Abstract
The adequate supply of vitamins A and E to newborns is essential. However, factors such as maternal nutritional status and nutrient interaction may limit its bioavailability. The aim of this study was to establish nutritional status for vitamins A and E and evaluate the correlation of retinol on colostrum alpha‐tocopherol in lactating women. A total of 103 lactating women were recruited at a Brazilian public maternity hospital. Fasting serum and colostrum samples were collected in the immediate post‐partum. Retinol and alpha‐tocopherol levels were determined by high‐performance liquid chromatography and nutritional status for these vitamins was defined from specific cut‐off points for serum and colostrum. Mean serum and colostrum retinol (1.49 µmol L−1, 2.18 µmol L−1) and alpha‐tocopherol (26.4 µmol L−1, 26.1 µmol L−1) indicated satisfactory biochemical status. However, we found a prevalence of subclinical deficiency of vitamin A and vitamin E in serum (15.5% and 16%) and colostrum (50% and 60%). Lactating women with serum retinol ≥ 1.05 µmol L−1 showed an inverse correlation between serum retinol and alpha‐tocopherol concentration in the colostrum (P = 0.008, r = −0.28). This association was not observed in serum level < 1.05 µmol L−1. The nutritional status of lactating women for vitamins A and E was adequate, although there is a risk of subclinical deficiency. The negative correlation of serum retinol on alpha‐tocopherol concentration in the colostrum must be carefully evaluated in situations of vitamin A supplementation, because alpha‐tocopherol bioavailability in maternal milk may be compromised.
Keywords: puerperal, vitamina A, vitamina E, serum, colostrum, HPLC
Introduction
Vitamins A and E are essential nutrients that play important roles in several biological processes. Lipid solubility is a characteristic that allows them to share similar mechanisms in the metabolism and during transfer to the offspring. Nevertheless, these vitamins also exhibit molecular aspects and completely different functions (Debier & Larondelle 2005).
The term vitamin A is used for all β‐ionone derivatives that display biological activity of all‐trans retinol. The retinoid class encompasses both natural forms of vitamin A and the many synthetic analogues of retinol, with or without biological activity (Blomhoff & Blomhoff 2006). Dietary vitamin A is ingested in two main forms: preformed vitamin A and provitamin A carotenoids. The first is provided by animal foods such as liver, eggs and dairy products, the latter are supplied by green and yellow or orange vegetables and some fruits (Ross & Harrison 2007).
The term vitamin E refers to a family of eight chemically related compounds: α‐, β‐, γ‐ and δ‐tocopherol, and tocotrienol, but which differ in structure and bioavailability. Alpha‐tocopherol is the most active form, while other natural forms do not contribute to the nutritional demands of vitamin E. Although they can be absorbed, they are not converted to alpha‐tocopherol by humans, in addition to being weakly recognised by liver alpha‐tocopherol transfer proteins (α‐TTP; Institute of Medicine 2000).
Vitamin E is widely distributed in foods such as cereal grains, green vegetables, oils, liver and dairy products. Because of this characteristic, its deficiency is rarely food‐based (Traber 2007). However, the insufficient supply of vitamin E may compromise immune and pulmonary system development in newborns, in addition to making them prone to haemolytic anaemia, mainly when they are premature and are of low‐weight (Antonakou et al. 2011).
By contrast, the primary cause of vitamin A deficiency (VAD), recognised as an important public health problem in developing countries, is a chronically insufficient diet. Preschoolers, newborns as well as pregnant and nursing women are the main risk groups (World Health Organization 2009).
In Brazil, biochemical studies confirm that VAD is a public health problem in several regions, including the Northeast (Ramalho et al. 2002). Periodic supplementation is the most widely adopted strategy to prevent and control VAD in developing countries. The Brazilian National Vitamin A Supplementation Program provides megadoses of 200 000 IU, administered in the immediate post‐partum to lactating women residing in high‐risk regions (Ministry of Health of Brazil 2009).
Vitamins A and E play a crucial role in the health and satisfactory development of newborns. Owing to limited placental transfer of liposoluble vitamins and diminished body reserves at birth, colostrum should provide them in amounts that ensure adequate body reserves in newborns (Debier & Larondelle 2005).
Certain aspects may affect the bioavailability of vitamins A and E in the organism. These include physiological factors, such as nutritional status, amount of fat in the diet, food matrix and interaction with other nutrients (Institute of Medicine 2000; Jeanes et al. 2004; Lodge et al. 2004; Lodge 2005).
With respect to the nutrient–nutrient competition aspect, studies conducted in cell cultures and animals suggest the antagonistic effects between vitamins A and E, showing that retinol may influence in reducing alpha‐tocopherol bioavailability in the organism (Eicher et al. 1994; Nonnecke et al. 1999; Ametaj et al. 2000). However, these results must still be confirmed in humans.
In light of the important role of vitamins A and E for both mother and child, the aim of the present study was to define the nutritional status of lactating women for the aforementioned vitamins and evaluate the correlation of retinol levels on serum and colostrum concentrations of alpha‐tocopherol in these women.
Key messages
-
•
This study showed the modulating effect of retinol on colostrum alpha‐tocopherol in lactating women with serum retinol ≥ 1.05 µmol L−1.
-
•
Megadose administration of vitamin A may compromise the bioavailability of alpha‐tocopherol in maternal milk, compromising the newborn's health.
-
•
The evaluation of maternal nutritional status and administration of massive doses of vitamin A in the immediate post‐partum should be conducted by qualified professionals using specific diagnostic criteria.
Materials and methods
Subjects
This cross‐sectional study was conducted between January and September 2010 with hospitalised lactating women at a reference state maternity hospital in the city of Natal, Brazil. During hospitalization, after delivery, mothers were recruited and gave written informed consent to take part in the study, which was approved by the Research Ethics Committee of the Federal University of Rio Grande do Norte (protocol no. 325/09).
Sample size was estimated based on the prevalence of a low serum vitamin A of the outcomes investigated by the study. It was observed a moderate prevalence for serum retinol deficiency (15.5%). For sample size calculation, a population of 300 was selected, with an expected frequency of 15.5% and a worst acceptable value of 6%, with a confidence level of 95%, a minimum sample size of 95 subjects was estimated (Statcalc software; Epi‐Info, version 3.5.1; CDC, Atlanta, GA, USA).
Sampling was obtained according to the following exclusion criteria: existence of associated pathologies, such as diabetes, eclampsia, neoplasias, gastrointestinal, hepatic and infectious diseases, cardiopathies, syphilis, HIV‐positivity; delivery occurring more than 12 h after blood collection; multiple conceptions; conception with malformation; use of supplements containing vitamin A or E during pregnancy or megadose vitamin A supplementation in the post‐partum. The final study sample was composed of 103 lactating women.
Data on obstetric, maternal and newborn characteristics were obtained from prenatal records in possession of the mothers and a questionnaire applied by the researchers. Pre‐pregnancy anthropometric nutritional status was determined by measuring body mass index (BMI), calculated from body weight before gestation and height (Institute of Medicine 1992). Gestational weight gain was obtained from the difference established between pre‐delivery and pre‐pregnancy body weight and evaluated using recommendations made depending on initial maternal nutritional status (Institute of Medicine 1992).
The newborn's gestational age was obtained through the information contained in hospital records and classified according to the recommendations of the World Health Organization (1995), which considers gestational age less than 37 weeks with an indication of prematurity. Thus, neonates were subdivided into term and preterm. For classification of birth weight we used the criteria of the World Health Organization (1995) establishes that body weight less than 2500 g as low birth weight, weighing 2500 and 2999 g as insufficient, weighing 3000 g and 3999 g as appropriate and 4000 g weight equal to or greater as overweight. Newborn classified as having low weight and insufficient weight were classified as insufficient weight in the single variable.
Biological material collection
Biological sample collection was performed after night‐time fasting on the first day post‐partum. Blood was collected (5 mL) on the first day and 2 mL of colostrum was collected for three consecutive days under the same conditions, in order to establish a colostrum pool. Colostrum was collected by manual expression of a single breast that had not been previously suckled and the first ejection was discarded to avoid fluctuations in fat content. Samples were collected in polypropylene tubes protected from light, transported under refrigeration and stored at −20°C until analyses. The serum obtained was kept under nitrogen atmosphere at −20°C until analyses.
After the third collection of colostrum at hospital discharge, the lactating women were supplemented with a megadose of vitamin A, in accordance with recommendations proposed by the Ministry of Health (Ministry of Health of Brazil 2009).
Biochemical analyses
Retinol was extracted from colostrum using an adaptation of the method described by Giuliano et al. (1992). At a 500 µL aliquot of colostrum pool was added 95% ethyl alcohol (Vetec, Rio de Janeiro, Brazil) and potassium hydroxide (50% v/v) (Vetec) for saponification at 45°C for 2 h under agitation. Retinol was then extracted with hexane p.a. (Merck, Rahway, NJ, USA) and evaporation in nitrogen atmosphere. The extract was resuspended in 500 µL of high‐performance liquid chromatography (HPLC)‐grade absolute ethanol (Vetec) and 20 µL was applied to a Shimadzu chromatograph with a Shimadzu LC‐20 AT pump coupled to a Shimadzu SPD‐20A/UV‐VIS detector (Shimadzu Corp., Kyoto, Japan) and Shim‐pack CLC‐ODS (M) column (4.6 mm × 15 cm). Data were processed using LC solution software (Shimadzu®). The mobile phase used was 100% methanol in an isocratic system with a flow rate of 1 mL min−1 and wavelength of 325 nm.
A 500‐µL aliquot of colostrum pool was used to extract alpha‐tocopherol using an adaptation of the method proposed by Ortega et al. (1998). Ethanol (95%) (Merck) was used for protein and hexane p.a. (Merck®) precipitation for extraction. After evaporation in nitrogen atmosphere, the extract was diluted in 250 µL of absolute ethanol (Vetec®) and 20 µL was applied to the HPLC. The mobile phase used was methanol and MiliQ® water (97:3) in an isocratic system with a flow rate of 1.5 mL min−1 and wavelength of 292 nm.
Analysis of serum retinol and alpha‐tocopherol followed the same method as that used for colostrum alpha‐tocopherol and detection was performed in the same chromatographic profile. A programmed change in wavelength from 325 to 292 nm occurred after 5 min of sample elution in 100% methanol and flow rate of 1 mL min−1.
Identification and quantification of retinol and alpha‐tocopherol in the samples was achieved by comparing the areas obtained in the chromatographic profile with those generated by all‐trans retinol and alpha‐tocopherol standards (Sigma, St Louis, MO, USA), respectively. Concentrations of the standards were confirmed by the specific extinction coefficient in absolute ethanol for retinol (ε 1%, 1 cm = 1780–325 nm) (Milne & Botnen 1986) and alpha‐tocopherol (ε 1%, 1 cm = 75.8–292 nm) (Nierenberg & Nann 1992). The accuracy of the method was evaluated, showing a recovery of 96%. Quantification and detection limits were obtained with 0.17 µmol L−1 and 0.08 µmol L−1 of retinol standard.
Specific cut‐off points were adopted to evaluate the biochemical nutritional status of lactating women as a function of retinol and alpha‐tocopherol. For retinol, 1.05 µmol L−1 in serum (West 2002) and 2.1 µmol L−1 in colostrum was adopted (Macias & Schweigert 2001), while for alpha‐tocopherol a cut‐off point of 16.2 µmol L−1 was used (Sauberlich et al. 1974). Since there is no previously defined cut‐off point for colostrum alpha‐tocopherol in the literature, values for this vitamin below the mean concentration found in the colostrum of individuals in this study who exhibited adequate levels of serum alpha‐tocopherol (≥16.2 µmol L−1) were classified as insufficient levels of vitamin E. Therefore, a cut‐off point of 26.1 µmol L−1 was established for colostrum.
Statistical analyses
Statistica 7 (StatSoft, Tulsa, OK, USA) software was used for statistical analyses. Vitamin concentrations in serum and milk were presented as mean and standard deviation. The differences between the means of parametric data were analysed using Student's t‐test for dependent and independent data. The chi‐squared test was used for categorical variables. The relationship between biochemical data and maternal characteristics was assessed using Pearson's correlation. Differences were considered significant for P < 0.05.
Results
The lactating women included in this study (n = 103) were aged between 14 and 41 years. Even though around 50% exhibited adequate pregestational nutritional status, with BMI suggestive of eutrophy, 36% displayed some degree of overweight prior to gestation, obtaining BMI indicative of overweight and obesity. The same situation was observed when analysing gestational weight gain. Half of the population (n = 56) maintained body weight gain within recommended levels. However, 21 women exceeded estimated mean weight based on pregestational nutritional status (Table 1).
Table 1.
Characterisation of the study population based on obstetric, maternal and newborn parameters established
| Characteristics | Total group (n = 103) |
|---|---|
| Maternal age (years) | 24 ± 7* |
| Adolescent [n (%)] | 25 (24) |
| Adult [n (%)] | 78 (76) |
| Maternal nutritional status † | |
| Low weight [n (%)] | 13 (13) |
| Eutrophy [n (%)] | 53 (51) |
| Excess weight [n (%)] | 37 (36) |
| Gestational weight gain ‡ (kg) | 12 ± 5* |
| Insufficient [n (%)] | 26 (25) |
| Adequate [n (%)] | 56 (54) |
| Excessive [n (%)] | 21 (20) |
| Number of previous deliveries § | |
| Primipara [n (%)] | 55 (53) |
| Multipara [n (%)] | 48 (47) |
| Type of delivery ¶ | |
| Caesarean [n (%)] | 53 (51) |
| Natural [n (%)] | 50 (49) |
| Newborn gestational age** (weeks) | 38 ± 3* |
| Preterm | 25 (24) |
| Term | 78 (76) |
| Birth weight †† (kg) | 3,1 ± 0,6* |
| Insufficient [n (%)] | 41 (40) |
| Adequate [n (%)] | 54 (52) |
| Excessive [n (%)] | 8.0 (7.0) |
*Mean ± standard deviation. †Maternal nutritional status classified according to pregestational body mass index (Institute of Medicine 1992). ‡Gestational weight gain classified according to the recommendations made from the initial maternal nutritional status. §primiparous: no previous pregnancy; multiparous: one or more previous pregnancies. ¶Type of delivery in the current pregnancy. **Pre‐term: gestational age < 37 weeks. Term: gestational age between 37 and 41 weeks and 6 days. ††Insufficient birth weight: ≤ 2999 g, Adequate birth weight: between 3000 and 3999 g. Excessive birth weight: ≥ 4000 g.
With respect to obstetric characteristics, half of the lactating women were primipara (n = 55) who underwent caesarean delivery (n = 53). It was found 61% of women classed as overweight and obesity according to BMI pre‐pregnancy and 33% of women who had excessive gestational weight gain had caesarean delivery. Mean gestational age of the newborns was 38 ± 3 weeks, 24% of which were preterm. Mean weight was 3.1 Kg; however, 40% had insufficient birth weight.
Analysis of the entire group of participants showed mean serum concentration of 1.49 ± 0.4 µmol L−1 of retinol and 26.4 ± 8.0 µmol L−1 of alpha‐tocopherol. Mean colostrum values were 2.18 ± 0.8 µmol L−1 for retinol and 26.1 ± 12.8 µmol L−1 for alpha‐tocopherol. An analysis of mean serum retinol and alpha‐tocopherol in the total group could suggest an adequate nutritional status. However, in individual analysis, serum retinol and alpha‐tocopherol levels of each of the lactating women indicated a moderate prevalence for serum retinol deficiency of 15.5% for vitamin A and 16% for vitamin E. The colostrum levels of these vitamins show higher prevalence of deficiency for both vitamin A (50%) and vitamin E (61%) (Table 2).
Table 2.
Biochemical levels and prevalence of retinol and alpha‐tocopherol deficiency in the total group of lactating women and in cut‐off points for serum
| Substance | Biochemical information | Total group (n = 103) | Serum cut‐off point | |
|---|---|---|---|---|
| <1.05 µmol L−1 (n = 16) | ≥1.05 µmol L−1 (n = 87) | |||
| ROH | Serum ROH* | 1.49 ± 0.4 | 0.84 ± 0.17 | 1.61 ± 0.35 |
| Colostrum ROH* | 2.18 ± 0.8 | 1.87 ± 0.72 | 2.25 ± 0.79 | |
| Serum deficiency † [n (%)] | 16 (16) | 16 (100) | 0.0 (0.0) | |
| Colostrum deficiency ‡ [n (%)] | 52 (50) | 12 (81) | 30 (34) | |
| <16.2 µmol L−1 (n = 15) | ≥16.2 µmol L−1 (n = 88) | |||
| Serum TOH* | 26.4 ± 8.0 | 12.8 ± 2.7 | 28.7 ± 6.08 | |
| TOH | Colostrum ROH* | 26.1 ± 12.8 | 25.7 ± 12.2 | 28.24 ± 16.1 |
| Serum deficiency § [n (%)] | 15 (16) | 15 (100) | 0.0 (0.0) | |
| Colostrum deficiency ¶ [n (%)] | 59 (61) | 10 (66) | 39 (44) | |
ROH, retinol; TOH, alpha‐tocopherol. *Mean ± standard deviation. †Prevalence of serum vitamin A deficiency based on a cut‐off point of 1.05 µmol L−1 for serum retinol. ‡Prevalence of colostrum vitamin A deficiency based on a cut‐off point of 2.09 µmol L−1 for colostrum retinol. §Prevalence of serum vitamin E deficiency based on a cut‐off point of 16.2 µmol L−1 for serum alpha‐tocopherol. ¶Prevalence of colostrum vitamin E deficiency based on a cut‐off point of 26.1 µmol L−1 established for colostrum alpha‐tocopherol.
In the group of lactating women with serum retinol levels above 1.05 µmol L−1, it was found that 34% exhibited colostrums retinol levels suggestive of subclinical deficiency. Similarly, those classified as having satisfactory nutritional status for vitamin E, based on their serum levels, had a subclinical deficiency prevalence of 44% for this vitamin when their colostrum levels were considered.
The correlation of all variables maternal, obstetric and newborn used in the study was tested for alpha‐tocopherol and retinol in serum and colostrum. However, none of the variables was associated with retinol and alpha‐tocopherol levels in the lactating women (P > 0.05). There was also no correlation between plasma retinol and alpha‐tocopherol (P = 0.53, r = 0.06), serum and colostrum retinol (P = 0.11, r = 0.15) or serum and colostrum alpha‐tocopherol (P = 0.22, r = −0.12). A positive correlation was observed only between colostrum vitamin A and E levels (P = 0.04, r = 0.18).
Biochemical serum and colostrum analyses in lactating women with serum retinol levels ≥ 1.05 µmol L−1 showed a negative correlation of serum retinol on colostrum alpha‐tocopherol (P = 0.008, r = −0.28) (Fig. 1). There was no correlation between serum and alpha‐tocopherol levels in this group of women (Table 3). Biochemical data on the lactating women group with serum retinol below the cut‐off point of 1.05 µmol L−1 revealed no relationship between serum retinol and alpha‐tocopherol levels in the colostrum.
Figure 1.
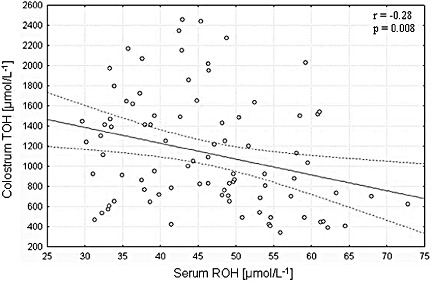
Relationship between serum retinol and colostrum alpha‐tocopherol concentrations of lactating women with serum retinol ≥ 1.05 µmol L−1. ROH, retinol; TOH, alpha‐tocopherol.
Table 3.
Correlations established between retinol and alpha‐tocopherol based on a cut‐off point for serum retinol
| Cut‐off point for serum retinol | Serum ROH × colostrum TOH | Serum ROH × serum TOH | Serum TOH × colostrum ROH | Colostrum ROH × colostrum TOH | ||||
|---|---|---|---|---|---|---|---|---|
| r * | P † | r | P | r | P | r | P | |
| <1.05 µmol L−1 | 0.25 | 0.364 | 0.31 | 0.235 | −0.33 | 0.21 | 0.49 | 0.055 |
| ≥1.05 µmol L−1 | −0.28 | 0.008 | 0.55 | 0.065 | 0.04 | 0.66 | 0.14 | 0.19 |
ROH, retinol; TOH, alpha‐tocopherol. r = correlation coefficient; P = significance level (P < 0.05).
*Correlation coefficient. †Significance level (P < 0,05).
Discussion
Lactating women have been considered a biologically vulnerable group, at risk of presenting with marginal or deficient nutritional status in a number of micronutrients and related composites. Maternal nutritional status is a critical factor of pregnancy outcome, for both the mother and the child. Thus, monitoring nutritional deficits and excesses is important for reducing the risks of gestational complications (Brasil & Demarchi 2003).
The percentage of excess weight in pregestational and gestational periods observed in the lactating women corroborated the results obtained in several countries (Walsh 2007). Our data confirm the worldwide tendency towards increased obesity in pregnant women, which is associated to a high risk of maternal and perinatal complications, including the need for caesarean delivery (Walsh 2007). This characteristic was found in the present study, as evidenced by the high prevalence of women that underwent this procedure.
The entire group of lactating women presented with a satisfactory biochemical nutritional status for vitamin A and E, based on their serum levels. Serum retinol levels corroborated similar studies (Ortega et al. 1997; Ribeiro et al. 2010), were higher than those in Africa (Semba et al. 2000) and lower than those obtained in German women (Schultz et al. 2007). Serum alpha‐tocopherol values were compatible with those observed in other studies (Ascherio et al. 1992; Garcia et al. 2010) and higher than in Cuban women (Rodriguéz et al. 2002).
Colostrum retinol levels also indicated satisfactory nutritional condition and agreed with values proposed by industrialised nations (Newman 1993). Alpha‐tocopherol content was similar to that obtained in other studies (Macias & Schweigert 2001; Garcia et al. 2010).
The prevalence of vitamin deficiency in the study population showed that for vitamin A, 15.5% had inadequate serum retinol, even though 50% exhibited insufficient colostrum levels. For vitamin E, the prevalence of alpha‐tocopherol levels indicative of deficiency also showed discrepancy between serum (16%) and colostrum (61%) levels, considering the cut‐off point established for alpha‐tocopherol in this study in consequence of lack of data in the literature.
These data allow us to assert that the amount of vitamins A and E obtained by maternal breastfeeding in our study population may have been nutritionally inadequate for the newborn, limiting its normal development and making it vulnerable to nutritional deficiency. A similar result was found in lactating women in Rio de Janeiro, where the prevalence of reduced colostrum retinol levels (42%) coincided with the unsatisfactory consumption of dietary vitamin A (Meneses & Trugo 2005). There is a lack of data about the prevalence of vitamin E deficiency in the colostrum.
It was found that 34% of women with serum retinol ≥ 1.05 µmol L−1 and 44% with serum alpha‐tocopherol ≥ 16.2 µmol L−1 showed reduced levels of vitamins A and E in colostrum, respectively. In accordance with previously discussed results, analysis of the prevalence of subclinical deficiency in groups of lactating women with serum values above the cut‐off point confirmed the greater sensitivity of colostrum in maternal nutritional status (World Health Organization 1996).
The World Health Organization (1996) recommends maternal milk level as the only indicator of the nutritional status of the mother and the newborn. Some studies show the use of milk retinol with this aim (Stoltzfus & Underwood 1995; Haskell & Brown 1999). However, even though alpha‐tocopherol content in milk has been the focus of several studies (Macias & Schweigert 2001; Schweigert et al. 2004; Azeredo & Trugo 2008; Dimenstein et al. 2010; Garcia et al. 2010), reports on the application of vitamin E as assessment parameter of nutritional status are scarce.
Vitamin concentration in maternal milk responds as a function of dietary ingestion and maternal supplementation, and when this ingestion is low, the concentration in milk may be more affected than plasma concentration, which is homeostatically controlled (Azeredo & Trugo 2008). For this reason, vitamin A and E levels in mother's milk are more responsible for changes in nutritional status, making them efficient parameters for diagnosing maternal and newborn nutritional status and good indicators for measuring the impact of intervention programmes.
The influence that the characteristics of the research subjects have on retinol levels remains contradictory in the literature, showing both positive (Mello‐Neto et al. 2009) and negative results (Dimenstein et al. 2003; Ahmed et al. 2004; Meneses & Trugo 2005). In this study, none of the obstetric, maternal and newborn was associated with retinol and alpha‐tocopherol levels of the lactating women.
As in other studies with Brazilian women (Azeredo & Trugo 2008; Garcia et al. 2010), a positive correlation was observed between retinol and alpha‐tocopherol in maternal milk. However, plasma retinol and alpha‐tocopherol were not related to their concentrations in milk, likely because of the alternative transport mechanism of these vitamins to the mammary gland, irrespective of their plasma concentrations (Li et al. 2000; Meneses & Trugo 2005).
The negative correlation of serum retinol on alpha‐tocopherol in colostrum, observed only in lactating women with serum retinol levels above the cut‐off point adopted (P = 0.008, r = −0.28), corroborates the aspect of competition between retinol and alpha‐tocopherol currently under discussion in the literature.
Studies that consider the modulating effect of retinol on alpha‐tocopherol levels were established only under conditions of elevated vitamin A (Debier & Larondelle 2005). In studies conducted with calves, mares and humans (1994, 1997; Schelling et al. 1995; Nonnecke et al. 1999; Ametaj et al. 2000), the vitamin A supplied reached levels many times higher than the daily doses recommended for the study population.
The exact mechanism that supports these observations has not been fully explained. However, it is suggested that retinoic acid isomers may have a negative influence on the production of α‐TTPs, reducing its incorporation into very low density lipoprotein and thus, affecting tocopherol plasma levels (Ametaj et al. 2000). There is also evidence that these isomers could reduce the hepatic expression of mRNA apoliprotein A‐1 present in high‐density lipoprotein (HDL), an important carrier of plasma alpha‐tocopherol to the tissues (Berthou et al. 1998).
Considering the evidence in the mammary gland of both α‐TTP ‐related mechanisms (Lauridsen et al. 2002; Debier & Larondelle 2005) and scavenger receptor class B, type I involved in the supply of alpha‐tocopherol by means of the HDL, it is suggested that the aforementioned devices described can be extended to the influence established in maternal milk.
Despite the diagnosis of satisfactory nutritional status, an elevated risk of subclinical deficiency for vitamins A and E was revealed in maternal colostrum levels. Such a situation, added to the negative association of retinol levels on colostrum alpha‐tocopherol, reinforces the idea that the evaluation of maternal nutritional status and administration of massive doses of vitamin A in the immediate post‐partum should be conducted by qualified professionals using more specific diagnostic criteria. These measures are necessary because lactating women not at risk of vitamin A deficiency enrolled in supplementation programmes may exhibit reduced bioavailability of alpha‐tocopherol in maternal milk, compromising the newborn's nutritional status.
Source of funding
CNPq – Process number: 552373/2009‐5.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributions
All the authors actively participated in the conception of the study, data analysis and preparation of the article.
Acknowledgements
Januário Cicco Maternity School, Natal, Brazil for permitting data collection at this institution.
References
- Ahmed L., Nasrul Islam S.K., Mni K., Huque S. & Ahsan M. (2004) Antioxidant micronutrient profile (vitamin E, C, A, copper, zinc, iron) of colostrum: association with maternal characteristics. Journal of Tropical Pediatrics 50, 357–358. [DOI] [PubMed] [Google Scholar]
- Ametaj B.N., Nonnecke B.J., Franklin S.T., Horst R.L., Bidlack W.R., Stuart R.L. et al (2000) Dietary vitamin A modulates the concentrations of RRR‐a‐tocopherol in plasma lipoproteins from calves fed milk replacer. The Journal of Nutrition 130, 629–626. [DOI] [PubMed] [Google Scholar]
- Antonakou A., Chiou A., Andrikopoulos N.K., Bakoula C. & Matalas A.L. (2011) Breast milk tocopherol content during the first six months in exclusively breastfeeding Greek women. European Journal of Nutrition 50, 195–202. [DOI] [PubMed] [Google Scholar]
- Ascherio A., Stampfer M., Colditz G.A., Rimm E.B., Litin L. & Willett W.C. (1992) Correlations of vitamin A in E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. The Journal of Nutrition 122, 1792–1801. [DOI] [PubMed] [Google Scholar]
- Azeredo V.B. & Trugo N.M.F. (2008) Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition 24, 133–139. [DOI] [PubMed] [Google Scholar]
- Berthou L., Langouet S., Grude P., Denefle P., Branellec D. & Guillouzo A. (1998) Negative regulation of apo‐A‐1 gene expression by retinoic acid in rat hepatocytes maintained in a coculture system. Biochimica et Biophysica Acta 1391, 329–336. [DOI] [PubMed] [Google Scholar]
- Blomhoff R. & Blomhoff H.K. (2006) Overview of retinoid metabolism and function. Journal of Neurobiology 66, 606–630. [DOI] [PubMed] [Google Scholar]
- Brasil A.L.D. & Demarchi A.L.G. (2003) Nutrição na gestação e lactação In: Nutrição e dietética em clinica pediátrica (eds Lopez F.A. & Brasil A.L.D.), pp 3–16. Atheneu: São Paulo. [Google Scholar]
- Debier C. & Larondelle Y. (2005) Vitamins A and E: metabolism, roles and transfer to offspring. The British Journal of Nutrition 93, 153–174. [DOI] [PubMed] [Google Scholar]
- Dimenstein R., Medeiros A.C.P., Cunha L.R.F., Araújo K.F., Dantas J.C.O., Macedo T.M.S. et al (2010) Vitamin E in serum and human colostrum under fasting and postprandial conditions. The Journal of Pediatrics 86, 345–348. [DOI] [PubMed] [Google Scholar]
- Dimenstein R., Simplício J.L., Ribeiro K.D. & Melo I.L. (2003) Retinol levels in human colostrum: influence of child, maternal and socioeconomic variables. The Journal of Pediatrics 79, 513–518. [PubMed] [Google Scholar]
- Eicher S.D., Morrill J.L. & Blecha F. (1994) Vitamin concentration and function of leukocytes from dairy calves supplemented with vitamin A, vitamin E and beta‐carotene in vitro. Journal of Dairy Science 77, 560–565. [DOI] [PubMed] [Google Scholar]
- Eicher S.D., Morrill J.L. & Velazco J. (1997) Bioavailability of a‐tocopherol fed with retinol and relative bioavailability of d‐a‐tocopherol or dl‐a‐tocopherol acetate. Journal of Dairy Science 80, 393–399. [DOI] [PubMed] [Google Scholar]
- Garcia L., Ribeiro K., Araújo K., Pires J., Azevedo G. & Dimenstein R. (2010) Alpha‐tocopherol concentration in the colostrum of nursing women supplemented with retinyl palmitate alpha‐tocopherol. Journal of Human Nutrition and Dietetics 23, 529–534. [DOI] [PubMed] [Google Scholar]
- Giuliano A.R., Neilson E.M., Kelly B.E. & Canfield L.M. (1992) Simultaneous quantitation and separation of carotenoids and retinol in human milk by high‐performance liquid chromatography. Methods in Enzymology 213, 391–399. [DOI] [PubMed] [Google Scholar]
- Haskell M. & Brown K. (1999) Maternal vitamin A nutriture and vitamin A content of human milk. Journal of Mammary Gland Biology and Neoplasia 4, 243–257. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (1992) Nutrition During Pregnancy and Lactation: An Implementation Guide. National Academy Press: Washington, DC. [Google Scholar]
- Institute of Medicine (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press: Washington, DC. [PubMed] [Google Scholar]
- Jeanes Y.M., Hall W.L., Ellard S., Lee E. & Lodge J.K. (2004) The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. The British Journal of Nutrition 92, 575–579. [DOI] [PubMed] [Google Scholar]
- Lauridsen C., Engel H., Jensen S.K., Craig A.M. & Traber M.G. (2002) Lactating sows and suckling piglets preferentially incorporate RRR‐ over all‐rac‐a‐tocopherol into milk, plasma and tissues. The Journal of Nutrition 132, 1258–1264. [DOI] [PubMed] [Google Scholar]
- Li Y., Craft N.E., Handelman G.H., Nommensen‐Rivers L.A., Mccrory M.A. & Dewey K.G. (2000) Associations between serum and breast milk carotenoids, vitamin A and E. The FASEB Journal 14, A240. [Google Scholar]
- Lodge J.K. (2005) Vitamin E bioavailability in humans. Journal of Plant Physiology 162, 790–796. [DOI] [PubMed] [Google Scholar]
- Lodge J.K., Hall W.L., Jeanes Y.J. & Proteggente A.R. (2004) Physiological factors influencing vitamin E biokinetics. Annals of the New York Academy of Sciences 1031, 60–73. [DOI] [PubMed] [Google Scholar]
- Macias C. & Schweigert F.J. (2001) Changes in the concentration on of carotenoids, vitamin A, alpha‐tocopherol and total lipids in human milk throughout early lactation. Annals of Nutrition and Metabolism 42, 82–85. [DOI] [PubMed] [Google Scholar]
- Mello‐Neto J., Rondo P.H.C., Oshiiwa M., Morgano M.A., Zacari C.Z. & Domingues S. (2009) The influence of maternal factors on the concentration of vitamin A in mature breast milk. Clinical Nutrition 28, 178–181. [DOI] [PubMed] [Google Scholar]
- Meneses F. & Trugo N.M.F. (2005) Retinol, b‐carotene, and lutein + zeaxanthin in the milk of Brazilian nursing women: associations with plasma concentrations and influences of maternal characteristics. Nutrition Research 25, 443–451. [Google Scholar]
- Milne D.B. & Botnen J. (1986) Retinol, α‐tocoferol, lycopene and α‐ and β‐carotene simultaneously determined in plasma by isocratic liquid chromatography. Clinical Chemistry 32, 874–876. [PubMed] [Google Scholar]
- Ministry of Health of Brazil (2009) Boletim Carências Nutricionais: Deficiência de Vitamina A. Departamento de Atenção Básica. Brasília, DF Brazil.
- Newman V. (1993) Vitamin A and Breastfeeding: A Comparison of Data from Developed and Developing Countries‐Summary. Wellstart International: San Diego. [Google Scholar]
- Nierenberg D.W. & Nann S.L. (1992) A method for determining concentrations of retinol, tocopherol, and five carotenoids in human plasma and tissue samples. The American Journal of Clinical Nutrition 56, 417–426. [DOI] [PubMed] [Google Scholar]
- Nonnecke B.J., Horst R.L., Waters W.R., Dubeski P. & Harp J.A. (1999) Modulation of fat‐soluble vitamins concentrations and blood mononuclear leukocyte populations in milk replacer‐fed calves by dietary vitamin A and β‐carotene. Journal of Dairy Science 82, 2632–2641. [DOI] [PubMed] [Google Scholar]
- Ortega R.M., Andrés P., Martínez R.M. & López‐Sobaler A.M. (1997) Vitamin A status during the third trimester of pregnancy in Spanish women: influence on concentrations of vitamin A in breast milk. The American Journal of Clinical Nutrition 66, 564–568. [DOI] [PubMed] [Google Scholar]
- Ortega R.M., López‐Sobaler A.M., Martínez R.M., Andrés P. & Quintas M.E. (1998) Influence of smoking on vitamin E status during the third trimester of pregnancy and on breast‐milk tocopherol concentrations in Spanish women. The American Journal of Clinical Nutrition 68, 662–667. [DOI] [PubMed] [Google Scholar]
- Ramalho R.A., Flores H. & Saunders C. (2002) Hipovitaminose A no Brasil: um problema de saúde pública. Revista Panamericana de Salud Publica 12, 117–122. [DOI] [PubMed] [Google Scholar]
- Ribeiro K., Araujo K., Souza H., Soares F., Pereira M. & Dimenstein R. (2010) Nutritional vitamin A status in northeast Brazilian lactating mothers. Journal of Human Nutrition and Dietetics 23, 154–161. [DOI] [PubMed] [Google Scholar]
- Rodriguéz G.P., Alonso D.P., Sintes G.S., Matos C.M., Hernandez A.C., Enríques Y.R. et al (2002) Vitaminas antioxidantes en un grupo de embarazadas y recién nacidos durante un año de estudio. Revista Cubana de Alimentación y Nutrición 16, 85–94. [Google Scholar]
- Ross A.C. & Harrison E.H. (2007) Vitamin A: nutritional aspects of retinoids and carotenoids In: Handbook of Vitamins (eds Zempleni J., Rucker R.B., McCormick D.B. & Suttie J.W.), 4th edn, pp 1–40. CRC Press: Cleveland, OH. [Google Scholar]
- Sauberlich H.E., Dowdy R.P. & Skala J.H. (1974) Laboratory Tests for the Assessment of Nutritional Status, pp 74–80. CRC Press: Cleveland, OH. [DOI] [PubMed] [Google Scholar]
- Schelling G.T., Roeder R.A., Garber M.I. & Pumfrey W.M. (1995) Bioavailability and interaction of vitamin A and vitamin E in ruminants. The Journal of Nutrition 125, 1799S–1803S. [DOI] [PubMed] [Google Scholar]
- Schultz C., Engel U., Kreienberg R. & Biesalski H.K. (2007) Vitamin A and b‐carotene supply of women with gemini or short birth intervals: a pilot study. European Journal of Nutrition 46, 12–20. [DOI] [PubMed] [Google Scholar]
- Schweigert F.J., Bathe K., Chen F., Büscher U. & Dudenhausen J.W. (2004) Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. European Journal of Nutrition 43, 39–44. [DOI] [PubMed] [Google Scholar]
- Semba R.D., Kumwenda N., Taha T.E., Mtimavalye L., Broadhead R., Miotti P.G. et al (2000) Plasma and breast milk vitamin A as indicators of vitamin A status in pregnant women. International Journal for Vitamin and Nutrition Research 70, 271–277. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J. & Underwood B.A. (1995) Breast milk vitamin A as an indicator of the vitamin A status of women and infants. Bulletin of the World Health Organization 73, 703–711. [PMC free article] [PubMed] [Google Scholar]
- Traber M.G. (2007) Vitamin E In: Handbook of Vitamins (eds Zempleni J., Rucker R.B., McCormick D.B. & Suttie J.W.), 4th edn, pp 153–174. CRC Press: Cleveland, OH. [Google Scholar]
- Walsh S.W. (2007) Obesity: a risk factor for preeclampsia. Trends in Endocrinology and Metabolism 18, 365–370. [DOI] [PubMed] [Google Scholar]
- West K.P. Jr (2002) Extent of vitamin A deficiency among preschool children and women of reproductive age. The Journal of Nutrition 132, 2857S–2866S. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1995) The World Health Organization's infant feeding recommendation. The Bulletin of the World Health Organization 73, 165–174. 7743587 [Google Scholar]
- World Health Organization (1996) Indicators for Assessing Vitamin A Deficiency and Their Application for Monitoring and Evaluating Interventions Programmes: Micronutrient Series. WHO/UNICEF: Geneva, Switzerland. [Google Scholar]
- World Health Organization (2009) Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005. WHO Global Database on Vitamin A Deficiency. WHO: Geneve. [Google Scholar]


