Abstract
The aim of this article is to describe the main methodological challenges in the monitoring of dietary intake in the Norwegian Mother and Child Cohort Study (MoBa), a pregnancy cohort aiming to include 100 000 participants. The overall challenge was to record dietary patterns in sufficient detail to support future testing of a broad range of hypotheses, while at the same time limiting the burden on the participants. The main questions to be answered were: which dietary method to choose, when in pregnancy to ask, which time period should the questions cover, which diet questions to include, how to perform a validation study, and how to handle uncertainties in the reporting. Our decisions were as follows: using a semi‐quantitative food frequency questionnaire (FFQ) (in use from 1 March 2002), letting the participants answer in mid‐pregnancy, and asking the mother what she has eaten since she became pregnant. The questions make it possible to estimate intake of food supplements, antioxidants and environmental contaminants in the future. Misreporting is handled by consistency checks. Reports with a calculated daily energy intake of <4.5 and >20 MJ day−1 are excluded, about 1% in each end of the scale. A validation study confirmed that the included intakes are realistic. The outcome of our methodological choices indicates that our FFQ strikes a reasonable balance between conflicting methodological and scientific interests, and that our approach therefore may be of use to others planning to monitor diet in pregnancy cohorts.
Keywords: pregnancy cohort, dietary methods, food frequency questionnaire (FFQ), foetal and child nutrition
Introduction
There has been a rapid development and profound changes in our understanding of the impact of fetal nutrition on development and health of the child. Three decades ago, the consensus was that the fetus was protected and lived more or less as a parasite on the mother, only being affected by maternal nutrition if this was extremely unbalanced or insufficient. The mother would pay the nutritional price of a pregnancy, rarely the child (Susser & Stein 1994). Subsequent research has modified this picture towards a much more important role for the mother's diet during pregnancy. The diet has an impact on all systems of the body, including the fetus, and can modulate different functions far beyond the levels that are connected to malnutrition, e.g. expression of genes, hormone levels, the developing nervous system and risk of diseases later in life (Godfrey & Barker 2001; Moore & Davies 2005). Knowledge is also accumulating about the effects of non‐nutrients and toxic dietary substances on fetal development and health later in life. As a consequence of this development in understanding, monitoring of dietary intake has become an integral part of pregnancy and birth cohort studies.
The Norwegian Mother and Child Cohort Study (MoBa) is a prospective pregnancy cohort that in the period 1999 to March 2007 has included more than 83 000 pregnancies, and that aims to include 100 000 by the end of 2008 (Magnus et al. 2006). Pregnant women are recruited to the study by postal invitation after they have signed up for the routine ultrasound examination in their local hospital. Participants are asked to provide biological samples and to answer questionnaires covering a wide range of information up to age 7 for the child. The cohort database is linked to the Medical Birth Registry of Norway (Irgens 2000).
The first 8954 women included in MoBa answered a food frequency questionnaire (FFQ) (old FFQ) that had been developed for general, nationwide dietary surveys in the 1990s. It had been validated, but not in pregnant women (Nes et al. 1992; Solvoll et al. 1993; Andersen et al. 1999). Its main focus was on energy and nutrient intake. There was an increasing discontent with this FFQ because of several limitations in the ability to mirror current research interests. In June 2001, we decided to develop a new FFQ especially for the MoBa cohort, with the aim to be more flexible about the types of hypotheses that can be tested, both with regard to components in the diet and with regard to dietary patterns and profiles.
Among the problems connected with assessment of diet in pregnancy are the large intra‐individual variations due to pregnancy complications that may influence eating habits, e.g. nausea, vomiting, constipation and bed rest. Furthermore, the time periods of interest may vary, i.e. preconceptional, by trimester or by critical windows for fetal organ/tissue development. These were problems we brought with us into the basic challenges of designing a new dietary survey, i.e. which dietary method to go for, include or not include portion sizes, when in pregnancy to ask, how many questions on foods, how detailed should we be on the supplement questions, how perform a validation study, and how handle uncertainties in the reporting.
The aim of this paper is twofold: (1) to describe our challenges in the development, validation and utilization of dietary monitoring in the MoBa study; and (2) to explain and discuss our design decisions.
Setting
Until very recently, the Norwegian population has been rather homogenous, culturally and ethnically speaking. In 2001, when we started to develop a new dietary assessment instrument for the MoBa study, non‐Western immigrants comprised 4.5% of a total population of approximately 4 600 000 people, Pakistanis being the largest group (Statistics Norway, 1 January 2005). Economic constraints have influenced many of the decisions made in MoBa, including the decision to use Norwegian as the only language of the study. Diet‐wise, this implied that the chances of enrolling many non‐ethnic Norwegians as participants were considered limited, and hence we could pay main attention to a dietary profile representative of the general population.
Furthermore, the high employment rate for women in Norway had to be taken into account. In 2001, 82% of women aged 25–40 years were employed. More than half worked full‐time, and about 70% worked 20 h or more per week (Statistics Norway 2006). Because of the high employment rate among women, we had to take into consideration the limited time frame many women have for ‘extras’ in their lives. Also, preliminary analysis of the first 10 000 women included in the study showed that approximately half of the participants already had one or more children when entering MoBa – emphasizing the need to limit the reporting burden of the women.
In a quality‐assured data file of 40 786 women in MoBa 1 answering the new FFQ, our considerations were confirmed: 55% of participants reported working full‐time (>30 h weekly), and additionally 27% reported working part‐time (<30 h weekly) in pregnancy. Only 7% reported to be ‘home‐stayers’ prior to and in the present pregnancy. More than half of the participants (55%) had at least one child prior to entering the study.
Which dietary method to use?
All dietary methods that assess the food intake of an individual or a group have their limitations (Cade et al. 2002). The most important criterion as to the appropriateness of a method is the purpose and/or research question to be addressed. The objective of MoBa is to provide a basis for studies on aetiology of disease and pregnancy outcome, be it genetic, dietary or lifestyle factors, aiming at prevention. The basic planning was not made on the basis of any single hypothesis or even any set of hypotheses, as one cannot foresee the specific research questions that will emerge 10–50 years ahead. The strategy has therefore been to collect data on as many relevant exposures and health outcomes as feasible. Diet‐wise, this implied covering as many known aspects of the diet as possible, on an individual level, over a restricted time period during pregnancy. Even though several dietary assessment methods were discussed, i.e. diet records and repeated 24‐h recalls, we came to the conclusion that there was no realistic alternative to a FFQ, taken the large number of participants into account (Willett & Hu 2006).
In many respects, FFQs are relatively low‐precision instruments (Byers 2001; Kristal et al. 2005). However, some types of criticism are probably less relevant for our questionnaire. We are only asking about a relatively short time period, which results in less recalling, estimation and abstraction for the participants. Good classification, rather than precise numerical estimation, has been a goal, and recent validation studies of FFQs in pregnant women generally indicate that FFQs can be used to classify women according to their nutritional intake with a reasonable degree of accuracy. This may, though, vary according to the population and the number of food items (Table 1) on the instrument (de Vries et al. 1994; Brown et al. 1996; Robinson et al. 1996; Erkkola et al. 2001; Parra et al. 2002; Siega‐Riz et al. 2004).
Table 1.
Description of the new MoBa food frequency questionnaire
| Number of questions | Comment | |
|---|---|---|
| Total | 340 questions grouped into 40 overall questions, of which 26 are related to food intake | Comprising 255 food items grouped according to the Norwegian meal pattern |
| Dietary supplements | 13 on specific supplements 6 for filling in product and brand name | Reported on the level of name, brand, frequency and dose |
| Portion sizes | Applied to questions on bread and drinks | Omitted to permit more food item questions |
| Types of meals | 8 | Breakfast, lunch, dinner, supper, evening meal and snacks |
| Dietary profile | 6 | Carnivore, vegetarian, vegan, partly vegetarian diets (fish – no meat) |
| Organic food preferences | 6 | Covers major food groups |
| Fast‐food purchases | 3 | Food bought at petrol stations kiosks, fast‐food stores |
| Genetically modified foods | 4 | Open questions, brand names to be filled in |
| Fermented foods | 5 milk products, 1 (2) cheese | |
| Coffee, caffeine intake | 5 coffee, 1 tea | In addition comes herb teas (2) and coffee alternatives (2) |
| Foods with high risk of being contaminated | 15 | Including: fish, game, organ meats and seagulls eggs |
| Raw vs. cooked vegetables | Raw vegetables: 1 global, 8 on specific foods Cooked vegetables: 2 global, 19 specific | In addition comes the foods almost always eaten raw, e.g. cucumber, avocado and salads |
| Open questions | 4 | Not connected to any specific food group |
| Diet changes after becoming pregnant | 17 | 11 solid food groups, 6 types of drinks |
| Questions related to pregnancy | 7 | Nausea, vomiting, pica |
A FFQ challenges respondents with rather complex cognitive tasks. People willing to participate in a cohort study may be more interested in health and health practices than the average individual and, therefore, may have different dietary practices from the general population. The target population of MoBa is all women who give birth in Norway. The overall participation rate of 44% is a matter of concern and will influence the prevalence of exposures and diseases. It is likely that there is a socio‐economic gradient that influences prevalence estimates and observed dietary patterns. However, the aim of MoBa is to estimate associations between exposures and disease in nested case–control studies, and these associations are less influenced by selection bias (Magnus et al. 2006). Basic characteristics of the women, such as age and parity, and birth outcomes such as gestational age, prevalence of preterm births and birthweight are not different between the study population and the total pregnancy population (Magnus et al. 2006). Details on the smoking, educational level and martial status of the participants are given in the diet validation article and show a wide range of variation (Brantsæter et al. 2008). 2, 3 convey a broad range of food and nutrient intakes. Estimates of participation, follow‐up and withdrawal are discussed in the paper by Magnus et al. (2006).
Table 2.
Daily intake of macronutrients and selected vitamins* and minerals* calculated by the food frequency questionnaire in 40 108 MoBa participants †
| Nutrients | Mean | SD | Median | 5th percentile | 95th percentile |
|---|---|---|---|---|---|
| Total energy (MJ) | 9.80 | 2.61 | 9.44 | 6.15 | 14.75 |
| Fat (g) | 80.3 | 24.6 | 76.6 | 47.1 | 126.2 |
| Protein (g) | 86.8 | 21.4 | 84.3 | 56.3 | 125.7 |
| Carbohydrate (g) | 313.9 | 94.0 | 300.6 | 184.9 | 491.0 |
| Dietary fibres (g) | 30.6 | 10.4 | 29.2 | 16.2 | 49.8 |
| Added sugar (g) | 65.1 | 41.4 | 55.3 | 19.6 | 143.7 |
| Energy % fat | 33.2 | 4.6 | 33.1 | 25.9 | 41.1 |
| Energy % protein | 15.3 | 2.1 | 15.2 | 11.9 | 18.9 |
| Energy % carbohydrate | 51.5 | 6.7 | 51.7 | 40.0 | 62.2 |
| Energy % added sugar | 10.9 | 5.3 | 10.1 | 4.2 | 20.6 |
| Vitamin D (μg) | 3.5 | 2.6 | 3.1 | 1.0 | 7.1 |
| Retinol (μg) | 869 | 664 | 666 | 237 | 2181 |
| Folate (μg) | 272 | 96 | 258 | 146 | 447 |
| Vitamin C (mg) | 166 | 93 | 147 | 57 | 340 |
| Calcium (mg) | 1044 | 428 | 972 | 486 | 1844 |
| Iron (mg) | 11.2 | 3.4 | 10.7 | 6.5 | 17.5 |
| Iodine (µg) | 131 | 63 | 121 | 50 | 247 |
Does not include dietary supplements.
Excluding 278 participants (0.7%) with energy intake <4.5 MJ and 400 participants (1.0%) with energy intake >20 MJ.
Table 3.
Daily intake of selected foods and food groups calculated by the food frequency questionnaire in 40 108 MoBa participants*
| Food and food groups | Mean | SD | Median | 5th percentile | 95th percentile |
|---|---|---|---|---|---|
| Milk for drinking (g) | 382 | 349 | 400 | 0 | 1013 |
| All dairy products (g) | 503 | 369 | 460 | 72 | 1225 |
| Bread (g) | 228 | 101 | 218 | 68 | 409 |
| Meat (g) | 112 | 34.2 | 111 | 59 | 168 |
| Fish** and seafood † (g) | 45 | 26.6 | 42 | 7 | 92 |
| Fruit (g) | 258 | 188 | 215 | 47 | 610 |
| Juice (g) | 175 | 195 | 135 | 0 | 507 |
| All vegetables except potatoes (g) | 159 | 101 | 138 | 44 | 349 |
| Margarine/butter (g) | 18 | 15.4 | 16 | 0 | 44 |
| Chocolates/sweets (g) | 46 | 40.4 | 36 | 8 | 121 |
| Soft drinks ‡ (ml) | 323 | 407 | 181 | 8 | 1097 |
| Tea § (ml) | 179 | 255 | 71 | 0 | 607 |
| Coffee (ml) | 102 | 173 | 16 | 0 | 441 |
Excluding 278 participants (0.7%) with energy intake <4.5 MJ and 400 participants (1.0%) with energy intake >20 MJ.
**Including fish products.
† Roe, crabs, scallops, prawns.
‡ Including both sugar sweetened and artificially sweetened.
§ Black, green and herb tea.
When in pregnancy to ask?
The earliest time possible for the women in MoBa to fill in a FFQ was when they received the invitation to participate in the study, at approximately week 14 of pregnancy. Questionnaire 1 in the survey, asking about background variables, has from the beginning of MoBa been posted together with the invitation to participate.
We made the decision to post the FFQ together with the invitation and the first general questionnaire, similar to what had been done with the old FFQ. The average time point for filling in the new FFQ turned out to be in weeks 17–18 of pregnancy. This went on from the introduction of the new FFQ 1 March 2002 till the spring of 2004 (11 May 2004). From the latter date and onwards, the FFQ has been posted separately to the women in week 22 of pregnancy (Magnus et al. 2006), and average time point for filling in the questionnaire after this change has been weeks 23–24 of pregnancy. The decision was taken in the hope that this would increase the participation rate of the study.
Which time period in pregnancy to cover?
Until recently, there has been little knowledge about potential dietary changes over the course of a pregnancy or whether a woman changes her diet at all. Two recent studies indicate that dietary changes do occur (Olafsdottir et al. 2006; Rifas‐Shiman et al. 2006), but these were not available at the time we planned our FFQ. Thus, it was an open question to us whether to ask about the pre‐pregnancy diet (as in the old MoBa FFQ) or ask about diet in pregnancy. We also had to decide which time window the diet questions should cover.
Today we know that the diet in first trimester may be more important to development of various organs, while the diet later in pregnancy may be important for overall fetal growth as well as for brain development. This had to be considered closely when planning both the time point for posting the FFQ and the time period to ask about. In a similarly large pregnancy cohort in Denmark (Olsen et al. 2001), the women were asked about their diet the previous month, i.e. covering the diet approximately between weeks 21 and 25 of pregnancy (Mikkelsen et al. 2006). Because birth defects caused by possible exposures early in pregnancy were a major driving force behind the initiation of MoBa study, we had to consider the dietary exposure to foods and nutrients from the beginning of pregnancy.
Hence we made the decision to ask the women about her diet since she became pregnant, and accordingly, the new FFQ covers her diet the first 4 or 5 months of pregnancy.
Analysis of the 17 questions pertaining to changes in diet after becoming pregnant, fully confirmed that a number of changes appear to take place for a large percentage of pregnant women. The most dramatic changes are seen for coffee and alcohol intake, but large changes are also seen for the other major food groups, as shown in Table 4. It is only partly possible to test the reliability of the answers to our questions on diet changes; however, 80% of the women stating that they stopped drinking coffee in pregnancy reported no intake of coffee after becoming pregnant. Likewise, 94% of the women who stated that they had stopped drinking alcohol in pregnancy reported no intake of alcohol. These findings indicate that the questions on dietary changes in pregnancy can be trusted.
Table 4.
Percentage of women reporting pregnancy‐related changes in consumption of food and beverages (n = 40 786)
| Did not eat/drink before pregnancy (%) | Same as before (%) | More than before (%) | Less than before (%) | Stopped (%) | |
|---|---|---|---|---|---|
| Milk/dairy products | 2.6 | 52.8 | 38.7 | 5.6 | 0.3 |
| Fish | 2.1 | 76.3 | 15.4 | 5.8 | 0.4 |
| Vegetables | 0.3 | 64.0 | 33.0 | 2.6 | 0 |
| Fruit | 0.2 | 37.4 | 60.8 | 1.6 | 0 |
| Chocolate | 1.4 | 47.6 | 15.5 | 33.7 | 1.8 |
| Sweets | 2.6 | 50.4 | 13.6 | 31.7 | 1.7 |
| Soft drinks – sugar | 13.8 | 40.3 | 6.8 | 34.6 | 4.6 |
| Soft drinks – artificial sweetener | 32.3 | 30.7 | 3.0 | 25.2 | 8.9 |
| Coffee | 31.9 | 13.8 | 0.6 | 40.1 | 13.6 |
| Alcohol | 9.9 | 1.5 | 0 | 10.2 | 78.3 |
How many food questions?
When designing the diet questionnaire, we were especially concerned about transcending the traditional focus on food and nutrient intake, and make possible the monitoring of non‐nutrients, e.g. antioxidants (like flavonoids) and environmental toxins (Kroes et al. 2002). We also had the aim to make it possible to support the testing of current (and possibly future) hypotheses on diet–health relationships. This is in line with the explicit aim in the MoBa project to provide data for support or refutation of conjectures and hypotheses about associations between pregnancy exposures and future health outcomes.
The starting point for all food questions in the development of our FFQ was our knowledge about the habitual food intake presented in earlier nationwide dietary surveys (Johansson et al. 1997). The number of food categories that must be included in a FFQ in order to capture the variation between persons within a population varies for different foods and nutrients (Overvad et al. 1991; Mark et al. 1996). Stepwise regression analyses have been applied to the Norwegian food intake data to identify the most discriminating food items (Mosdøl et al. 2000). Thus we feel confident that our food questions cover the major foods and food patterns representative of the majority of the Norwegian population. With rapidly changing food habits due to an ever‐increasing variety of foods in our grocery shops, we also had to take ‘new’ foods into consideration, e.g. burritos, ciabattas, etc., not traditionally part of the Norwegian diet.
We tried to open for future research on the impact of diet on gut health (e.g. microflora, constipation), including questions about fermented foods and vegetables eaten raw vs. cooked. As there are widely differing views about the impact of organic food on health, and the sales are increasing, we saw the opportunity to ask about the use of such foods. Questions on the use of genetically modified foods were included in spite of such foods not being allowed to sell in Norway at the time the FFQ was developed. With harmonization of European Union regulations, we anticipated that this ban would be lifted before the inclusion period of the study was over. However, this has not happened as to this date (April 2007), implying that these questions probably will have limited, if any, value in the future.
As to the ambition to test future hypotheses connected to the exposure to environmental contaminants, we included questions about rarely eaten foods that have an exposure impact on those few who eat them. In small cohorts, it is difficult to make use of a consumption prevalence which is very low. In a large cohort like MoBa, 1.5% stating that they eat, for example, seagulls eggs more than 10 times per year, gives 600 persons in the 40 786 file 1 . Such a number is more than sufficient to use in a nested case–control design to assess eventual negative health outcomes connected to this very polychlorinated biphenyl (PCB)‐rich food item.
The new FFQ ended up consisting of 340 questions organized into 40 groups according to the Norwegian meal pattern. Three of the groups had questions about dietary patterns and 23 about the use of 255 specific food items, with the goal of monitoring energy intake, nutrients, non‐nutrients, foods and food groups. Two groups of questions were of a more global character, to be used for correction of under‐ or over‐reporting of the food‐specific questions. The frequency of consumption was given per day, per week and/or per month, depending on the food item. Details are shown in Table 1. Several experts have pointed out the dangers of having a too detailed questionnaire, most of all because of increased risk of participant dropout (Willett 1998; Cade et al. 2002). We will never be able to know exactly how our 12‐page FFQ influenced the participation rate or the quality of the information we obtained, but 93% of the women participating in MoBa answered the FFQ (Magnus et al. 2006). Thus, generally, our population of FFQ respondents may be assumed to be as representative as the MoBa study population.
How monitor dietary supplement use?
A preliminary analysis of the first 2800 women participating in MoBa and answering the old FFQ indicated that 70% were using dietary supplements. Thus, another challenge became to design questions making it possible to assess supplement intake with a high degree of accuracy.
Based on information about the most sold food supplements in Norway, we decided to include 13 questions on specifically named items, and leave six lines open for the women to fill in themselves. The content of substances in food supplements has been unregistered in Norway. For estimation of the amounts of nutrients and compounds supplied by dietary supplements in MoBa, we are compiling a database containing details of the declared content of more than 1000 supplements. We have observed that changes in content have taken place during the study period. These are taken into account continuously. For substances satisfactory declared, such as vitamins and minerals, the exact content is entered into the database, while for substances not satisfactory declared, like herbal extracts, the presence of these substances, but no amounts, is recorded.
Analysis of the 2006 file with 40 786 participants showed that 81.4% reported taking dietary supplements during pregnancy. 1 This clearly supports our choice to ask about supplements at a brand‐name level, including frequency and dose. Supplements thus provide a substantial amount of vitamins and minerals to eight out of 10 participants, and no intake estimation of nutrients will give a true picture of the dietary exposure without the supplements included.
Include portion sizes, or not?
One of the main design decisions was whether or not to use portion sizes. Portions sizes in reality doubles the number of questions, and thus significantly increase the reporting burden. The first years of the MoBa inclusion period, we received a considerable amount of complaints about the complexity of the old FFQ, which included portion sizes. By renouncing on portion size data, we would be able to include a larger number of food questions, which allows a more precise description of food intake patterns and food habits. Several studies have shown that although there is a minor improvement in validity when allowing subjects to specify their own portion sizes, this does not necessarily justify the extra cost and time involved for the participants (Tjonneland et al. 1991; Haraldsdottir et al. 1994). Precision in the assessment of energy, fat and protein intake had to be weighed against the need for more detailed descriptions of the diet, and the best way to cater for future needs may be to record as many details as possible.
Hence we decided to sacrifice portion sizes for all foods apart from units of fruits, bread (slices) and liquids (cups/glasses) to be able to include more food questions, with the specific aim to capture the intake of non‐nutrients more accurately. For dinners, vegetables, cakes and snacks, standard Norwegian portion sizes are used (Blaker & Aarsland 1989), although adjusted for some fruit and vegetable portion sizes reported in our validation study (food diary; FD), and also adjusted for potatoes, rice and cereals according to more recent portion size estimations (National Association for Nutrition and Health 2004). The new semi‐quantitative FFQ is described in Table 1 and downloadable from the website http://www.fhi.no/morogbarn/.
How to compute nutrients and non‐nutrients?
Norway has for years had its own national food database comprising most nutrients (http://matportalen.no/matvaretabellen), but with no data on fatty acids, iodine and non‐nutrients (e.g. flavonoids or toxicants). To be able to comply with the broad approach of MoBa, we decided to gather data on several nutrients and non‐nutrients which are presently not included in the official Norwegian composition food tables, i.e. fatty acids, iodine and antioxidants (2002, 2006). Furthermore, work is in progress obtaining and organizing data about the mercury, cadmium, acrylamide, polybrominated diphenylethers (PBDEs), PCB and dioxin content of Norwegian foods.
Eighty‐six women answered both the old and the new questionnaire in a satisfactory way, thus enabling us to estimate whether we were successful in achieving a better detection of non‐nutrient intake. The Pearson correlations for energy and protein intake estimations were r = 0.634 (P < 0.001) and r = 0.721 (P < 0.001), respectively. The dispersion of the estimated antioxidant capacities was similar with the two questionnaires, reflecting that both the old and the new questionnaire had many questions on fruit and vegetable intake (results not shown). With the new questionnaire, the shape of the estimated intake distribution for cadmium and mercury was much more skewed, and the dispersion was significantly larger when compared with the old FFQ. In Fig. 1, this is shown with mercury intake estimations.
Figure 1.
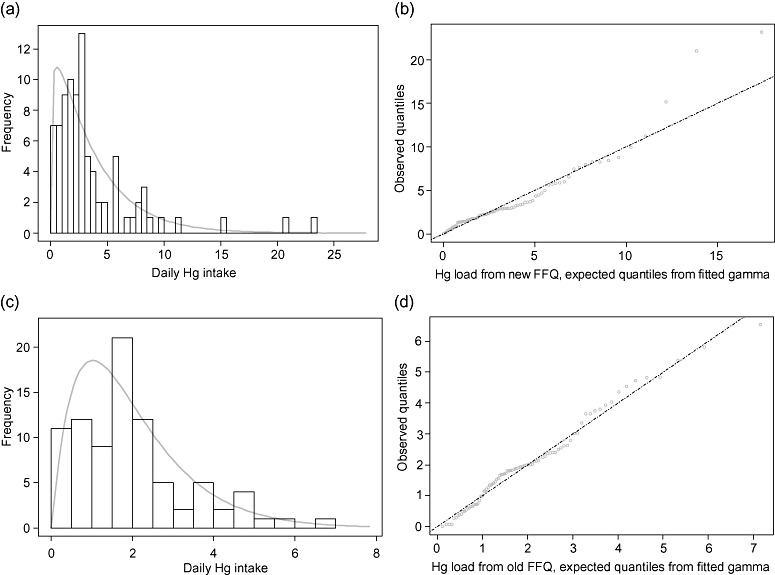
(a–d) Mercury intakes (μg day−1) calculated from the old and new questionnaires, with gamma distributions fitted by maximum likelihood estimation (n = 86). The fitted distributions are used for computing confidence intervals. (a) Daily Hg intake calculated from the new questionnaire, with gamma distribution fitted. (b) Plot of observed values vs. expected values from the gamma fitted in (a). (c) Daily Hg intake calculated from the old questionnaire, with gamma distribution fitted. (d) Plot of observed values vs. expected values from the gamma fitted in (c).
The calculated average mercury intakes with the old and new FFQs were 2.0 and 3.7 μg day−1, respectively. When the calculated intakes (n = 86) were fitted with gamma distributions by maximum likelihood estimation (MLE), the standard deviations were 1.4 and 3.4 μg day−1. Ninety‐five per cent confidence intervals (CIs) were 0.3–5.5 for the old questionnaire and 0.1–12.7 for the new one. Excluding the upper and lower 10% of intakes, we achieve for the 80% CIs 0.5–3.9 and 0.5–8.1, respectively. As Fig. 1a–d shows, the gamma MLE estimates fit the empirical data fairly well. Figure 1b and d is so‐called ‘quantile–quantile plots’ (‘Q–Q plots’) of the observed data and fitted distributions from Fig. 1a and c, respectively. The closer this plot follows the straight line, the better the fit. We see from Fig. 1b that the MLE gamma fits are in general quite good, but the new questionnaire produces some outliers at the upper end. Still, the fit is far better than those obtained with other actual two‐parameter families of distributions, like the normal and the lognormal, and it could be improved for the outliers by using alternate methods of estimation.
These preliminary calculations on mercury exposure confirmed that we were able to capture a broader range of dietary mercury exposures when including high‐mercury foods in the FFQ, e.g. perch, pike and tuna. With the old questionnaire, the intake of the 90 percentile was estimated as only half of the 8.1 μg day−1 estimated from the new one, indicating that the new design allows for a much more precise identification of higher loads. Thus, the risk for a (possibly) high load passing undetected is now generally reduced.
To our knowledge, non‐nutrient databases have been virtually non‐existent also in most other countries and seem to be project‐driven.
How perform a validation of the FFQ?
Validation of a FFQ should always be performed in the same target group as it is to be used. There are two main challenges that affect a validation of a FFQ designed for pregnant vs. non‐pregnant women: there has to be a time lag between the time point of filling in the FFQ and the validation study, whatever method used, which may imply dietary changes due to nausea or alterations in appetite and food preferences. Furthermore, a time lag implies a weight increase in most women (fetal weight increase + plasma volume expansion) that may influence the results. In general, weighed records or diet records are recommended to be the first method of choice for validating FFQs (Cade et al. 2002). Few validation studies pertaining to the use of FFQs in pregnant populations have been published, as opposed to the large number of validation studies in non‐pregnant populations (National Cancer Institute 2006).
Four‐day weighed FD was chosen as the dietary reference method in our validation study, which is considered sufficient if the sample size is large (Stram et al. 1995). As several biomarkers and activity registration (in the form of a motion sensor) also were included as reference methods, 4 days was considered the acceptable burden that could be imposed on the validation study participants. The validation study is described in detail in an accompanying article in this journal, so here only a general outline of findings is given (Brantsæter et al. 2008).
Validation of the MoBa FFQ resulted in correlations between the two dietary methods (FFQ and FD) (Brantsæter et al. 2008) in the range observed in other validation studies in pregnant women (Robinson et al. 1996; Erkkola et al. 2001; Mikkelsen et al. 2006), but lower than those reported in non‐pregnant populations (Byers 2001; Subar et al. 2001; Thompson et al. 2004). Even more important, the degree of misclassification is small (Brantsæter et al. 2008).
A number of biomarkers in blood and urine were included in our validation study, enabling us to apply the method of triads to calculate validity coefficients when three pairwise correlations of measurements were available. In spite of some possibly confounding factors, such as the well‐known plasma volume expansion in pregnancy, and biological samples not being taken fasting, the biomarkers complemented our dietary record validation and strengthened our results (2007a, 2007b, 2008).
Although the goal was to perform the validation study as close in time to answering the FFQ as possible, a number of practical hindrances prevented us from including women immediately after they had filled in the FFQ. Some women had filled in the FFQ immediately after receiving the questionnaire in week 14, while their appointment to the ultrasound examination, when they were recruited to the validation study, was several weeks later, on average week 17. This resulted in an average time lag of 24 days, with a range from 1 to 59 days, between filling in the FFQ and the FD. In this time period, 65% of the participants in the validation study had more than 1‐kg weight gain. Excluding participants with a weight change >1 kg improved the correlation for total energy intake between the FFQ and FD from r = 0.27 to r = 0.52 (Brantsæter et al. 2007b), indicating that we should have used excess days between answering the FFQ and recruitment to the validation study as an exclusion criterion.
How handle uncertainties in the reported dietary intake estimates
Some FFQ reports will be of poor quality due to missing data or gross misreporting of intake. Exclusion criteria of subjects reporting biologically implausible intakes have to be established for each study population. Characteristics of persons under‐reporting dietary intake have been identified in several populations, including pregnant women (Forsum et al. 1992; Goldberg et al. 1993; Maurer et al. 2006). Furthermore, over‐reporting occurs to a certain degree (Johansson et al. 1998; Black & Cole 2001). As most nutrients are strongly correlated to total energy intake, different approaches for excluding reports of poor quality based on the calculated energy intake have been developed.
In MoBa, all questionnaires are optically read and undergo a systematic quality control to ensure that the final data files correspond to the written self‐reports. Reported intakes of foods and supplements are converted into daily intakes by FoodCalc (Lauritsen 2005) and the Norwegian food composition table (Rimestad et al. 2001).
The detailed questions about dinners and fruits are preceded by global (control) questions on how often the mother has eaten meat, fish, vegetarian or fruits per day, per week and per month since she became pregnant. The information from the control questions is used to calibrate the intake of foods in these meals. For bread spreads, intake was calibrated to the number of bread slices recorded.
Furthermore, when frequency of consumption is given as a range, for example 5–6, 3–4 or 1–2 times weekly, the highest frequency in each range is used for foods commonly known to be under‐reported, such as cakes, sweets and snacks (Poppitt et al. 1998).
Although under‐reporting is a well‐known challenge in, and also has also been shown in studies of, pregnant women, relatively little work has been conducted on applying cut‐off points based on estimated energy expenditure to identify improbable under‐ and over‐reporters in pregnant populations. Results of the energy expenditure assessment in the validation study indicated that both the FFQ and the FD underestimated energy intake, and that under‐reporting on the group level was smaller with the FFQ than with the FD, while under‐reporting on the individual level was greater with the FFQ than with the FD.
Without further reason than being biologically improbable, a lower limit of 2.5 MJ day−1 and an upper limit of 15 MJ day−1 (alternatively 500 and 3500 kcal) have been applied as cut‐offs for reported energy intakes in women in the Nurses' Health Study (Schulze et al. 2004), in the European Prospective Investigation into Cancer and Nutrition study (Davey et al. 2003), and in the Norwegian Women and Cancer Study (Hjartaker & Lund 1998).
In an attempt to establish energy cut‐off values, we examined energy intake and expenditure in the validation group (n = 112) and compared their energy intake with the larger pregnancy population (n = 40 786). The averages of energy consumption as estimated from movement sensor data, 10.02 ± 1.02 MJ day−1, corresponded well with intake means calculated from FDs (9.09 ± 1.35 MJ day−1) and FFQs (9.61 ± 2.43 MJ day−1) for the validation group. The range of FFQ calculated intakes was 5.00–16.67 MJ day−1, while the range of energy expenditures was 7.87–12.88 MJ day−1. The mean FFQ intake also corresponded well with the population mean (9.93 MJ day−1) (n = 40 786), while the standard deviation in the entire study population was somewhat higher, 3.30 MJ day−1. This indicates that the validation group did not fully mirror the variation in the whole population, but the validation group data may still be used for establishing cut‐off points. Figure 2a shows the histogram of the FFQ intakes with a gamma curve fitted by MLE (after translation). Figure 2b shows observed values vs. those expected from the fitted gamma distribution, indicating that the gamma fit is indeed a good one, with no clearly identifiable group of outliers. A 99.8% CI from this curve is 4.52–19.70 MJ day−1, and when 4.5 and 20 MJ day−1 are used as cut‐off points for the population data, we exclude about 1% at each end. The 99.8% CI from the validation group thus becomes a 98% CI for the population. 2, 3 show the intake of the major nutrients and food groups when this cut‐off interval of 4.5–20 MJ day−1 has been applied. With this cut‐off, we omit 278 participants in the low end and 400 participants in the high end of energy intake. In our study, a limit of 15 MJ would exclude about 5.4% of all subjects, and our energy expenditure data indicate that an intake on this level is not improbable. It should be kept in mind that exclusion of subjects with improbable energy intakes will not correct for any misreporting or bias among the included subjects. As in other epidemiological studies, exclusion of dietary reports based on relative misreporting is, generally, not going to be feasible in MoBa, and energy‐adjusted intakes have to be considered when food composition is more appropriate than absolute intakes.
Figure 2.
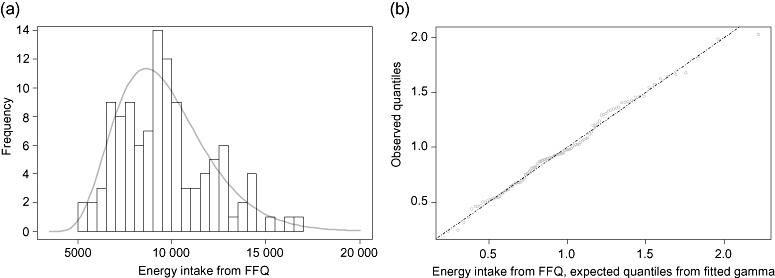
(a) The distribution of energy intakes (kJ/day) calculated from the food frequency questionnaire (FFQ), with gamma distribution fitted by maximum likelihood estimation. For estimation of gamma parameters, numbers were translated by 3000 kJ. This value gives the best fit for the data, and may be considered as a lower limit for the intakes in the ‘validation population’. The intakes in this group had a considerably lower dispersion than the whole MoBa population, the 99.8% confidence interval (CI) in this group roughly corresponding to the 98% CI of the whole population. (b) Plot of observed values vs. expected values from the gamma fitted in Fig. 1a.
Concluding remarks
In this article, we have described our challenges and considerations when given the opportunity to monitor the diet in a large pregnancy cohort in Norway. The data collected by a semi‐quantitative FFQ in MoBa will be used in investigations for decades to come. We therefore knew that, whatever compromises we decided to go for, the possibility of testing some hypotheses would have to be sacrificed on behalf of others. In theory, precision might have been improved by other, more time‐consuming dietary methods, like asking the women to keep a FD, but was ruled out as an option when considering the social setting of the participants, the possibility of validating the individual records and the economy of MoBa. Instead, we decided to obtain as much relevant information as possible out of a FFQ, including dietary components and factors that hitherto have seldom been covered. Our validation study shows that the MoBa FFQ is a valid tool for ranking pregnant women according to dietary intake (Brantsæter et al. 2008). Thus, we hope that we are building a foundation for a number of future studies investigating the impact of diet in pregnancy on fetal development and the health of the child both early and later in life.
Acknowledgements
The authors would like to thank all the women participating in the validation study. We also acknowledge the contribution in the initial phase of the FFQ planning from Kari Almendingen, Lene Frost Andersen and Anette Hjartåker. The study was financially supported by the Research Council of Norway and the European Commission, 6th Framework Programme, Priority 5 on Food Quality and Safety (FOOD Contract No. 016320 Integrated Project), ‘Newborns and Genotoxic Exposure Risk: Development and application of biomarkers of dietary exposure to genotoxic chemicals and of biomarkers of early effects, using mother–child birth cohorts and biobanks (NewGeneris)’. None of the authors had a conflict of interest.
Footnotes
The sample used here includes 40 786 participants who had completed questionnaire 1 and the new MoBa FFQ, using version II of the quality‐assured data files made available for research in March 2006 (Magnus et al. 2006). Anthropometric, sociodemographic and other background variables in the sample are presented in Brantsæter et al. (2008).
References
- Andersen L.F., Solvoll K., Johansson L.R., Salminen I., Aro A. & Drevon C.A. (1999) Evaluation of a food frequency questionnaire with weighed records, fatty acids, and alpha‐tocopherol in adipose tissue and serum. American Journal of Epidemiology 150, 75–87. [DOI] [PubMed] [Google Scholar]
- Black A.E. & Cole T.J. (2001) Biased over‐ or under‐reporting is characteristic of individuals whether over time or by different assessment methods. Journal of the American Dietetic Association 101, 70–80. [DOI] [PubMed] [Google Scholar]
- Blaker B. & Aarsland M. (1989) Mål og vekt for matvarer. Landsforeningen for kosthold og helse: Oslo. [Google Scholar]
- Brantsæter A.L., Haugen M., Hagve T.‐A., Aksnes L., Rasmussen S.E., Julshamn K. et al. (2007a) Self‐reported dietary supplement use is confirmed by biological markers in the Norwegian Mother and Child Cohort Study (MoBa). Annals of Nutrition and Metabolism 51, 146–154. [DOI] [PubMed] [Google Scholar]
- Brantsæter A.L., Haugen M., Rasmussen S.E., Alexander J., Samuelsen S.O. & Meltzer H.M. (2007b) Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutrition 10, 274–283. [DOI] [PubMed] [Google Scholar]
- Brantsæter A.L., Haugen M., Alexander J. & Meltzer H.M. (2008) Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Maternal and Child Nutrition 4, 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.E., Buzzard I.M., Jacobs D.R. Jr, Hannan P.J., Kushi L.H., Barosso G.M. et al. (1996) A food frequency questionnaire can detect pregnancy‐related changes in diet. Journal of the American Dietetic Association 96, 262–266. [DOI] [PubMed] [Google Scholar]
- Byers T. (2001) Food frequency dietary assessment: how bad is good enough? American Journal of Epidemiology 154, 1087–1088. [DOI] [PubMed] [Google Scholar]
- Cade J., Thompson R., Burley V. & Warm D. (2002) Development, validation and utilisation of food‐frequency questionnaires – a review. Public Health Nutrition 5, 567–587. [DOI] [PubMed] [Google Scholar]
- Davey G.K., Spencer E.A., Appleby P.N., Allen N.E., Knox K.H. & Key T.J. (2003) EPIC‐Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat‐eaters and 31 546 non meat‐eaters in the UK. Public Health Nutrition 6, 259–269. [DOI] [PubMed] [Google Scholar]
- Erkkola M., Karppinen M., Javanainen J., Rasanen L., Knip M. & Virtanen S.M. (2001) Validity and reproducibility of a food frequency questionnaire for pregnant Finnish women. American Journal of Epidemiology 154, 466–476. [DOI] [PubMed] [Google Scholar]
- Forsum E., Kabir N., Sadurskis A. & Westerterp K. (1992) Total energy expenditure of healthy Swedish women during pregnancy and lactation. American Journal of Clinical Nutrition 56, 334–342. [DOI] [PubMed] [Google Scholar]
- Godfrey K.M. & Barker D.J. (2001) Fetal programming and adult health. Public Health Nutrition 4, 611–624. [DOI] [PubMed] [Google Scholar]
- Goldberg G.R., Prentice A.M., Coward W.A., Davies H.L., Murgatroyd P.R., Wensing C. et al. (1993) Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. American Journal of Clinical Nutrition 57, 494–505. [DOI] [PubMed] [Google Scholar]
- Halvorsen B.L., Holte K., Myhrstad M.C., Barikmo I., Hvattum E., Remberg S.F. et al. (2002) A systematic screening of total antioxidants in dietary plants. Journal of Nutrition 132, 461–471. [DOI] [PubMed] [Google Scholar]
- Halvorsen B.L., Carlsen M.H., Phillips K.M., Bohn S.K., Holte K., Jacobs D.R. Jr et al. (2006) Content of redox‐active compounds (i.e. antioxidants) in foods consumed in the United States. American Journal of Clinical Nutrition 84, 95–135. [DOI] [PubMed] [Google Scholar]
- Haraldsdottir J., Tjonneland A. & Overvad K. (1994) Validity of individual portion size estimates in a food frequency questionnaire. International Journal of Epidemiology 23, 786–796. [DOI] [PubMed] [Google Scholar]
- Hjartaker A. & Lund E. (1998) Relationship between dietary habits, age, lifestyle, and socio‐economic status among adult Norwegian women. The Norwegian Women and Cancer Study. European Journal of Clinical Nutrition 52, 565–572. [DOI] [PubMed] [Google Scholar]
- Irgens L.M. (2000) The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstetrica et Gynecologica Scandinavica 79, 435–439. [PubMed] [Google Scholar]
- Johansson L., Solvoll K., Opdahl S., Bjorneboe G.E. & Drevon C.A. (1997) Response rates with different distribution methods and reward, and reproducibility of a quantitative food frequency questionnaire. European Journal of Clinical Nutrition 51, 346–353. [DOI] [PubMed] [Google Scholar]
- Johansson L., Solvoll K., Bjorneboe G.E. & Drevon C.A. (1998) Under‐ and over‐reporting of energy intake related to weight status and lifestyle in a nationwide sample. American Journal of Clinical Nutrition 68, 266–274. [DOI] [PubMed] [Google Scholar]
- Kristal A.R., Peters U. & Potter J.D. (2005) Is it time to abandon the food frequency questionnaire? Cancer Epidemiology, Biomarkers and Prevention 14, 2826–2828. [DOI] [PubMed] [Google Scholar]
- Kroes R., Muller D., Lambe J., Lowik M.R., Van Klaveren J., Kleiner J. et al. (2002) Assessment of intake from the diet. Food and Chemical Toxicology 40, 327–385. [DOI] [PubMed] [Google Scholar]
- Lauritsen J. (2005) FoodCalc. Current Version. 1 August. Available at: http://www.ibt.ku.dk/jesper/foodcalc (accessed 5 July 2005).
- Magnus P., Irgens L.M., Haug K., Nystad W., Skjærven R., Stoltenberg C. & the MoBa Study Group (2006) Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). International Journal of Epidemiology 35, 1146–1150. [DOI] [PubMed] [Google Scholar]
- Mark S.D., Thomas D.G. & Decarli A. (1996) Measurement of exposure to nutrients: an approach to the selection of informative foods. American Journal of Epidemiology 143, 514–521. [DOI] [PubMed] [Google Scholar]
- Maurer J., Taren D.L., Teixeira P.J., Thomson C.A., Lohman T.G., Going S.B. et al. (2006) The psychosocial and behavioral characteristics related to energy misreporting. Nutrition Reviews 64, 53–66. [DOI] [PubMed] [Google Scholar]
- Mikkelsen T.B., Osler M. & Olsen S.F. (2006) Validity of protein, retinol, folic acid and n‐3 fatty acid intakes estimated from the food‐frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutrition 9, 771–778. [DOI] [PubMed] [Google Scholar]
- Moore V.M. & Davies M.J. (2005) Diet during pregnancy, neonatal outcomes and later health. Reproduction Fertility and Development 17, 341–348. [DOI] [PubMed] [Google Scholar]
- Mosdøl A., Holmboe‐Ottesen G., Bjørge‐Løken E., Solvoll K., Johansson L. & Thelle D.S. (2000) Contribution of food categories to absolute nutrient intake and between‐person variation within a representative sample of 2677 Norwegian men and women. Norwegian Journal of Epidemiology 10, 25–30. [Google Scholar]
- National Association for Nutrition and Health (2004) Mat på data (a Nutrient Intake Calculation Program), Version 4.0. Landsforeningen for kosthold og helse [National Association of Nutrition and Health].
- National Cancer Institute (2006) Risk Factor Monitoring and Methods: Validation Studies in Pregnant Populations. Version Current 1 August. Available at: http://riskfactor.cancer.gov/tools/children/review/agegroups/pregnancy/ (accessed 25 August 2006).
- Nes M., Frost A.L., Solvoll K., Sandstad B., Hustvedt B.E., Lovo A. et al. (1992) Accuracy of a quantitative food frequency questionnaire applied in elderly Norwegian women. European Journal of Clinical Nutrition 46, 809–821. [PubMed] [Google Scholar]
- Olafsdottir A.S., Skuladottir G.V., Thorsdottir I., Hauksson A. & Steingrimsdottir L. (2006) Maternal diet in early and late pregnancy in relation to weight gain. International Journal of Obesity 30, 492–499. [DOI] [PubMed] [Google Scholar]
- Olsen J., Melbye M., Olsen S.F., Sorensen T.I., Aaby P., Andersen A.M. et al. (2001) The Danish National Birth Cohort – its background, structure and aim. Scandinavian Journal of Public Health 29, 300–307. [DOI] [PubMed] [Google Scholar]
- Overvad K., Tjonneland A., Haraldsdottir J., Ewertz M. & Jensen O.M. (1991) Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. International Journal of Epidemiology 20, 900–905. [DOI] [PubMed] [Google Scholar]
- Parra M.S., Schnaas L., Meydani M., Perroni E., Martinez S. & Romieu I. (2002) Erythrocyte cell membrane phospholipid levels compared against reported dietary intakes of polyunsaturated fatty acids in pregnant Mexican women. Public Health Nutrition 5, 931–937. [DOI] [PubMed] [Google Scholar]
- Poppitt S.D., Swann D., Black A.E. & Prentice A.M. (1998) Assessment of selective under‐reporting of food intake by both obese and non‐obese women in a metabolic facility. International Journal of Obesity and Related Metabolic Disorders 22, 303–311. [DOI] [PubMed] [Google Scholar]
- Rifas‐Shiman S.L., Rich‐Edwards J.W., Willett W.C., Kleinman K.P., Oken E. & Gillman M.W. (2006) Changes in dietary intake from the first to the second trimester of pregnancy. Paediatric and Perinatal Epidemiology 20, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimestad A.H., Borgejordet Å., Vesterhus K.N., Sygnestveit K., Løken E.B., Trygg K. et al. (2001) Den store matvaretabellen. Gyldendal: Oslo. [Google Scholar]
- Robinson S., Godfrey K., Osmond C., Cox V. & Barker D. (1996) Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. European Journal of Clinical Nutrition 50, 302–308. [PubMed] [Google Scholar]
- Schulze M.B., Manson J.E., Ludwig D.S., Colditz G.A., Stampfer M.J., Willett W.C. et al. (2004) Sugar‐sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle‐aged women. Journal of the American Medical Association 292, 927–934. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A.M., Savitz D.A., Zeisel S.H., Thorp J.M. & Herring A. (2004) Second trimester folate status and preterm birth. American Journal of Obstetrics and Gynecology 191, 1851–1857. [DOI] [PubMed] [Google Scholar]
- Solvoll K., Lund‐Larsen K., Søyland E., Sandstad B. & Drevon C.A. (1993) A quantitative food frequency questionnaire evaluated in a group of dermatologic outpatients. Scandinavian Journal of Nutrition 37, 150–155. [Google Scholar]
- Statistics Norway (2005) Statistics Norway, 1 January 2005. Available at: http://www.ssb.no
- Statistics Norway (2006) Available at: http://www.ssb.no
- Stram D.O., Longnecker M.P., Shames L., Kolonel L.N., Wilkens L.R., Pike M.C. et al. (1995) Cost‐efficient design of a diet validation study. American Journal of Epidemiology 142, 353–362. [DOI] [PubMed] [Google Scholar]
- Subar A.F., Thompson F.E., Kipnis V., Midthune D., Hurwitz P., McNutt S. et al. (2001) Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. American Journal of Epidemiology 154, 1089–1099. [DOI] [PubMed] [Google Scholar]
- Susser M. & Stein Z. (1994) Timing in prenatal nutrition: a reprise of the Dutch Famine Study. Nutrition Review 52, 84–94. [DOI] [PubMed] [Google Scholar]
- Thompson F.E., Midthune D., Subar A.F., Kahle L.L., Schatzkin A. & Kipnis V. (2004) Performance of a short tool to assess dietary intakes of fruits and vegetables, percentage energy from fat and fibre. Public Health Nutrition 7, 1097–1105. [DOI] [PubMed] [Google Scholar]
- Tjonneland A., Overvad K., Haraldsdottir J., Bang S., Ewertz M. & Jensen O.M. (1991) Validation of a semiquantitative food frequency questionnaire developed in Denmark. International Journal of Epidemiology 20, 906–912. [DOI] [PubMed] [Google Scholar]
- De Vries J.H., Zock P.L., Mensink R.P. & Katan M.B. (1994) Underestimation of energy intake by 3‐d records compared with energy intake to maintain body weight in 269 nonobese adults. American Journal of Clinical Nutrition 60, 855–860. [DOI] [PubMed] [Google Scholar]
- Willett W. (1998) Nutritional Epidemiology, 2nd edn Oxford University Press: New York. [Google Scholar]
- Willett W.C. & Hu F.B. (2006) Not the time to abandon the food frequency questionnaire: point. Cancer Epidemiology, Biomarkers and Prevention 15, 1757–1758. [DOI] [PubMed] [Google Scholar]


