Abstract
This study was designed to assess brain‐derived neurotrophic factor (BDNF) as a possible candidate for enhanced cognition in breastfed infants. The study was conducted on 42 infants, 4–6 months old, who were classified according to their feeding pattern into breastfed group, formula‐fed group and mixed‐feeding group. Each infant was subjected to history taking, clinical examination, estimation of the level of BDNF by Enzyme‐Linked Immunosorbent Assay (ELISA) technique and assessment by Bayley scale of infant development‐second edition (BSID‐II). The current study revealed that breastfed group had higher BSID‐II scores followed by mixed‐fed group then formula‐fed one, yet these results reached statistical significance only in total behaviour rating scale (TBRS) and Motor Quality Percentile rank values. Additionally, breastfed infants had significantly higher values of BDNF when compared to those receiving formula milk. Negative correlations between BDNF and both weight for age and weight for length scores were detected. Furthermore, significant positive correlation was detected between BDNF and TBRS. Regression analysis studies revealed that breastfeeding is the most determinant factor for BDNF, TBRS and Motor Quality Percentile rank values. Serum BDNF levels are significantly higher in breastfed infants and shows positive correlations with the results of BSID‐II. Given that simultaneous increase in brain BDNF occurs due to onsite production, transport from the periphery or both, it is prudent to hypothesize that BDNF could be one of the factors responsible for the enhanced cognition detected in breastfed infants.
Keywords: brain‐derived neurotrophic factor, brain function, breastfeeding, cognition, development, formula
Introduction
Infants must be provided with food containing all the necessary nutrients for proper growth and development (Gong et al. 2008). The human brain growth spurt begins during the third trimester of pregnancy and continues throughout the first 2 years of life, coinciding with the lactation period (Viberg et al. 2008).
The World Health Organization (WHO) and the American Dietetic Association suggest that infants should be exclusively breastfed until 6 months, which is expected to be the ideal feeding mode for infants (Gong et al. 2008). Breastfeeding, as numerous investigators have reported, might impart many advantages for the development of infants, including reduced risk of necrotizing enterocolitis, gastroenteritis, respiratory infection and immunologically based diseases, in addition to improved cognitive development (Schack‐Nielsen & Michaelsen 2007).
Brain‐derived neurotrophic factor (BDNF), a vital trophic protein for neuronal survival and differentiation in the developing nervous system, has been implicated in synaptic plasticity. Additionally, BDNF is involved in different aspects of learning and memory processing, in particular, in memory persistence and storage (Bekinschtein et al. 2008a).
There is experimental evidence that BDNF can cross the blood–brain barrier (Poduslo & Curran 1996; Pan et al. 1998). According to these results, BDNF changes within the central nervous system and cerebrospinal fluid might be paralleled by changes of BDNF serum levels. Additionally, Song et al. (2008) suggested that BDNF is anterogradely transported by axons; thus, peripherally derived and/or applied BDNF may act on the regeneration of central axons of ascending sensory neurons. Hypothetically, this may also apply centrally as well as peripherally.
To explore one of the possible candidates behind the enhanced cognition in breastfed infants and in line with the previous hypotheses, we designed the current study to measure the serum BDNF together with neuro‐developmental assessment in relation to pattern of feeding in the first 6 months of life.
Key messages
-
•
Brain derived neurotrophic factor (BDNF) levels were significantly higher in breastfed infants compared to those artificially fed.
-
•
Serum BDNF levels were positively correlated with Bayley Scale of Infant Development scores (BSID‐II).
-
•
BDNF could be one of the factors responsible for the enhanced cognition detected in breastfed infants.
Subjects and methods
Subjects
The present cross‐sectional study was conducted on 42 clinically healthy infants whose ages ranged between 4 and 6 months, recruited from the outpatient clinic, Children's Hospital, Faculty of Medicine, Ain Shams University in the period from April 2008 to December 2008. They were 28 males and 14 females recruited at random among those presenting for dietetic advice, vaccination or circumcision (in males). Preterm infants and those with any neurological disorder or those with history of perinatal problems such as pre‐eclampsia, obstructed labour or cyanosis at birth were excluded. We started with a randomly chosen number; sampling started with number 4 in the list of patients attending the clinic and fulfilling the inclusion criteria then every 5th subsequent child and this was done for the assigned period. The enrolled infants were divided into three groups according to their feeding patterns. The first group comprised 14 breastfed infants; the second group comprised 14 artificially fed infants, while the third group comprised 14 infants on mixed feeding.
Methodology
After the approval of the study procedures by the Children's Hospital Board, an informed consent was obtained from the parents or care givers. All infants were then subjected to full history taking laying stress on the perinatal history, developmental history and socio‐economic standard scoring according to Park & Park (1979). Dietetic history was taken especially type of feeding, age of weaning and type of weaning foods.
All infants underwent complete clinical examination with special emphasis on the anthropometric measurements and neurological examination. The weight was measured using an electronic scale with subject wearing light clothes and approximated to the nearest 0.5 kg. The length was measured on a wooden scale with subject barefooted and approximated to the nearest 0.5 cm. The skull circumference was measured with a measuring tape and approximated to the nearest 0.5 cm. The weight, length for age and weight for length Z‐scores were calculated based on the growth charts supplied by the WHO (2006). The mid‐arm circumference was measured at the mid‐arm point, midway between the tip of acromial process and the epicondyle of the humerus and approximated to the nearest 0.5 cm.
Two millilitres of venous blood were withdrawn from each infant under complete aseptic technique in one test tube and left to clot at room temperature for 30 min then centrifuged at 3000 rpm for 15 min. The separated serum was stored at −20°C until time of assay of BDNF by Enzyme‐Linked Immunosorbent Assay (ELISA) technique using the Quantikine Human BDNF Immunoassay supplied by R&D Systems, Inc. Minneapolis, USA.
Neurodevelopment assessment was carried out using Bayley scale of infant development‐second edition (BSID‐II) (Bayley 1993). The examiners were blinded to the subject groups and their BDNF levels.
Statistical analysis
The data were coded, entered and processed on an IBM‐PC compatible computer using SPSS 15.0 (SPSS Inc, Chicago, IL, USA). Data were tested for skewness and kurtosis to assess normality using Kolmogorov–Smirnov test.
Data are presented as median and range for continuous variables and as count and percentage for categorical variables. Groups of patients were compared using Fisher's exact test for categorized variables and using analysis of variance (ANOVA) test for continuous variables. To control for multiple comparisons, a Bonferroni adjustment was used. Pearson correlation coefficient was used to evaluate associations for continuous variables. Multiple regression was used to adjust the difference between variables for significant factors; results are presented as regression coefficients and 95% confidence intervals. All P‐values represented were two‐sided, and statistical significance was declared at P < 0.05.
Results
There were no statistical differences in age (F = 0.92 and P > 0.05), sex [χ2 = 1.50 and P > 0.05 (Table 1)] or socioeconomic standard (χ2 = 0.57 and P > 0.05) between the breastfed, artificially fed and mixed‐feeding groups.
Table 1.
Comparison between the three groups as regards the sex distribution
| Sex | Breastfed group | Formula‐fed group | Mixed‐fed group | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Male | 11 | 78.6% | 9 | 64.3% | 8 | 57.1% | 1.50 | 0.47 |
| Female | 3 | 21.4% | 5 | 35.7% | 6 | 42.9% | ||
There were no statistical differences in the anthropometric measurements among the three studied groups (P > 0.05, respectively) as shown in Table 2.
Table 2.
Comparison between the three studied groups as regards Anthropometric measurements:
| Breastfed group | Formula‐fed group | Mixed‐fed group | F * | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Weight at birth | 3.24 | 0.25 | 3.45 | 0.31 | 3.21 | 0.28 | 3.05 | 0.06 |
| Weight (Kg) | 7.51 | 0.64 | 7.88 | 0.85 | 7.92 | 0.67 | 1.33 | 0.28 |
| Weight for age (Z‐score) | 0.20 | 0.58 | 0.57 | 0.92 | 0.57 | 0.61 | 1.21 | 0.31 |
| Length (cm) | 64.29 | 1.94 | 65.69 | 1.32 | 65.37 | 2.21 | 2.20 | 0.12 |
| Length for age (Z‐score) | −0.47 | 0.48 | 0.02 | 0.46 | −0.31 | 0.65 | 3.08 | 0.06 |
| Weight for length (Z‐score) | 0.69 | 0.73 | 0.85 | 1.19 | 1.06 | 0.75 | 0.58 | 0.56 |
| Skull circumference (cm) | 43.86 | 1.41 | 43.64 | 1.28 | 43.60 | 1.07 | 0.17 | 0.85 |
| Mid arm circumference(cm) | 14.27 | 1.16 | 13.85 | 0.70 | 14.37 | 0.85 | 1.26 | 0.30 |
*Analysis of variance test. SD, standard deviation.
Table 3 shows a trend of higher Bayley scale scores in the breastfed group than in the mixed‐fed group, which was also higher than those for the formula‐fed group, yet these results reach statistical significance only in total behaviour rating scale (TBRS) and motor quality percentile rank values. Furthermore, in Table 4, the multiple comparisons show a significant difference only between breastfed and formula‐fed groups regarding TBRS and motor quality percentile rank.
Table 3.
Comparison between the three groups as regards mean values of BSID‐II
| Breastfed group | Formula‐fed group | Mixed‐fed group | F * | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Mental developmental index | 103.29 | 12.24 | 98.71 | 11.08 | 100.36 | 6.80 | 0.71 | 0.50 |
| Psychomotor developmental index | 108.64 | 13.19 | 105.21 | 10.08 | 106.07 | 10.90 | 0.34 | 0.71 |
| Total behaviour rating scale | 34.86 | 9.96 | 24.93 | 7.42 | 29.07 | 7.69 | 4.90 | 0.01 |
| Motor quality percentile rank | 48.07 | 10.22 | 37.14 | 11.82 | 45.07 | 11.63 | 3.53 | 0.04 |
| Attention/arousal percentile rank | 46.38 | 16.95 | 30.33 | 14.45 | 35.00 | 17.15 | 1.82 | 0.19 |
| Orientation engagement percentile rank | 36.83 | 16.58 | 31.50 | 9.93 | 32.50 | 15.46 | 0.27 | 0.77 |
| Emotion regulation percentile rank | 43.33 | 14.72 | 36.13 | 16.52 | 36.13 | 16.52 | 0.44 | 0.65 |
*Analysis of variance test. SD, standard deviation.
Table 4.
Multiple comparisons between the three studied groups regarding total behaviour rating scale and motor quality percentile rank tests
| Dependent variable | Mean* difference | P | ||
|---|---|---|---|---|
| Total behaviour rating scale | Breastfed group | Formula‐fed group | 9.93 | 0.01 |
| Mixed‐fed group | 5.79 | 0.23 | ||
| Formula‐fed group | Mixed‐fed group | −4.14 | 0.60 | |
| Motor quality percentile rank | Breastfed group | Formula‐fed group | 10.93 | 0.04 |
| Mixed‐fed group | 3.00 | 1.00 | ||
| Formula‐fed group | Mixed‐fed group | −7.93 | 0.21 |
Bonferroni.
On applying the ANOVA test, the current study revealed statistical differences in the serum BDNF among the three studied groups (Fig. 1). Table 5 shows that it is the breastfed group that demonstrates significantly higher BDNF level compared to the formula‐fed group.
Figure 1.
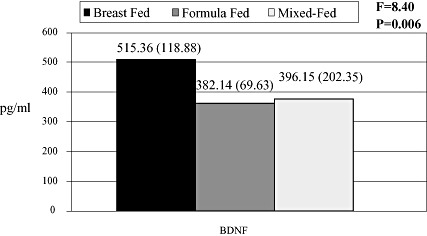
Mean values of brain‐derived neurotrophic factor (BDNF) serum levels in the three studied groups. N.B. Values are presented as mean (standard deviation).
Table 5.
Multiple comparisons of BDNF levels between the three studied groups
| Mean* difference | P | ||
|---|---|---|---|
| Breastfed group | Formula‐fed group | 133.21 | 0.04 |
| Mixed‐fed group | 119.20 | 0.09 | |
| Formula‐fed group | Mixed‐fed group | −14.01 | 1.00 |
*Bonferroni. BDNF, brain‐derived neurotrophic factor.
In a trial to correlate the BDNF levels to the anthropometric measurements; significant negative correlations were detected between both weight‐for‐age Z‐score (WAZ) and weight‐for‐length Z‐score (WLZ) and BDNF (2, 3, respectively). Additionally, Fig. 4 demonstrates a significant positive correlation between TBRS and BDNF.
Figure 2.
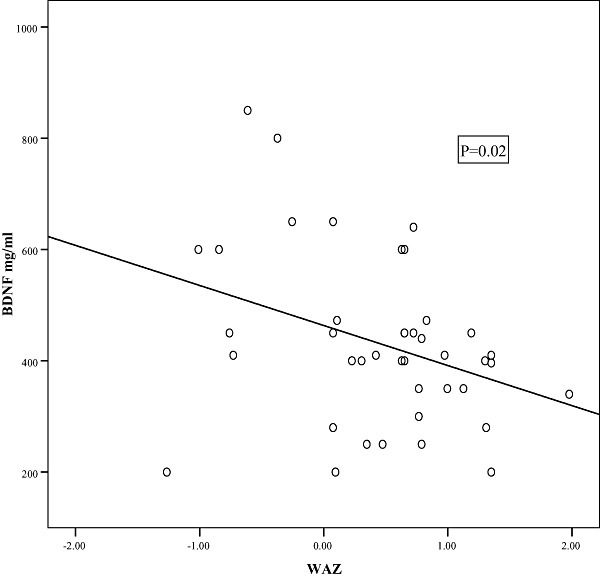
Scatter diagram showing correlation between weight‐for‐age Z‐score (WAZ) and brain‐derived neurotrophic factor (BDNF).
Figure 3.
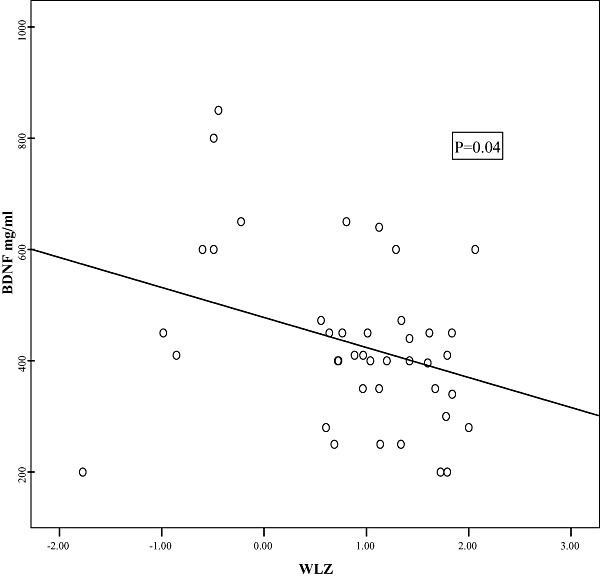
Scatter diagram showing correlation between weight‐for‐length Z‐score (WLZ) and brain‐derived neurotrophic factor (BDNF).
Figure 4.
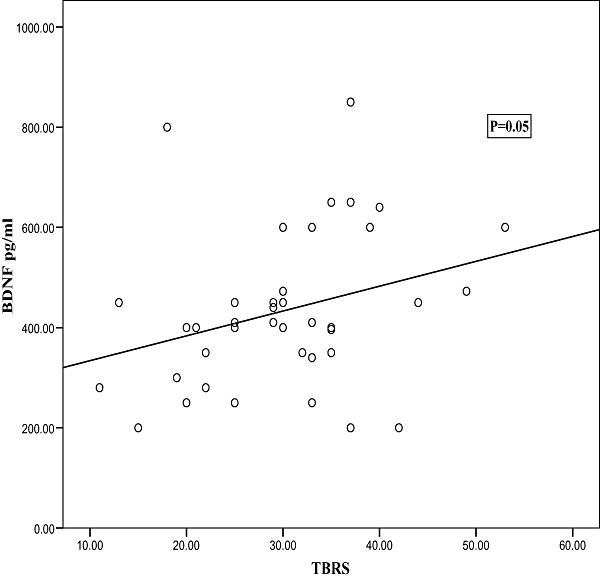
Scatter diagram showing correlation between total behaviour rating scale (TBRS) score and brain‐derived neurotrophic factor (BDNF).
Multiple regression analysis studies show that after adjustment, there is a closely significant increase in mean adjusted BNDF in breastfed children compared to the two other groups. Additionally, there was a significant increase in mean adjusted TBRS and motor quality percentile rank in breastfed children compared to the two other groups (6, 7, 8).
Table 6.
Effect of type of feeding and sex on BDNF
| Coefficient | 95% CI | P | |
|---|---|---|---|
| Breastfed group | |||
| Formula fed* | −122.0 | −228.0, −16.0 | 0.05 |
| Mixed fed* | −102.6 | −210.2, 5.0 | |
| Male | 0.10 | ||
| Female † | −77.5 | −171.0, 16.0 |
*Compared with breastfed group (reference group). † Compared to male.BDNF, brain‐derived neurotrophic factor.
Table 7.
Effect of type of feeding and social class on TBRS
| Coefficient | 95% CI | P | |
|---|---|---|---|
| Breastfed group | |||
| Formula fed* | −11.0 | −17.0, −5.0 | 0.004 |
| Mixed fed* | −7.0 | −13.2, −0.7 | |
| Low | 0.54 | ||
| Middle † | −2.78 | −9.9, 4.4 | |
| High † | −3.77 | −12.1, 4.6 | |
*Compared with breastfed group (reference group). † Compared with low class.TBRS, total behaviour rating scale.
Table 8.
Effect of type of feeding, sex and order of birth on motor quality percentile rank
| Coefficient | 95% CI | P | ||
|---|---|---|---|---|
| Breast fed | ||||
| Groups | Formula fed* | −11.2 | −19.2, −3.1 | 0.02 |
| Mixed fed* | −3.0 | −11.3, 5.3 | ||
| Sex | Male | 0.03 | ||
| Female † | 7.6 | 0.6, 15.0 | ||
| Order of birth | 1 | 0.11 | ||
| 2 ‡ | 2.24 | −5.3, 9.8 | ||
| 3 ‡ | 9.24 | 0.5, 18.0 |
Compared with breast fed group (reference group).
† Compared to male.
‡ Compared to 1.
Discussion
The present study revealed that breastfed group had higher Bayley Scale scores followed by the mixed‐fed group then the formula‐fed one, yet these results reached statistical significance only in TBRS and motor quality percentile rank values.
These results are concurrent with the meta‐analysis done by Anderson et al. (1999) which indicated that, after adjustment for appropriate key cofactors, breastfeeding was associated with significantly higher scores for cognitive development than was formula feeding. Moreover, Angelsen et al. (2001) found that the correlation between breastfeeding and cognitive ability increases with longer duration of breastfeeding. It was found that there is a mean difference of 2.4 points on cognitive test at the age of 6 months between children breastfed for less than 5 months, compared to children breastfed for at least 5 months.
In addition, Rao et al. (2002) found that infants who were exclusively breastfed >12 weeks had mean performance IQ 5 points higher at 5 years of age than children exclusively breastfed <12 weeks. Their study predicted that exclusively breastfed for 24 weeks would give small for gestational age children an advantage of 11 IQ points over those breastfed for ≤12 weeks.
Also, Mortensen et al. (2002) studied a cohort of 9125 individuals in Denmark born between 1959 and 1961, whose early feeding was recorded at 12 months of age. After adjusting for numerous socio‐demographic and birth characteristics, the authors suggested that there is a dose–response of duration of breastfeeding up to 9 months of age and higher adult intelligence. They concluded that human breast milk enhances brain development and improves cognitive development in ways that formula cannot.
Additionally, Morley et al. (2004) measured Mental Development Index (MDI) scores in a cohort of infants and found that at 18 months, breastfed infants had MDI scores 11.7 points higher. After adjusting for two sets of potential confounding variables, the association was ∼8 points higher among breastfed infants. However, the authors did not find a clear dose–response relation with either partial or exclusive breastfeeding. Furthermore, data from a non‐verbal intelligence test at 8.5 years of age in the Cebu Longitudinal Health and Nutrition survey in the Philippines indicated that there were 1.6‐ and 9.8‐point advantages in cognitive abilities for breastfeeding compared with no breastfeeding in normal birthweight and low‐birthweight infants, respectively (Daniels & Adair 2005).
From another perspective, Daniels & Adair (2005) stated that nearly all studies done previously have been conducted in developed countries in which women who breastfeed also exhibit other positive health‐related characteristics that may facilitate cognitive development (e.g. better socio‐economic standard, education, intelligence, responsiveness or home environment). The authors added that disentangling the positive effects of these other characteristics from those of breastfeeding has been a tremendous challenge. However, the current study, although performed in a developing country, still detected better cognitive values in breastfed infants.
In the present study, infants who received human milk showed statistically significant higher serum BDNF values when compared to those who received formula milk.
No previous studies have searched for the presence of BDNF in breast milk or breastfed infants. Breast milk contains many growth factors and although BDNF was not recovered from the breast milk, other growth factors were (Kverka et al. 2007 and Woo et al. 2009). Additionally, breastfeeding process itself may trigger the secretion of BDNF because it is the best pleasurable peaceful situation for the infant and the secretion of BDNF is known to be decreased by stress and increased by intellectual stimulation which is well documented among breastfed infants. Furthermore, infants feeding from the breast suckle strongly and this voluntary exercise also stimulates BDNF secretion (Russo‐Neustadt et al. 2000 and Gomez‐Pinilla et al. 2008).
Although this study did not measure brain BDNF levels, it seems plausible that brain BDNF is increased because of simultaneous onsite production, transport from the periphery or both. We hypothesize that BDNF may be one of the causative factors of higher cognitive functions in breastfed group especially that the regression analysis favours breastfeeding as a determinant factor for BDNF level.
Regarding the correlation studies in this work, negative correlations between BDNF and both WAZ and WLZ scores were detected. As the anthropometric measurements did not differ significantly with the pattern of feeding of the studied infants, the detected correlations could be explained by the studies in animal models which suggest that BDNF plays a key role in energy homeostasis; it is believed to act primarily within the ventromedial hypothalamus to regulate energy intake (Unger et al. 2007).
Although studies in animals provide support for a role of BDNF in energy homeostasis, data in humans are relatively limited. Some studies have shown an inverse association between the peripheral BDNF concentration and the body mass index in children and adults (El‐Gharbawy et al. 2006). Furthermore, Krabbe et al. (2007) added that a common BDNF polymorphism, Val66Met, has been inconclusively associated with altered body weight. Moreover, Yeo et al. (2004) described an obese child with hyperphagia and a heterozygous missense substitution resulting in impaired signalling of the cognate receptor of BDNF, TrkB.
In this study, distinct positive correlation was detected between BDNF values and all studied BSID‐II parameters, yet only the correlation with the TBRS reached statistical significance.
This could be explained by the work of Yamada & Nabeshima (2003) who reported that in the brain, BDNF is active in the hippocampus, cortex and basal forebrain, areas vital to learning, memory and higher thinking. Moreover, BDNF itself is important for long‐term memory (Bekinschtein et al. 2008b).
It is worth noting that in the current study, breastfeeding was a determinant factor for TBRS and motor quality percentile rank by regression analysis.
BDNF potentiates excitatory synaptic transmission in hippocampal neurons. It has been shown to play a critical role in learning outcome in a large number of studies and to have a role in hippocampal‐dependent cognitive performance. Thus, spatial memory was found to be superior in rats housed in enriched rather than in impoverished environments and after training. Disruption of BDNF expression by use of antisense oligonucleotides, in heterozygous BDNF knockout animals or by use of anti‐BDNF antibodies caused severe impairment in spatial memory and learning. Similarly, impaired learning was evident in the conditional TrkB knockout and in mice overexpressing the truncated form of TrkB (Allen & Dawbarn 2006).
Finally, the results of the current study show that girls have non‐significantly less BDNF values than boys. This difference is not enough for the different sex composition of the three groups to explain the evident difference in BDNF; however, it does show that sex differences exist and that future studies need to balance the number of girls and boys in each group to avoid confounding of sex with the feeding pattern.
In conclusion, serum BDNF levels are significantly higher in breastfed infants and shows positive correlations with the results of BSID‐II. Given that simultaneous increase in brain BDNF occurs due to onsite production, transport from the periphery or both, it is prudent to hypothesize that BDNF could be one of the factors responsible for the better cognition detected in breastfed infants. Larger‐scale studies are recommended to further prove this finding as well as other studies to measure the BDNF in breast milk and to explore other candidate factors for better cognition in breastfed infants.
Source of funding
None.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors acknowledge Dr Sahar Ezz‐Elarab who performed the statistical analysis in this study.
References
- Allen S.J. & Dawbarn D. ( 2006. ) Clinical relevance of the neurotrophins and their receptors . Clinical Science (London) 110 , 175 – 191 . [DOI] [PubMed] [Google Scholar]
- Anderson N.K. , Johnstone B.M. & Remley D.T. ( 1999. ) Breast feeding and cognitive development: a meta‐analysis . American Journal of Clinical Nutrition 70 , 525 – 535 . [DOI] [PubMed] [Google Scholar]
- Angelsen N.K. , Vik T. , Jacobsen G. & Bakketeig L.S. ( 2001. ) Breast feeding and cognitive development at age 1 and 5 years . Archives of Disease in Childhood 85 , 183 – 188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. ( 1993. ) Bayley Scales of Infant Development (Manual) , 2nd edn. The Psychological Corporation, Harcourt Brace & Company; : San Antonio . [Google Scholar]
- Bekinschtein P. , Cammarota M. , Izquierdo I. & Medina J.H. ( 2008a. ) BDNF and memory formation and storage . Neuroscientist 14 , 147 – 156 . [DOI] [PubMed] [Google Scholar]
- Bekinschtein P. , Cammarota M. , Katche C. , Slipczuk L. , Rossato J.I. , Goldin A. et al . ( 2008b. ) BDNF is essential to promote persistence of long‐term memory storage . Proceedings of the National Academy of Sciences of the United States of America 105 , 2711 – 2716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M.C. & Adair L.S. ( 2005. ) Breast‐feeding influences cognitive development in Filipino children . Journal of Nutrition 135 , 2589 – 2595 . [DOI] [PubMed] [Google Scholar]
- El‐Gharbawy A.H. , Adler‐Wailes D.C. , Mirch M.C. , Theim K.R. , Ranzenhofer L. , Tanofsky‐Kraff M. et al . ( 2006. ) Serum brain‐derived neurotrophic factor concentrations in lean and overweight children and adolescents . Journal of Clinical Endocrinology and Metabolism 91 , 3548 – 3552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Pinilla F. , Vayman S. & Ying Z. ( 2008. ) Brain‐derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition . European Journal of Neuroscience 28 , 2278 – 2287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.H. , Ji C.Y. , Zheng X.X. , Shan J.P. & Hou R. ( 2008. ) Correlation of 4‐month infant feeding modes with their growth and iron status in Beijing . Chinese Medical Journal (Beijing) 12 , 392 – 398 . [PubMed] [Google Scholar]
- Krabbe K.S. , Nielsen A.R. , Krogh‐Madsen R. , Plomgaard P. , Ramussen P. , Erikstrup C. et al . ( 2007. ) Brain‐derived neurotrophic factor (BDNF) and type 2 diabetes . Diabetologia 50 , 431 – 438 . [DOI] [PubMed] [Google Scholar]
- Kverka M. , Burianova J. , Lodinova‐Zadnikova R. , Kocourkova I. , Cinova J. , Tuckova L. et al . ( 2007. ) Cytokine profiling in human colostrums and milk by protein array . Clinical Chemistry 53 , 955 – 962 . [DOI] [PubMed] [Google Scholar]
- Morley R. , Fewtrell M.S. , Abbott R.A. , Stephenson T. , MacFadyen U. & Lucas A. ( 2004. ) Neurodevelopment in children born small for gestational age: a randomized trial of nutrient‐enriched versus standard formula and comparison with a reference breastfed group . Pediatrics 113 , 515 – 521 . [DOI] [PubMed] [Google Scholar]
- Mortensen E.L. , Michaelsen K.F. , Sanders S.A. & Reinisch J.M. ( 2002. ) The association between duration of breastfeeding and adult intelligence . Journal of the American Medical Association 287 , 2365 – 2371 . [DOI] [PubMed] [Google Scholar]
- Pan W. , Banks W.A. , Fasold M.B. , Bluth J. & Kastin A.J. ( 1998. ) Transport of brain‐derived neurotrophic factor across the blood–brain barrier . Neuropharmacology 37 , 1553 – 1561 . [DOI] [PubMed] [Google Scholar]
- Park J.E. & Park K. ( 1979. ) Text Book and Social Medicine , 7th edn, p 81 . Messers Banarsidas, Bhanot Publisher; : Napier Town, India . [Google Scholar]
- Poduslo J.F. & Curran G.L. ( 1996. ) Permeability at the blood–brain and blood‐nerve barriers of the neurotrophic factors: NGF, CNTF, NT‐3, BDNF . Brain Research. Molecular Brain Research (Amsterdam) 36 , 280 – 286 . [DOI] [PubMed] [Google Scholar]
- Rao M.R. , Hediger M.L. , Levine R.J. , Naficy A.B. & Vik T. ( 2002. ) Effect of breastfeeding on cognitive development of infants born small for gestational age . Acta Paediatrica 91 , 267 – 274 . [DOI] [PubMed] [Google Scholar]
- Russo‐Neustadt A.A. , Beard R.C. , Huang Y.M. & Cotman C.W. ( 2000. ) Physical activity and antidepressant treatment potentiate the expression of specific brain‐derived neurotrophic factor transcripts in the rat hippocampus . Neuroscience 101 , 305 – 312 . [DOI] [PubMed] [Google Scholar]
- Schack‐Nielsen L. & Michaelsen K.F. ( 2007. ) Advances in our understanding of the biology of human milk and its effects on the offspring . Journal of Nutrition 137 , 503S – 510S . [DOI] [PubMed] [Google Scholar]
- Song X.Y. , Li F. , Zhang F.H. , Zhong J.H. & Zhou X.F. ( 2008. ) Peripherally‐derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury . PLoS ONE 5 , e1707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T.J. , Calderon G.A. , Bradley L.C. , Sena‐Esteves M. & Rios M. ( 2007. ) Selective deletion of BDNF in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity . Journal of Neuroscience 27 , 14265 – 14274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H. , Mundy W. & Eriksson P. ( 2008. ) Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in change in BDNF, CaMKll and GAP‐43, biochemical substrates of neuronal survival, growth, and synaptogenesis . Neurotoxicology 29 , 152 – 159 . [DOI] [PubMed] [Google Scholar]
- WHO ( 2006. ) Child Growth Standards Length/Height for‐Age, Weight‐for‐Age, Weight‐for‐Length and Body Mass Index‐for‐Age: Methods and Development . World Health Organization; : Geneva . [Google Scholar]
- Woo J.R. , Guerrero M.L. , Altaye M. , Ruiz‐Palacios G.M. , Martin L.J. , Dubert‐Ferrandon A. et al . ( 2009. ) Human milk adiponectin is associated with infant growth in two independent cohorts . Breastfeeding Medicine 4 , 101 – 109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. & Nabeshima T. ( 2003. ) Brain‐derived neurotrophic factor/TrkB signaling in memory processes . Journal of Pharmacological Sciences 91 , 267 – 270 . [DOI] [PubMed] [Google Scholar]
- Yeo G.S. , Connie Hung C.C. , Rochford J. , Keogh J. , Gray J. , Sivaramakrishnan S. et al . ( 2004. ) A de novo mutation affecting human TrkB associated with severe obesity and developmental delay . Nature Neuroscience 7 , 1187 – 1189 . [DOI] [PubMed] [Google Scholar]


