Abstract
This study aimed to assess the effects of a 2‐month lifestyle modification trial on cardio‐metabolic abnormalities and C‐reactive protein (CRP) among obese adolescents with metabolic syndrome [phenotypically obese metabolically abnormal (POMA)] and obese adolescents without a cardio‐metabolic disorder [phenotypically obese metabolically normal (POMN)], as well as in normal‐weight adolescents with at least one cardio‐metabolic disorder [phenotypically normal metabolically obese (PNMO)].
The study comprised 360 adolescents assigned in three groups of equal number of POMN, POMA and PNMO. They were enrolled in a trial consisting of aerobic activity classes, diet and behaviour modification, and were recalled after 6 months.
Overall, 94.7% of participants completed the 2‐month trial, and 87.3% of them returned after 6 months. The mean CRP was not significantly different between the POMA and PNMO groups, but was higher than in the POMN group. After the trial, body mass index (BMI) and waist circumference (WC) decreased in obese participants, and the mean body fat mass decreased in all groups. At 2 months, the mean total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), triglycerides (TG) and CRP decreased in the POMA and PNMO groups. After 2 and 6 months, the decrease in mean TC, LDL‐C, TG, CRP and systolic blood pressure was greater in the POMA than in the POMN group. The magnitude of decrease in CRP correlated with that of BMI, WC, fat mass, TG, TC and LDL‐C.
Lifestyle modification programmes for primordial/primary prevention of chronic diseases would be beneficial at the population level and should not be limited to obese children.
Keywords: cardio‐metabolic disorders, obesity, adolescents, lifestyle modification
Introduction
Excessive amounts of adipose tissue in children comprise a growing health problem throughout the world (Weker 2006). Childhood obesity is, at least in part, the result of a continuing imbalance between energy intake and expenditure. Given the genetic background, major causative factors are inappropriate nutrition, psychological factors, lack of physical activity and their interactions. Low level of physical activity not only contributes to obesity development and maintenance, but also it is a negative effect of childhood obesity. Undesirable health effects of obesity of children such as dyslipidaemia, type 2 diabetes and metabolic syndrome (Eisenmann 2007) rationalize the need to look for efficient interventions for controlling overweight and its related cardio‐metabolic disorders.
Although cardio‐metabolic disorders usually exist among obese children [phenotypically obese metabolically abnormal (POMA)], studies have shown that some obese individuals have no associated metabolic abnormality. The term phenotypically obese metabolically normal (POMN) has been used to describe them. At the same time, there are normal‐weight subjects with a clustering of cardio‐metabolic abnormalities who may be at elevated risk for insulin resistance and elevated risk of cardiovascular disease, i.e. phenotypically normal metabolically obese (PNMO) (St‐Onge et al. 2004; Ramachandran et al. 2007; Kelishadi et al. 2008a).
Probably the most effective intervention for controlling cardio‐metabolic risk factors among youths is an integrative concept based on diet modification, behavioural change and encouraging physical activity (Stockli & Keller 2003). Weight loss reduces blood pressure (BP), serum triglyceride (TG) levels and probably low‐density lipoprotein cholesterol (LDL‐C) concentrations and increase high‐density lipoprotein cholesterol (HDL‐C) levels (Poirier & Despres 2001). An increase in physical activity along with weight loss profoundly influences peripheral lipoprotein metabolism and improves the atherogenic lipoprotein profile (Johnson et al. 2000).
It is suggested that some of the beneficial effects of lifestyle modification, notably physical activity, may be mediated through decreasing subclinical inflammation both in adults (Wannamethee et al. 2005) and in adolescents (de Ferranti et al. 2006). Previous studies have assessed the effects of lifestyle modification on cardio‐metabolic risk factors and markers of inflammation among obese children and adolescents (Roberts et al. 2006; Kelishadi et al. 2008b). This study, which to the best of our knowledge is the first of its kind, aimed to assess the short‐ and long‐term effects of a 2‐month lifestyle modification trial on cardio‐metabolic abnormalities and C‐reactive protein (CRP) among POMA, PNMO and POMN adolescents.
Key messages
-
•
The C‐reactive protein (CRP) level was higher in normal‐weight and obese adolescents with cardio‐metabolic disorder than in obese adolescents without such disorders.
-
•
Irrespective of the baseline weight status and weight reduction, improvement in dyslipidaemia and inflammation can be achieved by lifestyle modification among youths.
-
•
The magnitude of decrease in serum CRP after the 2‐month interventional period correlated significantly with the magnitude of decreases in body mass index, waist circumference and total fat mass, as well as total cholesterol, low‐density lipoprotein cholesterol and triglycerides, but not with the skinfold thickness and high‐density lipoprotein cholesterol (HDL‐C).
-
•
Dyslipidaemia in terms of low HDL‐C and high triglycerides should be considered not only in obese children, but also in normal‐weight children; this is of special concern in populations using hydrogenated fats and having low physical activity.
-
•
Lifestyle modification programmes designed for primordial/primary prevention of chronic diseases might be beneficial at the population level and should not be limited to obese children.
Methods
Study population
This trial was conducted in 2007 among 360 adolescents aged 12–16 years. The sample size was calculated based on a two‐sample t‐test, with a clinically important difference of 2.2 in the waist circumference (WC) of obese youths before and after participating in one of our previous lifestyle modification trials (Kelishadi et al. 2008b). By considering the estimated SDs of 4.7 and 4.1, the power of 95% and the statistical significance of 5%, the sample size was calculated as 105 in each group (total 315), but because of the attrition of participants during the follow‐up period, 360 individuals (120 in each group) were included in the trial.
Participants consisted of the following: 120 obese adolescents with normal blood glucose, lipid profile and BP were considered as the POMN group; 120 normal‐weight adolescents with an abnormal level of at least one cardio‐metabolic risk factor were considered as the PNMO group; and 120 obese adolescents with the criteria of the metabolic syndrome, i.e. at least three out of the following five risk factors: TGs ≥150 mg dL−1, HDL‐C <40 mg dL−1, fasting blood glucose (FBG) ≥100 mg dL−1, systolic BP (SBP) ≥130 mm Hg or diastolic BP (DBP) ≥85 mm Hg (Zimmet et al. 2007), were considered as the POMA group, respectively. The two obese groups were consecutively recruited from obese children who were referred to the Obesity and Metabolic Syndrome Research Clinic of the Pediatric Preventive Cardiology Department, Isfahan Cardiovascular Research Center (ICRC). The PNMO group was selected from the classmates of the first two groups. After screening 427 students, we reached the necessary number (i.e. 120) of subjects with at least one of the aforementioned risk factors, the most common being low serum HDL‐C followed by high TG level. The groups were balanced for age group and sex. Those subjects with syndromal obesity, mental retardation, endocrine disorders, presence of any physical disability, and or history of smoking, chronic medication use or infection during the 2 weeks before the study were not included in the trial.
The study was approved by the Ethics Committee of the ICRC [National Institute of Health (NIH) Code: FWA 0000t8578]. All parents of included subjects gave written informed consent after receiving information, and oral assent was obtained from the participants.
Measures
The calculated age from birth till the interview date was recorded. Weight and height were measured by a calibrated scale and stadiometer (Seca, Tokyo, Japan), with subjects lightly clothed and barefoot to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. BMI was categorized on the basis of the BMI reference curves of Iranian children and adolescents (Kelishadi et al. 2008c) that are relatively similar to the revised growth charts of the Centers for Disease Prevention and Control (CDC) (Kuczmarski et al. 2002). WC was measured at a point midway between the lower border of the ribcage and the iliac crest at the end of normal expiration (WHO 1995). WC percentiles were calculated according to the reference curves of Iranian children and adolescents (Kelishadi et al. 2007a). Triceps skinfold was measured by a skinfold caliper (Mojtahedi, Isfahan, Iran). Body fat mass was determined through dual energy absorptiometry using Omron body fat monitor instrument (Omron, Kyoto, Japan) that was validated in our previous studies (Kelishadi et al. 2008b).
SBP and DBPs were measured after a supine rest for 10 min using a mercury sphygmomanometer (Richter, Uttenreuth, Germany) under standard protocol. The readings at the first and the fifth Korotkoff phase were taken as SBP and DBP, respectively. The average of the two BP measurements at each visit was recorded and included in the analysis. All measurements were made by the same trained general practitioner and under the supervision of the same paediatrician. The percentiles were calculated according to the BP percentiles of Iranian children and adolescents (Kelishadi et al. 2006a) that are relatively similar to the US percentiles (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents 2004).
Children were asked to fast, 12 h before the laboratory assessments. Compliance with fasting was determined by interview on the morning of the examination. Blood samples were taken from the ante‐cubital vein between 8 a.m. and 9 a.m. while one of the parents accompanied his/her child. FBG, total cholesterol (TC), LDL‐C and HDL‐C, TG and CRP levels were measured in the ICRC central laboratory, which meets the national standards and is also under the quality control of the CDC, USA, and the Department of Epidemiology, St. Rafael University, Leuven, Belgium. For laboratory examinations, blood samples were centrifuged for 10 min at 3000 rpm within 30 min of venepuncture. FBG and lipid profile were measured by enzymatic method using an auto‐analyzer (Hitachi, Tokyo, Japan). HDL‐C was determined after dextran sulfate‐magnesium chloride precipitation of non‐HDL‐C (McNamara & Schaefer 1987). CRP was measured by the same auto‐analyzer; the kit had a minimum detection of less than 0.05 mg L−1 and a measurable concentration range of up to 160 mg L−1. The intra‐assay and inter‐assay variation coefficients were 0.8–1.3% and 1.0–1.5%, respectively.
The whole programme was offered free of charge. Testing sessions, including physical and biochemical examinations, were conducted at enrolment, plus 2 months (within 1 week after the end of the trial) and 6 months after baseline.
Intervention
The lifestyle modification trial, which was carried out with the same method in all groups, included three components of exercise, diet education and behaviour modification (2008b, 2008d). A physical therapist led the exercise interventions. Parents did not take part in the exercise sessions. During the 2‐month physical‐training period, aerobic activity classes were held 3 days a week. Each week, a 15‐min session was held before the beginning of the exercise to didactic presentations and/or discussion about the reasons for being active, ways of overcoming barriers to being active, and strategies for maintaining an active lifestyle and reduction of sedentary activities. The 40‐min sessions of physical training included 20 min of fitness‐oriented activities and 20 min of playing games and running.
A registered dietitian conducted the nutrition education sessions. The recommended diet was similar to our previous studies (2008b, 2008d) and based on a diet containing 30% energy fat, 15% energy proteins and 55% carbohydrates, with an energy content based on the calorie requirement for height (Lucas 2000). Necessary information about limiting the use of saturated and trans fatty acids was provided, and increase in the consumption of fruits and vegetables was encouraged. A licenced psychologist also carried out the behaviour modification sessions (2008b, 2008d). After the 2‐month trial, all participants and their parents were followed up by monthly telephone calls for 4 months, and then 6 months after the baseline survey; they were recalled to the clinic for the third step of the examinations.
Statistical analysis
SPSS for Windows (version 16.0, SPSS Inc., Chicago, IL, USA) was used for data analysis. The normality of the distribution of variables with a Kolmogorov–Smirnov test was verified and found to have no significant deviation from normality. The outcome measures on the dropouts prior to their withdrawal looked similar with those who stayed in the study. Consequently, no missing values were imputed and analyses are presented for participants in the study at each stage, in the group to which they were randomized. Analyses were initially stratified by gender, but as the chi‐square test showed that the differences were not significant, results are presented for girls and boys combined. Results are presented as mean ± SD unless mentioned otherwise. Analysis of variance (ANOVA) adjusted for age and gender with post hoc Bonferroni test was used to determine the significance of any baseline differences between different groups. In addition, repeated measures ANOVA with post hoc Bonferroni test was also used separately for comparison of within‐group and between‐group changes of variables studied at baseline and at each follow‐up study. Linear regression analysis was used to assess mean changes in anthropometric and biochemical parameters. In order to identify parameters associated with the change in CRP levels after the 2‐month interventional period, we performed bivariate regression analysis for the change in plasma CRP levels vs. the change in measures of adiposity and serum lipid profile. The significance level was set at P < 0.05.
Results
The flow of study participants from enrolment through the study completion is presented in Fig. 1. Overall, 94.7% of enrolled adolescents completed the 2‐month trial, and 87.3% of them (82.7% of the total sample enrolled) returned for the 6‐month study. The attendance rate for sessions ranged between 72 and 78%, without significant difference between groups. There was no significant difference between the characteristics of individuals who completed the study and those who dropped out in each group at 2‐ and 6‐month follow‐up studies, and the attrition of these individuals did not make any significant statistical change on the results.
Figure 1.
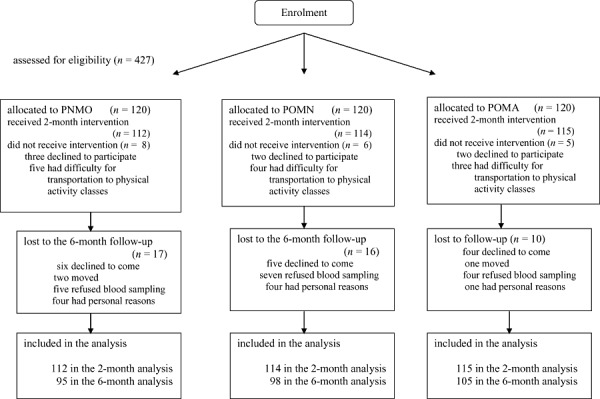
Participant retention vs. attrition. PNMO, phenotypically normal metabolically obese; POMA, phenotypically obese metabolically abnormal; POMN, phenotypically obese metabolically normal.
The mean age of the participants was 14.1 (1.7) years and the female : male ratio was 1.2:1, without a significant difference between groups under study. The mean BMI of the PNMO, POMN and POMA groups corresponded respectively to the 75th, 97th and 98th percentiles of Iranian children and adolescents (Kelishadi et al. 2008c). The corresponding figures for WC were 57th, 92nd and 96th percentiles, respectively (Kelishadi et al. 2007a). These percentiles were not significantly different between the POMN and POMA groups, but were significantly higher in these groups than in the PNMO group. Compared with the percentiles of Iranian children and adolescents (Kelishadi et al. 2006a), the mean SBP corresponded to the 75th percentile in the PNMO group, to the 90th percentile in the POMN group, and to the 95th percentile in the POMA group. The mean DBP corresponded to the 80th percentile in the PNMO group and to 90th percentile in the two other groups.
Table 1 presents the baseline characteristics of the three groups. The mean serum of TC, LDL‐C and TG was significantly higher, and that of HDL‐C was significantly lower in the POMA group followed by the PNMO and POMN groups. The mean CRP level was significantly lower in the POMN group than in the POMA and PNMO groups; in addition, it was lower in the PNMO than in the POMA group, but this difference was not statistically significant.
Table 1.
Baseline characteristics [mean (SD)] of participants
| PNMO (n = 120) | POMN (n = 120) | POMA (n = 120) | |
|---|---|---|---|
| Body mass index (kg m2) | 20.4 (0.5) | 26.4 (0.5)** | 26.2 (0.6)* |
| Waist circumference (cm) | 67.5 (1.4) | 80.5 (1.2)** | 82.8 (1.1) |
| Subcutaneous fat (mm) | 15.2 (0.4) | 18.5 (0.4)** | 18.2 (0.5)* |
| Body fat mass (kg) | 24.4 (1.4) | 30.4 (1.5)** | 30.6 (1.2)* |
| Total cholesterol (mg dL−1) | 172.8 (9.5) | 167.1 (8.5) | 179.7 (9.1) |
| LDL cholesterol (mg dL−1) | 114.2 (4.1) | 108.1 (6.4) | 116.4 (7.2) |
| HDL cholesterol (mg dL−1) | 35.7 (4.5) | 38.6 (4.2) | 32.7 (5.4) |
| Triglycerides (mg dL−1) | 131.5 (15.1) | 111.7 (14.6) | 139.7 (15.4) |
| Fasting blood glucose (mg dL−1) | 81.5 (5.2)* | 82.5 (7.1)** | 84.5 (6.2)* |
| C‐reactive protein (mg L−1) | 1.1 (0.04)* | 0.9 (0.05) | 1.4 (0.02) |
| Systolic blood pressure (mm Hg) | 117.2 (26.5)* | 122.5 (27.4) | 124.1 (25.1) |
| Diastolic blood pressure (mm Hg) | 74.2 (18.2) | 76.5 (19.2) | 77.1 (18.2)* |
HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PNMO, phenotypically normal metabolically obese; POMN, phenotypically obese metabolically normal; POMA, phenotypically obese metabolically abnormal. All differences are significant between groups except those marked by the following superscripts: *P > 0.05 vs. POMN group; **P > 0.05 vs. POMA group. Data are means (SD) compared between groups by analysis of variance adjusted for age and gender with post hoc Bonferroni test.
The within‐group and between‐group changes in the mean (SD) of the physical and biochemical variables assessed among the three groups studied at 2 months vs. baseline, and at 6 months vs. 2 months, are presented in Table 2. Within‐group comparisons showed that after the 2‐month trial, BMI and WC decreased significantly in obese participants, i.e. in the POMA and POMN groups, whereas the mean body fat mass decreased in all groups. The mean TC, LDL‐C, TG and CRP decreased significantly in the two groups with abnormal metabolic profile, i.e. the POMA and PNMO groups. In the last step of the study, conducted 6 months after the baseline survey, the mean WC and body fat mass, as well as the mean LDL‐C, TG and CRP, increased significantly compared with the 2‐month results; however, they remained lower than the baseline values. Comparison of the changes in variables studied between the three groups studied showed that both after 2 and 6 months, all differences were significant, except for the skinfold thickness, HDL‐C, FBG and DBP. After 2 months, the decrease in mean body fat mass, TC, LDL‐C, TG, CRP and SBP was greater in the POMA than in the POMN group. At 6 months, the decrease of these variables, except for body fat mass, remained significantly higher in the POMA than in the POMN group. Both at 2 and 6 months, the decrease in mean BMI, WC, body fat mass, TG and SBP was higher in the POMA than in the PNMO group.
Table 2.
Mean (SD) of within‐group and between‐group changes (Δ) in physical and biochemical variables in different steps of the study (2 months vs. baseline, and 6 months vs. 2 months)
| PNMO | POMN | POMA | P‐value between groups | |||||
|---|---|---|---|---|---|---|---|---|
| 2 months (n = 112) | 6 months (n = 95) | 2 months (n = 114) | 6 months (n = 98) | 2 months (n = 115) | 6 months (n = 105) | 2 months | 6 months | |
| Δ BMI (kg m2) | 0.2 (0.05) | 0.2 (0.04) | −1.1 (0.2)* | −0.7 (0.1) | −1.2 (0.3)* | −0.6 (0.1) | 0.01 § | 0.02 § |
| Δ WC (cm) | −0.4 (0.05) | −0.2 (0.04) | −2.1 (0.4)* | −1.4 (0.3) † | −2.5 (0.4)* | −1.1 (0.2) † | 0.01 § | 0.01 § |
| Δ Skinfold (mm) | −0.07 (0.001) | −0.05 (0.001) | −0.3 (0.001) | −0.1 (0.001) | −0.4 (0.001) | −0.1 (0.001) | NS | NS |
| Δ Fat mass (kg) | −1.7 (0.2)* | −0.4 (0.1) | −2.6 (0.3)* | −1.2 (0.1) † | −3.7 (0.5)* | −1.7 (0.2) † | 0.02 [Link] , [Link] | 0.01 § |
| Δ FBG (mg dL−1) | −0.7 (0.07) | −0.2 (0.01) | −1.1 (0.4) | −0.7 (0.05) | −3.2 (0.6) | −1.4 (0.1) | NS | NS |
| Δ TC (mg dL−1) | −3.1 (0.4)* | −2.4 (0.5) | −1.2 (0.1) | −0.8 (0.4) | −4.1 (0.4)* | −2.8 (0.6) | 0.01 ‡ | 0.02 ‡ |
| Δ LDL‐C (mg dL−1) | −3.2 (0.5)* | −1.8 (0.3) † | −0.7 (0.1) | −0.5 (0.03) | −3.1 (0.7)* | −1.7 (0.1) † | 0.03 ‡ | 0.01 ‡ |
| Δ HDL‐C (mg dL−1) | 1.2 (0.4) | 1.2 (0.2) | 0.7 (0.5) | 0.6 (0.02) | 1.7 (0.7) | 1.6 (0.2) | NS | NS |
| Δ TG (mg dL−1) | −11.6 (2.5)* | −7.2 (1.5) † | −5.4 (1.7) | −2.7 (0.5) | −18.4 (5.1)* | −12.4 (2.8) † | <0.0001 [Link] , [Link] | <0.0001 [Link] , [Link] |
| Δ CRP (mg L−1) | −0.4 (0.05)* | −0.3 (0.01) † | −0.1 (0.01) | −0.1 (0.02) | −0.5 (0.04)* | −0.3 (0.07) † | 0.04 ‡ | 0.04 ‡ |
| Δ SBP (mm Hg) | −0.7 (0.5) | −0.1 (0.1) | −0.4 (0.05) | −0.2 (0.01) | −1.2 (0.2)* | −0.8 (0.1) | 0.02 [Link] , [Link] | 0.04 [Link] , [Link] |
| Δ DBP (mm Hg) | −0.4 (0.5) | −0.2 (0.05) | −0.1 (0.01) | −0.1 (0.08) | −0.8 (0.1) | −0.4 (0.08) | NS | NS |
BMI, body mass index; CRP, C‐ reactive protein; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NS, not significant; PNMO, phenotypically normal metabolically obese; POMA, phenotypically obese metabolically abnormal; POMN, phenotypically obese metabolically normal; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference. *P < 0.05 for within‐group difference of 2 months after the beginning of the intervention vs. the baseline. † P < 0.05 for within‐group difference of 6 months vs. 2 months after the beginning of the intervention. ‡ P < 0.05 for comparison of POMN vs. POMA groups. § P < 0.05 for comparison of POMA vs. PNMO groups. All differences were significant between POMN and PNMO groups except those marked NS between the three groups.
The bivariate regression analysis for change in serum CRP vs. change in measurement of body composition and lipid profile showed that the magnitude of decrease in serum CRP after the 2‐month interventional period correlated significantly with the magnitude of decreases in BMI (β = −7.5, P = 0.04), WC (β = −10.1, P = 0.001) and total fat mass (β = −12.4, P < 0.0001), as well as with TC (β = −2.9, P = 0.03), LDL‐C (β = −2.7, P = 0.04) and TG (β = −6.4, P = 0.02), but not with the triceps' skinfold thickness and HDL‐C.
Discussion
In the current study, a multidisciplinary approach for lifestyle modification was applied in obese adolescents with and without cardio‐metabolic risk factors as well as in normal‐weight adolescents with at least one cardio‐metabolic risk factor. Results obtained indicated that irrespective of the baseline weight status and weight reduction, improvement in dyslipidaemia and inflammation can be achieved by lifestyle modification among youths.
We should acknowledge that consistent with our previous studies in children (Kelishadi et al. 2008d) and adolescents (Kelishadi et al. 2008b), this short‐term trial resulted in small changes in cardio‐metabolic risk factors; however, such favourable changes might have long‐term beneficial effects. Although we had near 20% of dropout in the current study, but as their outcome measures looked similar with those who completed the trial, we suggest that the missing values have not affected the findings of this trial.
The baseline serum TC and LDL‐C were not very high and consequently had small changes, whereas serum TG was considerably high and had greater decline than TC and LDL‐C did. Contrary to Western countries, where the prevalence of hypercholesterolaemia among children and adolescents is of concern, in our population, low HDL‐C and high TG are quiet prevalent. For instance, in 2005, a population‐based study reported that respectively 26 and 20% of rural children in Georgia, USA, had high TC and LDL‐C levels (Davis et al. 2005). At the same time, our national study in Iran revealed that 45.7% of children had dyslipidaemia, the most frequent ones were low HDL‐C (24.8%) and hypertriglyceridaemia (24.5%), followed by hypercholesterolaemia (6.4%) and high LDL‐C (6.3%), respectively (Kelishadi et al. 2006b). In the same study, the prevalence of high TC and LDL‐C was near 15.3 and 15.1%, respectively, among obese children and adolescents (Kelishadi et al. 2008a). We have also documented that the percentiles of TG are higher and the percentiles of HDL‐C are lower in Iranian children and adolescents than in Western populations such as US (Kelishadi et al. 2006b) and German children and adolescents (Kelishadi et al. 2008e). In the current study, there was no significant difference between the mean serum HDL‐C of the group without metabolic abnormality and the two other groups. This is suggested to be because of the considerably low serum level of HDL‐C in our region compared with Western countries, i.e. population‐based studies in the Middle East have shown a considerably high prevalence of low serum HDL‐C in this region (Al‐Lawati et al. 2003; Azizi et al. 2003; Khader et al. 2007). Our national studies in Iran showed that irrespective of weight status, 80% of adults (Delavari et al. 2009) and 25% of children and adolescents (2007b, 2008a) have low serum HDL‐C levels. The high prevalence of low HDL‐C, even in many individuals without excess weight, supports an ethnic predisposition to this lipid disorder. The findings of a recent study of the significant association between migration from Iran to Sweden and the prevalence of hypertension and smoking, but not dyslipidaemia (Koochek et al. 2008), provide further confirmatory evidence on the ethnic predisposition to low HDL‐C. This ethnic predisposition should be examined by future genetic studies. However, in spite of an ethnic predisposition to this type of dyslipidaemia, the improvement of the lipid profile in the current study suggests that a genetic environment interaction might explain this lipid disorder. Diets high in trans unsaturated fat may lower HDL‐C levels, increase TG levels and interfere with fatty acid metabolism. Our previous study (2004, 2008f) showed an association between the improper quality of the consumed fat (rich in saturated and trans fatty acids) and the high prevalence of dyslipidaemia in Iranian youths. The current study showed that lifestyle change was associated with improvement in cardio‐metabolic risk factors, notably some components of the metabolic syndrome, i.e. obesity, high TG and high BP. The current trial was conducted among youths with cardiovascular risk factors either in terms of obesity and/or cardio‐metabolic risk factors, hence, it included a diet recommendation by a dietitian as well as supervised physical activity with regular monitoring. However, more simple motivational lifestyle modification programmes with lower costs can be implemented at the population level in different community settings and can be beneficial not only for obese children and adolescents, but also for normal‐weight ones, either with ethnic predisposition to cardio‐metabolic disorders or with improper dietary and physical‐activity habits. Given that small changes at the individual level may result in large benefits at the population level, the magnitude of the impact of such intervention trials might have long‐term population‐level effects.
Obesity represents a chronic inflammatory status, and weight reduction is shown to normalize mediators of inflammation in obese children (Amati et al. 2007). It is well documented that the higher the CRP concentrations, the higher the possibility of having metabolic syndrome; and the lower the adiposity, the lower the CRP and probably the lower the risk of future cardiovascular events (Ford et al. 2005; de Ferranti et al. 2006). However, in the current study, the CRP level did not follow this trend, and irrespective of weight status, those children with cardio‐metabolic disorders had higher CRP levels. Several studies have demonstrated that obese children/adolescents with different ethnic backgrounds exhibited higher levels of CRP as a marker of inflammation (Ford et al. 2005; de Ferranti et al. 2006; Giannini et al. 2008). Serum CRP is associated with abdominal obesity in youths (Kelishadi et al. 2007c), and it is documented that children with metabolic syndrome have higher levels of CRP in comparison with obese children without this disorder (Soriano‐Guillen et al. 2008). Given the association of CRP with fasting plasma glucose and TG in obese children, it might be suggested that the microinflammation can be a pathogenic base of chronic diseases such as diabetes mellitus rather than their outcome. Then, serum high‐sensitivity CRP can be suggested as a simple and cheap predictor of early atherosclerosis, impaired glucose and lipid metabolism in obese children. Our findings were in line with aforementioned studies in showing high serum levels of CRP among obese adolescents, however, the novelty of our study was in showing the pro‐inflammatory state only in obese adolescents with abnormal metabolic profile (POMA) and not in POMN individuals. The other novelty of our study was in finding that the serum level of CRP in obese and normal‐weight adolescents with cardio‐metabolic risk factors was higher than in obese adolescents without a metabolic disorder (POMN). In addition, we found that irrespective of the weight status, the improvement of CRP and lipid profile was intercorrelated, and reduction in BMI, WC and body fat mass was associated with improvement of the CRP level. Longitudinal studies with long‐term follow‐up are necessary to confirm such associations and their possible clinical implications.
Some previous studies have shown that nutrition can affect inflammation; dietary patterns high in refined starches, sugar, and saturated and trans fatty acids, poor in natural antioxidants and fibre from fruits, vegetables, and whole grains, and poor in omega‐3 fatty acids may cause an activation of the innate immune system, most likely by an excessive production of pro‐inflammatory cytokines associated with a reduced production of anti‐inflammatory cytokines (Giugliano et al. 2006). However, the results of different studies are controversial; most studies confirmed such association, but few of them did not (Fredrikson et al. 2004). Previous studies in obese youths confirmed a significant association between dietary habits and the low‐grade systemic inflammation with the risk of type 2 diabetes (Syrenicz et al. 2006). Our finding about the existence of cardio‐metabolic disorders, notably high TG and low HDL‐C, which is consistent with previous studies in our community (Azizi et al. 2004; 2004, 2008a), might be, at least in part, because of improper dietary habits among families, especially using hydrogenated fats rich in saturated and trans fatty acids in preparing foods, as well as consumption of fast foods and snacks rich in trans fatty acids. Many efforts are currently being made at a national level to improve the quality of fat produced in Iran. Such efforts might improve the lipid profile at the population level.
In addition, levels of physical activity are negatively related to body fatness in children (Ruiz et al. 2006), however, cardiorespiratory fitness is shown to be more strongly correlated to metabolic risk than physical activity (Rizzo et al. 2007; McMurray et al. 2008). While an interaction of fitness and fatness on cardio‐metabolic risk factors in children has been shown (Eisenmann et al. 2007), and body fat appears to have a central role in the association of fitness with metabolic risk (Eisenmann et al. 2007; Rizzo et al. 2007), some studies demonstrated an inverse independent association between physical activity and metabolic risk (Brage et al. 2004). It is suggested that some of the beneficial effects of physical activity may be mediated through decreasing subclinical inflammation both in adults (Wannamethee et al. 2005) and in adolescents (de Ferranti et al. 2006). Hence, the low physical activity and sedentary lifestyle habits of children and adolescents in our community (Kelishadi et al. 2008f) might be another reason for the dyslipidaemia and pro‐inflammatory state documented in normal‐weight adolescents. The improvement of these metabolic disorders and the pro‐inflammatory state in PNMO adolescents may confirm such association.
The current trial was successful in the improvement of the cardio‐metabolic risk factors even in those individuals with normal triceps skinfold, BMI and WC at baseline (the PNMO group) who did not have extra adipose tissue to be reduced after the trial, and consequently, did not have significant change in their anthropometric indexes. This finding suggests that irrespective of changes in body composition, healthy lifestyle per se seems to have beneficial effects on the cardio‐metabolic disorders among children and adolescents.
Given that cardio‐metabolic risk factors track from early life to adulthood, and that childhood CRP value predicts adult CRP, and this association is found to be independent of other metabolic risk factors such as serum lipids, BP, smoking, obesity indices and insulin (Juonala et al. 2006), and that high CRP is one of the youth determinants of adult metabolic syndrome (Mattsson et al. 2008), all children and adolescents, and not only those with weight disorders, should be encouraged to have a healthier lifestyle for reducing the upcoming burden of chronic diseases.
Conclusion
The findings of the present study underscore the importance of healthy lifestyle from early life and represent practical directions for the future planning of programmes designed for primordial/primary prevention of chronic diseases. Such programmes should be implemented at the population level and should not be limited to obese children. Evaluation of other pro‐inflammatory and microinflammatory indices such as tumor necrosis factor‐alpha (TNF‐alpha), advanced glycation end products, anti‐food immunoglobulin G (IgG), circulating fatty acid‐binding protein aP2 and acute‐phase protein serum amyloid A and/or adiponectin in future studies might be useful for completing part(s) of the current puzzle of obesity and cardio‐metabolic disorders in children.
Source of funding
The study was funded by grant no. 185177 from the Vice Chancellery for Research, Isfahan University of Medical Sciences, Isfahan, Iran.
No conflicts of interest have been declared.
None declared.
References
- Al‐Lawati J.A., Mohammed A.J., Al‐Hinai H.Q. & Jousilahti P. (2003) Prevalence of the metabolic syndrome among Omani adults. Diabetes Care 26, 1781–1785. [DOI] [PubMed] [Google Scholar]
- Amati L., Chiloiro M., Jirillo E. & Covelli V. (2007) Early pathogenesis of atherosclerosis: the childhood obesity. Current Pharmaceutical Design 13, 3696–3700. [DOI] [PubMed] [Google Scholar]
- Azizi F., Mirmiran P. & Azadbakht L. (2004) Predictors of cardiovascular risk factors in Tehranian adolescents: Tehran Lipid and Glucose Study. International Journal for Vitamin and Nutrition Research 74, 307–312. [DOI] [PubMed] [Google Scholar]
- Azizi F., Salehi P., Etemadi A. & Zahedi‐Asl S. (2003) Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Research and Clinical Practice 61, 29–37. [DOI] [PubMed] [Google Scholar]
- Brage S., Wedderkopp N., Ekelund U., Franks P.W., Wareham N.J., Andersen L.B. et al. (2004) Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS). Diabetes Care 27, 2141–2148. [DOI] [PubMed] [Google Scholar]
- Davis C.L., Flickinger B., Moore D., Bassali R., Domel Baxter S. et al. (2005) Prevalence of cardiovascular risk factors in school children in a rural Georgia community. The American Journal of the Medical Sciences 330, 53–59. [DOI] [PubMed] [Google Scholar]
- Delavari A., Forouzanfar M.H., Alikhani S., Sharifian A. & Kelishadi R. (2009) First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for non‐communicable diseases of Iran. Diabetes Care 32, 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann J.C. (2007) Aerobic fitness, fatness and the metabolic syndrome in children and adolescents. Acta Paediatrica 96, 1723–1729. [DOI] [PubMed] [Google Scholar]
- Eisenmann J.C., Welk G.J., Wickel E.E. & Blair S.N. (2007) Combined influence of cardiorespiratory fitness and body mass index on cardiovascular disease risk factors among 8–18 year‐old youth: the Aerobics Center Longitudinal Study. International Journal of Pediatric Obesity 2, 66–72. [DOI] [PubMed] [Google Scholar]
- De Ferranti S.D., Gauvreau K., Ludwig D.S., Newburger J.W. & Rifai N. (2006) Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clinical Chemistry 52, 1325–1330. [DOI] [PubMed] [Google Scholar]
- Ford E.S., Ajani U.A. & Mokdad A.H. (2005) The metabolic syndrome and concentrations of C‐reactive protein among U.S. youth. Diabetes Care 28, 878–881. [DOI] [PubMed] [Google Scholar]
- Fredrikson G.N., Hedblad B., Nilsson J.A., Alm R., Berglund G. & Nilsson J. (2004) Association between diet, lifestyle, metabolic cardiovascular risk factors, and plasma C‐reactive protein levels. Metabolism 53, 1436–1442. [DOI] [PubMed] [Google Scholar]
- Giannini C., De Giorgis T., Scarinci A., Ciampani M., Marcovecchio M.L., Chiarelli F. et al. (2008) Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre‐pubertal children. Atherosclerosis 197, 448–456. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Ceriello A. & Esposito K. (2006) The effects of diet on inflammation: emphasis on the metabolic syndrome. Journal of the American College of Cardiology 48, 677–685. [DOI] [PubMed] [Google Scholar]
- Johnson M.S., Figueroa‐Colon R., Herd S.L., Fields D.A., Sun M., Hunter G.R. et al. (2000) Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics 106, E50. [DOI] [PubMed] [Google Scholar]
- Juonala M., Viikari J.S., Ronnemaa T., Taittonen L., Marniemi J. & Raitakari O.T. (2006) Childhood C‐reactive protein in predicting CRP and carotid intima‐media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arteriosclerosis, Thrombosis, and Vascular Biology 26, 1883–1888. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Pour M.H., Zadegan N.S., Kahbazi M., Sadry G., Amani A. et al. (2004) Dietary fat intake and lipid profiles of Iranian adolescents: Isfahan Healthy Heart Program – Heart Health Promotion from Childhood. Preventive Medicine 39, 760–766. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Ardalan G., Gheiratmand R., Majdzadeh R., Delavari A., Heshmat R. et al. (2006a) Blood pressure and its influencing factors in a national representative sample of Iranian children and adolescents: the CASPIAN Study. European Journal of Cardiovascular Prevention and Rehabilitation 13, 956–963. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Ardalan G., Gheiratmand R. & Ramezani A. (2006b) Is family history of premature cardiovascular diseases appropriate for detection of dyslipidemic children in population‐based preventive medicine programs? The CASPIAN Study. Pediatric Cardiology 27, 729–736. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Gouya M.M., Ardalan G., Hosseini M., Motaghian M., Delavari A. et al. (2007a) First reference curves of waist and hip circumferences in an Asian population of youths: CASPIAN Study. Journal of Tropical Pediatrics 53, 158–164. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Gheiratmand R., Ardalan G., Adeli K., Mehdi Gouya M., Mohammad Razaghi E. et al. (2007b) Association of anthropometric indices with cardiovascular disease risk factors among children and adolescents: CASPIAN Study. International Journal of Cardiology 117, 340–348. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Sharifi M., Khosravi A. & Adeli K. (2007c) Relationship between C‐reactive protein and atherosclerotic risk factors and oxidative stress markers among young persons 10–18 years old. Clinical Chemistry 53, 456–464. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Cook S.R., Motlagh M.E., Gouya M.M., Ardalan G., Motaghian M. et al. (2008a) Metabolically obese normal weight and phenotypically obese metabolically normal youths: the CASPIAN Study. Journal of the American Dietetic Association 108, 82–90. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Hashemi M., Mohammadifard N., Asgary S. & Khavarian N. (2008b) Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clinical Chemistry 54, 147–153. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Ardalan G., Gheiratmand R., Majdzadeh R., Hosseini M., Gouya M.M. et al. (2008c) Thinness, overweight and obesity in a national sample of Iranian children and adolescents: CASPIAN Study. Child: Care, Health and Development 34, 44–54. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Hashemipour M., Mohammadifard N., Alikhassy H. & Adeli K. (2008d) Short‐ and long‐term relationships of serum ghrelin with changes in body composition and the metabolic syndrome in prepubescent obese children following two different weight loss programs. Clinical Endocrinology (Oxford) 69, 721–729. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Schwandt P., Haas G.M., Hosseini M. & Mirmoghtadaee P. (2008e) Reference curves of anthropometric indices and serum lipid profiles in representative samples of Asian and European children. Archives of Medical Science 4, 329–335. [Google Scholar]
- Kelishadi R., Gouya M.M., Adeli K., Ardalan G., Gheiratmand R., Majdzadeh R. et al. (2008f) Factors associated with the metabolic syndrome in a national sample of youths: CASPIAN Study. Nutrition, Metabolism, and Cardiovascular Diseases 18, 461–470. [DOI] [PubMed] [Google Scholar]
- Khader Y., Bateiha A., El‐Khateeb M., Al‐Shaikh A. & Ajlouni K. (2007) High prevalence of the metabolic syndrome among Northern Jordanians. Journal of Diabetes and its Complications 21, 214–219. [DOI] [PubMed] [Google Scholar]
- Koochek A., Mirmiran P., Azizi T., Padyab M., Johansson S.E., Karlström B. et al. (2008) Is migration to Sweden associated with increased prevalence of risk factors for cardiovascular disease? European Journal of Cardiovascular Prevention and Rehabilitation 15, 78–82. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R.J., Ogden C.L., Guo S.S., Grummer‐Strawn L.M., Flegal K.M., Mei Z. et al. (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics 11, 1–190. [PubMed] [Google Scholar]
- Lucas B. (2000) Nutrition in childhood In: Kraue's Food, Nutrition & Diet Therapy (eds Mahan L.K. & Escott‐Stumps S.E.), 10th edn, pp. 242–245. Saunders: Philadelphia, PA. [Google Scholar]
- McMurray R.G., Bangdiwala S.I., Harrell J.S. & Amorim L.D. (2008) Adolescents with metabolic syndrome have a history of low aerobic fitness and physical activity levels. Dynamic Medicine 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J.R. & Schaefer E.J. (1987) Automated enzymatic standardized lipid analyses for plasma and lipid lipoprotein fractions. Clinica Chimica Acta 166, 1–8. [DOI] [PubMed] [Google Scholar]
- Mattsson N., Ronnemaa T., Juonala M., Viikari J.S. & Raitakari O.T. (2008) Childhood predictors of the metabolic syndrome in adulthood. The Cardiovascular Risk in Young Finns Study. Annals of Medicine 40, 542–552. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114, 555–576. [PubMed] [Google Scholar]
- Poirier P. & Despres J.P. (2001) Exercise in weight management of obesity. Cardiology Clinics 19, 459–470. [DOI] [PubMed] [Google Scholar]
- Ramachandran A., Snehalatha C., Yamuna A., Murugesan N. & Narayan K.M. (2007) Insulin resistance and clustering of cardiometabolic risk factors in urban teenagers in southern India. Diabetes Care 30, 1828–1833. [DOI] [PubMed] [Google Scholar]
- Rizzo N.S., Ruiz J.R., Hurtig‐Wennlof A., Ortega F.B. & Sjostrom M. (2007) Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European Youth Heart Study. The Journal of Pediatrics 150, 388–394. [DOI] [PubMed] [Google Scholar]
- Roberts C.K., Won D., Pruthi S., Kurtovic S., Sindhu R.K., Vaziri N.D. et al. (2006) Effect of a short‐term diet and exercise intervention on oxidative stress, inflammation, MMP‐9, and monocyte chemotactic activity in men with metabolic syndrome factors. Journal of Applied Physiology 100, 1657–1665. [DOI] [PubMed] [Google Scholar]
- Ruiz J.R., Rizzo N.S., Hurtig‐Wennlof A., Ortega F.B., Warnberg J. & Sjostrom M. (2006) Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. The American Journal of Clinical Nutrition 84, 299–303. [DOI] [PubMed] [Google Scholar]
- Soriano‐Guillen L., Hernandez‐Garcia B., Pita J., Dominguez‐Garrido N., Del Rio‐Camacho G. & Rovira A. (2008) High‐sensitivity C‐reactive protein is a good marker of cardiovascular risk in obese children and adolescents. European Journal of Endocrinology 159, R1–R4. [DOI] [PubMed] [Google Scholar]
- Stockli R. & Keller U. (2003) [Effectiveness of therapeutic interventions in obesity. Praxis (Bern 1994) 92, 1999–2006. [DOI] [PubMed] [Google Scholar]
- St‐Onge M.P., Janssen I. & Heymsfield S.B. (2004) Metabolic syndrome in normal‐weight Americans: new definition of the metabolically obese, normal‐weight individual. Diabetes Care 27, 2222–2228. [DOI] [PubMed] [Google Scholar]
- Syrenicz A., Garanty‐Bogacka B., Syrenicz M., Gebala A. & Walczak M. (2006) Low‐grade systemic inflammation and the risk of type 2 diabetes in obese children and adolescents. Neuro Endocrinology Letters 27, 453–458. [PubMed] [Google Scholar]
- Wannamethee S.G., Lowe G.D., Shaper A.G., Rumley A., Lennon L. & Whincup P.H. (2005) The metabolic syndrome and insulin resistance: relationship to haemostatic and inflammatory markers in older non‐diabetic men. Atherosclerosis 181, 101–108. [DOI] [PubMed] [Google Scholar]
- Weker H. (2006) [Simple obesity in children. A study on the role of nutritional factors. Medycyna Wieku Rozwojowego 10, 3–191. [PubMed] [Google Scholar]
- WHO (1995) Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. Technical Report Series no. 854. World Health Organization: Geneva. [PubMed] [Google Scholar]
- Zimmet P., Alberti K.G., Kaufman F., Tajima N., Silink M., Arslanian S. et al. (2007) The metabolic syndrome in children and adolescents: an IDF consensus report. Pediatric Diabetes 8, 299–306. [DOI] [PubMed] [Google Scholar]


