Abstract
Undernutrition is associated with poor cognitive development, late entry into school, decreased years of schooling, reduced productivity and smaller adult stature. We use longitudinal data from 1674 Peruvian children participating in the Young Lives study to assess the relative impact of early stunting (stunted at 6–18 months of age) and concurrent stunting (stunted at 4.5–6 years of age) on cognitive ability. Anthropometric data were longitudinally collected for children at 6–18 months of age and 4.5–6 years of age at which time verbal and quantitative ability were also assessed. We estimate that an increase in concurrent height‐for‐age z‐scores (HAZ) by one standard deviation was associated with an increase in a child's score on the Peabody Picture Vocabulary Test (PPVT) by 2.35 points [confidence interval (CI): 1.55–3.15] and a 0.16 point increase on the cognitive development assessment (CDA) (CI: 0.05–0.27). Furthermore, we report that the estimate for concurrent HAZ and PPVT is significantly higher than the estimate for early stunting and PPVT. We found no significant difference between early and concurrent estimates for HAZ and CDA. Children from older mothers, children whose mothers had higher education levels, children living in urban areas, children who attended pre‐school, children with fewer siblings and children from wealthier backgrounds scored higher on both assessments. Cognitive skills of children entering school were associated with early stunting but the strongest association was found with concurrent stunting suggesting that interventions preventing linear growth faltering should not only focus on the under 2s but include children up to 5 years of age.
Keywords: stunting, chronic undernutrition, cognitive development, pre‐school children, Peru
Introduction
Approximately 150 million children, or roughly one‐quarter of children worldwide, experience stunting (low height‐for‐age), while 20% are underweight (de Onis 2008). Children who are moderately to severely underweight have a 5–8 times greater risk of dying than well‐nourished children. Even children who are mildly underweight have a risk of death that is twice as high as well‐nourished children (Black et al. 2003). Undernutrition is responsible for 2.2 million deaths and 21% of Disability‐Adjusted Life‐Years (DALYs) among children less than 5 years of age. Maternal and child undernutrition is estimated to account for 3.5 million deaths and 11% of all global DALYs (Black et al. 2008). Micronutrient deficiencies account for 10% of deaths and DALYs in children less than 5 years of age (Bhutta et al. 2008).
Undernutrition is also associated with poor developmental outcomes (Adair 1999; Berkman et al. 2002; Li et al. 2003; Daniels & Adair 2004; Alderman et al. 2006; Victora et al. 2008). Children who experience stunting in early childhood are more likely to have vocabulary deficits (Sigman et al. 1991; Walker et al. 2000; Grantham‐McGregor 2002) and other deficiencies in school performance and intelligence (Grantham‐McGregor 1995). As Grantham‐McGregor and colleagues have noted (2007), with respect to poor outcomes associated with undernutrition, death is the tip of the iceberg.
While much is known about the association between undernutrition and cognitive development, gaps in knowledge persist. National level statistics on cognitive development in children are lacking. While there are numerous cross‐sectional studies indicating an association between concurrent undernutrition and cognitive ability, there are few longitudinal studies that do so (Grantham‐McGregor et al. 2007). The major longitudinal studies linking undernutrition to poor cognitive development evaluate the relationship between undernutrition and early development (e.g. motor development) as well as cognitive development in adolescence or later. Some longitudinal studies examine the association between early nutritional insult and cognitive abilities of children entering school (Cheung et al. 2001; Berkman et al. 2002; Kuklina et al. 2004; Cheung 2006) but additional longitudinal studies are needed.
In this paper, we describe the prevalence of undernutrition among Peruvian children in early infancy (6–18 months of age) and childhood (4.5–6 years of age). We test the hypothesis that early stunting (at 6–18 months of age) has a greater impact on cognitive abilities of children entering school than concurrent stunting (4.5–6 years of age).
Key messages
-
•
Factors that put children at risk of poor cognitive development include poverty, living in a rural environment, low maternal education and age, more siblings and failure to attend pre‐school.
-
•
In some settings, concurrent nutritional status may be at least as important as early nutritional status in predicting cognitive performance.
-
•
Stunting should be assessed beyond 18 months of age.
-
•
Broad‐based efforts to promote adequate nutrition should aim to reduce stunting during infancy and should continue at least to when children enter school.
-
•
Efforts to address stunting and poor cognitive performance must involve parents, clinicians, educators, programme planners and implementers, and policy makers.
Materials and methods
Study design and background
The Young Lives study, which uses a prospective cohort design, is a multi‐country research study investigating the consequences of childhood poverty, how poverty is passed from one generation to the next, and the effectiveness of poverty‐reduction policies (http://www.younglives.org.uk). The study has followed 12 000 children since 2002 and plans are to follow these children over a total period of 15 years. The study includes four countries: Ethiopia, India, Peru and Vietnam. These countries were selected to represent a broad range of political, social, geographical, and cultural contexts and circumstances. Each country follows two groups of children, one group beginning at approximately 1 year of age and the other enrolled at about 8 years of age. To meet our study objectives, we describe methods related to the sampling of children from the younger Peruvian cohort.
The study covers urban, peri‐urban and rural areas including respondents from all three main geographic regions in Peru – coastal, highland and jungle. Impoverished populations were oversampled. The study is managed in Lima, Peru, by researchers from the Instituto de Investigación Nutricional (IIN) and Grupo de Análisis para el Desarrollo.
Study participants
In the first round in 2002, 2052 children aged 6–17.9 months from 74 communities were recruited. A multistage sampling strategy was used. Districts were chosen by employing a list ranking of all the districts in Peru according to poverty level. The poverty rank was based on measures of infant mortality, schooling, housing and access to services. The highest‐ranking 5% of the districts were excluded to ensure that the study oversampled impoverished districts. Researchers then systematically selected 20 districts using a randomly selected individual starting point and fixed population interval. This randomization process was repeated 10 times until researchers arrived at a sampling framework that included the geographical areas of Amazon jungle, mountains and coast with a spread across the whole country. Using maps of each district, a house was then randomly chosen as the starting point and subsequent houses were visited in systematic fashion until 100 children were enrolled (Wilson et al. 2006). Because some districts were too small to yield 100 households with eligible children, contiguous districts were added until 100 children were enrolled.
This process resulted in a sample that approximates to 95% of the children in Peru excluding the wealthiest 5% of households. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the London South Bank University, London School of Hygiene and Tropical Medicine, University of Reading and the Ethics Committee of the IIN in Lima, Peru. Written informed consent was obtained from the parent or legal guardian of the children enrolled in the study. For illiterate respondents, verbal consent was obtained by interviewers prior to the interviews and was witnessed and formally recorded. Study approval was also obtained from Peru's Ministry of Health, and from local authorities and community leaders.
Data collection
Interview questionnaires were developed by international experts from several fields. The questionnaires consisted of a core survey used in all four countries participating in the Young Lives study. A community questionnaire was also used to assess population size (e.g. urban vs. rural) and geographic location (e.g. jungle, highland, coast). Investigators from each country added country‐specific questions. The questionnaire used in Peru included information on the following: household composition, child health (including acute and chronic illness), caregiver characteristics, livelihoods, socio‐economic status (assets), social capital, anthropometry of the child, child care and cognitive development.
Anthropometric data were obtained through the use of standardized digital platform scales (Soehnle, Nassau, Germany) accurate to 100 g and locally made rigid measures accurate to 2 mm, with fixed head and moveable foot piece. The core questionnaire was translated into Spanish, and then revised and modified through field testing and a pilot study before being used to collect data from the full cohort. Fieldworkers were trained extensively before interviewing began with special emphasis on economic sections of the questionnaire and how to properly use the questionnaire in rural areas. Members from each team were trained and standardized in anthropometric measurement according to World Health Organization (WHO) protocols. The training of fieldworkers culminated in a large pilot study where the data collection instrument, fieldworkers and entire data collection system were tested in a mixed rural‐urban district. The process of training, testing and revision took approximately 3 months.
First‐round data were collected in 2002 when children were 6–18 months of age. Second‐round data collection took place in 2006 and 2007 when the same children were 4.5–6 years old. Data collection was conducted by three teams consisting of six interviewers each. Field managers and study investigators oversaw all three teams. Each of the three teams worked in 6–7 districts. Interviews lasted 2–4 h and each district took 2–3 weeks to complete 100 interviews. In total, more than 40 000 households were contacted in order to find and enrol both the younger and older cohort of children.
Investigators used several methods to minimize the loss of participants at follow‐up. First, after the initial enrolment and baseline interview, participants received information about the study and at each subsequent visit they were given a present of a certificate with a photo of the child at each round, families were asked to give names and addresses or telephone numbers for family or friends with whom they were likely to maintain contact. The field staff visited the household once between the data gathering rounds in order to maintain contact and deliver the photos. Families were also asked to let the project team know if they moved and were given telephone numbers and a stamped addressed envelope to facilitate this. Finally, during round 2, interviewers returned to the home as many times as needed until they were able to conduct the interview, were turned away or were able to confirm the person no longer lived at the residence. Households with participating children were contacted almost annually to ensure continued involvement in the study. Additionally, children who did move were still contacted. If children moved within Peru, every effort was made to interview them. If children moved outside the country, they were contacted, but no effort was made to interview them. Only 3.5% of children were lost to follow‐up during the 4 years between round 1 and round 2.
Data from round 1 were entered using Delphi (Austin, TX, USA), a data entry software program, and then transferred into Microsoft Access (version 2000, Seattle, WA, USA). Data from round 2 were entered directly into Microsoft Access (round 2). Both data entry systems used pre‐programmed skip patterns and data and acceptable range controls. Additional information on the Young Lives Peruvian cohorts can be found at http://www.ninosdelmilenio.org/.
Cognitive outcomes
Several cognitive development assessments (CDAs) were pilot tested in the field. Based on these results, Young Lives researchers selected the Peabody Picture Vocabulary Test (PPVT) to assess listening comprehension and vocabulary skills, and the CDA to assess quantitative reasoning of children 4.5–6 years of age. The PPVT has been in use since 1959 and has several variations (PPVT‐I, PPVT‐II, PPVT‐III and PPVT‐Revised). The PPVT has shown a strong correlation with other intelligence measures such as the Wechsler and McCarthy Scales (Campbell 1998; Gray et al. 1999; Campbell et al. 2001). The PPVT‐R (Spanish version) consists of 125 items and was used for the Young Lives cohort (Dunn et al. 1986; Dunn & Dunn 1997). The PPVT has been used extensively as a measure of cognitive ability in many studies (Desai et al. 1989, Baydar & Brooks‐Gunn 1991; Blau & Grossberg 1992; Parcel & Menaghan 1994; Rosenzweig & Wolpin 1994; Grantham‐McGregor et al. 1997; Blau 1999; 2000, 2005; McCulloch & Joshi 2002; Kordas et al. 2004; Paxson & Norbert 2007). The Spanish version was reviewed by local language experts and then pilot tested to ensure that respondents were able to understand the questions.
The PPVT is orally administered, not timed and is given individually. During the PPVT, the child is presented with a stimulus word and a set of pictures, and is asked to select the picture that best represents the word's meaning. Test items are ordered from easiest to hardest. Each child is only given items within his or her critical range and is not given the entire set of questions. The critical range is established by the child's chronological age, which corresponds with a specific start point on the test. The test administrator begins at the starting point and proceeds until a ceiling (the point at which the respondent has 6 errors in a stretch of 8 responses) is reached. The PPVT yields raw scores that can then be standardized using a sample of PPVT scores for other Latin American countries (Dunn et al. 1986).
The CDA was developed by the International Evaluation Association for the purpose of studying the impact of attending pre‐school on cognitive development in 4‐year‐old children. The CDA has three main components or subtests: quantity, time and spatial relations. The Young Lives study used only the quantity subtest, which measures a child's notion of amount. Young Lives researchers pilot tested both the spatial relations and time subtests. The spatial relations subtest was not used in the Young Lives study because of the large amount of time it took to administer. The time subtest was not used because of low reliability among the Young Lives sample in Peru. The CDA was translated into Spanish for use in the Young Lives cohort. The translation was verified by a local Spanish language expert and then pilot tested.
The CDA quantity subtest consists of 15 items. Each item includes three or four images that the child is asked to consider in response to a question posed by the interviewer. For example, the interviewer shows the child a picture with several smaller images of cats and dogs, and says, ‘Look at the cats and dogs and point to the picture where the dog has less food than the cat.’ The child must then consider the different amounts of food and quantify them in order to determine the correct picture. Correct responses receive a score of 1 point while incorrect responses are given a score of 0.
Validity and reliability were established during pilot testing to ensure the appropriateness of using the PPVT and the CDA for the Young Lives sample. Young Lives researchers measured validity by assessing the degree to which evidence and theory supported the interpretations of test scores. Researchers estimated the correlation between each test score and variables such as age and educational level to determine whether these measures were supported by previous empirical evidence from the literature. For example, on average, children in higher grades of school should get better results than children in lower grades or children who no longer attend school. Furthermore, parental education should be positively correlated with scores on tests. Reliability was established according to Classical Test Theory (CTT) and Item Response Theory (IRT) (Crocker & Algina 1986; Baker & Kim 2004). The CTT was applied by using split‐half reliability coefficients to assess internal consistency. This was followed by the estimation of reliability coefficients using the Spearman‐Brown prophecy formula. The IRT was applied by using the person reliability index. Both assessments were found to have acceptable psychometric properties based on both CTT and IRT for children who spoke Spanish as their native language. Native Quechua speakers scored below the acceptable thresholds for both measures. For example, for Quechua speakers, Cronbach's alpha for the reliability coefficients of the CDA were below acceptable standards for the CTT (the acceptable level was 0.6 but the level for Quechua speakers was 0.4). The person reliability index among Quechua speakers (0.4) was also below the standard (0.5). The relatively poor performance of the tests in Quechua speakers was thought to be due to difficulties with the translation particularly due to the variation in the vocabulary of Quechua spoken in different parts of the country. As a result, only Spanish speakers (n = 1706) were included in our analyses.
Covariates
Height‐for‐age z‐scores from both rounds of data were used. Z‐scores are based on the international reference standard from the WHO (available at http://www.who.int/childgrowth/en/). The z‐score is calculated through an interpolation function that accounts for sex, age and height. We defined moderate stunting as a height‐for‐age z‐score (HAZ) between −2.0 and −2.99 standard deviations below the mean on the international reference standard. We defined severe stunting as an HAZ less than −3.0 standard deviations below the mean on the international reference standard. An HAZ less than −2.0 from round 1 was categorized as early stunting, while an HAZ less than −2.0 from round 2 was referred to as concurrent stunting, because it was concurrent with our cognitive assessments. Additionally, we calculated weight‐for‐age and weight‐for‐height z‐scores based on the same cut‐offs used for stunting. Because there were few children with low weight‐for‐height, we did not include this variable as a predictor of cognitive ability.
Using the conceptual framework outlined by Black and colleagues for the determinants and consequences of undernutrition, we controlled for potential confounding variables available in the Young Lives study (Black et al. 2008). These variables included age, sex, site (urban/rural), region (coast/highland/jungle), maternal education, maternal age, religion, pre‐school attendance, primary school attendance and a wealth index. The wealth index is based on work from the World Bank and is used in UNICEF's Multiple Indicator Cluster Surveys. The index is a continuous score from 0 to 1 that is a composite of housing quality, consumer durables and services (e.g. drinking water, toilet, electricity) (Filmer & Pritchett 2001).
Data analysis
Z‐scores were calculated with the EpiNut module of Epi Info (version 2000, Centers for Disease Control and Prevention, Atlanta, GA, USA). All statistical analyses were conducted using Statistical Analysis Systems statistical software version 9.1 (SAS Institute, Cary, NC, USA). Study participants and prevalence of stunting, wasting and underweight were described. More than 2000 children were enrolled at baseline; however, we only analysed data for children who had anthropometric information at baseline and follow‐up, and who spoke Spanish (n = 1674).
In order to test our hypothesis that early stunting (6–18 months of age) has a greater impact on the cognition of children entering school than concurrent stunting (4.5–6 years of age), we went through three processes. First, we reviewed the literature and conducted univariate analyses to identify variables that should be included in multivariate models. We then used mixed effects regression to determine the relative impact of early vs. concurrent stunting (when children were 6–18 months of age and 4.5–6 years of age, respectively) on each cognitive outcome. Finally, we compared the coefficients for early and concurrent stunting for each cognitive model using a Wald test. This test determined whether or not the coefficients for each model were significantly different. Mixed effects regression models are considered appropriate when sampling is based on clusters. We present unadjusted models as well as models that account for known confounders. With the MIXED procedure from SAS, we produced models with an unrestricted covariance structure for random effects, a random intercept for variation between clusters and fixed effects for all other variables in the model (e.g. early stunting, concurrent stunting, maternal age, maternal education, area population, pre‐school attendance, child age, wealth index and number of siblings). We retained or dropped variables from the models based on P‐values (<0.1) and conceptual considerations, and reported regression coefficients and 95% confidence intervals (CI) for all retained variables. We checked all models for interaction and for compliance with model assumptions. Interaction terms were checked for all retained variables for each model. None was retained based on P‐values (<0.1).
Results
Of all survey respondents, nearly three‐quarters lived in urban areas; most lived in coastal (40.2%) or highland (43.0%) regions compared with the jungle region (16.8%), and nearly half of all children were female (Table 1). Catholicism was the predominant maternal religion and nearly all mothers described themselves as mestizo. The mean age of children during the first round was 12 months, 86.1% of children attended pre‐school, and the mean score for wealth index was 0.42.
Table 1.
Characteristics of study participants
| Characteristics* | N = 1675 † |
|---|---|
| Child characteristics | |
| Child age (months) | 12.04 (3.55) ‡ |
| Sex, female (%) | 49.46 |
| Attended pre‐school, yes (%) | 86.14 |
| Mother characteristics | |
| Maternal age (years) | 26.69 (6.62) |
| Maternal education in years | 8.74 (3.98) |
| Maternal ethnicity (%) | |
| Caucasian | 4.96 |
| Mestizo | 91.88 |
| Indigenous § | 2.57 |
| Other | 0.60 |
| Household characteristics | |
| Region (%) | |
| Coast | 40.20 |
| Highland | 43.01 |
| Jungle | 16.79 |
| Area (%) | |
| Urban | 74.07 |
| Rural | 25.93 |
| Wealth index (score 0–1) | 0.42 (0.21) |
Data reported in table come from round 1 (when child was 6–18 months of age) except pre‐school attendance.
† Maximum missing value for any category was 11 (or 0.7%).
‡ Mean (standard deviation).
§ Indigenous could mean any of a variety of different ethnic groups living in the highlands, coastal areas or jungle.
Prevalence of stunting, wasting and underweight in round 1 are described in Table 2. The percentage of children who were stunted in round 2 rose approximately 5 percentage points while prevalence of underweight children in round 2 decreased slightly.
Table 2.
Prevalence of stunting, wasting and underweight by round
| Characteristics | Round 1 | Round 2 |
|---|---|---|
| N = 1675* | N = 1675* | |
| Stunted (%) | 22.94 | 27.80 |
| Wasted (%) | 1.63 | – |
| Underweight (%) | 4.82 | 4.31 |
Maximum missing value for any measure was 16 or (1.0%).
Figure 1 illustrates the distribution of HAZ for children from each round of data collection. The figure does not depict changes across time for individual children, but does display general trends in the population by age. During the first round of data collection, there is a general decline in HAZ from 6–18 months of age. Children from urban and rural areas appear to decline at a similar rate. Additional decline takes place between 18 and 56 months of age.
Figure 1.
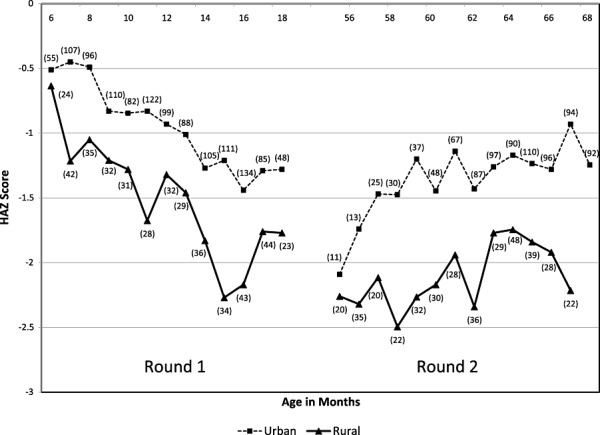
Cross‐sectional* data for median height‐for‐age z‐score (HAZ) by age at assessment and area for round 1 and round 2. *Child was measured once during round 1 and once during round 2; () = sample size for age group.
Unadjusted regression estimates between covariates and verbal score are presented in Table 3. Children from urban areas performed better on verbal cognitive tests than those from rural areas. Higher early and concurrent HAZ scores were also associated with better verbal scores. Those who attended pre‐school fared better than those who did not. Additional variables significantly associated with verbal score were wealth index, maternal age and number of siblings. A higher level of completed maternal education was also associated with a higher verbal score.
Table 3.
Linear regression results for predictors of verbal score
| Independent variable* | n | Unadjusted model † | Adjusted model ‡ | ||
|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||
| Intercept | – | – | 62.90 | 57.27, 68.53 | |
| Round 1 HAZ | 1649 | 2.63 | 1.90, 3.37 | 0.20 | −0.59, 0.99 |
| Round 2 HAZ | 1649 | 4.70 | 3.87, 5.53 | 2.23 | 1.29, 3.17 |
| Maternal age in years | 1649 | 0.06 | −0.06, 0.19 | 0.24 | 0.11, 0.38 |
| Maternal education in years | 1649 | 1.99 | 1.77, 2.20 | 1.39 | 1.15, 1.62 |
| Area population | |||||
| Rural | 421 | – | – | – | – |
| Urban | 1228 | 11.35 | 8.34, 14.35 | 4.58 | 1.81, 7.35 |
| Pre‐school attendance | |||||
| No | 226 | – | – | – | – |
| Yes | 1423 | 9.86 | 7.30, 12.43 | 3.69 | 1.29, 6.08 |
| Wealth index | 1649 | 44.26 | 37.32, 51.21 | 20.23 | 13.14, 27.31 |
| Number of siblings | 1649 | −2.11 | −2.68, −1.54 | −1.14 | −1.78, −0.50 |
CI, confidence interval; HAZ, height‐for‐age z‐score. *Data reported in table come from round 1 (when child was 6–18 months of age) except pre‐school attendance, wealth index and number of siblings. †Unadjusted models include an individual model for each independent variable. ‡Post‐regression Wald test comparing HAZ coefficients from both rounds = 6.97, P‐value = 0.0083.
Adjusted results demonstrate that children with higher concurrent z‐scores, children with older mothers, children with mothers who had increased education, children living in urban areas, children who attended pre‐school, children with fewer siblings and children from wealthier circumstances were more likely to score higher on the PPVT verbal assessment than their counterparts (Table 3).
What is most notable about findings presented in Table 3 is the large difference between adjusted estimates for early and concurrent stunting: improving HAZ of infants by one standard deviation increased their score on the PPVT by only 0.20 points (95% CI: −0.59, 0.99) but improving their concurrent HAZ (i.e. when they were 4.5–6 years of age) contributed to a 2.23 point increase (95% CI: 1.29, 3.17). Furthermore, we found that the differences in estimates were statistically significant (P‐value = 0.0083). Thus, concurrent stunting had a greater impact on vocabulary test scores than early stunting.
Table 4 displays unadjusted regression estimates between covariates (including HAZ) and quantitative scores. The child's age at round 1, maternal education, wealth index and number of siblings were all significantly associated with quantitative scores. In addition, higher early and concurrent HAZ scores were associated with higher quantitative scores. The adjusted model was similar except early stunting was no longer associated with quantitative score. As with verbal scores, the impact of concurrent stunting on CDA scores appeared greater than the impact of early stunting. However, the difference between coefficients was not significant. Thus, we cannot conclude that concurrent stunting had a significantly greater impact on CDA scores than early stunting despite the notable difference in magnitude.
Table 4.
Linear regression results for predictors of quantitative score
| Independent variable* | n | Unadjusted model † | Adjusted model ‡ | ||
|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||
| Intercept | – | – | 6.68 | 5.89, 7.47 | |
| Round 1 height‐for‐age z score | 1649 | 0.10 | 0.01, 0.20 | 0.03 | −0.09, 0.14 |
| Round 2 height‐for‐age z score | 1649 | 0.36 | 0.25, 0.47 | 0.15 | 0.02, 0.28 |
| Child Age | 1649 | 0.12 | 0.09, 0.15 | 0.11 | 0.08, 0.14 |
| Maternal age in years | 1649 | 0.01 | −0.01, 0.03 | 0.02 | 0.00, 0.04 |
| Maternal education in years | 1649 | 0.14 | 0.11, 0.17 | 0.09 | 0.05, 0.12 |
| Area population | |||||
| Rural | 421 | – | – | – | – |
| Urban | 1228 | 1.14 | 0.77, 1.51 | 0.56 | 0.20, 0.93 |
| Pre‐school attendance | |||||
| No | 226 | – | – | – | – |
| Yes | 1423 | 1.22 | 0.89, 1.55 | 0.54 | 0.21, 0.88 |
| Wealth index | 1649 | 3.67 | 2.77, 4.58 | 1.60 | 0.63, 2.57 |
| Number of siblings | 1649 | −0.15 | −0.23, −0.08 | −0.10 | −0.19, −0.02 |
CI, confidence interval; HAZ, height‐for‐age z‐score. *Data reported in table come from round 1 (when child was 6–18 months of age) except pre‐school attendance, wealth index and number of siblings. †Unadjusted models include an individual model for each independent variable. ‡Post‐regression Wald test comparing HAZ coefficients from both rounds = 1.26, P‐value = 0.2622.
Coefficients for models where the CDA test score is the dependent variable are small in comparison with coefficients for PPVT scores. However, it must be remembered that there were approximately 10 times as many items on the PPVT. Thus, multiplying coefficients in models predicting CDA test scores by 10 helps when comparing coefficients from both models.
Because of the very low prevalence of wasting at the early assessment, we did not model the relationship between wasting and the cognitive measures.
Discussion
We show that there are a variety of factors that predict verbal and quantitative test scores among young Peruvian children. These include wealth, living in an urban setting, increased maternal education and age, pre‐school attendance and fewer siblings. Our findings also indicate that children who demonstrate linear growth restriction concurrent with cognitive measurements when they enter school (4.5–6 years of age) are more likely to score poorly on cognitive tests than their better‐nourished peers. While our findings do not demonstrate an association between children who experience linear growth restriction at an early age (6–18 months) poor performance on cognitive tests when controlling for concurrent growth restriction, models with only early stunting (i.e. no concurrent stunting indicator in the model) do show a strong association between early HAZ and both cognitive outcomes (data not shown). What is more notable about our results is the greater impact of concurrent stunting than early stunting, both for verbal and for quantitative measures of cognition. These findings do not support our hypothesis that early stunting is more important in predicting cognition than concurrent stunting. Our results, though, are consistent with cross‐sectional studies that demonstrate associations between early nutritional insult and intelligence scores during the early school years (Agarwal et al. 1989; Beasley et al. 2000; Cheung 2006; Paxson & Norbert 2007) as well as longitudinal studies that report an association between early nutrition and later adolescent cognitive ability and academic achievement (Martorell et al. 1992; Grantham‐McGregor 1995; Grantham‐McGregor et al. 1997; Martorell 1997; Hack 1998; Mendez & Adair 1999; Beasley et al. 2000; Cheung et al. 2001; Glewwe et al. 2001; Berkman et al. 2002; Ivanovic et al. 2004; Kuklina et al. 2004). However, the results reported here examine intelligence at the time that students enter school, which has not been well studied using longitudinal data.
Our results illustrate the importance of assessing HAZ beyond 18 months of age. Specifically, in this sample, children continued to experience stunting past 18 months, sometimes falling an additional standard deviation between 18 and 56 months of age. This is consistent with findings that show that height‐for‐age scores begin faltering immediately after birth and continue into the third year of life (Martorell 1999; Shrimpton et al. 2001).
We found a strong association between wealth index and cognitive scores. This is similar to findings from a recent study in Ecuador that demonstrated an association between socio‐economic status and verbal scores among pre‐school children (Paxson & Norbert 2007). Additionally, our findings are similar to those of Daniels using data from the Philippines. She found associations between cognitive scores and birth order, maternal and paternal education, maternal height, household assets, number of siblings, household income, place of residence, presence of electricity and environmental cleanliness (Daniels & Adair 2004).
Stunting was common in this sample: more than 1 in 5 children were stunted when 6–18 months and 4.5–6 years old (22.9% and 27.8%, respectively). The level of stunting at 6–18 months of age (22.9%) was the same as that reported by a national survey (Demographic and Health Surveys 2000) for Peruvian children 6–23 months of age. In 2007, the percent of children in the Young Lives (YL) cohort who were stunted at 4.5–6 years of age was slightly higher than for children 48–59 months of age in the Demographic and Health Surveys (27.8% and 24.9%, respectively) (Demographic and Health Surveys 2008). Weight‐for‐height Z‐scores were similar for YL children 6–18 months old and DHS children 6–23 months of age (1.6% and 1.5%, respectively). For children at about 5 years of age, 0.8% were low weight‐for‐height among the DHS sample and 0% among YL children. While underweight was more common among DHS 1‐year‐olds than the YL cohort (8.7% vs. 4.8%), there were similar levels of underweight among 5‐year‐olds (4.8% DHS and 4.3% YL).
Our study suffers from several limitations. While this study benefits from a longitudinal design, the timing of assessment during the first round is problematic. Children received one anthropometric assessment sometime between 6 and 18 months of age. As a result, children who were assessed early were likely to experience more post‐assessment growth restriction than children who were assessed later in the 6–18 month period, thus limiting the comparability of the HAZ between children at round 1. Previous research suggests that height‐for‐age at 2 years is the best predictor of human capital (Victora et al. 2008). Because we lacked anthropometric measures at 2 years of age, we evaluated early and concurrent HAZ separately to determine how these time points might differ in predicting cognition. When comparing findings from HAZ measures at both time points, concurrent stunting had a noticeably greater impact on verbal and quantitative test scores than early stunting. It may be that concurrent stunting is a better predictor of cognition than early nutritional insult but additional research is needed to confirm or refute this conclusion.
An additional limitation of this research is the degree to which this sample represents Peru as a whole. Young Lives researchers deliberately oversampled from poorer communities. Also, because the reliability and validity of the cognitive assessments was not adequate for Quechua‐speaking children, nearly 300 indigenous children were excluded from the study. Based on Young Lives data, it can be noted that Quechua children were less likely than Spanish‐speaking children to attend pre‐school (73.6% vs. 85.5%, respectively). In addition, their mothers were more likely to have no education whatsoever (40.3% vs. 4.6%, respectively). However, while the nature of the sampling design and the exclusion of Quechua‐speaking children limit the generalizability of these findings to all Peruvian children, HAZ measures from our data are quite similar to nationally representative data collected by two demographic surveys conducted in the past decade.
It should also be noted that the quantitative assessment of cognition in Peru excluded the spatial relations and time subtests; consequently, the CDA reflected children's notions of quantity only. Lastly, despite the longitudinal design of this study, it is constrained by the observational nature of data collection. Thus, we are unable to fully control for unfavourable environmental factors such as caregiving behaviours that restrict both growth and delay or disrupt cognitive development (Grantham‐McGregor 2002).
For the past several decades, academicians, policy makers, donors, and programme planners and implementers have rightfully advocated for the prevention of undernutrition during infancy. However, our findings suggest that when cognition is the outcome of interest, it is equally important to ensure that older children are well nourished. These findings argue for a more comprehensive life course approach to addressing stunting and its negative sequela. In particular, if our findings are substantiated by others, raising awareness about the importance of concurrent stunting seems warranted. Efforts to address stunting could take a variety of forms but should involve multiple audiences, including parents, clinicians, educators, programme planners and implementers, and policy makers. Programme activities aimed at older children might include extra nutritional snacks at home or at school, pre‐school lunch programmes and regular growth monitoring – even as children enter pre‐school and begin their primary education. Growth monitoring should be accompanied by appropriate counselling for parents and teachers. As Glewwe has demonstrated, investments in children's nutrition can yield a threefold financial return in academic achievement (Glewwe et al. 2001).
Poor nutritional status alone does not account for children's cognitive deficits (Grantham‐McGregor 2002). Our study confirms that other factors such as wealth, maternal education, area of residence and number of siblings are also important determinants of verbal and quantitative ability. Consequently, programme and policies that reduce poverty, increase educational opportunities for adolescent girls and women, and promote birth spacing are also of importance. Additional efforts to improve children's cognition include psychosocial stimulation at a variety of levels including the home, neighbourhood and institutions such as day care, pre‐school and school; school readiness initiatives; and mental health support, among others (Committee on Integrating the Science of Child Development 2000).
Our findings suggest that broad‐based efforts to promote adequate nutrition need to aim to prevent stunting during infancy but should continue at least to when children enter school.
Source of funding
Young Lives is core‐funded by the UK Department for International Development (DFID) for the benefit of developing countries. Sub‐studies are funded by the Bernard van Leer Foundation, the Inter‐American Development Bank (in Peru), the International Development Research Centre (in Ethiopia) and the Oak Foundation. The views expressed here are those of the authors. They are not necessarily those of the Young Lives project, the University of Oxford, DFID or other funders.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We gratefully acknowledge the study participants who willingly gave their time. We also thank all those who participated in the preparation, collection and cleaning of the data.
This work was performed at the Department of Family and Preventive Medicine, University of Utah, 375 Chipeta Way, Salt Lake City, UT 84108, USA.
References
- Adair L.S. (1999) Filipino children exhibit catch‐up growth from age 2 to 12 years. The Journal of Nutrition 129, 1140–1148. [DOI] [PubMed] [Google Scholar]
- Agarwal D.K., Upadhyay S.K. & Agarwal K.N. (1989) Influence of malnutrition on cognitive development assessed by Piagetian tasks. Acta Paediatrica Scandinavica 78, 115–122. [DOI] [PubMed] [Google Scholar]
- Alderman H., Hoddinott J. & Kinsey W. (2006) Long‐term consequences of early childhood malnutrition. Oxford Economic Papers 58, 450–474. [Google Scholar]
- Baker F. & Kim S. (2004) Item Response Theory: Parameter Estimation Techniques, 2nd edn. Dekker: New York. [Google Scholar]
- Baydar N. & Brooks‐Gunn J. (1991) Effects of maternal employment and child‐care arrangements on pre‐schoolers' cognitive and behavioral outcomes: evidence from the children of the National Longitudinal Survey. Developmental Psychology 27, 932–945. [Google Scholar]
- Beasley N.M.R., Hall A., Tomkins A.M., Donnelly C., Ntimbwa P., Kivuga J. et al (2000) The health of enrolled and non‐enrolled children of school age in Tanga, Tanzania. Acta Tropica 76, 223–229. [DOI] [PubMed] [Google Scholar]
- Berkman D.S., Lescano A.G., Gilman R.H., Lopez S.L. & Black M.M. (2002) Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow‐up study. Lancet 359, 564–571. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Ahmed T., Black R.E., Cousens S., Dewey K., Giugliani E. et al (2008) What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440. [DOI] [PubMed] [Google Scholar]
- Black R.E., Morris S.S. & Bryce J. (2003) Where and why are 10 million children dying every year? Lancet 361, 2226–2234. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Blau D.M. (1999) The effect of income on child development. The Review of Economics and Statistics 81, 261–276. [Google Scholar]
- Blau F.D. & Grossberg A.J. (1992) Maternal labor supply and children's cognitive development. The Review of Economics and Statistics 743, 474–481. [Google Scholar]
- Campbell J.M. (1998) Review of the Peabody Picture Vocabulary Test, third edition. Journal of Psychoeducational Assessment 16, 334–338. [Google Scholar]
- Campbell J.M., Bell S.K. & Keith L.K. (2001) Concurrent validity of the Peabody Picture Vocabulary Test third edition as an intelligence and achievement screener for low SES African American children. Assessment 8, 85–94. [DOI] [PubMed] [Google Scholar]
- Cheung Y.B. (2006) Growth and cognitive function of Indonesian children: zero‐inflated proportion models. Statistics in Medicine 25, 3011–3022. [DOI] [PubMed] [Google Scholar]
- Cheung Y.B., Yip P.S.F. & Karlberg J.P.E. (2001) Fetal growth, early postnatal growth and motor development in Pakistani infants. International Journal of Epidemiology 30, 66–72. [DOI] [PubMed] [Google Scholar]
- Committee on Integrating the Science of Child Development (2000) From Neurons to Neighborhoods: The Science of Child Development. National Academy Press: Washington, DC. [Google Scholar]
- Crocker L. & Algina J. (1986) Introduction to Classical and Modern Test Theory. Holt, Rinehart and Winston: New York. [Google Scholar]
- Daniels M.C. & Adair L.S. (2004) Growth in young Filipino children predicts schooling trajectories through high school. The Journal of Nutrition 134, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Demographic and Health Surveys (2000) Peru 2000 Standard DHS . Calverton (MD): MEASURE DHS, Macro International. Available at: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=33 (accessed 9 March 2010).
- Demographic and Health Surveys (2008) Peru 2004–2008 Continuous DHS . Calverton (MD): MEASURE DHS, Macro International. Available at: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=33 (accessed 9 March 2010).
- Desai S.P., Chase‐Lansdale L. & Michael R.T. (1989) Mother or market? Effects of maternal employment on the intellectual ability of 4‐year‐old children. Demography 26, 545–561. [PubMed] [Google Scholar]
- Dunn L. & Dunn L. (1997) Examiner's Manual for the PPVT‐III. Form IIIA and IIIB. AGS: Circle Pines, MN. [Google Scholar]
- Dunn L., Padilla E., Lugo D. & Dunn L. (1986) Manual del examinador para el test de vocabulario en imágenes Peabody (Peabody Picture Vocabulary Test). Adaptación Hispanoamericana. AGS: Circle Pines, MN. [Google Scholar]
- Filmer D. & Pritchett L. (2001) Estimating wealth effects without expenditure data or tears: an application to educational enrolments in states of India. Demography 38, 115–132. [DOI] [PubMed] [Google Scholar]
- Glewwe P., Jacoby H.G. & King E.M. (2001) Early childhood nutrition and academic achievement: a longitudinal analysis. Journal of Public Economics 81, 345–368. [Google Scholar]
- Grantham‐McGregor S. (1995) A review of studies of the effect of severe malnutrition on mental development. The Journal of Nutrition 125, 2233S–2238S. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S. (2002) Linear growth retardation and cognition. Lancet 359, 542. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S.M., Walker S.P., Chang S.M. & Powell C.A. (1997) Effects of early childhood supplementation with and without stimulation on later development in stunted Jamaican children. The American Journal of Clinical Nutrition 66, 247–253. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S., Plante E., Vance R. & Henrichsen M. (1999) The diagnostic accuracy of four vocabulary tests administered to preschool‐age children. Language, Speech, and Hearing Services in Schools 30, 196–206. [DOI] [PubMed] [Google Scholar]
- Hack M. (1998) Effects of intrauterine growth retardation on mental performance and behavior, outcomes during adolescence and adulthood. European Journal of Clinical Nutrition 52 (Suppl. l), S65–S70. [PubMed] [Google Scholar]
- Ivanovic D.M., Perez H.T., Olivares M.D., Diaz N.S., Leyton B.D. & Ivanovic R.M. (2004) Scholastic achievement: a multivariate analysis of nutritional, intellectual, socioeconomic, sociocultural, familial, and demographic variables in Chilean school‐aged children. Nutrition 20, 878–889. [DOI] [PubMed] [Google Scholar]
- Kordas K., Lopez P., Rosado J.L., Vargas G.G., Rico J.A., Cebrián M.E. et al (2004) Blood lead, anemia, and short stature are independently associated with cognitive performance in Mexican school children. The Journal of Nutrition 134, 363–371. [DOI] [PubMed] [Google Scholar]
- Kuklina E.V., Ramakrishnan U., Stein A.D., Barnhart H.H. & Martorell R. (2004) Growth and diet quality are associated with the attainment of walking in rural Guatemalan infants. The Journal of Nutrition 134, 3296–3300. [DOI] [PubMed] [Google Scholar]
- Li H., Barnhart H.X., Stein A.D. & Martorell R. (2003) Effects of early childhood supplementation on the educational achievement of women. Pediatrics 112, 1156–1162. [DOI] [PubMed] [Google Scholar]
- Martorell R. (1997) Undernutrition during pregnancy and early childhood: consequences for cognitive and behavioral development In: Early Child Development: Investing in Our Children's Future (ed. Young M.E.), pp. 39–83. Elsevier Science BV: Amsterdam. [Google Scholar]
- Martorell R. (1999) The nature of child malnutrition and its long‐term implications. Food and Nutrition Bulletin 19, 288–292. [Google Scholar]
- Martorell R., Rivera J., Kaplowitz J. & Pollitt E. (1992) Long term consequences of growth retardation during early childhood In: Human Growth: Basic and Clinical Aspects (eds Hernandez M. & Argenta J.), pp. 143–149. Elsevier: Amsterdam. [Google Scholar]
- McCulloch A. & Joshi H.E. (2002) Child development and family resources: evidence from the second generation of the 1958 British birth cohort. Journal of Population Economics 15, 283–304. [Google Scholar]
- Mendez M.A. & Adair L.S. (1999) Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. The Journal of Nutrition 129, 1555–1562. [DOI] [PubMed] [Google Scholar]
- de Onis M. (2008) Child growth and development In: Nutrition and Health in Developing Countries (eds Semba R.D. & Bloem M.W.), 2nd edn, pp. 113–137. Humana Press: Totowa, NJ. [Google Scholar]
- Parcel T.L. & Menaghan E.G. (1994) Early parental work, family social capital and early childhood outcomes. American Journal of Sociology 99, 972–1009. [Google Scholar]
- Paxson C. & Norbert S. (2007) Cognitive development among young children in Ecuador: the roles of wealth, health and parenting. The Journal of Human Resources 42, 49–84. [Google Scholar]
- Rosenzweig M.R. & Wolpin K.I. (1994) Are there increasing returns to the intergenerational production of human capital? Maternal schooling and child intellectual achievement. The Journal of Human Resources 29, 670–693. [Google Scholar]
- Shrimpton R., Victora C., de Onis M., Costa Lima R., Blössner M. & Clugston G. (2001) Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics 107, 75–81. [DOI] [PubMed] [Google Scholar]
- Sigman M., McDonald M.A., Neumann C. & Bwibo N. (1991) Prediction of cognitive competence in Kenyan children from toddler nutrition, family characteristics and abilities. Journal of Child Psychology and Psychiatry, and Allied Disciplines 32, 307–320. [DOI] [PubMed] [Google Scholar]
- Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Ritcher L. et al (2008) Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.P., Grantham‐McGregor S.M., Powell C.A. & Chang S.M. (2000) Effects of growth restriction in early childhood on growth, IQ and cognition at age 11–12 years and the benefits of nutritional supplementation and psychosocial stimulation. The Journal of Pediatrics 137, 36–41. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Chang S.M., Powell C.A. & Grantham‐McGregor S.M. (2005) Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth‐stunted Jamaican children: prospective cohort study. Lancet 366, 1804–1807. [DOI] [PubMed] [Google Scholar]
- Wilson I., Huttly S.R.A. & Fenn B. (2006) A case study of sample design for longitudinal research: young lives. International Journal of Social Research Methodology 9, 351–356. [Google Scholar]


