Abstract
School‐based interventions are an effective way to treat childhood obesity. The purpose of the present study was to biologically validate an established school‐based intervention designed to reduce standardised body mass index (zBMI) over a period of 12 months. This intervention focused on a subset of Mexican‐American children who were participating in a larger clinical weight loss study. Plasma samples were analysed from self‐identified Mexican‐American children (12–14 years) who were randomised to either a school‐based intervention (IN, n = 152) or self‐help control (CN, n = 69). Treatment was 4 days week−1 of exercise (45 min day−1) and 1 day week−1 of nutritional counselling for 6 months. Fasting (>8 h) blood samples were collected at baseline, 6 months (end of active intervention) and 12 months (6 months after the end of the active intervention). Plasma resistin, adiponectin and leptin concentration were measured using a multiplex assay. Separate linear mixed models and a P < 0.05 were used to test for significance. Significant group × time interactions were found for resistin (P < 0.0001), adiponectin (P = 0.001) and leptin (P = 0.013). For resistin, IN was 12% lower at 6 months than CN. Adiponectin concentration in IN was greater at 6 months (26%) and 12 months (8%) than CN. Leptin concentration was 22% lower for IN at 12 months than CN. We have previously reported that our school‐based intervention reduced zBMI and now reported alterations in biologically relevant disease biomarkers. Some of the observed changes were only present at the end of the active intervention (resistin), while others persisted until 12 months (leptin and adiponectin). These changes underscore the effectiveness of our school‐based intervention at not only improving zBMI but also at reducing disease risk.
Keywords: childhood obesity, weight loss, resistin, adiponectin, leptin
Introduction
Obesity is an independent risk factor for the development of cardiovascular disease (CVD), which also often begins in childhood (Ferreira et al. 2007). CVD is a major concern within the Hispanic community (Karlamangla et al. 2010). Not only is CVD the leading cause of death for Hispanics (Karlamangla et al. 2010), but also Hispanics are more likely to die from CVD than other ethnic groups. Early prevention is critical as risk factors of CVD are shown to manifest at a younger age in Hispanic children (Kelly et al. 2004; Ogden et al. 2006; Patel et al. 2006; Pietrobelli et al. 2008). Thus, a school‐based monitoring and intervention programme is an obvious choice to target at‐risk children. Currently, 77% of Hispanic adults and 38% of Hispanic children are overweight or obese (Karlamangla et al. 2010), putting this population at greater risk for associated diseases including CVD, type 2 diabetes, hypertension and hyperlipidemia (Kelly et al. 2004; Ogden et al. 2006; Patel et al. 2006; Pietrobelli et al. 2008).
We have developed and validated a school‐based intervention (IN), which causes immediate reductions in standardised body mass index (zBMI) that is maintained for up to 2 years (2007a, 2007b, 2009). In an attempt to biologically validate our school‐based intervention model, we have previously demonstrated that specific inflammatory/disease biomarkers (i.e. sCD14, sTNF‐αR1, sTNF‐αR2, sIL‐6R and C‐reactive protein) are elevated in overweight and obese children (2007, 2009), suggesting that the deleterious effects of obesity are occurring even at young ages. We have also shown that some of these inflammatory biomarkers are reduced after our 6‐month intervention when zBMI decreases (McFarlin et al. 2009). In addition to the inflammatory biomarkers, others have shown that weight loss and disease risk are closely link to the plasma concentration of resistin, adiponectin and leptin (Bluher et al. 2005; Cambuli et al. 2008; Reinehr et al. 2009). As these biomarkers are produced by hypertrophied adipose tissue, their mechanistic link to obesity has already been established. In adults, weight gain is associated with an increase in plasma resistin and leptin, and a decrease in adiponectin (13). All of these biological markers have been demonstrated to play a role in hunger/satiety and thus have implications for body weight loss and maintenance (12). While these appear to be potentially promising markers in adults, very little information is known about how resistin, adiponectin and leptin respond in children whose zBMI has been reduced. Therefore, the purpose of this study was to further validate our school‐based intervention over a period of 12 months using plasma concentration of resistin, adiponectin and leptin as biological outcomes.
Key message
-
•
School‐based interventions are an effective way to treat childhood obesity.
-
•
While Mexican‐American children may be more prone to obesity, a reduction in zBMI through our school‐based intervention reduced biological indices of disease risk for up to 12 months after the start of the intervention.
-
•
Such improvements during childhood may reduce disease risk in adulthood.
Materials and methods
Subjects
The Institutional Review Board for Human Subjects at the Baylor College of Medicine approved all procedures involved in this study. The present study used a subset of participants from a larger study, whose parent gave written consent for their child to have their blood taken. The data in this study are part of a larger ongoing trial, and prior to this study, we had only published outcome data from the first two cohorts (2004–2005) (2007, 2009; 2007a, 2007b, 2009); however, the present investigation included data from the first three cohorts of this study (2004–2006). As subjects from the larger parent study, which was a clinical trial volunteered for participation in the present experiment, the present study does not necessarily adhere to the CONSORT standards or a true clinical trial. The subjects were students from an urban charter school in the city of Houston, TX, with a student population that was 95% Mexican‐American. Students who attend the school are chosen by a lottery system and do not pay tuition to attend the charter school. In order to enrol children in the study, parental consent was given, followed by child assent. Subjects were recruited in five cohorts over a period of 4 years (2004–2008). At each time point, a school nurse completed a standard Tanner test to determine pubertal status.
Intervention groups
In the parent study, subjects were randomly assigned to either an intervention (n = 152) or a self‐help (n = 69) group. Final subject counts represent the number of subjects who completed all three testing time points. Individuals assigned to the intervention group participated in a daily (Monday–Friday) instructor lead intervention for 3 months (four exercise sessions and one indoor nutrition counselling session each week), followed immediately by 3 months of biweekly intervention sessions. Participants in the self‐help group were provided a parent‐lead manual with information concerning weight loss and maintenance, but no formal instruction was provided. After the initial 6‐month active‐intervention period, subjects were encouraged to continue their programme; however, the study staff provided no assistance. Thus, the 6‐month sample marks the end of the active intervention, while the 12‐month sample marks the end of the self‐administered intervention. Baseline subject characteristics are presented in Table 1, characterised by group assignment.
Table 1.
Subject characteristics at baseline, stratified by treatment group
| Characteristic | Intervention (n = 152) | Control (n = 69) |
|---|---|---|
| Age (years) | 13 ± 1 | 13 ± 1 |
| Gender (% female) | 46% | 44% |
| Height (cm) | 150.4 ± 7.9 | 151.3 ± 9.3 |
| Weight (kg) | 50.9 ± 15.1 | 52.2 ± 13.3 |
| zBMI | 0.87 ± 0.99 | 0.99 ± 0.95 |
zBMI, standardised body mass index. Values represent the mean ± standard deviation. No significant group differences were found at baseline.
Intervention
The school‐based intervention used in the present study targeted both physical fitness and eating behaviours (2007a, 2007b, 2009). A biphasic approach was used to gradually build physical fitness over the first 3 months of the intervention. Phase 1 (first 1.5 months) used 8–10 exercise stations (e.g. stair stepping, shuttle runs and jump rope) designed to elicit 60–85% of age‐predicted maximum heart rate (Polar Electro, Kempele, Finland). Exercise sessions were completed in small groups with a 5:1 subject : staff ratio. Phase 2 (second 1.5 months) included team sports (basketball, soccer and softball) and leisure activities (jumping rope, dance and kickboxing) that were modified to include constant activity and elicit 60–85% of age‐predicted max heart rate (Polar). To address eating behaviours, subjects were educated to achieve the following learning objectives: (1) how to read nutrition labels; (2) how to determine portion size; and (3) how to categorise foods. Foods were categorised as either safety (most fruits and non‐starch vegetables), caution (low‐fat meats, low‐fat dairy and starches) or danger (foods consisting for more than 5 g of fat or 15 g of sugar/serving). Biweekly assessments and individualised follow‐up were used to reinforce the learning objectives.
Standardised body mass index
Body weight (digital scale) and height (stadiometer) were measured at baseline, 6 months and 12 months wearing light clothing and no footwear. BMI was calculated using measured weight and height. The zBMI was calculated using age and gender normative data from the Centers for Disease Control and Prevention (CDC) (Ogden et al. 2006). Using the CDC guidelines, subjects whose zBMI was <85th percentile were considered ‘normal weight’, in the 85th to 95th percentiles were considered ‘at‐risk for overweight’, or >95th percentile were considered ‘overweight’.
Venous blood collection
Blood samples (10 mL) were collected from a peripheral arm vein into ethylenediaminetetraacetic acid (EDTA) vacutainers following an overnight fast (>8 h) and abstention from exercise (>8 h). Plasma was separated by centrifugation, aliquoted and frozen (−80°C) until analysis. Blood samples were kept on ice, and all separations were completed within 2 h of blood collection.
Multiplex panel for disease biomarkers
Plasma samples were thawed only once prior to analysis to minimise freeze‐thaw effects. The present analysis was completed using a commercially available multiplex, flow cytometry‐based application (Flow Cytomix, eBioscience, San Diego, CA, USA). EDTA‐treated plasma was analysed for resistin, adiponectin and leptin. Controls (CN, commercially available and laboratory generated) were included with each sample analysis batch to determine inter‐ and intra‐assay coefficients of variability (CVs). Inter‐ and intra‐assay CVs were <7% and 5%, respectively, for all analyses. Samples were acquired on a flow cytometer equipped with a 488 nm laser (Millipore‐Guava EasyCyte Mini, Hayward, CA, USA). Sample analysis was completed offline using FCS Express (v. 3.00.0821, De Novo Software, Los Angeles, CA, USA) and unknown sample concentrations were calculated against known standard values.
Data analysis
All statistical testing was completed using PASW Statistics (v.18.0, IBM, Chicago, IL, USA). Prior to formal statistical testing, data were examined for normality and constant error variance using the explore function. From this analysis, we did not find that any of our variables required transformation. Separate linear mixed model (LMM) analyses with repeated measures on time were used to evaluate the data. The independent variable in all models was group (intervention or control). The LMM‐dependent variables were the leptin, adiponectin and resistin. Individual differences in the biomarkers was included as a random effect in the models to account for the correlated errors produced by including two measures for each subject on these variables. Age, gender and pubertal status were included as covariates in each model. Statistical significance was set at P < 0.05. Location of significant effects was completed using a Tukey's post‐hoc test. Biomarker data were presented as a percentage change from baseline within a respective condition to better represent the time course of change between intervention and control. Baseline values within each biomarker (i.e. resistin, adiponectin or leptin) were not significantly different between intervention and control and were included as a covariate in all statistical models.
Results
Anthropometrics
At 12 months, 43% of intervention subjects compared with 12% of control subjects had significantly reduced their zBMI. In the ‘overweight’ group, 54% of subjects decreased their zBMI. In the ‘at‐risk for overweight’ group, 41% of subjects decreased their body weight. Caution should be taken when examining body weight change in children and the CDC has suggested that the zBMI is more appropriate because it takes into account age, gender, body weight and height (Ogden et al. 2006). General subject characteristics at baseline are presented in Table 1; change in zBMI over the course of the intervention has been published elsewhere (2007a, 2007b). Different scores from baseline at both 6 and 12 months are presented in Table 2. Intervention subjects experienced significantly greater reductions in zBMI (P < 0.05), although changes in height and weight were not different between groups. There was no significant difference in Tanner status at any of the time points.
Table 2.
Difference scores for physical characteristics between baseline and 6 or 12 months
| Time | Characteristic | Intervention (n = 152) | Control (n = 69) |
|---|---|---|---|
| 6 months | Height (cm) | 3.63 ± 0.41 | 3.13 ± 0.39 |
| Weight (kg) | 1.31 ± 0.35 | 1.58 ± 0.33 | |
| zBMI | −0.211 ± 0.005* | −0.173 ± 0.004 | |
| 12 months | Height (cm) | 5.64 ± 0.88 | 5.07 ± 0.92 |
| Weight (kg) | 3.94 ± 0.48* | 4.43 ± 0.45 | |
| zBMI | −0.105 ± 0.004* | −0.068 ± 0.006 |
zBMI, standardised body mass index. Values represent the mean difference score from baseline ± standard deviation. *indicates different from control (P < 0.05).
Resistin
A significant interaction effect was found for plasma resistin at 6 months but not at 12 months. Plasma concentration of resistin was 26% lower in intervention compared with control subjects at 6 months (F = 12.536, P = 0.001; Fig. 1). Compared with baseline, the control group increased by 10% and the intervention group decreased by 16%.
Figure 1.
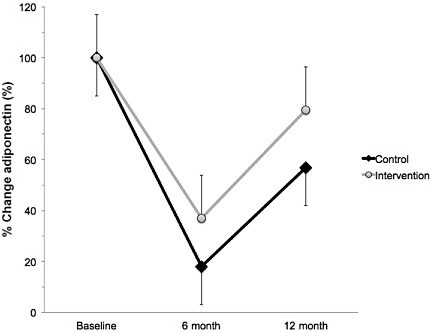
Percent change in plasma resistin from baseline in Mexican‐American children who were either randomised to an intervention (n = 152; grey circles) or control (n = 69, black diamonds) group. Intervention subjects completed a 6‐month supervised intervention that consisted of 4 days week−1 of exercise (45 min day−1) and 1 day week−1 of nutritional counselling. Compared with baseline, the control group increased by 10% and the intervention group decreased by 16%. Values represent the % change ± standard error of the mean. → indicates greater than intervention group (P < 0.05).
Adiponectin
A significant interaction effect was found for plasma adiponectin at both 6 and 12 months. At 6 months, adiponectin concentration was 26% greater in intervention than control (F = 9.137, P < 0.001; Fig. 2). At 12 months, adiponectin concentration was 18% greater in intervention compared with control (F = 9.137, P < 0.001; Fig. 2). The adiponectin response was interesting because there tended to be a trend towards decreased adiponectin at 6 months. While the adiponectin decreased in both groups, the control group experienced a greater decline than the intervention group. Compared with baseline, at 6 months, the control group decreased by 80% and the intervention group decreased by 64%. Compared with baseline, at 12 months, the control group decreased by 50% and the intervention group decreased by 32%.
Figure 2.
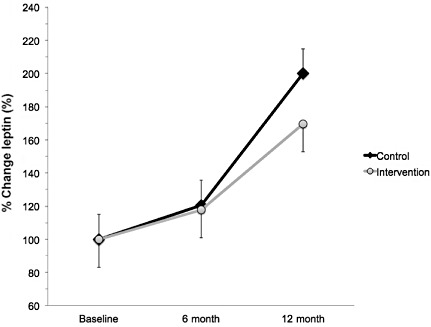
Percent change in plasma adiponectin from baseline in Mexican‐American children who were either randomised to an intervention (n = 152; grey circles) or control (n = 69, black diamonds) group. Intervention subjects completed a 6‐month supervised intervention that consisted of 4 days week−1 of exercise (45 min day−1) and 1 day week−1 of nutritional counselling. Compared with baseline, at 6 months, the control group decreased by 80% and the intervention group decreased by 64%. Compared with baseline, at 12 months, the control group decreased by 50% and the intervention group decreased by 32%. Values % change ± standard error of the mean. *indicates greater than control group (P < 0.05).
Leptin
A significant interaction effect was found for plasma leptin at 12 months. No significant effects were observed at 6 months. Specifically, leptin concentration was 22% lower in intervention compared with control at 12 months (F = 3.903, P = 0.013; Fig. 3). Over the course of the study there was a trend towards an increase in plasma leptin, which was partially attenuated in the intervention group. Compared with baseline, at 12 months, the control group increased by 100% and the intervention group increased by 78%.
Figure 3.
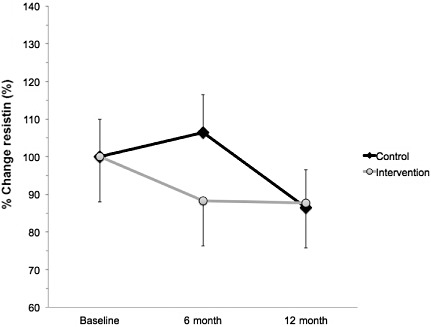
Percent change in plasma leptin from baseline in Mexican‐American children who were either randomised to an intervention (n = 152; grey circles) or control (n = 69, black diamonds) group. Intervention subjects completed a 6‐month supervised intervention that consisted of 4 days week−1 of exercise (45 min day−1) and 1 day week−1 of nutritional counselling. Compared with baseline, at 12 months, the control group increased by 100% and the intervention group increased by 78%. Values represent the % change ± standard error of the mean. → indicates greater than intervention group (P < 0.05).
Discussion
The present study sought to biologically validate the effect of a school‐based intervention to reduce zBMI and the resulting changes in plasma resistin, adiponectin and leptin in Mexican‐American children. These biomarkers were selected because they are produced by adipocytes and have previously been linked to disease risk and obesity in adults (Bluher et al. 2005; Cambuli et al. 2008; Reinehr et al. 2009). The subjects in this study were recruited from a larger study that has been documented to cause clinically relevant reductions in zBMI over a period of 6 months (2007a, 2007b) and maintain that loss for up to 2 years after the conclusion of the study (Johnston et al. 2009). We have also demonstrated that at baseline, children classified as at‐risk for overweight had significantly greater plasma inflammatory biomarkers than normal weight children (2007, 2009). The present study extends our previous findings by documenting that subjects who participated in our school‐based intervention had measurable improvements in plasma resistin, adiponectin and leptin compared with control subjects. We also found that while all three biomarkers are altered by a change in zBMI, the time course of their change is not the same. The relationship between zBMI and our biomarkers is not surprising because adipose tissue is the primary source of resistin, adiponectin and leptin (Cambuli et al. 2008). For instance, resistin and adiponectin appear to require only small changes in zBMI (differences observed at 6 months); however, larger changes in zBMI were needed to elicit changes in leptin (difference observed at 12 months).
In the present study, we found that at 6 months subjects in the intervention group who had decreased their zBMI also had significantly lower plasma resistin and higher adiponectin than control subjects. More interestingly, adiponectin was actually lower in both groups at 6 and 12 months. This response is potentially due to the fact that the initial intervention (and subsequent reduction in zBMI) stressed the system. In examining the change over 12 months, it is clear that adiponectin levels are starting to rebound and this response is significantly greater in IN than CN. Also, the calculated leptin : adiponectin ratio was lower. The leptin : adiponectin ratio provides an index of weight balance; lower values indicate weight loss has occurred, while higher values are observed during weight gain. In general, a lower plasma resistin and greater adiponectin is considered to be a positive effect in terms of body weight and disease risk. As adiponectin is closely linked to changes in body weight due to its role in energy balance in adults (Martos‐Moreno et al. 2010), our findings were anticipated. To our knowledge, this is the first study to longitudinally report improvements in resistin and trends towards eventual improvements in adiponectin after a 6‐month intervention in Mexican‐American children. It is important to note that in terms of a pattern of response, there was an overall trend towards a decrease followed by an increase in adiponectin. Also, this pattern of decrease was less pronounced in intervention subjects that decreased their zBMI more than control subjects. This is a very important population to study because Mexican‐American children are more prone to obesity, inflammation and disease than Caucasian and African‐American children (Dowd et al. 2010; Karlamangla et al. 2010). The exact reason(s) underlying this health disparity for Mexican‐Americans is not known, but is likely related to nurture and nature factors. Our findings at 6 months are consistent with what others have reported in adults and other race/ethnic groups (Cambuli et al. 2008; Holm et al. 2009; Amato et al. 2010; Martos‐Moreno et al. 2010; Reinehr et al. 2009). Martos‐Moreno et al. (2010) demonstrated that a multiplex method is a reliable means by which to detect changes in plasma leptin and adiponectin, but not resistin compared with traditional measurement techniques. Furthermore, Martos‐Moreno et al. demonstrated that decreased BMI in previously obese children was associated with decreased leptin and increased adiponectin (Martos‐Moreno et al. 2010). By combining previous studies with the present study, it is clear that regardless of age, gender or race/ethnicity, the most powerful predictor of change in leptin and adiponectin after an active intervention is the degree of weight loss or change in zBMI. Thus, it is clear from the present findings that after 6 months, our intervention resulted in biologically relevant changes in resistin and adiponectin, but not leptin. The most likely explanation for the disparity between the three biomarkers is that they may require different amounts of changes in zBMI in order to realise actual effects. It is also plausible to speculate that an active intervention is needed to sustain changes in resistin as resistin did not continue to decrease at 12 months. This study was not ideally designed to investigate the mechanisms underlying specific changes in zBMI, but that may be a target for future investigations.
It is important to note that the present results are part of a larger, ongoing study (Johnston et al. 2009), which has demonstrated that reductions in zBMI are maintained for up to 2 years after completion of the initial 6‐month randomised intervention. After the initial intervention, subjects are allowed to maintain their new lifestyle with little additional input from the study staff. The measurements that we make for 12 months are designed to provide information regarding how effective subjects are at maintaining a lower zBMI. In the case of the present study, we were also interested in determining if subjects in the intervention group could maintain biologically relevant changes in biomarkers beyond what was observed at the 6‐month time point. At 12 months, we detected a pattern of change for the key biomarkers such that we continued to observe a higher adiponectin in intervention subjects, but now we also observed a lower leptin than control subjects. We observed changes that appear to favour increased leptin over time; this upward trend was partially mitigated by participation in the intervention. Thus, despite an increase relative to baseline, it is likely that the negative effects associated with leptin were lower in intervention compared with control. The observed group change in resistin disappeared at 12 months. The fact that leptin differences were not observed until 12 months lends support to our hypothesis that a greater change in zBMI is needed to alter leptin than resistin or adiponectin. We did not observe a difference in resistin at 12 months, but are uncertain of an exact biological reason for this outcome. One possible explanation may be related to weight regain. The intervention subjects did experience a small amount of weight regain, but they still weighed less than control subjects. Cambuli et al. (2008) reported that after a 12‐month parent‐guided intervention, approximately 50% of obese children lost weight, resulting in a significant decrease in plasma leptin and an increase in adiponectin. In the present study, 43% of intervention subjects maintained weight loss at 12 months, which is consistent with the effects reported by Cambuli et al. Consistent with our own findings, Cambuli et al. noted that obese children, who did not lose weight, also did not experience any significant change in plasma leptin or adiponectin (Cambuli et al. 2008). Amato et al. (2010) reported that children who lost 0.5 standard deviation in BMI over a period of 6 months significantly decreased plasma leptin concentration. In the present study, we did not observe BMI improvements at the levels reported by Amato et al. until 12 months, which may explain the observed leptin response. Collectively, these findings combined with the present study demonstrate that change in zBMI is the most significant indicator of change in resistin, adiponectin and leptin over time in Mexican‐American children. Future interventions, whose goal is to improve biologically relevant outcomes, should strive to ensure that intervention subjects maintain lost body weight for a longer period of time.
In summary, children who participated in our school‐based intervention for 12 months experienced a decrease in zBMI, which was associated with decreased plasma leptin, increased adiponectin and decreased resistin relative to the control group. It is important to note that there was a diurnal trend towards a decreased adiponectin and an increased leptin that was partially countered by the intervention. All of the biomarkers measured in the present study have been previously associated with body weight in adults (Bluher et al. 2005; Reinehr et al. 2005; Cambuli et al. 2008) and are known to play a role in disease onset and progression (Pietrobelli et al. 2008). Based on the later relationship, it is reasonable to speculate that the children in the intervention group decreased their disease risk over time. The key findings of the present study may also be useful for future evaluation as clinical outcomes. In order to evaluate this effectiveness, clinical trials and clinical validation of the research‐based measurement techniques that we used in the present study would be needed. Others have speculated that such improvements during childhood are very likely to reduce disease risk in adulthood (Pietrobelli et al. 2008).
Source of funding
This study was funded by a grant from the United States Department of Agriculture (ARS 2533759358). This funding was used to cover costs associated with sample analysis.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
Each author in the present study contributed substantially to the generation of this manuscript. BKM contributed to the analysis, interpretation and preparation of the present manuscript as the corresponding author. CJJ, KCC and JPF coordinated the exercise study, sample collection and contributed to manuscript writing. KCC, TD, KS and ELB contributed to sample processing, sample analysis and manuscript writing.
References
- Amato A., Santoro N., Calabro P., Grandone A., Swinkels D.W., Perrone L. et al (2010) Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. International Journal of Obesity 34, 1772–1774. [DOI] [PubMed] [Google Scholar]
- Bluher M., Fasshauer M., Tonjes A., Kratzsch J., Schon M.R. & Paschke R. (2005) Association of interleukin‐6, C‐reactive protein, interleukin‐10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Experimental and Clinical Endocrinology & Diabetes 113, 534–537. [DOI] [PubMed] [Google Scholar]
- Cambuli V.M., Musiu M.C., Incani M., Paderi M., Serpe R., Marras V. et al (2008) Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. The Journal of Clinical Endocrinology and Metabolism 93, 3051–3057. [DOI] [PubMed] [Google Scholar]
- Dowd J.B., Zajacova A. & Aiello A.E. (2010) Predictors of inflammation in U.S. children aged 3–16 years. American Journal of Preventive Medicine 39, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A.P., Oliveira C.E. & Franca N.M. (2007) Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance (HOMA‐IR). Jornal de Pediatria 83, 21–26. [DOI] [PubMed] [Google Scholar]
- Holm J.C., Gamborg M., Ward L., Ibsen K.K., Gammeltoft S., Sorensen T.I. et al (2009) Longitudinal analysis of leptin variation during weight regain after weight loss in obese children. Obesity Facts 2, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C.A., Tyler C., Fullerton G., Poston W.S., Haddock C.K., McFarlin B. et al (2007a) Results of an intensive school‐based weight loss program with overweight Mexican American children. Internaltional Journal of Pediatric Obesity 2, 144–152. [DOI] [PubMed] [Google Scholar]
- Johnston C.A., Tyler C., McFarlin B.K., Poston W.S., Haddock C.K., Reeves R. et al (2007b) Weight loss in overweight Mexican American children: a randomized, controlled trial. Pediatrics 120, e1450–e1457. [DOI] [PubMed] [Google Scholar]
- Johnston C.A., Tyler C., Fullerton G., McFarlin B.K., Poston W.S., Haddock C.K. et al (2009) Effects of a school‐based weight maintenance program for Mexican‐American children: results at 2 years. Obesity (Silver Spring) 18, 542–547. [DOI] [PubMed] [Google Scholar]
- Karlamangla A.S., Merkin S.S., Crimmins E.M. & Seeman T.E. (2010) Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Annals of Epidemiology 20, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.S., Wetzsteon R.J., Kaiser D.R., Steinberger J., Bank A.J. & Dengel D.R. (2004) Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. Jornal de Pediatria 145, 731–736. [DOI] [PubMed] [Google Scholar]
- Martos‐Moreno G.A., Burgos‐Ramos E., Canelles S., Argente J. & Barrios V. (2010) Evaluation of a multiplex assay for adipokine concentrations in obese children. Clinical Chemistry and Laboratory Medicine 48, 1439–1446. [DOI] [PubMed] [Google Scholar]
- McFarlin B.K., Johnston C.A., Tyler C., Hutchison A.T., Kueht M.L., Reeves R. et al (2007) Inflammatory markers are elevated in overweight Mexican‐American children. Internaltional Journal of Pediatric Obesity 2, 235–241. [DOI] [PubMed] [Google Scholar]
- McFarlin B.K., Johnston C.A., Tyler C., O'Connor D.P., Strohacker K.A., Reeves R. et al (2009) Relation between adiposity and disease risk factors in Mexican American children. Journal of Pediatric Gastroenterology and Nutrition 49, 450–455. [DOI] [PubMed] [Google Scholar]
- Ogden C.L., Carroll M.D., Curtin L.R., McDowell M.A., Tabak C.J. & Flegal K.M. (2006) Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association 295, 1549–1555. [DOI] [PubMed] [Google Scholar]
- Patel D.A., Srinivasan S.R., Xu J.H., Li S., Chen W. & Berenson G.S. (2006) Distribution and metabolic syndrome correlates of plasma C‐reactive protein in biracial (black‐white) younger adults: the Bogalusa Heart Study. Metabolism 55, 699–705. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A., Malavolti M., Battistini N.C. & Fuiano N. (2008) Metabolic syndrome: a child is not a small adult. Internaltional Journal of Pediatric Obesity 3 (Suppl. 1), 67–71. [DOI] [PubMed] [Google Scholar]
- Reinehr T., Stoffel‐Wagner B., Roth C.L. & Andler W. (2005) High‐sensitive C‐reactive protein, tumor necrosis factor alpha, and cardiovascular risk factors before and after weight loss in obese children. Metabolism 54, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Reinehr T., Kleber M., de Sousa G. & Andler W. (2009) Leptin concentrations are a predictor of overweight reduction in a lifestyle intervention. Internaltional Journal of Pediatric Obesity 4, 215–223. [DOI] [PubMed] [Google Scholar]


