Abstract
Adolescent pregnancy is a major public health challenge for many industrialized countries and is associated with significant medical, nutritional, social and economic risk for mothers and their infants. Despite this, relatively little is known about the nutritional status of this population. The aim of this paper was to conduct a systematic review of the current evidence relating to the biochemical markers of nutritional status of pregnant adolescents living in industrialized countries. Six papers were identified that fulfilled the inclusion criteria, the majority of which were conducted in the United States. The studies were of variable quality and most failed to control for potential confounders which may have strongly influenced the findings. Due to limited research, conclusions cannot be drawn about the zinc and calcium status of pregnant adolescents, and data on folate and vitamin B12 status appeared conflicting. There was some consensus among studies, however, to suggest that indicators of anaemia and iron status were compromised in pregnant adolescents, particularly during the third trimester of pregnancy. Chronological age did not appear to influence nutritional status, although there was some evidence to suggest that increasing gynaecologic age may positively influence plasma ferritin levels. Current research is limited by sampling and measurement bias, and research is urgently required to address these limitations. Further consideration should also be made of the influence of the role of socio‐economic support on pregnant adolescents’ nutritional status. The achievement of improved nutrition in pregnancy among adolescents requires multidisciplinary collaborations of adolescent healthcare providers, academics, professional organizations, policymakers, industry and service users. Only once this is achieved can adolescent nutrition, and adolescent nutrition in pregnancy, be significantly and sustainably optimized.
Keywords: nutritional status, adolescent, pregnancy, systematic review
Introduction
Adolescent pregnancy is a major public health challenge for many industrialized countries, with the United States having the highest rates of adolescent births in the developed world (more than twice the European average), and the United Kingdom having the highest adolescent birth rate in Europe (UNICEF 2001). Adolescent pregnancy is associated with significant medical, nutritional, social and economic risk for mothers and their infants throughout the world. Adolescent mothers are more likely than older mothers to come from unskilled manual backgrounds or live in areas with higher social deprivation (Teenage Pregnancy Unit 2004), a life circumstance long associated with poor dietary intake. In addition to the social and economic environment of adolescent pregnancy, factors such as gynaecologic immaturity, competition for nutrients, and the growth of the mother are likely to impact on the mother’s nutritional status (King 2003). Poor maternal nutritional status has been associated with short‐ and long‐term health implications for both mother and child. Short‐term health outcomes of inadequate maternal nutritional status include pregnancy and birth complications, poor postpartum nutritional status, poor lactational performance and increased mortality in mothers, and compromised fetal growth, increased risk of preterm births, spontaneous abortion, congenital abnormalities, and morbidity and mortality in the infant (IOM 1990).
For the adolescent mother, the long‐term effects of poor nutritional status are considerable. As adolescence is a critical period during which lifetime habits are established (Cavadini et al. 2000), the dietary habits acquired during adolescence have the potential to enhance or undermine health throughout life. For example, high fat intake during adolescence and into adulthood is associated with an increased risk of heart disease, and low calcium intake during adolescence is associated with low bone density and an increased risk of osteoporosis in later life (Lytle 2002). Poor maternal nutritional status could also have long‐term implications for the offspring. Impaired intrauterine growth and development due to inadequate nutritional status may ‘program’ the fetus for cardiovascular, metabolic or endocrine disease in adult life (Barker & Osmond 1986). Epidemiological associations have been found to exist between lower birth size and a greater risk of death in later life from cardiovascular disease (Barker & Osmond 1986) and type 2 diabetes mellitus (Ravelli et al. 1998). More recent work has suggested that adult disease risk has developmental origins, i.e. the concept that certain adult diseases come about as a consequence of the fetal response to its environment, rather than a causal role for birth size. Thus, the current working model is one whereby early life events (e.g. maternal nutrition), acting through the processes of developmental plasticity (i.e. the ability of the fetus to respond to environmental cues by choosing a trajectory of development that often has adaptive advantage), alter the development of the organism to such an extent that it affects its capacity to cope with the environment of adult life and therefore influences disease risk. Experimental data suggest that the period in which these early life events influence lifelong consequences can extend from conception (and possibly preconception) to infancy, depending on the organ system involved (Gluckman et al. 2005).
Adequate nutrition in adolescent pregnancy therefore presents many challenges for health professionals and policymakers alike. Despite this, relatively little is known about the nutrient status of this population. Nutritional surveys have shown that the highest prevalence of nutritional deficiencies occurs in adolescence, with most commonly noted deficiencies in non‐pregnant adolescents being calcium, iron, zinc, riboflavin, folate, and vitamins A and D (e.g. Gregory et al. 2000). A recent systematic review (Hall Moran 2007) reported that the nutrient intakes of pregnant adolescents appeared to be low in a number of nutrients recognized to be vital for fetal growth and development during pregnancy. Intakes of energy, iron, calcium, folate, vitamin E and magnesium were particularly low. These papers, however, did not assess whether such low nutrient intakes reflected a diminished nutritional status in pregnant adolescents. The aim of this paper is to review the current evidence on the nutritional status of pregnant adolescents, as assessed by biochemical indicators.
‘Adolescence’ can be described as the transitional stage of development between childhood and adulthood. It is a cultural and social phenomenon, and therefore its endpoints are not easily defined. The World Health Organization (WHO 1998) defines adolescence as a person between 10 and 19 years of age. For reasons of clarity, throughout this paper ‘adolescence’ refers to the ages 10–19 years inclusive (unless otherwise stated).
Methods
The research question applied to the systematic review was ‘what is the nature of the nutritional status, as measured by biochemical indicators, of pregnant adolescents living in industrialised countries?’ Papers that focused on adolescents living in developing countries were not included in this review due to the cultural and socio‐economic disparities between the industrialized and developing world, making synthesis of findings impractical. The systematic review protocol broadly followed the National Health Service (NHS) Centre for Reviews and Dissemination guidelines (2001). The main stages of the review are illustrated in Fig. 1.
Figure 1.
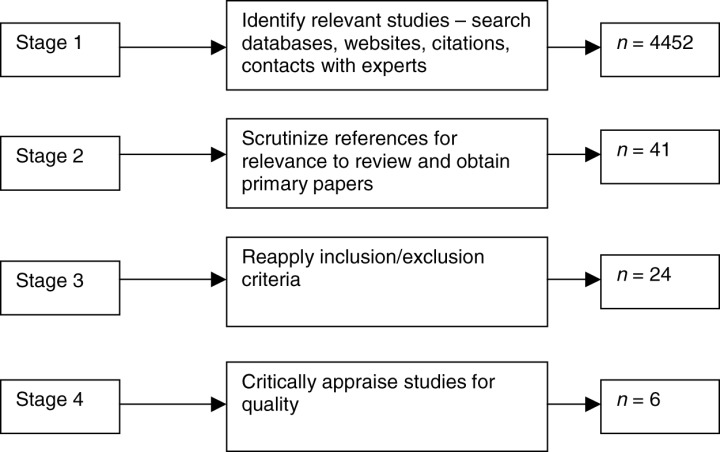
Main stages of the systematic review process.
Stage 1 of the review involved searching for publications using electronic databases, websites, citations, hand‐searching relevant journals and contacts with experts. The search terms used in the electronic databases were: pregnant, pregnancy, maternal, mother, nutritional status, nutrition assessment, biological markers, biological assays, adolescent, adolescence, teenage, young, child, and girl. The databases searched were: Ovid Medline, CINAHL, British Nursing Index, Proquest Nursing Journals, The Cochrane Library, and EBSCO Host Electronic Journals Service. The Journal of the American Dietetic Association and the British Journal of Nutrition were hand searched. Studies published in the English language between 1980 and 2006 were included in the review.
At stage 2 of the review, the title and abstracts were analysed for relevance to the research question; the inclusion and exclusion criteria were applied (see Table 1). Primary papers were then obtained and further scrutinized against the inclusion and exclusion criteria (stage 3). Studies that fulfilled the inclusion criteria were then assessed for quality (stage 4) using quality criteria for quantitative studies (CASP 2004).
Table 1.
Inclusion and exclusion criteria
| Aspect | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Types of paper | Research papers using any methods | Opinion papers |
| Letters to editor | ||
| Conference abstracts | ||
| Foreign language | ||
| Focus of study | Studies designed to assess the nutritional status of pregnant adolescents using biochemical markers | Papers evaluating nutritional status using anthropometric or dietary intake methods |
| Studies with emergent findings relating to nutritional status of pregnant adolescents using biochemical markers | Studies designed to assess the effect of vitamin and/or mineral supplementation on the nutritional status of pregnant adolescents 1 – 5 | |
| Participants | Healthy pregnant human adolescents | Non‐human studies |
| Pregnant adolescents living in industrialized countries | Non‐pregnant or unwell adolescents Pregnant adolescents living in developing countries 6 – 12 | |
| Presentation of data | Mean values and proportions of participants above/ below cut‐off values provided | Insufficient presentation of data or where data from adolescents and adults were combined 12 – 18 |
Analysis
This review describes the biochemical indicators of nutrient status in pregnant adolescents living in industrialized countries. Given the methodological variation in the included papers, the papers were tabulated and summarized narratively.
Findings
Six papers were identified that fulfilled the inclusion criteria (see Table 2). Two of the papers were based on the same cohort of pregnant adolescents (Wolfe et al. 1994; Gadowsky et al. 1995). The number and demographic characteristics of the adolescents participating in each of the two studies differed slightly as insufficient blood was available to complete all the biochemical assays on each blood sample. Four studies were conducted in the USA, and two were carried out in Canada. In three papers, the nutritional status of pregnant adolescents was the primary outcome of interest. Other primary outcome measures included the influence of adolescents’ nutritional status on birth outcome (Jackson & Mathur 1991; Chang et al. 2003a) and a comparison of adolescents’ nutritional status during pregnancy and in the early postpartum period (O’Brien et al. 2003). Birth outcome was also reported as a secondary outcome measure by Wolfe et al. (1994) (birthweight and birth length) and Gadowsky et al. (1995) (birthweight). It was not clear whether any of these studies were powered to examine the relationships between biochemical indicators and birth outcome, and therefore their findings should be treated with caution. Two studies compared nutritional status at different stages of pregnancy (Chang et al. 2003a; Iannotti et al. 2005), one did not clarify the stage of pregnancy when measurements from adolescent participants were obtained (Jackson & Mathur 1991), and the remainder took measurements during the third trimester. Three studies pooled data on adolescent nutritional status for analysis, regardless of ethnicity (Wolfe et al. 1994; Gadowsky et al. 1995; O’Brien et al. 2003). Two studies focused entirely on African American pregnant adolescents (Chang et al. 2003a; Iannotti et al. 2005), and one compared the nutritional status of black and non‐black adolescents (Jackson & Mathur 1991). Four studies sought to determine whether adolescent age was associated with nutritional status (Wolfe et al. 1994; Gadowsky et al. 1995; O’Brien et al. 2003; Iannotti et al. 2005).
Table 2.
Characteristics of included studies
| Author & Country | Participants | Methods | Results | ||
|---|---|---|---|---|---|
| Age/number | Eligibility criteria | Nutrient assessed | Participants | Nutritional status | |
| Jackson & Mathur (1991) USA | 98 black & 28 non‐ black pregnant adolescents aged 13–19 years (mean 17 years) | None specified | Iron status | All participated in the Special Supplementary Nutrition Program for Women, Infants and Children | Black adolescents: mean 33.4 (+2.6)%; 71% had haematocrit values ≤ 34%Non‐black adolescents: mean 34.5 (+2.8)%; 54% had haematocrit values ≤ 34% |
| Wolfe et al. (1994) Canada | 66 pregnant adolescents aged 14–19 years (17.1 + 0.15 years, mean + SEM) | None specified | Zinc | 92% Caucasian; 85% primigravida; 32.0–38.4 weeks’ gestation (35.9 + 0.2, mean + SEM); GA 4.9 + 0.20 years (mean + SEM); none married; 65% transient; 83% consumed prenatal vitamin and/or mineral supplement | None had plasma 1 or hair zinc 2 levels below cut‐off values; none exhibited alkaline phosphatase activity less than lower limit of normal range, but 36% had activities greater than the upper limit 3 |
| Gadowsky et al. (1995) Canada | 58 pregnant adolescents aged 14–19 years (17.0 + 0.17 years, mean + SEM) | None specified | FolateIron statusVitamin B12 Zinc | 88% Caucasian; 84% primigravida; 32.0–38.4 weeks’ gestation (35.9 + 0.2, mean + SEM); 98% had GA ≥ 2 years; none married; 67% transient; 61% smoked; 82% consumed prenatal vitamin and/or mineral supplement | Plasma folate: 7%/17.5% of adolescents had deficient/marginal status 4 Erythrocyte folate: 1%/7% of adolescents had deficient/marginal status 5 Vitamin B12: 20%/26% of adolescents had indeterminate/deficient status 6 Plasma ferritin: 78% had depleted iron stores 7 Haemoglobin: 22% were anaemic 8 |
| Chang et al. (2003a) USA | 918 pregnant adolescents aged 12–17 years (16.1 + 1.1, mean + SD) | African American adolescents who received prenatal care at an inner‐city maternity clinic; singleton pregnancy | Iron status | 82.2% primiparous; 99.7% unmarried; 10% were smokers; 6% had pre‐ eclampsia; measurements taken in 1st, 2nd and 3rd trimesters | As pregnancy progressed the prevalence of anaemia increased, as assessed by Hb and haematocrit levels: 10–13% in 1st trimester; 20–33% in 2nd trimester; 57–66% in 3rd trimester 9 |
| O’Brien et al. (2003) USA | 23 pregnant adolescents aged 13–18 years (16.5 + 1.4, mean + SD) | Healthy primiparous adolescents with uncomplicated singleton pregnancies. No medical problems, no medications known to influence Ca metabolism, no reported history of drug or alcohol abuse, non‐smokers | Calcium status | 32–36 weeks’ gestation (34.7 + 1.0, mean + SD); 20 African American, 3 white; 39% took prenatal supplements | Percentage calcium absorption averaged 53% during pregnancy. Calcium absorption was not significantly affected by age across the range of 13–18 years |
| Iannotti et al. (2005) USA | 80 pregnant adolescents aged 13–18 years (16.5 + 1.1, mean + SD) | African American adolescents attending an inner‐city maternity clinic with singleton pregnancy | Iron statusFolateVitamin B12 | 73% primigravida; 31% <16 years old; GA 4.9 + 1.8 years; prior to pregnancy 53% overweight (BMI > 26.1 kg m– 2) & 9% underweight (BMI < 19.8 kg m– 2; high prevalence of genitourinary infections; 23 participants were studied longitudinally for iron status in the 2nd and 3rd trimesters. Unclear at what stage of pregnancy folate and vitamin B12 was assessed | Iron status: rise in prevalence of compromised iron status from 2nd to 3rd trimester reflected by all indicators of iron status 10 Folate: 88% had levels indicative of positive folate balance; 1 adolescent showed early folate depletion 11 Vitamin B12: none had depleted or deficient status 12 |
BMI, body mass index; CDC, Centers for Disease Control and Prevention; GA, gynaecological age (i.e. age in years past onset of menarche); Hb, haemoglobin; sTfR, serum soluble transferrin receptors; WHO, World Health Organization. 1Based on 36‐week gestation cut‐off value (6.12 µmol L−1) (Hambidge et al. 1983). 2Based on cut‐off value of 1.07 µmol g−1. 3Based on lower and upper limits of normal range of <13 and >50 U L−1, respectively. 4Based on cut‐off values of 7.0 nmol L−1 indicating deficiency and 13.0 nmol L−1 indicating marginal status (Bailey et al. 1980). 5Based on cut‐off values of <317 nmol L−1 indicating deficiency and <362 nmol L−1 indicating marginal status (Bailey et al. 1980). 6Based on ranges of 118–148 pmol L−1 indicating indeterminate status and <118 pmol L−1 indicating deficient status. 7Based on cut‐off values of 26.6 pmol L−1. 8Based on the WHO classification system for anaemia in pregnancy (Hb < 110 g L−1) (Stolzfus & Dreyfuss 1998). 9Based on CDC criteria for anaemia in pregnancy, i.e. Hb cut‐off values of 110 g L−1 in first and third trimesters and 105 g L−1 during second trimester. Corresponding haematocrit cut‐off points were 0.33 during first and third trimesters and 0.32 during second trimester (CDC 1989). Hb concentrations were adjusted for smoking status using CDC criteria (1989). 10Adolescents classified as having iron‐deficiency anaemia if serum ferritin concentration was ≤15 µg L−1 and Hb concentration was<110 g L−1 (1st & 3rd trimesters) or<105 g L−1 (2nd trimester) (CDC 1989). CDC criteria (1989) were used to adjust for smoking status. Depleted iron stores were also indicated by serum ferritin concentrations ≤15 µg L−1 (Perry et al. 1995) and a sTfR : serum ferritin ratio >300 (van den Broek et al. 1998). Tissue iron deficiency was defined when sTfR concentrations exceeded 8.5 mg L−1 (Akesson et al. 1998). 11Serum folate concentrations <6.80 nmol L−1 were used to classify any of the stages of folate depletion or deficiency (Herbert 1967). 12Based on cut‐off values of <111 pmol L−1 for B12 depletion and <74 pmol L−1 for B12 deficiency (Herbert 1999).
Participants
Participants were aged between 12 and 19 years. Only one study randomly selected their participants (Jackson & Mathur 1991), one carried out a retrospective review of medical charts over a 10‐year period (Chang et al. 2003a), and the remainder used convenience samples. Eligibility criteria for participation were often limited, with only three studies employing exclusion criteria. O’Brien et al. (2003) excluded multigravida adolescents and those with complicated pregnancies, medical problems, a history of drug or alcohol abuse, smokers and adolescents taking medications known to influence nutrient metabolism. Chang et al. (2003a) and Iannotti et al. (2005) excluded adolescents with multiple pregnancies.
Few studies provided detailed information regarding the socio‐economic status of their participants. All participants in Jackson & Mathur’s study (1991) were enrolled on the Special Supplementary Nutrition Program for Women, Infants and Children, indicating 185% of poverty, and Wolfe et al. (1994) and Gadowsky et al. (1995) reported that 65% and 67% of participants (respectively) were not living with their parents and were transient, and that the majority of adolescents (55% and 56%, respectively) obtained their main source of income from governmental sources.
Data collection
Details of the included studies are given in Table 3. The measures utilized to ensure the validity of the measures of nutritional status were diverse and often inadequate. Only two studies stated how and when blood samples were separated and stored before analysis (Wolfe et al. 1994; Gadowsky et al. 1995). Other variables known to confound interpretations of the findings, including fluctuations due to diurnal variation and meal consumption, hydration status, medications, infection, inflammation and stress (Gibson 2005), were only partially addressed in the design and/or statistical analyses of the studies. Most studies either did not clarify whether participants had taken prenatal vitamin or mineral supplements, or did not incorporate their contribution into their calculations of nutritional status (see Table 3).
Table 3.
Quality of included studies
| Author | Measurement(s) | Sample | Fasting sample | Separation of sample | Sample storage | Sample characteristics | Variables controlled for in analyses |
|---|---|---|---|---|---|---|---|
| Jackson & Mathur (1991) | Haematocrit | Whole blood | Not stated | – | Not stated | Random sample; not stated whether participants took supplements; stage of pregnancy not stated | No variables controlled for in analysis of nutritional status. |
| Wolfe et al. (1994) | ZincAlkaline phosphatase | Plasma & hairPlasma | Overnight fast | Within 3‐h of taking sample | Frozen | Convenience sample; 4 had pre‐eclampsia and 1 had gestational diabetes | Pearson correlation analyses to test associations between nutrient intake from supplements, number of cigarettes smoked, chronological age and gynaecologic age with biochemical markers. |
| Gadowsky et al. (1995) | FolateVitamin B12 Methylmalonic acidHomocysteineFerritinHaemoglobinMean Cell Volume | Plasma & RBCPlasmaPlasmaPlasmaPlasmaWhole bloodWhole blood | Overnight fast | Sample transported on ice then separated | Frozen at −75°C | Convenience sample | Logistic regression analysis for associations between prenatal supplement use, oral contraceptive use, smoking status, chronological and gynaecologic age with biochemical markers. ANOVA used to test effect of living arrangement and main source of income on biochemical markers. |
| Chang et al. (2003a) | HaemoglobinHaematocrit | Whole blood | Non‐fasting samples | – | Not stated | 10‐year retrospective review of medical charts. All adolescents were prescribed daily prenatal supplements, and anaemic adolescents were prescribed additional iron supplements. However, data on prenatal supplement use or compliance were unavailable | Analysis of variance used to test effects of parity, pre‐pregnancy BMI and adequacy of prenatal care on Hb concentration. T‐tests used to test effects of sexually transmitted diseases, pre‐eclampsia, smoking status and drug use on Hb concentration. |
| O’Brien et al. (2003) | Calcium absorptionCalcium‐related hormones | UrineBlood | 24‐h urine postdosing & 3 spot daily urinesOvernight fast | Not stated | Not stated | Convenience sample. Only assessment of calcium intake included contribution of prenatal supplements | Simple regression analysis used to examine association between age and calcium intake with calcium absorption. T‐tests used to test effects of race on calcium‐related hormone levels. |
| Iannotti et al. (2005) | HaemoglobinHaematocritFerritinTransferrin receptorTfR : ferritin ratioBody ironFolic acidVitamin B12 | Whole bloodWhole bloodSerumSerum––SerumSerum | Non‐fasting samples | Not stated | Not stated | Convenience sample; not stated whether participants took supplements | Linear and logistic regression analyses for associations between maternal age, parity, pre‐pregnancy BMI, pregnancy weight gain, leptin concentration, infant birthweight, genitourinary infections, prenatal care visits, smoking status and iron status. |
BMI, body mass index; Hb, haemoglobin; RBC, red blood cell; TfR, transferrin receptors.
Inter‐relationships between biochemical status and other variables
Iron status and anaemia
Four studies described indices of iron status and anaemia in pregnant adolescents. Anaemia appeared to be prevalent among pregnant adolescents. Measuring haemoglobin (Hb) in 58 adolescents in the third trimester of pregnancy and using the WHO (Stolzfus & Dreyfuss 1998) classification system for anaemia (Hb < 110 g L−1), Gadowsky et al. (1995) reported that 22% of their mainly Caucasian participants were anaemic. Using Centers for Disease Control and Prevention (CDC 1989) criteria to identify anaemia in pregnant women (Hb < 110 g L−1 in the 1st and 3rd trimesters; <105 g L−1 in the 2nd trimester), Chang et al. (2003a) and Iannotti et al. (2005) found that, as pregnancy progressed, the prevalence of anaemia increased in African American pregnant adolescents. In a retrospective medical chart review of 918 adolescents, Chang et al. (2003a) reported the prevalence of anaemia to be 10% of pregnant adolescents in the first trimester, 20% in the second trimester and 57% in the third trimester. Similar findings were reported by Iannotti et al. (2005), with the prevalence of anaemia rising from 31% in the second trimester to 63% in the third trimester in 80 adolescents. Comparing black and non‐black pregnant adolescents, Jackson & Mathur (1991) reported that 71% of black (n = 98) and 54% of non‐black adolescents (n = 28) had a haematocrit (HCT) value of 34% or below. It is not clear how many participants had HCT values below the WHO (Stolzfus & Dreyfuss 1998) cut‐off values for anaemia of 33%.
Iannotti et al. (2005) measured other indicators of iron depletion and deficiency [serum ferritin, serum transferrin receptors (TfR) and body iron] and reported that, for all indicators, there was a rise in the prevalence of compromised iron status during the third trimester. Gadowsky et al. (1995) also assessed iron stores (plasma ferritin) and reported that, using the National Health And Nutrition Examination Survey (NHANES) III ferritin cut‐off value of 12.0 µg L−1 (Looker et al. 1997), 78% of participants had depleted stores at 36 weeks’ gestation, and that all participants demonstrating anaemia based on Hb concentration had low iron stores. In order to assess the relation between iron status indicators, infection and inflammation, Iannotti et al. (2005) conducted a number of analyses using the variables of white blood cell counts, the presence of sexually transmitted infection and various iron status indicators, with no significant correlations observed. Gadowsky et al. (1995) did not measure biochemical indicators of infection and, because the presence of infection can increase plasma ferritin levels, their findings may be confounded.
Chang et al. (2003a) found that lower Hb was associated with multiparity and inadequate prenatal care (in all trimesters), low pre‐pregnancy body mass index (BMI) (in the 1st and 2nd trimesters), and infection with sexually transmitted diseases and self‐reported cigarette use (during the 3rd trimester). Iannotti et al. (2005), on the other hand, reported that parity and age were risk factors for anaemia during the first trimester only, and that neither Hb nor any other iron status indicator was associated with smoking status, number of prenatal visits, BMI or serum leptin concentrations. While not found to be correlated with chronological age, Gadowsky et al. (1995) observed that gynaecologic age was positively correlated with plasma ferritin levels. Only one study investigated the influence of vitamin/mineral supplementation on iron status (Gadowsky et al. 1995). Despite adolescents’ consumption of approximately 31 mg of supplementary iron per day, based on self‐reported weekly supplement usage, no association was found to exist between iron intakes from prenatal supplements and Hb or plasma ferritin concentrations (Gadowsky et al. 1995). Data regarding the type and frequency of supplement use were determined during one prenatal interview, and therefore their accuracy may be questionable.
Indicators of iron status and/or anaemia were not found to be significantly associated with birthweight (Jackson & Mathur 1991; Gadowsky et al. 1995). Chang et al. (2003a) reported that while low Hb (≤105 g L−1) was not significantly associated with preterm delivery or low‐birthweight (LBW) infants, a high Hb (>120 g L−1) was associated with an increased risk of LBW in the second and third trimesters and with an increased risk of preterm delivery in the second trimester. No significant associations were observed to exist between Hb concentration and births that were small for gestational age.
Zinc status
One study investigated the zinc status of adolescents in the third trimester of pregnancy. Appropriate techniques were used in the treatment and storage of the samples (samples were collected after an overnight fast, separated within 3 h of collection and then frozen), avoiding artificially raised zinc values. Using a tentative 36‐week gestation cut‐off value (6.12 µmol L−1) suggested by Hambidge et al. (1983), Wolfe et al. (1994) reported that none of their 66 pregnant adolescent participants had plasma zinc values indicative of impaired zinc status. Similarly, no adolescents had hair zinc levels below the cut‐off value of 1.07 µmol g−1. Plasma zinc concentrations correlated negatively with reported birth length (but not birthweight) when chronological age was taken into account. Wolfe et al. (1994) also found evidence of a differential impact of type of prenatal supplement on zinc status. Adolescents who reported consuming a prenatal supplement with zinc sulphate had a significantly higher mean plasma zinc concentration than those not taking supplements or consuming supplements with zinc oxide. Wolfe et al. (1994) did not evaluate biochemical indicators of infection and therefore, as the presence of infection lowers serum zinc values, the reported values may be confounded.
Calcium
O’Brien et al. (2003) investigated the efficiency of fractional calcium absorption and changes in urinary calcium during (and after) pregnancy in a small sample (n = 23) of mainly African American adolescents. Fractional calcium absorption was determined by giving adolescents oral (46Ca or 44Ca) and intravenous (42Ca) stable calcium isotopes at 32–36 weeks’ gestation. A 24‐h urine collection was obtained postdosing, and three spot urine collections were obtained daily. Percentage calcium absorption averaged 53% in adolescents during pregnancy and was nearly 60% higher than values measured 3–4 weeks after delivery. Calcium absorption was not significantly affected by age across the range of 13–18 years, and the findings did not markedly differ from data reported for pregnant adults (Cross et al. 1995; Ritchie et al. 1998). Higher calcium intakes during pregnancy, however, did appear to be protective against loss of trabecular bone at the lumbar spine in the early postpartum period.
Folate status
Two studies investigated the folate status of pregnant adolescents. Using cut‐off criteria suggested by Bailey et al. (1980), Gadowsky et al. (1995) reported that 7% and 17.5% of participants (n = 50–57) had plasma folate concentrations indicative of deficient (<7.0 nmol L−1) and marginal (<13 nmol L−1) status, respectively, and 1% and 7% had erythrocyte concentrations indicative of deficiency (<317 nmol L−1) and marginal (<362 nmol L−1) folate status, respectively. A more recent study of African American pregnant adolescents (Iannotti et al. 2005) found that only one participant (of the 60 evaluated) had a serum folate concentration of <6.8 nmol L−1 (Herbert 1967). However, it was not stated whether suitable antioxidants were added to the blood samples in this study (a procedure utilized to prevent the oxidation of folate), and so these findings may be erroneous. Folate status was not found to be correlated with chronological or gynaecological age, infant birthweight (Gadowsky et al. 1995) or indicators of iron status (Iannotti et al. 2005). Erythrocyte folate concentrations, however, were negatively associated with the average number of cigarettes smoked per day during pregnancy (Gadowsky et al. 1995). Only Gadowsky et al. (1995) reported details of folate supplementation, with 45 (of the sample of 58) adolescents consuming supplemental folic acid and iron simultaneously. Limited information was available regarding the frequency of supplement use.
Vitamin B12
Two studies investigated the vitamin B12 status of pregnant adolescents. A study of African American pregnant adolescents (Iannotti et al. 2005) found that none of their 60 participants for whom measures were available, had serum vitamin B12 concentrations indicative of depletion (<111 pmol L−1) or deficiency (<74 pmol L−1) (Herbert 1999). Using different cut‐off values, Gadowsky et al. (1995) reported that 20% and 26% of participants had plasma B12 concentrations in the intermediate (118–148 pmol L−1) and deficient (<118 pmol L−1) range, respectively. Gadowsky et al. (1995) also measured plasma methylmalonic acid concentrations, as this accumulates in the plasma when the supply of vitamin B12 is reduced thereby providing a sensitive and specific marker of vitamin B12 deficiency (Gibson 2005). Four participants had elevated plasma methylmalonic acid concentrations, but only one of them had a plasma vitamin B12 concentration in the intermediate/low range. Vitamin B12 status was not found to be correlated with chronological or gynaecological age or infant birthweight (Gadowsky et al. 1995). Neither study collected data regarding vegetarianism, and only Gadowsky et al. (1995) reported details of vitamin B12 supplementation. Of their entire sample of 58 adolescents, only 22 consumed a supplement containing vitamin B12 during pregnancy. Limited information was available regarding the frequency of supplement use.
Discussion
The quality of the studies included in the review varied, and all suffered from methodological limitations, which should be considered when interpreting the results. Studies tended to use different reference cut‐off values to define nutritional adequacy, restricting the ability to make meaningful comparisons between studies. The application of exclusion criteria and control of potential confounding variables was limited in most studies, thereby limiting the reliability of the data. All of the studies were subject to sampling bias, with most utilizing convenience sampling methods. Only one study used random sampling in a study comparing black and non‐black pregnant adolescents (Jackson & Mathur 1991). However, the small sample number in the non‐black group (n = 28 versus 98 black adolescents) limits the generalizability of the findings. The participants in the included studies tended to be older adolescents, with a mean age of between 16 and 17 years. It is likely that the nutritional requirements of a younger adolescent group (which are needed to sustain their own growth, as well as the growth of their fetus) are different from an older adolescent group. Only four studies sought to determine whether adolescent age was associated with nutritional status (Wolfe et al. 1994; Gadowsky et al. 1995; O’Brien et al. 2003; Iannotti et al. 2005). While none of the indices of folate, vitamin B12 or calcium status were found to be associated with chronological age (Gadowsky et al. 1995; O’Brien et al. 2003), iron status did appear to be influenced by both chronological and gynaecologic age. Increasing chronological age had a significant protective effect against low Hb concentrations during the first trimester (Iannotti et al. 2005), and gynaecologic age correlated positively with plasma ferritin (Gadowsky et al. 1995). Wolfe et al. (1994) found that plasma zinc concentrations were only significantly correlated with chronological and gynaecologic age in adolescents who smoked. However, most of these studies suffered from small sample sizes and an over‐reliance on older pregnant adolescents, thereby potentially masking any further associations. There was limited evidence to support a significant influence of nutritional status on birth outcome. However, as no power calculations were conducted, it is likely that most, if not all, of the studies were underpowered for this analysis, and it is possible that small sample sizes played a role in the lack of significant associations observed.
Iron is needed for the rapid expansion of maternal blood volume and the deposition of iron in fetal tissues. Iron‐deficiency anaemia is the most common nutrient deficiency in pregnancy and has also been reported to be at its peak incidence between the ages of 15 and 19 years in non‐pregnant girls (Wahl 1999), related in part to the rapid growth associated with adolescence (Lifshitz et al. 1993). There does appear to be some consensus among the studies included in this review to suggest that anaemia was indeed prevalent among pregnant adolescents. Iron stores were also low in pregnant adolescents, particularly during the third trimester (Gadowsky et al. 1995; Iannotti et al. 2005). Previous studies have associated iron‐deficiency anaemia with reduced fetal oxygenation (Reifsnider & Gill 2000) and poor birth outcomes, such as a greater risk of LBW, prematurity and an increased perinatal mortality (Godfrey et al. 1991; Scholl et al. 1992; Tomashek et al. 2006). Despite this, there appeared to be little evidence that low iron status and/or the presence of anaemia were significantly associated with poor birth outcome among the adolescents in the included studies. The association between moderate anaemia and poor perinatal outcomes, however, has been found through epidemiological studies only, and the available evidence cannot establish this relationship as causal (Yip 2000). Indeed, anaemia may not be a direct cause of poor pregnancy outcomes, unless very severe. It is possible that a common factor could cause both anaemia and poor birth outcomes.
Each of the studies was limited, by varying degree, by measurement error. Many measures of anaemia and iron status are subject to confounders such as biological variation, ethnicity, smoking status and deficiencies in other micronutrients, such as vitamins A, B6 and B12, riboflavin, folic acid and copper (Gibson 2005). There is also considerable within‐subject day‐to‐day variation in plasma ferritin concentrations and other indicators of iron status such as plasma iron and transferrin saturation (Beard 1994). As a consequence, it has been argued that studies should include replicated measures and report within‐subject variability (Gibson 2005). Neither Gadowsky et al. (1995) nor Iannotti et al. (2005) accounted for within‐subject variability as plasma ferritin assessment relied on a single blood sample.
One of the studies combined measures of iron status from black and non‐black participants (Gadowsky et al. 1995). Black populations have been found to have a Hb concentration of 5.5–8.5 g L−1 lower than white populations (Perry et al. 1992), potentially leading to a misclassification on anaemia in these studies by using a single anaemia cut‐off value across ethnic groups. One study failed to control for smoking status (Jackson & Mathur 1991), which is associated with higher concentrations of Hb and a decrease in serum TfR concentration.
Misclassification of iron deficiency may also occur due to overlapping of normal and abnormal values if a single indicator is used. Thus, the use of several different indicators of iron status simultaneously provides a more valid assessment of iron status than any single measure. Moreover, multiple indicators differentiate the severity of iron deficiency more readily. A combination of three tests of iron status is commonly advocated, with abnormal values for at least two of the three tests indicating iron deficiency. At present, however, there is no consensus on the best definition of iron deficiency when based on multiple measurements. Gibson (2005) argued that a combination of serum TfR, serum ferritin and Hb measurements may be the most useful model for the measurement of iron status: serum ferritin levels reflect the decline and eventual exhaustion of body iron stores; serum TfR reflects the degree of deficiency in functional iron after the stores have been depleted; and Hb measures the presence of anaemia. Only one study (Iannotti et al. 2005) assessed each of these indices in pregnant adolescents, in addition to considering the effects of a variety of potential confounding factors, and reported an increased prevalence of compromised iron status during the third trimester compared with the second trimester. Unfortunately, they did not investigate how this impacted upon birth outcome.
During pregnancy, calcium is needed for the development of the fetal skeleton and the attainment of peak bone mass in adolescents. In a still‐growing adolescent, calcium intake may be limited by poor maternal diet and the need to retain enough calcium to mineralize two skeletons (Lenders et al. 2000). Low calcium intake has been significantly associated with low bone density and increased later risk of osteoporosis in adolescents (Lytle 2002) and decreased fetal femur length in utero in pregnant adolescents (Chang et al. 2003b). Only one study that aimed to characterize the calcium status of pregnant adolescents met the inclusion criteria of the current review (O’Brien et al. 2003). Even though adolescents in this study were unlikely to have achieved peak bone mass, calcium absorption did not markedly differ from data reported for pregnant adults in previous studies (Cross et al. 1995; Ritchie et al. 1998). Higher dietary calcium intakes during pregnancy were significantly associated with maternal lumar z‐scores in the early post‐natal period and improvements in estimated calcium balance, suggesting that higher calcium intakes were protective against bone loss in these adolescents. However, calcium intakes were substantially higher (approximately 1200 mg day−1) in this study than those reported by pregnant adolescents in previous studies (e.g. Endres et al. 1985; Job & Capra 1995; Pobocik et al. 2003). It has been suggested that, when calcium intake is low, the physiologic adaptation to pregnancy in adolescents may be different from that in adults, increasing the adolescents’ susceptibility to bone mineral loss (Bezerra et al. 2002). In a study of women and adolescents with mean calcium intakes of 400–500 mg day−1, Bezerra et al. (2002) found that the increased bone resorption associated with pregnancy was less pronounced in adolescents, possibly as a protective mechanism against excessive bone loss when calcium intake is low. Pregnancy also seemed to impair bone formation in adolescents, suggesting that skeletal growth may be suppressed. O’Brien et al.’s (2003) study was also limited by small sample size and the failure to address potential racial differences in calcium status.
Evidence on the folate status of pregnant adolescents was inconclusive, limited by interpretive difficulties. While Gadowsky et al. (1995) reported that 7% and 17.5% of their 58 participants had plasma folate concentrations indicative of deficient (<7.0 nmol L−1) and marginal (<13.0 nmol L−1) status, respectively, Iannotti et al. (2005) found that only one participant (of the 60 evaluated) had a serum folate concentration of <6.8 nmol L−1. As described earlier, because it was unclear whether the correct procedures had been followed in order to maintain the stability of the folate in the sample, these findings may be erroneous. An alternative explanation for the generally higher folate levels reported by Iannotti et al. (2005) could relate to the US policy of folate fortification of foods. The US government introduced the mandatory fortification of cereals in 1998, which consequently could have a marked effect on ‘normal’ reference ranges for folate (Klee 2000). Indeed, ecological studies have shown a concomitant rise in markers of folate status in similar populations during this period (CDC 2002). Other factors found to be significantly associated with folate status, including smoking status (which lowers serum and erythrocyte folate concentrations) and alcohol ingestion (which results in an acute drop in serum folate levels), were not measured comprehensively in Iannotti et al.’s (2005) study. While not found to be associated with plasma folate, Gadowsky et al. (1995) found that the number of cigarettes smoked per day averaged over the entire pregnancy was negatively associated with erythrocyte concentration. The influence of alcohol ingestion was not evaluated.
Other difficulties may also arise when attempting to interpret these findings. While the commonly used serum folate cut‐off value of <6.8 nmol L−1 (Herbert 1967) is known to be indicative of negative folate balance at the time of sampling, it does not necessarily indicate folate depletion unless the negative folate balance persists (Herbert 1987). Thus, as the included studies took measurements at a single time‐point, individuals who had serum folate concentrations below this cut‐off point may have had normal biochemical function and no evidence of tissue folate depletion (Gibson 2005). Furthermore, while this cut‐off point is used for low serum folate values during pregnancy, the data justifying its use are lacking (O’Connor 1994). Indeed, pregnancy usually results in a decline in serum folate values, due possibly to haemodilution and changes in renal tubular function (O’Connor 1994).
Gadowsky et al. (1995) also provided erythrocyte folate concentrations, which are less sensitive than serum folate levels to short‐term fluctuations in folate status and reflect stores of folate (Gibson 2005) and are a more reliable measure of folate status than serum folate (Senti & Pilch 1985). In their study of 58 adolescents, Gadowsky et al. (1995) reported that 1% and 7% of participants had erythrocyte concentrations indicative of deficient (<317 nmol L−1) and marginal (<362 nmol L−1) folate status, respectively. Although different cut‐off values were utilized, the folate status of this sample appears to be significantly better than that reported in large nutritional surveys of non‐pregnant adolescents. The UK National Diet and Nutrition Survey (NDNS) of Young People (Gregory et al. 2000), for example, reported that although no more than 1% of girls aged 4–18 years had erythrocyte folate levels reflecting severe folate deficiency (<230 nmol L−1), 39% aged 15–18 years had erythrocyte folate levels considered to indicate marginal status (<425 nmol L−1). The relatively low prevalence of suboptimal folate status among pregnant adolescents in Gadowsky and colleagues’ study may be attributed to the general practice of prescribing prenatal supplements to pregnant adolescents in the study area (Southern Ontario, Canada). The majority of participants were prescribed a prenatal supplement containing either 1.0‐ or 0.8‐mg folic acid, although compliance was reportedly low (18% consumed a prenatal supplement on average less than once per week) (Gadowsky et al. 1995). The NDNS also reported that erythrocyte folate concentrations declined from childhood through to adolescence (Gregory et al. 2000). In contrast, Gadowsky et al. (1995) found no correlation between plasma or erythrocyte folate concentration and chronological or gynaecologic age.
Cut‐off values to interpret erythrocyte folate concentrations during pregnancy remain ill‐defined. Similar to serum folate, pregnancy and increasing parity tend to be associated with lower erythrocyte folate levels (Gibson 2005). Thus, the use of generic cut‐off values, as utilized by Gadowsky et al. (1995), may be inappropriate. Furthermore, erythrocyte folate concentrations can underestimate the extent of folate depletion in pregnancy, when depletion may be rapid, because the concentration reflects folate stores at the time of red cell synthesis. Thus, during the progressive development of a deficiency state, erythrocyte folate will always lag behind more acute markers, such as serum folate (Gibson 2005).
Using a cut‐off value suggested by Hambidge et al. (1983), Wolfe et al. (1994) reported that pregnant adolescents did not appear to have plasma zinc values indicative of impaired zinc status. Revised cut‐off values for serum zinc have been more recently suggested by the International Zinc Consultative Group (Hotz & Brown 2004), who took into account potential confounding factors such as low serum albumin, elevated white blood cell counts, use of oral contraceptive agents, hormones or steroids, and the presence of diarrhoea. Although data for pregnant women were limited (n = 61), the lower limit for serum zinc in the third trimester was calculated at 7.6 µmol L−1, somewhat higher than the cut‐off value of 6.1 µmol L−1 used by Wolfe et al. (1994). Thus, if this higher cut‐off value were to be applied, it is possible that some adolescents did have a suboptimal zinc status. Indeed, there is evidence to suggest that adolescents are at risk of deficient zinc intake. The UK NDNS (Gregory et al. 2000) reported that one in three girls aged 11–14 years and one in 10 girls aged 15–18 years had a zinc intake below the lower reference nutrient intake (LRNI). By the age of 19–24 years, the proportion of women whose zinc intake fell below the LRNI was one in 20 (Henderson et al. 2003). The extent to which dietary zinc intake is associated with circulating zinc concentration, however, is unclear, and it has been suggested that plasma zinc concentrations are maintained at homeostatic levels over a wide range of zinc intakes (Pilch & Senti 1984). Rather, it is likely that poor zinc status is due to the presence of a condition or factor that alters zinc utilization, such as smoking, alcohol abuse, or an acute stress response to infection or trauma, rather than to the intake of a zinc‐poor diet (King 2000). In pregnancy, such factors could lower plasma zinc concentrations and reduce the amount of zinc available to the fetus.
The significance of plasma zinc concentrations below the lower limits for pregnancy is uncertain, and has varied with both the stage of gestation and the outcome variable measured (King 2000). Wolfe et al. (1994), controlling for chronological age, reported a negative correlation between plasma zinc concentrations and birth length, but not birthweight. A negative correlation between plasma zinc and chronological age was also observed, suggesting that younger pregnant adolescents may be more susceptible to zinc deficiency and its adverse outcomes. The majority of participants in Wolfe and colleagues’ study were aged 15–17 years (40%) and >17.1 years (53%), an age group said to have lower risk of serious adverse pregnancy outcomes than those aged <15 years (Olausson et al. 1999; Lenders et al. 2000).
The two studies that investigated the vitamin B12 status of pregnant adolescents, although using different cut‐off criteria, appeared to provide contrasting results. Whereas Iannotti et al. (2005) found no evidence of serum vitamin B12 depletion (<111 pmol L−1) or deficiency (<74 pmol L−1), Gadowsky et al. (1995) reported that 20% and 26% of subjects had plasma B12 concentrations in the intermediate (118–148 pmol L−1) and deficient (<118 pmol L−1) range, respectively. Gadowsky et al. (1995) assessed vitamin B12 status in the third trimester, but stage of pregnancy for this measurement was not clarified by Iannotti et al. (2005). This significantly limits the interpretation of this study, as serum vitamin B12 concentrations are known to decline steadily throughout the course of pregnancy due to the rapid transfer of the vitamin to the fetal circulation (Allen 1994). A recent study demonstrated that maternal plasma cobalamin levels declined significantly when tested at 18 (225 pmol L−1), 32 (172 pmol L−1) and 39 (161 pmol L−1) weeks’ gestation, and that 43% of women had cobalamin values <150 pmol L−1 at 39 weeks’ gestation (Milman et al. 2006).
Values that indicate vitamin B12 status, i.e. as ‘deficient’, ‘moderate’ and ‘normal’, are not well defined in the literature and vary with the analytical method used, the laboratory conducting the analysis, the sample size and the study design. It is recommended that each laboratory analysing serum or plasma vitamin B12 establish its own lower reference limits or cut‐off points based on its own assay method (Gibson 2005). Carmel et al. (1996) note that concentrations <74 pmol L−1 almost always indicate a vitamin B12 deficiency state, irrespective of the assay procedure used. Unfortunately, it is impossible to assess if any participants in Gadowsky et al.’s (1995) study had vitamin B12 concentrations below this cut‐off value.
Moderately low values for serum vitamin B12 concentrations are often defined as 100–150 pmol L−1. Such levels are much more difficult to interpret (Amos et al. 1994), however, as they can occur in association with megaloblastic anaemia produced by folate deficiency, in iron deficiency, and with other disease states and conditions (Gibson 2005). Because of these problems, many investigators recommend measuring concentrations of serum methylmalonic acid or serum homocysteine if serum vitamin B12 concentrations are <225 pmol L−1. These tests are more sensitive and specific indicators of functional vitamin B12 deficiency. Gadowsky et al. (1995) found that only four adolescents had an elevated plasma methylmalonic acid concentration and, of these four participants, only one had a suboptimal plasma vitamin B12 value.
Serum vitamin B12 levels may also be inversely related to BMI, although the evidence to support this relationship remains conflicting (Hanger et al. 1991; Weggemans et al. 1997; Wahlin et al. 2002). If such a relationship exists, then it could influence the interpretation of the serum vitamin B12 of the participants of the study of Iannotti et al. (2005), 53% of whom were overweight (i.e. BMI > 26.1 kg m−2).
Socio‐economic factors are known to exert a strong influence on nutrient intake and subsequent nutritional status. Adolescents in lower socio‐economic groups are more likely to consume whole milk, table sugar and sugar confectionary, and have lower biochemical status of folate, vitamin C, vitamin D and iron (Gregory et al. 2000). Barriers to good nutritional practices of women living in conditions of material deprivation include problems with access, cost and storage of food (Reid & Adamson 1997). It has been reported that a high proportion of low‐income pregnant adolescents miss meals and resort to buying less healthy ‘cheap filler’ foods when money runs out (Burchett & Seeley 2003). Over time, such ways of ‘managing’ poverty can become second nature, despite the potential costs to the adolescents’ physical and emotional well‐being (Attree 2005). In addition, while peer influences may become more dominant as adolescents get older (Buttriss 2002), the influence of the family on their food choices and eating behaviours should not be overlooked. A recent study has shown, for example, that parental presence at the evening meal has been positively associated with adolescents’ higher consumption of fruits, vegetables and dairy foods (Videon & Manning 2003). A recent survey of British pregnant adolescents revealed that over half had family members who shopped and cooked for them (Burchett & Seeley 2003), and that family influences were often positive, encouraging healthy eating and providing meals if the adolescent was not living at home. Few of the studies in this review provided sufficient amount of detail regarding the socio‐economic and living circumstances of their participants. Without this information, it is not possible to accurately identify adolescents who are in need of specific nutritional intervention.
Thus, it is clear that, at present, there is a paucity of high‐quality well‐controlled research to provide us with a clear description of pregnant adolescents’ nutritional status. Double‐blind randomized controlled trails are needed to confirm relationships between adolescents’ nutritional status and maternal and neonatal outcomes, with representative samples and a sample size appropriate for examining the outcomes to be investigated. Adolescent age, use of supplements, stage of pregnancy, ethnicity and the various socio‐cultural influences affecting the pregnant adolescent’s nutritional status should also be investigated further.
Nutrition in adolescent pregnancy must be viewed within a biopsychosocial context, because it has consistently been shown that there are multiple influencing factors that play a role in the eating behaviour and subsequent nutritional status of the pregnant adolescent. Eating behaviours are likely to be related to other, often ‘risky’, behaviours displayed in adolescents and should not be viewed in isolation (Irwin et al. 1997). Research has shown, for example, that adolescents who make less‐healthful food choices than their peers have lower physical activity patterns and are more likely to smoke cigarettes (Lytle et al. 1995), and that adolescents who have sex, drink and smoke are more likely to eat high‐salt, high‐fat and high‐sugar foods (Irwin et al. 1997).
Achieving dietary change and improving the nutritional status of this particularly vulnerable section of the population, many of whom are from disadvantaged backgrounds (Social Exclusion Unit 1999), presents a major public health challenge. Biopsychosocial factors often experienced by such groups, including low levels of disposable income, unemployment, poor housing, suboptimal mental and physical health, and limited access to a wide variety of reasonably priced foods, all contribute to difficulties in tacking behavioural change (Symon & Wrieden 2003). These factors, in turn, lead to increasing health inequalities (Acheson 1998). An important factor that should be considered when developing appropriate and effective strategies to promote healthy eating in pregnant adolescents is the heterogeneity of the group. Factors affecting food choices vary considerably, depending on the individual’s particular circumstances. Family and peers are likely to have a strong influence on the eating habits of most pregnant adolescents. Poverty is a significant factor that limits the ability of some pregnant adolescents to eat a healthy diet, even in those who aspire to it.
Overcoming the barriers in order to achieve improved nutrition in pregnancy among adolescents requires multidisciplinary collaborations of adolescent healthcare providers, academics, professional organizations, policymakers, industry and service users. Certainly, more needs to be done at a policy level, both with regards to enabling adolescents’ access to optimal nutrition and in modifying the nutrition message that adolescents receive. Governments around the world should ensure that there is consistency of food and nutrition messages in schools, for example, to include in the curriculum, food provision in the canteen, vending machine policies, breakfast clubs, snacking and lunchbox policies. Any intervention that aims to encourage changes in lifestyle should be multifactorial and incorporate measures to improve the socio‐economic circumstances of adolescents and their families. Only once this is achieved can adolescent nutrition, and adolescent nutrition in pregnancy, be significantly and sustainably optimized.
Acknowledgements
The author would like to thank Rosalind Gibson and Genevieve Becker whose insightful comments helped to improve this manuscript.
References
- Acheson D. [chairman] (1998) Independent Inquiry into Inequalities in Health. HMSO: London. [Google Scholar]
- Akesson A., Bjellerup P., Berglund M., Bremme K. & Vahter M. (1998) Serum transferring receptor: a specific marker of iron deficiency in pregnancy. American Journal of Clinical Nutrition 68, 1241–1246. [DOI] [PubMed] [Google Scholar]
- Allen L.H. (1994) Vitamin B12 metabolism and status during pregnancy, lactation and infancy In: Nutrient Regulation During Pregnancy, Lactation and Infant Growth (eds Allen L, King & J. B Lønnerdal), pp. 173–186. Plenum Press: New York. [DOI] [PubMed] [Google Scholar]
- Amos R.J., Dawson D.W.I., Fish D.I., Leeming R.J. & Linnell J.C. (1994) Guidelines on the investigation and diagnosis of cobalamin and folate deficiencies. Clinical and Laboratory Haematology 46, 101–115. [PubMed] [Google Scholar]
- Attree P. (2005) Low‐income mothers, nutrition and health: a systematic review of qualitative evidence. Maternal and Child Nutrition 1, 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L.B., Mahan C.S. & Dimperio D. (1980) Folacin and iron status in low‐income pregnant adolescents and mature women. American Journal of Clinical Nutrition 33, 1997–2001. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P. & Osmond C. (1986) Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081. [DOI] [PubMed] [Google Scholar]
- Beard J.L. (1994) Iron deficiency: assessment during pregnancy and its importance in pregnant adolescents. American Journal of Clinical Nutrition 59 (Suppl.), 502S–510S. [DOI] [PubMed] [Google Scholar]
- Bezerra F.F., Laboissière F.P., King J.C. & Donangelo C.M. (2002) Pregnancy and lactation affects markers of calcium and bone metabolism differently in adolescent and adult women with low calcium intakes. Journal of Nutrition 132, 2183–2187. [DOI] [PubMed] [Google Scholar]
- Van Den Broek N.R., Letsky E.A., White S.A. & Shenkin A. (1998) Iron status in pregnant women: which measurements are valid? British Journal of Haematology 103, 817–824. [DOI] [PubMed] [Google Scholar]
- Burchett H. & Seeley A. (2003) Good Enough to Eat? The Diet of Pregnant Teenagers. Maternity Alliance/Food Commission: London. [Google Scholar]
- Buttriss J. (2002) Nutrition, health and schoolchildren. Nutrition Bulletin 27, 275–305. [Google Scholar]
- Carmel R., Green R., Jacobsen D.W. & Qian G.D. (1996) Neutrophil nuclear segmentation in mild cobalamin deficiency: relation to metabolic tests of cobalamin status and observations on ethnic differences in neutrophil segmentation. American Journal of Clinical Pathology 106, 57–63. [DOI] [PubMed] [Google Scholar]
- Casanueva E., Jimenez J., Meza‐Camacho C., Mares M. & Simon L. (2003) Prevalence of nutritional deficiencies in Mexican adolescent women with early and late prenatal care. Archivos Latinoamericanos de Nutrición 53, 35–38. [PubMed] [Google Scholar]
- Cavadini C., Siega‐Riz A.M. & Popkin B.M. (2000) US adolescent food intake trends from 1965 to 1996. Archives of Disease in Childhood 83, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC ) (1989) CDC criteria for anemia in children and childbearing age women. Morbidity and Mortality Weekly Report 38, 400–404. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2002) Folate status in women of childbearing age, by race/ ethnicity – United States, 1999–2000. Morbidity and Mortality Weekly Report 51, 808–810. [PubMed] [Google Scholar]
- Chang S.‐C., O’Brien K.O., Schulman Nathanson M., Caulfield L.E., Mancini J. & Witter F.R. (2003a) Hemoglobin concentrations influence birth outcomes in pregnant African‐American adolescents. Journal of Nutrition 133, 2348–2355. [DOI] [PubMed] [Google Scholar]
- Chang S.‐C., O’Brien K.O., Schulman Nathanson M., Caulfield L.E., Mancini J. & Witter F.R. (2003b) Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. American Journal of Clinical Nutrition 77, 1248–1254. [DOI] [PubMed] [Google Scholar]
- Cherry F.F., Sandstead H.H., Rojas P., Johnson L.K., Batson H.K. & Wang X.B. (1989) Adolescent pregnancy: associations among body weight, zinc nutriture, and pregnancy outcome. American Journal of Clinical Nutrition 50, 945–954. [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme (CASP ) (2004) 12 Questions to Help You Make Sense of a Cohort Study. Public Health Resource Unit: Oxford. Available at: http://www.phru.nhs.uk/casp/critical_appraisal_tools.htm#cohort (accessed 11 July 2006). [Google Scholar]
- Cross N.A., Hillman L.S., Allen S.H., Krause G.F. & Vieira N.E. (1995) Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. American Journal of Clinical Nutrition 61, 514–523. [DOI] [PubMed] [Google Scholar]
- Dawson E.B. & McGanity W.J. (1989) Serum ferritin levels after multivitamin iron ingestion during teenage pregnancy. Clinical Therapeutics 11, 151–159. [PubMed] [Google Scholar]
- Dawson E.B., Albers J. & McGanity W.J. (1989) Serum zinc changes due to iron supplementation in teen‐age pregnancy. American Journal of Clinical Nutrition 50, 848–852. [DOI] [PubMed] [Google Scholar]
- Endres J.M., Poell‐Odenwald K., Sawicki M. & Welch P. (1985) Dietary assessment of pregnant adolescents participating in a supplemental‐food program. Journal of Reproductive Medicine 30, 10–17. [PubMed] [Google Scholar]
- Gadowsky S.L., Gale K., Wolfe S.A., Jory J., Gibson R. & O’Connor D.L. (1995) Biochemical folate, B12 and iron status of a group of pregnant adolescents accessed through the public health system in Southern Ontario. Journal of Adolescent Health 16, 465–474. [DOI] [PubMed] [Google Scholar]
- García‐Casal M.N., Osorio C., Landaeta M., Leets I., Matus P., Fazzino F. et al. (2005) High prevalence of folic acid and vitamin B12 deficiencies in infants, children, adolescents and pregnant women in Venezuela. European Journal of Clinical Nutrition 59, 1064–1070. [DOI] [PubMed] [Google Scholar]
- Geervani P. & Jayashree G. (1988) A study on nutritional status of adolescent and adult pregnant and lactating women and growth of their infants. Journal of Tropical Pediatrics 34, 234–237. [DOI] [PubMed] [Google Scholar]
- Gibson R.S. (2005) Principles of Nutritional Assessment, 2nd edn Oxford University Press: Oxford. [Google Scholar]
- Gluckman P.D., Hanson M.A. & Pinal C. (2005) The developmental origins of adult disease. Maternal and Child Nutrition 1, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K.M., Redman C.W.G., Barker D.J.P. & Osmond C. (1991) The effect of maternal anaemia and iron deficiency on the ratio of fetal weight to placental weight. British Journal of Obstetrics and Gynaecology 98, 886–891. [DOI] [PubMed] [Google Scholar]
- Gregory J., Lowe S., Bates C., Prentice A., Jackson L., Smithers G. et al. (2000) Report of the Diet and Nutrition Survey. Volume 1, National Diet and Nutrition Survey: Young People Aged 4 to 18 Years. The Stationery Office: London. [Google Scholar]
- Hall Moran V. (2007) A systematic review of dietary assessments of pregnant adolescents in industrialised countries. British Journal of Nutrition 97, 411–425. [DOI] [PubMed] [Google Scholar]
- Hambidge K.M., Krebs N.F., Jacobs M.A., Favier A., Guyette L. & Ikle D.N. (1983) Zinc nutritional status during pregnancy: a longitudinal study. American Journal of Clinical Nutrition 37, 429–442. [DOI] [PubMed] [Google Scholar]
- Hanger H.C., Sainsbury R., Gilchrist N.L., Beard M.E.J. & Duncan J.M. (1991) A community study of vitamin B12 and folate levels in the elderly. Journal of the American Geriatrics Society 39, 1155–1159. [DOI] [PubMed] [Google Scholar]
- Henderson J., Irving K., Gregory J., Bates C.J., Prentice A., Pervs J. et al. (2003) The National Diet and Nutrition Survey: Adults Aged 19–64 Years, Volume 3: Vitamin and Mineral Intakes and Urinary Analytes. The Stationery Office: London. [Google Scholar]
- Herbert V. (1967) Biochemical and haematological lesions in folic acid deficiency. American Journal of Clinical Nutrition 20, 562–569. [DOI] [PubMed] [Google Scholar]
- Herbert V. (1987) Making sense of laboratory tests of folate status: folate requirements to sustain normality. American Journal of Hematology 26, 199–207. [DOI] [PubMed] [Google Scholar]
- Herbert V. (1999) Folic Acid Modern Nutrition in Health and Disease, 9th edn Lippincott Williams & Wilkins: Philadelphia, PA. [Google Scholar]
- Hotz C. & Brown K.H. (eds) (2004) International Zinc Nutrition Consultative Group (IZiNCG). Technical Document No. 1: assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin 25 (Suppl. 2), S94–S204. [PubMed] [Google Scholar]
- Hunt I.F., Murphy N.J., Cleaver A.E., Faraji B., Swendseid M.E., Browdy B.L. et al. (1985) Zinc supplementation during pregnancy in low‐income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. American Journal of Clinical Nutrition 42, 815–828. [DOI] [PubMed] [Google Scholar]
- Iannotti L.L., O’Brien K.O., Chang S.‐C., Mancini J., Schulman Nathanson M., Liu S. et al. (2005) Iron deficiency anemia and depleted body iron reserves are prevalent among pregnant African‐American adolescents. Journal of Nutrition 135, 2572–2577. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM ) (1990) Nutrition During Pregnancy. National Academy Press: Washington, DC. [Google Scholar]
- Irwin C.E., Igra V., Eyre S. & Millstein S. (1997) Risk‐taking behavior in adolescents: the paradigm. Annals of the New York Academy of Sciences 817, 1–35. [DOI] [PubMed] [Google Scholar]
- Jackson E. & Mathur K. (1991) Adolescent pregnancy: effects of nutrients on hematocrit and birth weight in Orangeburg county. Journal of the South Carolina Medical Association 87, 8–11. [PubMed] [Google Scholar]
- Job J. & Capra S. (1995) Nutritional assessment of pregnant teenagers attending a metropolitan, public, maternity hospital in Brisbane 1. Nutrient intakes. Australian Journal of Nutrition and Dietetics 52, 76–82. [Google Scholar]
- King J.C. (2000) Determinants of maternal zinc status during pregnancy. American Journal of Clinical Nutrition 71, 1334S–1343S. [DOI] [PubMed] [Google Scholar]
- King J.C. (2003) The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. Journal of Nutrition 133, 1732S–1736S. [DOI] [PubMed] [Google Scholar]
- Klee G.C. (2000) Cobalamin and folate evaluation: measurement of methylalonic acid and homocysteine vs vitamin B12 and folate. Clinical Chemistry 46, 1277–1283. [PubMed] [Google Scholar]
- Lenders C.M., McElrath T.F. & Scholl T.O. (2000) Nutrition in adolescent pregnancy. Current Opinions in Pediatrics 12, 291–296. [DOI] [PubMed] [Google Scholar]
- Lifshitz F., Tarim O. & Smith M.M. (1993) Nutrition in adolescence. Adolescent Endocrinology 22, 673–683. [PubMed] [Google Scholar]
- Looker A.C., Dallman P.R., Carroll M.D., Gunter E.W. & Johnson C.L. (1997) Prevalence of iron deficiency in the United States. Journal of the American Medical Association 277, 973–976. [DOI] [PubMed] [Google Scholar]
- Lytle L.A. (2002) Nutritional issues for adolescents. Journal of the American Dietetic Association 102, S8–S12. [DOI] [PubMed] [Google Scholar]
- Lytle L.A., Kelder S., Perry C. & Klepp K. (1995) Covariance of adolescent health behaviours: the class of 1989 study. Health Education Research: Theory & Practice 19, 133–146. [Google Scholar]
- Martner‐Hewes P.M., Hunt I.F., Murphy N.J., Swendseid M.E. & Settlage R.H. (1986) Vitamin b‐6 nutriture and plasma diamine oxidase activity in pregnant Hispanic teenagers. American Journal of Clinical Nutrition 44, 907–913. [DOI] [PubMed] [Google Scholar]
- Massawe S.N., Ronquist G., Nyström L. & Lindmark G. (2002) Iron status and iron deficiency anaemia in adolescents in a Tanzanian suburban area. Gynecologic and Obstetric Investigation 54, 137–144. [DOI] [PubMed] [Google Scholar]
- Milman N., Byg K.E., Bergholt T., Eriksen L. & Hvas A.M. (2006) Cobalamin status during normal pregnancy and postpartum: a longitudinal study comprising 406 Danish women. European Journal of Haematology 76, 521–525. [DOI] [PubMed] [Google Scholar]
- NHS Centre for Reviews and Dissemination (2001) Undertaking Systematic Reviews of Research on Effectiveness: Guidance for Those Carrying Out or Commissioning Review. NHS CRD: York. [Google Scholar]
- O’Brien K., Schulman Nathanson M., Mancini J. & Witter F.R. (2003) Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. American Journal of Clinical Nutrition 78, 1188–1193. [DOI] [PubMed] [Google Scholar]
- O’Connor D.L. (1994) Folate status during pregnancy and lactation In: Nutrient Regulation During Pregnancy, Lactation and Infant Growth (eds Allen L, King J, Lønnerdal B.), pp. 157–173. Plenum Press: New York. [Google Scholar]
- Olausson P.O., Cnattingius S. & Haglund B. (1999) Teenage pregnancies and risk of late foetal death and infant mortality. British Journal of Obstetrics and Gynaecology 106, 116–121. [DOI] [PubMed] [Google Scholar]
- Perry G.S., Byers T., Yip R. & Margen S. (1992) Iron nutrition does not account for the hemoglobin differences between blacks and whites. Journal of Nutrition 122, 1417–1424. [DOI] [PubMed] [Google Scholar]
- Perry G.S., Yip R. & Zyrkowski C. (1995) Nutritional risk factors among low‐income pregnant US women: the Centers for Disease Control and Prevention (CDC) Pregnancy Nutrition Surveillance System, 1979 through 1993. Seminars in Perinatology 19, 211–221. [DOI] [PubMed] [Google Scholar]
- Pilch S.M. & Senti F.M. (1984) Assessment of the Zinc Nutritional Status of the US Population Based on Data Collected in the Second National Health and Nutrition Examination Survey, 1976–80. Life Sciences Research Office, Federation of the American Societies for Experimental Biology: Bethesda, MD. [Google Scholar]
- Pobocik R.S., Benavente J.C., Boudreau N.S. & Spore C.L. (2003) Pregnant adolescents in Guam consume diets low in calcium and other micronutrients. Journal of the American Dietetic Association 103, 611–614. [DOI] [PubMed] [Google Scholar]
- Ravelli A.C., Van Der Meulen J.H., Michels R.P., Osmond C., Barker D.J., Hales C.N. et al. (1998) Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177. [DOI] [PubMed] [Google Scholar]
- Reid M. & Adamson H. (1997) Opportunities for and Barriers to Good Nutritional Health in Women of Childbearing Age, Pregnant Women, Infants Under 1 and Children Aged 1 to 5. Health Education Authority: London. [Google Scholar]
- Reifsnider E. & Gill S.L. (2000) Nutrition for the childbearing years. Journal of Obstetrics and Gynecologic Neonatal Nursing 29, 43–55. [DOI] [PubMed] [Google Scholar]
- Ritchie L.D., Fung E.B., Halloran B.P., Turnlund J.R., Van Loan M.D., Cann C.E. et al. (1998) A longitudinal study of calcium homeostatis during human pregnancy and lactation and after resumption of menses. American Journal of Clinical Nutrition 67, 693–701. [DOI] [PubMed] [Google Scholar]
- Schneck M.E., Sideras K.S., Fox R.A. & Dupuis L. (1990) Low income pregnant adolescents and their infants: dietary findings and health outcomes. Journal of the American Dietetic Association 90, 555–558. [PubMed] [Google Scholar]
- Scholl T.O., Hediger M.L., Fischer R.L. & Shearer J.W. (1992) Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. American Journal of Clinical Nutrition 55, 985–988. [DOI] [PubMed] [Google Scholar]
- Schuster K., Bailey L.B. & Mahan C.S. (1981) Vitamin B6 status of low‐income adolescent and adult pregnant women and the condition of their infants at birth. American Journal of Clinical Nutrition 34, 1731–1735. [DOI] [PubMed] [Google Scholar]
- Senti F.R. & Pilch S.M. (1985) Assessment of the Folate Nutritional Status of the US Population Based on Data Collected in the Second National Health and Nutrition Examination Survey 1976–80 Life. Sciences Research Office, Federation of American Societies for Experimental Biology: Bethesda, MD. [Google Scholar]
- Shirima C.P. & Kinabo J.L. (2005) Nutritional status and birth outcomes of adolescent pregnant girls in Morogoro, Coast, and Dar es Salaam regions, Tanzania. Nutrition 21, 32–38. [DOI] [PubMed] [Google Scholar]
- Social Exclusion Unit (1999) Teenage Pregnancy: Report by the Social Exclusion Unit. HMSO: London. [Google Scholar]
- Sowers M.F., Scholl T., Harris L. & Jannausch M. (2000) Bone loss in adolescent and adult pregnant women. Obstetrics & Gynecology 96, 189–193. [DOI] [PubMed] [Google Scholar]
- Stolzfus R.J. & Dreyfuss M.L. (1998) Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia: International Nutritional Anemia Consultative Group (INACG), World Health Organization (WHO), United Nations Children’s Fund (UNICEF). International Life Sciences Institute Press: Washington, DC. [Google Scholar]
- Symon A.G. & Wrieden W.L. (2003) A qualitative study of pregnant teenagers’ perceptions of the acceptability of a nutritional education intervention. Midwifery 19, 140–147. [DOI] [PubMed] [Google Scholar]
- Teenage Pregnancy Unit (2004) Teenage Pregnancy: An Overview of the Research Evidence. Health Development Agency: London. [Google Scholar]
- Thame M., Wilks R., Matadial L. & Forrester T.E. (1999) A comparative study of pregnancy outcome in teenage girls and mature women. West Indian Medical Journal 48, 69–72. [PubMed] [Google Scholar]
- Tomashek K.M., Ananth C.V. & Cogswell M.E. (2006) Risk of stillbirth in relation to maternal haemoglobin concentration during pregnancy. Maternal and Child Nutrition 2, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (2001) A League Table of Teenage Births in Rich Nations Innocenti Report Card No. 3, UNICEF Innocenti Research Centre, Florence. Available at: http://www.unicef-icdc.org/publications/index.html (accessed 10 July 2006).
- Videon T.M. & Manning C.K. (2003) Influences on adolescent eating patterns: the importance of family meals. Journal of Adolescent Health 32, 365–373. [DOI] [PubMed] [Google Scholar]
- Wahl R. (1999) Nutrition in the adolescent. Pediatric Annals 28, 107–111. [DOI] [PubMed] [Google Scholar]
- Wahlin A., Backmän L., Hultdin J., Adolfsson R. & Nilsson L.‐G. (2002) Reference values for serum levels of vitamin B12 and folic acid in a population‐based sample of adults between 35 and 80 years of age. Public Health Nutrition 5, 505–511. [DOI] [PubMed] [Google Scholar]
- Weggemans R.M., De Groot L.C. & Haller J. (1997) Factors related to plasma folate and vitamin B12: the SENECA study. International Journal of Food Science and Nutrition 48, 141–150. [DOI] [PubMed] [Google Scholar]
- Wolfe S.A., Gibson R.S., Gadowsky S.L. & O’Connor D.L. (1994) Zinc status of a group of pregnant adolescents at 36 weeks gestation living in Southern Ontario. Journal of the American College of Nutrition 13, 154–164. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO ) (1998) The Second Generation. WHO: Geneva. [Google Scholar]
- Yip R. (2000) Significance of an abnormally low or high hemoglobin concentration during pregnancy: special considerations of iron nutrition. American Journal of Clinical Nutrition 72 (suppl), 2725–2795. [DOI] [PubMed] [Google Scholar]


