Abstract
Understanding of the importance of dietary fatty acids has grown beyond a simple source of energy to complex roles in regulating gene expression and cell and intracellular communication. This is important because the metabolic and neuroendocrine environment of the fetus and infant plays a key role in guiding the set point of neural receptors that regulate energy homeostasis and expression of genes that control energy storage and oxidation. Early deviations in these pathways have the potential to lead to lasting adaptations, termed metabolic programming, which may combine to increase the risk of metabolic syndrome in later life. The quality of fatty acids in human diets has undergone major changes in the last 50 years, characterized by an increase in ω‐6 and decrease in ω‐3 fatty acids. Evidence is accumulating to support the concept that the maternal intake of ω‐6 and ω‐3 fatty acids in gestation and lactation, possibly involving both excess ω‐6 and inadequate ω‐3 fatty acids, can impact the developing infant tissue lipids and neuroendocrine and metabolic pathways relevant to metabolic programming. Further work is needed to understand the needs for different ω‐6 and ω‐3 fatty acids during fetal and infant life, and their roles with respect to development of energy homeostasis and metabolism.
Keywords: metabolic programming, dietary fatty acids, pregnancy, lactation, docosahexaenoic acid, omega 6 fatty acids
Introduction
Evidence from epidemiological and experimental studies has accumulated to show that the nutrient substrate supply in utero and during infancy has long‐term implications for later development of metabolic diseases, including obesity, type 2 diabetes, cardiovascular disease (CVD) and hypertension (McMillian & Robinson 2005; Bouret 2009; Symonds et al. 2009; Bruce & Hanson 2010; Heerwagen et al. 2010). Dietary lipids, including their fatty acids, have effects that extend far beyond sources of metabolic and storage energy to pivotal roles in regulating cell growth and function, coordination of inter‐ and intracellular communication, and as modulators of the expression of genes that integrate the nutrient substrate supply and respond to the metabolic environment (Bordoni et al. 2006; Desvergne et al. 2006; Jump 2008; Tontonoz & Spiegelman 2008; Bradshaw et al. 2009; Di Marzo et al. 2009; Maccarrone et al. 2010). Central to concerns over the importance of dietary lipids in shaping early development, the fatty acids transferred across the placenta and secreted in mother's milk are not only variable, but highly dependent on the mother's diet (Jensen 1999; Innis 2005). This review integrates advances in understanding the essential and regulatory functions of dietary fatty acids with changes in dietary patterns to provide a framework to consider the question of whether dietary fatty acids may play a role in metabolic programming associated with chronic metabolic disease.
Key Messages
-
•
Fatty acids are more than energy and essential fatty acids: they regulate gene expression, protein function and function in signal molecules that regulate appetite, energy balance and inflammation.
-
•
The ω‐6, ω‐3 and monounsaturated fatty acids transferred across the placenta and in mother's milk are variable and depend on the mother's diet.
-
•
Nutrient deficiencies and excesses during development can reprogram how cells divide, differentiate and respond to their hormone and nutrient environment: these effects can be long‐lasting and increase risk of later chronic diseases, such as diabetes and obesity.
-
•
A global increase in diabetes and obesity has occurred concurrent with an increased consumption of ω‐6 linoleic acid form vegetable oils, not with an increase in saturated or animal fats.
-
•
Biological pathways and experimental data link high ω‐6 linoleic acid and low ω‐6 fatty acids to programming of appetite and energy metabolism; there is a lack of good studies to address this in humans.
Metabolic programming
Evidence that nutritional experiences during early life can have long‐lasting effects emerged over 50 years ago from classic studies that showed life‐time changes in growth and body size due to early abundant or restricted maternal milk in rodents raised in small or large litters (Widdowson & McCance 1963). A few years later, seminal work in Norway highlighted the association between poverty in childhood followed by later prosperity and higher blood cholesterol and arteriosclerotic heart disease (Forsdahl 1978). Subsequent epidemiological studies by Barker and coworkers solidified the concept of the developmental origins of health and disease through studies that linked low birth weight to an increased risk of coronary heart disease, hypertension and type 2 diabetes among almost 16 000 men and women in the UK. (Osmond & Barker 2000). However, concerns over chronic disease have shifted from a focus on coronary heart disease to the growing problems of metabolic syndrome now affecting greater numbers of people, including infants and youth (Amemiya et al. 2007; Zimmet et al. 2007). Metabolic syndrome, which is characterized by a cluster of risk factors for CVD and type 2 diabetes, including obesity (particularly abdominal), dyslipidaemia (elevated triglycerides and free fatty acids, and decreased HDL cholesterol), hypertension, insulin resistance and glucose intolerance, was recently estimated to affect a quarter of the world's adult population with a growing prevalence in developing nations as they continue socio‐economic development towards westernized lifestyles (Amemiya et al. 2007). Metabolic syndrome is also occurring with increasing frequency among children and youth in both developed and developing nations, with an increase in the prevalence of obesity and type 2 diabetes in children worldwide that is particularly evident from the mid‐ to late 1970s (Shaw 2007; Zimmet et al. 2007). In 2004, the World Health Organization (WHO) estimated that 22 million children under 5 years of age, and 10% of all children 5–17 years of age were overweight or obese, and predicted that childhood obesity will continue to increase in both developed and developing countries (Zimmet et al. 2007).
While there is no doubt that energy intake in excess of need plays a central role in overweight and obesity, scientific evidence has accumulated to show that pre‐ and post‐natal metabolic stressors can predispose the individual to later increased risk of characteristics of the metabolic syndrome. Among these early life events, low and high birthweight, maternal obesity and diabetes during pregnancy and lactation, and rapid post‐natal weight gains particularly in preterm infants, are all associated with an increased risk of obesity, glucose intolerance, insulin resistance, dyslipidaemia and elevated blood pressure in later life (McMillian & Robinson 2005; Symonds et al. 2009; Bruce & Hanson 2010; Heerwagen et al. 2010). Strong biological evidence for the concept that nutritional deficiencies and excesses in the maternal diet can ‘reprogram’ the way in which the offspring's cells divide, differentiate and respond to their metabolic milieu has come from studies in animals showing that high fat, high saturated fat and high carbohydrate diets, and deficiencies of protein and several vitamins in pregnancy and lactation, all lead to offspring at increased risk for characteristics of the metabolic syndrome (McMillian & Robinson 2005; Taylor & Poston 2007; Symonds et al. 2009; Bruce & Hanson 2010; Heerwagen et al. 2010). Although diverse, these early nutritional/endocrine adversities all converge on disturbances in appetite/energy sensing and/or inappropriate metabolic‐endocrine responses to nutrient intake involving the brain, adipose, muscle, liver, pancreas and other organs (Fig. 1). While the mechanism(s) remain incompletely understood, they can be broadly grouped as insults arising from structural alterations in cells and organs, metabolic including neuroendocrine changes, and alterations in gene expression including epigenetic mechanisms. Cross‐talk between mechanisms and organs is expected, and in many cases, the effects of the early nutritional milieu on later health outcomes may well be multifactorial.
Figure 1.
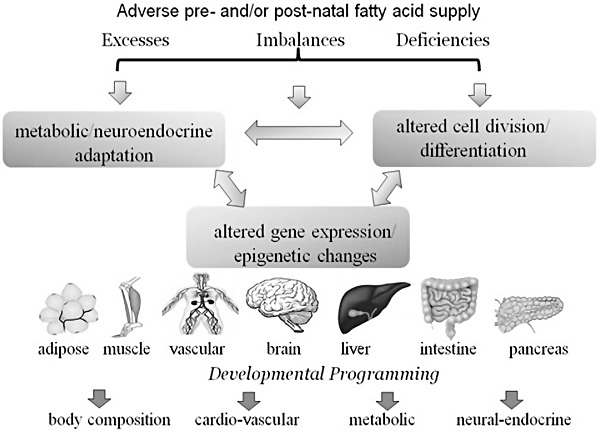
Schematic to illustrate the concept that deficiency, excesses or imbalances in fatty acids at key stages of development may have lasting consequences due to alterations in cellular development, gene expression or the metabolic and neuroendocrine response to the fatty acid supply. Effects are expected to involve multiple mechanisms and organs and cross‐talk among organs and mechanisms. Adapted from Symonds et al. (2009).
Several lines of evidence suggest that inadequate, unbalanced or excess supplies of specific fatty acids during early development may contribute to metabolic programming. Major changes in fatty acid nutrition have occurred in a time frame consistent with the increase in many chronic diseases, maternal diet impacts the quality of fatty acids transferred across the placenta and secreted in breast milk, fatty acid accumulation in fetal and infant tissues, including the brain, is influenced by the pre‐ and post‐natal fatty acid supply, and fatty acids and their metabolites play critical roles in cell growth, differentiation and integration of the cellular response to the metabolic and neuroendocrine environment (Innis 2010), as discussed in the following sections.
Critical periods of nutritional vulnerability: setting the stage for developmental programming
The concept of ‘critical’ periods when nutritional adversity leads to effects that persist into adulthood is a key feature of developmental programming, because the same adversity in the older child or adult is expected to cause only transient impairments that are reversible following restitution of an adequate diet. As for other nutrients, the potential for ‘programming’ due to inadequate/inappropriate fatty acid supplies is likely to be greatest when occurring at key stages of cell growth and differentiation, or establishment of the set point for response to nutritional, metabolic or endocrine cues, such as occuring during embryonic, fetal and infant development (Rice & Barone 2000; Georgieff & Innis 2005). Before birth, placental fatty acid transfer is relatively low and increases to about 11% of the energy supply by term gestation, with only low rates of fatty acid oxidation occurring in the fetal liver (Böhme et al. 1983; Herrera & Amusquivar 2000). At birth, the infant must ‘adapt’ to enable digestion, absorption and processing of the milk diet which provides about 50% energy from fat, with about half of this being saturated fatty acids (Jensen 1999). The axis of nutrient delivery shifts from the transfer of fatty acids directly to the fetal liver via the umbilical vein to transfer via the intestine with the transport of fatty acids in chylomicrons triacylglycerols. At the same time, the major energy substrate for muscle changes from glucose and amino acids to fatty acids, and the nutrient supply becomes episodic and regulated by the infant, rather than the mother. Remarkable structural, metabolic and neuroendocrine adaptation is orchestrated across organs, including liver, intestine, pancreas, kidney, adipose, muscle and brain, with development of regulatory networks involved in appetite and energy sensing, and circadian rhythm. The roles of fatty acids, not only in membrane glycerophospholipids, but also in a large number of lipid‐signalling molecules, several of which are involved in appetite and energy metabolism (Bradshaw et al. 2009; Di Marzo et al. 2009; Maccarrone et al. 2010), and as metabolic sensors contributing the regulation of genes of energy storage and oxidation (Desvergne et al. 2006; Jump 2008), provide a rationale to consider that fatty acid nutrition may be a key variable contributing to metabolic programming.
Trends in dietary lipids, fatty acids and their sources: setting the stage for metabolic programming
An understanding of the fatty acid quality of human diets and recent trends in dietary fat intake is important because maternal dietary fat composition is one of the most important factors determining the quality of fatty acids transferred across the placenta and secreted in breast milk (Jensen 1999; Innis 2005). Thus, the changes in dietary fat intake over the last half‐century provide the framework to consider the possibility that inadequacies, imbalances or excesses in certain fatty acids are contributing to the increased prevalence of preventable metabolic diseases in developed nations and their emergence in developing nations. Although total energy and fat intakes are clearly lower in many developing than developed nations (Wolmarans 2009), it is clear that the global increase in obesity and type 2 diabetes worldwide has occurred concurrent with an increased consumption of refined vegetable oils rich in the ω‐6 fatty acid linoleic acid (LA, 18:2ω‐6), and not with an increase in the intake of saturated fatty acids or animal fat. Information recently published by the Food and Agricultural Organization and provided in Fig. 2 shows the large increase in vegetable oil consumption from 13.2 to 30.4 g person−1 day−1 and from 8.8 to 25.6 g person−1 day−1 in developed and developing countries, respectively, over the period 1961–1963 to 2001–2003 (Wolmarans 2009). Over the same time period, animal fat intakes fell from 21 to 15.5 g person−1 day−1 in developed countries with a change from 2.2 to 4.6 g person−1 day−1 in developing countries. Compatible with world data, survey data in the USA show the average dietary fat intake decreased from 36.1% to 32.8% energy and saturated fat intake fell from 13.0% to 11.0% energy among US women from 1971–1974 to 1999–2000, while data on the secular trends in diet among US children from 1973 show an increase in carbohydrate, decrease in total and saturated fat, and a 26% increase in LA (Nicklas et al. 1993; Wolmarans 2009). The types of vegetable oils consumed also differ between developed and developing nations: the major dietary oil in North America is soybean oil, which typically has 58% LA and 6–7% of the ω‐3 fatty acid linolenic acid (ALA, 18:3ω‐3), while the consumption of peanut, cottonseed, coconut and palm kernel, which are all higher in saturated and monounsaturated fatty acids, is higher in developing countries. A further important difference is that while 80% of vegetable‐derived fat in North America is consumed as refined vegetable oils, 33–40% of vegetable‐derived fat consumed in Asia and Africa is consumed in plant foods (Wolmarans 2009). Vegetable fats and oils provide saturated and monounsaturated fatty acids, and LA and ALA, but the longer chain ω‐6 fatty acids such as arachidonic acid (ARA, 20:4ω‐6), and the ω‐3 fatty acids eicosapentaenoic acid (EPA, 20:5ω‐3) and docosahexaenoic acid (DHA, 22:6ω‐3) are naturally present in human diets only in animal tissue lipids. This means that the intakes of ARA, EPA and DHA depend on the types and amounts of protein in the diet, rather than fat. EPA and DHA are synthesized in aquatic phytoplankton and transferred up the aquatic food chain, and this leads to high EPA and DHA in fish and marine mammal fats. The fatty acid composition of ruminant and non‐ruminant meats, poultry and their products is more complex, because the fatty acids in animals' feeds contributes to the tissue saturated, monounsaturated and ω‐6 and ω‐3 fatty acids (Innis 2010). Overall, the trends in dietary fatty acids over the last 50 years have been towards a global increase in highly refined vegetable oils and increased intakes of ω‐6 LA. In westernized nations, LA now represents 80–90% of all dietary polyunsaturated fatty acids, accompanied by a lower intake and proportion of total ω‐3 fatty acids, and lower proportion of polyunsaturated fatty acids from ARA, EPA and DHA than occurred prior to the1900s. Although separating the effects of excess dietary energy and fat on chronic disease is always problematic, it seems clear that trends in fat and saturated fat intake in North America are not readily compatible with a hypothesis that high fat and saturated fatty acid intakes, or a need for higher ω‐6 fatty acids, are causal factors in metabolic programming. Rather, if fatty acids are involved, it seems more likely that the problem is excess ω‐6 fatty acids, inadequate ω‐3 fatty acids, imbalance in the dietary fatty acid profile or some other variable associated with high intakes of fat from refined triacylglycerols.
Figure 2.
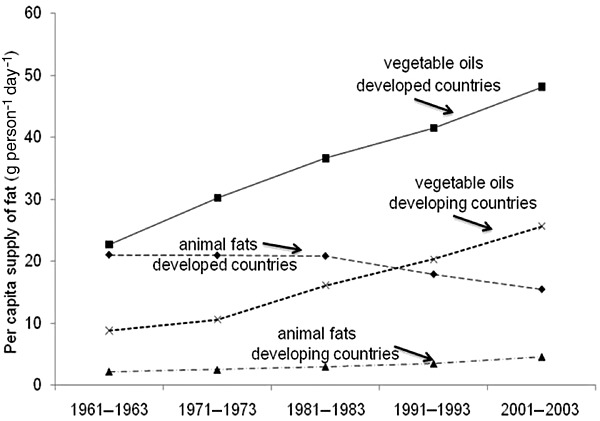
Per capita intakes of fats from vegetable oils and animal fats in developed and developing nations. Redrawn using data from Wolmarans (2009).
Much of the recent interest in fatty acid nutrition and infant development has focused on DHA particularly with regard to the developing brain and retina, as discussed in this volume by Makrides et al. (2011). However, the composition of other fatty acids in the maternal diet is also important with respect to the monounsaturated, ω‐6 fatty acids and other ω‐3 fatty acids transferred across the placenta and secreted in human milk (Jensen 1999; Innis 2004, 2005). In general, the shift to higher maternal‐to‐infant transfer of LA is accompanied by transfer of lower levels of monounsaturated oleic acid (18:1ω‐9) and ω‐3 fatty acids, including DHA, and this reflects the changes in the composition of dietary fatty acid intakes over the last 50 years. Trans fatty acids from partially hydrogenated vegetable oils are a clear example of the impact of modern food production practices on the fatty acid nutrition of the infant before birth and during breastfeeding. Prior to recent initiatives to reduce or eliminate industrially derived trans fatty acids, trans fatty acids as high as 12.8% were found in plasma triacylglycerols of newborn infants, with 13.8–18.7% trans fatty acids in human milk fatty acids in North America (Innis & King 1999; Elias & Innis 2001; Mosley et al. 2005). Although limited data on trans fatty acids exists for many developing countries, the association between oxidative stress, elevated inflammatory cytokines and metabolic programming (Heerwagen et al. 2010) suggests that hydrogenation should not be overlooked when considering the quality of dietary fats and oils in developing countries and their effects on infant development.
A large number of experimental studies have shown that maternal diet fat composition during gestation and lactation impacts not only the composition of fetal and infant blood lipids, but also the fatty acids in developing tissues, although effects do differ among different organs and cells (Innis 2010). Unfortunately, relatively little information is available on the effects of dietary fatty acids on the tissue fatty acids, other than blood cells in human infants. Classic studies by Widdowson and colleagues in 1975, however, described the adipose tissue fatty acid composition of British and Dutch infants at birth and during the first months of life (Widdowson et al. 1975). At that time, infant formulas in the UK were based largely on unmodified cows' milk fat, while infant formula in the Netherlands had been prepared with corn oil for about a decade. The Dutch formula fat had 10.7% 16:0 and 58.2% LA (18:2ω‐6), while the British formula had 30% 16:0 and 1.8% LA and British human milk had 23.7% 16:0, 9.0% LA and 3.4% ALA (18:3ω‐3). At birth, LA represented 1–3% of adipose tissue fatty acids in both the British and Dutch infants, but increased to 23% and 32–37% in the Dutch formula‐fed infants at 1 and 2 months of age, respectively, and never exceeded 3% in the British formula‐fed infants at any age, or 5% fatty acids in breastfed British infants (Widdowson et al. 1975). Clearly, the quality of the dietary fat has profound effects on the quality of fatty acids stored in infant adipose tissues. The exceedingly low LA in the adipose tissue of infants, including breastfed infants in the 1970s is remarkable. Regardless of diet, the brain is notable for very low LA, but high amounts of ARA, adrenic acid (22:4ω‐6) and DHA (Innis 2010). Autopsy studies by Farquharson et al. (1995) showed 17.6% and 19.6% ARA, 12.0% and 14.3% 22:4ω‐6, 3.2% and 7.0% 22:5ω‐6 and 17.7% and 11.6% DHA in the brain cortex ethanolamine lipids of infants who had been breastfed or fed formula with 14.5% LA and 0.4%ALA (with no ARA or DHA). These results demonstrate that the young infant brain accumulates excess long chain ω‐6 fatty acids but low DHA when the infant diet is high in LA but limiting in ω‐3 fatty acids. Overall, it is clear that regardless of pathways that result in tissue‐specific ω‐6 and ω‐3 fatty acid patterns, infant tissues are not protected from substantial shifts in fatty acid composition that result entirely from the quality of the dietary fatty acid supply.
Metabolic programming by dietary fatty acids
Much of the recent interest in metabolic programming has focused on maternal overnutrition, and this is relevant in developed nations where many women enter pregnancy overweight or obese and/or with diabetes (Heerwagen et al. 2010). Elevated maternal body weight and plasma triglycerides has been linked to a concentration‐dependent increase in maternal‐to‐fetal fatty acid transfer, increased infant birth weight and adiposity, elevated inflammatory cytokines and leptin, all of which set the stage for hyperphagia, insulin resistance and development of diabetes and obesity (Heerwagen et al. 2010). Similarly, studies with animals fed high fat or high carbohydrate diets during pregnancy and lactation have shown increased muscle, adipose and liver lipid deposition, altered hepatic gene expression, pancreatic β‐cell development and insulin and leptin secretion in young offspring (McMillian & Robinson 2005; Bouret 2010; Bruce & Hanson 2010). Later on, these offspring show metabolic programming involving multiple organs, including the adipose, muscle, liver and pancreas, and reprogramming of hypothalamic appetite/energy sensing pathways, all evidence that an early inappropriate nutrient‐substrate‐endocrine milieu has lasting consequences. Although many of these studies have focused on programming by maternal high fat diets compared with chow, or diets with lower energy density and higher protein, they provide important knowledge of the metabolic and neuroendocrine pathways susceptible to long‐term programming. Among these, early neuroendocrine and epigenetic changes are among the best understood mechanisms leading to long‐term metabolic programming.
Regulation of energy balance and appetite is complex, and involves not only input to the hypothalamus by hormones from the adipose, pancreas and intestine, but also the response of orexigenic neuropeptides including neuropeptide Y and agouti‐related peptide, and the anorexigenic peptides propiomelanocortin (POMC) and cocaine‐ and amphetamine‐regulated transcript (CART) (McMillian & Robinson 2005; Bouret 2010). The role of hormones as neurotrophic signals in establishing neural receptor set‐points is one of the best known mechanisms through which the early environment has lasting effects on the organism. A well‐known example relevant to maternal undernutrition and involving both neuroendocrine and epigenetic mechanisms is the effect of elevated prenatal glucocorticoid exposure in programming heightened stress responses, together with hyperglycemia, insulin resistance and epigenetic modification of the hippocampal glucorticoid receptor, and hepatic gluconeogenic enzymes such as phosphoenolpyruvate carboxykinase (McMillian & Robinson 2005). Leptin, a small polypeptide hormone synthesized by the adipose tissue, and insulin from the pancreas both act as neural regulatory peptides during a short window of development to coordinate development of the hypothalamic neuro‐regulatory network that regulates feeding behaviour and energy balance (Symonds et al. 2009; Bouret 2010). Deviations from the correct leptin and/or insulin input during short developmental windows alters development of neuro‐regulatory circuitry controlling feeding, and this is believed to contribute to later hyperphagia and excess body weight. Evidence that maternal high fat diets also alter hypothalamic peptides including POMC and agouti‐related protein in the fetal brain has also been published (Page et al. 2009; Grayson et al. 2010).
Although studies on maternal overnutrition and high fat feeding have not focused specifically on dietary fatty acid quality, information is accumulating to suggest that fatty acid quality is relevant to the development of regulatory networks involved in feeding and energy balance. However, the effect of dietary fatty acids is complex and likely to involve multiple tissues and metabolic pathways (Fig. 1), and may be both direct and indirect. For example, hypothalamic POMC neurons also respond to dopamine (Cone et al. 2001), which is also altered in the DHA‐deficient fetal and infant brain (Innis 2007). The N‐acyl amino acids, neurotransmitters, and ethanolamides are fatty acid‐linked signal molecules which have important roles in behaviour, cognition and memory, and regulation of food intake and energy metabolism (Fride 2008; Bradshaw et al. 2009; Maccarrone et al. 2010; Patel et al. 2010). These endogenously synthesized metabolites involve a fatty acid conjugated to an amino acid, such as glycine, a neurotransmitter such as dopamine or ethanolamide and several are ligands for canabinoid receptors and peroxisome proliferator‐activated receptor α (PPAR α) and peroxisome proliferator‐activated receptor (PPAR γ) (O'Sullivan & Kendall 2010). Some of the best known are arachidonyl‐ethanolamide (anandamide), palmitoyl ethanolamide (PEA), oleoyl‐ethanolamide (OEA)‐ethanolamide, and arachidonoyl‐, palmitoyl‐, stearoyl‐ and oleoyl‐glycine. OEA in particular is known for its anorectic role and expands consideration of dietary fat quality to the monounsaturated fatty acids. Emerging evidence is beginning to show that diet fatty acid composition influences several acyl‐signal molecules in the brain and intestine, with implications for neural function, eating behaviours and obesity‐related problems (Di Marzo et al. 2009; Maccarrone et al. 2010; O'Sullivan & Kendall 2010). This field of fatty acids research is likely to see important advances linking changes in membrane phospholipids and their fatty acids, which are precursors for synthesis of some of these acylated molecules, to the effects of diet on neural functions and energy homeostasis, and the immune system.
Although it is clear that dietary fat quality impacts neural membrane fatty acids and several neurotransmitters and lipidated signal molecules, programming effects arising from altered adipose tissue mass, composition or metabolism are also possible. ARA‐derived eicosanoids play key roles in adipocyte differentiation through interaction with PPAR γ (Tontonoz & Spiegelman 2008), raising the possibility that increased fetal and/or neonatal adipose tissue ω‐6 fatty acids foster increased adipose tissue differentiation and leptin signalling. In this regard, LA and ARA have been shown to decrease insulin‐stimulated adipocyte leptin secretion and mRNA expression (Pérez‐Matute et al. 2007), while EPA and DHA increased leptin production in adult male rats (Peyron‐Caso et al. 2002). Recent studies focusing on the dietary fatty acids and the accumulation of fat mass reported that a diet with 35% fat energy from fat with 18% energy from LA and 0.6% energy from ALA fed over four generations led to a gradual increase in fat mass due to combined adipose tissue hyperplasia and hypertrophy, with trans‐generational alterations in adipokines, adipose tissue gene expression and hyperinsulinaemia (Massiera et al. 2010). Other studies suggest that dietary ω‐6 and ω‐3 fatty acids alter hypothalamic peptides. In rats naturally susceptible to metabolic syndrome, an isoenergetic diet with higher EPA and DHA, and lower LA increased hypothalamic mRNA levels of the anorexigenic peptide CART (Burghardt et al. 2010). Consistent with this, early dietary ω‐3 fatty acid deficiency with high LA (safflower oil) during gestation has been reported to result in long‐term programming with impaired gluco‐regulatory networks causing excess food intake following short‐term food or glucose deprivation (Mathai et al. 2004).
The liver plays a key role in integration of the nutrient energy supply, releases metabolic energy as glucose or fatty acids for other organs and undergoes a fundamental shift in pathways for fatty acid oxidation, glycolysis and gluconeogenesis in the first hours and days following birth (Böhme et al. 1983; Herrera & Amusquivar 2000). Saturated and ω‐6 and ω‐3 fatty acids differ in effects on lipid metabolism, and although still incompletely understood, this may involve unique effects of fatty acids or their metabolites on transcription factors that regulates expression of genes for regulatory enzymes of gluconeogenesis, glycolysis and fatty acid synthesis and oxidation (Desvergne et al. 2006; Jump 2008). Epigenetic regulation of gene transcription involving DNA methylation, histone modifications or micro RNAs are key aspects of early development through which specific phenotypes are achieved, maintained and potentially transferred to future generations (McMillian & Robinson 2005; Heerwagen et al. 2010). DNA methylation is currently one of the best understood epigenetic processes that is sensitive dietary variables. Methylation of cytosine bases that are followed by a guanine, termed 5′‐CpG‐3′ sites or CpG islands that span the 5′ end of the regulatory region of genes, blocks the binding of transcription factors to the DNA, and this usually leads to gene silencing; hypomethylation, on the other hand, leads to increased gene expression. Sequential gene silencing and expression is a fundamental aspect of normal development; however, in some select genes, methylation is triggered by metabolic and neuroendocrine cues and these genes are thus susceptible to epigenetic programming by the nutritional or endocrine milieu. Fatty acids are part of the regulatory network through which the liver, and also skeletal muscle and adipose, sense and respond to the energy substrate supply to coordinate fatty acid oxidation and storage. The liver plays an additional key role through its capacity to convert excess metabolic energy to fatty acids and in gluconeogenesis. Methylation of gene promoter regions for PPAR α, the glucorticoid receptor and phosphoenoyl carboxykinase (a key regulatory enzyme of gluconeogenesis), and gene expression (mRNA) of hepatic nuclear factor (HNF) 4α and its gene targets phosphoenoyl carboxykinase, fructose 1,6 bisphosphatase and PPAR γ coactivator 1α (PGC1 α) in the fetal liver are known to be sensitive to maternal dietary cues (McMillian & Robinson 2005; Burdge et al. 2007; Lillycrop et al. 2008; McCurdy et al. 2009; Nijland et al. 2010). DHA has been suggested to have a unique role among fatty acids in decreasing the nuclear abundance of sterol regulatory element binding protein‐1, which activates genes involved in fatty acid synthesis, potentially explaining in part the triacylglycerol lowering action of long chain ω‐3, but not ω‐6 fatty acids (Jump 2008). HNF4α, on the other hand, is also a master regulator of hepatic gluconeogenesis and fatty acid and cholesterol metabolism, and regulates PPAR expression as well as genes involved in cell cycle, immune function, apoptosis and stress response (Bolotin et al. 2010). Recent studies identified LA as the endogenous, reversible ligand for HNF4α (Hwang‐Verslues & Sladek 2010). In proteomic analysis, 3‐day‐old offspring of rats fed iso‐energetic diets differing only in fatty acids showed high liver protein abundance of key enzymes of gluconeogenesis, including fructose‐1,6‐bisphosphatase, fatty acid oxidation, redox balance and nitric oxide signalling with higher liver LA, EPA and DHA, showing that the maternal dietary ω‐3 fatty acid supply directly impacted metabolic development in the neonatal liver (Novak et al. 2009). Whether specific fatty acids or their metabolites are metabolic cues that contribute to epigenetic regulation of gene transcription, culminating in a phenotype‐favouring energy retention in fat stores or increased oxidation, remains to be determined.
Some, although inconsistent, information relating dietary ARA and DHA to growth and measures of blood pressure is available from studies with term gestation breastfed infants and formula‐fed infants. Interpretation of studies comparing groups of breastfed and formula‐fed infants, however, is complicated by the possibility that maternal dietary fatty acids may contribute to variability within groups of breastfed infants. Complex interactions among genotype and children's diets and activity patterns, which are avoided in studies with animals, may also mask important effects of early diet in contributing to a phenotype with increased characteristics of metabolic syndrome that in themselves may be expressed only in some dietary conditions such as excess energy, carbohydrate or fat. In the largest ever randomized trial involving over 17 000 children who were breastfed or formula fed (prior to the addition of ARA and DHA to infant formulas), higher rates of formula feeding did not appear to increase adiposity or blood pressure (Kramer et al. 2007; Kramer et al. 2009), although longer exclusive breastfeeding led to higher child IQ scores when assessed at 6.5 years of age (Kramer et al. 2008). Helland et al. (2008) found no relationship between fatty acid status at birth or during the first 3 months of life and body mass index among 7‐year‐old children of mothers who took cod liver oil as a source of EPA and DHA, or a corn oil placebo during pregnancy and lactation. On the other hand, Lauritzen et al. (2005) reported that children 2.5 years of age whose mothers took 4.5 mL fish oil daily during the first 4 months of lactation had a larger waist circumference than children whose mothers took olive oil, although no difference in growth were present at 7 years of age (Lauritzen et al. 2005). However, the 7‐year‐old boys, but not girls whose mothers had taken fish oil had a higher diastolic and mean arterial blood pressure and lower physical activity than boys whose mothers had taken olive oil (Lauritzen et al. 2005). In contrast, a small multicentre study reported that the mean diastolic blood pressure was higher among 6‐year‐old children who had been fed formula without ARA and DHA than among children who had been breastfed or fed formula with ARA and DHA (Forsyth et al. 2003). In the longest follow‐up study to date, preterm girls fed formula containing 0.31% ARA and 0.17% DHA showed increased adiposity at 10 years of age when compared with preterm girls who had been fed a formula with no ARA and DHA (Kennedy et al. 2010). Although the data are limited, it should be clear that the ω‐6 and ω‐3 fatty acids are bioactive nutrients, which in the short and potentially long term regulate metabolic signalling and cellular differentiation, with the potential to increase or decrease lipid synthesis and storage, and potentially other physiological processes such as blood pressure.
Summary
This review has highlighted the evidence that fatty acids play key roles in lipid mediators and regulation of genes and proteins broadly relevant to the integration of metabolic substrate utilization, including lipid oxidation and storage, feeding response and maintenance of energy homeostasis. Diets have undergone major changes in fatty acid content over the last 50 years, best described as an increase in highly refined vegetable oil rich in ω‐6 LA, with a decrease in animal fats and loss of ω‐3 fatty acids from the food supply. At the same time, preventable metabolic diseases such as obesity and type 2 diabetes have increased. The economic and cultural transitions occurring in many developing countries include a shift towards westernized dietary patterns and increased consumption of refined vegetable oils. Programming of lipogenic and gluconeogenic pathways favouring energy storage and disturbances in central pathways that control feeding behaviour as a result of inadequate ω‐3 and/or high ω‐6 fatty acids in the modern westernized diet is an attractive hypothesis regarding the increasing prevalence of metabolic syndrome. However, information in direct support of this concept is limited, and most is drawn from animal studies in which the dietary interventions may have much greater effects, be irrelevant or miss the end points and mechanisms relevant to human health. Regardless, it should be clear that fatty acids are far more than sources of metabolic energy, and as such they may be key metabolic cues that contribute to the foundation of health human growth and development.
Conflicts of interest
The author has declared no potential conflicts.
References
- Amemiya S., Dobashi K., Urakami T., Sugihara S., Ohzeki T. & Tajima N. (2007) Metabolic syndrome in youths. Pediatric Diabetes 8, 48–54. [DOI] [PubMed] [Google Scholar]
- Böhme H.J., Sparmann G. & Hofmann E. (1983) Biochemistry of liver development in the perinatal period. Experientia 39, 473–483. [DOI] [PubMed] [Google Scholar]
- Bolotin E., Liao H., Ta T.C., Yang C., Hwang‐Verslues W., Evans J.R. et al (2010) Integrated approach for the identification of human hepatocyte nuclear factor 4alpha target genes using protein binding microarrays. Hepatology 51, 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni A., Di Nunzio M., Danesi F. & Biagi P.L. (2006) Polyunsaturated fatty acids: from diet to binding to ppars and other nuclear receptors. Genes Nutrition 1, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S.G. (2009) Early life origins of obesity: role of hypothalamic programming. Journal of Pediatric Gastroenterology and Nutrition 48, 531–538. [DOI] [PubMed] [Google Scholar]
- Bouret S.G. (2010) Role of early hormonal and nutritional experiences in shaping feeding behavior and hypothalamic development. Journal of Nutrition 140, 653–657. [DOI] [PubMed] [Google Scholar]
- Bradshaw H.B., Rimmerman N., Hu S.S., Burstein S. & Walker J.M. (2009) Novel endogenous N‐acyl glycines identification and characterization. Vitamins and Hormones 81, 191–205. [DOI] [PubMed] [Google Scholar]
- Bruce K.D. & Hanson M.A. (2010) The developmental origins, mechanisms and implications of metabolic syndrome. Journal of Nutrition 140, 648–652. [DOI] [PubMed] [Google Scholar]
- Burdge G.C., Slater‐Jefferies J., Torrens C., Phillips E.S., Hanson M.A. & Lillycrop K.A. (2007) Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. The British Journal of Nutrition 97, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt P., Kemmerer E.S., Buck B.J., Osetek A.J., Yan C., Koch L.G. et al (2010) Dietary n‐3 : n‐6 fatty acid ratios differentially influence hormonal signature in a rodent model of metabolic syndrome relative to healthy controls. Nutrition & Metabolism 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R.D., Cowley M.A., Butler A.A., Fan W., Marks D.L. & Low M.J. (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. International Journal of Obesity and Related Metabolic Disorders 25, S63–S67. [DOI] [PubMed] [Google Scholar]
- Desvergne B., Michalik L. & Wahli W. (2006) Transcriptional regulation of metabolism. Physiological Reviews 86, 465–514. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Ligresti A. & Cristino L. (2009) The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. International Journal of Obesity (2005) 33, S18–S24. [DOI] [PubMed] [Google Scholar]
- Elias S.L. & Innis S.M. (2001) Infant plasma trans, n‐6, and n‐3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. The American Journal of Clinical Nutrition 73, 807–814. [DOI] [PubMed] [Google Scholar]
- Farquharson J., Jamieson E.C., Abbasi K.A., Patrick W.J., Logan R.W. & Cockburn F. (1995) Effect of diet on the fatty acid composition of major phospholipids of infant cerebral cortex. Archives of Disease in Childhood 72, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdahl A. (1978) Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. The cardiovascular survey in Finnmark 1974–75. Journal of Epidemiology and Community Health 32, 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth J.S., Willatts P., Agostoni C., Bissenden J., Casaer P. & Boehm G. (2003) Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ 326, 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E. (2008) Multiple roles for the endocannabinoid system during the earliest stages of life: pre‐ and postnatal development. Journal of Neuroendocrinology 1, 75–81. [DOI] [PubMed] [Google Scholar]
- Georgieff M.K. & Innis S.M. (2005) Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatric Research 57, 94R–103R. [DOI] [PubMed] [Google Scholar]
- Grayson B.E., Levasseur P.R., Williams S.M., Smith M.S., Marks D.L. & Grove K.L. (2010) Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high‐fat diet. Endocrinology 151, 1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerwagen M.J., Miller M.R., Barbour L.A. & Friedman J.E. (2010) Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 299, R711–R722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland I.B., Smith L., Blomén B., Saarem K., Saugstad O.D. & Drevon C.A. (2008) Effect of supplementing pregnant and lactating mothers with n‐3 very‐long‐chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 122, e472–e479. [DOI] [PubMed] [Google Scholar]
- Herrera E. & Amusquivar E. (2000) Lipid metabolism in the fetus and the newborn. Diabetes/Metabolism Research and Reviews 16, 202–210. [DOI] [PubMed] [Google Scholar]
- Hwang‐Verslues W.W. & Sladek F.M. (2010) HNF4α‐role in drug metabolism and potential drug target? Current Opinion in Pharmacology 10, 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis S.M. (2004) Polyunsaturated fatty acids in human milk: an essential role in infant development. Advances in Experimental Medicine and Biology 554, 27–43. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2005) Essential fatty acid transfer and fetal development. Placenta 26, S70–S75. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2007) Dietary n‐3 fatty acids and brain development. The Journal of Nutrition 137, 855–859. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2010) The developing brain and dietary omega‐3 fatty acids In: International Handbook of Behavior, Diet, and Nutrition(ed. Preedy V.R., Watson R., Martin C.R.), Chapter 3.2, pp. Springer Publishing. [Google Scholar]
- Innis S.M. & King D.J. (1999) Trans fatty acids in human milk are inversely associated with concentrations of essential all‐cis n‐6 and n‐3 fatty acids and determine trans, but not n‐6 and n‐3, fatty acids in plasma lipids of breast‐fed infants. The American Journal of Clinical Nutrition 70, 383–390. [DOI] [PubMed] [Google Scholar]
- Jensen R.G. (1999) Lipids in human milk. Lipids 34, 1243–1271. [DOI] [PubMed] [Google Scholar]
- Jump D.B. (2008) N‐3 fatty acid regulation of hepatic gene transcription. Current Opinion in Lipidology 19, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K., Ross S., Isaacs E.B., Weaver L.T., Singhal A., Lucas A. et al (2010) The 10‐year follow‐up of a randomised trial of long chain polyunsaturated fatty acid supplementation in preterm infants: effects on growth and blood pressure. Archives of Disease in Childhood 95, 588–595. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Matush L., Vanilovich I., Platt R.W., Bogdanovich N., Sevkovskaya Z. et al (2007) Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. The American Journal of Clinical Nutrition 86, 1717–1721. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Aboud F., Mironova E., Vanilovich I., Platt R.W., Matush L. et al (2008) Breastfeeding and child cognitive development: new evidence from a large randomized trial. Archives of General Psychiatry 65, 578–584. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Matush L., Vanilovich I., Platt R.W., Bogdanovich N., Sevkovskaya Z. et al (2009) A randomized breast‐feeding promotion intervention did not reduce child obesity in Belarus. The Journal of Nutrition 139, 417S–421S. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Hoppe C., Straarup E.M. & Michaelsen K.F. (2005) Maternal fish oil supplementation in lactation and growth during the first 2.5 years of life. Pediatric Research 58, 235–242. [DOI] [PubMed] [Google Scholar]
- Lillycrop K.A., Phillips E.S., Torrens C., Hanson M.A., Jackson A.A. & Burdge G.C. (2008) Feeding pregnant rats a protein‐restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. The British Journal of Nutrition 100, 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M., Gasperi V., Catani M.V., Diep T.A., Dainese E., Hansen H.S. et al (2010) The endocannabinoid system and its relevance for nutrition. Annual Review of Nutrition 30, 423–440. [DOI] [PubMed] [Google Scholar]
- McCurdy C.E., Bishop J.M., Williams S.M., Grayson B.E., Smith M.S., Friedman J.E. et al (2009) Maternal high‐fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. Journal of Clinical Investigation 119, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian I.C. & Robinson J.S. (2005) Developmental origins of the metabolic syndrome prediction, plasticity, and programming. Physiological Reviews 85, 571–633. [DOI] [PubMed] [Google Scholar]
- Makrides M., Collins C.T. & Gibson R.A. (2011) Impact of fatty acid status on growth and neurobehavioural development in humans. Maternal and Child Health 7 (Suppl. 2), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massiera F., Barbry P., Guesnet P., Joly A., Luquet S., Moreilhon‐Brest C. et al (2010) A Western‐like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. Journal of Lipid Research 51, 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai M.L., Soueid M., Chen N., Jayasooriya A.P., Sinclair A.J., Wlodek M.E. et al (2004) Does perinatal omega‐3 polyunsaturated fatty acid deficiency increase appetite signalling? Obesity Research 12, 1886–1894. [DOI] [PubMed] [Google Scholar]
- Mosley E.E., Wright A.L., McGuire M.K. & McGuire M.A. (2005) Trans fatty acids in milk produced by women in the United States. The American Journal of Clinical Nutrition 82, 1292–1297. [DOI] [PubMed] [Google Scholar]
- Nicklas T.A., Webber L.S., Srinivasan S.R. & Berenson G.S. (1993) Secular trends in dietary intakes and cardiovascular risk factors of 10‐y‐old children: the Bogalusa Heart Study (1973–1988). The American Journal of Clinical Nutrition 57, 930–937. [DOI] [PubMed] [Google Scholar]
- Nijland M.J., Mitsuya K., Li C., Ford S., McDonald T.J., Nathanielsz P.W. et al (2010) Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. Journal of Physiology 588, 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak E.M., Lee E.K., Innis S.M. & Keller B.O. (2009) Identification of novel protein targets regulated by maternal dietary fatty acid composition in neonatal rat liver. Proteomics 73, 41–49. [DOI] [PubMed] [Google Scholar]
- Osmond C. & Barker D.J. (2000) Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environmental Health Perspectives 108, S545–S553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan S.E. & Kendall D.A. (2010) Cannabinoid activation of peroxisome proliferator‐activated receptors: potential for modulation of inflammatory disease. Immunology 215, 611–616. [DOI] [PubMed] [Google Scholar]
- Page K.C., Malik R.E., Ripple J.A. & Anday E.K. (2009) Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high‐fat diet in male offspring. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 297, R1049–R1057. [DOI] [PubMed] [Google Scholar]
- Patel K.D., Davison J.S., Pittman Q.J. & Sharkey K.A. (2010) Cannabinoid CB(2) receptors in health and disease. Current Medicinal Chemistry 17, 1393–1410. [DOI] [PubMed] [Google Scholar]
- Pérez‐Matute P., Martínez J.A., Marti A. & Moreno‐Aliaga M.J. (2007) Linoleic acid decreases leptin and adiponectin secretion from primary rat adipocytes in the presence of insulin. Lipids 42, 913–920. [DOI] [PubMed] [Google Scholar]
- Peyron‐Caso E., Taverna M., Guerre‐Millo M., Véronèse A., Pacher N., Slama G. et al (2002) Dietary (n‐3) polyunsaturated fatty acids up‐regulate plasma leptin in insulin‐resistant rats. Journal of Nutrition 132, 2235–2240. [DOI] [PubMed] [Google Scholar]
- Rice D. & Barone S. Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives 108, 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. (2007) Epidemiology of childhood type 2 diabetes and obesity. Pediatric Diabetes 8, 7–15. [DOI] [PubMed] [Google Scholar]
- Symonds M.E., Sebert S.P., Hyatt M.A. & Budge H. (2009) Nutritional programming of the metabolic syndrome. Nature Reviews. Endocrinology 5, 604–610. [DOI] [PubMed] [Google Scholar]
- Taylor P.D. & Poston L. (2007) Developmental programming of obesity in mammals. Experimental Physiology 92, 287–298. [DOI] [PubMed] [Google Scholar]
- Tontonoz P. & Spiegelman B.M. (2008) Fat and beyond: the diverse biology of PPARgamma. Annual Review of Biochemistry 77, 289–312. [DOI] [PubMed] [Google Scholar]
- Widdowson E.M. & McCance R.A. (1963) The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proceedings of the Royal Society of London. Series B, Biological Sciences 158, 329–342. [DOI] [PubMed] [Google Scholar]
- Widdowson E.M., Dauncey M.J., Gairdner D.M., Jonxis J.H. & Pelikan‐Filipkova M. (1975) Body fat of British and Dutch infants. British Medical Journal 1, 653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolmarans P. (2009) Background paper on global trends in food production, intake and composition. Annals of Nutrition & Metabolism 55, 244–272. [DOI] [PubMed] [Google Scholar]
- Zimmet P., Alberi K.G., Kaufman F., Tajima N., Silink M., Arslanian S. et al (2007) The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatric Diabetes 8, 299–306. [DOI] [PubMed] [Google Scholar]


