Abstract
An estimated 165 million children are stunted due to the combined effects of poor nutrition, repeated infection and inadequate psychosocial stimulation. The complementary feeding period, generally corresponding to age 6–24 months, represents an important period of sensitivity to stunting with lifelong, possibly irrevocable consequences. Interventions to improve complementary feeding practices or the nutritional quality of complementary foods must take into consideration the contextual as well as proximal determinants of stunting. This review presents a conceptual framework that highlights the role of complementary feeding within the layers of contextual and causal factors that lead to stunted growth and development and the resulting short‐ and long‐term consequences. Contextual factors are organized into the following groups: political economy; health and health care systems; education; society and culture; agriculture and food systems; and water, sanitation and environment. We argue that these community and societal conditions underlie infant and young child feeding practices, which are a central pillar to healthy growth and development, and can serve to either impede or enable progress. Effectiveness studies with a strong process evaluation component are needed to identify transdisciplinary solutions. Programme and policy interventions aimed at preventing stunting should be informed by careful assessment of these factors at all levels.
Keywords: complementary feeding; healthy growth and development; stunting, transdisciplinary approaches; conceptual framework; World Health Organization
Introduction
Healthy growth is a term that has gained currency in tandem with the policy shift from the previous predominant concern with reducing underweight to one focused on reducing impaired linear growth (stunting) (Piwoz et al. 2012). The term may also encompass the absence of excessive weight gain or obesity. For the purposes of this review, however, we will focus on linear growth. Linear growth in early childhood is considered as a marker of healthy growth given its association with risk of short‐term morbidity and mortality, non‐communicable diseases in later life, and learning capacity and productivity (Black et al. 2013). It also is closely linked with child development in several domains including cognitive, language and sensory‐motor capacities (McDonald et al. 2013). An adequate supply of nutrients, the prevention of infections, and opportunities for social interaction, play and stimulation are among the factors that contribute positively to the achievement of a child's full potential for growth and development.
Linear growth faltering occurs when a child is not growing in length or height in accordance with his/her potential. A child is considered to be stunted when his/her length/height‐for‐age falls below −2 standard deviations (SDs) of the World Health Organization (WHO) child growth standard median (WHO Multicentre Growth Reference Study Group 2006). However, many children with length‐for‐age above −2 SD have experienced some degree of linear growth faltering. This process is thought to begin prior to birth. Maternal undernutrition, along with maternal stunting (often due to past undernutrition), infections and other forms of deprivation, contributes to intrauterine growth restriction (IUGR). A recent analysis of Demographic and Health Surveys (DHS) data from 54 developing countries has shown that the average newborn's length‐for‐age is close to −0.5 SD of the WHO growth standard (reflecting IUGR) and declines to almost −2 SD by the end of the second year (Victora et al. 2010).
Although stunted growth has been described as a risk factor to child development (Walker et al. 2007a), both are in fact outcomes of the same biological and psychosocial deprivation, with long‐term effects on physical stature and the brain's structural and functional capacity (Grantham‐McGregor et al. 2007). Impaired development in association with stunted growth has been detected in infants from as early as age 7 months (Abubakar et al. 2010) and persisting into later childhood and adolescence (Walker et al. 2007b). Nutrient deficiencies can affect neuroanatomy, neurochemistry and neurophysiology, with potentially long‐term changes in form and function occurring if the deficiency changes the trajectory of brain development beyond a period when repair can occur (Georgieff 2007).
Key messages
Complementary feeding is one of the central pillars supporting healthy growth and development.
Stunting, with characteristic features of linear growth faltering, increased susceptibility to infection and impaired neurocognitive function, may arise from a complex array of causal and contextual factors.
Research, programmes and policies should be informed by careful assessment of the contextual determinants of stunting in order to design comprehensive, transdisciplinary approaches to promote healthy growth and development.
Complementary feeding within a broader framework to address stunting
As mentioned previously, stunting often begins very early in life, typically in utero, and generally continues during the first two post‐natal years. Most of the decline in length‐for‐age occurs during the complementary feeding period, between 6 and 24 months of age (Dewey & Huffman 2009; Victora et al. 2010). Indeed poor complementary feeding has been identified as a risk factor associated directly with stunting (Bhutta et al. 2013). Dewey & Huffman (2009) estimated that the cumulative difference in stature between Malawian children and the WHO growth standard median was 10 cm by age 3 years. Of this, 20% was already present at birth, 20% was added in the first 6 months, 50% occurred at 6–24 months and the remaining 10% in the third year. The 6–24 months age period is important because as the child is introduced to foods other than breast milk and becomes increasingly more independent and mobile, the environmental factors influencing growth and development multiply.
The WHO conceptual framework on Childhood Stunting: Context, Causes and Consequences presented in this paper (Fig. 1) builds on the UNICEF framework on causes of malnutrition (UNICEF 1990). Numerous other adaptations of the UNICEF framework have been developed over the years, most notably by the Lancet Maternal and Child Nutrition Series (Black et al. 2008, 2013), to serve a variety of purposes. In the present adaptation, stunted growth and development are coupled at the core of the framework in recognition of the fact that they share common causes and the highly sensitive period from −9 to 24 months. Strategies that promote and protect healthy growth are likely to benefit children's physical, mental, socio‐emotional, and intellectual growth and development.
Figure 1.
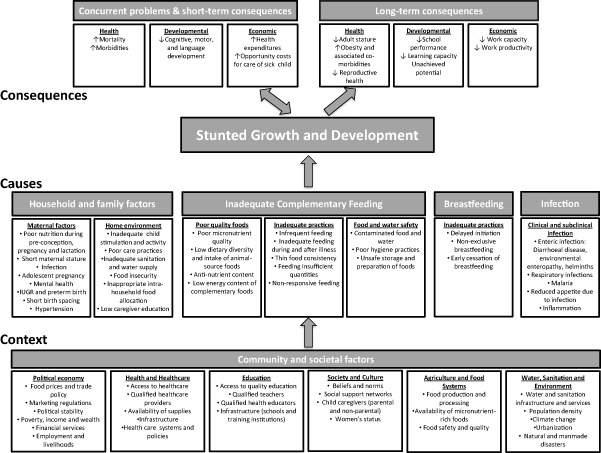
WHO conceptual framework on Childhood Stunting: Context, Causes, and Consequences, with an emphasis on complementary feeding.
This framework is timely, given the recognized need to strengthen the complementary feeding component of infant and young child feeding (IYCF) programmes (Piwoz et al. 2003; Daelmans et al. 2009). However, complementary feeding cannot be addressed in isolation from the promotion of exclusive breastfeeding during the first 6 months (critical for survival and the foundation of healthy growth in early infancy) and continued breastfeeding (to age 2 years or beyond). Considerable progress has been made towards development and implementation of policies and programmes designed to protect, promote and support breastfeeding over the past several decades (WHO et al. 2008; WHO & UNICEF 2009), though much remains to be done. By contrast, attention to complementary feeding practices lagged behind, in part because indicators for measuring such practices were not in place until 2008 (WHO 2008) and the scientific basis for efficacy and effectiveness of various strategies to improve complementary feeding was limited (Dewey & Adu‐Afarwuah 2008). Complementary feeding is a complex set of behaviours, comprising timing of introduction, food choices and dietary diversity, preparation methods, quantity, feeding frequency, responsiveness to infant cues, and safe preparation and storage of foods. Each behaviour may have context‐specific barriers, making recommendations for changes in behaviour difficult to apply as a ‘one package fits all’ model. For this reason, the various components of inadequate complementary feeding are delineated in the conceptual framework, to highlight how they might contribute to stunted growth.
The household and family factors identified in the causes category include maternal factors during pregnancy or prior to pregnancy that may exert a trans‐generational influence on offspring growth and development. Factors described within the home environment domain expand on care as depicted in one adaptation of the UNICEF framework (Engle et al. 1997). The home environment should ideally provide a clean, safe and stimulating environment to adequately nurture the mother and child.
The contextual layer (community and societal factors) is analogous to, but expands on the underlying and basic causes of malnutrition illustrated in the UNICEF framework. The sectors involved in the contextual factors shown in the WHO framework are home to the stakeholders responsible for the nutrition‐sensitive programmes mentioned in the Scaling Up Nutrition (SUN) movement's framework for action (Scaling Up Nutrition 2011) and by Black et al. (2013). Here, however, sub‐elements within each contextual category are itemized and can thus inform context‐specific considerations when developing or evaluating nutrition‐sensitive strategies for stunting reduction.
The importance of various underlying causes of stunting is influenced by different contextual factors. This implies that for programmes to be effective in preventing or reducing stunting, they should reach across disciplinary boundaries (Piwoz et al. 2012). This is referred to as transdisciplinarity (Dubé et al. 2012), the integrative process whereby novel conceptual and methodological approaches extend beyond discipline‐specific perspectives and theories to produce innovative solutions (Haire‐Joshu & McBride 2013). The transdisciplinary notions of vertical (cells to society) and horizontal (health, agriculture, economics, etc.) integration should be applied for problem solving during the complementary feeding period and thus are reflected in our framework. The paper by Casanovas et al. in this Supplement discusses the importance of a multisectoral approach that should achieve such transdisciplinarity (Casanovas et al. 2013), and the one by Pelletier et al. demonstrates how the framework might be applied to influence positive change (Pelletier et al. 2013). In this paper the different contextual factors will be considered with specific reference to their possible influence on complementary feeding and thereby, stunting. This is of interest to policy makers and programme managers seeking to strengthen the complementary feeding component of IYCF or to capitalize on its interactive links with household/contextual factors that could enhance the success of nutrition‐sensitive programmes aiming to reduce stunting.
Consequences of stunted growth and development
There is strong evidence that stunting has both immediate and long‐term consequences on health and development. A thorough review of this topic is beyond the scope of this paper which will summarize a few key issues only, but the reader is referred to a number of comprehensive reviews on this topic (Stein et al. 2005; Black et al. 2008; Victora et al. 2008; Dewey & Begum 2011). For some outcomes, particularly reproductive health outcomes among women, stunting is a direct risk factor. For other outcomes such as susceptibility to infection, poor schooling, reduced intellectual performance and economic productivity, stunted growth is reflective of and highly correlated with other underlying biologic processes that are likely to be more directly involved in the causal pathway.
Concurrent problems and short‐term consequences
Poor nutrition and frequent infection feedback upon each other, leading to a ‘vicious cycle’ (Scrimshaw et al. 1968; Brown 2003; Solomons 2007) that might be more aptly described as a downward spiral of worsening nutritional status and increasing susceptibility to infection. Infection impairs nutritional status through reduced appetite, impaired intestinal absorption, increased catabolism, and direction of nutrients away from growth and towards immune response. In turn, malnutrition increases the risk of infection by its negative impact on the epithelial barrier function and altered immune response (Brown 2003). Concurrent developmental problems and short‐term consequences consist of poorer psychomotor and mental development (Abubakar et al. 2010; McDonald et al. 2013), while the economic consequences relate to health expenditures and the opportunity costs incurred in caring for sick children. At the immediate level, stunting is associated with infectious diseases that increase household expenditures for the care of a sick child. Although the data on this are sparse, one study from Nepal estimated this to be as large as 4% of per capita annual household expenditures (Pokhrel & Sauerborn 2004).
Long‐term consequences
Individuals who are stunted at 2 years of age are likely to grow up to be stunted adults (Adair et al. 2013). There may be some opportunity for catch‐up growth during childhood, either due to improved nutrition or through a delay in skeletal maturation and the pubertal growth spurt that results in a longer overall period for growth in height (Coly et al. 2006; Prentice et al. 2013). Using pooled data from five birth cohorts in Brazil, Guatemala, India, the Philippines, and South Africa, Adair et al. found that a 1 SD lower height‐for‐age at 2 years was associated with 3.2 cm lower adult height while a 1 SD lower height‐for‐age during mid‐childhood was associated with a 1.9 cm lower adult height (Adair et al. 2013). Additionally, these authors estimated that a 1 SD increase in height at age 2 years was associated with a 77% reduction in short adult stature (OR 0.23, 95% CI 0.20, 0.25) (Adair et al. 2013).
Among women, shorter adult stature has important implications for pregnancy outcomes. Maternal stunting (<145 cm) is a consistent risk factor for perinatal mortality (Lawn et al. 2005), likely due to an increased risk of obstructed labour and asphyxia at birth. In Nepal, for example, stunted mothers had a 50% increased risk of having a baby with symptoms of birth asphyxia, and larger babies (median 3.3 kg) born to stunted mothers had a near fourfold risk of asphyxia compared with babies of median weight (2.6 kg) born to non‐stunted mothers (Lee et al. 2009). A pooled analysis of data from 109 DHS datasets concluded that children born to stunted women had a nearly 60% increased risk of neonatal mortality compared with those born to women 160 cm or taller (Ozaltin et al. 2010).
Stunting may have effects also on adult health and chronic disease risk (Uauy et al. 2008). Studies of infants born with low birthweight have demonstrated consistent associations with elevated blood pressure, renal dysfunction and altered glucose metabolism (Huxley et al. 2000; Whincup et al. 2008). The evidence linking stunting with obesity risk or altered energy expenditure has been mixed (Stettler 2007; Wilson et al. 2012; Adair et al. 2013). While it is unclear whether stunting may be a risk factor for obesity per se, rapid weight gain, particularly after the age of 2–3 years among individuals born small at birth, is thought to lead to a particularly high risk of chronic disease in later life (Gluckman et al. 2007).
Shorter adult stature has been linked to poorer schooling and economic productivity (Martorell 1996). Data from the COHORTS study showed that controlling for socio‐economic status, gender and maternal education, adults who at age 2 were stunted completed nearly 1 year less of schooling compared with non‐stunted individuals (Martorell et al. 2010). In other analyses, a 1 SD increase in height at age 2 years was associated with a 24% reduced risk of non‐completion of secondary school (Adair et al. 2013). Stunting has important economic consequences at the individual, household and community level, described in more detail in the paper by Hoddinott et al. in this Supplement (Hoddinott et al. 2013). It has been estimated that stunted children earn 20% less as adults (Grantham‐McGregor et al. 2007) compared with non‐stunted individuals. In World Bank estimates, a 1% loss in adult height due to childhood stunting is associated with a 1.4% loss in economic productivity (World Bank 2006).
Stunted development due to deficiencies of certain micronutrients, such as iodine and iron, can have long‐term and irreversible effects on neural and cognitive development (Lozoff et al. 2006; Georgieff 2007; Beard 2008; Lukowski et al. 2010; Zimmermann 2012), even if growth is not affected. Both iron and iodine are classified as ‘type 1’ nutrients, those that while essential and important for many biologic functions, are not thought to contribute to growth faltering unless deficiencies are severe (Golden 1995). This is important because population‐level interventions designed to correct iron or iodine deficiency are unlikely to impact the height‐for‐age indicator, yet have potential to impact neurodevelopmental outcomes, which are more difficult to measure (Fernald et al. 2009).
Causes of stunted growth and development
The most proximal factors contributing to stunted growth and development include household and family factors, inadequate complementary feeding, inadequate breastfeeding practices, and infection. These exposures appear individually in the framework, but in reality, they overlap and interact to compromise growth and development. The framework includes factors with the potential to be modified. Genetic factors, which are more static, are not listed despite their important contribution to growth and development. However, it is acknowledged that the environmental determinants operate in tandem with genetics through epigenetic mechanisms and longer‐term selection processes (Wells & Stock 2011).
Household and family factors
Periconceptional conditions including the pre‐pregnancy nutritional status of the mother, as well as her energy and nutrient intake, influence the early processes of growth and development (Gluckman & Pinal 2003). The maternal milieu sets physical and biological limits for offspring growth, but may also be signalling an unhealthy environment that adjusts growth trajectories and later reproductive viability (Kuzawa 2007). In addition to nutrition, other maternal factors play a role in determining offspring growth and development. Maternal infection related to malaria, helminths, HIV/AIDS and other conditions may lead to IUGR and later stunted growth in the infant (Luxemburger et al. 2001; Crompton & Nesheim 2002; Kuzawa et al. 2012). Adolescent pregnancy interferes with nutrient availability to the foetus due to the competing demands of ongoing maternal growth (Prakash et al. 2011). Short birth spacing increases the risk for depleted maternal reserves in subsequent pregnancies, with negative consequences for both mother and child (Dewey & Cohen 2007). Hypertension during pregnancy may also lead to adverse nutrition outcomes for the offspring (Thangaratinam et al. 2012). Recent studies have explored the impact of maternal mental health on child growth and development, with mixed findings (Harpham et al. 2005; Surkan et al. 2011; Vazir et al. 2013).
Within the home environment, several economic and caring determinants are associated with stunted growth. Some of these factors relate closely to context, but have been included here to highlight the importance of addressing modifiable household factors. Low caregiver education shows a strong and consistent relationship with poor child nutrition outcomes, and likely drives other caring practices associated with stunted development and growth (Semba et al. 2008; Imdad et al. 2011). Dietary intake may be affected by caregiver neglect or absence. Inadequate child stimulation and activity can interact with poor nutrition to impede development through multiple pathways. Household poverty may lead to food insecurity (Hong 2007), and more specifically micronutrient deficiencies arising from poor quality diets (Iannotti et al. 2012). Food may be available in the household but allocated preferentially to certain members, with harmful implications for vulnerable age/gender groups.
Inadequate complementary feeding
At the centre of the conceptual framework, three aspects of complementary feeding have been delineated to represent its contribution to stunted growth and development. Poor quality foods is the first category of determinants negatively impacting infant and young child growth. Inadequacies in micronutrient nutrition may arise from low dietary diversity (Onyango et al. 1998; Arimond & Ruel 2004), limited or no intake of animal source foods (Marquis et al. 1997; Bwibo & Neumann 2003; Krebs 2007), and high anti‐nutrient content such as phytates and polyphenols in the plant‐based diets of many poor populations (Gibson et al. 2010; Roos et al. 2013). The second category is inadequate practices. These include infrequent feeding, excessively dilute feeds with low energy density, inadequate feeding during illness, providing insufficient quantities of food and non‐responsive feeding (Umeta et al. 2003; Dewey & Adu‐Afarwuah 2008; Islam et al. 2008; Aboud & Akhter 2011).
The third category, food and water safety, relates primarily to the infection pathway to stunted growth, but may also contribute through inorganic contaminants and environmental pollutants (Weisstaub & Uauy 2012). Household‐level hygiene practices such as hand washing, safe water source and storage, and sanitation conditions affect the risk of diarrhoea and other morbidities interfering with growth (Checkley et al. 2004; Fink et al. 2011). Complementary foods may be stored in open or contaminated containers or left at temperatures supporting microbial growth (Black et al. 1982; Kimmons et al. 1999). Food preparation techniques such as inadequate cleaning or cooking time can also increase the risk of contamination. Recently there has been renewed interest in the role of mycotoxins, such as aflatoxin, in child growth faltering (Khlangwiset et al. 2011; Smith et al. 2012). Exposure to these toxins occurs particularly via maize and groundnuts contaminated with fungi during production, storage or food processing. Two studies from West Africa have reported a dose‐response association between serum aflatoxin and height‐for‐age z‐score (Gong et al. 2002, 2004). Smith et al. have proposed that gut inflammation may be one mechanism linking mycotoxin exposure to poor child growth (Smith et al. 2012).
Barriers to changing many of the practices just described may exist at many levels and will vary by context. Behaviour change messages in the absence of consideration of these barriers may have limited success in changing practices. For example, infrequent feeding may be due to caregiver time constraints. Dilute feeds may be fed due to fears of infant choking. Inadequate feeding during illness may be due to a loss of appetite and food refusals by the infant. Non‐responsive feeding patterns may arise from misinterpretations of infant cues. Unsafe preparation and storage of foods may arise from inadequate access to electricity for refrigeration, poor access to cooking fuel for proper re‐heating of meals or difficulty in accessing sufficient quantities of safe water for proper hygiene practices. Dietary diversity may be limited by access and affordability of higher‐quality foods. In households where both the mother and father work outside of the home, reliance upon other family members and oftentimes older children within the home may limit caregivers’ abilities to carry out their infant feeding intentions.
Inadequate breastfeeding
After birth, breastfeeding practices have an immediate effect on newborn health. Delayed initiation of breastfeeding, not breastfeeding and non‐exclusive breastfeeding all increase the risk of morbidity (Black et al. 2008; Kramer & Kakuma 2012), which may compromise growth in disadvantaged populations (Engebretsen et al. 2008). Early cessation of breastfeeding can also lead to stunted growth and development through multiple pathways including inadequate energy intake, nutrient deficiencies and removal of passive immunity provided by human milk (Onyango et al. 1999; Simondon et al. 2001; Arpadi et al. 2009).
Infections
Infection can be a critical proximal cause of both stunted growth and development (Adair & Guilkey 1997; Berkman et al. 2002). Diarrhoeal disease, respiratory illnesses, malaria, fever and helminth infection are known determinants acting variously through inflammation and nutrient diversion, sequestration or loss (Guerrant et al. 1992; Checkley et al. 2003; Wamani et al. 2006; Hall et al. 2008). Checkley et al. have estimated that 25% of the burden of stunting could be attributed to five or more episodes of diarrhoea occurring prior to the age of 2 years (Checkley et al. 2008). Severe infectious disease can lead to wasting (low weight‐for‐height), which may have longer‐term consequences on linear growth, particularly if there is insufficient food availability to recover after a bout of infection (Black et al. 2013). Sub‐clinical infection is also a likely contributor to poor growth and development. Though it occurs without outward cues, it may cause chronic, sustained insults to growth and development over time (Checkley et al. 1998; Campbell et al. 2003; Dewey & Mayers 2011). Environmental enteropathy is one example of a subclinical condition in which repeated exposure to pathogenic microorganisms leads to abnormalities in the structure and function of the small intestine. This condition, first described over 40 years ago as an enteropathy found among individuals living in tropical regions (Schenk et al. 1968; Lindenbaum et al. 1971), is characterized by villous atrophy, crypt hyperplasia, infiltration of the lamina propria by inflammatory T‐cells and increased permeability to enteric pathogens (Lunn et al. 1991). In studies from The Gambia, children experienced altered intestinal permeability, an indicator of environmental enteropathy, on 76% of observed days. This factor explained 43% variability in linear growth over a 9‐month period and corresponded directly with the peak period of linear growth faltering (Lunn 2000). Studies of children with altered intestinal permeability have suggested poorer absorption of zinc (Manary et al. 2010) and vitamin A (Chen et al. 2003). An emerging area of research is focused on understanding how the bacterial communities in the gut contribute to undernutrition in children (Gordon et al. 2012). While a relationship to acute malnutrition has been demonstrated (Smith et al. 2013), there is not yet evidence of an association between differences in the gut microbiota and child stunting.
Contextualising stunted growth and development
Recognising that direct nutrition interventions alone are often inadequate to prevent stunting, the concept of nutrition‐sensitive development has been incorporated in global advocacy for reducing malnutrition. In addition to supporting the scale‐up of direct nutrition interventions to prevent and treat undernutrition, the SUN framework for action is driven by multisectoral platforms promoting nutrition‐sensitive strategies in agriculture, education, health and social protection (Scaling Up Nutrition 2011). The Framework for Actions to Achieve Optimum Fetal and Child Nutrition and Development (Black et al. 2013), also inspired by the UNICEF framework, details nutrition‐specific interventions and programmes that should target direct causes of malnutrition. It also outlines nutrition‐sensitive programmes and approaches to address underlying causes, and actions related to use of knowledge and evidence, politics, governance and leadership to ensure the success of programmes.
Taking into account these contextual factors when designing programmes and their evaluation may increase the likelihood of success in reducing stunting. Evidence for the role of contextual factors is relatively limited compared with individual and household‐level determinants of stunting, in part because of an inherent attribution problem associated with studies examining community and societal factors. In this section, we attempt to trace more explicitly the relationships between the contextual factors and stunted growth and development during the complementary feeding period. Better characterisation of the pathways to maternal and household factors may in turn lead to more effective programming and policy.
Political economy
Government and other power structures influencing economic policies, markets and services play a major role with regard to food insecurity and undernutrition in populations (Maxwell 1999; Milman et al. 2005; Petrou & Kupek 2010). Macro‐ and micro‐economic conditions, closely linked to politics, similarly play influential roles in young child nutrition (World Bank 2006). Economic shocks such as the recent food price and financial crises have been examined in relation to household nutrition (Sari et al. 2010; Iannotti et al. 2012). Food prices were found to increase the probability of zinc and folate intake inadequacies, while reductions in income were associated with vitamin A and B12 intake inadequacies, disproportionately affecting poorer households (Iannotti et al. 2012). ‘Luxury’ foods with higher price‐nutrient and income‐nutrient elasticities also tend to be the nutrient‐rich complementary foods such as meat, fish, eggs and milk.
The bi‐directional pathway between poverty and undernutrition is well known. Disparities in stunting prevalence by wealth strata reflect this relationship (Black et al. 2013), as do country‐level trend data on changes in gross domestic product in relation to child malnutrition prevalence (Deaton 2010). However, evidence linking poverty alleviation interventions to nutrition outcomes is limited, coming largely from pilot studies or reviews of cash transfer (conditional and unconditional) types of programmes (Leroy et al. 2009). These social protection programmes have demonstrated a positive impact on poverty reduction, but effects on linear growth have been minimal (Manley et al. 2012; Ruel et al. 2013). Employment and livelihoods, access to financial services, and income and wealth are other factors under the category of political economy interacting to enable household access to higher‐quality foods, health care, and other more proximal determinants of growth and development.
More directly related to nutrition within the political sphere are international and national policies such as national plans of action for nutrition or regulatory policies protecting IYCF. The Code of Marketing of Breast‐milk Substitutes and food safety regulations in compliance with Codex Alimentarus are important instruments for regulating two aspects of IYCF. Appropriate marketing of complementary food requires an adequate regulatory framework (Bruyeron et al. 2010; Sun et al. 2011). Concerns about inappropriate marketing of complementary foods that could interfere with breastfeeding or encourage consumption of nutritionally inadequate foods illustrate the need for clear guidance (Soekarjo & Zehner 2011; Sweet et al. 2013). In May 2010, the World Health Assembly (WHA) noted that ‘promotion of breast‐milk substitutes and some commercial foods for infants and young children undermines progress in optimal infant and young child feeding’ and called on Member States to ‘end inappropriate promotion of food for infants and young children’. This was followed by a WHA request to WHO to ‘provide clarification and guidance’ on inappropriate promotion (World Health Assembly 2012), a task that the Organization is currently undertaking. Issues under consideration include the need to provide nutritionally adequate diets for children in food‐insecure populations, the potential risk of displacing breast milk and locally available nutritious foods by commercial products, and the need to protect children from feeding practices that could increase risk of overweight. Additional policy guidance will be required on how to proceed with complementary feeding products proven efficacious in research trials with potential for scale‐up.
For caregivers to be able to act on recommendations about healthy complementary food choices, they need to have confidence that products available in the market are safe and that health claims on labels and advertisements are accurate. Community infrastructure constraints, such as inadequate access to electricity or piped water and lack of paved roads that facilitate transportation to markets, all may serve as barriers to caregivers to act on complementary feeding practice recommendations. Thus, this contextual domain cuts across many of the proximal causal factors in the framework.
Health and health care
The health care system underlies multiple causal factors in the pathway to child growth and development, and is also responsible for screening and identification of inadequate growth and development. Because caregivers generally trust the guidance given by health professionals regarding their children's care and feeding, preventive and treatment services can converge to assure healthy growth and development. A community trial in Peru has demonstrated the important roles that health facility staff can play in improving child feeding practices (Penny et al. 2005). In many countries, community‐based maternal and child health clinics, child health days, and lay community health workers are common delivery platforms for nutrition‐specific interventions (Bhutta et al. 2013). However, overburdened health systems with too few trained providers are likely to lead to too little time spent on counselling on appropriate feeding practices. Further, although stunting has been flagged as a problem, its assessment remains a challenge for many countries due to lack of skills and time to assess linear growth (de Onis et al. 2012) and link assessment with counseling on appropriate complementary feeding. Finally, within this domain, it is important to consider the general health status of potential caregivers. In particular, conditions such as HIV/AIDS, chronic disease and mental health disorders will influence their capacity to care for their young children (Lartey et al. 2012; McDonald et al. 2012), potentially constraining household resources for the purchase of higher‐quality foods and hindering the ability of caregivers to follow infant feeding practice recommendations.
Education
Caregiver education is an important predictor of child health and nutritional outcomes. It has been estimated that improved female education was responsible for nearly 43% of the total reduction in undernutrition between 1971 and 1995 (Smith & Haddad 2000). A number of pathways have been proposed through which parental education may affect child health (Glewwe 1999; Frost et al. 2005). With regard to complementary feeding specifically, higher educational attainment can improve caregivers’ ability to understand and respond to nutrition behaviour change messages, to be more receptive to alternative food preparation methods or recipes, and to read and interpret food labels correctly.
A number of efficacy and effectiveness trials have focused on improving caregiver knowledge about complementary feeding as a means to improve child growth and nutrition. Interventions designed to improve education on optimal complementary feeding practices have been associated with reductions in stunting and improved linear growth (Bhutta et al. 2013). In locations where access to high‐quality food is sufficient, improved knowledge or skills building may be an important route to improving complementary feeding practices. Two studies of education alone that had a large impact on height‐for‐age were conducted in Peru (Penny et al. 2005) and China (Guldan et al. 2000). Both contexts were considered to be food secure, with access to affordable, high‐quality foods.
A lack of education and training opportunities for frontline health care workers can contribute to poor child growth. WHO estimates that there is a shortage of as many as 4.2 million trained health care workers in developing countries (WHO 2006).
Society and culture
In complementary feeding interventions, often the quality and quantity of food are thoughtfully considered. Yet other dimensions of feeding, such as how the food is fed, when and where it is fed, and who feeds the child are also important (Pelto et al. 2003). Cultural beliefs, knowledge and perceptions influence food behaviours to varying degrees (Kuhnlein & Pelto 1997). Deeply held beliefs may exist about the types of foods or preparation methods that are healthy or unhealthy for young children, when and what types of complementary foods should be first introduced, who can and should feed young children, how to feed children when they are sick, how to feed a child who does not want to eat, or how food will help a baby sleep or not (Dettwyler 1986; Paul et al. 2011). These beliefs are heavily influenced by the individuals who surround the primary caregiver – husbands, mothers‐in‐law, grandmothers, other family or neighbors within the community, and the health care providers upon whom the caregivers depend for help and support (McLorg & Bryant 1989; Kerr et al. 2008; Fouts & Brookshire 2009). Further, older siblings, family members or childcare facilities may play a role in feeding especially when the primary caregiver works outside the home.
The capacity of caregivers to respond to infant feeding recommendations will also depend on their ability to make decisions about infant feeding, seeking health care and use of household resources (Shroff et al. 2009). Men may serve as caregivers and may also directly or indirectly contribute to complementary feeding decision making through their control over household finances and choices over food purchases (Alive & Thrive 2010) or through decisions about allocation of foods to family members (Kuhnlein & Pelto 1997). Because women are usually the primary caregivers, female empowerment may be an important contextual factor underlying healthy child growth and development. Involvement of other members of the mother's social support network in complementary feeding programming therefore should be considered (Aubel et al. 2004; Affleck & Pelto 2012).
Food recipes and flavour preferences are heavily culturally rooted. The complementary feeding period is a time during which the infant is learning about foods and flavour combinations common to their families and cultural groups (Uvere & Ene‐Obong 2013). Repeated exposure to a variety of foods facilitates acceptance, establishes food preferences and makes it more likely that the child will consume those foods in later life (Mannella & Trabulsi 2012). Thus, promoting a varied diet in infancy is likely to establish lifelong healthier eating patterns. However, in resource‐constrained settings, caregivers may worry that providing higher‐cost foods such as meat or eggs to young children may lead them to develop unrealistic food preferences that cannot be sustained within the household budget (Colecraft et al. 2006).
Health and nutrition communication and behaviour change interventions intended to improve complementary feeding practices or influence the choice of high‐quality foods need to be designed with these cultural considerations in mind. Popular media can be powerful allies (or barriers) in public education, engagement of the wider public and social mobilisation around complementary feeding.
Agriculture and food systems
The agriculture sector encompasses food and cash crop cultivation and livestock production. It interacts with other contextual conditions such as the environment and political economy to drive food availability and access. Food systems similarly underlie nutritional outcomes through food processing, markets and food safety pathways. Numerous reviews have compiled the evidence examining the relationships between agriculture, food systems and nutrition, though few have focused on impact pathways during the complementary feeding period (Berti et al. 2004; Randolph et al. 2007; World Bank 2007; Girard et al. 2012; Hoddinott et al. 2012; Masset et al. 2012). The consensus across reviews is that the studies are highly heterogeneous and lack methodological rigour. There are some suggested features for agriculture programming to better ensure positive child growth outcomes: explicit nutrition objectives; behaviour change communication strategies; and access by women to credit, extension services and other inputs (Berti et al. 2004; Masset et al. 2012).
Trends towards mono‐cropping and heavy dependency on grains worldwide may be contributing to a lack of dietary diversity and consequent micronutrient deficiencies. In recent years, efforts have been directed at the agriculture sector to produce higher‐quality foods. Orange‐flesh sweet potatoes introduced in Mozambique were shown to improve vitamin A status (Low et al. 2007). Biofortification of plants with micronutrients is another strategy with potential to improve food quality, but evidence is limited in terms of the downstream impacts on child growth (Ruel et al. 2013). Other efforts are under way to promote sustainable diets, with consideration to biodiversity and use of indigenous foods, which may in turn lead to dietary diversification and improved infant and young child nutrition (IYCN) (Burlingame & Dernini 2011).
Another promising area of agriculture with high potential for impacts on growth and development in young children is small livestock development. Milk and eggs are nutrient‐rich complementary foods with some evidence for effects on IYCN (Iannotti et al. 2013). Poorer households in both rural and urban areas can feasibly raise poultry or goats with relatively low inputs. Other animal source foods produced through small enterprise development or available from wild sources, such as fish (Roos et al. 2007) or insects (van Huis et al. 2013; Kinyuru et al. 2013), may similarly provide critical nutrients to young children. Thus, there are many potential linkages between the agricultural sector and complementary feeding, primarily through access and availability to high‐quality foods. Yet increased access may be insufficient if not paired with a behaviour change component that works to ensure that high‐quality foods are fed to young children. Yet there is a clear need to generate higher‐quality evidence on the linkages to reduction of stunting from the agriculture sector.
Water, sanitation, and environment
Environmental determinants of infection, inflammation and undernutrition are important underlying factors contributing to unhealthy growth and development. Contaminated water and poor sanitation have been estimated to cause 5.4 billion cases of diarrhoea and 1.6 million deaths per year (Hutton & Haller 2004). Humphrey has speculated that the provision of toilets, improvements in hand washing practices and improvements in water quality are important tools to prevent environmental enteropathy and thereby reduce the risk of stunting (Humphrey 2009). Indeed, recent cross‐country comparisons of DHS data have suggested that open defecation can account for a large proportion of the gradient in child height, even after accounting for socio‐economic and other potentially confounding differences (Spears 2013). Fecal pollution in developing countries is pervasive (Kimani‐Murage & Ngindu 2007; Knappett et al. 2011). Environmental contamination is of direct import for infants and toddlers learning to feed themselves. Contamination of floors and the ground surrounding the house are particularly important to young children exploring their environments through crawling, early walking and by putting objects in their mouths. Proper disposal of faeces, removal of animal waste and hand hygiene are all critical during this sensitive age period. Insufficient access to safe water may serve as an important barrier to appropriate hygiene practices and safe preparation of complementary foods.
Finally, in considering the environment broadly, other factors such as population density (Spears 2013), degree of urbanisation and climate change (UNSCN 2010) are also important contextual factors that may contribute to worsening rates of malnutrition.
Conclusions
Since the release of the Lancet Series on Maternal and Child Nutrition in 2008 (Black et al. 2008), there has been tremendous momentum to refocus investment in programmes and research on improving maternal and child nutrition during the ‘first 1000 days’ of life. Indeed, the SUN Movement has served to build this impetus at the country level and among international partners by focusing on delivering programmes with proven efficacy at scale. As described in the paper by de Onis et al. in this Supplement, progress is being made at reducing undernutrition worldwide (de Onis et al. 2013). Adequate complementary feeding is one of the central pillars supporting healthy growth and development, yet much work is needed to build evidence documenting what works, in which contexts, and why programmes succeed or struggle. For this reason, we propose this conceptual framework to help guide policy and programme planning and evaluation in the arena of IYCF. It is not intended to replace the widely utilised and time‐tested UNICEF conceptual framework. Rather, it is intended to focus and highlight the unique considerations, issues and contextual factors important to promoting healthy growth through adequate complementary feeding.
The review supporting this framework demonstrates why well‐considered transdisciplinary action is needed. Programmes and policies tend to concentrate in the centre of the framework on proximal causal factors, through IYCF directly and to a lesser extent infection and other household and family factors. The conditions identified under Context in this framework, however, can ultimately act to impede or enable progress. The challenge, as is recognised in the dialogue on nutrition‐sensitive development, lies in designing approaches that best leverage positive contextual conditions or creatively circumvent the barriers.
Source of funding
The conceptual framework was developed for the World Health Organization with funds from the Bill and Melinda Gates Foundation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
Authors contributed sections for the first draft and all collaborated in harmonizing, editing and refining to produce the paper.
Disclaimer
AWO is a staff member of the World Health Organization. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Acknowledgements
We thank the Healthy Growth Project's Advisory Committee members for their contributions to the development and refinement of the conceptual framework, and Constanza Vallenas for her thoughtful reading and comments on an earlier draft of the manuscript.
The World Health Organization retains copyright and all other rights in the manuscript of this article as submitted for publication.
References
- Aboud F.E. & Akhter S. (2011) A cluster‐randomized evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 127, e1191–e1197. [DOI] [PubMed] [Google Scholar]
- Abubakar A., Holding P., Van De Vijver F.J., Newton C. & Van Baar A. (2010) Children at risk for developmental delay can be recognised by stunting, being underweight, ill health, little maternal schooling or high gravidity. Journal of Child Psychology and Psychiatry, and Allied Disciplines 51, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair L.S. & Guilkey D.K. (1997) Age‐specific determinants of stunting in Filipino children. The Journal of Nutrition 127, 314–320. [DOI] [PubMed] [Google Scholar]
- Adair L.S., Fall C.H.D., Osmond C., Stein A.D., Martorell R., Ramirez‐Zea M. et al, for the COHORTS Group (2013) Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet published online March 28. Available at: http://dx.doi.org/ 10.1016/S0140-6736(13)60103-8 (Accessed 8 August 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affleck W. & Pelto G. (2012) Caregivers’ responses to an intervention to improve young child feeding behaviors in rural Bangladesh: a mixed method study of facilitators and barriers to change. Social Science & Medicine 75 (4), 651–658. [DOI] [PubMed] [Google Scholar]
- Alive & Thrive (2010) IYCF practices, beliefs and influences in the SNNP region, Ethiopia. Addis Ababa, Ethiopia.
- Arimond M. & Ruel M.T. (2004) Dietary diversity is associated with child nutritional status: evidence from 11 Demographic and Health Surveys. The Journal of Nutrition 134, 2579–2585. [DOI] [PubMed] [Google Scholar]
- Arpadi S., Fawzy A., Aldrovandi G.M., Kankasa C., Sinkala M., Mwiya M. et al (2009) Growth faltering due to breastfeeding cessation in uninfected children born to HIV‐infected mothers in Zambia. The American Journal of Clinical Nutrition 90, 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubel J., Touré I. & Diagne M. (2004) Senegalese grandmothers promote improved maternal and child nutrition practices; the guardians of tradition are not averse to change. Social Science & Medicine 59, 945–959. [DOI] [PubMed] [Google Scholar]
- Beard J.L. (2008) Why iron deficiency is important in infant development. The Journal of Nutrition 138, 2534–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman D.S., Lescano A.G., Gilman R.H., Lopez S.L. & Black M.M. (2002) Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow‐up study. Lancet 359, 564–571. [DOI] [PubMed] [Google Scholar]
- Berti P.R., Krasevec J. & FitzGerald S. (2004) A review of the effectiveness of agriculture interventions in improving nutrition outcomes. Public Health Nutrition 7, 599–609. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Das J.K., Rizvi A., Gaffey M.F., Walker N., Horton S. et al (2013) Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382, 452–477. [DOI] [PubMed] [Google Scholar]
- Black R.E., Brown K.H., Becker S., Alim A.R. & Merson M.H. (1982) Contamination of weaning foods and transmission of enterotoxigenic Escherichia coli diarrhoea in children in rural Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 76, 259–264. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. et al (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Brown K.H. (2003) Diarrhea and malnutrition. The Journal of Nutrition 133, 328S–332S. [DOI] [PubMed] [Google Scholar]
- Bruyeron O., Denizeau M., Berger J. & Treche S. (2010) Marketing complementary foods and supplements inBurkina Faso, Madagascar, and Vietnam: lessons learned from the Nutridev program. Food and Nutrition Bulletin 31 (2 Suppl.), S154–S167. [DOI] [PubMed] [Google Scholar]
- Burlingame B. & Dernini S. (2011) Sustainable diets: the Mediterranean diet as an example. Public Health Nutrition 14, 2285–2287. [DOI] [PubMed] [Google Scholar]
- Bwibo N.O. & Neumann C.G. (2003) The need for animal source foods by Kenyan children. The Journal of Nutrition 133 (11 Suppl. 2), 3936S–3940S. [DOI] [PubMed] [Google Scholar]
- Campbell D.I., Elia M. & Lunn P.G. (2003) Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. The Journal of Nutrition 133, 1332–1338. [DOI] [PubMed] [Google Scholar]
- Casanovas M.C., Lutter C., Mangasaryan N., Mwadime R., Hajeebhoy N., Aguilar A.M. et al (2013) Multisectoral policies and programmes to consider in healthy growth promotion. Maternal & Child Nutrition 9 (Suppl. 2), 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W., Epstein L.D., Gilman R.H., Black R.E., Cabrera L. & Sterling C.R. (1998) Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch‐up growth. American Journal of Epidemiology 148, 497–506. [DOI] [PubMed] [Google Scholar]
- Checkley W., Epstein L.D., Gilman R.H., Cabrera L. & Black R.E. (2003) Effects of acute diarrhea on linear growth in Peruvian children. American Journal of Epidemiology 157, 166–175. [DOI] [PubMed] [Google Scholar]
- Checkley W., Gilman R.H., Black R.E., Epstein L.D., Cabrera L., Sterling C.R. et al (2004) Effect of water and sanitation on childhood health in a poor Peruvian peri‐urban community. Lancet 363, 112–118. [DOI] [PubMed] [Google Scholar]
- Checkley W., Buckley G., Gilman R.H., Assis A.M., Guerrant R.L., Morris S.S. et al (2008) Multi‐country analysis of the effects of diarrhea on childhood stunting. International Journal of Epidemiology 37, 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Soares A.M., Lima A.A., Gamble M.V., Schorling J.B., Conway M. et al (2003) Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. Journal of Health, Population, and Nutrition 21, 309–315. [PubMed] [Google Scholar]
- Colecraft E., Marquis G.S., Aryeetey R., Sakyi‐Dawson O., Lartey A., Ahunu B. et al (2006) Constraints on the use of animal source foods for young children in Ghana: a participatory rapid appraisal approach. Ecology of Food and Nutrition 45 (5), 351–377. [Google Scholar]
- Coly A.N., Milet J., Diallo A., Ndiaye T., Benefice E., Simondon F. et al (2006) Preschool stunting, adolescent migration, catch‐up growth, and adult height in young senegalese men and women of rural origin. The Journal of Nutrition 136, 2412–2420. [DOI] [PubMed] [Google Scholar]
- Crompton D.W. & Nesheim M.C. (2002) Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition 22, 35–59. [DOI] [PubMed] [Google Scholar]
- Daelmans B., Mangasaryan N., Martines J., Saadeh R., Casanovas C. & Arabi M. (2009) Strengthening actions to improve feeding of infants and young children 6 to 23 months of age: summary of a recent World Health Organization/UNICEF technical meeting, Geneva, 6–9 October 2008. Food and Nutrition Bulletin 30 (Suppl.), S236–S238. [DOI] [PubMed] [Google Scholar]
- Deaton A. (2010) Measuring Development: Different Data, Different Conclusions? Measure for measure: how well do we measure development. Proceedings of the 8th AFD‐EUDN Conference, Paris.
- Dettwyler K.A. (1986) Infant feeding in Mali, West Africa: variations in belief and practice. Social Science & Medicine 23, 651–664. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. & Adu‐Afarwuah S. (2008) Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition 4 (Suppl. 1), 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Begum K. (2011) Long‐term consequences of stunting in early life. Maternal & Child Nutrition 7 (Suppl. 3), 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Cohen R.J. (2007) Does birth spacing affect maternal or child nutritional status? A systematic literature review. Maternal & Child Nutrition 3, 151–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Huffman S.L. (2009) Maternal, infant, and young child nutrition: combining efforts to maximize impacts on child growth and micronutrient status. Food and Nutrition Bulletin 30, S187–S189. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. & Mayers D.R. (2011) Early child growth: how do nutrition and infection interact? Maternal & Child Nutrition 7 (Suppl. 3), 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé L., Pingali P. & Webb P. (2012) Paths of convergence for agriculture, health, and wealth. Proceedings of the National Academy of Sciences of the United States of America 109, 12294–12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen I.M., Tylleskär T., Wamani H., Karamagi C. & Tumwine J.K. (2008) Determinants of infant growth in Eastern Uganda: a community‐based cross‐sectional study. BMC Public Health 8, 418 doi: 10.1186/1471-2458-8-418. Accessed 9 August 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle P., Lhotská L. & Armstrong H. (1997) The Care Initiative: Assessment, Analysis and Action to Improve Care for Nutrition. UNICEF: New York.
- Fernald L.C.H., Kariger P., Engle P. & Raikes A. (2009) Examining early child development in low‐income countries: a toolkit for the assessment of children in the first five years of life . The World Bank: Washington, DC.
- Fink G., Gunther I. & Hill K. (2011) The effect of water and sanitation on child health: evidence from the demographic and health surveys 1986–2007. International Journal of Epidemiology 40, 1196–1204. [DOI] [PubMed] [Google Scholar]
- Fouts H.N. & Brookshire R.A. (2009) Who feeds children? A child's‐eye‐view of caregiver feeding patterns among the Aka foragers in Congo. Social Science & Medicine 69, 285–292. [DOI] [PubMed] [Google Scholar]
- Frost M.B., Forste R. & Haas D.W. (2005) Maternal education and child nutritional status in Bolivia: finding the links. Social Science & Medicine 60, 395–407. [DOI] [PubMed] [Google Scholar]
- Georgieff M.K. (2007) Nutrition and the developing brain: nutrient priorities and measurement. The American Journal of Clinical Nutrition 85 (Suppl.), 614S–620S. [DOI] [PubMed] [Google Scholar]
- Gibson R.S., Bailey K.B., Gibbs M. & Ferguson E.L. (2010) A review of phytate, iron, zinc, and calcium concentrations in plant‐based complementary foods used in low‐income countries and implications for bioavailability. Food and Nutrition Bulletin 31 (2 Suppl.), S134–S146. [DOI] [PubMed] [Google Scholar]
- Girard A., Self J., McAuliff C. & Olude O. (2012) The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review. Paediatric and Perinatal Epidemiology 26 (Suppl. 1), 205–222. [DOI] [PubMed] [Google Scholar]
- Glewwe P. (1999) Why does mother's schooling raise child health in developing countries. The Journal of Human Resources 34, 124–136. [Google Scholar]
- Gluckman P.D. & Pinal C.S. (2003) Regulation of fetal growth by the somatotrophic axis. The Journal of Nutrition 133 (5 Suppl. 2), 1741S–1746S. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. & Beedle A.S. (2007) Early life events and their consequences for later disease: a life history and evolutionary perspective. American Journal of Human Biology : The Official Journal of the Human Biology Council 19, 1–19. [DOI] [PubMed] [Google Scholar]
- Golden M.H. (1995) Specific deficiencies versus growth failure: type I and type II nutrients. SCN News 12, 10–14. [PubMed] [Google Scholar]
- Gong Y., Hounsa A., Egal S., Turner P.C., Sutcliffe A.E., Hall A.J. et al (2004) Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environmental Health Perspectives 112, 1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.Y., Cardwell K., Hounsa A., Egal S., Turner P.C., Hall A.J. et al (2002) Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. British Medical Journal 325, 20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J.I., Dewey K.G., Mills D.A. & Medzhitov R.M. (2012) The human gut microbiota and undernutrition. Science Translational Medicine 4, 137ps12. doi: 10.1126/scitranslmed.3004347. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R.L., Schorling J.B., McAuliffe J.F. & de Souza M.A. (1992) Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch‐up growth and malnutrition increases diarrhea frequency and duration. The American Journal of Tropical Medicine and Hygiene 47 (1 Pt 2), 28–35. [DOI] [PubMed] [Google Scholar]
- Guldan G.S., Fan H.C., Ma X., Ni Z.Z., Xiang X. & Tang M.Z. (2000) Culturally appropriate nutrition education improves infant feeding and growth in rural Sichuan, China. The Journal of Nutrition 130, 1204–1211. [DOI] [PubMed] [Google Scholar]
- Haire‐Joshu D. & McBride T. (eds) (2013) Transdisciplinary Problem Solving Public Health: Research, Education, and Practice. Jossey‐Bass, A Wiley Brand: San Franscisco, CA. [Google Scholar]
- Hall A., Hewitt G., Tuffrey V. & de Silva N. (2008) A review and meta‐analysis of the impact of intestinal worms on child growth and nutrition. Maternal & Child Nutrition 4 (Suppl. 1), 118–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpham T., Huttly S., De Silva M.J. & Abramsky T. (2005) Maternal mental health and child nutritional status in four developing countries. Journal of Epidemiology and Community Health 59, 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J., Rosegrant M.W. & Torero M. (2012) Investments to Reduce Hunger and Undernutrition . Challenge Paper on Hunger and malnutrition. Copenhagen Consensus Center: Lowell, MA, USA. Available at: http://www.copenhagenconsensus.com/sites/default/files/Hunger+and+Malnutrition.pdf (Accessed 8 August 2013).
- Hoddinott J., Alderman H., Behrman J.R., Haddad L. & Horton S. (2013) The economic rationale for investing in stunting reduction. Maternal & Child Nutrition 9 (Suppl. 2), 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong R. (2007) Effect of economic inequality on chronic childhood undernutrition in Ghana. Public Health Nutrition 10, 371–378. [DOI] [PubMed] [Google Scholar]
- van Huis A., Itterbeeck J.V., Klunder H., Mertens E., Halloran A., Muir G. & Vantomme P. (2013) Edible insects: future prospects for food and feed security. Food and Agriculture Organization of the UN (FAO). Rome, Italy. E‐ISBN 978‐92‐5‐107596‐8.
- Humphrey J. (2009) Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374 (9694), 1032–1035. [DOI] [PubMed] [Google Scholar]
- Hutton G. & Haller L. (2004) Evaluation of the costs and benefits of water and sanitation improvements at the global level . World Health Organization: Geneva, Switzerland.
- Huxley R.R., Shiell A.W. & Law C.M. (2000) The role of size at birth and postnatal catch‐up growth in determining systolic blood pressure: a systematic review of the literature. Journal of Hypertension 18, 815–831. [DOI] [PubMed] [Google Scholar]
- Iannotti L., Muehlhoff E. & McMahon D. (2013) Review of milk and dairy programmes affecting nutrition. Journal of Development Effectiveness 5 (1), 82–115. [Google Scholar]
- Iannotti L.L., Robles M., Pachon H. & Chiarella C. (2012) Food prices and poverty negatively affect micronutrient intakes in Guatemala. The Journal of Nutrition 142, 1568–1576. [DOI] [PubMed] [Google Scholar]
- Imdad A., Yakoob M.Y. & Bhutta Z.A. (2011) Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 11 (Suppl. 3), S25 doi: 10.1186/1471-2458-11-S3-S25. Accessed 9 August 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.M., Khatun M., Peerson J.M., Ahmed T., Mollah M.A., Dewey K.G. et al (2008) Effects of energy density and feeding frequency of complementary foods on total daily energy intakes and consumption of breast milk by healthy breastfed Bangladeshi children. The American Journal of Clinical Nutrition 88, 84–94. [DOI] [PubMed] [Google Scholar]
- Kerr R.B., Dakishoni L., Shumba L., Mzachi R. & Chirwa M. (2008) ‘We grandmothers know plenty’: breastfeeding, complementary feeding, and the multifaceted role of grandmothers in Malawi. Social Science & Medicine 66, 1095–1105. [DOI] [PubMed] [Google Scholar]
- Khlangwiset P., Shephard G.S. & Wu F. (2011) Aflatoxins and growth impairment: a review. Critical Reviews in Toxicology 41, 740–755. [DOI] [PubMed] [Google Scholar]
- Kimani‐Murage E.W. & Ngindu A.M. (2007) Quality of water the slum dwellers use: the case of a Kenyan slum. Journal of Urban Health 84, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmons J.E., Brown K.H., Lartey A., Collison E., Mensah P.P. & Dewey K.G. (1999) The effects of fermentation and/or vacuum flask storage on the presence of coliforms in complementary foods prepared for Ghanaian children. International Journal of Food Sciences and Nutrition 50, 195–201. [DOI] [PubMed] [Google Scholar]
- Kinyuru J.N., Konyole S.O., Owuor B.O., Kenji G.M., Onyango C.A., Estambale B.B. et al (2013) Nutrient composition of four selected winged termites in western Kenya. Journal of Food Composition and Analysis doi: 10.1016/J.JFCA.2013.02.008. [DOI] [Google Scholar]
- Knappett P.S., Escamilla V., Layton A., McKay L.D., Emch M., Williams D.E. et al (2011) Impact of population and latrines on fecal contamination of ponds in rural Bangladesh. The Science of the Total Environment 409, 3174–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.S. & Kakuma R. (2012) Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews (8), CD003517. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs N.F. (2007) Food choices to meet nutritional needs of breast‐fed infants and toddlers on mixed diets. The Journal of Nutrition 137, 511S–517S. [DOI] [PubMed] [Google Scholar]
- Kuhnlein H.V. & Pelto G.H. (1997) Culture, Environment and Food to Prevent Vitamin A Deficiency. International Nutrition Foundation for Developing Countries: Boston, MA. [Google Scholar]
- Kuzawa C.W. (2007) Developmental origins of life history: growth, productivity, and reproduction. American Journal of Human Biology 19, 654–661. [DOI] [PubMed] [Google Scholar]
- Kuzawa C.W., Tallman P.S., Adai L.S., Lee N. & McDade T.W. (2012) Inflammatory profiles in the non‐pregnant state predict offspring birth weight at Cebu: evidence for inter‐generational effects of low grade inflammation. Annals of Human Biology 39, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartey A., Marquis G.S., Mazur R., Perez‐Escamilla R., Brakohiapa L., Ampofo W. et al (2012) Maternal HIV is associated with reduced growth in the first year of life among infants in the Eastern region of Ghana: the Research to Improve Infant Nutrition and Growth (RIING) Project. Maternal & Child Nutrition doi: 10.1111/j.1740-8709.2012.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn J.E., Cousens S. & Zupan J. (2005) 4 million neonatal deaths: when? where? why? Lancet 365, 891–900. [DOI] [PubMed] [Google Scholar]
- Lee A.C., Darmstadt G.L., Khatry S.K., LeClerq S.C., Shrestha S.R. & Christian P. (2009) Maternal‐fetal disproportion and birth asphyxia in rural Sarlahi, Nepal. Archives of Pediatrics & Adolescent Medicine 163, 616–623. [DOI] [PubMed] [Google Scholar]
- Leroy J.L., Ruel M. & Verhofstadt E. (2009) The impact of conditional cash transfer programmes on child nutrition: a review of evidence using a programme theory framework. Journal of Development Effectiveness 1, 103–129. [Google Scholar]
- Lindenbaum J., Gerson C.D. & Kent T.H. (1971) Recovery of small‐intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Annals of Internal Medicine 74, 218–222. [DOI] [PubMed] [Google Scholar]
- Low J.W., Arimond M., Osman N., Cunguara B., Zano F. & Tschirley D. (2007) Ensuring the supply of and creating demand for a biofortified crop with a visible trait: lessons learned from the introduction of orange‐fleshed sweet potato in drought‐prone areas of Mozambique. Food and Nutrition Bulletin 28 (2 Suppl.), S258–S270. [DOI] [PubMed] [Google Scholar]
- Lozoff B., Beard J., Connor J., Barbara F., Georgieff M. & Schallert T. (2006) Long‐lasting neural and behavioral effects of iron deficiency in infancy. Nutrition Reviews 64 (5 Pt 2), S34–S43; discussion S72–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski A.F., Koss M., Burden M.J., Jonides J., Nelson C.A., Kaciroti N. et al (2010) Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long‐term deficits in executive function and recognition memory. Nutritional Neuroscience 13, 54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn P.G. (2000) The impact of infection and nutrition on gut function and growth in childhood. The Proceedings of the Nutrition Society 59, 147–154. [DOI] [PubMed] [Google Scholar]
- Lunn P.G., Northrop‐Clewes C.A. & Downes R.M. (1991) Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338, 907–910. [DOI] [PubMed] [Google Scholar]
- Luxemburger C., McGready R., Kham A., Morison L., Cho T., Chongsuphajaisiddhi T. et al (2001) Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. American Journal of Epidemiology 154, 459–465. [DOI] [PubMed] [Google Scholar]
- Manary M.J., Abrams S.A., Griffin I.J., Quimper M.M., Shulman R.J., Hamzo M.G. et al (2010) Perturbed zinc homeostasis in rural 3–5‐y‐old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatric Research 67, 671–675. [DOI] [PubMed] [Google Scholar]
- Manley J., Gitter S. & Slavchevska V. (2012) How effective are cash transfer programmes at improving nutritional status? A rapid evidence assessment of programmes effects on anthropometric outcomes. London EPPI Centre. Social Research Science Unit. Institute of Education. London: University of London.
- Mannella J.A. & Trabulsi J.C. (2012) Complementary foods and flavor experiences: setting the foundation. Annals of Nutrition & Metabolism 60 (Suppl. 2), 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis G.S., Habicht J.P., Lanata C.F., Black R.E. & Rasmussen K.M. (1997) Breast milk or animal‐product foods improve linear growth of Peruvian toddlers consuming marginal diets. The American Journal of Clinical Nutrition 66, 1102–1109. [DOI] [PubMed] [Google Scholar]
- Martorell R. (1996) The role of nutrition in economic development. Nutrition Reviews 54 (4 Pt 2), S66–S71. [DOI] [PubMed] [Google Scholar]
- Martorell R., Horta B.L., Adair L.S., Stein A.D., Richter L., Fall C.H. et al (2010) Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low‐ and middle‐income countries. The Journal of Nutrition 140, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masset E., Haddad L., Cornelius A. & Isaza‐Castro J. (2012) Effectiveness of agricultural interventions that aim to improve nutritional status of children: systematic review. British Medical Journal 344, d8222. doi: 10.1136/bmj.d8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D. (1999) The political economy of urban food security in Sub‐Saharan Africa. World Development 27 (11), 1939–1953. [Google Scholar]
- McDonald C.M., Kupka R., Manji K.P., Okuma J., Bosch R.J., Aboud S. et al (2012) Predictors of stunting, wasting and underweight among Tanzanian children born to HIV‐infected women. European Journal of Clinical Nutrition 66, 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C.M., Manji K.P., Kupka R., Bellinger D.C., Spiegelman D., Kisenge R. et al (2013) Stunting and wasting are associated with poorer psychomotor and mental development in HIV‐exposed Tanzanian infants. The Journal of Nutrition 143, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLorg P.A. & Bryant C.A. (1989) Influence of social network members and health care professionals on infant feeding practices of economically disadvantaged mothers. Medical Anthropology 10, 265–278. [DOI] [PubMed] [Google Scholar]
- Milman A., Frongillo E.A., de Onis M. & Hwang J.Y. (2005) Differential improvement among countries in child stunting is associated with long‐term development and specific interventions. The Journal of Nutrition 135, 1415–1422. [DOI] [PubMed] [Google Scholar]
- de Onis M., Onyango A., Borghi E., Siyam A., Blössner M., Lutter C. & for the WHO Multicentre Growth Reference Study Group (2012) Worldwide implementation of the WHO Child Growth Standards. Public Health Nutrition 15, 1603–1610. [DOI] [PubMed] [Google Scholar]
- de Onis M., Dewey K.G., Borghi E., Onyango A.W., Blössner M., Daelmans B. et al (2013) The World Health Organization global target on childhood stunting by 2025. Maternal & Child Nutrition 9 (Suppl. 2), 6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango A., Koski K.G. & Tucker K.L. (1998) Food diversity versus breastfeeding choice in determining anthropometric status in rural Kenyan toddlers. International Journal of Epidemiology 27, 484–489. [DOI] [PubMed] [Google Scholar]
- Onyango A.W., Esrey S.A. & Kramer M.S. (1999) Continued breastfeeding and child growth in the second year of life: a prospective cohort study in western Kenya. Lancet 354, 2041–2045. [DOI] [PubMed] [Google Scholar]
- Ozaltin E., Hill K. & Subramanian S.V. (2010) Association of maternal stature with offspring mortality, underweight, and stunting in low‐ to middle‐income countries. Journal of the American Medical Association 303, 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K.H., Muti M., Khalfan S.S., Humphrey J.H., Caffarella R. & Stoltzfus R.J. (2011) Beyond food insecurity: how context can improve complementary feeding interventions. Food and Nutrition Bulletin 32, 244–253. [DOI] [PubMed] [Google Scholar]
- Pelletier D., Haider R., Hajeebhoy N., Mangasaryan N., Mwadime R. & Sarkar S. (2013) The principles and practices of nutrition advocacy: evidence, experience and the way forward for stunting reduction. Maternal & Child Nutrition 9 (Suppl. 2), 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelto G.H., Levitt E. & Thairu L. (2003) Improving feeding practices: current patterns, common constraints, and the design of interventions. Food and Nutrition Bulletin 24 (1), 45–57. [DOI] [PubMed] [Google Scholar]
- Penny M.E., Creed‐Kanashiro H.M., Robert R.C., Narro M.R., Caulfield L.E. & Black R.E. (2005) Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: a cluster‐randomised controlled trial. Lancet 365, 1863–1872. [DOI] [PubMed] [Google Scholar]
- Petrou S. & Kupek E. (2010) Poverty and childhood undernutrition in developing countries: a multi‐national cohort study. Social Science & Medicine 71, 1366–1373. [DOI] [PubMed] [Google Scholar]
- Piwoz E., Sundberg S. & Rooke J. (2012) Promoting healthy growth: what are the priorities for research and action? Advances in Nutrition (Bethesda, Md.) 3, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwoz E.G., Huffman S.L. & Quinn V.J. (2003) Promotion and advocacy for improved complementary feeding: can we apply the lessons learned from breastfeeding? Food and Nutrition Bulletin 24, 29–44. [DOI] [PubMed] [Google Scholar]
- Pokhrel S. & Sauerborn R. (2004) Household decision‐making on child health care in developing countries: the case of Nepal. Health Policy Plan 19, 218–233. [DOI] [PubMed] [Google Scholar]
- Prakash R., Singh A., Pathak P.K. & Parasuraman S. (2011) Early marriage, poor reproductive health status of mother and child well‐being in India. The Journal of Family Planning and Reproductive Health Care 37, 136–145. [DOI] [PubMed] [Google Scholar]
- Prentice A.M., Ward K.A., Goldberg G.R., Jarjou L.M., Moore S.E., Fulford A.J. et al (2013) Critical windows for nutritional interventions against stunting. The American Journal of Clinical Nutrition 95, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph T., Schelling E., Grace D., Nicholson C., Leroy J., Cole D. et al (2007) Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. Journal of Animal Science 85 (11), 2788–2800. [DOI] [PubMed] [Google Scholar]
- Roos N., Wahab M., Chamnan C. & Thilsted S.H. (2007) The role of fish in food‐based strategies to combat vitamin A and mineral deficiencies in developing countries. The Journal of Nutrition 137, 1106–1109. [DOI] [PubMed] [Google Scholar]
- Roos N., Sorensen J.C., Sorensen H., Rasmussen S.K., Briend A., Yang Z. et al (2013) Screening for anti‐nutritional compounds in complementary foods and food aid products for infants and young children. Maternal & Child Nutrition 9 (Suppl. 1), 47–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel M.T., Alderman H. & the Maternal and Child Nutrition Study Group (2013) Nutrition‐sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet published online, doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- Sari M., de Pee S., Bloem M.W., Sun K., Thorne‐Lyman A.L., Moench‐Pfanner R. et al (2010) Higher household expenditure on animal‐source and nongrain foods lowers the risk of stunting among children 0–59 months old in Indonesia: implications of rising food prices. The Journal of Nutrition 140, 195S–200S. [DOI] [PubMed] [Google Scholar]
- Scaling Up Nutrition (2011) A Framework for Action . Reprint April 2011. Available at: http://scalingupnutrition.org/wp-content/uploads/2013/05/SUN_Framework.pdf (Accessed 8 August 2013).
- Schenk E.A., Samloff I.M. & Klipstein F.A. (1968) Morphology of small bowel biopsies. The American Journal of Clinical Nutrition 21, 944–961. [DOI] [PubMed] [Google Scholar]
- Scrimshaw N.S., Taylor C.E. & Gordon A.J.E. (1968) Interactions of Nutrition and Infection . WHO monograph series no. 57. World Health Organization: Geneva, Switzerland. [PubMed]
- Semba R.D., de Pee S., Sun K., Sari M., Akhter N. & Bloem M.W. (2008) Effect of parental formal education on risk of child stunting in Indonesia and Bangladesh: a cross‐sectional study. Lancet 371, 322–328. [DOI] [PubMed] [Google Scholar]
- Shroff M., Griffiths P., Adair L., Suchindran C. & Bentley M. (2009) Maternal autonomy is inversely related to child stunting in Andhra Pradesh, India. Maternal & Child Nutrition 5, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simondon K.B., Simondon F., Costes R., Delaunay V. & Diallo A. (2001) Breast‐feeding is associated with improved growth in length, but not weight, in rural Senegalese toddlers. The American Journal of Clinical Nutrition 73, 959–967. [DOI] [PubMed] [Google Scholar]
- Smith L.C. & Haddad L. (2000) Explaining Child Malnutrition in Developing Countries. International Food Policy Research Institute: Washington, DC. [Google Scholar]
- Smith L.E., Stoltzfus R.J. & Prendergast A. (2012) Food chain mycotoxin exposure, gut health, and impaired growth: a conceptual framework. Advances in Nutrition (Bethesda, Md.) 3, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J. et al (2013) Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekarjo D. & Zehner E. (2011) Legislation should support optimal breastfeeding practices and access to low‐cost, high‐quality complementary foods: Indonesia provides a case study. Maternal & Child Nutrition 7 (Suppl. 3), 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomons N.W. (2007) Malnutrition and infection: an update. The British Journal of Nutrition 98 (Suppl. 1), S5–10. [DOI] [PubMed] [Google Scholar]
- Spears D. (2013) How much international variation in child height can sanitation explain? Policy Research Working Paper 6351. World Bank: Washington, DC.
- Stein A.D., Thompson A.M. & Waters A. (2005) Childhood growth and chronic disease: evidence from countries undergoing the nutrition transition. Maternal & Child Nutrition 1, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler N. (2007) Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. International Journal of Obesity 31 (7), 1035–1043. [DOI] [PubMed] [Google Scholar]
- Sun J., Dai Y., Zhang S., Huang J., Yang Z., Huo J. et al (2011) Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China. Maternal & Child Nutrition 7 (Suppl. 3), 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan P.J., Kennedy C.E., Hurley K.M. & Black M.M. (2011) Maternal depression and early childhood growth in developing countries: systematic review and meta‐analysis. Bulletin of the World Health Organization 89, 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet L., Jerling J. & Van Graan A. (2013) Field‐testing of guidance on the appropriate labelling of processed complementary foods for infants and young children in South Africa. Maternal & Child Nutrition 9 (Suppl. 1), 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaratinam S., Rogozinska E., Jolly K., Glinkowski S., Roseboom T., Tomlinson J.W. et al (2012) Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta‐analysis of randomised evidence. British Medical Journal 344, e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R., Kain J., Mericq V., Rojas J. & Corvalan C. (2008) Nutrition, child growth, and chronic disease prevention. Annals of Medicine 40, 11–20. [DOI] [PubMed] [Google Scholar]
- Umeta M., West C.E., Verhoef H., Haidar J. & Hautvast J.G. (2003) Factors associated with stunting in infants aged 5–11 months in the Dodota‐Sire District, rural Ethiopia. The Journal of Nutrition 133, 1064–1069. [DOI] [PubMed] [Google Scholar]
- UNICEF (1990) Strategy for improved nutrition of children and women in developing countries . UNICEF Policy Review 1990. UNICEF: New York.
- UNSCN (2010) Climate change and nutrition security . United Nations Standing Committee on Nutrition: New York.
- Uvere P.O. & Ene‐Obong H.N. (2013) Complementary local foods for infants in developing countries In: Nutrition in Infancy, Volume 1 (eds Watson R.R., Grimble G., Preedy V.R. & Zibadi S.), pp 75–93. Humana Press: New York. [Google Scholar]
- Vazir S., Engle P., Balakrishna N., Griffiths P.L., Johnson S.L., Creed‐Kanashiro H. et al (2013) Cluster‐randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Maternal & Child Nutrition 9, 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Richter L. et al (2008) Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., de Onis M., Hallal P.C., Blossner M. & Shrimpton R. (2010) Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–e480. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Wachs T.D., Gardner J.M., Lozoff B., Wasserman G.A., Pollitt E. et al (2007a) Child development: risk factors for adverse outcomes in developing countries. Lancet 369, 145–157. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Chang S.M., Powell C.A., Simonoff E. & Grantham‐McGregor S.M. (2007b) Early childhood stunting is associated with poor psychological functioning in late adolescence and effects are reduced by psychosocial stimulation. The Journal of Nutrition 137, 2464–2469. [DOI] [PubMed] [Google Scholar]
- Wamani H., Astrom A.N., Peterson S., Tumwine J.K. & Tylleskar T. (2006) Predictors of poor anthropometric status among children under 2 years of age in rural Uganda. Public Health Nutrition 9, 320–326. [DOI] [PubMed] [Google Scholar]
- Weisstaub G. & Uauy R. (2012) Non‐breast milk feeding in developing countries: challenge from microbial and chemical contaminants. Annals of Nutrition & Metabolism 60, 215–219. [DOI] [PubMed] [Google Scholar]
- Wells J.C. & Stock J.T. (2011) Re‐examining heritability: genetics, life history and plasticity. Trends in Endocrinology and Metabolism 22, 421–428. [DOI] [PubMed] [Google Scholar]
- Whincup P.H., Kaye S.J., Owen C.G., Huxley R., Cook D.G., Anazawa S. et al (2008) Birth weight and risk of type 2 diabetes: a systematic review. Journal of the American Medical Association 300, 2886–2897. [DOI] [PubMed] [Google Scholar]
- WHO (2006) World Health Report: Working Together for Health. World Health Organization: Geneva. [Google Scholar]
- WHO (2008) Indicators for Assessing Infant and Young Child Feeding Practices‐ Part II: Measurement. World Health Organization: Geneva. [Google Scholar]
- WHO and UNICEF (2009) Baby Friendly Hospital Initiative: Revised, Updated and Expanded for Integrated Care. World Health Organization: Geneva. [PubMed] [Google Scholar]
- WHO, UNICEF and AED (2008) Learning from Large‐Scale Community‐Based Programmes to Improve Breastfeeding Practices. World Health Organization: Geneva. [Google Scholar]
- WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica (Oslo, Norway : 1992). Supplement 450, 76–85. [DOI] [PubMed] [Google Scholar]
- Wilson H.J., Dickinson F., Hoffman D.J., Griffiths P.L., Bogin B. & Varela‐Silva M.I. (2012) Fat free mass explains the relationship between stunting and energy expenditure in urban Mexican Maya children. Annals of Human Biology 39, 432–439. [DOI] [PubMed] [Google Scholar]
- World Bank (2006) Repositioning Nutrition as Central to Development: A Strategy for Large‐Scale Action. The World Bank: Washington, DC. [Google Scholar]
- World Bank (2007) From agriculture to nutrition: Pathways, synergies and outcomes . No. 40196‐ GLB. Agriculture and Rural Development Department, The World Bank: Washington, DC.
- World Health Assembly (2012) Agenda Item 13.3 of the Sixty‐Fifth World Health Assembly: Maternal, Infant and Young Child Nutrition . Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_R6-en.pdf (Accessed 11 July 2013).
- Zimmermann M.B. (2012) The effects of iodine deficiency in pregnancy and infancy. Paediatric and Perinatal Epidemiology 26 (Suppl. 1), 108–117. [DOI] [PubMed] [Google Scholar]


