Abstract
This study was aimed to compare the subjective and objective nutritional assessments and to analyse the performance of subjective global assessment (SGA) of nutritional status in diagnosing undernutrition in paediatric patients. One hundred and forty children (aged 2–12 years) hospitalized consecutively in Tabriz Paediatric Hospital from June 2008 to August 2008 underwent subjective assessment using the SGA questionnaire and objective assessment, including anthropometric and biochemical measurements. Agreement between two assessment methods was analysed by the kappa (κ) statistic. Statistical indicators including (sensitivity, specificity, predictive values, error rates, accuracy, powers, likelihood ratios and odds ratio) between SGA and objective assessment method were determined. The overall prevalence of undernutrition according to the SGA (70.7%) was higher than that by objective assessment of nutritional status (48.5%). Agreement between the two evaluation methods was only fair to moderate (κ = 0.336, P < 0.001). The sensitivity, specificity, positive and negative predictive value of the SGA method for screening undernutrition in this population were 88.235%, 45.833%, 60.606% and 80.487%, respectively. Accuracy, positive and negative power of the SGA method were 66.428%, 56.074% and 41.25%, respectively. Likelihood ratio positive, likelihood ratio negative and odds ratio of the SGA method were 1.628, 0.256 and 6.359, respectively. Our findings indicated that in assessing nutritional status of children, there is not a good level of agreement between SGA and objective nutritional assessment. In addition, SGA is a highly sensitive tool for assessing nutritional status and could identify children at risk of developing undernutrition.
Keywords: children, undernutrition, nutritional assessment, objective assessment, subjective global assessment
Introduction
Malnutrition and, in particular, undernutrition, is a common finding in hospitalized patients, especially in hospitalized children (Hankard et al. 2001; Pirlich et al. 2005), and is reported as varying between 21% and 80% in proportion with the level of development of a country (Dogan et al. 2005). Undernutrition has considerable health impacts on the physical, social and economical status of patients (Waitzberg et al. 2001; Correia & Waitzberg 2003). It may also affect the quality of life of patients (Wakahara et al. 2007). Therefore, it is essential that patients who are undernourished or at risk of developing undernutrition be identified soon after admission so that nutritional support may be provided to correct nutritional changes and improve the patient's prognosis(Yamauti et al. 2006).
Current methods of assessing nutritional status in children rely on a combination of objective anthropometric, dietary, biochemical and immunologic measures that are considerably time‐consuming and costly (Secker & Jeejeebhoy 2007). Ideally, nutritional assessment should be practical, easy to perform, non‐invasive, requiring no use of devices or supplementary examinations, applicable at the bedside, show appropriate sensitivity and specificity, and yield immediate result (Yamauti et al. 2006).
To complement the usual methods of nutritional evaluation, the subjective global assessment (SGA) was proposed by Baker et al. in 1982 as a manual skill for nutritional assessment and as a screening tool to detect patients at risk of developing undernutrition (Wakahara et al. 2007). SGA is a comprehensive screening tool for undernutrition based on the features of a medical history and physical examination of the patients (Shirodkar & Mohandas 2005; Heimburger 2006). The SGA classification technique has been used to evaluate the nutritional status of surgical patients with digestive tract diseases, cancer patients, AIDS patients, dialysis patients and liver transplant patients (Sacks et al. 2000). However, there are limited studies regarding its use in paediatric patients, and this nutritional assessment technique has not been validated thoroughly for its ability to identify undernutrition and or nutrition‐associated complications in paediatric patients. Further work in establishing the applicability of SGA in paediatric patients should be done to enable clinicians and researchers to properly use this nutritional assessment tool.
This study was aimed to compare the SGA with the objective assessment of nutritional status including anthropometric and biochemical measurements and to analyse the performance of subjective global assessment of nutritional status in paediatric patients.
Key messages
-
•
SGA, by combining both objective and subjective measures, represents an explicit model of the thought process to be used in assessing nutritional status. In other words, the tool emphasizes the use of simple clinical data to identify a patient as nutritional‐at‐risk for further detailed evaluation and proper therapy in order to prevent from associated increases in hospital and home‐care costs.
-
•
From a practical view, after completing the SGA and identifying the malnourished child, the clinician has determined whether the child's nutritional status is likely to improve or get worse, has established the potential cause or causes of malnutrition, and identified where to target the intervention.
-
•
SGA is a simple, practical, non‐invasive, easy‐to‐apply and cost‐effective method for assessing nutritional status in children and identifying those at higher risk of nutrition‐associated complications and prolonged hospitalizations; Therefore, using SGA, there is no need to impose the discomfort and cost of laboratory tests in assessing the nutritional status of patients.
Materials and methods
Subjects
The study was approved by the Ethics Committee of the Tabriz University of Medical Sciences. All subjects were made aware of the content of the study and written informed consent was obtained from the responsible caregivers of the children.
One hundred and forty children (aged 2–12 years) hospitalized consecutively in the Pediatric Hospital of Tabriz University of Medical Sciences, including Surgical, Infectional, Oncology, ENT, and Internal Medicine, including Gastroenterology, Nephrology, Respiratory, Neurology, Cardiology and Metabolic disorder wards between June 2008 and August 2008 were studied. Patients hospitalized in emergency, newborn intensive care and newborn special care units were excluded from the study. Pediatric Hospital, located in Tabriz, is the only specialty and subspecialty centre for children in the north‐west of Iran, and it serves secondary and tertiary care for paediatric patients. All patients underwent subjective assessment using the SGA questionnaire and objective assessment including anthropometric and biochemical measurements. Both the subjective and objective assessment of nutritional status of each patient was performed by one nutritionist during the first 3 days of hospitalization.
SGA
SGA is a nutritional assessment tool based on the history and physical examination of the individual patient. A patient's history consisted of weight change (during the preceding 6 months and 2 weeks), changes in dietary intake, gastrointestinal symptoms, functional capacity and the assumed metabolic demand of the underlying disease. A patient's physical examination consisted of loss of subcutaneous fat, muscle wasting and the presence of ankle/sacral oedema (Fig. 1) (Heimburger 2006). Degree of metabolic stress of current illness or disease is shown in Appendix 1.
Figure 1.
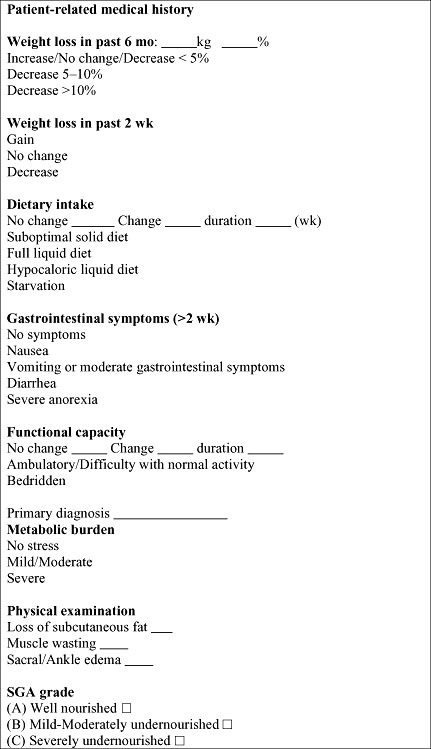
Subjective Global Assessment questionnaire.
Each of these features was rated separately as well nourished or undernourished. Based on a study previously done in Turkey (Nursal et al. 2005), the determined cut‐off score for SGA was 10; a score of less than 10 points is regarded as well nourished and a score of 10 points or higher is regarded as undernourished.
Objective assessment
All patients were assessed for anthropometric parameters [weight, height, triceps skinfold thickness and mid‐arm circumference (MAC)] and biochemical measurements (serum albumin, serum transferrin and total lymphocyte count). Measurements were performed with the use of calibrated equipment and standardized techniques.
Body weight of subjects was measured with barefoot and light clothing to the nearest 0.1 kg with a Seca scale. Height of subjects was measured with barefoot and using a mounted tape with the subject's arm hanging freely at their sides and recorded to the nearest 0.1 cm. In order to evaluate a patient's nutritional status, weight for age (W/A), height for age (H/A) and weight for height (W/H) were calculated (Dogan et al. 2005).
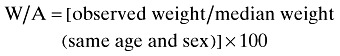 |
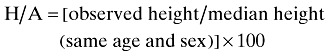 |
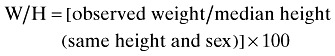 |
Undernutrition was defined by the criteria established by Waterlow that is below 90% of the median of sex‐specific reference values of weight for age of this population; below 95% of the median of sex‐specific reference values of height for age of this population; and below 90% of the median of sex‐specific reference values of weight for height of this population, respectively (Needleman 2000).
MAC was measured with a tape measure to the nearest 0.1 cm and triceps skinfold thickness (TSF) with a skinfold caliper (Harpenden, London, UK) to the nearest 0.1 mm at the midpoint of the arm between the tips of the acromion process and the olecranon process. The mean of three measurements was used.
The criteria used to define undernutrition and severe undernutrition, respectively, are MAC and TSF values <10th or <5th centiles of reference data based on the results of the US National Health and Nutrition Examination Survey I (NHANES I) (Pawellek et al. 2008).
A blood sample for measurement of serum albumin and transferrin was obtained. Biochemical analysis was performed with the use of the bromocresol green dye‐binding method for serum albumin and immunonturbidimetry for serum transferrin (Johnson et al. 1999). Automatic cell counter was used for measuring white blood cells and lymphocytes. Total lymphocyte count (TLC) was calculated using the formula (Heimburger 2006): TLC = white blood cells × % lymphocytes.
The criteria used to define undernutrition based on each parameter, respectively, are albumin values below 3.9 g dL−1, transferrin values below 200 mg dL−1 and total lymphocyte count below 1500 cell mm−3, respectively (Loughrey & Duggan 2000; Nicholson & Pesce 2000).
Finally, the nutritional status of each patient was determined according to the five major parameters, including weight for age, height for age, weight for height, triceps skinfold thickness and serum transferrin. Patients were classified as undernourished when at least two parameters were subnormal (Christensson et al. 2002).
Statistical analysis
Statistical analysis was performed with spss software. Data presented are means ± SD, and P less than 0.05 is considered to indicate statistical significance. Agreement between the SGA and the objective assessment was assessed using the kappa (κ) statistic. Also, statistical indicators including (sensitivity, specificity, positive and negative predictive values, error rates, accuracy, positive and negative powers, likelihood ratios and odds ratio) between SGA and objective assessment method were determined according to the formulas described in Table 1.
Table 1.
Statistical indicators between subjective global assessment and objective assessment
| Indicators | Formulas | Values* |
|---|---|---|
| Sensitivity |
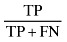
|
88.235 |
| Specificity |
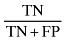
|
45.833 |
| Positive predictive value (PV+) |

|
60.606 |
| Negative predictive value (PV‐) |
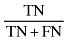
|
80.487 |
| False positive error rate |
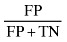
|
54.167 |
| False negative error rate |
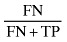
|
11.765 |
| Accuracy |
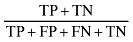
|
66.428 |
| Positive Power (Po+) |
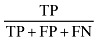
|
56.074 |
| Negative Power (Po‐) |
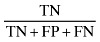
|
41.25 |
| Likelihood ratio positive (LR+) |
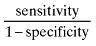
|
1.628 |
| Likelihood ratio negative (LR‐) |
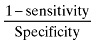
|
0.256 |
| Odds ratio (OR) |
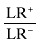
|
6.359 |
Values are presented as percentage (%) except for LR+, LR‐ and Odds ratio.
Results
The study sample comprised 67 girls (47.9%) and 73 boys (52.1%). The mean age (±SD) of the subjects was 6.43 ± 2.77 years. Patients anthropometric and laboratory data are shown in Table 2. According to SGA, 29.3% (n = 41) of patients were not at risk of undernutrition and 70.7% (n = 99) were at risk of undernutrition. Based on objective assessment of nutritional status, 51.4% (n = 72) of patients were well nourished and 48.5% (n = 68) were undernourished.
Table 2.
Patient anthropometric and laboratory data by nutritional status (n = 140)*
| Variables | SGA | Objective assessment | ||
|---|---|---|---|---|
| Not at risk of undernutrition (n = 41) | At risk of undernutrition (n = 99) | Well nourished (n = 72) | undernourished (n = 68) | |
| Weight (kg) | 20.34 ± 8.82 | 20.08 ± 7.62 | 21.53 ± 9.01 | 18.69 ± 6.42 |
| Height (cm) | 109.41 ± 19.39 | 114.13 ± 17.85 | 112.63 ± 19.00 | 112.88 ± 17.82 |
| Weight for age (%) | 99.21 ± 17.84 | 86.15 ± 14.34 | 101.19 ± 13.32 | 78.10 ± 9.91 |
| Height for age (%) | 98.49 ± 4.41 | 96.14 ± 5.71 | 99.61 ± 3.45 | 93.88 ± 5.65 |
| Weight for height (%) | 101.14 ± 14.05 | 93.27 ± 11.22 | 101.61 ± 12.77 | 89.19 ± 8.71 |
| TSF (mm) | 10.77 ± 3.28 | 9.67 ± 3.03 | 11.09 ± 3.15 | 8.83 ± 2.67 |
| MAC (cm) | 17.23 ± 2.59 | 15.95 ± 2.36 | 17.16 ± 2.60 | 15.45 ± 2.03 |
| Albumin (g dL−1) | 5.32 ± 0.43 | 5.25 ± 0.65 | 5.26 ± 0.57 | 5.29 ± 0.62 |
| Transferrin (mg dL−1) | 261.98 ± 55.65 | 238.81 ± 62.60 | 248.11 ± 60.00 | 242.93 ± 63.13 |
| Lymphocytes (cell mm−3) | 3416.16 ± 1743.90 | 3022.03 ± 1555.24 | 3161.61 ± 1573.98 | 3111.87 ± 1671.57 |
Data presented are means ± SD.
The ability of the SGA to predict objective assessment of nutritional status is shown in Table 3. Thirty‐three patients were correctly classified by the SGA as being well nourished (true negatives) and sixty patients were correctly classified as being undernourished (true positives). Agreement between the two different evaluation methods can be described as fair to moderate in 93 patients (κ = 0.336, P < 0.001).
Table 3.
Classification of 140 patients according to the SGA and the objective assessment of nutritional status
| SGA | Objective assessment | Total | |
|---|---|---|---|
| Undernourished | Well nourished | ||
| At risk of undernutrition (+) | TP (60) | FP (39) | 99 |
| Not at risk of undernutrition (−) | FN (8) | TN (33) | 41 |
| Total | 68 | 72 | 140 |
κ = 0.336, P < 0.001. TP, true positive; FP, false positive; TN, true negative; FN, false negative.
The sensitivity and specificity of the SGA method for screening undernutrition in this population were 88.235% and 45.833%, respectively (Table 1). Our findings indicate that subjective global assessment method can diagnose 88.235% of undernourished patients and 45.833% of well‐nourished patients correctly. Positive and negative predictive values of SGA indicate that 60.606% of patients who were diagnosed as undernourished by SGA were really undernourished, and 80.487% of patients who were diagnosed as well nourished by SGA were really well nourished. The reliability indicators including (accuracy, positive power and negative power) of the SGA method were 66.428%, 56.074% and 41.25%, respectively (Table 1).
Further evaluation of test properties of using SGA as a predictor of nutritional status was performed using LRs and odds ratio. Likelihood ratio positive (LR+), likelihood ratio negative(LR‐) and odds ratio (OR) of the SGA method were 1.628, 0.256 and 6.359, respectively (Table 1).
Discussion
According to the anthropometry assessment study done previously in Iran, moderate/severe underweight and moderate/severe stunting was reported in 11% and 15% of the under 5‐year‐old children, respectively. In addition, the results of prevalence of malnutrition among under 6‐year‐old children over 10 years showed that 4.7%, 3.7% and 5.2% of the children in Iran were stunted, underweight and wasted, respectively (Bayegi et al. 2006; Safavi et al. 2006).
This study is one of the first investigations that was conducted to assess the applicability of a SGA method for screening undernutrition in children referred to the Tabriz Pediatric Hospital in the north‐west region of Iran. In this study, the prevalence of undernutrition according to the SGA was about 70.7%, which was 22.2% higher than that by objective assessment. The prevalence of undernutrition within our study population, according to the SGA, is different from that found by Secker et al. (51%) (Secker & Jeejeebhoy 2007) in Canada and by Rojratsirikul et al. (35.9%) (Rojratsirikul et al. 2004) in Thailand. Many factors, including variations between the countries that conducted those studies, diversity in dietary patterns, socioeconomic status, co‐morbidities and medical care at hospitals from one country to another or even within the same country, play a role in causing variations in the prevalence of undernutrition in different studies.
SGA classification is one comprehensive assessment technique that has been shown to be a valid screening tool in the early identification of under‐nutrition in various patient populations. The original validation study of SGA classification was performed in 59 hospitalized surgical patients (Baker et al. 1982) in which SGA classification was compared with objective measurements of nutritional status and was noted to be the most sensitive (82%) and the most specific (72%) technique applied.
In our study, SGA showed good sensitivity (88.235%) in identifying patients diagnosed as undernourished by objective assessment (anthropometry and laboratory tests), but its specificity is suboptimal (45.833%). The sensitivity value found (88.235%) within our study was similar to that found by Ek et al. (85.2%) (Ek et al. 1996) in the study of elderly patients and by Detsky et al. (82%) (Baker et al. 1982) in the study of surgical patients but the specificity value found (45.833%) within our study was lower than that reported by Detsky et al. (72%) (Baker et al. 1982) in the same study. Several previous studies have compared SGA with objective parameters of nutritional assessment such as anthropometry, bioelectrical impedance and biochemical measures in various patient populations and have reported different sensitivity and specificity values with the SGA. The sensitivity (88.235%) and specificity (45.833%) values found with the SGA within our study were lower in comparison with the sensitivity of 96% and specificity of 83% found by Thoresen et al. (Thoresen et al. 2002) and with the sensitivity of 93% and specificity of 61% reported by Christensson et al. (Christensson et al. 2002). In a study of surgical patients, Mourão et al. (Mourão et al. 2004) showed that SGA was highly sensitive (100%) and specific (69%) than within our study. In a study of patients with rheumatoid arthritis, Elkan et al. (Elkan et al. 2008) evaluated diagnostic instruments for assessment of nutritional status in relation to objective anthropometrical measurements and found 46% sensitivity and 82% specificity for SGA. In a study of cardiac patients, Yamauti et al. (Yamauti et al. 2006) found that SGA sensitivity was 70.4% (76.2% in men and 50% in women) based on anthropometry tests and its accuracy among male patients was 70.3% and among female patients, 37.5%. In this study, it was also determined that SGA sensitivity was 76.5% (85.7% in men and 33.3% in women) based on anthropometry and laboratory tests, and its accuracy was 67.6% in men and 31.3% in women. Two studies have conducted to assess the applicability of SGA for screening undernutrition in children and have indicated that SGA is a valid (sensitive and specific) tool for assessing nutritional status in children and identifying those at higher risk of nutrition‐associated complications and prolonged hospitalizations (Rojratsirikul et al. 2004; Secker & Jeejeebhoy 2007).
It can be seen that studies conducted regarding validity and reliability of SGA have conflicting results. Some potential explanations may contribute to these contrary conclusions. First, patients studied by one researcher may represent a different population than the patients described by another researcher. This evidence suggests that nutritional assessment is more complicated than at first glance and appears to be population specific. Additional factors that influence nutritional evaluation is examiner's training and experience. Because SGA depends on the interviewer's training and on the interpretation of the collected data, and because training has perhaps the most significant impact on validity and reliability of SGA, a well‐trained SGA examiner was required if high sensitivity and specificity were to be attained and in order to be competent in nutritional assessment, a training period is proposed.
In the present study, SGA showed higher sensitivity than specificity, which means that more undernourished patients were correctly identified as being at nutritional risk than more non‐undernourished patients not being at nutritional risk. Low specificity means that many residents with false‐positive diagnoses of undernutrition might withdraw resources from those in real need of nutritional measures. When a tool with low specificity is used, it is most important that the disease/illness in focus commonly occurs in the population; otherwise, too many false‐positive diagnoses will be made. Low specificity, as found in this study, might indicate that SGA reflect poor health as well as nutritional status. SGA was originally developed for prediction of nutritionally associated complications but it is suggested that this tool may be equally likely to represent an index of sickness rather than nutrition (Jeejeebhoy et al. 1990). Accuracy, positive and negative power values in this study indicated that the use of SGA may give different results in various conditions and societies.
In conclusion, the present study showed that in assessing the nutritional status of paediatric patients, SGA differs with objective (anthropometry/laboratory) measurements. The result of our study also indicated that SGA is a highly sensitive tool for assessing nutritional status and could identify children at risk of developing undernutrition.
Source of funding
The authors wish to thank the Research Vice Chancellor of Tabriz University of Medical Sciences, Iran, for funding this project.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank the staff at Pediatric Medical Centre of Tabriz University of Medical Sciences, Iran, for their assistance in this project. We also wish to thank Hamed Nazermehr for his valuable assistance with laboratory measurements. In addition, we thank the children and their families for their participation in this research.
Appendix 1
Metabolic stress of current illness or disease
| Mild/Moderate metabolic stress | Severe metabolic stress |
|---|---|
| • Minor and non‐life threatening infection (e.g. UTI, peritonitis, CVL infection, bronchiolitis, gastroenteritis) • Co‐morbidity or concurrent disease that increases energy demand (e.g. malabsorption or chronic enteropathy, chronic cardiomyopathy, cystic fibrosis, persistent increased respiratory rate, sickle cell anaemia, spastic CP) • Fracture of a short bone • Minor surgery • Excessive physical activity | • Severe trauma • Major surgery • Burns • Severe sepsis/inflammation • Long‐bone fractures • Multi‐organ failure • Chronic illness with acute deterioration (e.g. acute flare of Crohns or ulcerative colitis) • Malignancy • AIDS with a secondary infection • Genetic metabolic disorder • Hyperthyroidism • Severe depression |
References
- Baker J.P., Detsky A.S., Wesson D.E., Wolman S.L., Stewart S., Whitewell J. et al. (1982) Nutritional assessment: a comparison of clinical judgment and objective measurements. New England Journal of Medicine 306, 969–972. [DOI] [PubMed] [Google Scholar]
- Bayegi F., Hagigiyan A. & Djazayeri A. (2006) Prevalence of malnutrition in children and interventions for poverty. 9th Iranian Nutrition Congress, Tabriz University of Medical Sciences, Tabriz, Iran, p. 32.
- Christensson L., Unosson M. & Ek A.‐C. (2002) Evaluation of nutritional assessment techniques in elderly people newly admitted to municipal care. European Journal of Clinical Nutrition 56, 810–818. [DOI] [PubMed] [Google Scholar]
- Correia M.I.T.D. & Waitzberg D.L. (2003) The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clinical Nutrition 22, 235–239. [DOI] [PubMed] [Google Scholar]
- Dogan Y., Erkan T., Yalvac S., Altay S., Aydin A., Cokuğraş F.C. et al. (2005) Nutritional status of patients hospitalized in pediatric clinic. The Turkish Journal of Gastroenterology 16, 212–216. [PubMed] [Google Scholar]
- Ek A.C., Unosson M., Larsson J., Ganowiak W. & Bjurulf P. (1996) Interrater variability and validity in subjective nutritional assessment of elderly patients. Scandinavian Journal of Caring Science 10, 163–168. [DOI] [PubMed] [Google Scholar]
- Elkan A.C., Engvall I.L., Tengstrand B., Cederholm T. & Hafström I. (2008) Malnutrition in women with rheumatoid arthritis is not revealed by clinical anthropometrical measurements or nutritional evaluation tools. European Journal of Clinical Nutrition 62, 1239–1247. [DOI] [PubMed] [Google Scholar]
- Hankard R., Bloch J., Martin P., Randrianasolo H., Bannier M.F., Machinot S. et al. (2001) Nutritional status and risk in hospitalized children. Archives of Pediatrics 8, 1203–1208. [DOI] [PubMed] [Google Scholar]
- Heimburger D.C. (2006) Adulthood In: Modern Nutrition in Health and Disease (eds Shils M.E., Shike M., Ross A.C., Caballero B., Cousins R.J.), pp. 830–842. Lippincott Williams and Wilkins: Philadelphia, PA. [Google Scholar]
- Jeejeebhoy K.N., Detsky A.S. & Barker J.P. (1990) Assessment of nutritional status. Journal of Parenteral Enternal Nutrition 14, 193–196. [DOI] [PubMed] [Google Scholar]
- Johnson A.M., Rohlfs E.M. & Silverman L.M. (1999) Proteins In: Teitz Textbook of Clinical Chemistry (eds Burtis C.A., Ashwoos E.R.), pp. 477–540. W.B. Saunders: Philadelphia, PA. [Google Scholar]
- Loughrey C.M. & Duggan C.H. (2000) Nutritional assessment: laboratory assessment of nutritional status In: Manual of Pediatric Nutrition (eds Hendricks K.M., Duggan C.H., Walker W.A.), pp. 66–76. BC Decker Hamilton: London. [Google Scholar]
- Mourão F., Amado D., Ravasco P., Vidal P.M. & Camilo M.E. (2004) Nutritional risk and status assessment in surgical patients: a challenge amidst plenty. Nutrition Hospital 19, 83–88. [PubMed] [Google Scholar]
- Needleman R.D. (2000) Assessment of growth In: Nelson Text book of Pediatrics (eds Behrman R.E., Kliegman R.M., Jenson H.B.), pp. 57–61. W.B. Saunders: Philadelphia, PA. [Google Scholar]
- Nicholson I.F. & Pesce M.A. (2000) Reference ranges for laboratory tests and procedures In: Nelson Text book of Pediatrics (eds Behrman R.E., Kliegman R.M., Jenson H.B.), pp. 2181–2229. W.B.Saunders: Philadelphia, PA. [Google Scholar]
- Nursal T.Z., Noyan T., Tarim A. & Karakayali H. (2005) A New weighted scoring system for Subjective Global Assessment. Nutrition 21, 666–671. [DOI] [PubMed] [Google Scholar]
- Pawellek I., Dokoupil K. & Koletzko B. (2008) Prevalence of malnutrition in paediatric hospital patients. Clinical Nutrition 27, 72–76. [DOI] [PubMed] [Google Scholar]
- Pirlich M., Schütz T., Kemps M., Luhman N., Minko N., Lübke H.J. et al. (2005) Social risk factors for hospital malnutrition. Nutrition 21, 295–300. [DOI] [PubMed] [Google Scholar]
- Rojratsirikul C., Sangkhathat S. & Patrapinyokul S. (2004) Application of subjective global assessment as a screening tool for malnutrition in pediatric surgical patients. Journal of Medical Association of Thai 87, 939–946. [PubMed] [Google Scholar]
- Sacks G.S., Dearman K., Replogle W.H., Cora V.L., Meeks M. & Canada T. (2000) Use of Subjective Global Assessment to Identify Nutrition‐Associated Complications and Death in Geriatric Long‐Term Care Facility Residents. Journal of American College of Nutrition 19, 570–577. [DOI] [PubMed] [Google Scholar]
- Safavi M., Sheikholeslam R., Naghavi M., Minaee M., Abdollahi Z. & Mohammadiyan S. (2006) Prevalence of malnutrition among under 6‐year‐old children over 10 years. 9th Iranian Nutrition Congress, Tabriz University of Medical Sciences, Tabriz, Iran, pp. 389–390.
- Secker D.J. & Jeejeebhoy K.N. (2007) Subjective global nutritional assessment for children. American Journal of Clinical Nutrition 85, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Shirodkar M. & Mohandas K.M. (2005) Subjective global assessment: a simple and reliable screening tool for malnutrition among Indians. Indian Journal of Gastroenterology 24, 246–250. [PubMed] [Google Scholar]
- Thoresen L., Fjeldstad I., Krogstad K., Kaasa S. & Falkmer U.G. (2002) Nutritional status of patients with advanced cancer: the value of using the subjective global assessment of nutritional status as a screening tool. Palliat Medicine 16, 33–42. [DOI] [PubMed] [Google Scholar]
- Waitzberg D.L., Caiaffa W.T. & Correia M.I.T.D. (2001) Hospital malnutrition: the Brazilian national survey(IBRANUTRI): a study of 4000 patients. Nutrition 17, 573–580. [DOI] [PubMed] [Google Scholar]
- Wakahara T., Shiraki M., Murase K., Fukushima H., Matsuura K., Fukao A. et al. (2007) Nutritional screening with Subjective Global Assessment predicts hospital stay in patients with digestive diseases. Nutrition 23, 634–639. [DOI] [PubMed] [Google Scholar]
- Yamauti A.K., Ochiai M.E., Bifulco P.S., De Araújo M.A., Alonso R.R., Cunha R.H. (2006) Subjective global assessment of nutritional status in cardiac patients. Arq Brasilian Cardiology 87, 707–712. [DOI] [PubMed] [Google Scholar]


