Abstract
This review summarizes the impact of stunting, highlights recent research findings, discusses policy and programme implications and identifies research priorities. There is growing evidence of the connections between slow growth in height early in life and impaired health and educational and economic performance later in life. Recent research findings, including follow‐up of an intervention trial in Guatemala, indicate that stunting can have long‐term effects on cognitive development, school achievement, economic productivity in adulthood and maternal reproductive outcomes. This evidence has contributed to the growing scientific consensus that tackling childhood stunting is a high priority for reducing the global burden of disease and for fostering economic development. Follow‐up of randomized intervention trials is needed in other regions to add to the findings of the Guatemala trial. Further research is also needed to: understand the pathways by which prevention of stunting can have long‐term effects; identify the pathways through which the non‐genetic transmission of nutritional effects is mediated in future generations; and determine the impact of interventions focused on linear growth in early life on chronic disease risk in adulthood.
Keywords: stunting, malnutrition, child growth, supplementary feeding, child development, economic development
Introduction
Children throughout the world can reach their growth potential if they are nurtured in healthy environments and their caregivers follow recommended health, nutrition and care practices. Stunting indicates a failure to achieve one's genetic potential for height (Golden 2009). A child is considered ‘stunted’ if his or her height is more than two standard deviations below the World Health Organization standard (WHO Multicentre Growth Reference Study Group, 2006). The main causes of stunting include intrauterine growth retardation, inadequate nutrition to support the rapid growth and development of infants and young children and frequent infections during early life (Frongillo 1999). Although a child may not be classified as ‘stunted’ until 2–3 years of age, the process of becoming stunted typically begins in utero. The result – a very short height – usually reflects the persistent, cumulative effects of poor nutrition and other deficits that often span across several generations. This review summarizes the impact of stunting, highlighting research findings published in the past 5 years.
Stunting affects one‐third of children under 5 in low‐income and middle‐income countries, for a total of 178 million children (Black et al. 2008). Stunting often goes unrecognized by families who live in communities where short stature is so common that it seems normal. Even among health workers, stunting generally does not receive the same attention as underweight or wasting (low weight for height), especially if height is not routinely measured as part of community health programmes. Many families, health workers and policy makers are unaware of the consequences of stunting so it may not be viewed as a public health issue.
The prevalence of stunting is highest in Africa (40%), and the largest number of stunted children is in Asia (112 million), mostly in South‐central Asia, as shown in Table 1. Ninety per cent of the overall global burden of child stunting is attributable to 36 countries. Stunting is found at many levels in society. In Bangladesh, for example, stunting in children less than 5 years of age was found in one‐fourth of the richest households [National Institute of Population Research and Training (NIPORT) et al. 2009]. In developing countries, stunting is more prevalent than underweight (low weight for age, 20%) or wasting (low weight for height, 10%) possibly because height gain is even more sensitive to dietary quality than is weight gain.
Table 1.
Stunting in children under 5 years of age, based on WHO Child Growth Standards
| Children <5 years in millions | Number stunted in millions | Percentage stunted | |
|---|---|---|---|
| Africa | 142 | 57 | 40 |
| Eastern | 49 | 24 | 50 |
| Middle | 20 | 8 | 42 |
| Northern | 22 | 5 | 25 |
| Southern | 6 | 2 | 30 |
| Western | 45 | 17 | 38 |
| Asia | 357 | 112 | 31 |
| Eastern | 95 | 14 | 15 |
| South‐central | 181 | 74 | 41 |
| Southeast | 55 | 19 | 34 |
| Western | 25 | 5 | 21 |
| Latin America and the Caribbean | 57 | 9 | 16 |
| Caribbean | 4 | 0.3 | 8 |
| Central America | 16 | 4 | 23 |
| South America | 37 | 5 | 37 |
| All developing countries | 556 | 178 | 32 |
Source: Black et al. 2008.
WHO, World Health Organization.
Key messages
-
•
Stunting is both a direct cause of short adult height and suboptimal function later in life and a key marker of the underlying processes in early life that lead to poor growth and other adverse outcomes.
-
•
Stunting is a risk factor for diminished survival, childhood and adult health, learning capacity and productivity.
-
•
Prevention of stunting should be made a priority. Intervention strategies should target the ‘window of opportunity’ from the pre‐conception period through the first 2 years of life and include interventions demonstrated to have a positive impact on linear growth.
-
•
Additional research is needed to confirm findings for other regions, to understand the pathways through which stunting can have long‐term effects and to identify pathways through which the non‐genetic transmission of nutritional effects is mediated in future generations.
Stunting often begins in utero
During fetal life and the first 2 years after birth, nutritional requirements to support rapid growth and development are very high. Average height‐for‐age z‐scores are already low at birth (below 0, the standard score or population average) in several regions and decline sharply during the first 24 months of life but show no further decline or any improvement thereafter (Victora et al. 2010), as illustrated in Fig. 1.
Figure 1.
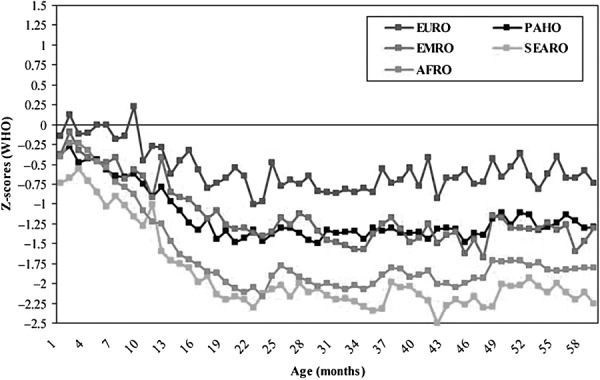
Timing of growth faltering: mean height‐for‐age z‐scores by age for 54 studies, relative to the WHO standard. WHO Regional Office for Europe (EURO); WHO Regional Office for the Eastern Mediterranean (EMRO); WHO Regional Office for Africa (AFRO); Pan American Health Organization (PAHO); WHO Regional Office for South‐East Asia (SEARO). Source: Victora et al., reproduced with permission from Pediatrics 125, e473–e480. Copyright 2010 by the AAP. AAP, American Academy of Pediatrics; WHO, World Health Organization.
Maternal undernutrition, anaemia, tobacco use and indoor air pollution can restrict fetal growth and result in low birthweight. Table 2 shows that the prevalence of low body mass index (BMI) among women 15–49 years of age may be as high as 35% in some countries. In many countries, more than half of all women of reproductive age are anaemic (Fig. 2). Diets of poor nutritional quality during pregnancy, infancy and early childhood lead to inadequate nutrient intake. Frequent infections during the first 2 years of life also contribute to the high risk of becoming stunted during this period.
Table 2.
Nutritional status of women: among women aged 15–49 years, the percentage with height under 145 cm and the percentage with body mass index <18.5 kg m‐ 2 (thin), DHS Surveys 2003–2009
| Country and year of DHS | Height: percentage under 145 cm | Body mass index: percentage thin (<18.5 kg m−2) |
|---|---|---|
| Sub‐Saharan Africa | ||
| Benin 2006 | 1.4 | 9.2 |
| Burkina Faso 2003 | 0.5 | 20.8 |
| Chad 2004 | 0.3 | 22.1 |
| Congo DR 2007 | 4.0 | 18.5 |
| Ethiopia 2005 | 3.2 | 26.5 |
| Ghana 2008 | 1.4 | 8.6 |
| Guinea 2005 | 1.2 | 13.2 |
| Kenya 2008–2009 | 1.2 | 12.3 |
| Liberia 2007 | 2.5 | 10 |
| Madagascar 2003–2004 | 6.5 | 19.2 |
| Malawi 2004 | 3.1 | 9.2 |
| Mali 2006 | 0.8 | 13.5 |
| Mozambique 2003 | 4.9 | 8.6 |
| Namibia 2006–2007 | 1.0 | 15.9 |
| Niger 2006 | 0.7 | 19.2 |
| Nigeria 2008 | 3.0 | 12.2 |
| Rwanda 2005 | 3.8 | 9.8 |
| Senegal 2005 | 0.4 | 18.2 |
| Tanzania 2004–2005 | 3.4 | 10.4 |
| Uganda 2006 | 1.9 | 12.1 |
| Zambia 2007 | 2.6 | 9.6 |
| Zimbabwe 2005–2006 | 0.7 | 9.2 |
| South/Southeast Asia | ||
| Bangladesh 2007 | 15.1 | 29.7 |
| Cambodia 2005 | 7.7 | 20.3 |
| India 2005–2006 | 11.4 | 35.6 |
| Nepal 2006 | 14.1 | 24.4 |
| Latin America and the Caribbean | ||
| Bolivia 2003 | 10.3 | 1.9 |
| Guatemala 2008–2009 | 29.4 | 1.3 |
| Haiti 2005–2006 | 1.2 | 15.5 |
| Honduras 2005–2006 | 9.8 | 4 |
| Peru 2004–2008 continuous | 11.2 | 1.8 |
DHS, Demographic and Health Surveys.
Figure 2.
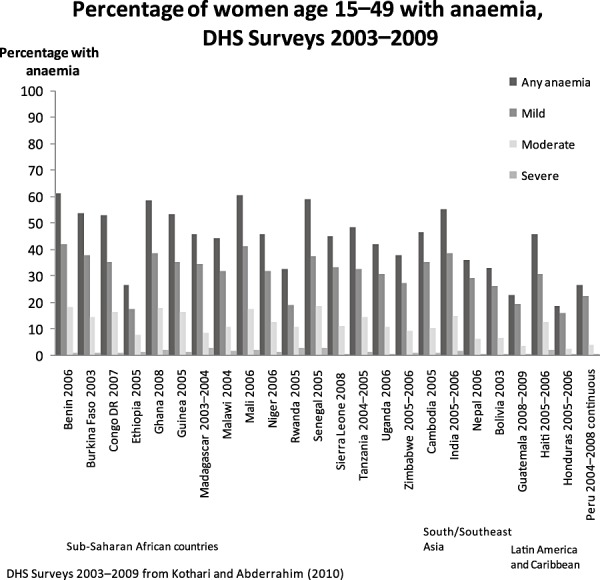
Percentage of women aged 15–49 with anaemia, DHS 2003–2009. Anaemia cut‐offs, non‐pregnant women: any, <12.0 g dL−1; mild, 10.0–11.9 g dL−1; moderate, 7.0–9.9 g dL−1; and severe, <7.0 g dL−1 and pregnant women: any, <11.0 g dL−1; mild, 10.0–10.9 g dL−1; moderate, 7.0–9.99 g dL−1; and severe, <7.0 g dL−1. DHS, Demographic and Health Surveys.
Children who are stunted usually grow up to be stunted adults (Martorell et al. 1994). An opportunity exists to make up some of the height deficit during adolescence because stunted children often experience a delay in skeletal maturation, lengthening the total period of time for growth in height. However, the potential for substantially reducing the height deficit during adolescence is limited because the maturational delays are usually shorter than 2 years (Martorell et al. 1994). Moreover, adolescents who enter this period stunted are often living under the same adverse nutritional, socio‐economic and environmental conditions that triggered stunting when they were young children.
Consequences of stunting
Childhood stunting is related to long‐term consequences in two ways:
-
•
as a direct cause of short adult height and suboptimal function later in life and
-
•
as a key marker of the underlying processes in early life that lead to poor growth and other adverse outcomes.
Scientific understanding of stunting as a direct cause of adverse consequences is incomplete, in part because most of the evidence comes from observational studies. Nonetheless, there is growing evidence of the connections between slow growth in height in early life and impaired health and educational and economic performance later in life.
The Maternal and Child Undernutrition Study Group (Victora et al. 2008) reviewed cohort studies from five low‐income and middle‐income countries: Brazil, Guatemala, India, Philippines and South Africa. The studies involved long‐term follow‐up of children into late adolescence and adulthood. The study group concluded that small size at birth and childhood stunting were linked with short adult stature, reduced lean body mass, less schooling, diminished intellectual functioning, reduced earnings and lower birthweight of infants born to women who themselves had been stunted as children. Recent evidence also indicates that children born to women who are stunted are at greater risk of dying than children of mothers with normal height (Ozaltin et al. 2010). The links between stunting and health, educational and economic outcomes are discussed below and illustrated in Fig. 3.
Figure 3.
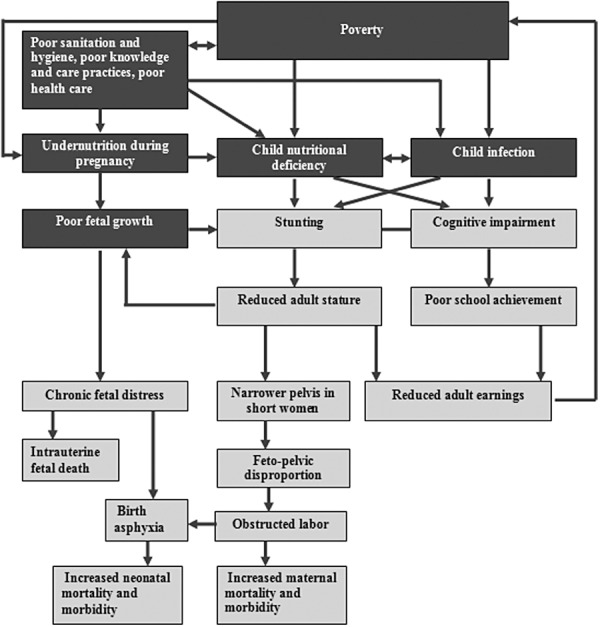
Potential causal pathways for long‐term consequences of stunting (Source: adapted from Grantham‐McGregor et al. 2007).
Long‐term health consequences of maternal stunting
A woman who is less than 145 cm or 4′7″ is considered to be stunted, which presents risks to the survival, health and development of her offspring. Table 2 shows the percentage of women of reproductive age who are stunted. The prevalence of stunting among women is highest in South/Southeast Asia (e.g. 15% in Bangladesh) and in parts of Latin America (e.g. 29% in Guatemala).
Maternal stunting can restrict uterine blood flow and growth of the uterus, placenta and fetus. Intrauterine growth restriction (IUGR) is associated with many adverse fetal and neonatal outcomes (Kramer 1987; Kramer et al. 1990; Black et al. 2008). During pregnancy, IUGR may lead to chronic fetal distress or fetal death. If born alive, the growth‐restricted infant is at higher risk for serious medical complications (Black et al. 2008). Infants with IUGR often suffer from delayed neurological and intellectual development, and their deficit in height generally persists to adulthood.
Maternal stunting is consistently associated with an elevated risk of perinatal mortality (stillbirths and deaths during the first 7 days after birth) (Lawn et al. 2009), mostly related to obstructed labour resulting from a narrower pelvis in short women. In a hospital‐based study in Nigeria, obstructed labour accounted for 53% of perinatal mortality (Omole‐Ohonsi & Ashimi 2007). Perinatal mortality from obstructed labour is largely the result of birth asphyxia. Mothers with height shorter than 145 cm are more likely to have an infant with birth asphyxia (Lee et al. 2009). Globally, birth asphyxia accounts for 23% of the four million neonatal deaths each year (Lawn et al. 2005). An estimated one million children who survive birth asphyxia live with chronic neuro‐developmental disorders, including cerebral palsy, mental retardation and learning disabilities (World Health Organization 2005).
In a recent analysis of 109 Demographic and Health Surveys (DHS) conducted between 1991 and 2008 in 54 countries, children (under 5 years of age) who were born to the shortest mothers (<145 cm) had a 40% increased risk of mortality after adjusting for multiple factors (Ozaltin et al. 2010). Although the percentage of mothers shorter than 145 cm is low in most countries, the analysis showed an elevated risk of child mortality with each lower category of maternal height, compared with mothers ≥160 cm in height (adjusted relative risks of 1.06, 1.13, 1.23 and 1.40 for the height categories of 155–159.9, 150–154.9, 145–149.9 and <145 cm, respectively). The effect of short maternal stature on child mortality was comparable to the effect of having no education or being in the poorest 20% of households. The likely explanations for this finding include an elevated risk of perinatal death, for the reasons explained above, as well as longer‐term effects of IUGR on child nutrition and immune function that increase the risk of child mortality.
As mentioned above, short maternal stature increases the risk of disparity in size between the baby's head and the mother's pelvis. Because of this disproportion, short mothers are less likely to have a successful spontaneous vaginal delivery (Kwawukume et al. 1993; Merchant et al. 2001), which increases the risk of maternal mortality and short‐ and long‐term disability. If timely referral to a well‐equipped hospital occurs, a Caesarean section can be performed; however, even a Caesarean section carries potential risks of complications that can jeopardize maternal and newborn health. Failure to deliver by Caesarean section in time may lead to more serious consequences of obstructed labour. These consequences can include injury to the birth passage, post‐partum haemorrhage, rupture of the uterus, genital sepsis or fistula, leading to urinary dribbling or incontinence. In the worst case scenario, obstructed labour can lead to maternal death, mostly because of ruptured uterus or puerperal sepsis. The percentages of maternal mortality attributable to obstructed labour are 4% in Africa, 9% in Asia and 13% in Latin America and the Caribbean (Khan et al. 2006). Mothers who survive but have long‐term disability due to complications such as fistula experience social, economic, emotional and psychological consequences that have an enormous impact on maternal health and well‐being (Ahmed & Holtz 2007).
Decreased maternal stature is also associated with an increased risk of underweight and stunting among offspring. In their analysis of DHS in 54 countries, Ozaltin et al. (2010) found that a 1‐cm decrease in height was associated with an increased risk of underweight and stunting. Compared with the tallest mothers (≥160 cm), each lower‐height category had a substantially higher risk of underweight and stunting among children, with the highest risk for mothers shorter than 145 cm. The association between maternal height and stunting was statistically significant in 52 of 54 countries (96%) analysed.
Growth restriction in early life is linked not only to short adult height but also to certain metabolic disorders and chronic diseases in adulthood. Data from the Maternal and Child Undernutrition Study Group (Victora et al. 2008) indicate that lower birthweight (which is strongly correlated with birth length) and undernutrition in childhood are risk factors for high glucose concentrations, blood pressure and harmful lipid profiles in adulthood after adjusting for adult height and BMI. The ‘developmental origins of health and disease’ hypothesis posits that the intrauterine and early post‐natal environment can modify expression of the fetal genome and lead to lifelong alterations in metabolic, endocrine and cardiovascular function (Gluckman et al. 2010). In this case, it is likely that the process of stunting is harmful and not necessarily short stature itself.
Long‐term educational and economic consequences of child stunting
The process of becoming stunted, due to restricted nutrient supply and/or frequent infection, is likely a common cause of both short stature and structural and functional damage to the brain, resulting in delay in the development of cognitive functions as well as permanent cognitive impairments (Kar et al. 2008). The Maternal and Child Undernutrition Study Group, using the same pooled cohort mentioned above, found that being stunted at 24 months was associated with a reduction in schooling of 0.9 year, an older age at school enrolment and a 16% increased risk of failing at least one grade in school after controlling for confounding variables such as sex, socio‐economic status and maternal schooling (Martorell et al. 2010a). Evidence from other developing countries also indicates that being stunted between 12 and 36 months of age is associated with poorer cognitive performance and lower school achievement in middle childhood (Grantham‐McGregor et al. 2007). Short stature has also been linked to lower economic productivity. For example, in a large cross‐sectional study in Brazil, a 1% increase in height was associated with a 2.4% increase in wages (Thomas & Strauss 1997). Taller men and women earned more even after controlling for education and other indicators of health such as BMI, per capita energy intake and per capita protein intake.
The most convincing evidence on these consequences comes from long‐term follow‐up studies of randomized trials, such as the large‐scale nutritional supplementation trial carried out in Guatemala between 1969 and 1977 (Box 1, Fig. 4 and Table 3), the only one of the five cohort studies examined by the Maternal and Child Undernutrition Study Group that used an experimental design. Several recent papers have evaluated the impact of nutrition supplementation in early life on stunting and on various aspects of the development of human capital in adulthood. The Institute of Nutrition of Central America and Panama (INCAP) Oriente Longitudinal Study was a large supplementary feeding trial targeted to pregnant and lactating women and their children from birth to 7 years of age, which was conducted in four rural Guatemalan villages (Martorell, 1992). Subsequent follow‐up studies occurred in 1988–2007 through backward tracing of the original population up to 40 years later (Ramirez‐Zea et al. 2010). The trial included two sets of two matched villages. One village in each set was randomly selected to receive either a high‐protein (6.4 g 100 mL−1), high‐energy (91 kcal 100 mL−1) supplement called ‘Atole’ or a non‐protein, low‐energy (33 kcal 100 mL−1) supplement called ‘Fresco’, the nutrient composition of which has been described elsewhere (Martorell et al. 1995; Ramirez‐Zea et al. 2010). Dry skim milk was the predominant source of energy and protein in Atole. From October 1971 until the end of the intervention in 1977, both supplements were fortified with several micronutrients (iron, fluoride, thiamine, riboflavin, niacin, ascorbic acid and vitamin A) in equal concentrations by volume (Martorell et al. 1995). Fresco was given as a control for social interaction associated with attending the feeding centre, which might have influenced certain outcomes such as cognitive development.
Figure 4.
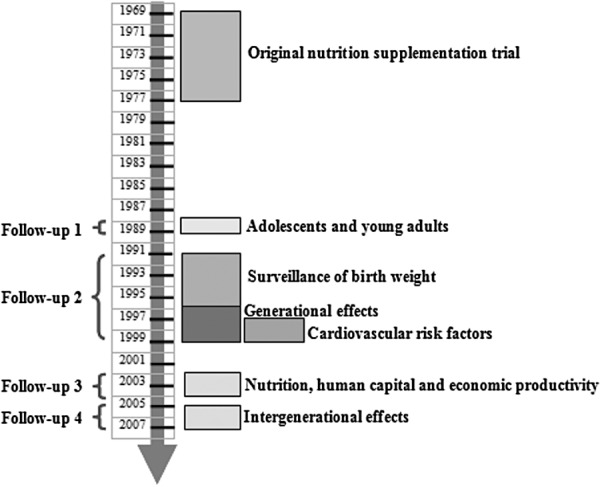
Prospective cohort studies nested in the follow‐up of the Institute of Nutrition of Central America and Panama trial (Source: adapted from Ramirez‐Zea et al. 2010).
Table 3.
Follow‐up studies for the INCAP longitudinal study
Box 1. The INCAP Longitudinal Study
In the Institute of Nutrition of Central America and Panama (INCAP) Longitudinal Study, supplements were made available to the villagers from four centrally located feeding stations, one in each village, where supplements were distributed daily at mid‐morning and mid‐afternoon. Attendance and supplement consumption were open to all villagers but were recorded only for the target population. Routine medical services in each village were established and maintained by INCAP. All women who were pregnant or lactating and all children from birth to 7 years of age living in the study villages between 1 January 1969 and 28 February 1977 were included in the original design of the study (Habicht & Martorell 1993). Supplementation was provided from 1 March 1969 to 28 February 1977. Children were followed through age 7 years or until the end of the study, whichever came first. Thus, all children were exposed either to Atole or to Fresco at different ages and for different periods of time: prenatally through supplement intake by the mother and post‐natally through the effects of maternal supplement intake on breast milk content of certain nutrients, as well as through the child's own consumption (Stein et al. 2008). The trial included 643 pregnant and lactating women and 2392 children 0–7 years of age who received supplementation. Several prospective follow‐up studies were conducted between 1988 and 2007, and some are still ongoing or being planned (Fig. 4). Table 3 provides descriptive information about the follow‐up studies conducted with this cohort.
Child length gain was greater in ‘Atole’ villages than in ‘Fresco’ villages during the first 3 years of life (+0.9 cm in the first year, +1.0 cm in the second year and +0.4 cm in the third year) (Schroeder et al. 1995). This effect persisted even after controlling for initial body size, diarrhoeal disease, socio‐economic status, gender and energy from home diets during the second year. No effect of Atole on length gain was observed when supplementation occurred between 3 and 7 years of age. The greater impact during the first 3 years of life is probably due to the greater growth potential, greater relative nutritional requirements and relatively frequent infections in younger children.
The first follow‐up study was conducted during 1988–1989 when the cohort was 11–26 years old. It documented that improved nutrition in early childhood had significant effects on body size and intellectual functioning (Martorell et al. 2010b). Specifically, during adolescence, subjects from Atole villages were taller, weighed more and had greater lean body mass than subjects from Fresco villages (Rivera et al. 1995). Subjects receiving Atole also scored significantly higher on tests of knowledge, numeracy, reading and vocabulary than those given Fresco (Pollitt et al. 1995).
Key findings from the 2002–2004 follow‐up study, when the cohort was 26–42 years of age, included the impact on school achievement (Maluccio et al. 2009) and economic productivity (Hoddinott et al. 2008). These studies showed that exposure to Atole supplementation before 3 years of age, but not after 3 years, increased years of schooling completed by 1.2 grades for women (but not for men). Reading comprehension and intelligence scores increased in both men and women. The impact of Atole supplementation on intelligence was independent of schooling (Stein et al. 2008). Wage rate (income earned per hour worked) increased by US$ 0.62–0.67 per hour in men (but not in women). In the subgroup exposed to Atole supplementation during the first 2 years of life, this represented a 46% increase in average wages. The lack of effect on income measures in women could be due to differences in economic activity between men and women. Virtually all men (99%) participated in at least one income‐generating activity, whereas the proportion was much less for women (70%) who were mostly engaged in activities that did not generate much income.
The 2006–2007 follow‐up study (Behrman et al. 2009a) of intergenerational effects found that compared with the offspring of women exposed to Fresco, the offspring of women exposed to Atole as children (starting before 7 years of age) had greater birthweight (+116 g), height (+1.3 cm), head circumference (+0.6 cm), height‐for‐age z‐score (+0.26) and weight‐for‐height z‐score (+0.20). The effects on height differed by sex of the offspring. Sons of women exposed to Atole were 2.0 cm taller than sons of women exposed to Fresco, whereas the difference for female offspring was only 0.6 cm. There were no significant differences in the measures of offspring adiposity (BMI, arm circumference, triceps skinfold thickness and sub‐scapular skinfold thickness). Paternal exposure to Atole was not associated with any of the 11 anthropometric indicators.
This unique, long‐term study demonstrated that nutritional intervention before 3 years of age has significant long‐term effects on height, as well as human capital and economic productivity in adulthood, and that nutritional supplementation of girls starting in early childhood has significant effects on body size of their offspring.
In a subsequent analysis of the pathways by which Atole supplementation benefited wage rates in men (Behrman et al. 2009b), it was found that adult lean body mass (which is usually correlated with height) and adult reading comprehension scores were both explanatory variables. However, when both variables were treated as ‘endogenous’ (i.e. potentially reflecting earlier choices), only the reading comprehension scores remained significant in explaining the impact on wage rates. This does not mean that early life nutrition was not important but that it worked through reading comprehension scores and not through adult lean body mass. The lack of impact via lean body mass is probably explained by the relatively low proportion of men in the follow‐up study who worked in physically demanding occupations. When analysis was restricted to men with such occupations, lean body mass remained important in explaining the impact of supplementation on wage rates. Thus, the relative importance of improvements in ‘brains’ vs. ‘brawn’ may depend on the types of employment available to adults.
Discussion
The studies discussed above provide strong evidence that stunting matters for two reasons. First, it strongly affects adult height, which among women has an impact on health and survival of their children, as well as their own reproductive health, and among men has been linked to economic productivity. Second, the process of stunting reflects damage that affects (in some cases, irreparably) health and development over the long term. The follow‐up studies of the INCAP trial in Guatemala demonstrate that a nutritional intervention in early life that improves linear growth also has sizeable effects on human capital formation and economic productivity in adulthood, as well as on growth of future generations. They also show that intervention needs to occur during the period when stunting usually occurs – the prenatal period and the first 3 years of life – in order to have a significant impact.
Thus, efforts to prevent stunting are likely to be of benefit for multiple outcomes, including cognitive development, school achievement and wages earned in adulthood. In developing countries, an estimated 99 million children of primary school age are not enrolled in school, and of those enrolled, only 78% complete primary school [United Nations Educational, Scientific and Cultural Organization (UNESCO) 2010]. About 200 million children under 5 years of age fail to reach their potential in cognitive development because of a combination of risk factors such as poverty, poor health and nutrition and inadequate caring practices (Grantham‐McGregor et al. 2007). These conditions play an important part in the intergenerational transmission of poverty (Grantham‐McGregor et al. 2007). Therefore, interventions to prevent stunting early in life should accelerate achievement of the Millennium Development Goals of achieving universal primary education, eradicating poverty, reducing mortality and improving maternal health.
Making prevention of stunting a priority, however, will require that certain actions be taken by policy makers and those responsible for the design and implementation of programmes. Specifically, interventions need to be targeted at the ‘window of opportunity’, which includes the pre‐conception period, pregnancy, lactation and the first 2 years of life (Bhutta et al. 2008; Dewey & Huffman 2009; Victora et al. 2010; Dewey & Adu‐Afarwuah 2008). The choice of intervention strategies should be guided by those that have been demonstrated to have a positive impact on linear growth, not just child weight. Similarly, evaluation of programme impact must include measures of child height, not just weight. Lastly, policy makers should shift towards an emphasis on stunting as an indicator of overall child health and nutrition rather than underweight. This is particularly important as the ‘nutrition transition’ towards greater overweight accelerates in many developing countries, which can lead to populations with low rates of underweight but persistently high rates of stunting.
Research priorities
Additional research, especially research from intervention trials, is needed to better understand the long‐term consequences of stunting in early life. Research from intervention trials in other regions is needed to add to the findings of the long‐term follow‐up of the intervention trial in Guatemala. We need to know whether interventions that improve linear growth of infants and young children in Africa and Asia are also beneficial for key outcomes later in life. The analysis by the Maternal and Child Undernutrition Study Group (Victora et al. 2008) using data from four prospective cohort studies from Brazil, India, Philippines and South Africa (in addition to the Guatemala trial) suggests that this will be the case; however, follow‐up of randomized intervention trials is the gold standard for drawing such conclusions.
Further research is also needed to understand the pathways by which prevention of stunting can have long‐term effects on cognitive development, school achievement and economic productivity in adulthood, particularly in populations where labour force participation among women is high. What are the direct effects of increased height, and for which outcomes is greater height simply a marker of improvement in other domains such as cognitive function? More information on the consequences for maternal reproductive outcomes and parental caregiving practices is needed.
Finally, research is needed to identify the pathways through which the non‐genetic transmission of nutritional effects is mediated in future generations and to determine the impact of interventions focused on linear growth in early life, rather than accelerated weight gain, on chronic disease risk in adulthood.
Source of funding
Bill & Melinda Gates Foundation to Academy for Educational Development – Applied Research and Technical Services for Alive & Thrive.
Conflicts of interest
No conflicts of interest have been declared.
Acknowledgements
A shorter version of this paper has previously been posted on the website for Alive & Thrive (http://www.aliveandthrive.org/). We thank Luann Martin, Alive & Thrive, Elizabeth Zehner and Global Alliance for Improved Nutrition for their assistance with the preparation of this paper.
References
- Ahmed S. & Holtz S.A. (2007) Social and economic consequences of obstetric fistula: life changed forever? International Journal of Gynecology & Obstetrics 99 (Suppl. 1), S10–S15. [DOI] [PubMed] [Google Scholar]
- Behrman J.R., Calderon M.C., Preston S.H., Hoddinott J., Martorell R. & Stein A.D. (2009a) Nutritional supplementation in girls influences the growth of their children: prospective study in Guatemala. The American Journal of Clinical Nutrition 90, 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman J.R., Hoddinott J., Maluccio J.A. & Martorell R. (2009b) Brains versus brawn: labor market returns to intellectual and health human capital in a poor developing country. Middlebury College Economics Discussion Paper No. 0907. Available at: http://sandcat.middlebury.edu/econ/repec/mdl/ancoec/0907.pdf (Accessed 17 May 2011).
- Bhutta Z.A., Ahmed T., Black R.E., Cousens S., Dewey K.G., Giugliani E. et al (2008) What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. & Adu‐Afarwuah S. (2008) Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal and Child Nutrition 4 (Suppl. 1), 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Huffman S.L. (2009) Maternal, infant, and young child nutrition: combining efforts to maximize impacts on child growth and micronutrient status. Food and Nutrition Bulletin 30 (2 Suppl.), S187–S189. [DOI] [PubMed] [Google Scholar]
- Frongillo E.A. Jr (1999) Symposium: causes and etiology of stunting. Introduction. The Journal of Nutrition 129 (2S Suppl.), 529S–530S. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. & Buklijas T. (2010) A conceptual framework for the developmental origins of health and disease. Journal of Developmental Origins of Health and Disease 1, 6–18. [DOI] [PubMed] [Google Scholar]
- Golden M.H. (2009) Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Bulletin 30 (3 Suppl.), S267–S342. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habicht J.P. & Martorell R. (1993) Objectives, research design, and implementation of the INCAP longitudianl study. Food and Nutrition Bulletin 14, 176–190. [Google Scholar]
- Hoddinott J., Maluccio J.A., Behrman J.R., Flores R. & Martorell R. (2008) Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet 371, 411–416. [DOI] [PubMed] [Google Scholar]
- Kar B., Rao S. & Chandramouli B. (2008) Cognitive development in children with chronic protein energy malnutrition. Behavioral and Brain Functions 4, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.S., Wojdyla D., Say L., Gulmezoglu A.M. & Van Look P.F.A. (2006) WHO analysis of causes of maternal deaths: a systematic review. Lancet 367, 1066–1074. [DOI] [PubMed] [Google Scholar]
- Kothari M. & Abderrahim N. (2010) Nutrition Update 2010. ICF Macro: Calverton, MD. [Google Scholar]
- Kramer M.S. (1987) Determinants of low birth weight: methodological assessment and meta‐analysis. Bulletin of the World Health Organization 65, 663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer M.S., Olivier M., McLean F.H., Willis D.M. & Usher R.H. (1990) Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics 86, 707–713. [PubMed] [Google Scholar]
- Kwawukume E.Y., Ghosh T.S. & Wilson J.B. (1993) Maternal height as a predictor of vaginal delivery. International Journal of Gynaecology and Obstetrics 41, 27–30. [DOI] [PubMed] [Google Scholar]
- Lawn J.E., Cousens S., Zupan J. & Lancet Neonatal Survival Steering T (2005) 4 million neonatal deaths: when? Where? Why? Lancet 365, 891–900. [DOI] [PubMed] [Google Scholar]
- Lawn J.E., Lee A.C., Kinney M., Sibley L., Carlo W.A., Paul V.K. et al (2009) Two million intrapartum‐related stillbirths and neonatal deaths: where, why, and what can be done? International Journal of Gynaecology and Obstetrics 107 (Suppl. 1), S5–S18, S19. [DOI] [PubMed] [Google Scholar]
- Lee A.C., Darmstadt G.L., Khatry S.K., LeClerq S.C., Shrestha S.R. & Christian P. (2009) Maternal‐fetal disproportion and birth asphyxia in rural Sarlahi, Nepal. Archives of Pediatrics & Adolescent Medicine 163, 616–623. [DOI] [PubMed] [Google Scholar]
- Maluccio J.A., Hoddinott J., Behrman J.R., Martorell R., Quisumbing A.R. & Stein A.D. (2009) The impact of improving nutrition during early childhood on education among Guatemalan adults. The Economic Journal 119, 734–763. [Google Scholar]
- Martorell R. (1992) Overview of long‐term nutrition intervention studies in Guatemala, 1968–1989. Food and Nutrition Bulletin 14, 270–277. [Google Scholar]
- Martorell R., Khan L.K. & Schroeder D.G. (1994) Reversibility of stunting: epidemiological findings in children from developing countries. European Journal of Clinical Nutrition 48 (Suppl. 1), S45–S57. [PubMed] [Google Scholar]
- Martorell R., Habicht J.P. & Rivera J.A. (1995) History and design of the INCAP longitudinal study (1969–1977) and its follow‐up (1988–1989). The Journal of Nutrition 125 (4 Suppl.), 1027S–1041S. [DOI] [PubMed] [Google Scholar]
- Martorell R., Horta B.L., Adair L.S., Stein A.D., Richter L., Fall C.H. et al (2010a) Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low‐ and middle‐income countries. The Journal of Nutrition 140, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell R., Melgar P., Maluccio J.A., Stein A.D. & Rivera J.A. (2010b) The nutrition intervention improved adult human capital and economic productivity. The Journal of Nutrition 140, 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant K.M., Villar J. & Kestler E. (2001) Maternal height and newborn size relative to risk of intrapartum Caesarean delivery and perinatal distress. BJOG: An International Journal of Obstetrics and Gynaecology 108, 689–696. [DOI] [PubMed] [Google Scholar]
- National Institute of Population Research and Training (NIPORT) , Mitra and Associates , & Macro International (2009) Bangladesh Demographic and Health Survey 2007. NIPORT, Mitra and Associates, and Macro International: Dhaka, Bangladesh and Calverton, MD.
- Omole‐Ohonsi A. & Ashimi A.O. (2007) Obstructed labour – a six year review in Aminu Kano teaching Hospital, Kano, Nigeria. The Nigerian Medical Practitioner 51, 59–63. [Google Scholar]
- Ozaltin E., Hill K. & Subramanian S.V. (2010) Association of maternal stature with offspring mortality, underweight, and stunting in low‐ to middle‐income countries. JAMA: The Journal of the American Medical Association 303, 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt E., Gorman K.S., Engle P.L., Rivera J.A. & Martorell R. (1995) Nutrition in early life and the fulfillment of intellectual potential. The Journal of Nutrition 125 (4 Suppl.), 1111S–1118S. [DOI] [PubMed] [Google Scholar]
- Ramirez‐Zea M., Melgar P. & Rivera J.A. (2010) INCAP Oriente longitudinal study: 40 years of history and legacy. The Journal of Nutrition 140, 397–401. [DOI] [PubMed] [Google Scholar]
- Rivera J.A., Martorell R., Ruel M.T., Habicht J.P. & Haas J.D. (1995) Nutritional supplementation during the preschool years influences body size and composition of Guatemalan adolescents. The Journal of Nutrition 125 (4 Suppl.), 1068S–1077S. [DOI] [PubMed] [Google Scholar]
- Schroeder D.G., Martorell R., Rivera J.A., Ruel M.T. & Habicht J.P. (1995) Age differences in the impact of nutritional supplementation on growth. The Journal of Nutrition 125 (4 Suppl.), 1051S–1059S. [DOI] [PubMed] [Google Scholar]
- Stein A.D., Wang M., DiGirolamo A., Grajeda R., Ramakrishnan U., Ramirez‐Zea M. et al (2008) Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Archives of Pediatrics & Adolescent Medicine 162, 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. & Strauss J. (1997) Health and wages: evidence on men and women in urban Brazil. Journal of Econometrics 77, 159–185. [DOI] [PubMed] [Google Scholar]
- United Nations Educational, Scientific and Cultural Organization (UNESCO) (2010) Education for All Global Monitoring Report 2010. United Nations Educational, Scientific and Cultural Organization: Paris, France. [Google Scholar]
- Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Richter L. et al (2008) Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., de Onis M., Hallal P.C., Blossner M. & Shrimpton R. (2010) Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–e480. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group (2006) Assessment of differences in linear growth among populations in the WHO Multicentre Growth Reference Study. Acta Paediatrica Supplement 450, 56–65. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2005) The World Health Report: 2005: Make Every Mother and Child Count. World Health Organization, Geneva, Switzerland. [Google Scholar]



