Abstract
The major aim of this trial was to compare the development of 18‐month‐old infants who received complementary feeding for 1 year either with lipid‐based nutrient supplements or micronutrient fortified corn‐soy porridge. Our secondary aim was to determine the socio‐economic factors associated with developmental outcomes in the same population. A total of 163 six‐month‐old rural Malawian children were enrolled in a randomized controlled trial where the control population received daily supplementation with 71 g corn‐soy flour [Likuni Phala (LP)] (282 kcal) and individuals in the intervention groups received daily either 50 g of lipid‐based nutrient supplement (FS50) (264 kcal) or 25 g of lipid‐based nutrient supplement (FS25) (130 kcal). The main outcome measures were Griffiths' developmental scores at 0–2 years. Independent comparison of study groups was carried out using analysis of variance (ANOVA) statistics where mean raw scores, quotients, or mental ages were compared. Association of developmental outcome with predictor variables were examined using multiple regression. At 18 months of chronological age, the mean ± standard deviation (SD) mental ages in the LP, FS50, and FS25 groups were 17.9 ± 1.3, 17.9 ± 1.3, and 17.9 ± 1.2 (P > 0.99), respectively. Likewise, the mean raw developmental scores and mean developmental quotients did not differ significantly. Length‐for‐age z‐score gain during the intervention period, and maternal education were associated with developmental outcome at 18 months (P = 0.03 and P = 0.04; respectively). In conclusion, rural Malawian infants receiving 12‐month daily supplementation of their diet either with the tested lipid‐based nutrient supplements or fortified corn‐soy flour have comparable development outcomes by 18 months of age.
Keywords: Lipid‐based nutrient supplements, child development, children, randomized controlled trial, complementary feeding
Introduction
Stunting affects approximately 170 million children under 5 years of age, with a prevalence of 40% in southern Asia and 50% in sub‐Saharan Africa (de Onis & Blössner 2003; UNICEF 2006). In early childhood, stunting is associated with poor child development including poor sensorimotor, cognitive–language development and social–emotional development (Meeks Gardner et al. 1999; Cheung et al. 2001; Siegel et al. 2005; Olney et al. 2007). Childhood stunting and poor child development are also associated with poor school performance, reduced physical performance, and reduced income in adulthood as well as lower birth weight of the next generation (Haas et al. 1995; Mendez & Adair 1999; Daniels & Adair 2004; Grantham‐McGregor et al. 2007; Victora et al. 2008).
Prenatal and early childhood undernutrition has been widely reported as a cause of stunting and poor development in early childhood (Cheung et al. 2001; Siegel et al. 2005; Olney et al. 2007). Because of the persistence of this effect on childhood development after the age of two (Chang et al. 2002; Victora et al. 2008), prevention strategies have been cited as a global health priority (Eagle et al. 2007; Grantham‐McGregor et al. 2007; Walker et al. 2007). Nutritional interventions targeted at the prevention of stunting or promotion of linear growth would be expected to promote child development. Results from long‐term follow‐up studies suggest that nutritional interventions in childhood promote cognitive performance, physical performance, and economic productivity (Haas et al. 1995; Eagle et al. 2007; Grantham‐McGregor et al. 2007; Walker et al. 2007; Hoddinot et al. 2008). To date, however, only a few nutritional intervention studies have assessed childhood development of under 2‐year‐old children as an outcome measurement in Africa (Oelofse et al. 2003; Adu‐Afarwuah et al. 2007; Grantham‐McGregor et al. 2007).
Recently, we reported results of a clinical trial suggesting that fortified spread promotes growth and prevents severe stunting in infancy when given as complementary food for 1 year (Phuka et al. 2008). Fortified spreads (FS) are micronutrient adulterated energy‐dense lipid‐based nutrient supplements (LNS), which are simple to produce, can be stored in warm conditions without supporting bacterial growth, and do not displace breast milk intake when given in infancy (Briend et al. 1999; Briend 2001; Galpin et al. 2007). In the present study, we hypothesized that nutritional intervention with 50 g of LNS would promote early child development more than iso‐energetic corn‐soy blend or half‐dose LNS when given as complementary food for 1 year starting from the age of 6 months. Hence, the primary aim was to compare the developmental outcomes between children who received either LNS or corn‐soy blend. And as secondary analysis, to identify other predictors of child development in the trial sample.
Key message
-
•
Stunting affects approximately 170 million children under 5 years of age worldwide.
-
•
Because of its association with poor childhood development after the age of two, prevention strategies have been cited as a global health priority.
-
•
Few nutritional intervention studies have assessed childhood development of under 2‐year‐old children.
-
•
This study investigated the impact on developmental outcomes after a year of complementary feeding with lipid‐based nutrient supplements or corn‐soy flour.
-
•
Developmental outcomes on the Griffiths scale are similar after 12 months supplementation with corn‐soy blend or lipid‐based nutrient supplements among Malawian infants.
Methods
Study area and timing
The present assessment was conducted between October and December, 2005 in Lungwena, a rural Malawian community with high prevalence of early childhood stunting and underweight (2003a, 2003b). The staple food, maize, was grown during the single rainy season between December and March. Exclusive breastfeeding for babies was almost non‐existent, and infant diet was typically complemented with thin maize porridge from as early as 2–6 months of age.
Eligibility criteria and enrolment
The study material consists of data from a recently reported clinical trial called Lungwena Child Nutrition Intervention Study 3 (LCNI‐3). Healthy infants who met inclusion criteria (age 5.50–6.99 months, resident in the study area, and whose guardian had signed an informed consent) were randomized to a control group, which was provided with a daily dose of 71 g micronutrient fortified corn‐soy flour [Likuni Phala (LP)], and the interventions groups, which were provided with LNS either as 50 g fortified spread (FS50) or 25 g micronutrient fortified spread (FS25). The micronutrient content was adjusted so that children in both FS groups received the same daily micronutrient doses from the supplement (Table 1). The supplements were home‐delivered every 3 weeks (at each food delivery three 500 g bags of LP, four 262 g jars of FS50, or two 262 g jars of FS25 were given). Sample sizes were based on expected values for one of the primary outcomes (weight gain) and predicted equal standard deviation in comparison groups based on an earlier preliminary dose‐finding trial (Kuusipalo et al. 2006). The sample sizes calculation was set to provide the trial with 80% power and 95% confidence to detect difference in weight gain between the supplementary groups. Finally, the sample sizes were adjusted to allow for approximately 5% attrition.
Table 1.
Energy and nutrient content of the daily ration of the food supplement used in the trial
| Variable | Intervention group | ||
|---|---|---|---|
| LP | FS50 | FS25 | |
| Weight, g | 71 | 50 | 25 |
| Energy, kcal | 282 | 264 | 130 |
| Protein, g | 10.3 | 7.6 | 3.8 |
| Carbohydrates, g | NA | 15.4 | 8.3 |
| Fat, g | 3.1 | 16.5 | 8.3 |
| Retinol, µg RE | 138 | 400 | 400 |
| Folate, µg | 43 | 160 | 160 |
| Niacin, mg | 3 | 6 | 6 |
| Pantothenic acid, mg | NA | 2 | 2 |
| Riboflavin, mg | 0.3 | 0.5 | 0.5 |
| Thiamin, mg | 0.1 | 0.5 | 0.5 |
| Vitamin B6, mg | 0.3 | 0.5 | 0.5 |
| Vitamin B12, µg | 0.9 | 0.9 | 0.9 |
| Vitamin C, mg | 48 | 30 | 30 |
| Vitamin D, µg | NA | 5 | 5 |
| Calcium, mg | 71 | 366 | 283 |
| Copper, mg | NA | 0.4 | 0.5 |
| Iodine, µg | NA | 90 | 90 |
| Iron, mg | 5 | 8 | 8 |
| Magnesium, mg | NA | 60 | 60 |
| Selenium, µg | NA | 17 | 17 |
| Zinc, mg | 3.6 | 8.4 | 8.4 |
FS25, fortified spread, 25 g day−1; FS50, fortified spread, 50 g day−1; LP, Likuni Phala; NA, not applicable; RE, retinol equivalents.
Measurement of outcome variables
Development was assessed using the Griffiths' developmental assessment tool for 0–2 years (Griffiths & Huntley 1996). This was adapted by removing three items from the locomotor section (climbs into a low chair, climbs to stand on a chair, and can seat self at table) and six items from the personal‐social skills (tries to turn doorknob or handle, shows shoes, likes adult to show book, can open a door, can take off shoes and socks, and at table uses spoon and fork together without help). These were items which were considered inappropriate in the rural Malawian setting. Two local research assistants were trained and certified in assessment and use of the Griffiths by Melissa Gladstone, one of the authors. A total of 267 of the total 276 items in the 0–2 Griffiths scales of mental development were used; 52 from personal/social skills, 56 were from hearing/language, 51 from the locomotor, and 54 from the two other subscales. The participant's raw total score as well as each subscale were expressed as the sum of all the items scored (Cheung et al. 2008). The sub‐ and total raw scores were then used to find developmental quotients from the Griffiths scales (Griffiths & Huntley 1996). The mental age column in the Griffiths scale was used to read the mental age corresponding to attained the subscale raw score, and general mental age was calculated as the mean of all subscale mental ages.
Explanatory variables of development
The individual and household factors hypothesized a priori to be associated with cognitive and motor development were included in multiple regression models as explanatory variables. These included complementary feeding groups, length‐for‐age z‐score (LAZ) at 18 months with adjustment for baseline LAZ, maternal education, number of under five siblings in the household, asset index, and morbidity. Maternal education was included in the model as a binary variable representing the capacity of the participants' mothers to write their names or not. Morbidity data were defined as the total number of days during the follow‐up when the participants had either diarrhoea or fever episodes. These days were a sum of days recorded daily by the mothers at home by checking against a symbol representing diarrhoea or fever. The mothers were trained on how to record daily episodes of diarrhoea or fever on a weekly morbidity calendar at the beginning of the study. The study field assistants collected the morbidity records from the participants' homes every week. Anthropometric indices weight‐for‐age z‐score (WAZ), LAZ, and weight‐for‐length z‐score (WLZ) were calculated with Epi‐Info 3.3.2 software (CDC, Atlanta, GA), based on the CDC 2000 growth reference (Kuczmarski et al. 2002).
At household level, the number of children and socio‐economic status were included in the regression model. The number of siblings aged under five in the household was included to evaluate possible effect of having competitors for care in the household. The total number of people in the household was excluded from analysis because of strong correlation with number of under five children. An asset index was created using principal components analyses on a set of assets available to assess long‐term economic welfare in the households (Filmer & Pritchet 2001). The index was included in the multiple regression models as a continuous explanatory variable.
Data management and analysis
Collected data were recorded on paper forms and double‐entered into a tailor‐made Microsoft Excel 2003 program (Microsoft Corp., Redmond, WA). The two entries were electronically compared and extreme values were confirmed or corrected. Statistical analysis was carried out using Stata 9.0 (StataCorp, College Station, TX). For continuous variables, the three groups were compared using analysis of variance (ANOVA). Attained mental ages were compared with chronological age using t‐test. Multiple regression analysis was used to test interaction and to identify factors associated with general developmental outcomes at 18 months. P‐values of <0.05 were considered significant.
Ethics, study registration, and participants' safety
The trial was performed according to International Conference of Harmonization – Good Clinical Practice guidelines (ICH‐GCP) and it adhered to the principles of Helsinki declaration and regulatory guidelines in Malawi. The trial protocol was reviewed and approved by the College of Medicine Research and Ethics Committee (University of Malawi) and the Ethical Committee of Pirkanmaa Hospital District (Finland). Key details of the protocol were published at the clinical trial registry of the National Library of Medicine, Bethesda, MD, USA (http://www.clinicaltrials.gov, trial identification is NCT00131209).
Results
Of 182 initially enrolled infants, 10 infants died during the 12‐month food supplementation period, two were not located at the end of follow–up, and seven did not show up for developmental assessment at the research centre. The remaining 163 participated in the developmental assessment study (Fig. 1). No statistically significant differences were observed in proportions of success to follow‐up, deaths, and dropouts across the trial groups (P = 0.24, P = 0.77 and P = 0.13, respectively). Reasons for not showing up were not established.
Figure 1.
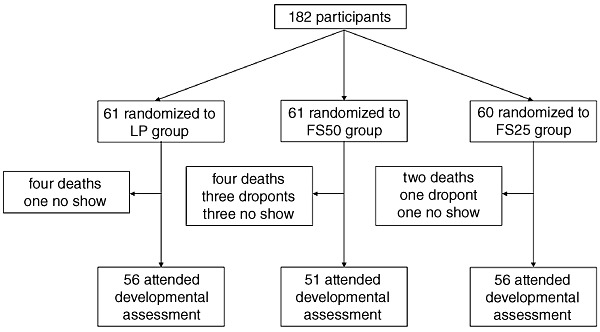
Flow of participants. FS25, fortified spread, 25 g day−1; FS50, fortified spread, 50 g day−1; LP, Likuni Phala.
The background anthropometric characteristics at developmental assessment and changes between enrolment and developmental assessment were mostly similar (Table 2). The age at developmental assessment was similar across the groups. The proportion of boys and mean WAZ, LAZ, and WLZ were lowest in LP group and highest in FS25. The lowest decrease in anthropometric indices during intervention period occurred in FS50 group except WLZ. However, none of these decreases reached statistical significance when compared between the groups (P = 0.80, P = 0.70, and P = 0.67 for WAZ, LAZ, and WLZ, respectively).
Table 2.
Background characteristics of the 163 participants, by intervention
| Variable | Intervention group | ||
|---|---|---|---|
| LP | FS50 | FS25 | |
| (n = 56) | (n = 51) | (n = 56) | |
| Characteristics at developmental assessment | |||
| Male sex, no. (%) | 23 (39.3) | 26 (54.1) | 32 (56.7) |
| Age, mean ± SD, month | 17.9 ± 0.5 | 18.0 ± 0.49 | 17.9 ± 0.4 |
| Weight‐for‐age z‐score, mean ± SD | −1,89 ± 1.17 | −1.72 ± 1.11 | −1.62 ± 1.06 |
| Length‐for‐age z‐score, mean ± SD | −1.71 ± 0.91 | −1.50 ± 0.87 | −1.42 ± 1.01 |
| Weight‐for‐length z‐score, mean ± SD | −0.47 ± 1.04 | −0.43 ± 1.17 | −0.37 ± 1.06 |
| Changes between enrolment and end of intervention (18 months) | |||
| 12 months cumulative diarrhoea days, mean ± SD | 5.8 ± 4.8 | 5.1 ± 3.3 | 5.0 ± 3.0 |
| 12 months cumulative fever days, mean ± SD | 10.6 ± 9.6 | 11.3 ± 8.5 | 9.7 ± 9.0 |
| WAZ, mean ± SD | −1.30 ± 0.63 | −1.24 ± 0.90 | −1.33 ± 0.66 |
| LAZ, mean ± SD | −0.54 ± 0.99 | −0.41 ± 0.81 | −0.48 ± 0.91 |
| WLZ, mean ± SD | −0.98 ± .80 | −1.05 ± 0.89 | −1.12 ± 0.74 |
FS25, fortified spread, 25 g day−1; FS50, fortified spread, 50 g day−1; LP, Likuni Phala; SD, standard deviation.; WAZ, weight‐for‐age z‐score; LAZ, length‐for‐age z‐score; WLZ, weight‐for‐length z‐score.
The mean ± standard deviation (SD) general raw Griffiths scores were 227 ± 9.0, 226 ± 11, and 226 ± 10 for the LP, FS50, and FS25 groups, respectively (P = 0.90) (Table 3). Correspondingly, mean general quotients were 101 ± 7, 100 ± 9, and 100 ± 8 (P = 0.88) and mean ± SD mental ages for each group were 17.9 ± 1.3, 17.9 ± 1.3 and 17.9 ± 1.2 (P > 0.99). Similar comparisons of the trial groups on all the developmental subscales showed no statistically significant differences (Table 3). There was no statistically significant differences between mean chronological age and general mental age among all groups or when all groups were combined; combined mean difference [95% confidence interval (CI)] were 0.01 (−0.19–0.21).
Table 3.
Griffiths' general raw scores, quotients, and mental ages with specific subscale mental age among the trial groups
| Griffiths characteristic | Intervention group | P‐value | ||
|---|---|---|---|---|
| LP | FS50 | FS25 | ||
| Griffiths general scales | ||||
| Raw score, mean ± SD | 226.5 (9.0) | 225.6 (10.7) | 225.7 (9.6) | 0.88 |
| Quotient, mean ± SD | 101 (7) | 100 (9) | 100 (8) | 0.88 |
| Mental age, mean ± SD | 17.9 ± 1.3 | 17.9 ± 1.3 | 17.9 ± 1.2 | >0.99 |
| Griffiths subscale mental ages | ||||
| Locomotor, mean ± SD | 15.91 ± 0.98 | 15.81 ± 0.78 | 16.01 ± 0.93 | 0.52 |
| Personal and social, mean ± SD | 16.30 ± 1.10 | 16.25 ± 1.03 | 16.26 ± 0.97 | 0.97 |
| Hearing and language, mean ± SD | 17.86 ± 2.91 | 18.01 ± 3.25 | 17.78 ± 2.03 | 0.90 |
| Eye–hand coordination, mean ± SD | 21.21 ± 1.36 | 21.17 ± 1.83 | 21.38 ± 1.49 | 0.76 |
| Performance, mean ± SD | 18.41 ± 2.31 | 18.25 ± 2.41 | 18.21 ± 2.46 | 0.89 |
FS25, fortified spread 25 g day−1; FS50, fortified spread 50 g day−1; LP, Likuni phala; P‐value Obtained by ANOVA.
No significant interaction was found between baseline length for age and intervention groups using general developmental scores as outcome variable (P = 0.10 and P = 0.40 for FS50 and FS25, respectively), or between baseline weight for age and intervention groups (P = 0.18 and P = 0.22, respectively).
In an exploratory analysis to determine predictors of development at 18 months, we fitted a multiple regression model for raw general score with all available individual and household‐level explanatory factors (Table 4). Of the all the variables modelled, only the capacity of mothers to write and LAZ at 18 months were significantly associated with general developmental score (P < 0.05). No interaction was found between capacity of mothers to write and supplementary groups.
Table 4.
Regression coefficient (95% CI) from multiple regression analysis of general mental score and explanatory characteristics among the 163 participants at 18 months of age
| Explanatory variables | General mental score | |
|---|---|---|
| Regression coefficient (95% CI) | P‐value | |
| Constant | 228 (222, 234) | <0.001 |
| FS25 group | −0.9 (−4.5, 2.8) | 0.64 |
| FS50 group | −0.4 (−4.1, 3.3) | 0.81 |
| LAZ at 18 months | 2.2 (0.2, 4.3) | 0.03 |
| LAZ at 6 months | −1.1 (−3.6, 1.4) | 0.38 |
| Maternal education | 3.7 (0.2, 7.3) | 0.04 |
| Number of under five | 0.7 (−1.1, 2.4) | 0.46 |
| Asset index | −0.6 (−1.6, 04) | 0.22 |
| 12 months cumulative diarrhoea days | −0.2 (−0.6, 0.2) | 0.42 |
| 12 months cumulative fever days | 0.01 (−0.2, 0.2) | 0.85 |
FS25, fortified spread 25 g day−1; FS50, fortified spread 50 g day−1; LAZ, Length‐for‐age z‐score; CI, confidence interval.
Discussion
The present study was designed to compare developmental outcomes associated with 1 year of complementary feeding in a randomized controlled trial, where participants received either corn‐soy blend (LP) or LNS (FS50 or FS25). The enrolment was population based where almost all eligible infants in the catchment area were recruited, group allocation was random, losses to follow‐up were minimal and our assessment methods were retrospectively validated through the regression analysis. As expected (Ivanans 1975; Pena et al. 2000; Cheung et al. 2001; Griffiths et al. 2004; Larrea & Kawachi 2005; Walker et al. 2005; Hatt & Waters 2006), maternal education and length gain of the infant during the follow‐up were both positively associated with the developmental score we used. Therefore, the observed results of comparable developmental outcomes between the LP and the FS50 or the FS25 trial groups are likely to be valid and representative of the target population. Thus, the findings do not reject the null hypothesis that complementary feeding with the tested LNS regimens equally promotes early childhood development more than corn‐soy blend.
The notion that different types of dietary supplementation schemes that are all fortified with multiple micronutrients result in comparable developmental outcomes is consistent with some earlier findings (Black et al. 2004; Adu‐Afarwuah et al. 2007). After a 6‐month supplementary intervention from the age of 6 months, a trial in Ghana showed no significant differences between micronutrient fortified LNS and micronutrient powder or micronutrient tablets in promoting motor development (Adu‐Afarwuah et al. 2007). Another trial in Bangladesh showed no significant difference in psychomotor development when a group receiving multiple micronutrients was compared with a group receiving combined iron and zinc supplements (Black et al. 2004). However, all micronutrient‐fortified supplements in Ghana, and multiple micronutrient or combined iron and zinc supplements in Bangladesh promoted motor development more than non‐supplementation or placebo groups, respectively (Black et al. 2004; Adu‐Afarwuah et al. 2007). Several other studies have also shown that micronutrient fortification of supplements (Moffat et al. 1994; Harahap et al. 2000; Olney et al. 2006) or unfortified supplementation (Husaini et al. 1991) promotes neurobehavioural development more than non‐supplementation in infants.
The three food supplements used in this study had different levels of essential fatty acids: the corn‐soy blend, FS50 and FS 25 provided an additional 0.3, 0.8, and 0.4 g of alpha linolenic acid (ALA) per day and 2.7, 7.3, and 3.6 g of linolenic acid (LA), respectively. A differential impact on development was plausible as essential fatty acids are needed for brain growth (Helland et al. 2003; Innis 2008, 2009). It is possible, however, that with the additional essential fatty acids provided by breast milk, the overall difference of intake was not sufficient to have an influence on development quotient. The adequate intake of LA and ALA was 4.6 and 0.5 g day−1 (Food and Nutrition Board 2005), and breast milk was estimated to provide at least 4.4 and 0.4 g day−1 given the minimum intake was 830 g day−1 (Galpin et al. 2007).
The major limitation of the present study was lack of non‐supplemented control group. Because of this omission, we cannot conclusively analyse if none of the interventions had an effect or if all three supplementation schemes had an equal positive effect on the developmental outcomes. The fact that the participants attained mean general mental ages comparable with their own chronological age is, however, consistent with the latter alternative. This possibility is further supported by the notion that the observed mental ages were similar to those from the reference population of the Griffiths scale (Black et al. 2004) and those attained by British children of similar age in a different study (Sutcliffe et al. 1999). Because the study area had a high prevalence of stunting, malaria is endemic and income level was low (Maleta et al. 2003a; UNICEF 2006), making it more likely that the participants would attain lower developmental outcomes when compared with reference population from a high‐income setting (Cheung et al. 2001; Kariger et al. 2005; Siegel et al. 2005; Grantham‐McGregor et al. 2007; Olney et al. 2007). In the earlier mentioned Ghana trial, using a somewhat similar approach to ours, a difference in motor development was noticed in an analysis that compared no intervention with one with LNS but not between two different supplement types (Adu‐Afarwuah et al. 2007).
The adaptation method of the locomotor and personal social subscales resulted in lower possible scores than in the total scores in the subscales. Attained raw score were likely to be lower than could normally be attained. No specific correction was performed on the raw score or the corresponding mental ages in these subscales. Thus, the lower mental ages than chronological ages attained in these specific subscales are likely and partly explained by the adaptation of the Griffiths tools. The lower mental ages in these subscales, however, should not have affected our group comparison as they affected all the participants.
In conclusion, developmental outcomes of a year‐long complementary feeding intervention to otherwise healthy infants with micronutrient‐fortified LNS does not differ from the outcome of intervening with micronutrient‐fortified corn‐soy flour. After these results, further trials on effects of complementary feeding interventions with LNS that include a non‐supplemented control group are warranted.
Source of funding
The trial was funded by grants from Academy of Finland (grants 200720 and 109796), Foundation for Paediatric Research in Finland, and Medical Research Fund of Tampere University Hospital. The micronutrient mixture used in the production of lipid‐based nutrient supplements was provided free of charge by Nutriset Inc. (Malaunay, France). John Phuka and Chrissie Thakwalakwa received personal stipends from Nestle Foundation.
Conflicts of interest
André Briend was a staff member of the World Health Organization. André Briend alone was responsible for the views expressed in this publication and they do not necessarily represent the decisions or stated policy of the World Organization.
André Briend was a consultant to Nutriset until December, 2003 and the company has also financially supported the planning of another research project by the same study team through Per Ashorn and the University of Tampere after the completion of this trial. Other authors declare no conflict of interest. The funders of trial had no role in the implementation, analysis or reporting.
Contributions
All authors except YBC designed the trial, PA wrote the protocol and raised its funding, JCP and CT were responsible for data collection, YBC designed the details of statistical analysis and JCP did the analysis and wrote the first draft of the manuscript under the supervision of PA, YBC, and KM. All authors commented on the analysis and participated in writing of the manuscript. JCP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We are grateful to the people of Lungwena, the staff at the Lungwena Training Health Centre and our research assistants for their positive attitude, support, and help in all stages of the study, and to Laszlo Csonka for designing the collection tools and data entry programs.
References
- Adu‐Afarwuah S., Lartey A., Brown K.H., Briend A., Zlotkin Z. & Dewey K.G. (2007) Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. The American Journal of Clinical Nutrition 86, 412–420. [DOI] [PubMed] [Google Scholar]
- Black M.M., Baqui A.H., Zaman K., Persson L.A., El Arifeen S., Le K. et al (2004) Iron and Zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. The American Journal of Clinical Nutrition 80, 903–910. [DOI] [PubMed] [Google Scholar]
- Briend A. (2001) Highly nutrient‐dense spreads: a new approach to delivering multiple micronutrients to high‐risk groups. The British Journal of Nutrition 85 (Suppl. 2), S175–S179. [PubMed] [Google Scholar]
- Briend A., Lacsala R., Prudhon C., Mounier B., Grellety Y. & Golden M.H. (1999) Ready‐to‐use therapeutic food for treatment of marasmus. Lancet 353, 1767–1768. [DOI] [PubMed] [Google Scholar]
- Chang S.M., Walker S.P., Grantham‐McGregor S. & Powel C.A. (2002) Early childhood stunting and late behaviour and school achievement. Journal of Child Psychology and Psychiatry, and Allied Disciplines 46, 775–783. [DOI] [PubMed] [Google Scholar]
- Cheung Y.B., Yip P.S.F. & Karlberg J.P.E. (2001) Fetal growth, early postnatal growth and motor development in Pakistani infants. International Journal of Epidemiology 30, 518–526. [DOI] [PubMed] [Google Scholar]
- Cheung Y.B., Gladstone M., Maleta K., Duan X. & Ashorn P. (2008) Comparison of four approaches to score development: a study of Malawian children. Tropical Medicine & International Health: TM & IH 13, 987–993. [DOI] [PubMed] [Google Scholar]
- Daniels M.C. & Adair L.S. (2004) Growth in young Filipino children predicts schooling trajectories through high school. The Journal of Nutrition 134, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Eagle P.L., Black M.M., Behrman J.R., de Mello M.C., Gertler P.J., Kapiriri L. et al (2007) Strategies to avoid the loss of development potential in more than 200 million children in the developing world. Lancet 369, 229–242. [DOI] [PubMed] [Google Scholar]
- Filmer D. & Pritchet L.H. (2001) Estimating wealth effects without expenditure data‐ or tears: an application to educational enrollments in states of India. Demography 38, 115–132. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board (FNB), Institute of Medicine, National Academies . (2005) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) . Available at: http://books.nap.edu/openbook.php?record_id=10490&page=1324 [DOI] [PubMed]
- Galpin L., Thakwalakwa C., Phuka J., Ashorn P., Maleta K., William W.W. et al (2007) Breast milk intake is not reduced more by the introduction of energy dense complementary food than by typical infant porridge. The Journal of Nutrition 137, 1828–1833. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. & Huntley M. (1996) GMDS 0‐2. Griffiths Mental Development Scales – Revised: Birth to 2 Years. Hogrefe: Oxford. [Google Scholar]
- Griffiths P., Madise N., Whitworth A. & Mathews Z. (2004) A tale of two continents: a multilevel comparison of determinants of child nutritional status from selected African and Indian regions. Health & Place 10, 183–199. [DOI] [PubMed] [Google Scholar]
- Haas J.D., Martinez E.J., Murdoch S., Conlisk E., Rivera J.A. & Martorell R. (1995) Nutritional supplementation during the preschool years and physical work capacity in adolescent and young adult Guatemalans. The Journal of Nutrition 125 (Suppl. 4) 1078S–1089S. [DOI] [PubMed] [Google Scholar]
- Harahap H., Jahari A.B., Nelson E.C., McClish D.K., Manuel M. & Chacon M.E. (2000) Effects of an energy and micronutrient supplement in iron deficiency anemia, physical activity and motor and mental development in undernourished children in Indonesia. European Journal of Clinical Nutrition 54 (Suppl. 2), S114–S119. [DOI] [PubMed] [Google Scholar]
- Hatt L.E. & Waters H.R. (2006) Determinants of child morbidity in Latin America: a pooled analysis of interactions between parental education and economic status. Social Science & Medicine 62, 375–376. [DOI] [PubMed] [Google Scholar]
- Helland I.R., Smith L., Saarem K., Saugstad M.D. & Drevon C.A. (2003) Maternal supplementation with very‐long chain n‐3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 111, e39–e44. [DOI] [PubMed] [Google Scholar]
- Hoddinot J., Maluccio J.A., Behrman J.R., Flores R. & Martorell R. (2008) Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet 371, 411–416. [DOI] [PubMed] [Google Scholar]
- Husaini M.A., Karyadi L., Husaini Y.K., Sandjaja, Karyadi D. & Pollitt E. (1991) Developmental effects of short‐term supplementary feeding in nutritionally‐at‐risk Indonesian infants. The American Journal of Clinical Nutrition 54, 799–804. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2008) Dietary omega fatty acids and developing brain. Brain Research 1237, 35–43. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2009) Omenga‐3 fatty acids and neural development to 2 years of age: do we know enough fro dietary recommendations? Journal of Pediatric Gastroenterology and Nutrition 48, S16–S24. [DOI] [PubMed] [Google Scholar]
- Ivanans I. (1975) Effect of maternal education and ethnic background on infant development. Archives of Disease in Childhood 5, 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariger P.K., Stoltzfus R.J., Olney D., Sazawal S., Black R., Tielsch J.M. et al (2005) Iron deficiency and physical growth predict attainment of walking but not crawling in poorly nourished Zanzibari Infants. The Journal of Nutrition 135, 814–819. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R.J., Ogden C.L., Guo S.S., Grummer‐Strawn L.M., Flegel K.M., Mei Z. et al (2002) 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat 11 (246), 1–190. [PubMed] [Google Scholar]
- Kuusipalo H., Maleta K., Briend A., Manary M. & Ashorn P. (2006) Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: A preliminary trial. J Pediatr Gastroenterol Nutr 43, 525–532. [DOI] [PubMed] [Google Scholar]
- Larrea C. & Kawachi I. (2005) Does economic inequality affect child malnutrition? The case of Ecuador. Social Science & Medicine 60, 165–178. [DOI] [PubMed] [Google Scholar]
- Maleta K., Virtanen S., Espo M., Kulmala T. & Ashorn P. (2003a) Child malnutrition and its predictors in Rural Malawi. Paediatric and Perinatal Epidemiology 17, 384–390. [DOI] [PubMed] [Google Scholar]
- Maleta K., Virtanen S., Espo M., Kulmala T. & Ashorn P. (2003b) Timing of growth faltering in rural Malawi. Archives of Disease in Childhood 88, 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks Gardner J.M., Grantham‐McGregor S., Himes J. & Chang S. (1999) Behaviour and development of stunted and nonstunted Jamaican children. Journal of Child Psychology and Psychiatry, and Allied Disciplines 40, 817–827. [DOI] [PubMed] [Google Scholar]
- Mendez M.A. & Adair L.S. (1999) Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. The Journal of Nutrition 125, 1555–1562. [DOI] [PubMed] [Google Scholar]
- Moffat M.E.K., Longstaffe S., Besant J. & Dureski C. (1994) Prevention of iron deficiency and psychomotor decline in high‐risk infants through use of iron‐fortified infants formula: a randomized clinical trial. The Journal of Pediatrics 125, 527–534. [DOI] [PubMed] [Google Scholar]
- Oelofse A., Van Raaij J.M., Benade A.J., Dhansay M.A., Tolboom J.J. & Hautvast J.G. (2003) The effect of a micronutrient‐fortified complementary food on micronutrient status, growth and development of 6 to 12‐month‐old disadvantaged urban South African infants. International Journal of Food Sciences and Nutrition 54, 399–407. [DOI] [PubMed] [Google Scholar]
- Olney D.K., Pollitt E., Kariger P.K., Khalfan S.S., Ali N.S., Tielsch J.M. et al (2006) Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibar infants 5 to 11 months old. The Journal of Nutrition 136, 2427–2434. [DOI] [PubMed] [Google Scholar]
- Olney D.K., Pollitt E., Kariger P.K., Khalfan S.S., Ali N.S., Tielsch J.M. et al (2007) Young Zanzibar children with iron deficiency, iron deficiency anemia, stunting, or malaria have lower motor activity scores and spend less time in locomotion. The Journal of Nutrition 137, 2756–2762. [DOI] [PubMed] [Google Scholar]
- de Onis M. & Blössner M. (2003) The World Health Organization Global Database on Child Growth and Malnutrition: methodology and applications. International Journal of Epidemiology 32, 518–526. [DOI] [PubMed] [Google Scholar]
- Pena R., Wall S. & Persson L.A. (2000) The effect of poverty, social inequality and maternal education on infant mortality in Nicaragua, 1988–1993. American Journal of Public Health 90, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuka J.C., Maleta K., Thakwalakwa C., Cheung Y.B., Brien A., Manary M.J. et al (2008) Complementary feeding with fortified spread and incidence of severe stunting in 6‐to 18‐month‐old rural Malawians. Archives of Pediatrics & Adolescent Medicine 162, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E.M., Stoltzfus R.J., Kariger P.K., Katz J., Khatry S.K. et al (2005) Growth indices, anemia and diet independently predict motor milestone acquisition of infants in South Central Nepal. The Journal of Nutrition 135, 2840–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe A.G., Taylor B., Li J., Thornton S., Grudzinskas J.G. & Lieberman B.S. (1999) Children born after intracytoplasmic sperm injection: population control study. BMJ 318, 704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . (2006) The State of the World's Children 2007. UNICEF House: New York. [Google Scholar]
- Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Richter L. et al (2008) Maternal and child undernutrition: consequences for adult and human capital. Lancet 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.P., Chang S.M., Powell C.A. & Grantham‐McGregor S.M. (2005) Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth stunted Jamaican children: prospective cohort study. Lancet 366, 1804–1807. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Wachs T.D., Meeks‐Gardner J., Lozoff B., Wasserman G.A., Pollit E. et al (2007) Child development: risk factors adverse outcomes in developing countries. Lancet 369, 145–157. [DOI] [PubMed] [Google Scholar]


