016 |
| Height‐for‐age | ||
| Intercept | −2.376 (−3.617, −1.135) | 0.0002 ‡ |
| Intervention | −0.137 (−0.293, 0.020) | 0.088 |
| Time | −0.142 (−0.171, −0.113) | <0.001 ‡ |
| Intervention × time | 0.037 (−0.004, 0.078) | 0.078 ‡ |
| Weight‐for‐height | ||
| Intercept | −1.710 (−3.058, −0.362) | 0.013 ‡ |
| Intervention | −0.119 (−0.291, −0.053) | 0.178 |
| Time | −0.061 (−0.092, −0.030) | <0.001 ‡ |
| Intervention × time | 0.050 (0.005, 0.095) | 0.030 |
All the outcomes were adjusted for parents' age, education, employment, number of children, and the child's birthweight and birth length.
‡ P value of multivariate t‐square test.
Discussion
We implemented an educational intervention based on locally accessible, affordable and acceptable resources in a rural area in China. The results showed that the intervention improved caregivers' knowledge, food selection, responsive feeding and hygiene behaviours, and ICFI, and children's physical growth. The effect size of the intervention is comparable with other studies. For example, a study in Bangladesh, which employed nutrition education and enhanced recipes found the WAZ score was 0.28 higher in the intervention sites (Roy et al. 2005). A study in Peru using similar strategies found WAZ score was 0.29 higher in the intervention group (Penny et al. 2005). These results provide evidence that promoting appropriate complementary feeding practices through health education and communication strategies can produce positive effects on children's growth outcomes.
In a recently published literature review, Shi and Zhang revealed that nutrition education combined with other strategies can improve growth and reduce malnutrition in developing countries (Shi & Zhang 2011). Several randomised controlled trials had similar design to our study, in that the intervention was developed based on formative research results and integrated the use of nutrition education and enhanced recipes (Santos et al. 2001; Bhandari et al. 2004; Aboud et al. 2008.). For example, Santos et al. (2001) also delivered similar intervention through health care providers. Our study has unique implications because of the intensity and sustainability of the intervention that could be achieved when it was delivered by health care providers. First, the intervention was delivered by the health care providers who have ready access to local families through routine health services. The primary health care providers delivered the intervention through multiple channels such as group training, home visit and individual counselling. These activities were scheduled to accommodate their regular child preventive health services, such as vaccination and regular home visit. This strategy ensures the intervention to be sustainable after the end of the study. Based on our communication with administrators of the local health department, they have integrated the education contents developed in our study into their regular child well visit schedule. In addition, according to our survey with local caregivers, they felt that the most trustful information source on child feeding is from health care professionals. This enhanced the effectiveness of the intervention when it was delivered by local health care providers. Second, the intervention strategies and educational messages were simple and effective, and cost little to disseminate. Therefore, our intervention can provide useful information on child feeding and nutrition in other developing countries with limited resources. It is noteworthy, however, that context and culture play an important role in infant and young child feeding behaviours; therefore caution is needed when replicating our study design in another location to make it adaptive to the local setting. Furthermore, the food availability alone is not a limiting factor in the study area because most families are able to afford recommended complementary foods. However, the traditional beliefs about the nature of foods and the digestive ability of young children made caregivers prefer foods with low protein such as flour and rice noodle, and avoid egg, meat and cooking oil, which were thought to be too heavy for infants to digest (Shi et al. 2010). Our study revealed that the educational messages were well taken by families and resulted in improved food selection practices such as incorporating animal‐source foods into complementary foods. Although this strategy is dependent on adequate family resources to acquire such foods, the findings from our study suggest that interventions to incorporate more animal source foods in the diets of older infants can be an important component of policies to improve growth in the 2 years of life. This strategy can be applied to food‐secure areas where dietary restriction beliefs result in poor quality (i.e. low protein and fat content) of complementary food.
Many nutrition educational intervention studies on complementary feeding have emphasised the consumption of animal‐source foods such as eggs, fish, chickens, meat, and of vegetables and fruits (Santos et al. 2001; Pachon et al. 2002; Bhandari et al. 2004). Because there were substantial variations on food resources and culture in different geographical regions, it is hard to compare the consumption of specific foods among different intervention studies. ICFI was developed and applied in Asia, Latin America and Africa as a comprehensive measure of feeding practices (Ruel & Menon 2002; Arimond & Ruel 2004; Ntab et al. 2005; Prosper et al. 2006; Moursi et al. 2009). ICFI summarises the recommended infant and young child feeding practices, with a higher score indicating better feeding practices. A few studies have tested the association between ICFI and child nutritional status (Ruel & Menon 2002; Arimond & Ruel 2004; Prosper et al. 2006; Moursi et al. 2009). Our study was the first attempt to apply ICFI to evaluate the nutritional intervention effects. The results showed that ICFI scores were significantly higher at 6, 9, 12, 15 and 18 months of age in the intervention groups than those in the control. It is expected that other educational intervention studies can use this ICFI to evaluate the intervention effects on complementary feeding behaviours and provide more evidence on the association between ICFI and child growth outcomes. We also evaluated other key complementary feeding behaviours according to WHO‐recommended guideline (WHO/UNICEF 1998; Dewey 2001). The results were consistent with the findings from a previous study in India (Bhandari et al. 2004).
This study found that the intervention group had higher z‐scores than that in the control. In a prior publication (Shi & Zhang 2011), the intervention group gained a faster growth velocity, but there were no significant difference in weight and length between the intervention and control groups at 12 months old. After an additional 6‐month follow‐up period, the improvement in weight and length in the intervention group were observed. The reason for the little impacts of the intervention on growth during the first year of life, but an effect at 18 months could be that children in rural China mainly depend on breast milk or formula in the first year and months of life; therefore, the effects of improved complementary foods may demonstrate at 18 months after most children switch to solid food. In this study, mixed model for longitudinal data analysis was applied to address the intraclass correlation of the repeated measures on the same child. Although z‐scores decreased over time in both groups, they decreased more slowly in the intervention group. These provide further evidence that educational intervention can effectively improve child growth.
There are some limitations in our study. Firstly, this study was not and could not be double‐blinded, because the same health care providers who implemented the educational intervention also measured the children, but the data collection was standardised and interviews were structured, which might limit any bias. We also clearly stated in the beginning of the study that the intervention effects including child's physical growth outcomes were not used as a measure to evaluate the job performance of the health care providers. Moreover, the children in both groups were enrolled into the study consecutively and measured repeatedly over the study period, and the primary outcome of interest was change of growth over time. This decreased the potential bias caused by health care providers to a certain degree. Secondly, about 20% of participants dropped out during the study period. Many rural labourers poured into cities for jobs because of the huge inequality in social and economic development between the rural and urban areas in China. However, the families lost to follow‐up were not significantly different from those who remained in the study with respect to baseline socio‐demographic characteristics. Lastly, the enhanced recipes developed in this study were based on locally available, affordable and acceptable foods, and included some animal organs such as bovine and chicken livers. These may contain dietary harmful ingredients such as polyamines. Animal organs and tissues contain a high content of spermine, which is associated with bacterial growth and tumour development. However, animal organs and tissues are widely used in the Chinese diet; in addition, polymines exist in a wide range of food items; therefore, it is difficult to avoid these food items in our enhanced recipes. Furthermore, polymines may be lost during the food storage and processing. Currently, there is no established evidence among human subjects for the harmful effects of dietary polyamines, and its safety level (Kalac 2009).
The strengths of our study are the randomised controlled design, well‐trained and supervised field workers, standardised evaluation procedures, and a carefully designed data analysis plan to take into account the clustering nature of the data. In addition, we followed up the subjects until 18 months of age, so we could observe the longer‐term impacts of the intervention. This study provides evidence on the effects of the educational intervention on complementary feeding practices and child's physical growth. Although the rate of malnutrition in this study setting is not high, malnutrition is still an important public health issue in most rural areas of China. Effective and sustainable interventions to reduce malnutrition and improve child growth are urgently needed in China and other developing countries. Therefore, this study has significant implications for prevention and intervention of child malnutrition in broad areas with similar social and economic conditions. The strategies employed in this study are ready to be replicated and adapted by future nutrition intervention studies.
Source of funding
This study was funded by the Proctor & Gamble Fellowship at Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. The funding source had no role in the study design, data analysis, data interpretation, or writing of the report.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
JZ wrote the first draft of the manuscript. LS, JZ, YW contributed to the design of the study and questionnaires. JZ, LS and JW contributed to the data collection and field supervision. D‐fC and LS contributed to the analysis.
Acknowledgement
The authors gratefully acknowledge the assistance of Laishui County Maternal and Child Health Hospital and the participating township hospitals in data collection, as well as the cooperation of the participants.
References
- Aboud F.E., Moore A.C. & Akhter S. (2008) Effectiveness of a community‐based responsive feeding programme in rural Bangladesh: a cluster randomized field trial. Maternal & Child Nutrition 4, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimond M. & Ruel M.T. (2004) Dietary diversity is associated with child nutritional status: evidence from 11 demographic and health surveys. Journal of Nutrition 134, 2579–2585. [DOI] [PubMed] [Google Scholar]
- Bhandari N., Mazumder S., Bahl R., Martines J., Black R.E., Bhan M.K. et al (2004) An educational intervention to promote appropriate complementary feeding practices and physical growth in infants and young children in rural Haryana, India. Journal of Nutrition 134, 2342–2348. [DOI] [PubMed] [Google Scholar]
- Chang S., He W., Jia F. & Chen C. (2007) Analysis on the changes of nutritional status in China anemia status of children under 5 in China. (in Chinese). Journal of Hygiene Research 36, 210–212. [PubMed] [Google Scholar]
- Chinese Ministry of Health (2004) A Study on Young Childrens' Nutrition and Health in Eight Provinces in China: Department of Maternal and Child Health.
- Dewey K.G. (2001) The challenges of promoting optimal infant growth. Journal of Nutrition 131, 1879–1880. [DOI] [PubMed] [Google Scholar]
- Fu Z., He W. & Chen C. (2000) Relationship between growth of young children and complementary feeding. (in Chinese). Journal of Hygiene Research 29, 279–282. [PubMed] [Google Scholar]
- Guldan G.S., Fan H.C., Ma X., Ni Z.Z., Xiang X. & Tang M.Z. (2000) Culturally appropriate nutrition education improves infant feeding and growth in rural Sichuan, China. Journal of Nutrition 130, 1204–1211. [DOI] [PubMed] [Google Scholar]
- He Y. & Zhai F. (2001) Complementary feeding practice in Chinese rural children. (in Chinese). Journal of Hygiene Research 30, 305–307. [PubMed] [Google Scholar]
- Kalac P. (2009) Recent advances in the research on biological roles of dietary polyamines in man. Journal of Applied Biomedicine 7, 65–74. [Google Scholar]
- Kilaru A., Griffiths P.L., Ganapathy S. & Ghosh S. (2005) Community‐based nutrition education for improving infant growth in rural Karnataka. Indian Journal of Pediatric 42, 425–432. [PubMed] [Google Scholar]
- Li L., Rao K., Kong L., Yao C., Xiang H., Zhai F. et al (2005) A description on the Chinese national nutrition and health survey in 2002. (in Chinese). Chinese Journal of Epidemiology 26, 478–484. [PubMed] [Google Scholar]
- Moursi M.M., Treche S., Martin‐Prevel Y., Maire B. & Delpeuch F. (2009) Association of a summary index of child feeding with diet quality and growth of 6–23 months children in urban Madagascar. European Journal of Clinical Nutrition 63, 718–724. [DOI] [PubMed] [Google Scholar]
- Ntab B., Simondon K.B., Milet J., Cisse B., Sokhna C., Boulanger D. et al (2005) A young child feeding index is not associated with either height‐for‐age or height velocity in rural Senegalese children. Journal of Nutrition 135, 457–464. [DOI] [PubMed] [Google Scholar]
- Pachon H., Schroeder D.G., Marsh D.R., Dearden K.A., Ha T.T. & Liang T.T. (2002) ) Effect of an integrated child nutrition intervention on the complementary food intake of young children in rural north Viet Nam. Food & Nutrition Bulletin 23(4 Suppl.):62–69. [PubMed] [Google Scholar]
- Pan American Health Organization/WHO (2001) Guiding principles for complementary feeding of the breastfed child. Pan American Health Organization, Washington, DC: PAHO/WHO.
- Penny M.E., Creed‐Kanashiro H.M., Robert R.C., Narro M.R., Caulfield L. & Black R. (2005) Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: a cluster‐randomised controlled trial. Lancet 365, 1863–1872. [DOI] [PubMed] [Google Scholar]
- Prosper S.S., Yves M., Mathilde S., Yves K., Pierre T., Alfred S.T. et al (2006) An infant and child feeding index is associated with the nutritional status of 6‐ to 23‐month‐old children in Rural Burkina Faso. Journal of Nutrition 136, 656–663. [DOI] [PubMed] [Google Scholar]
- Roy S.K., Fuchs G.J., Mahmud Z., Ara G., Islam S., Shafique S. et al (2005) ) Intensive nutrition education with or without supplementary feeding improves the nutritional status of moderately‐malnourished children in Bangladesh. Journal of Health, Population & Nutrition 23, 320–330. [PubMed] [Google Scholar]
- Ruel M.T. & Menon P. (2002) Child feeding practices are associated with child nutritional status in LatinAmerica: innovative uses of the Demographic and Health Surveys. Journal of Nutrition 132, 1180–1187. [DOI] [PubMed] [Google Scholar]
- Salehi M., Kimiagar S.M., Shahbazi M., Mehrebi Y. & Kolahi A.A. (2004) Assessing the impact of nutrition education on growth indices of Iranian nomadic children: an application of a modified beliefs, attitudes, subjective‐norms and enabling‐factors model. British Journal of Nutrition 91, 779–787. [DOI] [PubMed] [Google Scholar]
- Santos I., Victora C.G., Martines J., Gonçalves H., Gigante D.P., Valle N.J. et al (2001) Nutrition counseling increases weight gain among Brazilian children. Journal of Nutrition 131, 2866–2873. [DOI] [PubMed] [Google Scholar]
- Shen T., Habicht J.P. & Chang Y. (1996) Effect of economic reforms on child growth in urban and rural areas of China. New England Journal of Medicine 35, 400–406. [DOI] [PubMed] [Google Scholar]
- Shi L. & Zhang J. (2011) Recent evidence of the effectiveness of educational interventions for improving complementary feeding practices in developing countries. Journal of Tropical Pediatrics 57, 91–98. [DOI] [PubMed] [Google Scholar]
- Shi L., Zhang J., Wang Y., Caulfield L.E. & Guyer B. (2010) Effectiveness of an educational intervention on complementary feeding practices and growth in rural China: a cluster randomised controlled trial. Public Health Nutrition 13, 556–565. [DOI] [PubMed] [Google Scholar]
- UNICEF (2005) Nutrition issues: Micronutrient Deficiency .
- Waterlow J.C. (1988) Observations on the natural history of stunting In: Linear Growth Retardation in Less Developed Countries, pp 5–8. Nestle Nutrition S.A.: Vevey, Switzerland. [Google Scholar]
- World Health Organization (WHO)/UNICEF (1998) Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge. World Health Organization: Geneva. WHO/NUT/98.1. [Google Scholar]
- Zhang J., Shi L., Wang J. & Wang Y. (2009) An infant and child feeding index is associated with child nutritional status in rural China. Early Human Development 85, 247–252. [DOI] [PubMed] [Google Scholar]


