Abstract
Over the past two decades, there has been a marked shift in the fatty acid composition of the diets of industrialized nations towards increased intake of the n‐6 fatty acid linoleic acid (LA, 18:2n‐6), largely as a result of the replacement of saturated fats with plant‐based polyunsaturated fatty acid (PUFA). While health agencies internationally continue to advocate for high n‐6 PUFA intake combined with increased intakes of preformed n‐3 long‐chain PUFAs (LCPUFA) docosahexaenoic acid (DHA, 22:6n‐3) and eicosapentaenoic acid (EPA, 20:5n‐3) to reduce the incidence of cardiovascular disease (CVD), there are questions as to whether this is the best approach. LA competes with alpha‐linolenic acid (18:3n‐3) for endogenous conversion to the LC derivatives EPA and DHA, and LA also inhibits incorporation of DHA and EPA into tissues. Thus, high‐LA levels in the diet generally result in low n‐3 LCPUFA status. Pregnancy and infancy are developmental periods during which the fatty acid supply is particularly critical. The importance of an adequate supply of n‐3 LCPUFA for ensuring optimal development of infant brain and visual systems is well established, and there is now evidence that the supply of n‐3 LCPUFA also influences a range of growth, metabolic and immune outcomes in childhood. This review will re‐evaluate the health benefits of modern Western diets and pose the question of whether the introduction of similar diets to nations with emerging economies is the most prudent public health strategy for improving health in these populations.
Keywords: omega 3 fatty acids, LCPUFA, omega 6 fatty acids
Introduction
Despite the large‐scale availability of cheap foods, the overall health of the populations in many industrialized countries is on the decline. The incidence of diseases of affluence including obesity, diabetes, allergy, asthma and cardiovascular disease (CVD) are on the rise in the USA and Australia (WHO, 2006). While many of these diseases have a mixed aetiology and include lifestyle factors, diet has been strongly implicated. Dietary factors such as high‐glycaemic index foods and the low nutrient content of processed foods are considered important (Barclay et al. 2008), but dietary fats, including the polyunsaturated fats, also undoubtedly play a role (Ailhaud et al. 2008). This review attempts to interpret the available evidence that may explain how dietary fats may play a role in these diseases and discusses alternative fat supplies that may help chart a healthier future.
Key messages
-
•
Diets low in n‐6 polyunsaturated fatty acid (PUFA) allow better endogenous conversion of alpha‐linolenic acid to n‐3 long‐chain (LC) PUFA and permit better accumulation of n‐3 LCPUFA into tissues.
-
•
Consideration needs to be given to replacing the n‐6 fatty acid level of vegetable oils and spreads with monounsaturated fatty acids.
-
•
While increasing the n‐3 fatty acid status of individuals can be brought about by consuming fish or fish oils, this strategy is unsustainable globally.
What is a Western diet?
The typical Western diet has undergone a marked evolution over the past few decades, largely as a result of recommendations from health agencies to reduce the intake of saturated fats and to replace these with plant‐based polyunsaturated fatty acid (PUFA), which are mostly high in linoleic acid (LA). These recommendations have produced a notable shift in the food industry, with lard and butter being replaced with vegetable‐based oils and spreads based initially on sunflower oil but increasingly replaced with soy and canola oils. Most commonly used vegetable oils are rich in LA and lower in n‐3 PUFA. This FA profile contrasts markedly with that of their predecessors, as both butter and other animal fats contained little n‐6 PUFA and higher amounts of monounsaturated and saturated FAs (Gibson et al. 2009). Tracked over time, what emerges is a progressive and marked increase in LA intake, coupled with a progressive (but more modest) reduction in dietary intake of n‐3 long‐chain PUFA (LCPUFA) (Ailhaud et al. 2006). This review will discuss how this shift in dietary fat composition has the potential to substantially reduce both the extent to which dietary alpha‐linolenic acid (ALA) is converted to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as the extent to which n‐3 LCPUFA are incorporated into tissues. As a result, the amount of n‐3 LCPUFA that must be consumed before the beneficial health effects of these FAs are realized is likely to increase. Some scientists are suggesting that Western diets have created a conditional essentiality for n‐3 LCPUFA, which is unlikely to be sustainable in the context of the decline in global fish stocks (Racine & Deckelbaum 2007). As a result, it is pertinent to question whether modern Western diets do in fact represent a gold standard to which developing countries should aspire or whether there are deficits in the dietary profile that should be addressed and corrected before such diets are recommended as ‘model’ diets.
Dietary fats
Most of the dietary fats can be broken into unavoidable fats that are chiefly phospholipids and are integral to the food, and avoidable fats that are either visible on foods (such as the visible fats of untrimmed meat) or fats used in cooking. These latter fats are generally in the form of triglycerides. Regardless of their form, most fats contain FAs that can be either saturated (no double bonds), monounsaturated (one double bond per FA) or polyunsaturated (two or more double bonds). The PUFAs are further subdivided into omega 3 (n‐3) or omega 6 (n‐6) series.
FA pathways: what do they tell us?
The two 18‐carbon essential FAs, LA (18:2n‐6) (parent of the n‐6 series of FAs) and ALA (18:3n‐3) (parent of the n‐3 series), rely for much of their biological activity on being converted to 20‐ and 22‐carbon FAs (Sprecher et al. 1999). In the case of the n‐6 series, this primarily refers to the conversion of LA to the 20‐carbon arachidonic acid (AA, 20:4n‐6), while for the n‐3 series, ALA must be converted to a 20‐carbon FA, EPA (20:5n‐3), and a 22‐carbon FA, DHA (22:6n‐3). The pathways were established in the 1950s by Ralph Holman (1986) and have since been refined by Howard Sprecher et al. (1995). Some basic understandings have come from the research that has been done to date. First, there is only one set of enzymes, and all the FAs compete for the use of this single set. Second, a FA from one series cannot be converted to another, that is, a n‐6 FA cannot be converted to a n‐3 FA.
The diagrammatic representation of the pathway has undergone a number of iterations throughout the years, and current understanding of the pathway is depicted in Fig. 1. It should be noted that the delta 6 desaturase (D6D) is considered to be a key regulatory enzyme in the pathway because it is used twice in the conversion of ALA to DHA.
Figure 1.
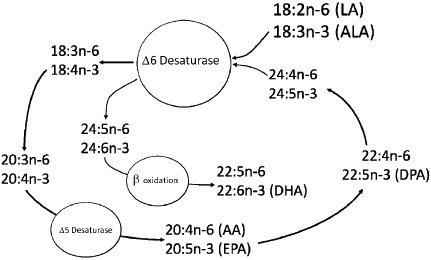
Diagrammatic representation of our current understanding of the synthetic pathway for n‐3 and n‐6 fatty acids. AA, arachidonic acid; ALA, alpha‐linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
The diagram leads to the formation of testable hypotheses.
-
•
First, when diets contain only 18‐carbon FAs (LA, ALA), levels of the 20‐carbon metabolites tend to be regulated by the relative amounts of these FAs in the diet. In other words, there is competition between the two substrates for the three enzymes involved, and the amount of 20‐carbon FAs that are produced are a result of simple zero‐order kinetics. This means that the more of any one essential FA in the diet, the more of that family's 20‐carbon FA will be present in tissues;
-
•
Second, at the final use of the D6D for the conversion of the 24‐carbon FA (24:5n‐3) through to 22‐carbon FAs including DHA, there is competition from both 18‐carbon precursors and 24:5n‐3 for D6D. As a result, we can hypothesize that diets that contain very high levels of PUFA can actually inhibit the conversion of ALA to DHA by inhibiting the access of 24:5n‐3 to the D6D enzyme. Importantly, the diagram suggests that both LA and ALA have the capacity to inhibit DHA synthesis when they are present at high levels in the diet. It also infers that diets that are low in both PUFA are likely to be able to enhance levels of DHA provided that there is still sufficient ALA present.
The balance of ALA and LA determines the balance of 20 carbon FAs (EPA and AA) in tissues
Many animal studies have confirmed that relative conversion of LA to AA and ALA to EPA, and thus the balance of EPA and AA in tissues, is determined by the ratio of LA and ALA carbon substrates in the diet (Lands et al. 1990). This is illustrated by the results of a study in which chickens were fed a range of diets varying in the LA : ALA ratio. At LA : ALA ratios above 5, the FAs in the liver phospholipids are rich in AA but poor in EPA. As the LA : ALA ratio drops below 5, there is an exchange of AA for EPA (Fig. 2; L.R. Kartikasari et al., unpublished observations). It is easy to see that LA‐dominant diets result in AA as the major accumulated 20‐carbon PUFA in tissues and that EPA can only accumulate when the LA : ALA ratios of diets are low.
Figure 2.
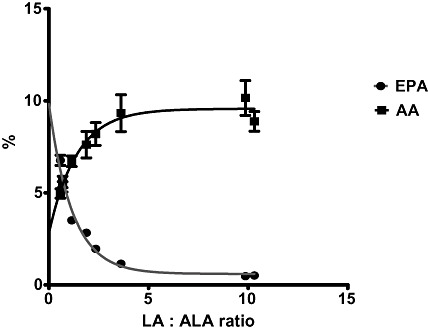
Results of an experiment illustrating the effect of feeding chickens a range of diets varying in the LA : ALA ratio on the EPA (close circles) and AA (closed squares) content of tissues as a % of total fatty acids. AA, arachidonic acid; ALA, alpha‐linolenic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
High‐LA diets inhibit synthesis of n‐3 LCPUFA
Over two decades ago, Lands et al. undertook a series of studies in which he measured the n‐3 LCPUFA content of red blood cells and tissues in rats consuming different ratios of LA and ALA, and used these measurements to develop equations that described the relationships between dietary FA composition and n‐3 LCPUFA synthesis and their incorporation into cell membranes (Lands et al. 1990). Although the levels of LCPUFA in tissues were used to infer endogenous synthesis rather than direct measure of synthesis, what was formalized in this work was the concept that diets that were high in LA would inhibit the synthesis of n‐3 LCPUFA by simple competitive inhibition. In other words, less ALA would have access to the D6D and other enzymes in the pathway if ALA was diluted out by excessive amounts of LA. As a result of this work, Lands has since proposed that the LA : ALA ratio of US diets should be drastically reduced (Lands 2008).
An example of the effect of dietary LA on the conversion of ALA to DHA was reported in formula‐fed infants (Clark et al. 1992). Conducted at a time before LCPUFA supplements were added to formulas, Clark and colleagues compared the FA profiles of term infants fed for 10 weeks on a commercial infant formula with a high ratio (19:1) of LA : ALA with comparable groups fed experimental formulas in which the LA : ALA ratio was reduced either by an increase in ALA (4:1; LA, 13%; ALA, 3.3%) or a decrease in LA (3:1; LA, 3.5%; ALA, 1.1%). In this study, decreasing the ratio of LA : ALA was associated with an increased incorporation of the C20 and C22 FAs in red cells compared with commercial formula. Importantly, the level of n‐3 LCPUFA achieved by simply decreasing the LA level in the formula from 13% to 3.5% and maintaining the ALA at around 1% was as effective as increasing the ALA to 3.3% but maintaining the high level of LA in the fat. These results confirm the importance of the LA : ALA ratio in determining the LCPUFA status in human tissues.
LA also inhibits incorporation of LCPUFA
In addition to the inhibition of n‐3 LCPUFA synthesis, there are data from both animals and humans that diets containing high amounts of LA are associated with reduced incorporation of preformed n‐3 LCPUFA into tissue phospholipids (McMurchie et al. 1990; Lands et al. 1992). In one of these studies, preformed EPA was given to non‐human primates consuming a background diet containing either low (7%) or high (33%) levels of LA, and incorporation of EPA into tissue phospholipids was significantly lower in the high‐LA group (McMurchie et al. 1990). Similarly, Anding and Hwang reported in 1986 that feeding rats increasing amounts of ALA in the diet was associated with a decrease in the AA content in brain phospholipids (Anding & Hwang 1986). In vitro studies of the enzyme kinetics of the key enzymes involved in the incorporation of FAs into cell membrane glycerophospholipids have also shown competitive aspects of n‐3 LCPUFA incorporation into liver microsomes when different concentrations of n‐6 and n‐3 PUFA are added to the culture media (Iritani & Narita 1984).
High‐ALA diets inhibit further conversion of ALA to DHA
As indicated in the synthetic pathway for n‐3 and n‐6 FAs (Fig. 1), increasing the level of dietary ALA will automatically provide more substrate for conversion to EPA. However, unlike the accumulation of EPA, the further metabolism of EPA to DHA is not simple, and high levels of dietary ALA actually have the capacity to inhibit one of the last steps in the synthesis of DHA, namely, the desaturation of 24:5n‐3. Support for this concept came from a rat study we conducted (Tu et al. 2010) in which the level of LA was held constant at a low but adequate level (1%en), while the level of ALA in the diet was increased from 0.1 to 2.8%en (Fig. 3). The data shown in the figure illustrate that while the relationship between dietary ALA content and EPA content in both plasma and liver is linear, DHA status has a curvilinear relationship with dietary ALA intake such that DHA status increases up to a dietary level of ALA of around 1%en, after which DHA level plateaus and then declines. Similar data have been reported in a piglet study (Blank et al. 2002) in which the background diet of LA was somewhat higher (∼2%en), and the ALA level was increased as high as 16%en. Accumulation of DHA in plasma phospholipids in piglets showed a similar trend as seen in rats, namely, that DHA accumulation was maximal at around 2%en ALA, and at higher concentrations, DHA accumulation was inhibited. The ability of high‐ALA diets to suppress DHA synthesis is further supported by work by Cleland and colleagues, which showed that the relative potencies of dietary oils for increasing the content of DHA in plasma and in heart tissues in rats actually increased as their ALA content decreased (Cleland et al. 1992).
Figure 3.
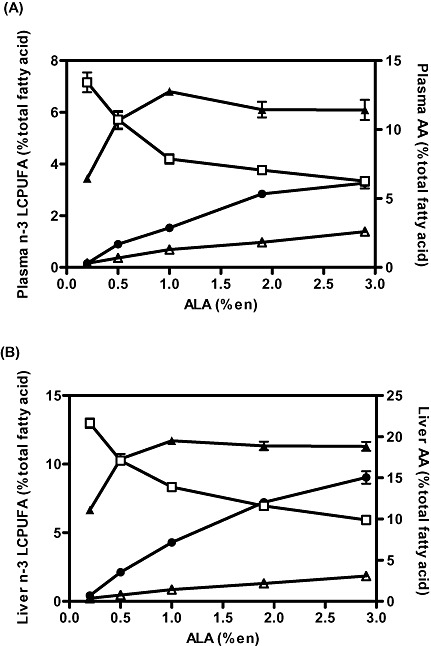
Effect of dietary ALA levels on (A) plasma and (B) liver phospholipid fatty acid content in rats fed diets containing various levels of ALA ranging from 0.2 to 2.9%en for 3 weeks. Fatty acids include EPA (circles), DPA (open triangles), DHA (closed triangles) and AA (open squares). Reprinted from Tu et al. (2010). AA, arachidonic acid; ALA, alpha‐linolenic acid; DHA, docosahexaenoic acid; DPA, Docosapentaenoic acid; EPA, eicosapentaenoic acid; LCPUFA, long‐chain polyunsaturated fatty acids.
The same phenomenon has been demonstrated in human studies. Chan and colleagues conducted a dose–response study in which the ALA content of the diet ranged from 0.8 to 13.4% energy and LA : ALA ratio ranged from 0.3 to 27, and showed a dose‐dependent increase in the concentrations of EPA, but not DHA (Chan et al. 1993). Mantzioris et al. reported similar findings in earlier studies (Mantzioris et al. 1994).
Summary
These data suggest that both the total PUFA content and the balance of LA to ALA consumed are critical determinants of LCPUFA status, and it therefore stands to reason that we need to pay close attention to the complete FA profile of the diet, in particular, the balance of n‐6 and n‐3 fats, when evaluating its potential health benefits. Furthermore, providing increasing amounts of ALA is not an effective strategy for increasing tissue DHA content. Counter‐intuitively, the data suggest that diets low in ALA are preferred so long as the level of LA in the diet is also low.
Effect of FA balance during pregnancy and infancy
The fetal and infant period is a particularly critical developmental window, and there is evidence that suggests that nutritional perturbations during this period have long‐term effects on metabolic and cardiovascular health (McMillen et al. 2009). That the FA supply during this time is important for the optimal development of the brain and visual system was established in studies in non‐human primates, which reported impaired cognitive function and visual acuity in offspring of mothers who were deficient in n‐3 PUFA that could not be rescued by post‐natal n‐3 LCPUFA supplementation even though n‐3 LCPUFA status was improved (Neuringer et al. 1986; Anderson et al. 2005). More recently, as we have become more aware of the importance of n‐3 and n‐6 FAs in health in adults, there has been a growing interest in how the balance of FAs present during the perinatal period affects the development of the systems regulating immune function, fat deposition and metabolism (Makrides & Gibson 2000, 2002; Carlson 2009). While there is evidence from human studies that increasing the n‐3 LPCUFA intake of preterm infants in the immediate post‐natal period is associated with improved cognitive outcomes (Makrides et al. 2009), the potential for maternal n‐3 LCPUFA supplementation during pregnancy to improve cognitive function in childhood is less well established, and there are several clinical trials under way that are attempting to address this question. What has been established in existing studies, and in systematic reviews, is that maternal n‐3 supplementation is associated with a small but significant increase in the length of gestation and a modest increase in infant birthweight (Makrides et al. 2006).
Lactation
The FA composition of breast milk reflects that of the maternal blood and, hence, maternal diet. Given the increase in dietary LA and the low intake of foods rich in n‐3 LCPUFA, it is perhaps not surprising that the composition of breast milk in the USA has undergone a marked shift in composition in parallel with the changes in the FA profile of typical Western diets; the mean LA content has increased from 6 to 15% of total FAs between 1944 and 1990, and has since remained at ∼16%, while the ALA content has declined (Ailhaud et al. 2006). A similar pattern has also been reported in the breast milk of Australian women, with an increase in LA content and decrease in DHA content between 1981 and 2000 (Fig. 4; Gibson & Kneebone 1981; Makrides et al. 2000).
Figure 4.
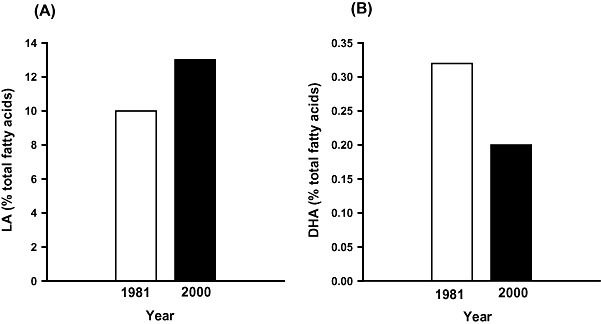
Histograms depicting the average (A) LA and (B) DHA content of breast milk in Australian women in 1981 and 2000 (Gibson and Kneebone, 1981; Makrides et al., 2000). DHA, docosahexaenoic acid; LA, linoleic acid.
Thus, breastfed infants in 2010 will receive a quite different nutrition to breastfed infants born in 1980. What are the implications of this? One of the key features of n‐6 fats is that they give rise to pro‐inflammatory compounds, which have been implicated in the development of allergies and asthma. In addition, n‐6 fats promote the differentiation of pre‐adipocytes, and exposure to excessive n‐6 fats during the period of fat cell formation (in utero and early infancy) has the potential to promote excess fat deposition in early life (Ailhaud et al. 1992). Ailhaud et al. has subsequently published a series of studies that have implicated the increasing dominance of n‐6 to n‐3 fats in Western diets as a contributing factor to the increased incidence of childhood obesity (2007, 2006; Massiera et al. 2010).
Infant formulas around the world are modelled on the composition of breast milk of US women, with high‐LA levels with small amounts of n‐3 LCPUFA. This is despite the fact that the breast milk of women in developing countries has very different FA profiles. A study conducted in Malaysian women reported clear differences in the composition of breast milk between different ethnicities (Kneebone et al. 1985). This study showed that the LA levels in the breast milk of Chinese women are higher than those in Indian and Malay women, while levels of DHA are lower. Importantly, the composition is markedly different to the typical breast milk composition in Western countries, with twofold to threefold higher DHA levels than those seen in typical breast milk in Australian women (Kneebone et al. 1985). These differences reflect the different FA profiles of the typical diets consumed by these ethnic groups. For example, the lower LA levels in these breast milks would suggest that all the women were consuming less LA in their diet than Australian or American women.
Direct evidence for the relationship between maternal n‐3 LCPUFA intake and the level of these FAs in breast milk comes from a randomized controlled trial in which lactating mothers were provided with either placebo or an oil containing DHA at a dose of between 0.2 and 1.3 g day−1 (Makrides et al. 1996). This study demonstrated a highly significant positive relationship between the maternal dose of DHA and the content of DHA in the breast milk (Fig. 5). Thus, the n‐3 status of the mother is a major determinant of the n‐3 status of her milk and her breastfed infants (Gibson et al. 1997).
Figure 5.
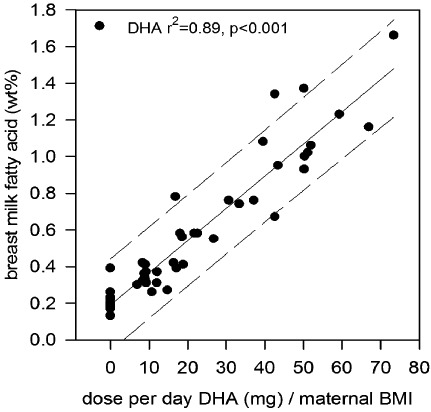
The effect of dietary dose of DHA corrected for BMI on the percentage of DHA in human breast milk (taken from Makrides et al. 1996).
If high‐LA diets are to be avoided, what is the alternative?
Mattson & Grundy (1985) demonstrated that replacing saturated fats in human diets with monounsaturated fats was as effective at lowering CVD risk factors as polyunsaturated fats. This result has been confirmed by Ashton and co‐workers, who have shown the protective effect against CVD risk factors of monounsaturated fat diets by demonstrating a significant increase in low‐density lipoprotein oxidation lag phase and the significantly higher levels of plasma high‐density lipoprotein cholesterol (Ashton et al. 2001). The effectiveness of diets where the main oil used is monounsaturated olive oil in conjunction with other healthy foods has been clearly demonstrated in the Lyon Heart Study (Kris‐Etherton et al. 2001). In addition, the advantages of highly monounsaturated fats over polyunsaturated fats in food preparation are many; monounsaturated fats are more stable because of the reduced number of double bonds and can attain a higher cooking temperature for longer without oxidizing. Importantly, monounsaturated fats can have very low levels of LA and thus do not interfere with endogenous conversion of ALA to DHA. Ideally, if we were trying to improve the n‐3 status of whole populations, cheap sources of a vegetable oil for cooking would need to be available that were high in monounsaturates and have low levels of total PUFA and a low LA : ALA ratio. Some oils are already available that meet some of the criteria. For example, macadamia and sunola (hybrid sunflower) oils have very high levels of monounsaturates and less than 5% LA, but neither of the two oils has meaningful amounts of ALA. Canola oil has a good balance of LA : ALA (2:1), but the total level of PUFA is high (∼30%). Perhaps an oil blend would provide the best alternative for meeting the criteria we have espoused, namely, a blend of sunola and small amounts of both linseed and canola oils to result in around 5% of the total FAs as PUFA and with an LA : ALA ratio of around 2:1 or less.
What is the effect of dietary fats that meet the requirement of low PUFA with a favourable LA : ALA ratio?
There are natural fats that comply with the dietary parameters indicated by the animal and human experiments cited above. Interestingly, dairy fat has very low levels of PUFA (∼3%), and the LA : ALA ratio would seem to be ideal (2:1) to allow maximal endogenous synthesis of DHA. Sinclair et al. demonstrated in butter fat‐fed rats that the level of DHA was high (Naughton et al. 1988). More important are the findings of others who have shown that infants fed formulas based on dairy fats (Sanders & Naismith 1979) or indeed evaporated milk (Courage et al. 1998) have LCPUFA status midway between those fed formulas enriched with LA‐rich vegetable oils and those fed breast milk. Finally, in their interesting monastery study, Lasserre and co‐workers (Baudet et al. 1984) reported that the level of n‐3 LCPUFA in nuns consuming a dairy fat‐based diet was superior to the same group when they consumed a sunflower oil‐based diet.
The use of cooking oils that are low in PUFA such as dairy and highly monounsaturated oil blends may confer some metabolic advantages in that they allow better endogenous conversion of ALA from vegetables through to EPA and DHA. Naturally, there may be negative consequences in terms of CVD risk if fats with a high saturated fat content are used. However, it is noted that there is increasing debate about the association between saturated fat intake and deaths from CVD (Siri‐Tarino et al. 2010).
When dietary fats are important and when they do not matter
The data from animal and human studies together provide clear evidence that the balance of n‐6 to n‐3 fats in the diets can have major effects on the FA profile of tissues. This has obvious relevance and importance in the context of typical Western diets, which are high in n‐6 and relatively low in n‐3 LCPUFA. The FA profile of dietary fats is less likely to be of critical importance if the remainder of the diets are rich in dietary sources of preformed EPA and DHA, such as Japanese‐style or traditional Inuit diets. In addition, in societies where the total fat content of the diet is less than that consumed in many developed countries (>30%en), the level of total PUFA in the major dietary fat can be higher. For this reason, the total PUFA needs to be expressed in terms of the total energy of the diet to have an appreciation of their likely effects. It is clear that close attention needs to be paid to not only the n‐3 and n‐6 levels of dietary fats but also the overall PUFA contents of diets in order to assess their likely impact on n‐3 status of consumers.
Concluding comments
Ensuring an adequate n‐3 FA status in individuals is increasingly seen as important for optimizing long‐term health outcomes. High dietary intakes of n‐6 FAs through vegetable oils and spreads in many developed countries have made an intake of preformed n‐3 LCPUFA conditionally essential to large numbers of people if they are to improve their n‐3 LCPUFA status. Low n‐3 LCPUFA status is particularly evident in low‐income groups, which tend to consume poorer quality diets than the more socially advantaged, and these low‐income groups have the highest incidence of diseases of affluence (obesity, diabetes, CVD). In contrast, in some developing countries with low fat diets and where a significant proportion of the protein comes from fish, the n‐3 LCPUFA status appears high. Care needs to be taken when recommending dietary change to communities, based on the dietary fat intake of many developed countries.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Source of funding
RG and MM are supported by senior research fellowships from the National Health and Medical Research Council of Australia (NHRMC). BM is supported by an NHMRC Peter Doherty postdoctoral training fellowship.
References
- Ailhaud G., Grimaldi P. & Negrel R. (1992) Cellular and molecular aspects of adipose tissue development. Annual Review of Nutrition 12, 207–233. [DOI] [PubMed] [Google Scholar]
- Ailhaud G., Guesnet P. & Cunnane S.C. (2008) An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? British Journal of Nutrition 100, 461–470. [DOI] [PubMed] [Google Scholar]
- Ailhaud G., Massiera F., Alessandri J. & Guesnet P. (2007) Fatty acid composition as an early determinant of childhood obesity. Genes and Nutrition 2, 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailhaud G., Massiera F., Weill P., Legrand P., Alessandri J.M. & Guesnet P. (2006) Temporal changes in dietary fats: role of n‐6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Progress in Lipid Research 45, 203–236. [DOI] [PubMed] [Google Scholar]
- Anderson G.J., Neuringer M., Lin D.S. & Connor W.E. (2005) Can prenatal n‐3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatric Research 58, 865–872. [DOI] [PubMed] [Google Scholar]
- Anding R.H. & Hwang D.H. (1986) Effects of dietary linolenate on the fatty acid composition of brain lipids in rats. Lipids 21, 697–701. [DOI] [PubMed] [Google Scholar]
- Ashton E.L., Best J.D. & Ball M.J. (2001) Effects of monounsaturated enriched sunflower oil on CHD risk factors including LDL size and copper‐induced LDL oxidation. Journal of the American College of Nutrition 20, 320–326. [DOI] [PubMed] [Google Scholar]
- Barclay A.W., Petocz P., McMillan‐Price J., Flood V.M., Prvan T., Mitchell P. et al (2008) Glycemic index, glycemic load, and chronic disease risk – a meta‐analysis of observational studies. American Journal of Clinical Nutrition 87, 627–637. [DOI] [PubMed] [Google Scholar]
- Baudet M., Dachet C., Lasserre M., Esteva O. & Jacotot B. (1984) Modification in the composition and metabolic properties of human low density and high density lipoproteins by different dietary fats. Journal of Lipid Research 25, 456–468. [PubMed] [Google Scholar]
- Blank C., Neumann M.A., Makrides M. & Gibson R.A. (2002) Optimizing DHA levels in piglets by lowering the linoleic acid to {alpha}‐linolenic acid ratio. Journal of Lipid Research 43, 1537–1543. [DOI] [PubMed] [Google Scholar]
- Carlson S.E. (2009) Early determinants of development: a lipid perspective. American Journal of Clinical Nutrition 89, 1523S–1529S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.K., McDonald B.E., Gerrard J.M., Bruce V.M., Weaver B.J. & Holub B.J. (1993) Effect of dietary alpha‐linolenic acid and its ratio to linoleic acid on platelet and plasma fatty acids and thrombogenesis. Lipids 28, 811–817. [DOI] [PubMed] [Google Scholar]
- Clark K.J., Makrides M., Neumann M.A. & Gibson R.A. (1992) Determination of the optimal ratio of linoleic acid to alpha‐linolenic acid in infant formulas. Journal of Pediatrics 120, S151–S158. [DOI] [PubMed] [Google Scholar]
- Cleland L., James M., Neumann M., D'Angelo M. & Gibson R. (1992) Linoleate inhibits EPA incorporation from dietary fish‐oil supplements in human subjects. American Journal of Clinical Nutrition 55, 395–399. [DOI] [PubMed] [Google Scholar]
- Courage M.L., McCloy U.R., Herzberg G.R., Andrews W.L., Simmons B.S., McDonald A.C. et al (1998) Visual acuity development and fatty acid composition of erythrocytes in full‐term infants fed breast milk, commercial formula, or evaporated milk. Journal of Developmental and Behavioral Pediatrics 19, 9–17. [DOI] [PubMed] [Google Scholar]
- Gibson R. & Kneebone G. (1981) Fatty acid composition of human colostrum and mature breast milk. American Journal of Clinical Nutrition 34, 252–257. [DOI] [PubMed] [Google Scholar]
- Gibson R.A., Makrides M., Smithers L.G., Voevodin M. & Sinclair A.J. (2009) The effect of dairy foods on CHD: a systematic review of prospective cohort studies. British Journal of Nutrition 102, 1267–1275. [DOI] [PubMed] [Google Scholar]
- Gibson R.A., Neumann M.A. & Makrides M. (1997) Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. European Journal of Clinical Nutrition 51, 578–584. [DOI] [PubMed] [Google Scholar]
- Holman R.T. (1986) Control of polyunsaturated acids in tissue lipids. Journal of the American College of Nutrition 5, 183–211. [DOI] [PubMed] [Google Scholar]
- Iritani N. & Narita R. (1984) Changes of arachidonic acid and n‐3 polyunsaturated fatty acids of phospholipid classes in liver, plasma and platelets during dietary fat manipulation. Biochimica et Biophysica Acta 793, 441–447. [PubMed] [Google Scholar]
- Kneebone G., Kneebone R. & Gibson R. (1985) Fatty acid composition of breast milk from three racial groups from Penang, Malaysia. American Journal of Clinical Nutrition 41, 765–769. [DOI] [PubMed] [Google Scholar]
- Kris‐Etherton P., Eckel R.H., Howard B.V., St. Jeor S. & Bazzarre T.L. (2001) Lyon diet heart study: benefits of a Mediterranean‐style, National Cholesterol Education Program/American Heart Association step I dietary pattern on cardiovascular disease. Circulation 103, 1823–1825. [DOI] [PubMed] [Google Scholar]
- Lands B. (2008) A critique of paradoxes in current advice on dietary lipids. Progress in Lipid Research 47, 77–106. [DOI] [PubMed] [Google Scholar]
- Lands W.E., Libelt B., Morris A., Kramer N.C., Prewitt T.E., Bowen P. et al (1992) Maintenance of lower proportions of (n‐6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n‐3) fatty acids. Biochimica et Biophysica Acta 1180, 147–162. [DOI] [PubMed] [Google Scholar]
- Lands W.E., Morris A. & Libelt B. (1990) Quantitative effects of dietary polyunsaturated fats on the composition of fatty acids in rat tissues. Lipids 25, 506–516. [DOI] [PubMed] [Google Scholar]
- Makrides M., Duley L. & Olsen S.F. (2006) Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre‐eclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews 2006 3, Art. No.: CD003402. DOI: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- Makrides M. & Gibson R.A. (2000) Long‐chain polyunsaturated fatty acid requirements during pregnancy and lactation. American Journal of Clinical Nutrition 71, 307S–311S. [DOI] [PubMed] [Google Scholar]
- Makrides M. & Gibson R.A. (2002) The role of fats in the lifecycle stages: pregnancy and the first year of life. Medical Journal of Australia 176, S111–S112. [PubMed] [Google Scholar]
- Makrides M., Gibson R.A., McPhee A.J., Collins C.T., Davis P.G., Doyle L.W. et al (2009) Neurodevelopmental outcomes of preterm infants fed high‐dose docosahexaenoic acid: a randomized controlled trial. Journal of the American Medical Association 301, 175–182. [DOI] [PubMed] [Google Scholar]
- Makrides M., Neumann M.A. & Gibson R.A. (1996) Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. European Journal of Clinical Nutrition 50, 352–357. [PubMed] [Google Scholar]
- Makrides M., Neumann M.A., Jeffrey B., Lien E.L. & Gibson R.A. (2000) A randomized trial of different ratios of linoleic to {alpha}‐linolenic acid in the diet of term infants: effects on visual function and growth1. American Journal of Clinical Nutrition 71, 120–129. [DOI] [PubMed] [Google Scholar]
- Mantzioris E., James M., Gibson R. & Cleland L. (1994) Dietary substitution with an alpha‐linolenic acid‐rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. American Journal of Clinical Nutrition 59, 1304–1309. [DOI] [PubMed] [Google Scholar]
- Massiera F., Barbry P., Guesnet P., Joly A., Luquet S., Moreilhon‐Brest C. et al (2010) A western‐like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. Journal of Lipid Research 51, 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson F. & Grundy S. (1985) Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. Journal of Lipid Research 26, 194–202. [PubMed] [Google Scholar]
- McMillen I.C., Rattanatray L., Duffield J.A., Morrison J.L., MacLaughlin S.M., Gentili S. et al (2009) The early origins of later obesity: pathways and mechanisms. Advances in Experimental Medicine and Biology 646, 71–81. [DOI] [PubMed] [Google Scholar]
- McMurchie E.J., Rinaldi J.A., Burnard S.L., Patten G.S., Neumann M., McIntosh G.H. et al (1990) Incorporation and effects of dietary eicosapentaenoate (20:5(n‐3)) on plasma and erythrocyte lipids of the marmoset following dietary supplementation with differing levels of linoleic acid. Biochimica et Biophysica Acta 1045, 164–173. [DOI] [PubMed] [Google Scholar]
- Naughton J.M., Sinclair A.J., O'Dea K. & Steel M.S. (1988) Effects of dietary butter enrichment on the fatty acid distribution of phospholipid fractions isolated from rat platelets and aortae. Biochimica et Biophysica Acta 962, 166–172. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Connor W.E., Lin D.S., Barstad L. & Luck S. (1986) Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America 83, 4021–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R.A. & Deckelbaum R.J. (2007) Sources of the very‐long‐chain unsaturated omega‐3 fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Current Opinion in Clinical Nutrition and Metabolic Care 10, 123–128. [DOI] [PubMed] [Google Scholar]
- Sanders T.A.B. & Naismith D.J. (1979) A comparison of the influence of breast‐feeding and bottle‐feeding on the fatty acid composition of the erythrocytes. British Journal of Nutrition 41, 619–623. [DOI] [PubMed] [Google Scholar]
- Siri‐Tarino P.W., Sun Q., Hu F.B. & Krauss R.M. (2010) Saturated fat, carbohydrate, and cardiovascular disease. American Journal of Clinical Nutrition 91, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher H., Chen Q. & Yin F.Q. (1999) Regulation of the biosynthesis of 22:5n‐6 and 22:6n‐3: a complex intracellular process. Lipids 34, S153–S156. [DOI] [PubMed] [Google Scholar]
- Sprecher H., Luthria D., Mohammed B. & Baykousheva S. (1995) Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. Journal of Lipid Research 36, 2471–2477. [PubMed] [Google Scholar]
- Tu W.C., Cook‐Johnson R.J., James M.J., Muhlhausler B.S. & Gibson R.A. (2010) Omega‐3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins, Leukotrienes, and Essential Fatty Acids 83, 61–68. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2006) Fact Sheet: Obesity and Overweight. Available at: http://www.Who.Int/mediacentre/factsheets/fs311/en/print.Html


