Abstract
Increasing concern has been recently raised on the possible effects of soy‐derived phyto‐oestrogens on the development of cognitive functions in infants. However, limited studies have been conducted to date, and no data have been made available for determining whether infant soy formula can affect normal development of the human brain. We compared electroencephalographic (EEG) spectral power derived from high‐density recordings of infants fed milk‐based or soy formula (46 fed milk‐based formula and 39 fed soy formula) at 3 and 6 months of age. The spectral parameters included absolute power, relative power and spectral edge frequency (SEF) at 85%, 90% and 95% levels. The frequency domain contained four bands (0.1–3, 3–6, 6–9 and 9–12 Hz). EEG signals were collected from eight brain areas in each hemisphere. The results showed that the highest spectral power was mainly distributed in the low‐frequency bands and was predominant in the frontal and anterior temporal areas. None of the spectral variables significantly differed between the soy‐ and milk‐fed infants (anova, all P > 0.2). However, significant effects were indicated on the SEFs for factors of sex, age and brain area (all P < 0.01). Hemispheric differences in the absolute and relative power were also indicated. Our results suggest that the EEG power spectral development of soy‐fed infants does not differ from that of infants fed milk‐based formula. In addition, EEG spectral development appears more advanced in female than in male infants at 6 months.
Keywords: electroencephalography, scalp distribution, spectral analysis
Introduction
Soy‐based infant formulas have been used for more than 70 years and were originally developed for infants who are unable to tolerate milk‐based infant formulas. Approximately 25% of all formulas sold in the USA are soy‐based (AAP Committee on Nutrition 1998). In the past decade, it has been estimated that about 18% of all infants born in the USA were fed soy formula during some period of the first year of life (Essex 1996). Several important modifications have been made in soy formulas since their introduction, making the nutrient composition similar to that of milk formulas (Venkataraman et al. 1992).
Soy formula contains many phytochemicals which are bioactive. For example, soy isoflavones are present in high concentrations in soy formulas and, under certain conditions, may have weak oestrogenic activity. Soy‐fed infants have circulating isoflavone concentrations about 13 000‐ to 22 000‐fold higher than endogenous oestrogens (Winter et al. 1976;Setchell et al. 1997;Setchell et al. 1998), and although the potency has been reported to be 1000–40 0000 times less than oestradiol, this concentration may be sufficient to affect infant health. This potential oestrogenic activity is at the root of a debate regarding the safety of soy formulas (Irvine et al. 1998; Chen & Rogan 2004).
Another concern is based on the oestrogen‐induced sexual differentiation of the brain. During very early development, brain differentiation is sexually dimorphic. Examples of nuclei sensitive to hormones include the sexually dimorphic nucleus of the preoptic area and the locus coeruleus in rodents (Dunn et al. 2004), and similar brain sexual dimorphism has been identified in humans (Swaab & Fliers 1985; Giedd et al. 1997). Critics suggest that soy formula may carry the risk of interrupting hormone‐mediated morphological and neural functional changes at critical developmental periods, and thus may cause long‐term modifications in physiology and behaviour. To date, such effects have not been observed in the more than 20 million infants fed soy formula, but a paucity of data exists with respect to the effects of soy formula on brain physiological functions.
Electroencephalography (EEG) is a commonly used and relatively sensitive, but non‐specific, measure of maturation of brain electrical activity which has been linked to brain development. For example, variations in EEG absolute or relative spectral power have been used to distinguish between normal and abnormal development (Gasser et al. 2003). Spectral edge frequency (SEF) is the frequency point that delimits a certain per cent of total power. It has been explained at various levels (Myers et al. 1993; Reulen et al. 1999; Nijland & Ross 2000; Pandin 2004) and used for monitoring brain physiological functions. SEF is considered to be more sensitive to the changes in EEG amplitude than conventional amplitude analysis (Inder et al. 2003). A decrease in SEF has been linked to delayed brain maturation and functional impairment (Victor et al. 2005). In a study investigating preterm infants with white matter injury, SEF was found useful in detecting and predicting the severity of brain injury (Inder et al. 2003).
The aim of this study was to determine whether brain electrical activity of infants fed soy formula differed from that of infants fed milk‐based formula by using power spectral analysis as an objective measure of resting EEGs at 3 and 6 months of age. The power spectral characteristics were compared from three different aspects: absolute power (μV), relative power and SEF. In this study, SEFs were calculated at 85%, 90% and 95% levels. Differences on these spectral variables (i.e. spectral power and SEFs) between soy formula‐fed and milk‐based formula‐fed infants would suggest a dietary influence on brain development. A male–female difference in spectral characteristics could reflect the influence of endogenous oestrogens, soy‐derived phyto‐oestrogens, or the interaction of these compounds.
Material and methods
Participants
Eighty‐five infants for whom acceptable EEG data were collected at 3 and 6 months of age were selected from a large pool of participants who had been recruited in an ongoing longitudinal, prospective project investigating long‐term health and developmental consequences of early infant diet. The criteria for inclusion in this study, along with demographic data, are summarized in Table 1. Each infant was healthy, full‐term, and had an unremarkable medical history. Their growth was assessed by measuring the head circumference, weight, length, motor milestones and mental development at each study visit. Parents selected formula type (milk‐based or soy). All infants received a commercially available infant formula containing docosahexaenoic acid. Prior to participation, informed consent was obtained from parents. The protocol of this study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences.
Table 1.
Inclusion criteria and demographic data
| Inclusion criteria |
| Healthy full‐term infants |
| Weight at birth >2700 g |
| Must have been on the same type of formula (milk‐based or soy formula) for at least 1 month before the first study visit at 3 months |
| Must take no additional nourishment except water through the ages of 4 months |
| No medical diagnosis at birth to affect normal growth and development |
| No significant intake of soy food by mothers during pregnancy or lactation |
| No alcohol, tobacco or drug used by mothers during pregnancy or lactation |
| Demographic data |
| Gestation age: 39 ± 1 weeks |
| Birthweight: 3457 ± 437 g |
| Birth length: 51 ± 3 cm |
| Number of infants in the milk formula group |
| Male: 24 |
| Female: 22 |
| Number of infants in the soy formula group |
| Male: 2 |
| Female: 17 |
EEG recordings
Resting EEG signals were recorded in the morning while infants were awake with eyes open. The infants were seated in a dimly lighted, electrically shielded and sound‐attenuated testing room. The same recording condition would be used in several follow‐up study visits. The resting EEGs obtained in this study served as a baseline measure of brain activity preceding other paradigms (such as auditory‐evoked potentials). To keep the whole study visit period short, only 5‐min EEG signals were recorded from each subject.
The signals were recorded from 124 electrode sites on the scalp (Electrical Geodesic Inc., Eugene, OR, USA). Eye movement was detected by electrodes placed at the outer canthi of both eyes, and above and below one eye. The bandpass of the analogue filter was 0.1–100 Hz, with a sampling rate of 250 Hz. Offline, the signals were re‐referenced to the mean of those from both mastoids. Signal segments contaminated with movement artefacts were excluded. For the spectral analyses, artefact‐free and non‐overlapping EEG segments were analysed. Each segment contained 2048 data points (about 8 s).
Measurements and statistics
Approximately 6–8 non‐overlapping segments were obtained from each EEG record for the spectral analysis. These segments were determined by scanning the entire EEG record. First, about 580 segments were randomly selected. The onset time between any two segments differed for ≥0.5 s (note that many of these segments overlapped at this time). EEG segments were screened for artefacts from the 1st through the 124th channel. Segments containing artefacts in multiple channels were rejected. After the initial screening, all EEG segment candidates were examined again to exclude all overlapping segments. Initially, records from 151 infants were examined; 66 subjects were dropped because of either excessive artefacts or limited numbers of useful EEG segments at either 3 or 6 months.
A standard Fast Fourier Transformation was applied to estimate EEG power spectra. Linear trends of signals were removed before calculation. For each infant, the mean spectral power was calculated from all the segments. Grand averaged spectral power was then obtained from all the individuals. Visual inspection of the power spectra revealed that EEG power predominantly localized in the low‐frequency range. Because the curve of spectral power above 12 Hz was relatively flat and less variable, our analyses focused on the spectral power below 12 Hz. This frequency range was divided into four bands: 0.1–3, 3–6, 6–9 and 9–12 Hz. The absolute power for each band was calculated as the mean power per Hz. The relative power was calculated as follows: (sum of spectral power in the band)/(sum of spectral power in all bands). In this study, SEF was defined as the frequency delimiting 85% (SEF85), 90% (SEF90) and 95% (SEF95) of the total power. Mean values were calculated from groups of electrodes placed in the same general brain area (i.e. in both the left and right hemispheres: prefrontal, frontal, central, parietal, occipital, anterior temporal, mid‐temporal and posterior temporal areas). These results were grouped because it was less practical to include all the 124 channels in one statistical evaluation. In addition, we were more interested in spontaneous brain activity at the regional level rather than at individual sites.
Differences in spectral power were examined in a mixed‐type analysis of variance (anova) using statistica (StatSoft, Tulsa, OK, USA) software. In this statistical model, factors investigated include both independent and dependent variables (http://www.statsoft.com for online documents). The independent factors (i.e. non‐repeated measures) included dietary group and sex. The dependent factors (i.e. repeated measures) included age, brain area, hemisphere and frequency band. The data were examined by two series of analyses. In one set of evaluations, all the factors were included to determine the main effects and interactions among the factors. Because EEG spectral power varies as a function of frequency band, to determine the effects on band‐specific EEG spectral power, a series of anova tests were performed – one for each frequency band. In addition to these analyses, the main effects and interactions were also visually examined. Post‐hoc tests were used for detecting differences between individual means.
The above analyses were applied to the measures of absolute power and relative power. A similar procedure was used for SEF85, SEF90 and SEF95. Before the anova tests, the histogram of each variable was inspected and Kolmogorov‐Smirov tests were performed to determine whether anova requirements regarding normal distributions were met. Other descriptive parameters, such as means and standard deviations, were calculated for each variable. Greenhouse–Geisser adjustments were performed when applicable. Significance was set at P < 0.05.
Results
The Kolmogorov‐Smirov tests showed normal distributions for all the estimated parameters (P > 0.05). Visual examination of these power spectra revealed several features common in all participants: (1) the highest power was present in low‐frequency bands (below 6 Hz at 3 months and 9 Hz at 6 months); (2) the anterior brain regions (frontal, central and anterior temporal areas) appeared to have higher spectral power than remaining brain areas; (3) a marked increase in 3‐ to 6‐Hz spectral power occurred at 6 months relative to 3 months; and (4) no apparent differences in power spectra were visually observed between the two groups of infants. This observation was further confirmed by the anova examination, in which significant effects were not detected between dietary groups for the absolute power (F = 0.054, P = 0.82), relative power (F = 1.4, P = 0.24), SEF85 (F = 0.098, P = 0.75), SEF90 (F = 0.20, P = 0.66), or SEF95 (F = 0.66, P = 0.42). Moreover, no group difference was indicated for any of the four frequency bands (for absolute power: F = 0.26, 0.18, 1.33 and 0.02, respectively; for relative power: F = 0.77, 0.82, 1.04 and 0.33, respectively; all P > 0.2). Because no interaction effects were found between group and other factors (e.g. sex, age, brain area, hemisphere and frequency band), the data from both groups were pooled in the further analyses. Examples of EEG power spectra recorded from the two infant groups are presented in Fig. 1.
Figure 1.
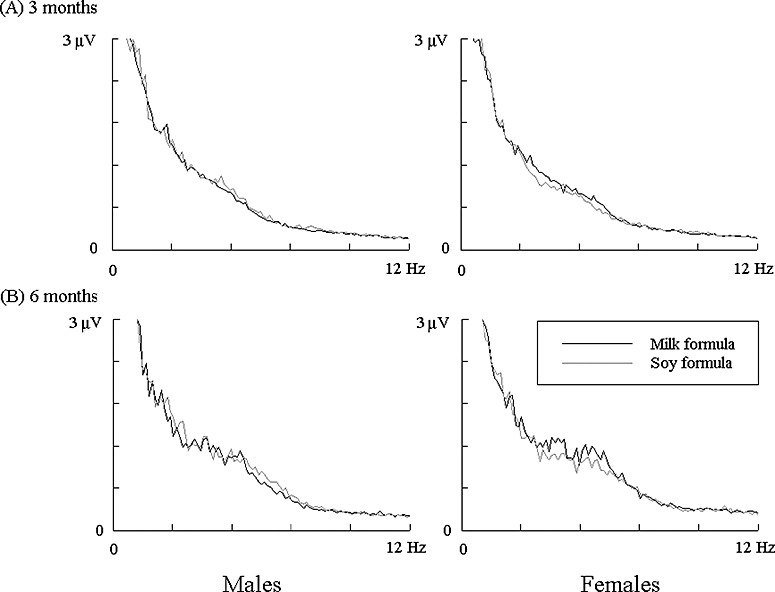
Average power spectra of resting EEG at 3 months (A) and 6 months (B) recorded from milk formula‐fed and soy formula‐fed infants. The results demonstrated were obtained in the left frontal area. EEG, electroencephalography.
The main effects and between‐factor interactions are summarized in Table 2. For absolute power, a significant effect was detected for age (P < 0.05), showing that the spectral power at 6 months was higher in general as compared with that at 3 months. Spectral power varied among the brain areas (P < 0.01). In general, the anterior brain regions (including prefrontal, frontal, central and anterior temporal areas) showed higher power than other brain areas. The left hemisphere had higher spectral power than the right hemisphere (P < 0.01). Post‐hoc analyses revealed that this effect was mainly determined by the band of 0.1–3 Hz (see Fig. 2). The highest spectral power was seen in the low‐frequency band, i.e. <6 Hz (P < 0.01). Further, the effect of brain area was modified by sex (P < 0.05); that is, while the spectral power in the anterior part of brain was similar for boys and girls, girls had lower mean spectral power in the parietal and occipital areas than the boys. Other interaction effects included those between age and brain area (P < 0.01, showing greater increase in spectral power from 3 to 6 months in the anterior part of the brain than in the occipital and posterior temporal areas), between age and frequency band (P < 0.01, showing that the increase in the spectral power from 3 to 6 months was greater in 3–6 Hz relative to other bands), and between brain area and frequency band (P < 0.01, with the greatest power spectral changes among brain areas in 0.1–3 Hz). See Table 2 for additional interaction effects detected from these power spectral parameters.
Table 2.
Effects of sex, age, brain area, hemisphere and frequency band on spectral parameters †
| Absolute power | Relative power | SEF85 | SEF90 | SEF95 | |
|---|---|---|---|---|---|
| Sex (F1) | n.s. | n.s. | 7.74** | 8.69** | 8.31** |
| Age (F2) | 43.4* | n.s. | 62.7** | 27.8** | 30.1** |
| Brain area (F3) | 619** | n.s. | 6.41** | 25.4** | 57.3** |
| Hemisphere (F4) | 25.0** | 9.15** | n.s. | n.s. | n.s. |
| Frequency band (F5) | 2482** | 7737** | |||
| F1 × F2 | n.s. | n.s. | n.s. | n.s. | n.s. |
| F1 × F3 | 2.43* | n.s. | n.s. | n.s. | n.s. |
| F1 × F4 | n.s. | n.s. | n.s. | n.s. | n.s. |
| F1 × F5 | n.s. | n.s. | |||
| F2 × F3 | 29.2** | n.s. | 17.8** | 19.7** | 13.7** |
| F2 × F4 | n.s. | n.s. | n.s. | n.s. | n.s. |
| F2 × F5 | 7.56** | 78.1** | |||
| F3 × F4 | 3.89** | n.s. | 5.63** | 11.2** | 17.5** |
| F3 × F5 | 349** | 65.6** | |||
| F4 × F5 | 12.3** | n.s. |
SEF, spectral edge frequency. n.s. means that the effect is not significant. *P < 0.05; **P < 0.01. †The data are the F‐values calculated in the analysis of variance. Blank entries indicate that the corresponding effect was not applicable to that variable. Effects of all the two‐factor interactions are reported.
Figure 2.
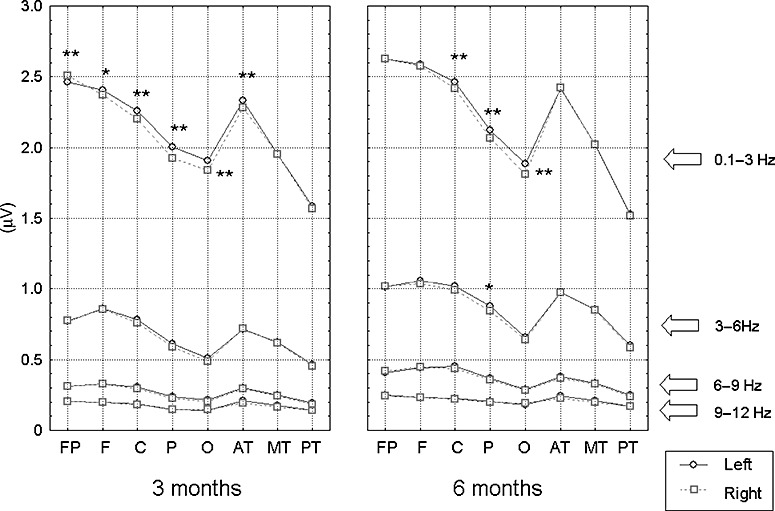
Comparisons of mean EEG spectral power estimated in four frequency bands. *P < 0.05; **P < 0.01. EEG, electroencephalography.
However, most of these effects and interactions were not present in the relative power analyses. The only significant effects for this measure were for hemisphere (P < 0.01) and frequency band (P < 0.01). The interaction effect between brain area and frequency band was significant (P < 0.01), showing that both the highest and lowest mean relative power was in the occipital areas, but in different frequency bands – the highest in 0.1–3 Hz and the lowest in 3–6 Hz.
anova results obtained from each frequency band are shown in Table 3. Again, the absolute power of male infants did not differ from that of the female infants (P > 0.05). However, the results significantly varied topographically in each frequency band (P < 0.05). Similar results were observed for the relative power. A between‐sex difference was found in the bands of 6–9 and 9–12 Hz (F = 8.89 and 5.87, respectively; P < 0.05; see Table 3).
Table 3.
Effects of sex, age, brain area and hemisphere in each frequency band †
| Absolute power (Hz) | Relative power (Hz) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.1–3 | 3–6 | 6–9 | 9–12 | 0.1–3 | 3–6 | 6–9 | 9–12 | |
| Sex (F1) | 8.89** | 5.87** | ||||||
| Age (F2) | 6.31* | 60.4** | 259** | 152** | 109** | 27.8** | 150** | 28.0** |
| Brain area (F3) | 775** | 654** | 676** | 387** | 69.8** | 123** | 65.4** | 77.5** |
| Hemisphere (F4) | 33.1** | 31.4** | 26.6** | 4.68* | ||||
| F1 × F2 | 4.03* | 6.07* | ||||||
| F1 × F3 | 2.99** | 4.38* | 4.25** | |||||
| F1 × F4 | ||||||||
| F2 × F3 | 21.1** | 33.9** | 79.0** | 18.4** | 7.77** | 10.8** | 29.6** | 23.4** |
| F2 × F4 | ||||||||
| F3 × F4 | 2.95** | 6.40** | 5.60** | 13.0** | 2.78** | 2.83** | 2.30* | 19.5** |
P < 0.05;
P < 0.01.
The data are the F‐values calculated in the analysis of variance. Blank entries represent non‐significance.
A sex effect was observed in the SEF data at all three levels (all P < 0.01). Post‐hoc tests revealed that female infants had higher SEFs than male infants in the parietal region at 3 months and in all brain areas at 6 months of age (P < 0.05, see Fig. 3).
Figure 3.
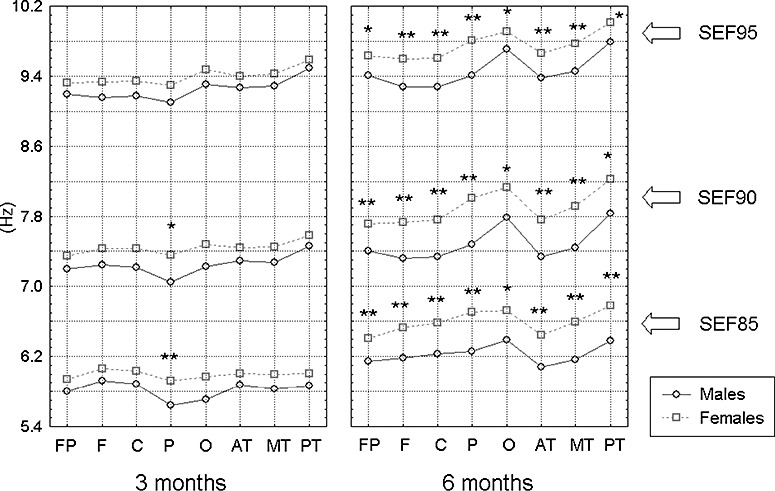
Differences in the spectral edge frequencies between male and female infants at 3 and 6 months of age. Only the results from the right hemisphere are demonstrated. The data were averaged for each group. *P < 0.05; **P < 0.01.
Although there were hemispheric effects in absolute and relative spectral power measures, there were no hemispheric differences for any of the three SEF levels.
The SEF values increased from 3 to 6 months (P < 0.01), and varied among the brain areas (P < 0.01). Post‐hoc tests indicated that, as compared with the SEF results at 3 months, these variables remarkably increased in the occipital and posterior temporal areas at 6 months in both male and female infants (see Fig. 3). However, the brain area effect was modified by hemisphere (P < 0.01), showing that the hemispheric differences were greater in the parietal and occipital areas than in other brain areas, and the SEFs were higher in the right parietal and occipital areas than in the left side.
Discussion
There has been limited investigation into the relationship between soy formula intake and brain development and/or brain function, and to our knowledge, this is the first longitudinal study to investigate the impact of soy formula feeding on neurophysiological functions in infants. Because brain function is known to be affected by gonadal oestrogens, differences in the EEG power spectrum might be expected in infants who have high circulating levels of the potentially oestrogenic soy isoflavones compared with infants fed milk‐based formula. If such concentration‐dependent effects are present, they might be expected to be most evident during the first half year after birth, when there is maximal intake of formula, the circulating isoflavone levels are highest, and infants are just beginning to be weaned to solid food.
To detect possible diet‐related effects on the development of brain electrical activity, we used an array of measures derived from spectral analyses of EEG activity, including: absolute power, which provides an estimate of the amplitude of neural activity; relative power, which describes the distribution of spectral power across frequency bands; and SEF, the frequency point below which a specified amount of power is present.
One of the important findings of this study is the absence of evidence in support of the contention that soy formula may adversely affect brain development or neurophysiological functions as compared with milk‐based formula (at least at 3 and 6 months of age). Similar robust EEG spectral characteristics were observed in infants fed soy formula and in those fed milk‐based formula for all the spectral parameters investigated, indicating that the development of brain physiological functions as reflected by these measures may not differ between these two infant groups.
It is possible that soy formula‐induced brain effects are too subtle for the present methods to detect. Another possibility is that there may be a lag between the exposure and physiological effects in terms of brain function alterations; that is, although no effects were observed in the present study, physiological effects of soy formula could occur in later childhood and preadolescence. The longitudinal nature of this study makes it possible to address this consideration because these subjects will be followed until they reach puberty.
One of the possible limitations of this study is the relatively small sample size of the dietary groups. All the 85 infants had a relatively sufficient amount of artefact‐free EEG segments (mean: 6–8) to be included for power spectral analysis. We anticipate the extension of the present conclusions in future reports with larger sample sizes as more subjects are recruited into this ongoing investigation. There is another solution that may be used for increasing the sample size – to clean the existing EEG data to produce more useful EEG segments. One technique is to remove channels that are contaminated with artefacts, and then interpolate those signals. However, this will result in correlation between signals. Thus, the reliability of this technique remains to be determined.
One of the most extensive studies of the long‐term effects of soy formula on health outcomes was reported by Strom et al. (2001). In that study, adults (n = 248) who as infants were fed soy formula were compared with adults (n = 563) who were fed milk‐based formula as infants. This retrospective study employed a questionnaire to obtain health outcome data for more than 30 variables (including pubertal maturation, menstrual and reproductive history, height and usual weight, and current health), and revealed no significant differences between the groups in either women or men. The authors concluded that exposure to soy formula may not lead to general health or reproductive outcomes different from exposure to milk formula.
There have been few studies documenting age‐based sex‐related brain physiological differences in infancy using EEG power spectral analysis. The current study found that a sex difference is strongly indicated in all the brain areas by the SEF parameters at 6, but not at 3 months of age. Female infants generally showed higher SEFs than male infants across levels. These results suggest that female infants have greater spectral power in the higher‐frequency range than their male counterpart, and the male infants have greater spectral power in the lower‐frequency range than female infants. Considering the relationship between SEF and brain functional maturation (Victor et al. 2005), our data suggest that female infants appear to be more advanced than their male counterparts in EEG development at 6 months of age. A paper reporting a sex effect on human EEG activity has been published recently (Thordstein et al. 2006). In that study, full‐term neonates (10 boys and 10 girls) were examined and EEG spectral power was obtained during quiet sleep and wakefulness stages. The investigators found that, during sleep, the frequency activity in the range of 0–0.5 Hz was the most prominent, and the spectral power was higher in boys than in girls; however, this between‐sex difference was absent when the neonates were awake. Their results indicated an earlier maturation in girls than in boys. Our present data show that the advance in EEG spectral power in girls may remain at least until 6 months of age. Sex‐related differences have also been reported in studies investigating auditory brainstem responses. It was found that female newborns have shorter V‐wave latencies than male newborns (Stuart & Yang 2001). Similarly, Pravitha et al. (2005) reported a difference on linear complexity of adult EEG between male and female individuals. Our results generally parallel those previous findings.
Additional developmental changes in EEG power spectra seen in this study include: (1) an increase of mean spectral power in the higher‐frequency bands (>3 Hz) at 6 months, particularly in the band of 3–6 Hz; and (2) in both male and female infants, the SEFs were lowest in parietal areas and the highest in occipital areas at 3 months of age. But at 6 months, the SEFs in the occipital areas increased markedly, with the lowest SEF values in the frontal and anterior temporal areas. These results indicate a greater increase of spectral power in the high‐frequency range in the posterior part of the brain with development – findings generally consistent with previous results (Epstein 1980; Ishiwa et al. 1991; Mandelbaum et al. 2000). In one early longitudinal study, Benninger et al. (1984) followed EEG development of 96 healthy children (47 boys and 49 girls) for up to 7 years, and found that the power spectral changes in the occipital areas were nearly twice as great as those in the central areas, particularly in girls. Their study also showed that girls under 6 years of age have more theta, but less alpha, power than boys, and the spectral changes per year remain higher in girls than in boys for a long period.
Dynamic changes of SEFs in infants during the first 6 months have been investigated in only a few studies. The normal range of 95% SEF in premature neonates was reported by Victor et al. (2005). As far as we know, normal ranges of SEF have not been determined for infants at 3 and 6 months of age. The SEF values estimated in our samples are comparable to early results obtained from healthy neonates (Bell et al. 1991; Inder et al. 2003; Thordstein et al. 2004). For example, Bell et al. (1991) reported that the mean SEF (95%) computed in a group of full‐term (37–41 weeks) healthy neonates was in a range of about 10–14 Hz at 3 days of age. These findings were also reported in another study based on the infants with similar gestation age (Victor et al. 2005).
In summary, EEG power spectral characteristics were measured in infants fed soy formula or milk‐based formula. Our results showed that the EEG spectral power of soy‐fed infants does not differ from that of infants fed milk‐based formula at 3 and 6 months of age. Whether other more subtle effects on brain function occur in infants fed soy formula must await further research. Another interesting and potentially important finding of this study is that female infants seem to be more advanced in EEG power spectral development than male infants at 6 months. The short‐ and long‐term consequences of these latter effects are not apparent at this time.
Acknowledgements
This work was supported by USDA CRIS 6251‐51000‐004‐01S.
References
- American Academy of Pediatrics (AAP) Committee on Nutrition (1998) Soy protein‐based formulas: recommendations for use in infant feeding. Pediatrics 101, 148–153. [PubMed] [Google Scholar]
- Bell A.H., McClure B.G., McCullagh P.J. & McClelland R.J. (1991) Spectral edge frequency of the EEG in healthy neonates and variation with behavioural state. Biology of the Neonate 60, 69–74. [DOI] [PubMed] [Google Scholar]
- Benninger C., Matthis P. & Scheffner D. (1984) EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalography and Clinical Neurophysiology 57, 1–12. [DOI] [PubMed] [Google Scholar]
- Chen A. & Rogan W.J. (2004) Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annual Review of Nutrition 24, 33–54. [DOI] [PubMed] [Google Scholar]
- Dunn A.J., Swiergiel A.H. & Palamarchouk V. (2004) Brain circuits involved in corticotropin‐releasing factor–norepinephrine interactions during stress. Annals of the New York Academy of Sciences 1018, 25–34. [DOI] [PubMed] [Google Scholar]
- Epstein H.T. (1980) EEG developmental stages. Developmental Psychobiology 13, 629–631. [DOI] [PubMed] [Google Scholar]
- Essex C. (1996) Phytoestrogen and soy based infant formula. British Medical Journal 313, 507–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T., Rousson V. & Schreiter Gasser U. (2003) EEG power and coherence in children with educational problems. Journal of Clinical Neurophysiology 20, 273–282. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Castellanos F.X., Rajapakse J.C., Vaituzis A.C. & Rapoport J.L. (1997) Sexual dimorphism of the developing human brain. Progress in Neuropsychopharmacology and Biological Psychiatry 21, 1185–1201. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Buckland L., Williams C.E., Spencer C., Gunning M.I., Darlow B.A. et al. (2003) Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants. Pediatrics 111, 27–33. [DOI] [PubMed] [Google Scholar]
- Irvine C.H., Fitzpatrick M.G. & Alexander S.L. (1998) Phytoestrogens in soy‐based infant foods: concentrations, daily intake, and possible biological effects. Proceedings of the Society for Experimental Biology and Medicine 217, 247–253. [DOI] [PubMed] [Google Scholar]
- Ishiwa S., Ogawa T. & Sonoda H. (1991) Developmental characteristics of topographic EEG in the newborn using an autoregressive model. Brain Topography 4, 23–30. [DOI] [PubMed] [Google Scholar]
- Mandelbaum D.E., Krawciw N., Assing E., Ostfeld B., Washburn D., Rosenfeld D. et al. (2000) Topographic mapping of brain potentials in the newborn infant: the establishment of normal values and utility in assessing infants with neurological injury. Acta Paediatrica 89, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Myers M.M., Stark R.I., Fifer W.P., Grieve P.G., Haiken J., Leung K. et al. (1993) A quantitative method for classification of EEG in the fetal baboon. American Journal of Physiology 265(3 Pt 2), R706–R714. [DOI] [PubMed] [Google Scholar]
- Nijland M.J. & Ross M.G. (2000) Ovine hourly fetal urine production: relation to fetal electrocortical activity. Journal of Maternal Fetal Medicine 9, 267–272. [DOI] [PubMed] [Google Scholar]
- Pandin P.C. (2004) Can electrophysiological assessments of brain function be useful to the intensive care physician in daily clinical practice? Critical Care 8, 437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravitha R., Sreenivasan R. & Nampoori V.P. (2005) Complexity analysis of dense array EEG signal reveals sex difference. International Journal of Neuroscience 115, 445–460. [DOI] [PubMed] [Google Scholar]
- Reulen J.P., Gavilanes A.W., Van Mierlo D., Blanco C., Spaans F. & Vles J.S. (1999) The Maastricht Cerebral Monitor (MCM) for the neonatal intensive care unit. Journal of Medical Engineering and Technology 23, 29–37. [DOI] [PubMed] [Google Scholar]
- Setchell K.D., Zimmer‐Nechemias L., Cai J. & Heubi J.E. (1997) Exposure of infants to phyto‐oestrogens from soy‐based infant formula. Lancet 350, 23–27. [DOI] [PubMed] [Google Scholar]
- Setchell K.D., Zimmer‐Nechemias L., Cai J. & Heubi J.E. (1998) Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. American Journal of Clinical Nutrition 68(6 Suppl.), 1453S–1461S. [DOI] [PubMed] [Google Scholar]
- Strom B.L., Schinnar R., Ziegler E.E., Barnhart K.T., Sammel M.D., Macones G.A. et al. (2001) Exposure to soy‐based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 286, 807–814. [DOI] [PubMed] [Google Scholar]
- Stuart A. & Yang E.Y. (2001) Gender effects in auditory brainstem responses to air‐ and bone‐conducted clicks in neonates. Journal of Communication Disorders 34, 229–239. [DOI] [PubMed] [Google Scholar]
- Swaab D.F. & Fliers E. (1985) A sexually dimorphic nucleus in the human brain. Science 228, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Thordstein M., Flisberg A., Lofgren N., Bagenholm R., Lindecrantz K., Wallin B.G. et al. (2004) Spectral analysis of burst periods in EEG from healthy and post‐asphyctic full‐term neonates. Clinical Neurophysiology 115, 2461–2466. [DOI] [PubMed] [Google Scholar]
- Thordstein M., Lofgren N., Flisberg A., Lindecrantz K. & Kjellmer I. (2006) Sex differences in electrocortical activity in human neonates. Neuroreport 17, 1165–1168. [DOI] [PubMed] [Google Scholar]
- Venkataraman P.S., Luhar H. & Neylan M.J. (1992) Bone mineral metabolism in full‐term infants fed human milk, cow milk‐based, and soy‐based formulas. American Journal of Diseases of Children 146, 1302–1305. [DOI] [PubMed] [Google Scholar]
- Victor S., Appleton R.E., Beirne M., Marson A.G. & Weindling A.M. (2005) Spectral analysis of electroencephalography in premature newborn infants: normal ranges. Pediatric Research 57, 336–341. [DOI] [PubMed] [Google Scholar]
- Winter J.S., Hughes I.A., Reyes F.I. & Faiman C. (1976) Pituitary‐gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. Journal of Clinical Endocrinology and Metabolism 42, 679–686. [DOI] [PubMed] [Google Scholar]


