Abstract
The United Nations Convention on the Rights of the Child holds governments responsible to ensure children's right to the highest attainable standard of health by providing breastfeeding support, and access to nutritious foods, appropriate health care, and clean drinking water. International experts have identified key child care practices and programmatic activities that are proven to be effective at reducing infant and young child undernutrition, morbidity, and mortality. Nevertheless, progress towards reducing the prevalence of undernutrition has been sporadic across countries of the Sahel sub‐region of Sub‐Saharan Africa. In view of this uneven progress, a working group of international agencies was convened to ‘Reposition children's right to adequate nutrition in the Sahel.’ The first step towards this goal was to organize a situational analysis of the legislative, research, and programmatic activities related to infant and young child nutrition (IYCN) in six countries of the sub‐region: Burkina Faso, Chad, Mali, Mauritania, Niger, and Senegal. The purposes of this introductory paper are to review current information concerning the nutritional and health status of infants and young children in the Sahel and to summarize international guidelines on optimal IYCN practices. These guidelines were used in completing the above‐mentioned situational analyses and encompass specific recommendations on: (i) breastfeeding (introduction within the first hour after birth, exclusivity to 6 months, continuation to at least 24 months); (ii) complementary feeding (introduction at 6 months, use of nutrient dense foods, adequate frequency and consistency, and responsive feeding); (iii) prevention and/or treatment of micronutrient deficiencies (vitamin A, zinc, iron and anaemia, and iodine); (iv) prevention and/or treatment of acute malnutrition; (v) feeding practices adapted to the maternal situation to reduce mother‐to‐child transmission of HIV; (vi) activities to ensure food security; and (vii) the promotion of hygienic practices concerning food preparation and storage and environmental sanitation. The following papers in this issue will present results of the situational analyses for the individual countries.
Keywords: breast feeding, complementary feeding, infant and young child nutrition, micronutrient malnutrition, nutritional intervention, Sub‐Saharan Africa
Background
With the introduction of the Millennium Development Goals (MDG) in 1990 and adoption of the Millennium Declaration in 2000, the international community acknowledged the need to ‘spare no effort to free our fellow men, women, and children from the abject and dehumanizing conditions of extreme poverty’. Infants and young children are at particular risk of the effects of poverty and lack of education. Francophone countries of the Sahel region of Africa – including Senegal, Mauritania, Mali, Burkina Faso, Niger, and Chad – are positioned along the southern fringe of the Sahara Desert (Fig. 1) where year‐to‐year and seasonal variations in rainfall often lead to precarious food harvests and chronic strain on national financial stability. These and other factors contribute to food insecurity in the region and to high rates of malnutrition and mortality among infants and young children (Table 1).
Figure 1.
Map of the Sahelian countries included in the situational analysis. Map courtesy of OCHA, Dakar, Senegal Regional Office.
Table 1.
National under 5 mortality rates and selected indicators of nutritional status among children less than 5 years of age in six Sahel countries†
| Country | MDG 4.1. < 5 years mortality/1000 live births (1) | Stunting (%) ‡ | MDG 1.8. under‐weight (%) ‡ | Exclusive breastfeeding <6 months (%) | Complementary feeding at 6–9 months ¶ (%) | Anaemia (<110 g/L haemoglobin) (%) | Vitamin A deficiency* (%) | Iodine deficiency disorder § (% goitre) |
|---|---|---|---|---|---|---|---|---|
| Burkina Faso a | 188 | 45 | 37 | 8 | 50 | 83 | 46 | 29 |
| Chad b | 191 | 45 | 34 | 2 | 68 | 76 | 45 | 24 |
| Mali c | 191 | 39 | 28 | 38 | 30 | 81 | 47 | 42 |
| Mauritania d | 122 | 29 | 23 | 11 | 40 | 74 | 17 | 21 |
| Niger e | 198 | 55 | 40 | 14 | 78 | 84 | 41 | 20 |
| Senegal f | 121 | 20 | 15 | 34 | 69 | 83 | 61 | 23 |
| Cut off indicative of public health problem** | ≥20 | ≥10 | Rating of ‘good’ is ≥50 | Rating of ‘good’ is ≥80 (2) | ≥5 | ≥20 | ≥5 |
Rates reported depend on data collection methods and proximity in time to supplementation programmes (4).
† Sudan was not included due to the political conflicts that hindered on‐site data collection and analyses.
Anthropometric data reflect the new WHO Growth Reference Standards, as reported in the WHO Global Database (3). Stunting is defined as <−2 SD of the WHO standard for height‐for‐age and underweight is defined as <−2 SD for weight‐for‐age.
§ Goitre rate.
% of infants 6–9 months of age who received complementary foods in addition to breast milk and/or other liquids in the 24 h prior to the survey.
While the causes of malnutrition are complex, there is a consensus about the two immediate causes of malnutrition: insufficient intake of nutritious food and increased incidence of infections, which are often associated with inadequate child care practices (Fig. 2).
Figure 2.

Conceptual framework of malnutrition (11). Figure adapted from original source (11) by the United Nations Children's Fund (UNICEF) in the 1998 State of the World's Children and printed here with permission from UNICEF West and Central Africa Regional Office.
With current estimates that malnutrition is an underlying cause of one‐third (12) to one‐half (13) of deaths among young children less than 5 years of age, the global community has emphasized the critical need to protect the health and well‐being of young children by implementing appropriate actions to improve the nutritional situation. Examples of internationally recognized initiatives, declarations, and conventions aimed at improving the health and nutrition of infants and young children include the United Nations Convention on the Rights of the Child (14), the Millennium Declaration from which the MDGs (15) are derived, the International Code of Marketing of Breast milk Substitutes (16), the Ten‐Year Strategy for the Reduction of Vitamin and Mineral Deficiencies (17), REACH: Ending Child Hunger and Undernutrition/Renewed Efforts Against Child Hunger (18) and the International Labour Organization Convention on Maternity Protection No. 183 (19), which provides for maternity leave during pregnancy and lactation.
The United Nations Convention on the Rights of the Child holds governments responsible to ensure children's right to the highest attainable standard of health by providing breastfeeding support, adequate nutritious foods, appropriate health care, and clean drinking water. The Sahelian countries have ratified some or all of the United Nations Convention on the Rights of the Child and other international codes aimed at protecting the health and well‐being of children (Table 2); and these countries are working towards improving the health and nutrition of infants and young children. However, recent surveys reveal that there is still a substantial need to strengthen and expand these efforts.
Table 2.
Documentation, by country, of adoption or ratification of specific international conventions and codes aimed at protecting the health and well‐being of infants and children
| International conventions and codes | Burkina Faso | Chad | Mali | Mauritania | Niger | Senegal |
|---|---|---|---|---|---|---|
| The UN Convention on the Rights of the Child (14), * | 31 Aug 1990 | 2 Oct 1990 | 20 Sept 1990 | 16 May 1991 | 30 Sept 1990 | 31 Jul 1990 |
| International Code of Marketing of Breast Milk Substitutes (16), ‡ | Law | No action | Being studied | Being studied | Many provisions ratified as law | Many provisions ratified as law |
| ILO Convention on Maternity Protection, No. 183, 2000 (22), † | – | – | 5 June 2008 | – | – | – |
| International Covenant on Economic, Social and Cultural Rights (20) | 4 Apr 1999 a | 9 Sept 1995 a | 3 Jan 1976 a | – | 7 June 1986 a | 13 May 1978 |
| Convention on the Elimination of All Forms of Discrimination Against Women (21) | 13 Nov 1987 a | 9 July 1995 a | 10 Oct 1985 | 9 Jul 2001 a | 7 Nov 1999 a | 7 Mar 1985 |
Date of accession, not ratified.
Mali – Reservation: The Government of the Republic of Mali declares that, in view of the provisions of the Mali Family Code, there is no reason to apply article 16 of the Convention. Mauritania – Upon signature, Reservation: ‘In signing this important Convention, the Islamic Republic of Mauritania is making reservations to articles or provisions which may be contrary to the beliefs and values of Islam, the religion of the Mauritania People and State.’
Includes laws on maternity leave for women (24).
Status of the Code in Africa – 2006 (23) and 2008.
The International Covenant on Economic, Social, and Cultural Rights states that everyone has the right to adequate food and the highest attainable standards of health (Articles 11 and 12) (20). Women have the right to equality in employment, access to natural resources and other services, including a special right to adequate nutrition during pregnancy and lactation (Articles 11, 12, and 14) (21). Promoting the right to adequate food and applying human rights approaches to nutrition contribute to tackling immediate, underlying, and root causes of malnutrition. Rights‐based advocacy entails focusing on better nutrition as well as the process of achieving this outcome through increased participation, empowerment, and non‐discrimination.
The two MDGs that are most directly related to infant and young child nutritional status are: Goal 1 to eradicate extreme poverty and hunger 1 ; and Goal 4 to reduce child mortality 2 . Several of the indicators used to evaluate these MDGs include the proportion of the population living on less than US$1 per day (% population < US$1/day), the proportion of children less than 5 years of age who are suffering from moderate and severe underweight (per cent of children with weight‐for‐age Z‐score < −2), the mortality rate among infants and children less than 5 years of age and the per cent of children less than 5 years who have been immunized against measles.
International progress towards these MDGs varies by country, region, and the indicator measured. Sub‐Saharan Africa is not keeping up with most improvements observed among developing regions with respect to key infant and young child health indicators (Table 3). The exception is the relative increase in the per cent of infants who are immunized against measles.
Table 3.
Regional and international progress towards selected Millennium Development Goals, Sub‐Saharan Africa and All Developing Regions, values weighted by population size (25)
| Sub‐Saharan Africa | All developing regions | |
|---|---|---|
| MDG I: eliminate extreme poverty and hunger | ||
| Indicator 1.1: reduce by half the % population living on <US$1/day – methodology was changed, so the available indicator presented is the % of employed people living below $1 (PPP) a day | ||
| 1990 (% of population) | 55.5 | 30.6 |
| 2007 (% of population) | 51.4 | 20.4 |
| % change | −7 | −33 |
| Indicator 1.8: reduce by half the % under‐fives suffering from underweight | ||
| ∼1990 value (% of population) | 32 | 33 |
| ∼2006 value (% of population) | 28 | 26 |
| % change | −13 | −21 |
| MDG IV: reduce mortality among children less than 5 years | ||
| Indicator 4.2: Reduce by 2/3 the mortality rate among children <5 years | ||
| ∼1990 value (deaths/1000 live births) | 184 | 103 |
| ∼2006 value (deaths/1000 live births) | 157 | 80 |
| % change | −15 | −22 |
| Indicator 4.3: increase the % of infants immunized against measles (goal not cited) | ||
| ∼1990 value (% of population) | 56 | 71 |
| Recent value (% of population) | 72 | 78 |
| % change | 29 | 10 |
MDG, Millennium Development Goal.
In the Sahel region, progress towards indicator 1.8 of MDG I, to reduce poverty and hunger by half is very limited, and in some situations seems to be worsening (Fig. 3). Some countries in the Sahel have made substantial progress towards the reduction of mortality rates among children less than 5 years of age (MDG IV, Fig. 4). However, improvements in other indicators have been very limited in the Sahel region as a whole, and current trends are not sufficient to reach the desired goals by 2015.
Figure 3.
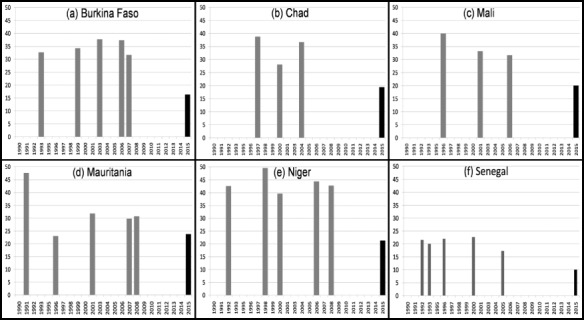
Prevalence of children <5 years of age suffering from moderate and severe underweight (weight‐for‐age z‐score <−2) and Millennium Development Goal (MDG 1.8) in six countries of the Sahel*†. *Final columns indicate MDG 1.8 of a 50% underweight reduction between 1990 and 2015. †Data from Demographical and Health Surveys and Multiple Indicator Cluster Survey5 as presented in the World Health Organization (WHO) Global Database on Child Growth and Malnutrition (3), using WHO child growth standards. Data: Millenium Development Goals Indicators, Official UN site, http://unstats.un.org/unsd/mdg/Data.aspx.
Figure 4.
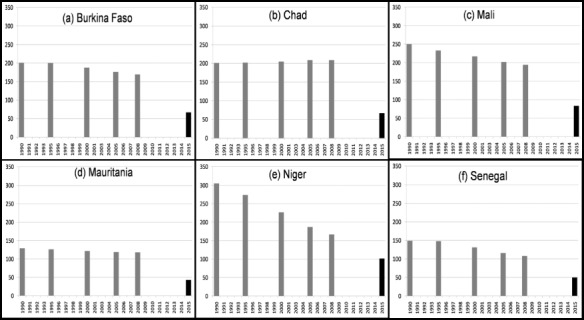
Mortality rate among children <5 years of age and Millennium Development Goal (MDG 4.1) in six countries of the Sahel*†. *Final columns indicate a 2/3 reduction (MDG 4.1) between 1990 and 2015. †1990 estimates were obtained 13 February 2009 from the United Nations Children's Fund, Childinfo.org (26). Data: Childinfo.org for mortality rate among <5 years/1000 live births, by year of survey.
Among the other markers that are measured to evaluate national and international progress towards optimal infant and young child health are national rates of breastfeeding (early introduction, exclusivity to 6 months and continuation to 24 months as complementary foods are introduced), complementary feeding practices (the timing of introduction, quality, and quantity of complementary foods), use of nutrient supplements or fortified foods, use of insecticide treated bed nets (to reduce the risk of malaria and thus anaemia) and appropriate management of illness, particularly diarrhoea. Some progress has been achieved in increasing the prevalence of exclusive breastfeeding in the Sahel region, but, these improvements have not been sustained in most countries (Fig. 5).
Figure 5.
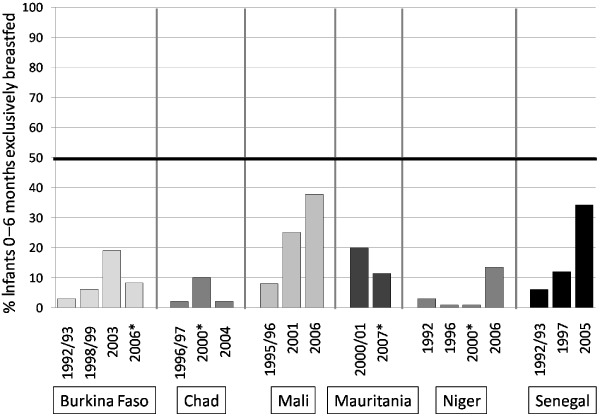
Trends in prevalence of exclusive breastfeeding during the first 6 months of life, by country and years of survey, horizontal line indicates j50% prevalence. *Data with asterisk from Multiple Indicator Cluster Survey5 data all others from Demographic and Health Survey data.
Regional infant and young child nutrition (IYCN) initiative
The data presented above demonstrate that although some progress has been achieved in the Sahel region, there is need for continued support of effective actions and/or for implementing new programmes in geographic areas not currently covered. To evaluate the current situation and guide appropriate actions to address the problem of malnutrition among infants and young children in the Sahel region, the Food and Agriculture Organization of the United Nations (FAO), Helen Keller International (HKI), the Micronutrient Initiative (MI), the United Nations Children's Fund (UNICEF) and the World Food Programme (WFP) launched an IYCN initiative to ‘Restore children's right to adequate nutrition in the Sahel.’
The Initiative's aim is to achieve optimal feeding of >80% of children 0–24 months in the Sahel. The Initiative will propose options to enhance infant and young child feeding during the first two years of life (i.e. breastfeeding and complementary feeding) with the ultimate goal to improve the nutritional status, growth and development, health, survival and quality of life of children in the Sahel. To facilitate the development of appropriate intervention strategies, additional quantitative and qualitative information is required on food availability, food, and nutrient intakes and behavioural aspects of child feeding as well as current public policies and programmes.
The first step of this initiative was to organize a situational analysis, to compile, analyse, and interpret available information on infant and young child feeding practices and relevant policies, research, training, and intervention programmes in the six target countries of Burkina Faso, Chad, Mali, Mauritania, Niger, and Senegal. Sudan was not included due to the level of political unrest, which prevented data collection. Additional aims were to assess the barriers to optimal feeding (knowledge, cultural, environmental, and socio‐political factors and food availability), to develop recommendations on how to overcome these barriers and to establish a framework of essential large‐scale interventions.
This paper summarizes internationally accepted, evidence‐based guidelines for improving the health and well‐being of infants and young children. These recommendations were used in the situational analyses to assess each country's progress, as described in the following papers in this issue 27, 28, 29, 30, 31, 32.
The organizing members of this Initiative hope that these findings will serve as a guide to support national efforts and will establish a foundation upon which governments, international agencies and non‐governmental organizations can build collaboration within and across countries to refine and expand their actions to improve the health and nutrition of young children in the region.
Overview of international recommendations for IYCN practices
Key documents that form the foundation for promoting improved IYCN internationally include the Global Strategy for Infant and Young Child Feeding (33), the Global Strategy's planning guide (34) and tools for assessing national practices, policies, and programmes (2), the Guiding Principles for Complementary Feeding of the Breastfed Child (35), Guiding Principles for Feeding Non‐Breastfed Children 6–24 months of age (36) and the Essential Nutrition Actions approach 37, 38, 39– including Behaviour Change Communication (BCC) and Information Education Communication (IEC) guidance. In addition, we incorporated key findings and recommendations from the Lancet's series on Maternal and Child Undernutrition 12, 40, 41, 42. The key IYCN practices that were examined in the situational analysis of each country are listed in Box 1.
Box 1. Key actions to support infant and young child nutrition
-
1
Promotion of optimal breastfeeding practices
-
a.
Early introduction of breastfeeding (in the first hour of life)
-
b.
Exclusive breastfeeding during the first 6 months of life
-
c.
Continued breastfeeding to at least 24 months of age
-
a.
-
2
Promotion of optimal complementary feeding practices
-
a.
Timely introduction of complementary foods at 6 months of age
(Provision of complementary foods that are dense in the key nutrients of vitamin A, iron, and zinc, with an emphasis on animal source foods)
-
b.
Responsive feeding practices to encourage and enhance the feeding process
-
c.
Progressive increase in the amount and consistency of complementary foods, without supplanting breastfeeding
-
a.
-
3
Micronutrient assessment, prevention, and treatment of deficiencies
-
a.
Vitamin A assessment and supplementation for children 6–59 months and mothers within 6 weeks postpartum
-
b.
Assessment and prevention of zinc deficiency, as appropriate; promotion of the use of zinc in the treatment of diarrhoea
-
c.
Assessment, prevention, and/or treatment of nutritional anaemia related to deficiencies of iron and other micronutrients, and non‐nutritional anaemia related to malaria and other parasites
-
d.
Assessment of the risk of iodine deficiency, quality control of the production and/or importation of adequately iodized salt, promotion of the use of iodized salt, additional measures to treat iodine deficiency when necessary
-
e.
Dietary assessments for development of nutrition education programmes and food fortification
-
a.
-
4
Other nutritional support
-
a.
Identification and treatment of acute malnutrition at the clinic and/or community level
-
b.
Nutritional guidance and support for HIV‐positive mothers and children, including specific support for feeding options and other treatment to reduce mother‐to‐child transmission of HIV
-
c.
Support for food insecure populations
-
d.
Sanitation services and personal hygiene practices related to water, food, and the local surroundings
-
a.
-
5
Organizational support
-
a.
Identification of champions of nutrition in the government and other agencies crucial to infant and young child nutrition (IYCN) including a national working group of key stakeholders
-
b.
Clear national IYCN policies, strategies, plans of action, training manuals, programmes, monitoring, and evaluation of programmes and formative research to specifically address the above practices
-
c.
Information sharing among agencies and across countries
-
a.
The following sections provide brief summaries of current knowledge regarding the international guidelines and recommendations highlighted in Box 1 on breastfeeding, complementary feeding, micronutrients, other nutritional, and organizational support. These topics are discussed as they apply to the six countries included in the situational analyses to follow in this issue 27, 28, 29, 30, 31, 32 and Supporting Information Appendix.
Methods used in collecting and analysing national data for comparison with international recommendations
From May to July 2008, a coordinator was selected in each country to contact nutrition focal points in each organization and agency conducting nutrition‐related activities in the respective countries. The individual focal points were then asked to complete a questionnaire summarizing their scope of organizational IYCN activities and to provide any relevant documents describing those activities. We evaluated each document for consistency with the aforementioned key infant and young child feeding guidelines, as outlined in Box 1. The categories of documents obtained were: (i) national policies, strategies, and plans of action; (ii) research reports related to BCC/IEC and other formative research conducted in each country regarding some aspect of the IYCN practices; (iii) in‐service and pre‐service training manuals and protocols and other curricula developed for programme implementation and for students studying health‐related topics in universities and vocational schools; (iv) reports of programmes, including the intended and actual coverage of those programmes; and (v) programme monitoring and impact evaluation reports, whether specific to the programme or general through nutritional surveys. We also completed PubMed bibliographic searches for additional peer‐reviewed research that fit the categories of ‘nutrition’ and either ‘child’ or ‘women’ for each country. It was expected that these sets of documents would indicate each country's progress towards improving IYCN practices as illustrated in Fig. 6.
Figure 6.
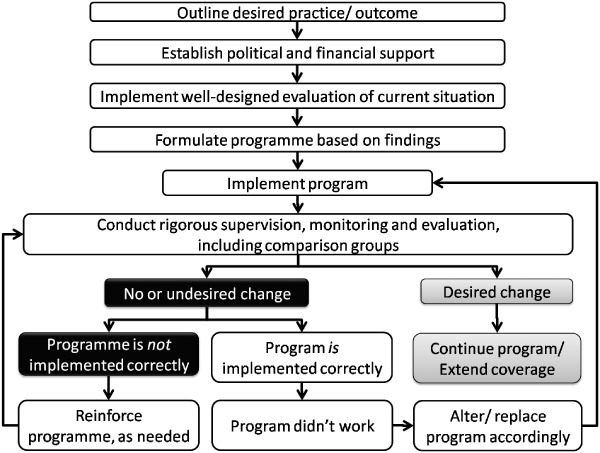
Optimal steps in moving a desired practice from conception through programme implementation and eventual national coverage of successful programmes.
Summary of current knowledge and international recommendations with respect to IYCN practices
The following summaries of key IYCN recommendations were prepared to assist in the review of current national policies and programmes, and to serve as a basis for evaluating progress achieved in each of the specific nutrition‐related domains. The summaries are based on current international guidelines concerning specific IYCN policies and programmes, and they are not meant to provide comprehensive reviews of existing literature on each topic.
Breastfeeding
International recommendations for breastfeeding (Table 4) have been well substantiated through a number of research studies and reviews 12, 13, 43. More recently, Edmund et al. (44) found that the risk of mortality was 2.4‐fold lower among rural Ghanaian infants, 2 to 28 days old, who commenced breastfeeding in the first 24 h following birth compared with those who commenced breastfeeding later than the first day (P < 0.0001). The risk of mortality was also 3.8‐fold lower among neonates who were exclusively breastfed compared with those who were partially breastfed, even after adjusting for the timing of initiating breastfeeding, and for socioeconomic and health‐related factors at birth. In Bangladesh, researchers found significant associations between breastfeeding practices and the prevalence of respiratory infections and/or diarrhoea 45, 46. Similar benefits have also been identified among infants of HIV‐positive women 47, 48. In the 2008 Lancet series on Maternal and Child Undernutrition (12), it was estimated that 10% of the disease burden among children less than 5 years of age is attributable to suboptimal breastfeeding practices, especially non‐exclusive breastfeeding in the first 6 months of life.
Table 4.
Summary of international breastfeeding recommendations
| Practice | Specific recommendations |
|---|---|
| Early initiation of breastfeeding | Breastfeeding should commence within the first 30–60 min after birth. |
| No other liquids or concoctions should be provided prior to initiation of breastfeeding. | |
| Exclusive breastfeeding to 6 months | Breastfeeding should be on demand or at least 8 times per day. |
| No additional water, food or other liquids should be given during this time to avoid the risk of introducing contaminants and to ensure the infant receives adequate amounts of breast milk. | |
| Continued breastfeeding to 24 months | Beyond 6 months, the infant should continue to receive breast milk on demand and complementary foods should be added to the breastfeeding regimen rather than replacing breastfeeding. (Some older infants may reduce the frequency or number of times they breastfeed as sucking becomes more efficient and capacity of the stomach increases.) |
Baby friendly hospital initiative
The Baby Friendly Hospital Initiative (BFHI) promotes activities in hospitals, clinics, and communities that support women in their efforts towards successful breastfeeding. The 10 fundamental action areas, required for hospital or community certification, include:
-
1
Have a written breastfeeding policy that is routinely communicated to all health care staff.
-
2
Train all health care staff in skills necessary to implement this policy.
-
3
Inform all pregnant women about the benefits and management of breastfeeding.
-
4
Help mothers initiate breastfeeding within one half‐hour of birth.
-
5
Show mothers how to breastfeed and maintain lactation, even if they should be separated from their infants.
-
6
Give newborn infants no food or drink other than breast milk, unless medically indicated.
-
7
Practice rooming‐in – that is, allow mothers and infants to remain together 24 h a day.
-
8
Encourage breastfeeding on demand.
-
9
Give no artificial teats or pacifiers (also called dummies or soothers) to breastfeeding infants.
-
10
Foster the establishment of breastfeeding support groups and refer mothers to them upon discharge from the hospital or clinic.
A revised BFHI package was developed by the World Health Organization (WHO) that includes five sections: (i) background and implementation of the BFHI; (ii) a course for decision‐makers to strengthen and sustain the BFHI; (iii) a 20‐h course for maternity staff on breastfeeding promotion and support; (iv) hospital self‐appraisal and monitoring; and (v) external assessment and reassessment (49).
Breastfeeding promotion research
Several authors and agencies have recently reviewed breastfeeding promotion programmes and factors associated with their success 50, 51, 52. The findings of one such review sponsored by the WHO are summarized in Box 2. Another review of large‐scale programmes promoting exclusive breastfeeding in various countries found a positive dose–response relationship between the number of contacts with mothers or the number of different methods employed in disseminating messages and the adoption of appropriate breastfeeding practices (51). Therefore, appropriate breastfeeding practices should be promoted through as many channels as possible.
Box 2. Key findings from large‐scale community‐based programmes to improve breastfeeding practices (52)
Factors associated with successful breastfeeding promotion programmes
-
1
The community offers indispensable resources for breastfeeding promotion and support, and these resources need continual mentoring and encouragement.
-
2
Multiple approaches are used in community‐based breastfeeding promotion and support.
-
3
Breastfeeding practices can change over a relatively short period and need continued reinforcement to be sustained.
-
4
Effective communication and advocacy are vital to set policy priorities, influence community norms, and improve household practices.
-
5
During training more attention needs to be given to interpersonal counselling skills.
-
6
Partnerships, leadership, proof of concept, and adequate resources facilitate programme scale‐up.
-
7
Monitoring and evaluation is critical to measure progress, identify successful, and unsuccessful strategies, and make appropriate programme adjustments. Those programmes that carried out baseline and end‐line surveys as well as annual rapid assessments in programme areas were best positioned to spot problem areas and adjust programmes accordingly.
Complementary feeding
Although breast milk provides adequate nutrition for the first 6 months of an infant's life, research has shown that breast milk is no longer adequate as the sole source of nutrition beyond 6 months of age 53, 54, 55. Therefore, at the age of 6 months (26 weeks), infants must commence and gradually increase consumption of nutrient‐dense complementary foods, while continuing breastfeeding, in order to meet their increasing needs for energy and specific nutrients (e.g. iron, zinc, vitamin A, etc.). WHO feeding recommendations to meet the nutritional needs of breastfed (and non‐breastfed) infants and young children are listed in Table 5, 35, 36.
Table 5.
Summary of guiding principles for complementary feeding for both the breastfed and non‐breastfed infant and child, adapted from references: 35, 36)
| Recommendation | Age in months | ||
|---|---|---|---|
| 6–8 | 9–11 | 12–24 | |
| Energy needed from complementary food assuming average breast milk intake, kcal/day | 200 | 300 | 550 |
| Kcal/day for the non‐breastfed infant/child | 600 | 700 | 900 |
| Number of meals and snacks/day in addition to breast milk | 2–3 meals | 3–4 meals | 3–4 meals |
| 1–2 snacks | 1–2 snacks | 1–2 snacks | |
| Number of meals and snacks/day for the non‐breastfed infant/child, includes breast milk substitutes (36) | 4–5 meals | 4–5 meals | 4–5 meals |
| 1–2 snacks | 1–2 snacks | 1–2 snacks | |
| Consistency | Pureed, mashed and semi‐solid foods | Also finger foods | Increase consistency as the child's eating skills improve to consume family foods |
| Responsive feeding | Spoon feed infants to start, and assist to develop eating skills. Be sensitive to hunger and satiety cues. Feed slowly and patiently. Encourage but do not force to eat. Experiment with a variety of foods. Interact with child during meals, but minimize distractions | ||
| Good hygiene and proper food handling | Wash caregivers' and children's hands before food preparation and eating. Store foods safely and serve foods immediately after preparation. Use clean utensils to prepare and serve food. Use clean cups and bowls when feeding children, and avoid the use of feeding bottles, which are difficult to keep clean. | ||
| Variety | Meat, poultry, fish or eggs daily or as often as possible; vitamin A‐rich fruits and vegetables daily; diet with adequate, but not excessive, fat content | ||
| Fortified complementary foods or vitamin/mineral supplements | As needed* | As needed* | As needed* |
| During and after illness | Increased fluids, more frequent breastfeeding. Encourage soft, varied, appetizing, favourite foods | ||
Based on quantitative studies of dietary intake and/or surveys of micronutrient status.
Assessment, prevention and treatment of micronutrient deficiencies
The recent Lancet series on Maternal and Child Undernutrition assessed the global risk of mortality and morbidity in children less than 5 years of age attributed to micronutrient deficiencies (12). The authors concluded that the most critical micronutrient deficiencies associated with deaths and disability‐adjusted life years (DALYs) in children less than 5 years were vitamin A deficiency (6.5 and 5.3%, respectively), zinc deficiency (4.4 and 3.8%, respectively), iron deficiency (0.2% and 0.5%, respectively) and iodine deficiency (0.03 and 0.6%, respectively) (12). The present review and the situational analyses are limited to these four key micronutrients.
Vitamin A
The importance of vitamin A in reducing mortality and morbidity among young children and women of child bearing age has been well established 56, 57, 58, 59, 60, 61, 62, 63, 64. Programmes to provide universal vitamin A supplements (VAS) to high‐risk populations of children 6–59 months and to women within 6 weeks postpartum are promoted by WHO (65) and are being implemented in a large number of countries.
Assessment of vitamin A status
Methods that have been employed to evaluate population vitamin A status include measurement of serum retinol concentration (<0.7 µmol L−1 is considered inadequate) in a representative sample of the selected population, the prevalence of clinical signs of deficiency, such as night blindness or xerophthalmia, and the under‐five mortality rate (66). Few countries in the Sahel have undertaken assessments of serum retinol concentration or of xerophthalmia among a representative population sample. However, the reported prevalence of night blindness among pregnant women and population mortality rates among children less than 5 years of age are regularly assessed.
Prevention of vitamin A deficiency
In areas where the intake of vitamin A is inadequate, WHO recommends a combination of breast feeding, dietary improvement, food fortification, and VAS to prevent vitamin A deficiency among young children 67, 68. Recommended doses and schedules for administering VAS, according to the Annecy Accords, the International Vitamin A Consultative Group (IVACG) and WHO are listed in Table 6.
Table 6.
Schedule and doses of routine vitamin A supplementation in vitamin A‐deficient populations, as recommended in the Annecy Accords to assess and control vitamin A deficiency 67, 68
| Population | Amount of vitamin A to be administered | Time of administration |
|---|---|---|
| Infants 0–5 months | 150 000 IU as three doses of 50 000 IU with at least a 1‐month interval between doses | At each DTP immunization contact (6, 10 and 14 weeks) (otherwise at other opportunities) |
| Infants 6–11 months | 100 000 IU as a single dose every 4–6 months | At any opportunity (e.g. measles immunization or other clinic visits) |
| Children 12 months and older | 200 000 IU as a single dose every 4–6 months | At any opportunity or six monthly mass distribution campaign |
| Postpartum women | 400 000 IU as two doses of 200 000 IU at least 1 days apart | As soon after delivery as possible and not more than 6 weeks later |
| and/or 10 000 IU daily or 25 000 IU weekly | and/or during the first 6 months after delivery* |
DTP, diphtheria‐tetanus‐pertussis combination vaccine; IU, International Units. *Due to possible teratrogenic effects of vitamin A supplements on the developing fetus, any vitamin A supplementation beyond the initial 6 weeks postpartum should be administered with caution and under supervision.
Zinc
Systematic reviews have highlighted the critical importance of zinc in preventing childhood illnesses, such as diarrhoea and pneumonia, and in supporting adequate growth 69, 70. The Lancet Series on Child Survival (60) and Maternal and Child Undernutrition (12) concluded that about 4% of disease burden in young children is attributable to zinc deficiency.
Assessment of zinc status
WHO, UNICEF, the International Atomic Energy Agency (IAEA), and the International Zinc Nutrition Consultative Group (IZiNCG) have published guidelines on the assessment of population zinc status (71). The three main types of zinc status assessment that were considered included biochemical, dietary, and functional methods. Serum or plasma zinc concentration is considered the best available biomarker of risk of zinc deficiency in populations 72, 73. When the prevalence of low plasma or serum zinc concentration is >20%, zinc deficiency is considered a public health concern. Inadequate dietary intake of absorbable zinc is one of the major causes of zinc deficiency. Therefore, dietary zinc intakes can be assessed among a representative sample of individuals from the target population to determine the risk of zinc deficiency (74). When the prevalence of inadequate dietary zinc intake is >25% the risk of zinc deficiency is considered to be elevated and of public health concern. The easiest‐to‐measure outcome of zinc deficiency is stunting (height‐for‐age z‐score < −2) among preschool children. Although this is the least reliable method of estimating the risk of zinc deficiency, data are already available for most countries throughout the world, which can be used as a preliminary assessment of the risk of zinc deficiency. WHO considers a prevalence of stunting > 20% to be elevated and of public health concern (75). When the prevalence of stunting is high, IZiNCG recommends programmatic action, while conducting further assessments of the level of zinc deficiency in the target population.
Prevention of zinc deficiency
Studies have found that preventive zinc supplements can decrease the incidence of diarrhoea and pneumonia and reduce the prevalence of stunting among vulnerable populations 76, 77. Recommended doses of zinc supplements have been proposed by IZiNCG for various age groups in populations at risk of zinc deficiency (69). Adoption of these recommendations by the WHO is pending. Other possible interventions among population groups at risk of zinc deficiency include targeted food fortification with zinc (78), biofortification of grains (79), dietary modification to increase consumption of zinc‐rich foods (particularly animal source foods such as meat, fish, and poultry, organ meats, and to a lesser extent dairy, eggs) and food processing (such as fermentation and soaking of grains) to reduce the phytate content and thereby increase zinc bioavailability (80).
Zinc in the treatment of diarrhoea
Zinc supplements given during an episode of acute diarrhoea reduce the duration and severity of the episode (81). Thus, WHO and UNICEF jointly recommend providing zinc in the treatment of diarrhoea along with oral rehydration salts solution (ORS) 82, 83, 84 (Table 7). Programmes to include zinc supplementation in diarrhoea treatment are being rolled out in several countries (85).
Table 7.
Recommended dose and duration of zinc supplementation for use in the treatment of diarrhoea in addition to oral rehydration salts (ORS) (83)
| Age of infant or child | ||
|---|---|---|
| 0–5 months | 6+ months | |
| Dose of zinc supplementation | 10 mg/day | 20 mg/day |
| Duration | 10–14 days | 10–14 days |
Iron and anaemia
Studies have demonstrated the value of preventing and/or treating anaemia among young children, and the importance of commencing preventive activities during prenatal development. Risks associated with iron deficiency include impaired cognitive development, and increased morbidity and mortality 12, 40, 88, 89, 90, 91.
Many nutritional surveys fail to differentiate iron deficiency anaemia from anaemia due to other causes. Specific assessment methods are required to confirm iron deficiency anaemia vs. other causes of anaemia, the term ‘iron deficiency anaemia is often inappropriately used interchangeably with ‘anaemia’ in national surveys that assess the prevalence of anaemia (low haemoglobin concentration of low haematocrit) but not iron status per se (92). This distinction is important because studies have shown that providing iron supplements to individuals who are not iron deficienct can cause severe adverse events, including increasing the risk of mortality (93).
Other nutritional factors involved in the aetiology of anaemia are deficiencies in, vitamin A, folate, vitamin B12 and possibly other micronutrients. Malaria, helminths, and other infections and haemoglobinopathies also play an important role (94).
Assessment of anaemia and iron status
Iron deficiency can be diagnosed by measuring serum ferritin, transferrin receptor or red blood cell zinc‐protoporphyrin concentrations in addition to measuring haemoglobin or haematocrit concentration 95, 96, 97. Iron stores are considered depleted when serum ferritin concentrations are <12 µg L−1 for children less than 5 years and <15 µg L−1 for adults and children more than 5 years of age unless infection is present (98). Because serum ferritin concentration increases in the presence of infection, WHO recommends measuring acute phase proteins, such as C‐reactive protein (CRP) and alpha‐1 acid glycoprotein (AGP), to help interpret data on serum ferritin. The presence of anaemia is assessed by measuring haemoglobin concentration or haematocrit. The cut‐offs for haemoglobin concentration recommended by WHO to define anaemia are: 110 g L−1 for preschool aged children and pregnant women and 120 g L−1 for non‐pregnant women (98). The prevalence of anaemia considered to be a public health problem is: 5–19%, mild public health problem; 20–39%, moderate public health problem; ≥40%, severe public health problem.
When biochemical assessments are not possible, alternative methods have been proposed to detect anaemia using: (i) the ‘Haemoglobin Colour Scale’; and (ii) assessment of extreme pallor in the lower conjunctiva of the eye, fingernail beds or in the palm of the hand 99, 100, 101. However, these assessments are not as accurate as measuring haemoglobin or haematocrit using laboratory methods.
Prevention of iron deficiency and anaemia
Strategies for the prevention and control of iron deficiency and anaemia include the use of iron supplements 96, 99, 102, 103, iron‐fortified foods 96, 104, point‐of‐use fortification (105), helminth control 106, 107, control of malaria and other infections (95) and reproductive and obstetric interventions, such as delayed‐cord clamping 108, 109. Due to the high prevalence of anaemia in the six countries included in the situational analyses 27, 28, 29, 30, 31, 32, iron deficiency is also likely to be prevalent. Therefore, provision of additional iron to infants and young children who are iron deficient should be a public health priority. However, these countries are also malaria‐endemic regions. The WHO recommends exercising caution to ensure that iron supplements (including point‐of‐use fortification products) are only given to iron deficient children, or iron is provided in small doses distributed over the day in processed complementary foods (Box 3, (95)). The WHO further recommends an integrated approach including the control and prevention of malaria, helminths, and other infectious diseases, such as HIV/AIDS and tuberculosis 110, 111.
Box 3. Summary of conclusions and recommendations of the World Health Organization Consultation on prevention and control of iron deficiency in infants and young children in malaria endemic areas (95)
-
1
Strategies to control iron deficiency should be carried out in the context of comprehensive and effective health care, including the provision of insecticide‐treated nets and vector control for the prevention of malaria, and prompt recognition and treatment of malaria and its complications with effective anti‐malarial therapy. They should also include control of other prevalent parasitic diseases and infections and the promotion of exclusive breastfeeding for the first 6 months of life, followed by consumption of nutrient‐dense and/or processed fortified complementary foods.
-
2
Children should be screened for iron deficiency prior to providing iron supplementation.
-
3
Processed complementary foods fortified with iron are an alternative method of providing iron that is likely safe. Although the safety in malaria‐endemic areas has not been documented, this approach has been considered safe because it would avoid the potential adverse effects of a large bolus of iron taken in a single dose, as the iron would be consumed in smaller amounts throughout the day and therefore absorbed more slowly.
-
4
Infants and young children who have malaria and are diagnosed with iron deficiency or severe anaemia, should be treated with an anti‐malarial and, where appropriate, antibiotic therapy as well as iron therapy, which should always be administered with food.
-
5
Iron preparations administered through home fortification of complementary foods for infants and young children, i.e. powders, crushable tablets, and fat‐based spreads, are not yet recommended alternative methods of providing iron until their safety has been confirmed. (Some researchers are evaluating whether it may be possible to administer these products in doses that approximate the amount of iron delivered in fortified complementary foods.)
-
6
The reservations concerning the harmful effects of universal iron supplementation do not diminish the need for adequate iron therapy when iron deficiency is diagnosed.
-
7
Because widespread folate deficiency is not known to be a problem in infants and young children, and supplemental folic acid may interfere with the efficacy of anti‐folate anti‐malarial drug therapy, supplemental folic acid should not be given to infants and young children in areas where anti‐folate anti‐malarial drugs are used (i.e. pyrimethamine, sulphadoxine and their combination in Fansidar).
‘Iron supplements’ refers to medicinal iron supplements given orally to population groups for the prevention and control of iron deficiency. ‘Iron therapy’ refers to medicinal iron supplements given orally for treatment of iron deficiency of individual patients. ‘Iron preparations for home fortification’ refers to iron mixed with foods at home. Such iron preparations may be in the form of a powder, crushable tablet or fat‐based spread. ‘Processed foods fortified with iron’ refers to food fortified with iron during food processing.
Treatment of iron deficiency and anaemia
Recommendations for the treatment of confirmed iron deficiency anaemia are similar for malaria endemic and non‐endemic regions (Table 8) except that folic acid treatment can interfere with some anti‐malaria medications, such as pyrimethamine and sulfadoxine. Therefore, this needs to be taken into consideration in malaria endemic regions to ensure that the folic acid does not prevent effective treatment of malaria.
Table 8.
Guidelines for oral iron and folate therapy to treat iron deficiency anaemia (99)
| Age group | Dose | Duration |
|---|---|---|
| <2 years | 25 mg iron + 100–400 µg folic acid daily | 3 months |
| Adolescents and adults, including pregnant women | 120 mg iron + 400 µg folic acid daily | 3 months |
Iodine
Iodine deficiency during pregnancy increases the risk of spontaneous abortion, stillbirths, and congenital abnormalities (112). Early pregnancy is the most critical period for impaired foetal brain development due to maternal iodine deficiency 113, 114. Iodine deficiency during childhood and adolescence can also affect mental function and cause delayed physical development. These consequences of iodine deficiency, which result from inadequate production of thyroid hormone, are termed iodine‐deficiency disorders (IDDs).
Assessment of iodine status
WHO recommends assessing population iodine status by measuring urinary iodine concentrations in school‐age children (115). The population is considered at risk when the median urinary iodine concentration in school‐aged children falls below 100 µL. Due to variability of urinary iodine concentrations, this is not a reliable method of assessing iodine status in an individual. The presence of an enlarged thyroid, or goitre, is used to assess the severity of iodine deficiency in individuals and the long‐term impact of programmes after there has been sufficient time to prevent the development of goitre in subsequent groups of school‐aged children. The preferred method for assessing thyroid size is ultrasonography. When ultrasonography is not possible, the presence of goitre can also be assessed by inspection and palpation. However, this method has poor sensitivity and specificity and is therefore not ideal in areas of mild iodine deficiency (116). Thyroid‐stimulating hormone is a useful indicator of iodine nutrition in newborns, but not in other age groups. Recently a dried blood spot assay for thyroglobulin concentration has been developed, and reference values for school children have been proposed (116).
Prevention of iodine deficiency
Universal salt iodization is considered to be the most cost‐effective method of controlling IDD (115). The recommended level of iodine in salt, at the point of production, is 20–40 ppm (115). Salt iodization programmes are monitored based on the per cent of households that are using adequately iodized salt, as measured in a nationally representative sample of households. The goal is to reach 90% of households with salt that is iodized to 15–40 ppm (115).
In some countries, however, implementation of salt iodization programmes may not be feasible in all areas, resulting in insufficient access to iodized salt for some population groups (117). In these cases, besides strengthening the salt iodization programmes, complementary strategies should be considered to ensure optimal iodine nutrition for the susceptible groups. In particular, WHO et al. (118) recommend increasing iodine intake by providing iodine supplements (Table 9) or iodine fortified foods to pregnant and lactating women and children 7–24 months of age.
Table 9.
Recommended dosages of daily and annual iodine supplementation (115)
| Population group | Daily dose of iodine supplement (µg/day) | Single annual dose of iodized oil supplement (mg/yr) |
|---|---|---|
| Pregnant women | 250 | 400 |
| Lactating women | 250 | 400 |
| Women of reproductive age (15–49 years) | 150 | 400 |
| Children <2 years*, † | 90 | 200 |
For children 0–6 months of age, iodine supplementation should be given through breast milk. This implies that the child is exclusively breastfed and that the lactating mother received iodine supplements as indicated above.
These figures for iodine supplements are given in situations where complementary food fortified with iodine is not available, in which case iodine supplementation is required for children of 7–24 months of age.
Treatment of iodine deficiency
In diagnosed cases of iodine deficiency, the WHO recommends that iodized oil can be used to promptly correct hypothyroidism (119).
Other nutrition support: management of acute malnutrition, ensuring food security, prevention of mother‐to‐child transmission (PMTCT) of HIV, and promotion of improved personal hygiene, and environmental sanitation
Management of acute malnutrition
The Global Strategy for Infant and Young Child Feeding (33) recommends actively searching for malnourished infants and young children to permit early treatment. For the purposes of this report we will not discuss the specialized treatment guidelines for moderate and severe acute malnutrition (SAM), but will summarize the current recommendations for screening to detect acute malnutrition.
Growth monitoring
The international growth reference standards have been updated from the 1977 NCHS reference (120) to the 2006 WHO Child Growth Standards (121). The WHO recommends replacing the 1977 NCHS reference with these new standards. This also requires updating anthropometric survey data from older surveys to allow comparisons across time (122). The WHO provides reference charts and tables of the new growth standards for use in screening for malnutrition and in growth monitoring (http://www.who.int/childgrowth/en/).
The cut‐offs for SAM are higher and therefore more inclusive when using the new 2006 WHO Child Growth Standards 123, 124. Thus, more children are considered eligible for nutrition rehabilitation programmes when the WHO standards are applied than when the previous NCHS reference data were applied. Initially, this could place a strain on existing programmes as greater numbers of children are enrolled during the period of transition to the new WHO standards. However, researchers have found a positive impact on treatment outcomes when children are enrolled sooner because a shorter duration of treatment is required and a greater rate of recovery is likely to occur (123). Further, these children have fewer medical complications requiring inpatient care and lower mortality rates. Thus, the initial increased costs of treating the additional children identified with SAM may be offset by the reduced duration of treatment and lower costs for hospitalization.
Assessment and screening of acute malnutrition
The WHO has recently adopted new recommendations for the screening of moderate acute malnutrition (MAM) and SAM. Guidelines are available for the treatment of MAM and SAM among infants and children, whether in specialized care facilities or in the community 125, 126, 127, 128, 129, 130. International recommendations are to refer all infants and children with MAM or SAM with clinical complications, such as systemic infection, anorexia, or bilateral edema, to a hospital or nutrition rehabilitation centre where they can be observed, treated, and fed according to standardized protocols (10, 11).
Table 10.
Latest recommended criteria for assessing severe acute malnutrition (SAM) (130)
| Condition | Indicator | Cut‐off |
|---|---|---|
| Severe wasting † | Weight‐for‐height* | <−3 SD |
| Severe wasting † | MUAC | <115 mm |
| Bilateral oedema † | Clinical sign |
MUAC, mid‐upper arm circumference; SD, standard deviation. *Based on WHO Growth Standards (121). †bilateral oedema is an indicator of SAM independent of the anthropometric measures of low weight‐for‐height and/or low MUAC; the presence of bilateral oedema and/or severe wasting indicate the need for urgent action.
Table 11.
Classification of type and severity of malnutrition (125), World Health Organization (WHO) child growth standards (121)
| Indicator | Classification | |
|---|---|---|
| Moderate malnutrition | Severe malnutrition (type)* | |
| Symmetrical oedema | Not present | Present (oedematous malnutrition) |
| Weight‐for‐height | −3 < SD‐score <−2 † (or 70–79%) ‡ | SD‐score <−3 (or <70%) (severe wasting) |
| Height‐for‐age | −3 < SD‐score <−2 (or 85–89%) ‡ | SD‐score <−3 (or <85%) (severe stunting) |
The diagnoses are not mutually exclusive.
† Below the median World Health Organization (WHO) 2006 reference.
‡ Percentage of the median WHO 2006 reference.
Due to the successes found in some community‐based malnutrition treatment programmes, the SCN Informal Consultation determined that children 6–59 months of age with uncomplicated SAM can be treated in communities when there is access to ready‐to‐use therapeutic foods (RUTFs) (128) (Fig. 7). The effectiveness of these programmes is being tested in various countries (129). Children with MAM should be included in these community treatment programmes as resources are available.
Figure 7.
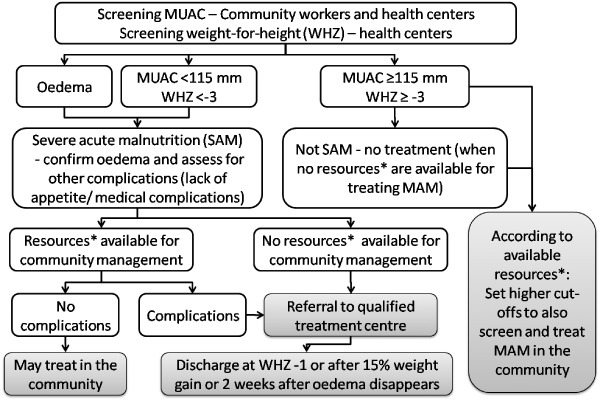
Decision tree for assessing, referring and/or treating severe acute malnutrition in the community when resources are not sufficient to also allow treatment of moderate malnutrition, based on recommendations from references 128, 130. *Resources include ready‐to‐use foods (RUTF). Figure based on recommendations from: WHO child growth standards and the identification of severe acute malnutrition in infants and children, 2009 and Community‐based management of severe acute malnutrition, May 2007; recommendations for children 6–60 months. MAM, moderate acute malnutrition; MUAC, mid‐upper arm circumference; WHO, World Health Organization.
Accurate assessment of malnutrition by weight‐for‐height requires training, practice, proper equipment and detailed international growth standards tables for comparison. Because these resources are not always available, particularly for screening, United Nations organizations also recommend measuring mid‐upper arm circumference (MUAC) using colour‐coded plastic tapes for assessing malnutrition among children 6–59 months 127, 128. The recommended MUAC cut‐off has recently changed from 110 to 115 mm (11.5 cm) for SAM (130). The new recommended criterion for MAM is a MUAC between 115 and 125 mm. Because the two screening methods, MUAC, and weight‐for‐height, each capture a certain number of high‐risk acutely malnourished children who are not captured by the other screening method (131), it is recommended to enrol children in treatment programmes who are eligible by either criterion (130).
Iron in the treatment of malnutrition
The use of iron in the treatment of acute malnutrition is of particular concern due to the health risk of providing iron during the initial stabilization phase (125). The 1998 WHO guidelines for the management of acute malnutrition assume that most children with malnutrition are iron deficient. Therefore, it is recommended to introduce iron supplementation in the second phase of treatment, after infections are successfully controlled. In the report of the WHO, UNICEF, WFP, UNHCR Consultation on the dietary management of moderate malnutrition in children <5 years (132), Golden (133) and Michaelsen et al. (134) recommend moderate amounts of iron in treating moderately acute malnourished children 6–59 months of age. Golden (133) proposed two sets of recommendations based on whether the iron requirements were to be met by unfortified local foods or with foods fortified specifically for use in treatment programmes for moderately acutely malnourished children. The proposed target dose of iron was 9 mg/1000 kcal for non‐fortified local diets, and 18 mg/1000 kcal when the food is specially fortified for this purpose. It is recommended to avoid therapeutic doses of iron when treating children with MAM. Updated recommendations specific to iron in the treatment of children with severe malnutrition in malaria endemic regions are not yet available.
PMTCT
It is estimated that 5% of adults in Sub‐Saharan Africa are infected with HIV. The prevalence of HIV among adults in the countries included in this issue's situational analyses is lower than the continent‐wide figure and generally ranges from 0.8 to 1.6%, except in Chad where the prevalence is 3.5%. The international recommendations surrounding the optimal feeding methods to prevent transmission of HIV mother‐to‐child (PMTCT) have recently been revised, as summarized in Box 4, 135, 136, 137, 138, 139.
Box 4. Selected excerpts from key principles in developing feeding recommendations, and the key feeding recommendations for infants of HIV positive women, from the World Health Organization (WHO) (136)
Selected key principles:
-
•
(Key Principle 4) Pregnant women and mothers known to be HIV‐infected should be informed of the infant feeding strategy recommended by the national or sub‐national authority to improve HIV‐free survival of HIV‐exposed infants and the health of HIV‐infected mothers, and informed that there are alternatives that mothers might wish to adopt;
-
•
(Key Principle 5) Skilled counselling and support in appropriate infant feeding practices and antiretroviral (ARV) interventions to promote HIV‐free survival of infants should be available to all pregnant women and mothers;
-
•
(Key Principle 6) Counselling and support to mothers known to be HIV infected, and health messaging to the general population, should be carefully delivered so as not to undermine optimal breastfeeding practices among the general population;
-
•
(Key Principle 7) Mothers who are known to be HIV uninfected or whose HIV status is unknown should be counselled to exclusively breastfeed their infants for the first 6 months of life and then introduce complementary foods while continuing breastfeeding for 24 months or beyond.
-
•
Mothers whose status is unknown should be offered HIV testing.
-
•
Mothers who are HIV uninfected should be counselled about ways to prevent HIV infection and about the services that are available such as family planning to help them to remain uninfected.
Selected recommendations:
-
•
(Recommendation 1) Mothers known to be HIV‐infected should be provided with lifelong ARV therapy or ARV prophylaxis interventions to reduce HIV transmission through breastfeeding, specific guidelines are clarified in: (138).
-
•
(Recommendation 2) Mothers known to be HIV‐infected (and whose infants are HIV uninfected or of unknown HIV status) should exclusively breastfeed their infants for the first 6 months of life, introducing appropriate complementary foods thereafter. Continuation of breastfeeding from 6 to 12 months is also recommended, but there is stronger evidence for continuation of breastfeeding during this age‐range when accompanied by ARV than when no ARV treatment is available for the uninfected infant.
-
•
(Recommendation 3) Mothers known to be HIV‐infected who decide to stop breastfeeding at any time should stop gradually within one month. Mothers or infants who have been receiving ARV prophylaxis should continue prophylaxis for one week after breastfeeding is fully stopped.
-
•
Stopping breastfeeding abruptly is not advisable.
-
•
-
•
(Recommendation 4) When mothers known to be HIV‐infected decide to stop breastfeeding at any time, infants should be provided with safe and adequate replacement feeds to enable normal growth and development.
-
•
Alternatives to breastfeeding include:
-
•
For infants less than 6 months of age:
-
○
Commercial infant formula milk as long as home conditions outlined in Recommendation #5 below are fulfilled,
-
○
Expressed, heat‐treated breast milk (see Recommendation #6 below),
Home‐modified animal milk is not recommended as a replacement food in the first 6 months of life.
-
○
-
•
For children over 6 months of age:
-
○
Commercial infant formula milk as long as home conditions outlined in Recommendation #5 below are fulfilled,
-
○
Animal milk (boiled for infants under 12 months), as part of a diet providing adequate micronutrient intake.
-
○
Meals, including milk‐only feeds, other foods and combination of milk feeds and other foods, should be provided four or five times per day (36).
-
○
-
•
-
•
All children need complementary foods from 6 months of age.
-
•
-
•
(Recommendation 5) Mothers known to be HIV‐infected should only give commercial infant formula milk as a replacement feed to their HIV uninfected infants or infants who are of unknown HIV status, when specific conditions are met (referred to as AFASS – affordable, feasible, acceptable, sustainable, and safe in the 2006 WHO recommendations on HIV and Infant Feeding (139)):
-
•
safe water and sanitation are assured at the household level and in the community, and,
-
•
the mother, or other caregiver can reliably provide sufficient infant formula milk to support normal growth and development of the infant, and,
-
•
the mother or caregiver can prepare it cleanly and frequently enough so that it is safe and carries a low risk of diarrhoea and malnutrition, and,
-
•
the mother or caregiver can, in the first 6 months, exclusively give infant formula milk, and,
-
•
the family is supportive of this practice and,
-
•
the mother or caregiver can access health care that offers comprehensive child health services.
-
•
-
•
(Recommendation 6) Mothers known to be HIV‐infected may consider expressing and heat‐treating breast milk as an interim feeding strategy:
-
•
In special circumstances such as when the infant is born with low birth weight or is otherwise ill in the neonatal period and unable to breastfeed; or
-
•
When the mother is unwell and temporarily unable to breastfeed or has a temporary breast health problem such as mastitis; or
-
•
To assist mothers to stop breastfeeding; or
-
•
If ARV drugs are temporarily not available.
-
•
-
•
(Recommendation 7) If infants and young children are known to be HIV‐infected, mothers are strongly encouraged to exclusively breastfeed for the first 6 months of life and continue breastfeeding, as per the recommendations for the general population that is up to two years or beyond.
It is notable that many of these recommendations are the same as the general feeding recommendations for infants and young children.
Support for food insecure populations
Food security is based on food availability, accessibility and utilization. At the household level, food security is guaranteed when livelihoods are sustainable and not easily affected by external shock. In the Sahel, regional organizations, including the Inter State Committee to Fight Drought in the Sahel, the WFP of the United Nations, and other partners have developed methods to evaluate the food availability at regional and national levels. An example is the Emergency Food Security Assessment methodology 140, 141 that appraises household food accessibility, resilience and food diversity. These evaluations allow decision makers to develop appropriate recommendations that target food insecure populations and regions through interventions such as food aid, subsidized food sales, food for work, cash for work, and restoration and strengthening of livelihoods. Integrated approaches are carried out to support families affected by malnutrition through nutrition interventions, such as nutrition and hygiene education and promotion of local food production.
Anthropometric assessments of preschool children are commonly included in surveys to assess the population's nutritional status and level of food insecurity. However, changes in the food availability can occur more rapidly than changes in anthropometric indicators, and malnutrition can be caused by both food insecurity and lack of access to adequate health care, along with other causes. Therefore, a variety of rapid assessment techniques have been developed to find and target those at highest risk during periods of food insecurity 140, 141, 142, 143, 144, 145, 146, 147.
The objectives of rapid assessments are to increase awareness of critical needs and improve intervention prioritization, particularly in resource poor situations. We found no specific international recommendations regarding a best method of rapidly assessing the nutritional situation of young children. However, there is general recognition of the need for these assessments to identify groups at risk of food insecurity, including those with HIV/AIDS, who need supplementary preventive and curative support during various crises (145).
There are also a variety of activities to prevent and/or respond to food insecurity, such as ensuring the availability of adequate stocks of basic food items and/or therapeutic foods in case of emergencies. For the purposes of the situational analyses in this issue, we only evaluated whether responses are planned and available, but not the type of response.
Optimal hygienic practices related to food, water, and the household environment
The importance of good water quality, sanitation, and hygiene is well established. Fewtrell et al. (148) conducted meta‐analyses of interventions focused on improving hygiene, sanitation, water supply, and water quality. Based on relative risk estimates among available scientifically sound studies, they found that handwashing had the most impact on reducing diarrhoea (44% reduction) compared with non‐intervention. Other interventions with a beneficial impact on reducing diarrhoea included water treatment at the point of use (35% reduction), multiple interventions (33% reduction), overall improvement in sanitation (32% reduction), overall improvement in water supply (25% reduction) and water treatment at the source, such as chemical treatment, boiling, pasteurization, and solar disinfection (11% reduction) (148). The importance of hand hygiene was also address by the WHO in a recently developed guidebook for hand hygiene in health care settings (149), and UNICEF joined the Global Public‐Private Partnership for Handwashing with Soap in support of this important practice for preventing the spread of disease (http://www.globalhandwashing.org). Additional guidelines promoted by international organizations to improve hygienic practices are listed in Box 5. For the situational analysis, we did not search for documents specifically related to sanitation and hygiene, but reviewed whether the points noted in Box 5 were addressed in the nutrition‐related documents. Therefore, some countries may have relevant programmes and activities in addition to those we identified.
Box 5. Key practices related to sanitation and hygiene are 150, 151
-
1
Dispose of faeces safely (toilet/latrine/buried sufficiently to prevent resurfacing),
-
2
Wash hands thoroughly with soap (or ash) and water after contact with faeces, before touching food, and before feeding children,
-
3
Wash the face with soap and water every day to help prevent eye infections
-
4
Use water from a safe source or that is purified and keep it covered
-
5
Wash or cook raw foods, reheat cooked foods thoroughly prior to eating and do not delay the consumption of these foods after their preparation,
-
6
Keep food, utensils and food preparation surfaces clean and store foods in covered containers,
-
7
Safely dispose of all refuse to prevent illness.
Overall organizational and programmatic support
To coordinate activities among various groups and provide guidance to national IYCN programmes, it is recommended to organize an official national committee(s) or commission(s) composed of representatives of relevant government ministries, international and bi‐lateral agencies, non‐governmental organizations, private sector companies, and civil society organizations. (2). The recommended frequency of these group meetings is at least semi‐annual, and more frequently as the situation might require. The purpose is to share information on current activities, avoid duplication of efforts and exchange technical or other assistance according to each agency's expertise. The global REACH initiative: Ending Child Hunger and Undernutrition (http://www.reach-partnership.org), has developed a toolkit for organizations ready to move forward with developing and strengthening multi‐sector partnerships with systematic planning and implementation of nutrition‐related activities. The planning guide by WHO and UNICEF also provides more specific guidance for national implementation of the Global Strategy for Infant and Young Child Feeding (34).
Summary
Countries in the Sahel region of Sub‐Saharan Africa have made limited or no progress towards improving infant and young child nutritional status and in reaching the related Millennium Development Goals.
As a first step towards furthering the fight against high rates of malnutrition in the Sahel, a situational analysis was commissioned to evaluate national policies, strategies, plans of action, research, training materials, programmes, surveys and monitoring, and evaluation activities in six countries (Burkina Faso, Chad, Mali, Mauritania, Niger and Senegal).
Key activities that have been found to be effective at improving infant and young child nutrition and are recommended internationally are: the promotion of optimal breastfeeding and complementary feeding practices, prevention and/or treatment of micronutrient deficiencies, management of acute malnutrition, prevention of mother‐to‐child transmission of HIV, promotion of food security and optimal hygienic practices, and support for national organizations charged with carrying out these activities.
Supporting information
Table S1. Appendix of selected internationally recognized guides to develop national IYCN policies and programmes, with internet links
Supporting info item
Footnotes
Goal 1 indicators include: Target 1a: Reduce by half the proportion of people living on less than a dollar a day: 1.1 Proportion of population below $1 (PPP) per day, and Target 1c: Reduce by half the proportion of people who suffer from hunger: 1.8 Prevalence of underweight children under‐5 years of age (http://www.undp.org/mdg/).
Goal 4 Indicators include: Target 4a: Reduce by two‐thirds the mortality rate among children under five: 4.1 – under‐five mortality rate; 4.2 –Infant mortality rate; 4.3 – proportion of 1‐year‐old children immunized against measles (http://www.undp.org/mdg/).
References
- 1. World Health Organization (2008) WHO Statistical Information System (WHOSIS). World Health Organization: Geneva. [cited 28 August 2008]; Available at: http://www.who.int/whosis/en/ [Google Scholar]
- 2. World Health Organization (2003) Infant and Young Child Feeding: A Tool for Assessing National Practices, Policies and Programs. World Health Organization: Geneva. [Google Scholar]
- 3. Global Database on Child Growth and Malnutrition (2010) Child Malnutrition Estimates by WHO Child Growth Standards [Database on the Internet]. World Health Organization: Geneva. Available at: http://www.who.int/nutgrowthdb/database/en/ [Google Scholar]
- 4. World Bank (2006) Repositioning Nutrition as Central to Development: A Strategy for Large‐Scale Action. The International Bank for Reconstruction and Development/The World Bank: Washington, DC. [Google Scholar]
- 5. Institut National de la Statistique et de la Démographie (INSD) Burkina Faso, United Nations Children's Fund (UNICEF) (2006) Multiple Indicator Cluster Survey (MICS) Burkina Faso.
- 6. Ouagadjio B., Nodjimadji K., Bagamla T., Madnodji R., Sibaye Tokindang J., Ngakoutou N. et al (2004) Enquête Démographique et de Santé Tchad 2004. INSEED et ORC Macro: Calverton, Maryland. [Google Scholar]
- 7. Cellule de Planification et de Statistique du Ministère de la Santé (CPS/MS) – Mali, Direction Nationale de la Statistique et de l’Informatique du Ministère de l’Économie de l’Industrie et du Commerc (DNSI/MEIC), Inc. MI. (2007) Enquête Démographique et de Santé du Mali 2006: Calverton, Maryland.
- 8. Office National de la Statistique (ONS) (2008) Mauritanie Enquête par Grappe à Indicateurs Multiples 2007 (MICS).
- 9. République du Niger, Institut National de la Statistique – Niger, Ministère de l'Économie et des Finances – Niger, Macro International Inc., United States Agency for International Development (USAID), Coordination Inter‐Sectorielle de Lutte contre les IST/VIH/SIDA (CISLS) – Niger et al (2007) Enquête Démographique et de Santé et à Indicateurs Multiples 2006. Available at: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=29
- 10. République du Sénégal, Ministère de la Santé et de la Prévention Médicale – Sénégal, Centre de Recherche pour le Développement Humain, Ndiaye S, Ayad M, ORC Macro (2005) Enquête Démographique et de la Santé Sénégal.
- 11. United Nations Children's Fund (UNICEF) (1990) A UNICEF Policy Review: Strategy for Improved Nutrition of Children and Women in Developing Countries. Available at: http://www.ceecis.org/iodine/01_global/01_pl/01_01_other_1992_unicef.pdf [DOI] [PubMed]
- 12. Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- 13. Bryce J., Boschi‐Pinto C., Shibuya K. & Black R.E. (2005) WHO estimates of the causes of death in children. Lancet 365, 1147–1152. [DOI] [PubMed] [Google Scholar]
- 14. Office of the United Nations High Commissioner for Human Rights (1989) Convention on the Rights of the Child: Adopted and Opened for Signature, Ratification and Accession by General Assembly, Resolution 44/25 of 20 November 1989, Entry into Force 2 September 1990, in Accordance with Article 49.
- 15. World Health Organization (2005) Health and the Millennium Development Goals. World Health Organization: Geneva. Available at: http://www.who.int/hdp/publications/en/ [Google Scholar]
- 16. World Health Organization (WHO) (1981) International Code of Marketing of Breast‐Milk Substitutes. World Health Organization: Geneva. Available at: http://www.unicef.org/nutrition/index_24805.html [Google Scholar]
- 17. Sanghvi T., Van Ameringen M., Baker J., Fiedler J., Borwankar R., Phillips M. et al (2007) Vitamin and mineral deficiencies technical situation analysis: a report for the Ten Year Strategy for the Reduction of Vitamin and Mineral Deficiencies. Food and Nutrition Bulletin (1 Suppl. Vitamin), S160–S219. [PubMed] [Google Scholar]
- 18. United Nations Children's Fund (UNICEF) (2006) REACH: Ending Child Hunger and Undernutrition Initiative: Oral Report. [cited 1 April 2008]; Available at: http://www.unicef.org/about/execboard/files/Ending_Child_Hunger_background_note.pdf
- 19. International Labor Organization (2007) Safe Maternity and the World of Work. International Labor Organization; Geneva. Available at: http://www.ibfan.org/site2005/Pages/article.php?art_id=550&iui=1&goto_news=1 [Google Scholar]
- 20. International Covenant on Economic, Social and Cultural Rights (1976) International Covenant on Economic, Social and Cultural Rights, Adopted and Opened for Signature, Ratification and Accession by General Assembly Resolution 2200A (XXI) of 16 December 1966, Entry into Force 3 January 1976, in Accordance with Article 27. Available at: http://www2.ohchr.org/english/law/pdf/cescr.pdf
- 21. Convention on the Elimination of All Forms of Discrimination against Women (1979) Convention on the Elimination of All Forms of Discrimination against Women New York, 18 December. Available at: http://www2.ohchr.org/english/law/cedaw.htm
- 22. Maternity Protection Convention (2000) The General Conference of the International Labour Organization. ILO Convention, C183 Maternity Protection Convention. Geneva 2000.
- 23. IBFAN Africa (2007) Status of the Code in Africa – 2006.
- 24. IBFAN Africa Mali Ratifies C183. Available at: http://www.ibfan.org/site2005/Pages/article.php?art_id=550&iui=1&goto_news=1
- 25. Department of Economic and Social Affaires of the United Nations Secretariat, International Labour Organization, Food and Agriculture Organization of The United Nations (FAO), United Nations Educational Scientific and Cultural Organization, World Health Organization (WHO), The World Bank et al (2009) The Millennium Development Goals Report 2008.
- 26. United Nations Children's Fund (UNICEF) (2009) Childinfo: Monitoring the Situation of Children and Women, Infant Feeding Patterns by Country. [updated December 2008; cited 2 February 2009]; Available at: http://www.childinfo.org/breastfeeding_infantfeeding.html
- 27. Wuehler S.E. & Biga Hassoumi A. (2011) Situational analysis of infant and young child nutrition policies and programmatic activities in Niger. Maternal & Child Nutrition 7 (Suppl. 1), in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wuehler S.E. & Coulibaly M. (2011) Situational analysis of infant and young child nutrition policies and programmatic activities in Mali. Maternal & Child Nutrition 7 (Suppl. 1), in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wuehler S.E. & Djasndibye N. (2011) Situational analysis of infant and young child nutrition policies and programmatic activities in Chad. Maternal & Child Nutrition 7 (Suppl. 1), in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wuehler S.E. & Ly Wane C.T. (2011) Situational analysis of infant and young child nutrition policies and programmatic activities in Senegal. Maternal & Child Nutrition 7 (Suppl. 1), in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wuehler S.E. & Ouedraogo A.W. (2011) Situational analysis of infant and young child nutrition policies and programmatic activities in Burkina Faso. Maternal & Child Nutrition 7 (Suppl. 1), in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wuehler S.E. & Ould Dehah C.M.E.H. (2011) Situational analysis of infant and young child nutrition policies and programmatic activities in the Islamic Republic of Mauritania. Maternal & Child Nutrition 7 (Suppl. 1), in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization (WHO), United Nations Children's Fund (UNICEF) (2003) Global Strategy for Infant and Young Child Feeding [Electronic]. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/topics/global_strategy/en/index.html [Google Scholar]
- 34. World Health Organization, UNICEF (2007) Planning Guide for National Implementation of the Global Strategy for Infant and Young Child Feeding. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/topics/global_strategy/en/index.html [Google Scholar]
- 35. Pan American Health Organization, World Health Organization (WHO) (2004) Guiding Principles for Complementary Feeding of the Breastfed Child. Available at: http://www.who.int/nutrition/publications/guiding_principles_compfeeding_breastfed.pdf
- 36. Department of Child and Adolescent Health and Development (CAH), World Health Organization (WHO) (2005) Guiding Principles for Feeding Non‐Breastfed Children 6–24 Months of Age. World Health Organization: Geneva. Available at: http://whqlibdoc.who.int/publications/2005/9241593431.pdf [Google Scholar]
- 37. Acharya K., Sanghvi T., Diene S., Stapleton V., Seumo E., Srikantiah S. et al (2004) Using ‘Essential Nutrition Actions’ to Accelerate Coverage with Nutrition Interventions in High Mortality Settings. USAID: Washington, DC. Available at: http://www.basics.org/documents/pdf/Using%20ENA.pdf [Google Scholar]
- 38. Basic Support for Institutionalizing Child Survival Project (BASICS II), U.S. Agency for International Development (USAID) (2003) Tools for Operationalizing Essential Nutrition Actions. Available at: http://www.basics.org/documents/ENA%20Toolkit_2003.pdf#search="essential_nutrition_actions"
- 39. Linkages Project (2009) Using the Essential Nutrition Actions to Improve the Nutrition of Infants, Young Children and Women: A Four Day Training Course for Program Managers and Pre‐service Instructors. [cited 15 January 2009]; Available at: http://www.linkagesproject.org/tools/training/essentialnutrition.php
- 40. Bhutta Z.A., Ahmed T., Black R.E., Cousens S., Dewey K., Giugliani E. et al (2008) What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440. [DOI] [PubMed] [Google Scholar]
- 41. Bryce J., Coitinho D., Darnton‐Hill I., Pelletier D. & Pinstrup‐Andersen P. (2008) Maternal and child undernutrition: effective action at national level. Lancet 371, 510–526. [DOI] [PubMed] [Google Scholar]
- 42. Morris S.S., Cogill B. & Uauy R. (2008) Effective international action against undernutrition: why has it proven so difficult and what can be done to accelerate progress? Lancet 371, 608–621. [DOI] [PubMed] [Google Scholar]
- 43. Roth D.E., Caulfield L.E., Ezzati M. & Black R.E. (2008) Acute lower respiratory infections in childhood: opportunities for reducing the global burden through nutritional interventions. Bulletin of the World Health Organization 86, 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edmond K.M., Zandoh C., Quigley M.A., Amenga‐Etego S., Owusu‐Agyei S. & Kirkwood B.R. (2006) Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics 117, e380–e386. [DOI] [PubMed] [Google Scholar]
- 45. Mihrshahi S., Ichikawa N., Shuaib M., Oddy W., Ampon R., Dibley M.J. et al (2007) Prevalence of exclusive breastfeeding in Bangladesh and its association with diarrhoea and acute respiratory infection: results of the multiple indicator cluster survey 2003. Journal of Health, Population, and Nutrition 25, 195–204. [PMC free article] [PubMed] [Google Scholar]
- 46. Mihrshahi S., Oddy W.H., Peat J.K. & Kabir I. (2008) Association between infant feeding patterns and diarrhoeal and respiratory illness: a cohort study in Chittagong, Bangladesh. International Breastfeed Journal of. 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koyanagi A., Humphrey J.H., Moulton L.H., Ntozini R., Mutasa K., Iliff P. et al (2009) Effect of early exclusive breastfeeding on morbidity among infants born to HIV‐negative mothers in Zimbabwe. The American Journal of Clinical Nutrition 89, 1375–1382. [DOI] [PubMed] [Google Scholar]
- 48. Quigley M.A., Kelly Y.J. & Sacker A. (2007) Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics 119, e837–e842. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organization (WHO), United Nations Children's Fund (UNICEF) (2009) Baby‐Friendly Hospital Initiative: Revised, Updated and Expanded for Integrated Care. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/publications/infantfeeding/9789241594950/en/index.html [PubMed] [Google Scholar]
- 50. Bhandari N., Bahl R., Mazumdar S., Martines J., Black R.E. & Bhan M.K. (2003) Effect of community‐based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 361, 1418–1423. [DOI] [PubMed] [Google Scholar]
- 51. Bhandari N., Kabir A.K. & Salam M.A. (2008) Mainstreaming nutrition into maternal and child health programmes: scaling up of exclusive breastfeeding. Maternal & Child Nutrition 4 (Suppl. 1), 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. World Health Organization (WHO), United Nations Children's Fund (UNICEF), Academy for Educational Development (AED) (2008) Learning from Large‐Scale Community‐Based Programmes to Improve Breastfeeding Practices. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/publications/infantfeeding/9789241597371/en/index.html [Google Scholar]
- 53. Brown K.H. (2007) Breastfeeding and complementary feeding of children up to 2 years of age. Nestle Nutrition Workshop Series Paediatric Programme 60, 1–10. discussion 3. [DOI] [PubMed] [Google Scholar]
- 54. Brown K.H., Dewey K.G. & Allen L.H. (1998) Complementary feeding of young children in developing countries – A review of current scientific knowledge. Geneva: World Health Organization.
- 55. Dewey K.G. & Brown K.H. (2003) Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food and Nutrition Bulletin 24, 5–28. [DOI] [PubMed] [Google Scholar]
- 56. Aguayo V.M., Garnier D. & Baker S.K. (2007) Drops of life: vitamin A supplementation for child survival. Progress and lessons learned in west and central Africa. UNICEF Regional Office for West and Central Africa.
- 57. Beaton G.H., Martorell R., Aronson K.A., Edmonston B., McCabe G., Ross A.C. et al (1994) Vitamin A supplementation and child morbidity and mortality in developing countries. Food and Nutrition Bulletin 15, 4. [Google Scholar]
- 58. Fawzi W.W., Chalmers T.C., Herrera M.G. & Mosteller F. (1993) Vitamin A supplementation and child mortality. A meta‐analysis. The Journal of the American Medical Association 269, 898–903. [PubMed] [Google Scholar]
- 59. Glasziou P.P. & Mackerras D.E. (1993) Vitamin A supplementation in infectious diseases: a meta‐analysis. BMJ 306, 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones G., Steketee R.W., Black R.E., Bhutta Z.A. & Morris S.S. (2003) How many child deaths can we prevent this year? Lancet 362, 65–71. [DOI] [PubMed] [Google Scholar]
- 61. Ramakrishnan U. & Martorell R. (1998) The role of vitamin A in reducing child mortality and morbidity and improving growth. Salud publica de Mexico 40, 189–198. [DOI] [PubMed] [Google Scholar]
- 62. Ross D.A., Kirkwood B.R., Binka F.N., Arthur P., Dollimore N., Morris S.S. et al (1995) Child morbidity and mortality following vitamin A supplementation in Ghana: time since dosing, number of doses, and time of year. American Journal of Public Health 85, 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Semba R.D., Ndugwa C., Perry R.T., Clark T.D., Jackson J.B., Melikian G. et al (2005) Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus‐infected children in Uganda: a controlled clinical trial. Nutrition 21, 25–31. [DOI] [PubMed] [Google Scholar]
- 64. Vijayaraghavan K. (1991) Vitamin A supplementation and child morbidity and mortality. Indian Pediatrics 28, 707–711. [PubMed] [Google Scholar]
- 65. World Health Organization (WHO), United Nations Children's Fund (UNICEF), International Vitamin A Consultative Group (IVACG) (1997) Vitamin A Supplements: A Guide to Their Use in the Treatment and Prevention of Vitamin A Deficiency and Xerophthalmia. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/publications/micronutrients/vitamin_a_deficieny/9241545062/en/index.html [Google Scholar]
- 66. Schultink W. (2002) Use of under‐five mortality rate as an indicator for vitamin A deficiency in a population. The Journal of Nutrition 132, 2881S–2883S. [DOI] [PubMed] [Google Scholar]
- 67. Alnwick D., Dary O., Sommer A., Davidson F.R., Tontisirin K., Smitasiri S. et al (2002) IVACG statement: the annecy accords to assess and control vitamin A deficiency summary of recommendations and clarifications. Annecy, FRANCE: International Vitamin A Consultative Group (IVACG).
- 68. Sommer A. & Davidson F.R. (2002) Assessment and control of vitamin A deficiency: the Annecy Accords. The Journal of Nutrition 132 (9 Suppl.), 2845S–2850S. [DOI] [PubMed] [Google Scholar]
- 69. Brown K.H., Rivera J.A., Bhutta Z., Gibson R.S., King J.C., Lonnerdal B. et al (2004) International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin (1 Suppl. 2), S99–203. [PubMed] [Google Scholar]
- 70. Brown K.H., Hess S.Y., Boy E., Gibson R.S., Horton S., Osendarp S.J. et al (2009) Setting priorities for zinc‐related health research to reduce children's disease burden worldwide: an application of the Child Health and Nutrition Research Initiative's research priority‐setting method. Public Health Nutrition 12, 389–396. [DOI] [PubMed] [Google Scholar]
- 71. de Benoist B., Darnton‐Hill I., Davidsson L., Fontaine O. & Hotz C. (2007) Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG Interagency Meeting on Zinc Status Indicators. Food and Nutrition Bulletin 28, (3 Suppl.), S480–S484. [DOI] [PubMed] [Google Scholar]
- 72. Hess S.Y., Peerson J.M., King J.C. & Brown K.H. (2007) Use of serum zinc concentration as an indicator of population zinc status. Food and Nutrition Bulletin 28, S403–S429. [DOI] [PubMed] [Google Scholar]
- 73. Lowe N., Fekete K. & Decsi T. (2009) Methods of assessment of zinc status in humans: a systematic review. The American Journal of Clinical Nutrition 89, 2040S–2051S. [DOI] [PubMed] [Google Scholar]
- 74. Hotz C. (2007) Dietary indicators for assessing the adequacy of population zinc intakes. Food and Nutrition Bulletin 28, S430–S453. [DOI] [PubMed] [Google Scholar]
- 75. World Health Organization (WHO). World Health Organization (1995) Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. Technical Report Series No. 854. World Health Organization: Geneva. [PubMed] [Google Scholar]
- 76. Bhutta Z.A., Black R.E., Brown K.H., Gardner J.M., Gore S., Hidayat A. et al (1999) Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. Journal of Pediatrics 135, 689–697. [DOI] [PubMed] [Google Scholar]
- 77. Brown K.H., Peerson J.M., Baker S.K. & Hess S.Y. (2009) Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food and Nutrition Bulletin 30, 1S. [DOI] [PubMed] [Google Scholar]
- 78. Brown K.H., Baker S.K., IZiNCG Steering Committee (2009) Galvanizing action: conclusions and next steps for mainstreaming zinc interventions in public health programs. Food and Nutrition Bulletin 30 (1 (Suppl.), S179–S184. [DOI] [PubMed] [Google Scholar]
- 79. Hotz C. (2009) The potential to improve zinc status through biofortification of staple food crops with zinc. Food and Nutrition Bulletin 30 (1 Suppl.), S172–S178. [DOI] [PubMed] [Google Scholar]
- 80. Gibson R.S. & Anderson V.P. (2009) A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food and Nutrition Bulletin 30 (1 Suppl.), S108–S143. [DOI] [PubMed] [Google Scholar]
- 81. Haider B.A. & Bhutta Z.A. (2009) The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food and Nutrition Bulletin 30 (1 Suppl.), S41–S59. [DOI] [PubMed] [Google Scholar]
- 82. United States Agency for International Development (USAID) from the American People, United Nations Children's Fund (UNICEF), World Health Organization (WHO) (2009) Diarrhoea treatment guidelines including new recommendations for the use of ORS and zinc supplementation for clinic‐based healthcare workers (Not yet field‐tested) 2005. 24 February.
- 83. World Health Organization (WHO), United Nations Children's Fund (UNICEF) (2004) WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. World Health Organization: Geneva. [Google Scholar]
- 84. World Health Organization (WHO), United Nations Children's Fund (UNICEF), Johns Hopkins Bloomberg School of Public Health, U.S. Agency for International Development (USAID) (2006) Implementing the New Recommendations on the Clinical Management of Diarrhoea: Guidelines for Policy Makers and Programme Managers. World Health Organization: Geneva. [Google Scholar]
- 85. Zinc Task Force (2010) Diarrhea treatment. Policy and product. updates 2010. Available at: http://www.izincg.org/treatment/country-exp
- 86. Asobayire F.S., Adou P., Davidsson L., Cook J.D. & Hurrell R.F. (2001) Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Cote d'Ivoire. The American Journal of Clinical Nutrition 74, 776–782. [DOI] [PubMed] [Google Scholar]
- 87. Renaudin P. & Lombart J.P. (1994) [Anemia in infants less than 1 year old in Moundou, Chad: prevalence and etiology]. Medicine Tropical (Mars) 54, 337–342. [PubMed] [Google Scholar]
- 88. Brabin B.J., Premji Z. & Verhoeff F. (2001) An analysis of anemia and child mortality. The Journal of Nutrition 131, 636S–645S. discussion 46S–48S. [DOI] [PubMed] [Google Scholar]
- 89. Hurrell R.F. (2007) Iron fortification: its efficacy and safety in relation to infections. Food and Nutrition Bulletin 28 (4 Suppl.), S585–S594. [DOI] [PubMed] [Google Scholar]
- 90. Stoltzfus R.J., Heidkamp R., Kenkel D. & Habicht J.P. (2007) Iron supplementation of young children: learning from the new evidence. Food and Nutrition Bulletin 28 (4 Suppl.), S572–S584. [DOI] [PubMed] [Google Scholar]
- 91. Wasantwisut E. (1997) Nutrition and development: other micronutrients' effect on growth and cognition. The Southeast Asian Journal of Tropical Medicine and Public Health 28 (Suppl. 2), 78–82. [PubMed] [Google Scholar]
- 92. Kasili E.G. (1990) Malnutrition and infections as causes of childhood anemia in tropical Africa. The American Journal of Pediatric Hematology/Oncology 12, 375–377. [DOI] [PubMed] [Google Scholar]
- 93. Sazawal S., Black R.E., Ramsan M., Chwaya H.M., Stoltzfus R.J., Dutta A. et al (2006) Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community‐based, randomised, placebo‐controlled trial. Lancet 367, 133–143. [DOI] [PubMed] [Google Scholar]
- 94. McLean E., Egli I., De Benoist B., Wojdyla D. & Cogswell M. (2007) Worldwide Prevalence of Anemia in Pre‐School Aged Children, Pregnant Women and Non‐Pregnant Women of Reproductive Age. Sight and Life Press: Basel. Available at: http://www.sightandlife.org/pdf/NAbook.pdf [Google Scholar]
- 95. World Health Organization (2007) Secretariat on behalf of the participants to the Consultation. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria endemic areas, Lyon, France, 12–14 June 2006. Food and Nutrition Bulletin 28, S621–S627. [DOI] [PubMed] [Google Scholar]
- 96. Lynch S., Stoltzfus R. & Rawat R. (2007) Critical review of strategies to prevent and control iron deficiency in children. Food and Nutrition Bulletin 28 (4 Suppl.), S610–S620. [DOI] [PubMed] [Google Scholar]
- 97. World Health Organization (WHO), Centers for Disease Control and Prevention (2005) Assessing the Iron Status of Populations. World Health Organization: Geneva. [Google Scholar]
- 98. World Health Organization (WHO). World Health Organization, United Nations Children's Fund, United Nations University (2001) Iron Deficiency Anemia: Assessment, Prevention, and Control. World Health Organization: Geneva. [Google Scholar]
- 99. Stoltzfus R.J. & Dreyfuss M.L. (1998) Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. ILSI Press: Washington D.C. [Google Scholar]
- 100. Stoltzfus R.J., Edward‐Raj A., Dreyfuss M.L., Albonico M., Montresor A., Dhoj Thapa M. et al (1999) Clinical pallor is useful to detect severe anemia in populations where anemia is prevalent and severe. The Journal of Nutrition 129, 1675–1681. [DOI] [PubMed] [Google Scholar]
- 101. Yurdakök K., Güner S.N. & Songül Yalçın S. (2008) Validity of using pallor to detect children with mild anemia. Pediatrics International 50, 232–234. [DOI] [PubMed] [Google Scholar]
- 102. World Health Organization (WHO) (2009) Weekly Iron–Folic Acid Supplementation (WIFS) in Women of Reproductive Age: Its Role in Promoting Optimal Maternal and Child Health. Position statement. Available at: http://www.who.int/nutrition/publications/micronutrients/weekly_iron_folicacid.pdf [PubMed]
- 103. Stoltzfus R.J. (2008) Research needed to strengthen science and programs for the control of iron deficiency and its consequences in young children. Journal of Nutrition 138, 2542–2546. [DOI] [PubMed] [Google Scholar]
- 104. Allen L., de Benoist B., Dary O. & Hurrell R. (2006) Guidelines on Food Fortification with Micronutrients. World Health Organization: Geneva. [Google Scholar]
- 105. Dewey K.G. (2007) Increasing iron intake of children through complementary foods. Food and Nutrition Bulletin 28 (4 Suppl.), S595–S609. [DOI] [PubMed] [Google Scholar]
- 106. World Health Organization (WHO) (2005) Report of the Third Global Meeting of the Partners for Parasite Control: Deworming for Health and Development Geneva, 29–30 November 2004. World Health Organization: Geneva. Available at: http://whqlibdoc.who.int/hq/2005/who_cds_cpe_pvc_2005.14.pdf [Google Scholar]
- 107. World Health Organization (WHO), United Nations Children's Fund (UNICEF) (2004) How to Add Deworming to Vitamin A Distribution. World Health Organization: Geneva. Available at: http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_PVC_2004.11.pdf [Google Scholar]
- 108. Allen L.H. (2000) Anemia and iron deficiency: effects on pregnancy outcome. The American Journal of Clinical Nutrition 71 (5 Suppl.), 1280S–1284S. [DOI] [PubMed] [Google Scholar]
- 109. Pan American Health Organization (PAHO), Pan American Health Organization: Washington, DC. United States Agency for International Development (USAID) (2007) Essential Delivery Care Practices for Maternal and Newborn Health and Nutrition. Available at: http://www.paho.org/english/ad/fch/ca/ca_delivery_care_practices_eng.pdf
- 110. World Health Organization (WHO) (2004) Focusing on Anaemia: Towards an Integrated Approach for Effective Anaemia Control. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/publications/micronutrients/WHOandUNICEF_statement_anaemia/en/index.html [Google Scholar]
- 111. World Health Organization (WHO) (2006) Iron Supplementation of Young Children in Regions Where Malaria Transmission is Intense and Infectious Disease Highly Prevalent. World Health Organization: Geneva. Available at: http://www.who.int/child_adolescent_health/documents/iron_statement/en/ [Google Scholar]
- 112. Zimmermann M.B., Jooste P.L. & Pandav C.S. (2008) Iodine‐deficiency disorders. Lancet 372, 1251–1262. [DOI] [PubMed] [Google Scholar]
- 113. de Escobar G.M., Obregon M.J. & del Rey F.E. (2007) Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutrition 10, 1554–1570. [DOI] [PubMed] [Google Scholar]
- 114. Williams G.R. (2008) Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of Neuroendocrinology 20, 784–794. [DOI] [PubMed] [Google Scholar]
- 115. World Health Organization (WHO), United Nations Children's Fund (UNICEF), International Council for Control of Iodine Deficiency Disorder (ICCIDD) (2007) Assessment of iodine deficiency disorders and monitoring their elimination, a guide for program managers, third edition World Health Organization: Geneva. [Google Scholar]
- 116. Zimmermann M.B. (2008) Methods to assess iron and iodine status. The British Journal of Nutrition 99 (3 Suppl.), S2–S9. [DOI] [PubMed] [Google Scholar]
- 117. Andersson M., de Benoist B., Delange F. & Zupan J. (2007) Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2‐years‐old: conclusions and recommendations of the Technical Consultation. Public Health Nutrition 10, 1606–1611. [DOI] [PubMed] [Google Scholar]
- 118. World Health Organization (WHO), United Nations Children's Fund (UNICEF) (2007) Reaching Optimal Iodine Nutrition in Pregnant and Lactating Women and Young Children. World Health Organization: Geneva. [Google Scholar]
- 119. World Health Organization (WHO) (2005) Iodine Deficiency Disorder. World Health Organization: Geneva. [Google Scholar]
- 120. Hamill P.V., Drizd T.A., Johnson C.L., Reed R.B. & Roche A.F. (1977) NCHS growth curves for children birth‐18 years. United States. Vital and Health Statistics Series 11 i–iv. 1–74. [PubMed] [Google Scholar]
- 121. World Health Organization (WHO) (2006) WHO Child Growth Standards: Methods and Development: Length/Height‐for‐Age, Weight‐For‐Age, Weight‐for‐Length, Weight‐For‐Height and Body Mass Index‐For‐Age. Geneva: World Health Organization. Available at: http://www.who.int/childgrowth/publications/technical_report_pub/en/index.html
- 122. World Health Organization (2008) Global Database on Child Growth and Malnutrition [Database on the Internet]. World Health Organization: Geneva. [cited 17 March 2008]. Available at: http://www.who.int/nutgrowthdb/database/en/ [Google Scholar]
- 123. Isanaka S., Villamor E., Shepherd S. & Grais R.F. (2009) Assessing the impact of the introduction of the World Health Organization growth standards and weight‐for‐height z‐score criterion on the response to treatment of severe acute malnutrition in children: secondary data analysis. Pediatrics 123, e54–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Seal A. & Kerac M. (2007) Operational implications of using 2006 World Health Organization growth standards in nutrition programmes: secondary data analysis. BMJ 334, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. World Health Organization (1999) Management of severe malnutrition: a manual for physicians and other senior health workers. World Health Organization: Geneva. [Google Scholar]
- 126. World Health Organization, UNICEF, WFP, UN System Standing Committee on Nutrition (2007) Joint Statement on the Community‐Based Management of Severe Acute Malnutrition. World Health Organization: Geneva. Available at: http://www.who.int/child_adolescent_health/documents/a91065/en/index.html [Google Scholar]
- 127. Prudhon C., Briend A., Weise Prinzo Z., Daelmans B. & Mason J.B. (2006) SCN Nutrition Policy Paper No. 21; WHO, UNICEF, and SCN Informal Consultation on Community‐Based Management of Severe Malnutrition in Children. [DOI] [PubMed]
- 128. World Health Organization (WHO), World Food Programme of the United Nations (WFP), United Nations System Standing Committee on Nutrition, United Nations Children's Fund (UNICEF) (2007) Community‐based management of severe acute malnutrition: A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/topics/Statement_community_based_man_sev_acute_mal_eng.pdf [Google Scholar]
- 129. United Nations System Standing Committee on Nutrition (2007) Innovative Approach Tackles Malnutrition in the Community. Geneva/New York/Rome [updated 12 January 2009] 7th June 2007.
- 130. World Health Organization (WHO), United Nations Children's Fund (UNICEF) (2009) WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children's Fund. World Health Organization: Geneva. Available at: http://www.who.int/nutrition/publications/severemalnutrition/9789241598163/en/index.html [PubMed] [Google Scholar]
- 131. Myatt M., Khara T. & Collins S. (2006) A review of methods to detect cases of severely malnourished children in the community for their admission into community‐based therapeutic care programs. Food and Nutrition Bulletin 27 (3 Suppl.), S7–23. [DOI] [PubMed] [Google Scholar]
- 132. World Health Organization (WHO), United Nations Children's Fund (UNICEF), World Food Programme (WFP), United Nations High Commissioner for Refugees (UNHCR) (2009) WHO, UNICEF, WFP and UNHCR consultation on the dietary management of moderate malnutrition in under‐5 children. Food and Nutrition Bulletin 30, S265. 19998862 [Google Scholar]
- 133. Golden M.H. (2009) Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Bulletin 30 (3 Suppl.), S267–S342. [DOI] [PubMed] [Google Scholar]
- 134. Michaelsen K.F., Hoppe C., Roos N., Kaestel P., Stougaard M., Lauritzen L. et al (2009) Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food and Nutrition Bulletin 30 (3 Suppl.), S343–S404. [DOI] [PubMed] [Google Scholar]
- 135. World Health Organization (2007) HIV and infant feeding: update based on the technical consultation held on behalf of the Inter‐agency Team (IATT) on prevention of HIV infections in pregnant women, mothers and their infants, Geneva, 25–27 October 2006.
- 136. World Health Organization (WHO) (2009) Rapid Advice: Revised WHO Principles and Recommendations on Infant Feeding in the Context of HIV – November. World Health Organization: Geneva. Available at: http://www.who.int/child_adolescent_health/documents/9789241598873/en/index.html [Google Scholar]
- 137. World Health Organization (WHO), Joint United Nations Programme on HIV/AIDS (UNAIDS), United Nations Children's Fund (UNICEF) (2009) Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector – Progress Report. World Health Organization: Geneva. Available at: http://www.who.int/hiv/pub/2009progressreport/en/index.html [Google Scholar]
- 138. World Health Organization (WHO), USAID, United Nations Population Fund (UNFPA), United Nations Children's Fund (UNICEF) (2010) Guidelines on HIV and infant feeding 2010, Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. World Health Organization: Geneva. [Google Scholar]
- 139. World Health Organization (WHO) (2006) Alimentation infantile et VIH: outils pour le conseil: guide de référence. World Health Organization: Geneva. Available at: http://www.who.int/child_adolescent_health/documents/9241592494/en/index.html [Google Scholar]
- 140. World Food Programme of the United Nations (WFP), Emergency Needs Assessment Branch (ODAN) (2005) Emergency Food Security Assessment Handbook – First Edition. World Food Programme: Rome. Available at: http://www.wfp.org/content/emergency-food-security-assessment-handbook [Google Scholar]
- 141. World Food Programme of the United Nations (WFP), Emergency Needs Assessment Branch (ODAN) (2009) Emergency Food Security Assessment Handbook – Second Edition. Available at: http://documents.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp203246.pdf; http://www.wfp.org/content/emergency-food-security-assessment-handbook
- 142. Maire B. & Delpeuch F. (2005) Nutrition Indicators for Development: Reference Guide. Available at: http://www.fao.org/docrep/008/y5773e/y5773e00.HTM
- 143. Food and Agriculture Organization (FAO) (2007) Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access, and Dietary Diversity (DD) Questionnaire. Food and Agriculture Organization: Rome. Available at: http://www.fivims.org/index.php?option=com_content&task=blogcategory&id=22&Itemid=38 [Google Scholar]
- 144. World Health Organization (1995) Field Guide on Rapid Nutritional Assessment in Emergencies. World Health Organization: Geneva. Available at: http://whqlibdoc.who.int/emro/1994-99/9290211989.pdf [Google Scholar]
- 145. United Nations Population Fund (UNFPA) (2003) Meetings on food crisis to highlight impact of HIV/AIDS as new type of famine threatens Africa.
- 146. World Food Programme Vulnerability and Analysis Mapping Branch (ODAV‐VAM) (2005) Integrating ‘Livelihoods’ into Food Security and Vulnerability Analysis: Some Initial Guidance. World Food Programme of the United Nations: Rome. Available at: http://home.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp204204.pdf [Google Scholar]
- 147. World Food Programme of the United Nations (WFP) (2008) Comprehensive Food Security and Vulnerability Assessment (CFSVA) Guidelines. World Food Programme: Rome. Available at: http://home.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp203193.pdf [Google Scholar]
- 148. Fewtrell L., Kaufmann R.B., Kay D., Enanoria W., Haller L. & Colford J.M. Jr (2005) Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta‐analysis. The Lancet Infectious Diseases 5, 42–52. [DOI] [PubMed] [Google Scholar]
- 149. World Health Organization (WHO) (2006) WHO Guidelines on Hand Hygiene in Health Care (Advanced Draft). World Health Organization: Geneva. Available at: http://www.who.int/patientsafety/information_centre/guidelines_hhad/en/index.html [Google Scholar]
- 150. Rehydration Project (2008) Hygiene, Hand Washing and Clean Water: What Every Family and Community Has a Right to Know about Hygiene [cited 4 February 2009]; 31 December 2008. Available at: http://rehydrate.org/hygiene/
- 151. The Global Public Private Partnership for Handwashing (2005) The Handwashing Handbook: A Guide for Developing a Hygiene Promotion Program to Increase Handwashing with Soap. The International Bank for Reconstruction and Development; The World Bank: Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Appendix of selected internationally recognized guides to develop national IYCN policies and programmes, with internet links
Supporting info item


