Abstract
The influence of childhood nutrition on the development of constipation beyond the period of weaning and breastfeeding is relatively understudied. In addition, eating patterns in childhood can be highly correlated with overweight and sedentary behaviour, which may also have an influence on constipation. The aim of this study was to assess whether common dietary patterns, sedentary behaviour and childhood overweight are associated with constipation in childhood. The study was embedded in a population‐based prospective birth cohort. Information on dietary intake was obtained by a food frequency questionnaire at the child's age of 14 months (n = 2420). The adherence scores on a ‘Health conscious’ and ‘Western‐like’ diet were extracted from principal component analysis. At the age of 24, 36 and 48 months, information on constipation and sedentary behaviour, and weight and height was obtained by parental‐derived questionnaires and from the child health centres, respectively. Adherence to a ‘Western‐like’ dietary pattern was associated with a higher prevalence of constipation up to 48 months [adjusted odds ratio (aOR); 95% confidence interval (CI): 1.39; 1.02–1.87], which was not mediated by overweight or sedentary behaviour. Adherence to a ‘Health Conscious’ dietary pattern was only associated at short term, with a lower prevalence of constipation at 24 months (aOR; 95%CI: 0.65; 0.44–0.96). No association was found between overweight, sedentary behaviour and constipation. Our results suggest that specific dietary patterns in early childhood could be associated with higher or lower risks for constipation, but these effects are time‐dependent. Overweight and sedentary behaviour seem to not have a major role on constipation in childhood.
Keywords: overweight, dietary patterns, constipation, children
Introduction
Constipation is frequently seen in the paediatric population with a prevalence varying from 0.7% to 30% (Mugie et al. 2011). Several factors including infant nutrition as potential causes for constipation in childhood have been suggested. Previously, we have found that timing of introduction of gluten and a history of cow's milk allergy are associated with constipation in childhood (Kiefte‐de Jong et al. 2010). Another study demonstrated that constipation was more frequent in infants who were not exclusively breastfed (Tunc et al. 2008). The influence of childhood nutrition on the development of constipation shortly beyond the period of weaning and breastfeeding is relatively understudied. In adults and schoolchildren, it is known that increasing dietary fibre and fluid intake can be effective in amelioration of symptoms of constipation (Kokke et al. 2008; Maffei & Vicentini 2011). However, in pre‐school children, this approach has been controversial as the results of studies regarding dietary fibre and constipation in very young children are inconsistent (Roma et al. 1999; Aguirre et al. 2002). Several studies have demonstrated that the prevalence of overweight and sedentary behaviour is increased in children with functional bowel disorders (Pashankar & Loening‐Baucke 2005; Misra et al. 2006; Teitelbaum et al. 2009; Chien et al. 2011). As dietary fibre intake and physical activity can be important determinants of both constipation and overweight (Zanovec et al. 2010; Maffei & Vicentini 2011) but unhealthy eating patterns also cluster with sedentary behaviour (Gubbels et al. 2009), the association between diet and constipation can be easily mediated by overweight or with the level of physical activity.
Although most studies regarding nutrition and health focused on single nutrients, the fact that people do not eat isolated nutrients but a variety of foods that may have a biological interaction in the human body should be considered. For instance, dietary fibre intake may have an interaction with carbohydrate and dietary fat absorption (Burton‐Freeman 2000). Accordingly, a new approach within nutritional research has been developed by using dietary pattern analysis, taking into account that the intake of foods can be highly intercorrelated (Hu 2002). A benefit of this approach is that cumulative effects of nutrients and nutrient interactions can be detected much easier than the effect of a single nutrient as the effects of single nutrients are often very small (Hu 2002). Finally, dietary patterns capture the totality of a child's diet and give greater insight in overall lifestyle choices as dietary patterns incorporate with non‐dietary behaviours as well (Gubbels et al. 2009). Studying dietary patterns in relation to constipation can improve understanding of dietary practice in young children and may provide guidance for nutritional recommendations in children with constipation.
The aim of this study was to determine in a population‐based sample whether adherence to common dietary patterns in early childhood is associated with constipation between 24 and 48 months of age. The second aim was to explore whether overweight and TV watching, as proxy for sedentary behaviour, are associated with constipation in childhood.
Key messages
-
•
The influence of dietary patterns shortly beyond the weaning and lactation period on childhood constipation is relatively unknown.
-
•
Dietary patterns in young children correlate with overweight and sedentary behaviour, which also has a link with constipation.
-
•
This study shows that a ‘Western‐like’ dietary pattern of the child is longitudinally associated with an increased risk of constipation, whereas adherence to a ‘Health conscious’ diet is associated with a lower risk of constipation, but this is time‐dependent.
-
•
In a population‐based setting, overweight and sedentary behaviour are not associated with constipation.
Materials and methods
This study was embedded in the Generation R study, a population‐based prospective cohort study from fetal life until young adulthood, and has been described in detail previously (Jaddoe et al. 2010). In total, 5088 mothers with a delivery date between April 2002 and January 2006 provided consent for post‐natal follow‐up and received a food frequency questionnaire (FFQ) for their child at the age of 14 months. Ethic approval for the study was obtained from the Medical Ethical Committee of the Erasmus MC, University Medical Center Rotterdam.
Dietary assessment
Out of 5088 mothers who received the FFQ to assess the child's dietary intake, 3643 (72%) completed the FFQ [mean: 14 months; standard deviation (SD): 2 months] and were eligible for analysis. The FFQ was developed in cooperation with the division of Human Nutrition of Wageningen University, the Netherlands, and is based on an existing validated food questionnaire developed and described in detail previously (Feunekes et al. 1993). This FFQ was modified on the basis of foods frequently consumed in early childhood according to a Dutch food consumption survey among young children (Hulshof & Breedveld 2002). The FFQ was validated against three‐day 24 h recalls in Dutch children aged 14 months (n = 32) with the following intra‐class correlation coefficients for macronutrients: total energy: 0.4, total protein: 0.7, total fat: 0.4, carbohydrates: 0.4 and dietary fibre: 0.7. The FFQ consisted of 211 food items and included questions on the frequency of consumption of these food items over the last month, the amount and type of the food item, and preparation methods. Dietary pattern analysis was restricted to children from parents who were both born in the Netherlands (n = 2420) (Swertz et al. 2004) after random exclusion of siblings within the Generation R cohort.
Dietary patterns
All 211 food items from the FFQ data of all Dutch children (n = 2420) were classified into 21 food groups (Supplementary Table 1). Subsequently, we applied principal component analysis (PCA) on 21 food groups (in g per day) of the children to construct overall dietary patterns by explaining the largest proportion of variation in the food group intake (Hu 2002). To reduce correlation between the factors, the varimax method by maximising the sum of the variance of the loading components was used (Kaiser 1958). To reduce bias as a result of multiple testing and to better identify common dietary patterns, only the dietary patterns with an eigenvalue of ≥1.5 were extracted (n = 2), which accounted for 24.5% of the variability in food consumption within our study population. Dietary pattern 1 represented a ‘Health conscious’ dietary pattern characterised of high intake of fruit, vegetables, legumes and fish (Table 1). Dietary pattern 2 represented a ‘Western‐like’ dietary pattern comprising high intakes of savoury and snacks, animal fats, confectionary and sugar‐containing beverages (Table 1). Nutrient characteristics of the dietary patterns are presented in Table 1. Accordingly, each participant was assigned two personalised adherence scores (z‐scores) for these dietary components, which is a linear composite of the optimally weighted food items by factor loadings constructed for the two dietary patterns derived from the PCA.
Table 1.
Correlation of foods and macronutrients for ‘Western‐like’ and ‘Health conscious’ dietary pattern scores in Dutch children aged 14 months (retaining r > 0.2 or r < − 0.2)
| Mean intake (g day−1) | Western‐like dietary pattern | Health conscious dietary pattern | |
|---|---|---|---|
| Food group | Pearson's correlation coefficient | Pearson's correlation coefficient | |
| Refined bread and breakfast cereals | 15 | 0.57 | – |
| Whole bread and breakfast cereals | 62 | – | – |
| Pasta and rice | 23 | – | 0.62 |
| Dairy | 626 | – | – |
| Fruit | 162 | – | 0.32 |
| Soy substitutes | 4 | – | – |
| Vegetables | 52 | – | 0.74 |
| Potatoes | 34 | – | 0.61 |
| Soups and sauces | 9 | 0.23 | – |
| Savoury and snacks | 4 | 0.59 | – |
| Confectionary | 28 | 0.72 | – |
| Vegetable oils | 1 | – | 0.50 |
| Animal fats | 11 | 0.58 | – |
| Fish | 8 | – | 0.22 |
| Shellfish | 0.3 | – | – |
| Meat | 26 | 0.27 | 0.21 |
| Eggs | 2 | – | – |
| Legumes | 4.0 | – | 0.59 |
| Sugar‐containing beverages | 198 | 0.59 | – |
| Non‐sugar‐containing beverages | 56 | – | – |
| Composite dishes | 102 | – | – |
| Eigenvalue* | 1.7 | 3.4 | |
| Variance explained (%) | 8.2 | 16.3 | |
| Nutrients | |||
| Energy (kcal) | 1275 kcal | 0.5 | 0.3 |
| Proteins (g) | 41 | 0.3 | 0.4 |
| Fat (g) | 40 | 0.6 | 0.2 |
| Saturated fat (g) | 14 | 0.3 | – |
| Monounsaturated fat (g) | 12 | 0.3 | – |
| Polyunsaturated fat (g) | 7 | 0.4 | 0.2 |
| Carbohydrates (g) | 188 | 0.5 | 0.3 |
| Mono‐ and disaccharides (g) | 111 | 0.5 | – |
| Polysaccharides (g) | 76 | 0.4 | 0.5 |
| Dietary fibre (g) | 18 | – | – |
Principal component analysis was used as an extraction method in which the Pearson's correlation coefficients represent the relative contribution of that food group to the identified dietary pattern. *The eigenvalue was used as indicator of the amount of variation explained by each dietary pattern.
Overweight and sedentary behaviour
Height and weight were measured with standardised methods at visit to the child health centre at the age of 24, 30 and 36 months (response: 69%, 75% and 65%, respectively). Body mass index (BMI) was calculated as weight (kg)/height (m)2, and overweight and obesity was defined according to the age‐ and gender‐dependent cut‐off points for childhood BMI of the International Obesity Task Force (Cole et al. 2000). Information on TV watching as a proxy for sedentary behaviour was obtained by questionnaire at the child's age of 24, 36 and 48 months (response rates: 70%, 64% and 63%, respectively) and was categorised into ≥2 h and <2 h a day, according to the American Academy of Pediatrics (American Academy of Pediatrics. Committee on Public, E 2001).
Constipation during childhood
At the age of 24, 36 and 48 months, stool pattern of the child was assessed by using a parental‐derived questionnaire (response rates: 70%, 64% and 63%, respectively) consisting of the following questions: ‘Has your child had the following for at least 2 weeks?’ (1) A bowel movement twice or less per week (yes vs. no), and (2) predominantly hard/firm feces (yes vs. no). The outcome of constipation was considered as present if at least one of the above symptoms of ROME II was reported (Rasquin‐Weber et al. 1999).
Covariates
Several medical, behavioural and socio‐demographic characteristics obtained from a combination of pre‐ and post‐natal questionnaires, community midwife and hospital registries were used as potential confounder or used as predictor in the multiple imputation procedure.
In early, mid‐ and second trimester of pregnancy (response: 91%, 81% and 77%, respectively) information by questionnaire was obtained on maternal educational level (low: no education, primary school or less than 3 years of secondary school; mid: more than 3years of secondary school or higher vocational training or bachelor's degree; and high: academic education), household income (≥2000 euro vs. <2000 euro month−1), marital status (living together vs. living alone), maternal smoking (yes vs. no), maternal alcohol use (yes vs. no), folic acid supplementation (yes vs. no), history of intestinal disorders, atopic disease, diabetes mellitus, hypertension, and hypercholesterolemia (yes vs. no), and parity.
During visits at one of the community midwife research centres in the first, second and third trimester (response: 76%, 93% and 93%, respectively), maternal anthropometrics were measured. Information on pregnancy complications was obtained from medical records as described in detail previously (Jaddoe et al. 2010), which was available in 99% of the enrolled mothers. In all children, information about gender, birthweight and gestational age was available from the obstetric records from the hospitals and midwife practices.
From post‐natal questionnaires at the age of 6 and 12 months (response: 73% and 71%, respectively), data were available on timing of introduction of solids (>6 months vs. ≤6 months of age; after the age of 6 months, all children received complementary feeding), breastfeeding duration and infant history of food allergy in the first year of life as described in detail previously (Kiefte‐de Jong et al. 2010). Post‐natal questionnaires at the age of 24, 36 and 48 months (response: 76%, 72% and 73%, respectively) included information on wheezing, atopic dermatitis, and infectious disease, and day‐care attendance in the previous year. Information on the child anthropometrics prior to 24 months was collected at each routine visit to the child health centres at the age of 6, 11, 14 and 18 months (response varied from 60% to 82%). The level of parental stress was assessed by the Nijmeegse Ouderlijke Stress Index‐Kort (De Brock et al. 1992)), the Dutch version of the Parental Stress Index‐Short Form (Abidin 1983) at the child age of 18 months (response: 75%).
Statistical analysis
Univariate analyses were performed by using Chi‐square tests for categorical variables and the Student's t‐test for continuous variables to compare difference in diet score and prevalence of overweight between children with and without constipation. Subsequently, to assess how the child's dietary patterns, overweight and TV watching were associated with functional constipation, logistic generalised estimating equations (GEEs) with an exchangeable correlation structure was performed. Briefly, GEE analysis assesses the longitudinal association between variables by correction for the within subject's dependence as a result of the repeated observations on constipation, overweight and TV watching (Twisk 2004).
The primary independent variables in the GEE model were (1) adherence score (z‐scores) to the ‘Health conscious’ and ‘Western‐like’ dietary pattern after stratification into tertiles with the first tertile (lowest z‐score) as reference category; (2) overweight, divided into being overweight and being obese with normal weight as reference category; or (3) sedentary behaviour defined as TV watching of at least 2 h a day with less than 2 h a day as reference category. All models were adjusted for time, and the analyses concerning the dietary patterns were all adjusted for total energy intake (Willett et al. 1997) and age of food assessment. We created multivariate models with stepwise adjustment for potential confounders as maternal age, household income, marital status, maternal educational background, maternal BMI, maternal smoking, maternal alcohol consumption, maternal co‐morbidity, maternal folic acid supplementation, maternal history of intestinal disorders, parity, birthweight, gestational age, gender, timing of introduction of solids, breastfeeding duration and history of food allergy. These confounders were selected on the basis of variables associated with constipation or dietary patterns in young children described in previous studies (North & Emmett 2000; Kiefte‐de Jong et al. 2010; Mugie et al. 2011).
In case of ≥10% alteration in effect estimate, the potential confounder was kept in the final multivariate model, as described by Greenland & Mickey (1989). Additionally, to assess whether overweight and TV watching had any influence on the association between the dietary patterns and constipation, these variables were added separately to the final multivariate models.
To reduce bias associated with missing data, multiple imputation of the data (n = 5 imputed datasets) as applied. The multiple imputation was based on the correlation between each variable with missing values (varying from 0% to 28%; Table 2) with the following subject characteristics: maternal age, household income, marital status, maternal educational background, maternal BMI, maternal smoking, maternal alcohol consumption, maternal co‐morbidity, maternal folic acid supplementation, maternal history of intestinal disorders, history of atopic disease, pregnancy complications (i.e. diabetes gravidarum, hypertension), parity, birthweight, gestational age, gender, all anthropometric measurements, timing of introduction of solids, breastfeeding duration, watching TV, history of food allergy, symptoms of wheezing, atopic dermatitis, infectious disease and constipation in previous year, any day‐care attendance, parental stress score, total kcal intake, dietary pattern z‐score (used as predictor only). This procedure has been described in detail by Sterne et al. (2009). Data were imputed according to the Markov chain Monte Carlo method as no monotone missing pattern was found. GEE analysis was then performed in each dataset separately to obtain the desired effect sizes and standard errors. Results of the five imputed datasets were pooled by taking the average of the regression coefficients. The pooled standard error was then calculated by using Rubin's rules (Rubin & Schenker 1991): √[W + (1 + 1/m) × B], where W is the mean variance of the effect size within the imputed datasets, B is the variance of the effect sizes between the imputed datasets and m is the number of imputed datasets (n = 5), which takes into account the uncertainty associated with missing data (Sterne et al. 2009). Analyses were performed in the original data and after the multiple imputation procedure. As we found similar effect estimates, the final results in our paper are presented as the pooled odds ratio (OR) with its 95% confidence interval (95%CI) after the multiple imputation procedure. A P‐value <0.05 was considered as statistically significant. Statistical analyses and the multiple imputation procedure were performed by using PASW Statistics version 17.0 (SPSS Inc., Chicago, IL, USA).
Table 2.
Child and mother characteristics of the study population (n = 2420)
| Original data | Imputed data | |||
|---|---|---|---|---|
| n | % | n | % | |
| Mother | ||||
| Maternal educational background | ||||
| Low | 39 | 2% | 41 | 2% |
| Mid | 1662 | 69% | 1703 | 70% |
| High | 658 | 27% | 677 | 28% |
| Missing | 61 | 2% | – | – |
| Household income per month | ||||
| <2000 euro | 294 | 12% | 301 | 12% |
| ≥2000 euro | 1804 | 75% | 2119 | 88% |
| Missing | 322 | 13% | – | – |
| Marital status | ||||
| Married/living together | 2239 | 93% | 2298 | 95% |
| No partner | 121 | 5% | 122 | 5% |
| Missing | 60 | 3% | – | – |
| Smoking during pregnancy | 432 | 18% | 508 | 21% |
| Missing | 399 | 17% | – | – |
| Alcohol consumption during pregnancy | 1159 | 48% | 1420 | 59% |
| Missing | 296 | 12% | – | – |
| Body mass index at intake (mean; SD; kg/m2) | 24; | SD: 4 | 24; | SD: 4 |
| Missing | 214 | 9% | – | – |
| Perinatal folic acid supplementation | 1685 | 70% | 2230 | 92% |
| Missing | 593 | 25% | – | – |
| Maternal age at intake (mean, SD; years) | 32.0; | SD: 4.2 | 32.0; | SD: 4.2 |
| Missing | – | – | – | – |
| Nulliparous | 1454 | 60% | 1498 | 62% |
| Missing | 59 | 2% | – | – |
| History of intestinal disorders | 70 | 3% | 78 | 3% |
| Missing | 232 | 10% | – | – |
| History of diabetes mellitus, hypertension or hypercholesterolemia | 51 | 2% | 243 | 10% |
| Missing | 683 | 28% | – | – |
| Child | ||||
| Male gender | 1201 | 50% | 1201 | 50% |
| Missing | – | – | – | – |
| Birthweight (mean; SD; g) | 3503; | SD: 570 | 3503; | SD: 570 |
| Missing | – | – | – | – |
| Gestational age (mean, SD; weeks) | 39.9; | SD: 1.7 | 39.9; | SD: 1.7 |
| Missing | – | – | – | – |
| Breastfeeding | ||||
| Never breastfeeding | 231 | 10% | 302 | 13% |
| Partial breastfeeding until 4 months of age | 1314 | 54% | 1439 | 59% |
| Exclusive breastfeeding until 4 months of age | 630 | 26% | 679 | 28% |
| Missing | 245 | 10% | – | – |
| Timing of introduction of solids ≤6 months of age | 1620 | 67% | 1628 | 67% |
| Missing | 14 | 1% | – | – |
| History of food allergy in first year of life | 144 | 6% | 152 | 6% |
| Missing | 87 | 4% | – | – |
| Institutional and non‐institutional care in first year of life >16 h week−1 | 1301 | 54% | 1640 | 68% |
| Missing | 524 | 22% | – | – |
| Body mass index in second year of life | 16.5 | SD: 1.4 | 17.1; | SD: 1.3 |
| Missing | 536 | 22% | – | – |
| TV watching ≥2 h a day in second year of life | 223 | 9% | 245 | 10% |
| Missing | 166 | 7% | – | – |
SD, standard deviation.
Results
Child and mother characteristics are presented in Table 2. The prevalence of constipation ranged from 8% to 13% and significantly increased between 24 and 48 months (P < 0.01 for difference in prevalence of constipation between 24 and 36 months and between 24 and 48 months).
The prevalence of overweight remained stable around 10% between 24 and 36 months of age (P = 0.34 for difference in prevalence relative to 24 months) but slightly decreased to 8% at 48 months (P = 0.01 for difference in prevalence between 24 and 48 months).
The prevalence of overweight was almost similar in children with and without constipation (8% vs. 11%; P = 0.46, 13% vs. 10%; P = 0.10 and 8% vs. 9%; P = 0.60 at the age of 24, 36 and 48 months, respectively). TV watching of at least 2 h a day was slightly more frequent in children with constipation than in children without constipation at the age of 36 months (10% vs. 11%; P = 0.49, 4% vs. 7%; P = 0.02, 5% vs. 6%; P = 0.70 at the age of 24, 36 and 48 months, respectively).
Mean [standard deviation (SD)] score of a ‘Western‐like’ dietary pattern score was 0.07 (1) vs. 0.01 (1), 0.13 (1) vs. 0.02 (1) and 0.05 (0.8) vs. 0.01 (1) in children with constipation relative to those without symptoms at the age of 24, 36 and 48 months, respectively (P = 0.35, P = 0.04 and P = 0.55 for 24, 36 and 48 months, respectively). Mean (SD) score of a ‘Health conscious’ dietary pattern score was −0.14 (1) vs. 0.01 (1), −0.07 (1) vs. 0.01 (1) and −0.05 (1) vs. 0.01 (1) in children with constipation relative to those without symptoms at the age of 24, 36 and 48 months (P = 0.04, P = 0.22 and P = 0.45 for 24, 36 and 48 months, respectively).
Mean (SD) dietary fibre intake was 17 (9) g day−1 vs. 18 (9) g day−1 for children with and without constipation, respectively, at the age of 24, 36 and 48 months, which was not significantly different between groups (P > 0.5 for between group difference). Mean total energy intake was similar among children with and without constipation (mean difference in total energy intake of 8–12 kcal day−1 at the age of 24, 36 and 48 months; P > 0.7). Children with constipation consumed slightly higher energy percentage from saturated fat than children without constipation at the age of 36 months but this was not statistically significant (11% vs. 10%, P = 0.15). Also, a slightly lower energy percentage from polysaccharides and a slightly higher energy percentage from mono‐ and disaccharides was consumed by children with constipation relative to those without it (24% vs. 23%, P = 0.06, and 35% vs. 34%, P = 0.12 at the age of 48 months).
No difference was found between other macronutrient consumption and constipation at the age of 24, 36 and 48 months (data not shown). Also, no difference was found in total food volume per day between children with and without constipation (data not shown)
On short term, high adherence to the ‘Health conscious’ dietary pattern was significantly associated with a lower prevalence of constipation at the age of 24 months, whereas no association was found between high adherence to a ‘Western‐like’ dietary pattern and constipation at the age of 24 months (Fig. 1).
Figure 1.
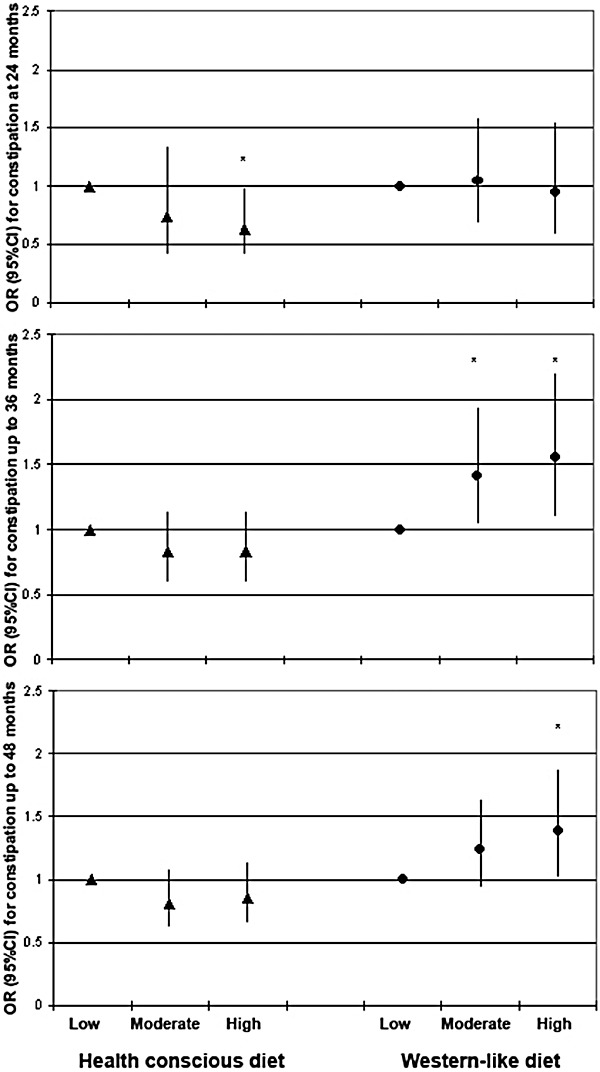
Time‐specific (i.e. at 24 and up to 36 and 48 months) association between dietary patterns and constipation (n = 2420; *P < 0.05 after the multiple imputation procedure). 95%CI, 95% confidence interval; OR, odds ratio.
Longitudinal analyses revealed that adherence to the ‘Health conscious’ dietary pattern did not remain significantly associated with constipation up to 36 and 48 months of age (Table 3, Fig. 1), whereas high adherence to a ‘Western‐like’ dietary pattern was longitudinally associated with a significant higher prevalence of constipation up to 36 months and up to 48 months (Table 3; Fig. 1). Additional adjustment for other potential confounders as maternal folic acid supplementation, maternal education, maternal co‐morbidity and marital status did not alter these results (data not shown).
Table 3.
Longitudinal analyses on the child's dietary patterns, overweight, and sedentary behaviour and childhood constipation after the multiple imputation procedure (n = 2420)
| Univariate | Multivariate † | |
|---|---|---|
| OR (95%CI) | OR (95%CI) | |
| Health conscious diet | ||
| Low adherence (n = 807) | Reference | Reference |
| Moderate adherence (n = 807) | 0.87 (0.69–1.10) | 0.81 (0.62–1.07) ‡ |
| High adherence (n = 806) | 0.83 (0.64–1.07) | 0.85 (0.65–1.13) ‡ |
| Western‐like diet | ||
| Low adherence (n = 807) | Reference | Reference |
| Moderate adherence n = 807) | 1.16 (0.91–1.47) | 1.24 (0.94–1.63) ‡ |
| High adherence (n = 806) | 1.31 (1.02–1.70)* | 1.39 (1.02–1.87)*, ‡ |
| Nutritional status | ||
| Normal weight ( n = 2161) | Reference | Reference |
| Overweight ( n = 237) | 0.98 (0.98–1.07) | 0.95 (0.64–1.40) |
| Obese (n = 22) | 1.37 (0.67–2.79) | 1.01 (0.69–1.46) |
| Sedentary behaviour | ||
| TV watching <2 h a day (n = 2173) | Reference | Reference |
| TV watching ≥2 h a day (n = 247) | 1.11 (0.97–1.55) | 1.07 (0.74–1.57) |
OR, odds ratio; 95%CI, 95% confidence interval derived after the multiple imputation procedure. *P < 0.05. †Adjusted for time, maternal smoking, maternal alcohol consumption, maternal history of intestinal disorders, maternal body mass index, household income, parity, gender birthweight, gestational age, duration of breastfeeding, timing of introduction of solids and history of food allergy. ‡Additionally adjusted for age of food assessment and total energy intake.
No association was found between overweight and constipation and between TV watching and constipation (Table 3). Additional adjustment for overweight and TV watching did not have any influence on the results between adherence to a ‘Health conscious’ or ‘Western‐like’ dietary pattern and constipation between 24 and 48 months of age (data not shown).
Discussion
This study shows that high adherence to a ‘Western‐like’ dietary pattern is longitudinally associated with constipation, which was independent of the presence of overweight or sedentary behaviour in pre‐school children. More interestingly, adherence to a ‘Health conscious’ dietary pattern was only associated with a lower risk of constipation at 24 months, whereas no association was found between overweight and constipation and between TV watching and constipation.
The association between high adherence to a ‘Western‐like’ dietary pattern and an increased prevalence of constipation in childhood can be explained by several components. This dietary pattern was characterised by foods with high fat content (Table 1). In healthy adults, it is known that infusion of fat into the small intestine reduces gastric emptying and is associated with lower intestinal motility that may be a trigger for constipation (Stewart et al. 2011). Furthermore, studies show that foods high in fat content causes gut problems in subjects with the irritable bowel syndrome (Simren et al. 2001; Saito et al. 2005).
High intake of confectionary and sugar‐containing beverages has been shown to be associated with poor diet quality (Libuda et al. 2009). In children, high intake of confectionary and sugar‐containing beverages may lead to early satiety, which may cause poor compliance to meals containing starchy foods and vegetables. In addition, another study in children aged 9–18 months demonstrated that children who were frequently fed with confectionary and sugar‐containing beverages had less‐frequent intakes of healthy foods as fruit, vegetables, potatoes and bread (Brekke et al. 2007). Hence, this leads to a lower dietary fibre intake but may also reflect a less‐regular eating pattern.
Strikingly, we did not find a longitudinal association between adherence to a ‘Health conscious’ dietary pattern and constipation in childhood as the effect only concerned constipation at the age of 24 months. The ‘Health conscious’ dietary pattern was characterised by high intake of fruits, vegetables, potatoes, starchy foods and legumes. As these food products have high dietary fibre content, we also expected a longitudinal protective effect of high adherence to this dietary pattern and childhood constipation. The role of dietary fibre in very young children with constipation is controversial. There are concerns that a high‐fibre diet in children under the age of 5 years may lead to growth faltering due to decreased energy density of the diet and altered mineral absorption (Edwards & Parrett 2003). However, these concerns are not well supported by evidence (Edwards & Parrett 2003). Besides, studies have shown that constipation in children was associated with low dietary fibre intake (Jennings et al. 2009; Maffei & Vicentini 2011) and low consumption of fruit and vegetables (Lee et al. 2008). Although the ORs implied that this dietary pattern was overall associated with a lower prevalence of constipation, this effect was not statistically significant in the long run. An explanation for this short‐term impact of the ‘Health conscious’ diet might be that a healthy diet at pre‐school age may change more towards a diet with components of a ‘Western‐like’ dietary pattern when the child gets older. Indeed, from the Bogalusa Heart Study, it is known that the intake of sugar‐sweetened beverages, snacks and confectionary increases during childhood with an overall decrease in diet quality over the years (Demory‐Luce et al. 2004). This may weaken our association between a ‘Health conscious diet’ and a lower prevalence of constipation in later childhood in our study.
Several studies reported an increased prevalence of overweight or obesity in children with constipation (Pashankar & Loening‐Baucke 2005; Misra et al. 2006; Teitelbaum et al. 2009; Vd Baan‐Slootweg et al. 2011). Lower prevalence of overweight is associated with high dietary fibre consumption (Zanovec et al. 2010), whereas high prevalence of overweight is associated with high consumption of sugar‐containing beverages (Libuda & Kersting 2009). Nonetheless, the association between the dietary patterns and constipation was not influenced by the presence of overweight in our study. Also, we were not able to confirm the association between overweight and constipation. This might be explained by the fact that most studies concerning overweight and constipation in children have been performed in a secondary‐care setting (Pashankar & Loening‐Baucke 2005; Misra et al. 2006; Teitelbaum et al. 2009; Vd Baan‐Slootweg et al. 2011), and the association between overweight and constipation might not be so apparent in primary care‐ or population‐based studies.
Although increasing physical activity has shown to be effective in the amelioration of symptoms of constipation in adults (De Schryver et al. 2005), other studies regarding the association between physical activity and constipation in adults show inconsistent results (Dukas et al. 2003; Tuteja et al. 2005). However, the role of sedentary behaviour or physical activity in constipation is very much understudied in the paediatric population. Only two studies on physical activity and constipation in children have been published. One study reported that sedentary time during a school day rather than moderate physical activity time was significantly associated with low defecation frequency in children aged 10–18 years (Chien et al. 2011). In another study among children aged 7–10 years, constipation was more prevalent in children with high physical activity levels (Jennings et al. 2009). Although these studies can be barely compared with our study group because it concerns different age groups, we found no association between sedentary behaviour, as measured by at least 2 h of TV watching per day, and constipation. Nonetheless, we did not have comprehensive data on physical activity; thus, our study precludes final conclusions on physical activity and constipation in children.
The strength of this study is the use of a large‐scale and population‐based study group. However, a possible drawback of this study can be that most data were obtained by parental‐derived questionnaires and no additional information from medical records or physical examinations was available. Therefore, some subjects may be misclassified concerning the outcome of constipation. However, only if this misclassification is also related to the child's diet, sedentary behaviour or overweight, this misclassification would have markedly influenced our results. We did not have data on potential metabolic or physiological causes of constipation. Although the prevalence of these diseases can be expected to be low in our population, potential influence of, e.g. food hypersensitivity or celiac disease on constipation cannot be fully ruled out. Also, we did not have data on constipation at the age of 14 months. Parents of children with constipation may be more likely to change their child's diet towards a ‘Health conscious diet’. However, only if this was also related to the presence of constipation at the age of 24 months onwards, this would have influenced our results.
Another challenge is the identification of dietary patterns in young children. This involves several decisions such as in the division of food items to food groups and the selected method to define these patterns and the labelling of these components that may have a influence on the final content of the dietary pattern in this study population (Hu 2002). The amount of variance (24.5%) explained by the dietary patterns is small but is comparable with previous studies (North & Emmett 2000; Robinson et al. 2007). Nevertheless, this may have consequences on the generalisability of our results on diet and constipation in other populations. Also, the dietary patterns may vary among other ethnic groups and culture; therefore, replication of our study in other ethnic populations is necessary.
In conclusion, a ‘Western‐like’ dietary pattern is longitudinally associated with an increased prevalence of constipation up to 48 months, which was not mediated by the presence of overweight or sedentary behaviour. A time‐specific protective effect on constipation seems applicable when the child adheres to a ‘Health conscious’ dietary pattern.
Clinicians should not focus on one specific nutrient in the case of childhood constipation, but a combination of dietary changes as eliminating fat‐rich foods, sugar‐containing beverages, confectionary and refined grains may be worth trying to explore in children with constipation. Further studies are needed to clarify whether the association between overweight and constipation is applicable to primary‐care settings and to what extent physical activity and the ‘Health conscious’ diet play a role in childhood constipation in the long run.
Source of funding
This phase of the Generation R study was supported by the Erasmus Medical Center, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (Zon Mw) and Europe Container terminals B.V.
Conflicts of interests
The authors declare that they have no conflicts of interest.
Contributions
HAM, HR, VWVJ, AH and JCK‐dJ designed, planned, conducted the study and collected data. Statistical analyses and data interpretation was done by JCK‐dJ and HAM. JHdV and JCK‐dJ acquired nutritional data. JCK‐dJ, HAM and JCE drafted the final manuscript. All authors critically reviewed the manuscript.
Supporting information
Table S1. Division of food items into food groups.
Supporting info item
Acknowledgements
The Generation R study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service–Rotterdam Metropolitan Area, the Rotterdam Homecare Foundation and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond. We acknowledge the contributions of children and parents, general practitioners, hospitals and midwives in Rotterdam. Also, we thank Saskia Meyboom, Corine Perenboom and Els Siebelink of the Department of Human Nutrition, Wageningen University, the Netherlands, for their contribution in processing the food consumption data.
References
- Abidin R.R. (1983) Parenting Stress Index – manual. University of Virginia Press: Charlottesville, VA. [Google Scholar]
- Aguirre A.N., Vitolo M.R., Puccini R.F. & de Morais M.B. (2002) Constipacao em lactentes: influencia do tipo de aleitamento e da ingestao de fibra alimentar [Constipation in infants: influence of type of feeding and dietary fiber intake]. Jornal de Pediatria 78, 202–208. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Committee on Public, E (2001) American academy of pediatrics: children, adolescents, and television. Pediatrics 107, 423–426. [DOI] [PubMed] [Google Scholar]
- Brekke H.K., van Odijk J. & Ludvigsson J. (2007) Predictors and dietary consequences of frequent intake of high‐sugar, low‐nutrient foods in 1‐year‐old children participating in the ABIS study. The British Journal of Nutrition 97, 176–181. [DOI] [PubMed] [Google Scholar]
- Burton‐Freeman B. (2000) Dietary fiber and energy regulation. The Journal of Nutrition 130, 272S–275S. [DOI] [PubMed] [Google Scholar]
- Chien L.Y., Liou Y.M. & Chang P. (2011) Low defaecation frequency in Taiwanese adolescents: association with dietary intake, physical activity and sedentary behaviour. Journal of Paediatrics and Child Health 14, 381–386. [DOI] [PubMed] [Google Scholar]
- Cole T.J., Bellizzi M.C., Flegal K.M. & Dietz W.H. (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clinical Research ed.) 320, 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brock A.J.L.L., Vermulst A.A., Gerris J.R.M. & Abidin R. (1992) Nijmeegse Ouderlijke Stress Index (NOSI) – manual. Swets en Zeitlinger: Lisse. [Google Scholar]
- De Schryver A.M., Keulemans Y.C., Peters H.P., Akkermans L.M., Smout A.J., De Vries W.R. et al (2005) Effects of regular physical activity on defecation pattern in middle‐aged patients complaining of chronic constipation. Scandinavian Journal of Gastroenterology 40, 422–429. [DOI] [PubMed] [Google Scholar]
- Demory‐Luce D., Morales M., Nicklas T., Baranowski T., Zakeri I. & Berenson G. (2004) Changes in food group consumption patterns from childhood to young adulthood: the Bogalusa Heart Study. Journal of the American Dietetic Association 104, 1684–1691. [DOI] [PubMed] [Google Scholar]
- Dukas L., Willett W.C. & Giovannucci E.L. (2003) Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. The American Journal of Gastroenterology 98, 1790–1796. [DOI] [PubMed] [Google Scholar]
- Edwards C.A. & Parrett A.M. (2003) Dietary fibre in infancy and childhood. The Proceedings of the Nutrition Society 62, 17–23. [DOI] [PubMed] [Google Scholar]
- Feunekes G.I., Van Staveren W.A., De Vries J.H., Burema J. & Hautvast J.G. (1993) Relative and biomarker‐based validity of a food‐frequency questionnaire estimating intake of fats and cholesterol. The American Journal of Clinical Nutrition 58, 489–496. [DOI] [PubMed] [Google Scholar]
- Greenland S. & Mickey R.M. (1989) Re: ‘The impact of confounder selection criteria on effect estimation’. American Journal of Epidemiology 129, 125–137. [DOI] [PubMed] [Google Scholar]
- Gubbels J.S., Kremers S.P., Stafleu A., Dagnelie P.C., de Vries S.I., de Vries N.K. et al (2009) Clustering of dietary intake and sedentary behavior in 2‐year‐old children. Jornal de Pediatria 155, 194–198. [DOI] [PubMed] [Google Scholar]
- Hu F.B. (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Current Opinion in Lipidology 13, 3–9. [DOI] [PubMed] [Google Scholar]
- Hulshof K. & Breedveld B. (2002) Results of the study on nutrient intake in young toddlers 2002. Zeist, the Netherlands: TNO Nutrition.
- Jaddoe V.W., van Duijn C.M., van der Heijden A.J., Mackenbach J.P., Moll H.A., Steegers E.A. et al (2010) The Generation R study: design and cohort update 2010. European Journal of Epidemiology 25, 823–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings A., Davies G.J., Costarelli V. & Dettmar P.W. (2009) Dietary fibre, fluids and physical activity in relation to constipation symptoms in pre‐adolescent children. Journal of Child Health Care 13, 116–127. [DOI] [PubMed] [Google Scholar]
- Kaiser H. (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrika 22, 187–200. [Google Scholar]
- Kiefte‐de Jong J.C., Escher J.C., Arends L.R., Jaddoe V.W., Hofman A., Raat H. et al (2010) Infant nutritional factors and functional constipation in childhood: the Generation R study. The American Journal of Gastroenterology 105, 940–945. [DOI] [PubMed] [Google Scholar]
- Kokke F.T., Scholtens P.A., Alles M.S., Decates T.S., Fiselier T.J., Tolboom J.J. et al (2008) A dietary fiber mixture versus lactulose in the treatment of childhood constipation: a double‐blind randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 47, 592–597. [DOI] [PubMed] [Google Scholar]
- Lee W.T., Ip K.S., Chan J.S., Lui N.W. & Young B.W. (2008) Increased prevalence of constipation in pre‐school children is attributable to under‐consumption of plant foods: a community‐based study. Journal of Paediatrics and Child Health 44, 170–175. [DOI] [PubMed] [Google Scholar]
- Libuda L., Alexy U., Buyken A.E., Sichert‐Hellert W., Stehle P. & Kersting M. (2009) Consumption of sugar‐sweetened beverages and its association with nutrient intakes and diet quality in German children and adolescents. The British Journal of Nutrition 101, 1549–1557. [DOI] [PubMed] [Google Scholar]
- Libuda L. & Kersting M. (2009) Soft drinks and body weight development in childhood: is there a relationship? Current Opinion in Clinical Nutrition and Metabolic Care 12, 596–600. [DOI] [PubMed] [Google Scholar]
- Maffei H.V. & Vicentini A.P. (2011) Prospective evaluation of dietary treatment in childhood constipation: high dietary fiber and wheat bran intake are associated with constipation amelioration. Journal of Pediatric Gastroenterology and Nutrition 52, 55–59. [DOI] [PubMed] [Google Scholar]
- Misra S., Lee A. & Gensel K. (2006) Chronic constipation in overweight children. JPEN. Journal of Parenteral and Enteral Nutrition 30, 81–84. [DOI] [PubMed] [Google Scholar]
- Mugie S.M., Benninga M.A. & Di Lorenzo C. (2011) Epidemiology of constipation in children and adults: a systematic review. Best Practice & Research. Clinical Gastroenterology 25, 3–18. [DOI] [PubMed] [Google Scholar]
- North K. & Emmett P. (2000) Multivariate analysis of diet among three‐year‐old children and associations with socio‐demographic characteristics. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Study Team. European Journal of Clinical Nutrition 54, 73–80. [DOI] [PubMed] [Google Scholar]
- Pashankar D.S. & Loening‐Baucke V. (2005) Increased prevalence of obesity in children with functional constipation evaluated in an academic medical center. Pediatrics 116, e377–e380. [DOI] [PubMed] [Google Scholar]
- Rasquin‐Weber A., Hyman P.E., Cucchiara S., Fleisher D.R., Hyams J.S., Milla P.J. et al (1999) Childhood functional gastrointestinal disorders. Gut 45 (Suppl. 2), II60–II68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S., Marriott L., Poole J., Crozier S., Borland S., Lawrence W. et al Southampton Women's Survey Study, G. (2007) Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. The British Journal of Nutrition 98, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Roma E., Adamidis D., Nikolara R., Constantopoulos A. & Messaritakis J. (1999) Diet and chronic constipation in children: the role of fiber. Journal of Pediatric Gastroenterology and Nutrition 28, 169–174. [DOI] [PubMed] [Google Scholar]
- Rubin D.B. & Schenker N. (1991) Multiple imputation in health‐care databases: an overview and some applications. Statistics in Medicine 10, 585–598. [DOI] [PubMed] [Google Scholar]
- Saito Y.A., Locke G.R., 3rd , Weaver A.L., Zinsmeister A.R. & Talley N.J. (2005) Diet and functional gastrointestinal disorders: a population‐based case‐control study. The American Journal of Gastroenterology 100, 2743–2748. [DOI] [PubMed] [Google Scholar]
- Simren M., Mansson A., Langkilde A.M., Svedlund J., Abrahamsson H., Bengtsson U. et al (2001) Food‐related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 63, 108–115. [DOI] [PubMed] [Google Scholar]
- Sterne J.A., White I.R., Carlin J.B., Spratt M., Royston P., Kenward M.G. et al (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical Research ed.) 338, b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.E., Feinle‐Bisset C. & Keast R.S. (2011) Fatty acid detection during food consumption and digestion: associations with ingestive behavior and obesity. Progress in Lipid Research 50, 225–233. [DOI] [PubMed] [Google Scholar]
- Swertz O., Duimelaar P. & Thijssen J. (2004) Migrants in the Netherlands 2004. Voorburg/Heerlen: Statistics Netherlands.
- Teitelbaum J.E., Sinha P., Micale M., Yeung S. & Jaeger J. (2009) Obesity is related to multiple functional abdominal diseases. Jornal de Pediatria 154, 444–446. [DOI] [PubMed] [Google Scholar]
- Tunc V.T., Camurdan A.D., Ilhan M.N., Sahin F. & Beyazova U. (2008) Factors associated with defecation patterns in 0–24‐month‐old children. European Journal of Pediatrics 167, 1357–1362. [DOI] [PubMed] [Google Scholar]
- Tuteja A.K., Talley N.J., Joos S.K., Woehl J.V. & Hickam D.H. (2005) Is constipation associated with decreased physical activity in normally active subjects? The American Journal of Gastroenterology 100, 124–129. [DOI] [PubMed] [Google Scholar]
- Twisk J.W. (2004) Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. European Journal of Epidemiology 19, 769–776. [DOI] [PubMed] [Google Scholar]
- Vd Baan‐Slootweg O.H., Liem O., Bekkali N., van Aalderen W.M., Rijcken T.H., Di Lorenzo C. et al (2011) Constipation and colonic transit times in children with morbid obesity. Journal of Pediatric Gastroenterology and Nutrition 52, 442–445. [DOI] [PubMed] [Google Scholar]
- Willett W.C., Howe G.R. & Kushi L.H. (1997) Adjustment for total energy intake in epidemiologic studies. The American Journal of Clinical Nutrition 65, 1220S–1228S. [DOI] [PubMed] [Google Scholar]
- Zanovec M., O'Neil C.E., Cho S.S., Kleinman R.E. & Nicklas T.A. (2010) Relationship between whole grain and fiber consumption and body weight measures among 6‐ to 18‐year‐olds. Jornal de Pediatria 157, 578–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Division of food items into food groups.
Supporting info item


