Abstract
The European Micronutrient Recommendations Aligned (EURRECA) Network aims to provide standardized approaches to reveal and beneficially influence variability within the European Union in micronutrient recommendations for vulnerable population groups. Characterization of the ‘vulnerability’ together with the ‘variability’ of micronutrient needs represents the first step to creating guidelines for setting micronutrient recommendations within target populations. This paper describes some of the key factors and characteristics relevant to assess micronutrient requirements and formulate recommendations of micronutrients in pregnancy. Nutritional requirements during pregnancy increase to support fetal growth and development as well as maternal metabolism and tissue accretion. Micronutrients are involved in both embryonal and fetal organ development and overall pregnancy outcomes. Several factors may affect directly or indirectly fetal nourishment and the overall pregnancy outcomes, such as the quality of diet including intakes and bioavailability of micronutrients, maternal age, and the overall environment. The bioavailability of micronutrients during pregnancy varies depending on specific metabolic mechanisms because pregnancy is an anabolic and dynamic state orchestrated via hormones acting for both redirection of nutrients to highly specialized maternal tissues and transfer of nutrients to the developing fetus. The timing of prenatal intakes or supplementations of specific micronutrients is also crucial as pregnancy is characterized by different stages that represent a continuum, up to lactation and beyond. Consequently, nutrition during pregnancy might have long‐lasting effects on the well‐being of the mother and the fetus, and may further influence the health of the baby at a later age.
Keywords: EURRECA, pregnancy, vulnerability, recommendation, requirement, bioavailability, dietary factors, micronutrient intakes
Background
Nutrient recommendations are part of the basis for food policy and food‐based dietary guidelines, and are used in nutrition labelling. The historical development of the concept of dietary recommendations for populations or groups has been reported by Aggett et al. (1997). This evolution happened as a consequence of the understanding of the role of nutrients not only in the avoidance of clinical deficiencies but also in the reduction of the risk of chronic degenerative diseases.
A large heterogeneity of micronutrient recommendations exists across Europe, both quantitatively and qualitatively; therefore, a common agreement should be sought on the different uses and applications of nutrient recommendations, as critically discussed by Pijls et al. (2009). Table 1 collates differences in the recommendations of some micronutrients for pregnant women from several European countries. The European Micronutrient Recommendations Aligned (EURRECA) Network aims at providing standardized approaches to reveal variability within the European Union in micronutrient recommendations for population groups (Doets et al. 2008), with particular interest in ‘vulnerable populations’. ‘Vulnerable groups’ are defined as ‘population groups in a healthy population having a higher requirement’.
Table 1.
Recommendations of some micronutrients for pregnant women and their related footnotes within some European countries (adapted from the original tables in references)
| Vit A | Vit D | Vit B12 | Folate | Iodine | Zinc | Iron | |
|---|---|---|---|---|---|---|---|
| (µg day−1) | (mg day−1) | ||||||
| United Kingdom (COMA 1991) | 700 | 10 | 1.5 § | 300 | 140 § | 7 § | 14.8 [Link] , [Link] |
| Italy (LARN 1996) | 700 | 10 †† | 2.2 | 400 †† | 175 | 7 | 30 †† |
| Nordic countries (NNR 2004) | 800 | 10 | 2.0 | 500 | 175 | 9 §§ | – [Link] , [Link] |
| Spain (Moreiras et al. 2007) † | 800 | 10 | 2.2 | 600 ‡ | 135 | 20 | 18 |
| D‐A‐CH (2000) | 1.1 mg RE | 5 | 3.5 | 600 | 230 (CH: 200) | 10 | 30 |
Nordic Countries, Denmark, Finland, Iceland, Norway, Sweden; D‐A‐CH, Germany, Austria, Switzerland; RE, retinol equivalent; Vit D, 10 µg day−1 corresponds to 400 IU day−1, 5 µg day−1 corresponds to 200 IU day−1; †from the second half of pregnancy; ‡first and second half of gestation; §no increment. ¶Insufficient for women with high menstrual losses where the most practical way of meeting iron requirements is to take iron supplements. ††Dietary supplements or fortified foods may be required. ‡‡The composition of the meal influences the utilization of dietary iron. The availability increases if the diet contains abundant amounts of vitamin C and meat or fish daily, while it is decreased at simultaneous intake of e.g. polyphenols or phytic acid. §§The utilization of zinc is negatively influenced by phytic acid and positively by animal protein. The recommended intakes are valid for a mixed animal/vegetable diet. For vegetarian cereal‐based diets, a 25–30% higher intake is recommended. ¶¶Iron balance during pregnancy requires iron stores of approximately 500 mg at the start of pregnancy. The physiological need of some women for iron cannot be satisfied during the last two‐thirds of pregnancy with food only, and supplemental iron is therefore needed.
Pregnant women are considered a ‘vulnerable group’, as their nutritional requirements increase to support fetal and infant growth and development as well as maternal metabolism and tissue accretion. The estimated average requirement (EAR) is the daily intake value that is estimated to meet this requirement, as defined by the specific indicator of adequacy, in half of the individuals in a life‐stage or gender group (WHO/FAO 2004). The estimated total nutrient requirements during pregnancy can be derived from nutrients and energy accumulated in maternal tissues plus those necessary for products of pregnancy and lactation in addition to the baseline requirements for non‐pregnant, non‐lactating women. Determination of nutrient needs during pregnancy is a complex task because of the alteration of nutrient levels in tissues and fluids as a result of the hormone‐induced changes in metabolism, shifts in plasma volume and changes in renal function as well as in patterns of urinary excretion.
When assessing micronutrient recommendations for pregnant women, besides physiological variation, environmental factors must be defined and explored. Macro‐level factors such as socio‐economic and political contexts, and food availability along with micro‐level factors such as local cultural practices, norms, lifestyles, attitudes and beliefs influence food consumption (Pelto 1987; Hall Moran & Dykes 2009). Moreover, application of any future nutritional guidelines should also consider new evidence for biological role of micronutrients.
The aim of this paper is to discuss the nutritional specificities of pregnant women and the approaches underlying the definition of micronutrient dietary reference values and recommendation, i.e. the physiological and environmental factors influencing the bioavailability of micronutrients in pregnancy.
This narrative review develops from several integrating meetings and activities within the EURRECA Network through evidence‐based opinion and explorative work ( http://www.eurreca.org). Publications were searched using electronic databases and websites, hand searching relevant journals, contact with experts. The databases searched were Embase, Medline and PubMed databases, and Google‐indexed scientific literature and periodics from on‐line University of Milan Library Service. We used combinations of the following keywords: micronutrient, requirement, intake, supplement, status, malnutrition, deficiency, excess, overload, food, dietary patterns, pregnancy, pregnancy need, pregnancy health, pregnancy disease, pregnancy outcome, fetus, placenta, newborn and mother. Only human studies were considered, both original studies and reviews. Moreover, official and national documents were used.
Key messages
-
•
Nutrition during pregnancy may have long‐lasting effects on the well‐being of the mother and the fetus, and may further influence the health of the baby at a later age.
-
•
Targeted recommendations must be given to guide pregnant women in their food choice and dietary supplement use.
-
•
When assessing micronutrient recommendations for pregnant women, besides physiological variation, environmental factors must be defined and explored.
Factors influencing micronutrient recommendations in pregnancy
The physiological requirement for a nutrient should be the basis for calculating a reference intake. The ideal definition of a physiological requirement is the amount and chemical form of a nutrient that is needed systematically to maintain normal health and development without disturbance of the metabolism of any other nutrient. The corresponding dietary requirement would be the intake sufficient to meet the physiological requirement.
When assessing recommendations, quality of diet, genetics, physiological stress, pre‐pregnancy body mass index, body composition, gestational weight gain, time of gestation, maternal age, lifestyle, socio‐economic status, culture, ethnicity, etc. must be taken into account. This means that the bioavailability of nutrients, depending not only on the composition of diet or the chemical form of the nutrient but also on the nutritional status or physiological stage, is a crucial issue.
Pregnancy physiological–metabolic factors
Pregnancy is an anabolic state in which the body undergoes significant physiological and anatomical changes (Munro & Eckerman 1998). Hormones act towards a redirection of nutrients to highly specialized maternal tissues (placenta and mammary gland) and for the transfer of nutrients to the developing fetus. Biochemical, metabolic and physiological adjustments of the maternal organism meet the extra demands of the developing fetus and placenta (Kalhan 2000; Lain & Catalano 2007; Carlin & Alfirevic 2008) and support the homeostasis of micronutrients such as iodine (Zimmermann 2009), iron (Milman 2006) and calcium (Kovacs 2008). Body composition changes dramatically with maternal fat accretion. Maternal fat storage increases in early to mid‐gestation and, during late gestation, these maternal energy reserves are mobilized, following changes in maternal insulin production, to provide an increased supply of energy to the fetus. Improved availability of substrates and precursors for fetal–placental metabolism and hormone production is mediated through increments in dietary intake and endocrine changes that increase the availability of nutritional substrates (Weissgerber & Wolfe 2006).
Pregnancy is characterized by different stages that represent a continuum (Fig. 1) both in a life cycle context and from a nutritional point of view. In details, the first trimester is the time for the fetus when organogenesis (embryogenesis) takes place, and tissue patterns and organ systems are established; in the second trimester, the fetus undergoes major cellular adaptation and an increase in body size; during the third trimester, organ systems mature, and there is a significant increase in fetal body weight (Mullis & Tonella 2008). Nutritional deficiencies occurring during pregnancy might have long‐lasting effects on both maternal and infantile and adult health. In particular, the periconceptional period, which encompasses preconception, conception, implantation, placentation and embryo‐ or organogenesis, is a stage of pregnancy representing a critical step in determining fetal development (reviewed in Cetin et al. 2010). Later on, placental function regulates fetal growth and development (Desoye & Hauguel‐De Mouzon 2007). Several fetal diseases originate in the placenta and develop only later on in the fetus (Pardi & Cetin 2006). The ‘fetal’ or ‘early’ origins of adult disease hypothesis suggests that environmental factors, particularly nutrition, act through the processes of developmental plasticity (i.e. the ability of the fetus to respond to environmental cues by choosing a trajectory of development that often offers an adaptive advantage) to alter the development of the organism to such an extent that affects its capacity to cope with the environment in adult life, and therefore influences disease risk in adult life (Gluckman et al. 2005; McMillen & Robinson 2005).
Figure 1.
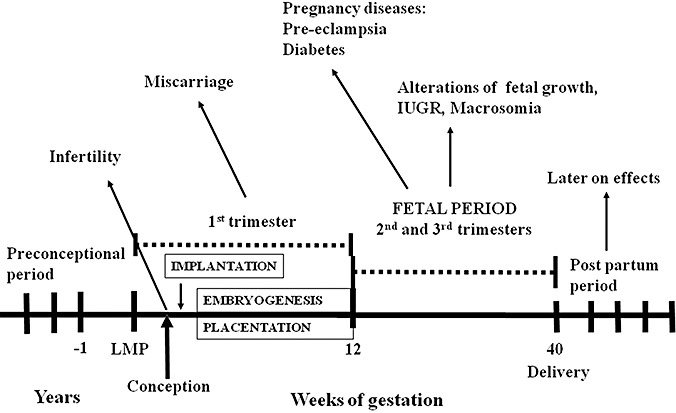
Different pregnancy stages from the preconceptional to the post‐partum period. Several specific malformations and pregnancy‐related disorders may originate during each phase. IUGR, Intrauterine growth restriction. LMP, last menstrual period. Adapted from Cetin et al. (2010).
The main characteristics of pregnancy that need to be highlighted from the nutritional perspective are as follows:
-
1
Pregnancy is characterized by a three‐compartment model, i.e. mother/placenta/fetus. Each of them has different metabolism; placenta transport function determines the composition of the umbilical cord blood providing nutrients and oxygen to the fetus to assure appropriate fetal growth (Cetin et al. 2005). Fetal growth is regulated by the balance between fetal nutrient demand and maternal–placental nutrient supply. Maternal nutrition and metabolism, utero‐placental blood flow, size and transfer capabilities of the placenta all determine the maternal–placental supply of nutrients (Pardi & Cetin 2006).
-
2
Pregnancy is a dynamic state, during which adjustments in nutrient metabolism evolve continuously as the mother switches from an anabolic condition during early pregnancy to a catabolic state during late pregnancy (Catalano et al. 2002; Hauguel‐de Mouzon et al. 2006). This switch is illustrated, e.g. in lipid metabolism going from fat deposition as a result of both hyperphagia and enhanced lipogenesis during the first and second trimesters to fat breakdown during the third trimester (Herrera 2000). Consequently, qualitative differences in dietary requirements exist during early and late pregnancy.
-
3
Maternal stores that have been developed before and throughout pregnancy will influence the composition of breast milk during lactation (Picciano 2003).
Period of gestation
Adequate maternal micronutrient status and intake prior to conception and throughout the entire pregnancy is critical to ensure satisfactory birth outcomes (reviewed in Picciano 2003, and Allen 2005). Timing of maternal nutritional intake and status impacting specifically and differently on the embryonal/fetal organ development, time of initiation and dose of prenatal supplementation influencing maternal micronutrient status, as well as the role of the interaction between the pre‐ and post‐natal environment in determining final health outcomes, are important issues (Gardiner 2007). Preconceptional nutritional status appears to be crucial for an optimal onset and development of pregnancy (reviewed by Cetin et al. 2010), suggesting the importance of adequate micronutrient intake of all women of childbearing age. This may be, for instance, of particular concern for calcium; if adequate bone mass has not been accrued before pregnancy and the intake of calcium from maternal diet is low, calcium is taken from the maternal skeleton (Thomas & Weisman 2006).
Specifying the micronutrient recommendations for specific periods of gestation may improve the overall outcome of pregnancy. In this regard, mineral recommendations by WHO/FAO (2004) are separated for the first, second and third trimesters of pregnancy. Spain recommends increased intakes of calcium, iodine, magnesium, niacin and vitamin E from the second half of pregnancy and an increased folate intake in the first half of gestation (Moreiras et al. 2007). In the UK, increased thiamin intake is recommended for the last trimester of pregnancy only (COMA 1991). However, micronutrient recommendations in most European countries do not differentiate between specific periods of gestation.
Effect of prenatal micronutrients on pregnancy outcomes
The biological role and reliable functional markers or indicators of status of the micronutrients that are of considerable public health significance during pregnancy are shown in Table 2.
Table 2.
The biological role and the reliable biomarkers or indicators of status of micronutrients of considerable public health significance
| Micronutrient | Function | Indicators of status | References |
|---|---|---|---|
| Folate | Involvement in the DNA cycle (cell replication); methylation cycle (aminoacids cysteine and methionine cycle) | Erythrocyte folate † ; serum/plasma folate † ; serum/plasma total homocysteine † | WHO/FAO 2004; McNulty & Scott 2008 |
| Vitamin B12 | Conversion of homocysteine to methionine as cofactor of the methionine synthase | Serum/plasma vitamin B12 † ; serum/plasma methylmalonic acid (MMA) † | Ryan‐Harshman & Aldoori 2008; Hoey et al. 2009 |
| Vitamin A | Growth and differentiation of a number of cells and tissues | Serum retinol † | Ross 2006 |
| Vitamin D | Bone resorption, intestinal calcium transport (calcium and bone homeostasis), modulation of transcription of cell cycle proteins, and cell‐differentiating, anti‐inflammatory and immunomodulatory properties | Plasma 25‐hydroxyvitamin‐D [25(OH)D] | WHO/FAO 2004 |
| Iodine | Synthesis of thyroid hormones | Urinary Iodine excretion in 24 h † ; serum thyroid‐stimulating hormone (TSH) † | WHO/FAO 2004; Zimmerman 2008; Ristic‐Medic et al. 2009 |
| Iron | Haematopoiesis; nucleic acid metabolism; carrier of oxygen to the tissues by red blood cell haemoglobin; transport medium for electrons within cells; integrated part of important enzyme systems | Haemoglobin † ; serum ferritin † ; serum transferrin receptor † | WHO/FAO 2004; Wood & Ronnenberg, 2006; Zimmerman 2008 |
| Zinc | Structural, regulatory and catalytic functions as cofactor for numerous metalloenzymes | Plasma/serum zinc † ; prevalence of inadequate intakes of dietary zinc † | McCall et al. 2000; Lowe et al. 2009 |
| Selenium | Protection of body tissues against oxidative stress, maintenance of defences against infection, and modulation of growth and development | Plasma/serum selenium † ; platelet or erythrocyte selenium † ; selenium‐related proteins † | WHO/FAO 2004; Sunde et al. 2008 |
Indicators of status were taken from a table compiled by the Biomarkers of Status Working Party, which comprised a group of international micronutrient experts and EURRECA partners (Fairweather‐Tait 2008), and successive updates†. Biomarkers reported here are those rated Excellent or Good according to a star rating used to classify the range of biomarkers available for each mineral/vitamin in relation to the limitations of the method.
Also reviewed in ‘Biomarkers of status/exposure. Minerals and vitamins’. RA1.2 Status Methods/IA3 Individuality, Vulnerability and Variability. July, 2008; ‘BIOMARKERS OF STATUS/EXPOSURE. Iron, Zinc, Vitamin A, Vitamin B12, Folate, Iodine & Selenium’. RA1.2 Status Methods/IA3 Individuality, Vulnerability and Variability. February, 2010 (http://www.eurreca.org).
Increasing the intake of folic acid before and during the first weeks of pregnancy can reduce birth defects (MRC 1991; Czeizel & Dudás 1992; Czeizel et al. 1999); hence, periconceptional folic acid supplements in doses of 4000 and 400 µg daily are recommended in addition to adequate dietary folate to prevent, respectively, recurrence and occurrence of neural tube defects (NTDs) (de Bree et al. 1997). Folate and/or vitamin B6/B12 deficiencies as a result of deregulation of their normal metabolism and/or low dietary intake (reviewed in Steen et al. 1998, and Tamura & Picciano 2006) may induce elevation in plasma total homocysteine or hyperhomocysteinemia as a consequence of decrease in the methylation cycle. Some ‘placenta events’ are postulated to arise from deficiencies of either folate and/or vitamin B12 or defect within the methionine–homocysteine metabolic pathways (Goddijn‐Wessel et al. 1996; Ray & Laskin 1999; Braekke et al. 2007; Dodds et al. 2008). Moreover, altered homocysteine metabolism leading to hyperhomocysteinemia has been proposed as the mechanism involved in NTDs (Locksmith & Duff 1998).
Prenatal vitamin A or beta‐carotene supplementation or fortification may reduce maternal mortality in vitamin A‐deficient mothers (West et al. 1999). Although an excessive intake has been shown to be teratogenic (McCaffery et al. 2003; Williamson 2006), adequate maternal vitamin A status is crucial for fetal lung development and maturation (reviewed in Strobel et al. 2007). Interestingly, liver stores of retinol in human fetuses were found to increase with the progress of gestation and to vary with maternal retinol levels, with the influence of maternal status being greater in later pregnancy than in the earlier stages (Shah et al. 1987). Consequently, supplementation after mid‐pregnancy at physiological levels can improve fetal stores without the risk of teratogenic effects. Insufficient vitamin A intake seems to be associated to low birthweight (LBW) (Strobel et al. 2007).
Dietary antioxidants (i.e. vitamin C, vitamin E, selenium, zinc, beta‐carotene) enhance many aspects of the immune response and limit pathological aspects of the cytokine‐mediated response (Bendich 2001; Arrigoni & De Tullio 2002). Recent reports associate poor maternal selenium status as a nutritional factor predisposing mothers to pre‐eclampsia, as women who develop pre‐eclampsia have a lower selenium status (Rayman et al. 2003). Through the selenoproteins, selenium plays a critical role in regulating the antioxidant status. The various demands of pregnancy impose oxidative, metabolic and inflammatory stresses on the mother (Redman et al. 1999). When occurring during embryogenesis and in the placenta, oxidative stress causes adverse pregnancy outcomes such as birth defects, early pregnancy failure, miscarriage and pre‐eclampsia (Agarwal et al. 2005; Jauniaux et al. 2006; Forges et al. 2007). Oxidative stress and inflammatory mediators seem to be involved in the abnormal implantation associated with pre‐eclampsia (Roberts et al. 2003; Vanderlelie et al. 2005). Dietary antioxidants seem to play a crucial role in regulating the antioxidant status, thereby aiding in maintaining health. As an example, when comparing women with a higher level of prenatal vitamin C (≥11.734 µg mL−1) to women with a lower level of prenatal vitamin C (<8.997 µg mL−1), a significant lower trophoblast expression for the endothelial scavenger receptor low‐density lipoprotein receptor‐1 and the apoptotic index in normal full‐term pregnancy was detected in women with a higher level of prenatal vitamin C (Ahn et al. 2007). This seems to indicate that placental oxidative stress and apoptotic activity were associated with the gestational vitamin C status.
Increasing calcium intake can reduce the risk of pregnancy‐induced hypertensive disorders (Thomas & Weisman 2006). A significant association was also observed between low 25‐hydroxyvitamin D [25(OH)D] concentrations in early pregnancy and subsequent pre‐eclampsia (Bodnar et al. 2007). Moreover, a significant association was found between maternal plasma 25(OH)D concentrations in mid‐gestation and the risk of developing gestational diabetes mellitus (Clifton‐Bligh et al. 2008; Maghbooli et al. 2008). Adequate maternal calcium intake can affect positively both maternal and fetal bone health because the fetus is dependent on maternal sources for the total calcium load. On the contrary, maternal bone loss during pregnancy might lead to osteoporosis and fracture either contemporaneously or by reducing peak bone mass in later life (Prentice 1994). It was shown that whole body bone mineral content of fetus increases between 32 and 33 and 40–41 weeks of gestation. Several findings suggest that the greatest fetal calcium accumulation occurs during the third trimester. Hence, calcium consumption should be encouraged during pregnancy to replace maternal skeletal calcium stores that are depleted during this period (Thomas & Weisman 2006). Similarly, 25 mg day−1 of vitamin D should be given during the last 3 months of pregnancy or 2500 mg day−1 in one dose at the beginning of the last trimester in countries where sunshine exposure is negligible (i.e. in northern countries) or to women avoiding dairy products for cultural or dietary reasons (Salle et al. 2000).
The role of iron supplementation during pregnancy is more controversial. An adequate iron intake is mandatory for normal fetal growth and development, although evidence for either a beneficial or harmful effect of iron prophylaxis on pregnancy outcomes is inconclusive and routine supplementation in pregnancy is a matter of debate (Breymann 2002). Iron‐deficiency anaemia (IDA), early in pregnancy, has been found to be inversely related to placental size and associated with reduced infant growth and increased risk of adverse pregnancy outcomes (Scholl & Hediger 1994; Hindmarsh et al. 2000; Ronnenberg et al. 2004; Buckley et al. 2005). Moreover, maternal anaemia during the second trimester has been associated with an increased risk of preterm delivery (Scholl 2005). New insights are emerging into the role of iron on neurocognitive and neurobehavioural development of the fetus during the last two‐thirds of gestation and into the long‐term consequences of their perinatal deficiency (Beard 2003; Beard 2008). Human brain growth spurt begins in the latter part of the second trimester (Lukas & Campbell 2000), but its peak velocity is during the last trimester of gestation and the first post‐natal months (reviewed in Innis 2003). As the physiological needs of some women for iron are not achieved during the last two‐thirds of pregnancy with food only, supplemental iron is therefore needed (Beaton 2000). Pregnant women using iron supplements have a better iron status and a lower frequency of IDA compared with women receiving no supplement (Makrides et al. 2003; Milman et al. 2005). Hence, pre‐partum IDA seems to be prevented by oral iron supplements (30–40 mg day−1) taken from the 20th week of gestation until delivery (Milman et al. 2005). Women supplemented with iron presented a higher mean birth weight and a lower preterm delivery incidence compared with the control group (Cogswell et al. 2003; Siega‐Riz et al. 2006). On the contrary, high dose of iron supplement (more than 100 mg day−1) was observed to be significantly associated to gestational diabetes (Bo et al. 2009).
Iodine intake is required to prevent the onset of subclinical hypothyroidism of mother and fetus during pregnancy, thus to prevent the possible risk of brain damage of the fetus (WHO/FAO 2004). Maternal iodine deficiency leads to fetal hypothyroidism results in cretinism as thyroid hormones are critical for normal brain development and maturation (WHO/FAO 2004). However, if hypothyroidism develops late in pregnancy, the neurological damage is not as severe as when it is already present in early pregnancy (WHO/FAO 2004). Third trimester pregnant women with urinary iodine concentrations below 50 µg L−1 are significantly more likely to have a small‐for‐gestational‐age (SGA) infant. Higher levels of thyroid‐stimulating hormone were also associated with a higher risk of having an SGA or LBW newborn (Alvarez‐Pedrerol et al. 2009). A randomized trial showed that a daily dose of 200 µg iodine starting from 16–20th week of gestation in marginal iodine deficiency appeared to be effective in preventing gestational goiter without enhancing the frequency of post‐partum thyroiditis (Antonangeli et al. 2002).
Docosahexaenoic acid (DHA; n‐3) and arachidonic acid are essential for fetal and neonatal growth and development (Eilander et al. 2007), as long‐chain polyunsaturated fatty acids are involved in modifications of neuronal membrane fluidity, function of neuronal membrane ionic channels and production of neurotransmitters and brain peptides (Innis 2007). If maternal DHA supply is limited, the fetus is particularly vulnerable to developmental deficits in the third trimester. Adequate maternal DHA intake or supplementation from the second trimester seems to be crucial in avoiding the potential perturbation of cellular environments in the offspring (reviewed in Innis 2003). The PeriLip Steering Committee and the Project Coordinating Committee of the early Nutrition Programming project stated that pregnant women should aim to achieve an average dietary intake of at least 200 mg DHA day−1, and women of childbearing age should be recommended to consume one or two portions of sea fish per week, including oily fish such as salmon, herrings, etc. (Koletzko et al. 2008). Beneficial effects on subsequent infant visual function and neurodevelopment were also reported (reviewed in Judge et al. 2007, and Innis & Friesen 2008). Potential benefit of enhanced supply of n‐3 fatty acids in preventing pre‐eclampsia has been suggested in a recent prospective cohort study (Oken et al. 2007). Moreover, associations between maternal long‐chain polyunsaturated fatty acids supplementation and a small reduction of risk of early preterm delivery in women with high‐risk pregnancies (Horvath et al. 2007) as well as small increment in the duration of pregnancy (Szajewska et al. 2006) have been observed in several studies.
Effect of prenatal micronutrients on lactation and post‐natal outcomes
The timing of prenatal micronutrient status and intake has been observed to condition breast milk composition. In particular, maternal vitamin A status from the second trimester of gestation seems to influence both retinol (vitamin A) concentration in breast milk (Muslimatun et al. 2001) and newborn development, and inadequacies during pregnancy are not compensated by post‐natal supplementation (Strobel et al. 2007). Similarly, levels of vitamin E in transitional milk seem to be dependent on vitamin E and polyunsaturated fatty acids intakes during the third trimester (Ortega et al. 1999).
The timing of prenatal nutrition seems to impact differently on the nature of adult diseases by programming post‐natal pathophysiology. Accumulating data suggest that the early environment may modify the effects of the genome (Newnham et al. 2002; Fleming et al. 2004; Buckley et al. 2005; de Boo & Harding 2006; Gluckman et al. 2008). Molecular, cellular, metabolic, neuroendocrine and physiological adaptations in the early nutritional environment may cause a permanent alteration of the developmental pattern of cellular proliferation and differentiation in tissue and organ systems that may result in pathological consequences in adult life (Koletzko et al. 1998; McMillen & Robinson 2005). Studies in the offspring of women exposed to the Dutch Winter Famine showed that the nutrient challenge in the first trimester of pregnancy was linked to increased prevalence of coronary heart disease and obesity, and to raised blood lipids (Ravelli et al. 1999; Roseboom et al. 2000; Roseboom et al. 2001), whereas famine occurring during late gestation led to decreased glucose tolerance in adult life (Ravelli et al. 1998).
Poor maternal vitamin D status early in pregnancy may result in impaired maternal–fetal transfer of 25(OH)D and consequently reduced bone mineral content during infancy and childhood (Javaid et al. 2006). There are also arguments that low maternal vitamin D intake from the second trimester of pregnancy may be associated with the risk of recurrent wheeze at 3 or 5 years, suggesting that childhood asthma may be influenced by maternal diet during pregnancy (Camargo et al. 2007; Devereux et al. 2007). Maternal IDA during the last two‐thirds of gestation is suggested to result in irreversible effects on neurochemistry and neurobiology (Beard 2003) such as schizophrenia in later life (Brown & Susser 2008; Insel et al. 2008). Data collected from a population‐based cohort born from 1959 to 1967, and followed up for development of schizophrenia spectrum disorders from 1981 through to 1997, suggested that second and third trimester exposure to maternal haemoglobin concentrations ≤10.0 g dL−1 was associated with a fourfold significantly increased rate of schizophrenia disorders in adult offspring (Insel et al. 2008). Similarly, in a cohort of births from 1978 to 1998, and followed from their 10th birthday, cohort members whose mothers were diagnosed with anaemia during pregnancy had a 1.60‐fold increased risk of schizophrenia (Sørensen et al. 2010). It may be proposed that low haemoglobin concentrations compromise oxygen delivery to the developing fetus. In addition, insufficient iron in utero exposure may crucially disrupt neurodevelopment given that iron is essential for several metabolic processes involved in the development of brain structures and functions (i.e. dopaminergic neurotransmission, myelination and energy metabolism).
Birth spacing
It has been suggested that short birth intervals, by giving the mother insufficient time to recover from the nutritional burden of pregnancy, could adversely affect the nutritional status of both mother and child (King 2003). This nutritional burden may increase significantly when pregnancy overlaps with lactation, a period of very high maternal nutritional demand (Adair 1993). In a recent systematic review, Dewey & Cohen (2007) reported that, in studies conducted in developing countries, longer birth interval has been associated with a lower risk of child malnutrition in some populations. Where such a significant relationship was shown, the reduction in stunting associated with a pervious birth interval of 35 months ranged from ∼10–50%, although considerable residual confounding variables existed in the studies. One study suggested a possible increased risk of maternal anaemia associated with short interpregnancy interval (Conde‐Agudelo & Belizán 2000), but iron supplementation during pregnancy was not accounted for in the analysis. There was no clear evidence of a link between interpregnancy interval and maternal anthropometric status, perhaps due in part to changes in hormonal regulation of nutrient partitioning between the malnourished mother and the fetus (Dewey & Cohen 2007). Considering the methodological limitations apparent in the majority of current studies on birth interval and maternal and child nutritional status, there is a clear and urgent need for further research.
Maternal diet
Eating patterns
Eating habits (e.g. vegetarian diet, fast food frequency, breakfast skipping) impact the adequacy of nutrient intakes. Some studies showed an association between dietary patterns and pregnancy outcomes. A reduction in the risk of early delivery has been associated with a maternal mid‐pregnancy Mediterranean‐type diet rich in fruit and vegetables, that is characterized by high vitamin C, folate, α‐tocopherol, magnesium, calcium, iron and vitamin D intake and low sugar and cholesterol intake (Mikkelsen et al. 2008). Vujkovic and colleagues (2007) found an increased risk of cleft lip or palate and high plasma total homocysteine levels with a maternal periconceptional Western diet that was high in meat, pizza, legumes and potatoes, and low in fruits.
Vegans may be at risk of vitamin B12 deficiency as they do not consume any animal products (ADA Report 2009). A long‐term ovo‐lacto vegetarian diet has been shown to result in significantly lower serum vitamin B12 and higher plasma total homocysteine concentrations during pregnancy and in an increased risk of vitamin B12 deficiency with respect to a Western diet (Koebnick et al. 2004).
Similarly, meal patterns seem to be related to pregnancy outcomes. It is recommended that pregnant women ‘eat small to moderate‐sized meals at regular intervals, and eat nutritious snacks’ in order to meet the increased nutritional needs. Prolonged periods of time without food can cause hypoglycemia, thus a physiological stress. In a prospective cohort study of risk factors for preterm birth, women were asked to indicate how many meals and snacks they usually ate per day and the time of consumption (Siega‐Riz et al. 2001). Results showed that consuming food at a lower optimal frequency was associated to a slightly increased risk for delivering preterm mainly after premature rupture of the membranes.
Consumption behaviour in pregnancy is influenced by a complex range of psychological, socio‐demographic and cultural factors. For any given community, an understanding of these variables is required when transferring recommendations into action. Social class may affect the quality of diet. On the whole, low‐income groups consume a poor‐quality diet, and diet‐related diseases, such as obesity and diabetes, have begun increasing among lower‐ and middle‐income groups (Popkin 2003). High palatability, high convenience, and the low cost of energy‐dense foods in conjunction with large portions and low satiating power may be the principal reasons for overeating and weight gain (Drewnowski & Darmon 2005). In particular, a review undertaken by Darmon & Drewnowski (2008) about the relationship between socio‐economic status and eating behaviour showed that studies on the plasma biomarkers of dietary exposure provide evidence that socio‐economic status affects vitamin intakes, and that low‐income pregnant or breastfeeding women are at greater risk of insufficient vitamin and mineral intakes. For instance, a dietary survey undertaken in UK showed that diet of low‐income pregnant women did not meet the EAR for folate, calcium and iron (Mouratidou et al. 2006). In addition, maternal education seems to correlate to food choices. As demonstrated in a large population‐based birth cohort study in Finland, pregnant women with higher education levels had higher daily consumption of vegetables, fruits and berries, leading to higher intakes of dietary fibres, and of some vitamins (Arkkola et al. 2006). Similarly, the Pregnancy, Infection and Nutrition Study in North Carolina, involving 2063 pregnant women, showed that high school graduates had significantly higher Diet Quality Index for Pregnancy scores, and that higher percentages of recommended vegetable servings were consumed by better‐educated women (Bodnar & Siega‐Riz 2002).
Geographic factors
The micronutrient status of an entire community may be influenced by region and seasonal variation impacting the availability of micronutrients. Iodine and selenium deficiencies tend to be geographically specific because of deficiencies in the soil and therefore the food chain (Ladipo 2000; WHO/FAO 2004). The majority of vitamin D comes from sunlight exposure. In most situations, during summer, approximately 30 min of skin exposure to sunlight in the middle of the day can provide 50 000 IU (1.25 mg) of vitamin D to people with white skin. Latitude and season as well as skin pigmentation and ethnicity influence the ability of the skin to provide the total vitamin D needs of the individual (WHO/FAO 2004; Yu et al. 2009). This means that in locations around the equator, the most physiologically relevant and efficient way of acquiring vitamin D is to synthesize it endogenously in the skin (Hollis & Wagner 2004), whereas during winter at latitudes higher than 42°, vitamin D synthesis is virtually zero (WHO/FAO 2004). Taken together, these findings suggest that not routinely sun‐replete individuals or persons with darker pigmentation should correct their vitamin D status by consuming the amounts of vitamin D appropriate for their population. Unfortunately, a recent review by Hollis & Wagner (2004) indicated that, currently, the appropriate dose of vitamin D during pregnancy is unknown, although it appears to be greater than the current dietary reference intake of 5–10 µg day−1, and that further studies are necessary to determine optimal vitamin D intakes for pregnancy as a function of latitude and race. This concern was confirmed by a prospective randomized controlled study that took place in the UK comparing the effects of a single dose of 200 000 IU vitamin D (calciferol) and of a daily dose of 800 IU vitamin D (ergocalciferol) from the 27th week to delivery on pregnant women and their baby at delivery (Yu et al. 2009). Results showed that despite supplementation enhanced significantly the 25(OH)D levels within supplemented groups with respect to the untreated group, vitamin D sufficiency >50 nmol L−1 was achieved only in 30% of supplemented women, and only 8% of babies were vitamin D sufficient in the supplement group.
Micronutrient bioavailability and diet
Appropriate intake of micronutrients depends not only on the quality of diet but also on their bioavailability. Lack of accurate data on micronutrients' bioavailability from natural food sources may be an ongoing concern for policy‐makers for setting dietary recommendations. As an example, the recommended nutrient intakes for dietary zinc (mg day−1) to meet the normative storage requirements from diets by the Joint Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) Expert Consultation on Human Vitamin and Mineral Requirements (WHO/FAO 2004) are stated according to different bioavailabilities (Table 3).
Table 3.
Recommended nutrient intakes for dietary zinc (mg day−1) in pregnancy to meet the normative storage requirements from diets differing in zinc bioavailability and principal dietary characteristics for categorizing diets according to the potential bioavailability of their zinc † (adapted from WHO/FAO 2004)
| Trimester | High bioavailability | Moderate bioavailability | Low bioavailability |
|---|---|---|---|
| Refined diets low in cereal fibre and phytic acid content, with phytate–zinc molar ratio <5; adequate protein content principally from non‐vegetable sources, such as meats and fish. | Mixed diets containing animal‐fish protein. Lacto‐ovo, ovo‐vegetarian, or vegan diets not based primarily on unrefined cereal grains or high‐extraction‐rate flours. Phytate–zinc molar ratio of total diet = 5–15, or not >10 if more than 50% of the energy intake is accounted for by unfermented, unrefined cereal grains and flours and the diet is fortified with inorganic calcium salts. Availability of zinc improves when the diet includes animal protein or milks, or other protein sources or milks. | Diets high in unrefined, unfermented and ungerminated cereal grain, especially when fortified with inorganic calcium salts and intake of animal protein is negligible. Phytate–zinc molar ratio of total diet >15. High‐phytate, soya–protein products as the primary protein source. Diets in which approximately 50% of the energy intake is accounted for by the following high‐phytate foods: high‐extraction‐rate (≥90%) wheat, rice, maize, grains and flours, oatmeal and millet; chapatti flours and tanok; sorghum, cowpeas, pigeon peas, grams, kidney beans, black‐eyed beans and groundnut flours. High intakes of inorganic calcium salts, either as supplements or as adventitious contaminants, potentiate the inhibitory effects, and low intakes of animal protein exacerbate these effects. | |
| First | 3.4 | 5.5 | 11.0 |
| Second | 4.2 | 7.0 | 14.0 |
| Third | 6.0 | 10.0 | 20.0 |
At intakes adequate to meet the average normative requirements for absorbed zinc, the three availability levels correspond to 50%, 30% and 15% absorption.
Dietary factors such as food matrix, chemical form, processing and cooking methods may modify micronutrient bioavailability (Hotz & Gibson 2007). They may limit absorption through nutritional interactions (e.g. fibre/phytate–minerals complexes), mineral–mineral interactions involved in the same metabolism, competition for a common transport site or transport ligand (e.g. zinc/copper, iron/manganese), and effects of drugs or chemicals on the metabolism of the nutrient (Keen et al. 2003). Alternatively, they may enhance absorption as in the case of iron if the diet contains abundant amounts of vitamin C and meat/fish (Gibson et al. 2006), or for carotenoids in the presence of dietary fats (van Het Hof et al. 2000), or for zinc by germination of cereals and legumes (Gibson et al. 2006). Interestingly, based on this assumption, the footnote to the original table in the Nordic Country Recommendation (NNR 2004) (see Table 1) states: ‘The composition of the meal influences the utilization of dietary iron. The availability increases if the diet contains abundant amounts of vitamin C and meat or fish daily, while it is decreased at simultaneous intake of, e.g. polyphenols or phytic acid; the utilization of zinc is negatively influenced by phytic acid and positively by animal protein. The recommended intakes are valid for a mixed animal/vegetable diet. For vegetarian cereal based diets, a 25–30% higher intake is recommended’.
Similarly, the form of micronutrient influences its bioavailability: i.e. haem‐ iron is absorbed better than non‐haem iron. On the contrary, there is conflicting evidence as to whether the extent of conjugation of polyglutamyl folate is a limiting factor in folate bioavailability. Estimates of the extent of lower bioavailability of food folates compared with folic acid show great variation, depending on the methodological approach used (McNulty & Pentieva 2004). The EAR for vitamin A, expressed as mg retinol equivalents (mg RE), should account for the proportionate bioavailability of preformed vitamin A (about 90%) and provitamin A carotenoids from a diet that contains sufficient fat (WHO/FAO 2004).
Maternal age
Maternal age represents a critical factor in micronutrient requirement. Accordingly, the dietary recommended intake for micronutrients should take into account maternal age; e.g. US recommendations for vitamin K, vitamin C, calcium, phosphorus, magnesium and zinc in pregnancy are separated for age >18 or ≤18 years by the Institute of Medicine's Food and Nutrition Board ( http://www.usda.gov). Adolescent pregnancy, as defined by WHO as pregnancy in those aged 10–19 years (WHO 2004), appears to be a risk factor for micronutrient deficiencies (Lenders et al. 2000; Hall Moran 2007a). A systematic review of the nutrient intakes of pregnant adolescents living in industrialized countries suggested that, compared with US dietary reference intake values, intake of energy, iron, folate, calcium, vitamin E and magnesium were lower than those currently recommended (Hall Moran 2007b).
In recent decades, adolescent pregnancy has become an important public health issue because of associated poor obstetric outcomes, particularly with respect to fetal growth restriction and preterm delivery. Approximately a fifth of all births worldwide are to adolescent mothers (Population Reference Bureau 2000) and, although the general trend over the past 20 years in Europe is that of declining adolescent pregnancy and birth rate, the distributions across Europe are large, ranging from 42.69 live births per 1000 women aged 15–19 in Tajikistan to 5.39 live births per 1000 women in Switzerland (Avery & Lazdane 2008). Despite this, relatively little is known about nutrient intakes of adolescents during pregnancy, and few prospective studies have been conducted in this population. One recent prospective, observational study of 500 adolescents conducted in the UK highlighted the extent of poor vitamin D status in pregnant adolescents and suggested a clear relationship between maternal folate and iron status and the incidence of SGA birth and preterm delivery in this cohort (Baker et al. 2009).
Despite the increasing prevalence of pregnancy in women over the age of 40 years as a result of recent advances in assisted reproductive technology, to our knowledge, there are no studies related to nutritional needs and reference values in this population of pregnant women. This lack of knowledge is reflected in the European micronutrient recommendations for pregnancy, the vast majority of which do not differentiate for maternal age.
Conclusion
Targeted recommendations must be given to guide pregnant women in their food choice and dietary supplement use so that they may obtain adequate nutritional status and meet the increased need for nutrients. The term ‘vulnerability’ represents a key concept in assessing nutrient needs and defining recommended nutrient intakes for target populations at risk of low intake. Several physiological and metabolic factors characterize pregnant women such as adaptation and timing of gestation, and determine their nutritional requirements. In addition, environmental and demographic variables seem to influence the overall quality of diet and the adequacy of micronutrient intake during pregnancy. Unfortunately, a large number of European recommendations do not consider these factors. Moreover, current research is limited by sampling and measurement bias, and findings are often inconclusive or contradictory. Thereby, further studies and actions are urgently warranted to address limitations and to:
-
•
determine optimal biomarkers and concentrations even with regards to non‐classic actions of micronutrients on maternal and fetal outcomes;
-
•
investigate of the most effective way to supply micronutrients, including appropriate timing and dosage. In this context, strategies of supplementation and dietary intervention are currently under discussion. Several studies are ongoing to evaluate the effect of different timing in pregnancy (i.e. early or late pregnancy) as well as the different frequencies of supplementation (i.e. daily or weekly). Forms of micronutrient supplement/intake are also of interest as it is well acknowledged that micronutrient status is influenced by both the content and the bioavailability of the micronutrient in the diet;
-
•
explore the influence of age and of role of socio‐economic factors on the nutrient requirements of pregnant women.
Sources of funding
The work reported herein has been carried out within the EURRECA Network of Excellence (http://www.eurreca.org) which is financially supported by the Commission of the European Communities, specific Research, Technology and Development (RTD) Programme Quality of Life and Management of Living Resources, within the Sixth Framework Programme, contract no. 036196. This report does not necessarily reflect the Commission's views or its future policy in this area.
Conflict of interest
The authors have declared no conflict of interest.
References
- ADA Report (2009) Position of the American Dietetic Association: vegetarian diets. Journal of the American Dietetic Association 109, 1266–1282. [DOI] [PubMed] [Google Scholar]
- Adair S‐R. (1993) Biological determinants of pregnancy weight gain: a longitudinal study of Filipino women. American Journal of Clinical Nutrition 57, 365–372. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Gupta S. & Sharma R.K. (2005) Role of oxidative stress in female reproduction. Reproductive Biology and Endocrinology 3, 28. doi:10.1186/1477‐7827‐3‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggett P.J., Bresson J., Haschke F., Hernell O., Koletzko B., Lafeber H.N. et al (1997) Recommended dietary allowances (RDAs), recommended dietary intakes (RDIs), recommended nutrient intakes (RNIs), and population reference intakes (PRIs) are not ‘recommended intakes’. Journal of Pediatric Gastroenterology and Nutrition 25, 236–241. [DOI] [PubMed] [Google Scholar]
- Ahn Y., Kim Y., Park H., Park B. & Lee H. (2007) Prenatal vitamin C status is associated with placental apoptosis in normal‐term human pregnancies. Placenta 28, 31–38. [DOI] [PubMed] [Google Scholar]
- Allen L.H. (2005) Multiple micronutrients in pregnancy and lactation: an overview. American Journal of Clinical Nutrition 81, 1206S–1212S. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Pedrerol M., Guxens M., Mendez M., Canet Y., Martorell R., Espada M. et al (2009) Iodine levels and thyroid hormones in healthy pregnant women and birth weight of their offspring. European Journal of Endocrinology 160, 423–429. [DOI] [PubMed] [Google Scholar]
- Antonangeli L., Maccherini D., Cavaliere R., Di Giulio C., Reinhardt B., Pinchera P. et al (2002) Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: a longitudinal study. European Journal of Endocrinology 147, 29–34. [DOI] [PubMed] [Google Scholar]
- Arkkola T., Uusitalo U., Pietikäinen M., Metsälä J., Kronberg‐Kippilä C., Erkkola M. et al (2006) Dietary intake and use of dietary supplements in relation to demographic variables among pregnant Finnish women. British Journal of Nutrition 96, 913–920. [DOI] [PubMed] [Google Scholar]
- Arrigoni O. & De Tullio M.C. (2002) Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta 1569, 1–9. [DOI] [PubMed] [Google Scholar]
- Avery L. & Lazdane G. (2008) What do we know about sexual and reproductive health of adolescents in Europe? The European Journal of Contraception and Reproductive Health Care 13, 58–70. [DOI] [PubMed] [Google Scholar]
- Baker P.N., Wheeler S.J., Sanders T.A., Thomas J.E., Hutchinson C.J., Clarke K. et al (2009) A prospective study of micronutrient status in adolescent pregnancy. American Journal of Clinical Nutrition 89, 1114–1124. [DOI] [PubMed] [Google Scholar]
- Beard J. (2003) Iron deficiency alters brain development and functioning. Journal of Nutrition 133, 1468S–1472S. [DOI] [PubMed] [Google Scholar]
- Beard J.L. (2008) Why iron deficiency is important in infant development. Journal of Nutrition 138, 2534–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton G.H. (2000) Iron needs during pregnancy: do we need to rethink our targets? American Journal of Clinical Nutrition 72, 265S–271S. [DOI] [PubMed] [Google Scholar]
- Bendich A. (2001) Micronutrients in women's health and immune function. Nutrition 17, 858–867. [DOI] [PubMed] [Google Scholar]
- Bo S., Menato G., Villois P., Gambino R., Cassader M., Cotrino I. et al (2009) Iron supplementation and gestational diabetes in midpregnancy. American Journal of Obstetrics and Gynecology 201, 158.e1–158.e6. [DOI] [PubMed] [Google Scholar]
- Bodnar L.M. & Siega‐Riz A.M. (2002) A diet quality index for pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutrition 5, 801–809. [DOI] [PubMed] [Google Scholar]
- Bodnar L.M., Catov J.M., Simhan H.N., Holick M.F., Powers R.W. & Roberts J.M. (2007) Maternal vitamin D deficiency increases the risk of preeclampsia. Journal of Clinical Endocrinology Metabolism 92, 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braekke K., Ueland P.M., Harsem N.K., Karlsen A., Blomhoff R. & Staff A.C. (2007) Homocysteine, cysteine, and related metabolites in maternal and fetal plasma in preeclampsia. Pediatric Research 62, 319–324. [DOI] [PubMed] [Google Scholar]
- De Bree A., Van Dusseldorp M., Brouwer I.A., Van Het Hof K.H. & Steegers‐Theunissen R.P.M. (1997) Folate intake in Europe: recommended, actual and desired intake. European Journal of Nutrition 51, 643–660. [DOI] [PubMed] [Google Scholar]
- Breymann C. (2002) Iron supplementation during pregnancy. Fetal and Maternal Medicine Review 13, 1–29. [Google Scholar]
- Brown A.S. & Susser E.S. (2008) Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophrenia Bulletin 34, 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A.J., Jaquiery A.L. & Harding J.E. (2005) Nutritional programming of adult disease. Cell and Tissue Research 322, 73–79. [DOI] [PubMed] [Google Scholar]
- Camargo C.A. Jr, Rifas‐Shiman S.L., Litonjua A.A., Litonjua A.A., Rich‐Edwards J.W., Weiss S.T. et al (2007) Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. American Journal of Clinical Nutrition 85, 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin A. & Alfirevic Z. (2008) Physiological changes of pregnancy and monitoring. Best Practice & Research Clinical Obstetrics and Gynaecology 22, 801–823. [DOI] [PubMed] [Google Scholar]
- Catalano P.M., Nizielski S.E., Shao J., Preston L., Qiao L. & Friedman J.E. (2002) Downregulated IRS‐1 and PPARγ in obese women with gestational diabetes: relationship to FFA during pregnancy. American Journal of Physiology. Endocrinology and Metabolism 282, E522–E533. [DOI] [PubMed] [Google Scholar]
- Cetin I., Alvino G., Radaelli T. & Pardi G. (2005) Fetal nutrition: a review. Acta Pædiatrica 94, 7S–13S. [DOI] [PubMed] [Google Scholar]
- Cetin I., Berti C. & Calabrese S. (2010) Role of micronutrients in the periconceptional period. Human Reproduction Update 16, 80–95. [DOI] [PubMed] [Google Scholar]
- Clifton‐Bligh R.J., McElduff P. & McElduff A. (2008) Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabetic Medicine 25, 678–684. [DOI] [PubMed] [Google Scholar]
- Cogswell M.E., Parvanta I., Ickes L., Yip R. & Brittenham G.M. (2003) Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. American Journal of Clinical Nutrition 78, 773–781. [DOI] [PubMed] [Google Scholar]
- COMA (1991) Panel on DRVs of the Committee on Medical Aspects of Food Policy. Dietary reference values (DRVs) for food energy and nutrients for the UK, Report on Health and Social Subjects 41. [PubMed]
- Conde‐Agudelo A. & Belizán J.M. (2000) Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. British Medical Journal 321, 1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel A.E. & Dudás I. (1992) Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. The New England Journal of Medicine 327, 1832–1835. [DOI] [PubMed] [Google Scholar]
- Czeizel A.E., Tímár L. & Sárközi A. (1999) Dose‐dependent effect of folic acid on the prevention of orofacial clefts. Pediatrics 104, e66. [DOI] [PubMed] [Google Scholar]
- D‐A‐CH (2000) German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research. Reference Values for Nutrient Intake (D‐A‐CH). Frankfurt am Main.
- Darmon N. & Drewnowski A. (2008) Does social class predict diet quality? American Journal of Clinical Nutrition 87, 1107–1117. [DOI] [PubMed] [Google Scholar]
- De Boo H.A. & Harding J.E. (2006) The development origins of adult disease (Barker) hypothesis. Australian New Zealand Journal of Obstetrics and Gynaecology 46, 4–14. [DOI] [PubMed] [Google Scholar]
- Desoye G. & Hauguel‐De Mouzon S. (2007) The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 30, S120–S126. [DOI] [PubMed] [Google Scholar]
- Devereux G., Litonjua A.A., Turner S.W., Craig L.C.A., McNeill G., Martindale S. et al (2007) Maternal vitamin D intake during pregnancy and early childhood wheezing. American Journal of Clinical Nutrition 85, 853–859. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. & Cohen R.J. (2007) Does birth spacing affect maternal or child nutritional status? A systematic literature review. Maternal & Child Nutrition 3, 151–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds L., Fell D.B., Dooley K.C., Armson B.A., Allen A.C., Nassar B.A. et al (2008) Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clinical Chemistry 54, 326–334. [DOI] [PubMed] [Google Scholar]
- Doets E.L., De Wit L.S., Dhonukshe‐Rutten R.A.M., Cavelaars A.E.J.M., Raats M.R., Timotijevic L. et al (2008) Current micronutrient recommendations in Europe: towards understanding their differences and similarities. European Journal of Nutrition 47, S17–S40. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. & Darmon N. (2005) Food choices and diet costs: an economic analysis. Journal of Nutrition 135, 900–904. [DOI] [PubMed] [Google Scholar]
- Eilander A., Hundscheid D.C., Osendarp S.J., Transler C. & Zock P.L. (2007) Effects of n‐3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: A review of human studies. Prostaglandins, Leukotrienes and Essential Fatty Acids 76, 189–203. [DOI] [PubMed] [Google Scholar]
- Fairweather‐Tait S.J. (2008) Biomarkers of micronutrient status. British Journal of Nutrition 99, S1. [Google Scholar]
- Fleming T.P., Kwong W.Y., Porter R., Ursell E., Fesenko I., Wilkins A. et al (2004) The embryo and its future. Biology of Reproduction 1046–1054. [DOI] [PubMed] [Google Scholar]
- Forges T., Monnier‐Barbarino P., Alberto J.M., Guéant‐Rodriguez R.M., Daval J.L. & Guéant J.L. (2007) Impact of folate and homocysteine metabolism on human reproductive health. Human Reproduction Update 13, 225–238. [DOI] [PubMed] [Google Scholar]
- Gardiner H.M. (2007) Early environmental influences on vascular development. Early Human Development 83, 819–823. [DOI] [PubMed] [Google Scholar]
- Gibson R., Perlas L. & Hotz C. (2006) Improving the bioavailability of nutrients in plant foods at the household level. Proceedings of the Nutrition Society 65, 160–168. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. & Pinal C.S. (2005) The developmental origins of adult disease. Maternal & Child Nutrition 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Cooper C. & Thornburg K.L. (2008) Effect of in utero and early‐life conditions on adult health and disease. The New England Journal of Medicine 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddijn‐Wessel T.A.W., Wouters M.G.A.J., Vd Molen E.F., Spuijbroek M.D.E.H., Steegers‐Theunissen R.P.M., Blom H.J. et al (1996) Hyperhomocysteinemia: a risk factor for placental abruption or infarction. European Journal of Obstetrics & Gynecology and Reproductive Biology 66, 23–29. [DOI] [PubMed] [Google Scholar]
- Hall Moran V. (2007a) Nutritional status in pregnant adolescents: a systematic review of biochemical markers. Maternal & Child Nutrition 3, 74–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Moran V. (2007b) A systematic review of dietary assessments of pregnant adolescents in industrialised countries. British Journal of Nutrition 97, 411–425. [DOI] [PubMed] [Google Scholar]
- Hall Moran V. & Dykes F. (2009) Complex challenges to implementing the Global Strategy for Infant and Young Child Feeding In Dykes F. and Hall Moran V. (eds) Infant and Young Child Feeding: Challenges to implementing a Global Strategy. Oxford: Wiley‐Blackwell. [Google Scholar]
- Hauguel‐de Mouzon S., Lepercq J. & Catalano P. (2006) The known and unknown of leptin in pregnancy. American Journal of Obstetrics and Gynecology 194, 1537–1545. [DOI] [PubMed] [Google Scholar]
- Herrera E. (2000) Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. European Journal of Clinical Nutrition 54, S47–S51. [DOI] [PubMed] [Google Scholar]
- Hindmarsh P.C., Geary M.P.P., Rodeck C.H., Jackson M.R. & Kingdom J.C.P. (2000) Effect of early maternal iron stores on placental weight and structure. Lancet 356, 719–723. [DOI] [PubMed] [Google Scholar]
- Hoey L., Strain J.J. & McNulty H. (2009) Studies of biomarker responses to intervention with vitamin B‐12: a systematic review of randomized controlled trials. American Journal of Clinical Nutrition 89, 1981S–1996S. [DOI] [PubMed] [Google Scholar]
- Van Het Hof K.H., West C.E., Weststrate J.A. & Hautvast J.G.A. (2000) Dietary factors that affect the bioavailability of carotenoids. Journal of Nutrition 130, 503–506. [DOI] [PubMed] [Google Scholar]
- Hollis B.W. & Wagner C.L. (2004) Assessment of dietary vitamin D requirements during pregnancy and lactation. American Journal of Clinical Nutrition 79, 717–726. [DOI] [PubMed] [Google Scholar]
- Horvath A., Koletzko B. & Szajewska H. (2007) Effect of supplementation of women in high‐risk pregnancies with long‐chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. British Journal of Nutrition 98, 253–259. [DOI] [PubMed] [Google Scholar]
- Hotz C. & Gibson R.S. (2007) Traditional food‐processing and preparation practices to enhance the bioavailability of micronutrients in plant‐based diets. Journal of Nutrition 137, 1097–1100. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2003) Perinatal biochemistry and physiology of long‐chain polyunsaturated fatty acids. Journal of Pediatrics 143, S1–S8. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2007) Fatty acids and early human development. Early Human Development 83, 761–766. [DOI] [PubMed] [Google Scholar]
- Innis S.M. & Friesen R.W. (2008) Essential n‐3 fatty acids in pregnant women and early visual acuity maturation in term infants. American Journal of Clinical Nutrition 87, 548–557. [DOI] [PubMed] [Google Scholar]
- Insel B.J., Schaefer C.A., McKeague I.W., Susser E.S. & Brown A.S. (2008) Maternal iron deficiency and the risk of schizophrenia in offspring. Archives of General Psychiatry 65, 1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E., Poston L. & Burton G.J. (2006) Placenta‐related diseases of pregnancy: involvement of oxidative stress and implication in human evolution. Human Reproduction Update 12, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid M.K., Crozier S.R., Harvey N.C., Taylor P., Inskip H.M., Godfrey K.M. et al (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367, 36–43. [DOI] [PubMed] [Google Scholar]
- Judge M.P., Harel O. & Lammi‐Keefe C.J. (2007) Maternal consumption of a docosahexaenoic acid‐containing functional food during pregnancy: benefit for infant performance on problem‐solving but not on recognition memory tasks at age 9 mo. American Journal of Clinical Nutrition 85, 1572–1577. [DOI] [PubMed] [Google Scholar]
- Kalhan S.C. (2000) Protein metabolism in pregnancy. American Journal of Clinical Nutrition 71, S1249–S1255. [DOI] [PubMed] [Google Scholar]
- Keen C.L., Clegg M.S., Hanna L.A., Lanoue L., Rogers J.M., Daston G.P. et al (2003) The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. Journal of Nutrition 133, 1597S–1605S. [DOI] [PubMed] [Google Scholar]
- King J.C. (2003) The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. Journal of Nutrition 133, 1732S–1736S. [DOI] [PubMed] [Google Scholar]
- Koebnick C., Hoffmann I., Dagnelie P.C., Heins U.A., Wickramasinghe S.N., Ratnayaka I.D. et al (2004) Long‐term ovo‐lacto. vegetarian diet impairs vitamin B‐12 status in pregnant women. Journal of Nutrition 134, 3319–3326. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Aggett P.J., Bindels J.G., Bung P., Ferré P., Gif A. et al (1998) Growth, development and differentiation: a functional food science approach. British Journal of Nutrition 80, S5–S45. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Lien E., Agostoni C., Böhles H., Campoy C., Cetin I. et al (2008) The roles of long‐chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. Journal of Perinatal Medicine 36, 5–14. [DOI] [PubMed] [Google Scholar]
- Kovacs C.S. (2008) Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. American Journal of Clinical Nutrition 88, S520–S528. [DOI] [PubMed] [Google Scholar]
- Ladipo O.A. (2000) Nutrition in pregnancy: mineral and vitamin supplements. American Journal of Clinical Nutrition 72, 280S–290S. [DOI] [PubMed] [Google Scholar]
- Lain K.Y. & Catalano P.M. (2007) Metabolic changes in pregnancy. Clinical Obstetrics and Gynecology 50, 938–948. [DOI] [PubMed] [Google Scholar]
- LARN (1996) Livelli di Assunzione Raccomandati di Energia e Nutrienti per la Popolazione Italiana. Revision. Societá Italiana di Nutrizione Umana (SINU).
- Lenders C.M., McElrath T.F. & Scholl M.T. (2000) Nutrition in adolescent. Current Opinion in Pediatrics 12, 291–296. [DOI] [PubMed] [Google Scholar]
- Locksmith G.J. & Duff P. (1998) Preventing Neural Tube Defects: the importance of periconceptional folic acid supplements. Obstetrics and Gynecology 91, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Lowe N.M., Fekete K. & Decsi T. (2009) Methods of assessment of zinc status in humans: a systematic review. American Journal of Clinical Nutrition 89, 2040S–2051S. [DOI] [PubMed] [Google Scholar]
- Lukas W.D. & Campbell B.C. (2000) Evolutionary and ecological aspects of early brain malnutrition in humans. Human Nature 11, 1–26. [DOI] [PubMed] [Google Scholar]
- Maghbooli Z., Hossein‐Nezhad A., Karimi F., Shafaei A.R. & Larijani B. (2008) Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes/Metabolism Research and Reviews 24, 27–32. [DOI] [PubMed] [Google Scholar]
- Makrides M., Crowther C.A., Gibso R.A., Gibson R.S. & Skeaff C.M. (2003) Efficacy and tolerability of low‐dose iron supplements during pregnancy: a randomized controlled trial. American Journal of Clinical Nutrition 78, 145–153. [DOI] [PubMed] [Google Scholar]
- McCaffery P.J., Adams J., Maden M. & Rosa‐Molinar E. (2003) Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. European Journal of Neuroscience 18, 457–472. [DOI] [PubMed] [Google Scholar]
- McCall K.A., Huang C‐C. & Fierke C.A. (2000) Function and mechanism of zinc metalloenzymes. Journal of Nutrition 130, 1437S–1446S. [DOI] [PubMed] [Google Scholar]
- McMillen C. & Robinson J.S. (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological Reviews 85, 571–633. [DOI] [PubMed] [Google Scholar]
- McNulty H. & Pentieva K. (2004) Folate bioavailability. Proceedings of the Nutrition Society 63, 529–536. [DOI] [PubMed] [Google Scholar]
- McNulty H. & Scott J.M. (2008) Intake and status of folate and related B‐vitamins: considerations and challenges in achieving optimal status. Intake and status of folate and related B‐vitamins: considerations and challenges in achieving optimal status. British Journal of Nutrition 99, S48–S54. [DOI] [PubMed] [Google Scholar]
- Mikkelsen T.B., Østerdal M.L., Knudsen V.K., Haugen M., Meltzer H.M., Bakketeig L. et al (2008) Association between a Mediterranean‐type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstetricia et Gynecologica Scandinavica 87, 325–330. [DOI] [PubMed] [Google Scholar]
- Milman N. (2006) Iron and pregnancy – a delicate balance. Annals of Hematology 85, 559–565. [DOI] [PubMed] [Google Scholar]
- Milman N., Bergholt T., Eriksen L., Byg K‐E., Graudal N., Pedersen P. et al (2005) Iron prophylaxis during pregnancy – How much iron is needed? A randomized dose‐response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstetricia et Gynecologica Scandinavica 84, 238–247. [DOI] [PubMed] [Google Scholar]
- Moreiras O., Carbajal A., Cabrera L. & Cuadrado C. (2007) Ingestas recomendadas de energía y nutrientes para la población española In: Tablas de composicion de alimentos, 11a edición revisada y ampliada, pp. 227–230. Ediciones Pirámide/Grupo Anaya: SA. [Google Scholar]
- Mouratidou T., Ford F., Prountzou F. & Fraser R. (2006) Dietary assessment of a population of pregnant women in Sheffield, UK. The British Journal of Nutrition 929–935. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338, 131–137. [PubMed] [Google Scholar]
- Mullis P‐E. & Tonella P. (2008) Regulation of fetal growth: consequences and impact of being born small. Best Practice & Research Clinical Endocrinology & Metabolism 22, 173–190. [DOI] [PubMed] [Google Scholar]
- Munro N.B. & Eckerman K.F. (1998) Impacts of physiological changes during pregnancy on maternal biokinetic modelling. Radiation Protection Dosimetry 79, 327–333. [Google Scholar]
- Muslimatun S., Schmidt M.K., West C.E., Schultink W., Hautvast J.G.A.J. & Karyadi D. (2001) Weekly Vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. Journal of Nutrition 131, 2664–2669. [DOI] [PubMed] [Google Scholar]
- Newnham J.P., Moss T.J.M., Nitsos I., Sloboda D.M. & Challis J.R.G. (2002) Nutrition and the early origins of adult disease. Asia Pacific Journal of Clinical Nutrition 11, S537–S542. [DOI] [PubMed] [Google Scholar]
- NNR (Nordic Nutrition Recommendations) (2004) Integrating nutrition and physical activity, 4th edn. Nordic Council of Ministers: Copenhagen. [Google Scholar]
- Oken E., Ning Y., Rifas‐Shiman S.L., Rich‐Edwards J.W., Olsen S.F. & Gillman M.W. (2007) Diet during pregnancy and risk of preeclampsia or gestational hypertension. Annals of Epidemiology 17, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega R.M., López‐Sobaler A.M., Andrés P., Martínez R.M., Quintas M.E. & Requejo A.M. (1999) Maternal vitamin E status during the third trimester of pregnancy in Spanish women: influence of breast milk vitamin E concentration. Nutrition Research 19, 25–36. [Google Scholar]
- Pardi G. & Cetin I. (2006) Human fetal growth and organ development: 50 years of discoveries. American Journal of Obstetrics and Gynecology 194, 1088–1099. [DOI] [PubMed] [Google Scholar]
- Pelto G. (1987) Cultural issues in maternal and child health and nutrition. Social Science and Medicine 25, 553–559. [DOI] [PubMed] [Google Scholar]
- Picciano M.F. (2003) Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. Journal of Nutrition 133, 1997S–2002S. [DOI] [PubMed] [Google Scholar]
- Pijls L., Ashwell M. & Lambert J. (2009) EURRECA – a Network of Excellence to align European micronutrient recommendations. Food Chemistry 113, 748–753. [Google Scholar]
- Popkin B.M. (2003) The nutrition transition in developing world. Development Policy Review 21, 581–597. [Google Scholar]
- Population Reference Bureau (2000) The World's Youth 2000. Population Reference Bureau: Washington DC. Available at: http://www.phishare.org/files/249_WorldsYouth_Eng.pdf#xml=http://www.phishare.org/cgibin/texis/webinator/elibsearch/xml.txt?query=world+youth&pr=phishare&order=r&cq=&id=4446a9d06 [Google Scholar]
- Prentice A. (1994) Maternal calcium requirements during pregnancy and lactation. American Journal of Clinical Nutrition 59, 477S–483S. [DOI] [PubMed] [Google Scholar]
- Ravelli A.C.J., Van Der Meulen J.H.P., Michels R.P.J., Osmond C., Barker D.J.P., Hales C.N. et al (1998) Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177. [DOI] [PubMed] [Google Scholar]
- Ravelli A.C.J., Van Der Meulen J.H.P., Osmond C. & Barker D.J.P. & Bleker O.P. (1999) Obesity at the age of 50 y in men and women exposed to famine prenatally. American Journal of Clinical Nutrition 70, 811–816. [DOI] [PubMed] [Google Scholar]
- Ray J.G. & Laskin C.A. (1999) Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre‐eclampsia and spontaneous pregnancy loss: a systematic review. Placenta 20, 519–529. [DOI] [PubMed] [Google Scholar]
- Rayman M.P., Bode P. & Redman C.W.G. (2003) Low selenium status is associated with the occurrence of the pregnancy disease preeclampsia in women from the United Kingdom. American Journal of Obstetrics and Gynecology 189, 1343–1349. [DOI] [PubMed] [Google Scholar]
- Redman C.W.G., Sacks G.P. & Sargent I.L. (1999) Preeclampsia: an excessive maternal inflammatory response to pregnancy. American Journal of Obstetrics and Gynecology 180, 499–506. [DOI] [PubMed] [Google Scholar]
- Ristic‐Medic D., Piskackova Z., Hooper L., Ruprich J., Casgrain A., Ashton K. et al (2009) Methods of assessment of iodine status in humans: a systematic review. American Journal of Clinical Nutrition 89, 2052S–2069S. [DOI] [PubMed] [Google Scholar]
- Roberts J.M., Balk J.L., Bodnar L.M., Belizán J.M., Bergely E. & Martinez A. (2003) Nutrient involvement in preeclampsia. Journal of Nutrition 133, 1684S–1692S. [DOI] [PubMed] [Google Scholar]
- Ronnenberg A.G., Wood R.J., Wang X., Xing H., Chen C., Chen D. et al (2004) Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. Journal of Nutrition 134, 2586–2591. [DOI] [PubMed] [Google Scholar]
- Roseboom T.J., Van Der Meulen J.H.P., Osmond C., Barker D.J.P., Ravelli A.C.J., Schroeder‐Tanka J.M. et al (2000) Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84, 595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom T.J., Van Der Meulen J.H.P., Ravelli A.C.J., Osmond C., Barker D.J.P. & Bleker O.P. (2001) Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Molecular and Cellular Endocrinology 185, 93–98. [DOI] [PubMed] [Google Scholar]
- Ross A.C. (2006) Vitamin A and carotenoids In: Modern Nutrition in Health and Disease (eds Shils M.E., Shike M., Ross A.C., Caballero B. & Cousins R.J.), 10th edn, pp. 351–375. Lippincott Williams & Wilkins: Baltimore, USA. [Google Scholar]
- Ryan‐Harshman M. & Aldoori W. (2008) Vitamin B12 and health. Canadian Family Physician 54, 536–541. [PMC free article] [PubMed] [Google Scholar]
- Salle B.L., Delvin E.E., Lapillonne A., Bishop N.J. & Glorieux F.H. (2000) Perinatal metabolism of vitamin D. American Journal of Clinical Nutrition 71, S1317–S1324. [DOI] [PubMed] [Google Scholar]
- Scholl T.O. (2005) Iron status during pregnancy: setting the stage for mother and infant. American Journal of Clinical Nutrition 81, 1218S–1222S. [DOI] [PubMed] [Google Scholar]
- Scholl T.O. & Hediger M.L. (1994) Anemia and iron‐deficiency anemia: compilation of data on pregnancy outcome. American Journal of Clinical Nutrition 59, 492S–501S. [DOI] [PubMed] [Google Scholar]
- Shah R.S., Rajalakshmi R., Bhatt R.V., Hazra M.N., Patel B.C., Swamy N.B. & Patell T.V. (1987) Liver stores of vitamin A in human fetuses in relation to gestational age, fetal size and maternal nutritional status. British Journal of Nutrition 58, 181–189. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A.M., Herrmann T.S., Savitz D.A. & Thorp J.M. (2001) Frequency of eating during pregnancy and its effect on preterm delivery. American Journal of Epidemiology 153, 647–652. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A.M., Hartzema A.G., Turnbull C., Thorp J., McDonald T. & Cogswell M.E. (2006) The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. American Journal of Obstetrics and Gynecology 194, 512–519. [DOI] [PubMed] [Google Scholar]
- Sørensen H.J., Nielsen P.R., Pedersen C.B. & Mortensen P.B. (2010) Association between prepartum maternal iron deficiency and offspring risk of schizophrenia: population‐based cohort study with linkage of Danish national registers. Schizophrenia Bulletin doi:10.1093/schbul/sbp167. [DOI] [PMC free article] [PubMed]
- Steen M.T., Boddie A.M., Fisher A.J., Macmahon W., Saxe D., Sullivan K.M. et al (1998) Neural‐tube defects are associated with low concentrations of cobalamin (Vitamin B12) in amniotic fluid. Prenatal Diagnosis 18, 545–555. [PubMed] [Google Scholar]
- Strobel M., Tinz J. & Biesalski H‐K. (2007) The importance of b‐carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. European Journal of Nutrition 46, I/1–I/20. [DOI] [PubMed] [Google Scholar]
- Sunde R.A., Paterson E., Evenson J.K., Barnes K.M., Lovegrove J.A. & Gordon M.H. (2008) Longitudinal selenium status in healthy British adults: assessment using biochemical and molecular biomarkers. British Journal of Nutrition 99, S37–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajewska H., Horvath A. & Koletzko B. (2006) Effect of n–3 long‐chain polyunsaturated fatty acid supplementation of women with low‐risk pregnancies on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition 83, 1337–1344. [DOI] [PubMed] [Google Scholar]
- Tamura T. & Picciano M.F. (2006) Folate and human reproduction. American Journal of Clinical Nutrition 83, 993–1016. [DOI] [PubMed] [Google Scholar]
- Thomas M. & Weisman S.M. (2006) Calcium supplementation during pregnancy and lactation: effects on the mother and the fetus. American Journal of Obstetrics and Gynecology 194, 937–945. [DOI] [PubMed] [Google Scholar]
- Vanderlelie J., Venardos K., Clifton V.L., Gude N.M., Clarke F.M. & Perkins A.V. (2005) Increased biological oxidation and reduced anti‐oxidant enzyme activity in pre‐eclamptic placentae. Placenta 26, 53–58. [DOI] [PubMed] [Google Scholar]
- Vujkovic M., Ocke M.C., Van Der Spek P.J., Yazdanpanah N., Steegers E.A. & Steegers‐Theunissen R.P.M. (2007) Maternal Western dietary patterns and the risk of developing a cleft lip with or without a cleft palate. Obstetrics and Gynecology 110, 378–384. [DOI] [PubMed] [Google Scholar]
- Weissgerber T.L. & Wolfe L.A. (2006) Physiological adaptation in early human pregnancy: adaptation to balance maternal–fetal demands. Applied Physiology, Nutrition, and Metabolism 31, 1–11. [DOI] [PubMed] [Google Scholar]
- West J.K.P., Katz J., Khatry S.K., LeClerq S.C., Pradhan E.K., Shrestha S.R. et al (1999) Double blind, cluster randomised trial of low dose supplementation with vitamin A or β‐carotene on mortality related to pregnancy in Nepal. British Medical Journal 318, 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson C.S. (2006) Nutrition in pregnancy. Nutrition Bulletin 31, 28–59. [Google Scholar]
- Wood R.J. & Ronnenberg A.G. (2006) Iron In: Modern Nutrition in Health and Disease (eds Shils M.E., Shike M., Ross A.C., Caballero B. & Cousins R.J.), 10th edn, pp. 248–270. Lippincott Williams & Wilkins: Baltimore, USA. [Google Scholar]
- World Health Organization (2004) Adolescent pregnancy. Issues in adolescent health and development WHO Discussion Papers on Adolescence. World Health Organization: Geneva, Switzerland. [Google Scholar]
- World Health Organization and Food and Agriculture Organization of the United Nations (2004) Folate and folic acid In: Vitamin and Mineral Requirements in Human Nutrition (FAO/WHO Report), 2nd edn, pp 289–302. World Health Organization and Food and Agriculture Organization of the United Nations: Rome. [Google Scholar]
- Yu C.K.H., Sykes L., Sethi M., Teoh T.G. & Robinson S. (2009) Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology 70, 685–690. [DOI] [PubMed] [Google Scholar]
- Zimmerman M.B. (2008) Methods to assess iron and iodine status. British Journal of Nutrition 99, S2–S9. [DOI] [PubMed] [Google Scholar]
- Zimmermann M.B. (2009) Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. American Journal of Clinical Nutrition 89, S668–S672. [DOI] [PubMed] [Google Scholar]


