Abstract
The aim of the present study was to examine the relative validity of foods and nutrients calculated by a new food frequency questionnaire (FFQ) in the Norwegian Mother and Child Cohort Study (MoBa). Reference measures were a 4‐day weighed food diary (FD), a motion sensor for measuring total energy expenditure, one 24‐h urine collection for analysis of nitrogen and iodine excretion, and a venous blood specimen for analysis of plasma 25‐hydroxy‐vitamin D and serum folate. A total of 119 women participated in the validation study, and 112 completed the motion sensor registration. Overall, the level of agreement between the FFQ and the FD was satisfactory, and significant correlations were found for all major food groups and for all nutrients except vitamin E. The average correlation coefficient between the FFQ and the FD for daily intake was 0.48 for foods and 0.36 for nutrients, and on average, 68% of the participants were classified into the same or adjacent quintiles by the two methods. Estimated total energy expenditure indicated that under‐reporting of energy intake was more extensive with the FD than with the FFQ. The biological markers confirmed that the FFQ was able to distinguish between high and low intakes of nutrients, as measured by vitamin D, folate, protein and iodine. This validation study indicates that the MoBa FFQ produces reasonable valid intake estimates and is a valid tool to rank pregnant women according to low and high intakes of energy, nutrients and foods.
Keywords: validation, dietary assessment, pregnant women, food frequency questionnaire (FFQ), weighed food diary
Introduction
The importance of maternal nutrition for the health of both mother and child has long been recognized (Godfrey & Barker 2001). Therefore, assessment of maternal diet has become an important exposure variable in pregnancy cohorts. A questionnaire for dietary assessment in pregnant women was developed for use in the Norwegian Mother and Child Cohort Study (MoBa) (Meltzer et al. 2008). Food frequency questionnaires (FFQs) have been shown to be an appropriate method for assessing diet in a wide variety of epidemiological settings, including studies among pregnant women (Greeley et al. 1992; Brown et al. 1996; Robinson et al. 1996; Erkkola et al. 2001). In comparison with short‐term records, the FFQ provides a better approximation of the habitual diet over a longer period (Willett 1998). However, there are errors associated with the use of all dietary assessment instruments, and validation is required (Nelson 1997; Cade et al. 2002). Among the feasible comparative methods available for validating a FFQ, food records are likely to have the smallest correlated errors (Cade et al. 2002). In addition, biological markers of food or nutrient intake are useful as objective measures in validation studies (Hunter 1998). However, few biological markers are directly related to intake, and use is limited by high cost and detailed/laborious procedures (Bates et al. 1997).
A FFQ provides a representation or ‘image’ of food consumption over a designated period of time (Drewnowski 2001). Although FFQs are not considered appropriate for estimating true nutrient intake at the individual level, they can be used in epidemiological studies to rank individuals along the distribution of intake, so that individuals with low intakes can be separated from those with high intakes (Masson et al. 2003). Only the relative validity of the test method can be assessed, and use of several reference measures and statistical methods is recommended. Furthermore, the relative validity is a matter of degree and is limited by the reference methods (Nelson 1997; Willett 1998; Cade et al. 2002). The aim of the present study was to compare intakes of foods and nutrients by the MoBa FFQ and a 4‐day weighed food diary (FD) and independent measures (motion sensor and biological markers), and to assess the relative validity of the MoBa FFQ.
Subjects and methods
Validation study subjects and design
The present study is a subproject in MoBa initiated by the Norwegian Institute of Public Health (Magnus et al. 2006). Healthy pregnant women participating in MoBa who were referred to Bærum Hospital (Norway), were invited to take part in the validation study when they came for routine ultrasound examination at 17–18 weeks of gestation. Exclusion criteria were hyperemesis and anorexia. Before inclusion, the subjects had to have completed the MoBa FFQ. The inclusion period lasted from 15 January 2003 to 1 February 2004.
The women participating in the validation study were asked to keep a 4‐day weighed FD, to wear a motion sensor for the same 4 days, and to provide one 24‐h urine collection and a blood sample. They were given detailed information and materials for data collection at a meeting with the project nutritionist (A.L.B.) in groups of 5–10. The weight, height and age were recorded. Data pertaining to parity, marital status, smoking and education were collected from a separate questionnaire in which MoBa participants answered questions related to lifestyle and demographic factors.
Of the 120 women enrolled in the study, one dropped out due to illness and 119 completed the FD and the 24‐h urine collection, while 112 completed the motion sensor assessment according to the instructions. The average time interval between completion of the FFQ and participation in the study was 24 days [±12 days (SD)].
The study protocol was approved by the Regional Ethics Committee of Southern Norway, and informed written consent was obtained from all participants.
Dietary assessment
The MoBa FFQ (available at: http://www.fhi.no/dav/011fbd699d.pdf) was mailed to all participants around the 15th week and completed around 16–18th weeks of gestation. It is a semi‐quantitative questionnaire that asks about the intake of 255 food items and is designed to capture dietary habits and intake of dietary supplements during the first 4 months of pregnancy. More details of the FFQ are presented in an accompanying paper in this journal (Meltzer et al. 2008). Respondents were asked to fill in the mean intake of the food items eaten since becoming pregnant. The frequency intervals ranged from never to more than eight times a day. The questionnaires were checked for completeness and optically read. In the FFQ, portion size was only given for units of fruit, bread (slices) and liquids (cups/glasses). When portion sizes were not given in the questionnaire, consumption frequencies were converted into food amounts (g day−1) by the use of standard Norwegian portion sizes for women (Blaker & Aarsland 1989).
In the validation study, participants were asked to weigh and record all foods, beverages and dietary supplements consumed during three consecutive weekdays and one weekend day. Each participant was given a FD and a digital balance (Philips Essence HR 2389, Budapest, Hungary), and were asked to eat her normal diet. Upon collection, each FD was checked for completeness by the nutritionist (A.L.B.).
In the present study, FoodCalc (Lauritsen 2005) and the Norwegian food composition table (Rimestad et al. 2001) were used for calculating the daily intake of nutrients from foods. For the calculation of nutrients from dietary supplements, a database containing details of the declared content of supplements was used.
Before calculating correlation coefficients between total folate intake and serum folate, supplementary folic acid were expressed as dietary folate equivalents: 1.0 μg food folate = 0.6 μg folic acid from supplements (Yates et al. 1998).
Validation study participants were compared with a nationwide sample of MoBa participants. The sample used here includes 40 786 women who had completed the MoBa FFQ, using version II of the quality‐assured data files made available for research in 2006 (Magnus et al. 2006).
Other reference measures
There are few biomarkers directly related to dietary intake, and very few that have been validated for use in pregnant women. The choice of biomarkers was, therefore, with the exception of urinary iodine excretion, based on published studies in non‐pregnant subjects. The biological markers assessed in the validation study were: 24‐h urinary nitrogen excretion for comparison with protein intake (Bingham 2003), 24‐h urinary iodine excretion for iodine intake (Brussaard et al. 1997), plasma 25‐hydroxy‐vitamin D [25(OH)D] concentration for vitamin D intake (Bates et al. 1997), and serum folate concentration for comparison with total folate intake (Jacques et al. 1993).
At the end of the FD period, each participant provided one 24‐h urine collection taken on a weekday. On the first morning of the urine collection, participants were asked to discard their first urine specimen and, from then on, to collect all specimens for the next 24 h, including the first urine specimen of the next day. Participants had been provided with a funnel and bottles. All urine was pooled for each participant, and the samples were stored at −20°C within 8 h of collection. Total urinary nitrogen was determined by the Kjeldahl technique at the Norwegian Institute for Food and Environmental Analysis, Oslo, Norway. For evaluation of nitrogen excretion vs. protein intake, urinary protein was calculated as: (urinary nitrogen + 2) × 6.25 g protein per day (Isaksson 1980). Urinary iodine excretion was determined by inductively coupled mass spectrometry at the Institute of Nutrition and Seafood Research, Bergen, Norway (Dahl et al. 2003).
Non‐fasting blood samples were drawn at the time of recruitment, then separated into aliquots of serum and plasma within 2 h of venipuncture, and stored at −70°C until analysis. Plasma 25(OH)D concentrations were determined by high‐performance liquid chromatography at the Department of Clinical Medicine, Section for Pediatrics, University of Bergen, Norway (Aksnes 1994), and serum folate concentration was determined at Fürst Medical Laboratory using a standardized immunoassay technique (Centaur, Abbott Laboratories, Abbott Park, IL, USA).
Daily total energy expenditure was computed from the motion sensor ActiReg® data with ActiCalc software (Hustvedt et al. 2004). Resting energy expenditure (REE) was calculated with the World Health Organization (1985) expert group standard equation, using weight and height at the time of the motion sensor assessment to account for the increased REE with pregnancy.
Statistical analysis
The significance level was set at 5%, and all analyses were performed using spss, version 14 (SPSS Inc., Chicago, IL, USA). Fisher's exact test was used to compare the number of participants classified according to age, body mass index (BMI), parity, marital status, smoking habits, education and nausea in the validation study sample and in a nationwide MoBa sample.
Most nutrient and food intakes were not normally distributed, and are presented as medians, 5 and 95 percentiles. Nutrient intakes are presented as total intake including dietary supplements and as nutrients from food only.
The differences between food and nutrient intakes estimated by the FFQ and the FD were tested with Wilcoxon signed rank test (paired data).
The agreement between methods was analysed as proposed by Bland & Altman (1986), using a plot of the differences between the measurements by the two methods for each subject, against their mean. This was performed for all nutrients and food groups, and two plots are presented because no plots differed clearly from these.
The relationship between the two dietary methods is also presented by Spearman rank correlation coefficient. This was also performed for intake estimates and biomarker concentrations. Reference measures (FD, motion sensor and biomarkers) were examined by quintiles of FFQ intake. Differences in reference measures between the lowest and highest FFQ quintile were examined using the Mann–Whitney U‐test, and P for trend in reference measures across increasing quintiles of FFQ intake was assessed by regression. Furthermore, agreement on category level was examined by classification of food and nutrient intakes into same or adjacent quintile (correct classification) and into extreme opposite quintiles (misclassification) by the FFQ and the FD for both crude (amount per day) and energy adjusted (amount per 10 MJ) intakes.
The observed correlation between the 4‐day FD and the FFQ will be attenuated by the day‐to‐day variation in the types of food consumed in the FD period. Thus, we calculated attenuation factors to correct the observed correlation for the attenuating effect of random within‐person error. Attenuation factors were calculated for intake of energy, protein, fat, saturated fatty acids, mono‐unsaturated fatty acids, polyunsaturated fatty acids, calcium and vitamin D. Variance component analysis was used to calculated the within‐ and between‐person variation in the FD. The correction of the observed correlations for the attenuating effect of random within‐person error was computed according to the equation: 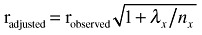 , where λx is the ratio of the within‐ and between‐person variances for x, and n
x is the number of replicates for the x variable (Willett 1998). For this study, n = 4 represents each recording day.
, where λx is the ratio of the within‐ and between‐person variances for x, and n
x is the number of replicates for the x variable (Willett 1998). For this study, n = 4 represents each recording day.
Results
Among the 119 participants in the validation study, there was large dispersion with regard to age and pre‐pregnant BMI (Table 1). The validation study sample participants were slightly older, thinner, included more married women and women without previous children, were better educated, and included fewer smokers than the nationwide sample of MoBa participants (Table 1). The incidence of pregnancy‐related nausea did not differ between the two samples.
Table 1.
Characteristics of the participants included in the validation study and of a nationwide sample of participants in the Norwegian Mother and Child Cohort Study (MoBa)*
| Validation study (n = 119) | MoBa (n = 39 375) | |
|---|---|---|
| Mean ± SD (min., max.) | Mean ± SD (min., max.) | |
| Age (years) | 31.2 ± 4.1 (23, 44) | 29.6 ± 4.6 (14, 47) |
| BMI prior to pregnancy (kg m−2) | 23.2 ± 3.6 (17, 43) | 24.2 ± 4.4 (13, 56) |
| n (%) | n (%) | |
| Age in categories (years) | ||
| <20 | 0 | 1039 (2.6) |
| 20–24.9 | 6 (5.0) † | 6259 (15.9) |
| 25–29.9 | 49 (41.2) | 15 598 (39.6) |
| 30–34.9 | 45 (37.8) | 12 746 (32.4) |
| 35+ | 19 (16.0) † | 3729 (9.5) |
| Missing data | 0 | 4 (0) |
| BMI (kg m−2) prior to pregnancy, in categories | ||
| <20 | 19 (16.0) | 4556 (11.6) |
| 20–24.9 | 75 (63.0) † | 21 020 (53.4) |
| 25–29.9 | 19 (16.0) | 8682 (22.0) |
| 30+ | 6 (5.0) | 3936 (10.0) |
| Missing data | 0 | 1181 (3) |
| Parity | ||
| 0 | 66 (55.5) † | 17 757 (45.1) |
| 1 | 25 (21.0) † | 14 005 (35.6) |
| 2+ | 28 (23.5) | 7613 (19.3) |
| Marital status | ||
| Married | 72 (60.5) † | 19 522 (49.6) |
| Cohabitants | 45 (37.8) | 18 378 (46.7) |
| Single | 2 (1.7) | 898 (2.3) |
| Missing data | 0 | 577 (1.5) |
| Smoking status prior to pregnancy | ||
| Never | 95 (79.8) † | 27 254 (69.2) |
| Occasional | 13 (10.9) | 3775 (9.6) |
| Daily | 11 (9.2) † | 8011 (20.3) |
| Missing data | 0 | 335 (0.9) |
| Education | ||
| ≤12 years | 20 (16.8) † | 15 487 (39.3) |
| 13–16 years | 58 (48.7) | 15 999 (40.6) |
| >16 years | 41 (34.5) † | 6964 (17.7) |
| Missing data | 0 | 925 (2.3) |
| Nausea during pregnancy* | ||
| Yes | 91 (76.5) | 29 815 (73.1) |
| Nausea at time of FFQ* | ||
| Yes | 18 (15.1) | 5914 (14.5) |
BMI, body mass index; FFQ, food frequency questionnaire. *The MoBa file included FFQ data for 40 786 subjects, while background variables were available for 39 375 (96.5%) of these subjects. † P < 0.05 (Fisher's exact test).
The differences between the FFQ and the FD for absolute intakes of nutrients and foods were examined using Bland–Altman plots. The plots for all nutrients and foods were similar to the plot of energy intake (Fig. 1), with the exception of the plots for fruit, juice and vegetables, for which the plot of the sum of these is presented (Fig. 2). For all nutrients and food groups, the mean difference between the methods (bias) was small, whereas the confidence limits were wide. For energy, nutrients and most food groups, but not for fruit and vegetables, there seemed to be a systematic increase in the difference between the two methods with increasing intake. The observed differences were both negative and positive, implying that participants both under‐ and over‐reported intakes with the FFQ compared with the FD. Using the Bland–Altman method for comparison of energy intake and expenditure (motion sensor) showed that the level of agreement was better for the FFQ (mean difference: −406 kJ) than for the FD (mean difference: −930 kJ), whereas, the bias of agreement was larger for the FFQ (SD: 2510 kJ) than for the FD (SD: 1302 kJ).
Figure 1.
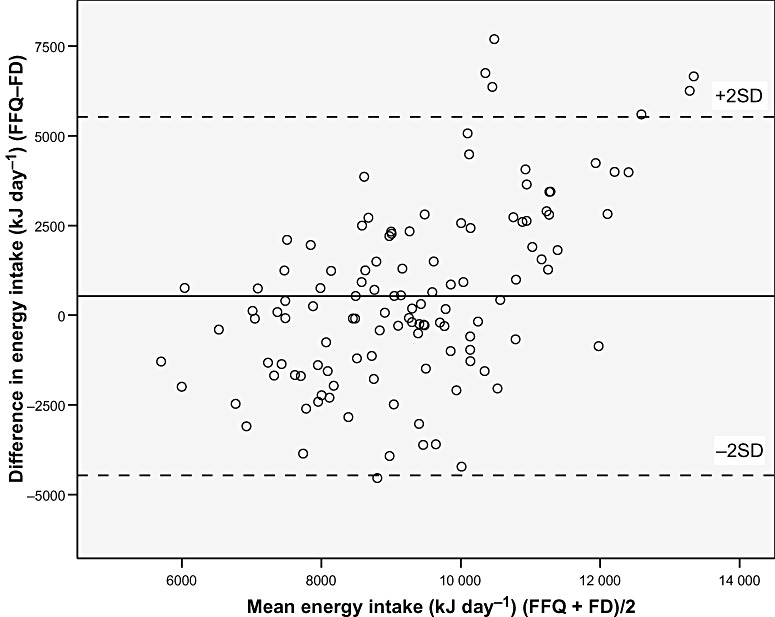
Bland–Altman plot between the food frequency questionnaire (FFQ) and the food diary (FD) methods for measuring daily energy intake. The solid line represents the mean difference between the two methods, and the dashed lines represent the limits of agreement corresponding to ±2 (SD).
Figure 2.
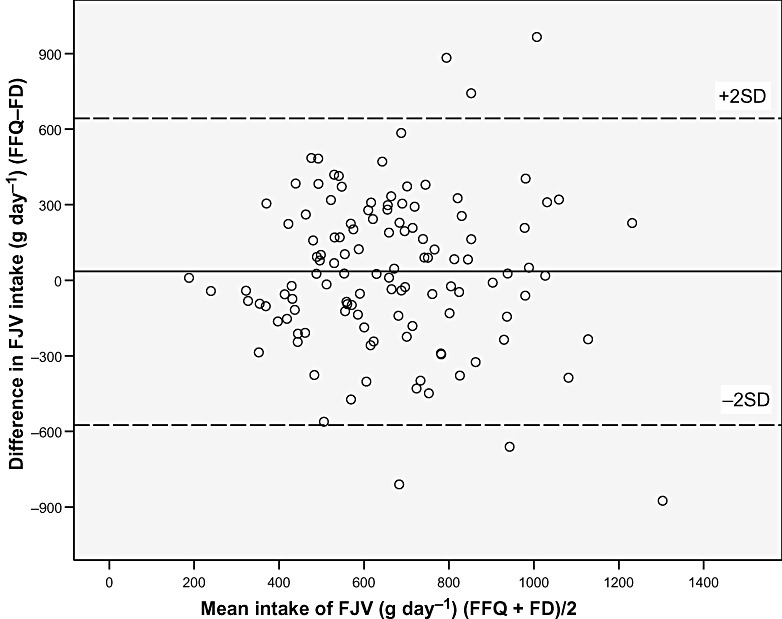
Bland–Altman plot between the food frequency questionnaire (FFQ) and the food diary (FD) methods for measuring the intake of fruit, juice and vegetables (FJV) The solid line represents the mean difference between the two methods, and the dashed lines represent the limits of agreement corresponding to ±2 (SD).
The median intake of most nutrients was larger calculated by the FFQ than by the FD (Table 2). The average correlation of absolute intakes between the two dietary assessments was 0.36, ranging from r = 0.13 for vitamin E in food to r = 0.55 for vitamin D in food plus supplements. The average energy‐adjusted correlation was 0.37, ranging from r = 0.10 for thiamine in food to r = 0.54 for dietary fibre. The correlations were generally stronger when nutrients supplied by dietary supplements were included in the intake estimates. All correlations were statistically significant except for vitamin E in food (crude correlation) and for thiamine in food (energy‐adjusted correlation)
Table 2.
Calculated daily intake of energy and nutrients by the food frequency questionnaire (FFQ) and by the weighed food diary (FD) and Spearman correlations (n = 119)
| Nutrients | FFQ intake per day Median (P5, P95) | FD intake per day Median (P5, P95) | Spearman r | Energy‐adjusted r |
|---|---|---|---|---|
| Energy (MJ) | 9.3 (6.3, 14.2) | 9.2 (6.7, 11.1) | 0.27 | |
| Protein (g) | 86 ‡ (59, 130) | 81 (60, 110) | 0.28 | 0.44 |
| Fat (g) | 75 (45, 120) | 78 (55, 110) | 0.23 | 0.39 |
| Carbohydrate (g) | 300 ‡ (170, 460) | 270 (190, 370) | 0.34 | 0.36 |
| Added sugar (g) | 54 (19, 140) | 48 (22, 110) | 0.36 | 0.29 |
| Fibre (g) | 29 ‡ (16, 48) | 24 (14, 36) | 0.42 | 0.54 |
| Beta‐carotene in food (μg) | 1900 ‡ (1000, 7400) | 1700 (490, 6600) | 0.29 | 0.33 |
| Beta‐carotene total* (μg) | 2000 ‡ (1000, 7500) | 1700 (490, 6800) | 0.34 | 0.37 |
| Retinol in food (μg) | 610 (230, 2100) | 610 (270, 1600) | 0.22 | 0.19 |
| Retinol total* (μg) | 950 ‡ (290, 2400) | 820 (300, 1800) | 0.32 | 0.25 |
| Vitamin D in food (μg) | 3.3 (1.2, 7.7) | 2.7 (0.7, 7.8) | 0.30 | 0.34 |
| Vitamin D total* (μg) | 8.4 ‡ (2.0, 21.2) | 7.1 (1.3, 16.2) | 0.55 | 0.50 |
| Vitamin E † in food (mg) | 10 (6, 16) | 9 (6, 14) | 0.13 ns | 0.37 |
| Vitamin E † total* (mg) | 18 (7, 42) | 18 (7, 44) | 0.45 | 0.50 |
| Thiamine in food (mg) | 1.5 ‡ (0.9, 2.3) | 1.4 (1.0, 2.0) | 0.35 | 0.10 ns |
| Thiamine total* (mg) | 2.0 ‡ (0.9, 4.2) | 1.6 (0.9, 3.8) | 0.49 | 0.43 |
| Riboflavin in food (mg) | 1.8 (1.0, 3.5) | 1.7 (1.1, 2.5) | 0.43 | 0.35 |
| Riboflavin total* (mg) | 2.5 ‡ (1.1, 6.0) | 2.0 (1.1, 4.3) | 0.46 | 0.37 |
| Niacin equivalents in food (mg) | 31 ‡ (21, 45) | 28 (20, 39) | 0.23 | 0.45 |
| Niacin equivalents total* (mg) | 38 ‡ (23, 62) | 32 (21, 53) | 0.43 | 0.42 |
| Vitamin B6 in food (mg) | 1.6 ‡ (1.0, 2.3) | 1.4 (1.0, 2.1) | 0.30 | 0.47 |
| Vitamin B6 total* (mg) | 2.1 ‡ (1.2, 6.4) | 1.7 (1.0, 5.4) | 0.48 | 0.50 |
| Folate in food (μg) | 280 ‡ (150, 470) | 230 (160, 370) | 0.22 | 0.25 |
| Folate total* (μg) | 450 ‡ (160, 870) | 330 (170, 690) | 0.31 | 0.41 |
| Vitamin C in food (mg) | 160 ‡ (59, 300) | 130 (45, 250) | 0.28 | 0.28 |
| Vitamin C total* (mg) | 180 (70, 350) | 150 (60, 380) | 0.25 | 0.27 |
| Calcium in food (mg) | 930 ‡ (490, 1900) | 920 (560, 1400) | 0.37 | 0.33 |
| Calcium total* (mg) | 980 ‡ (510, 2000) | 950 (580, 1400) | 0.41 | 0.35 |
| Phosphorous in food (mg) | 1600 ‡ (1100, 2700) | 1500 (1100, 2000) | 0.43 | 0.48 |
| Potassium in food (g) | 3.8 ‡ (2.6, 6.0) | 3.4 (2.5, 4.6) | 0.42 | 0.32 |
| Magnesium in food (mg) | 380 ‡ (250, 590) | 350 (230, 490) | 0.45 | 0.40 |
| Magnesium total* (mg) | 390 ‡ (250, 610) | 360 (240, 580) | 0.48 | 0.40 |
| Iron in food (mg) | 11 ‡ (7, 19) | 10 (7, 15) | 0.27 | 0.42 |
| Iron total* (mg) | 13 (8, 66) | 13 (8, 94) | 0.29 | 0.25 |
| Zinc in food (mg) | 11 ‡ (7, 17) | 10 (7, 14) | 0.43 | 0.39 |
| Copper in food (mg) | 1.3 (0.9, 2.1) | 1.2 (0.9, 1.9) | 0.38 | 0.44 |
| Iodine in food (μg) | 120 (54, 300) | 120 (54, 200) | 0.46 | 0.42 |
| Iodine total* (μg) | 140 (54, 300) | 130 (54, 250) | 0.48 | 0.40 |
| Selenium in food (μg) | 63 ‡ (40, 90) | 52 (29, 88) | 0.28 | 0.32 |
| Selenium total* (μg) | 67 ‡ (41, 138) | 57 (29, 124) | 0.33 | 0.30 |
Total = food and dietary supplements;
Vitamin E = alpha‐tocopherol equivalents.
Test of difference between the FFQ and the FD, P < 0.05 (Wilcoxon signed rank test).
P > 0.05, all other P < 0.05.
Several independent measures of dietary intake were examined in this study. Total energy expenditure correlated with the FD energy intake (r FD = 0.43, P < 0.001), but not with the FFQ energy intake (r FFQ = 0.14, P = 0.130). The protein content of the 24‐h urine sample correlated with both the FD and the FFQ protein intake (r FD = 0.65, P < 0.001 and r FFQ = 0.27, P = 0.004). Plasma 25(OH)D correlated with both the FD and the FFQ vitamin D intake (r FD = 0.43, P < 0.001 and r FFQ = 0.32, P < 0.001). Serum folate concentration correlated with both the FD and the FFQ intake of dietary folate equivalents (r FD = 0.57, P < 0.001 and r FFQ = 0.26, P = 0.005), and finally, the iodine content of the 24‐h urine sample correlated with both the FD and the FFQ iodine intake (r FD = 0.46, P < 0.001 and r FFQ = 0.38, P < 0.001). A significant increase in intake across increasing quintiles of FFQ was found for all nutrients calculated by the FD, while a significant increase in biomarker levels across increasing quintiles of FFQ intakes was found for protein, vitamin D and iodine. Significant differences between the upper and lower quintile of FFQ intakes were found for all FD intakes, as well as for urinary nitrogen, plasma 25(OH)D, serum folate and urinary iodine excretion (Table 3).
Table 3.
Calculated intakes of energy and nutrients from the 4‐day food diary (FD) and independent measures (activity registration and biomarkers in plasma and urine) by the MoBa food frequency questionnaire (FFQ) quintiles
| Nutrient | Correctly classified (%) | Grossly misclassified (%) | Levels in quintiles of the MoBa FFQ (mean ± SD) | Q1 vs. Q5 P‐value † | P for trend ‡ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 23) | Q2 (n = 24) | Q3 (n = 24) | Q4 (n = 24) | Q5 (n = 24) | |||||
| Energy | |||||||||
| Intake from FD (kJ day−1) | 66 | 13 | 8408 ± 1 365 | 8791 ± 1595 | 9387 ± 1 091 | 9178 ± 1412 | 9322 ± 1 173 | <0.001 | 0.012 |
| Energy expenditure (kJ day−1) | 58 | 17 | 10 100 ± 1 098 | 9507 ± 829 | 10 344 ± 1 325 | 9995 ± 740 | 10 203 ± 883 | 0.575 | 0.248 |
| Protein | |||||||||
| Intake from FD (g day−1) | 62 | 13 | 78 ± 13 | 79 ± 15 | 78 ± 11 | 87 ± 14 | 89 ± 15 | 0.028 | 0.001 |
| Urinary protein (g per 24 h) | 59 | 11 | 79 ± 12 | 74 ± 15 | 78 ± 17 | 89 ± 16 | 89 ± 17 | 0.038 | 0.001 |
| Vitamin D* | |||||||||
| Intake from FD (μg day−1) | 71 | 0 | 4.6 ± 2.6 | 5.5 ± 3.7 | 7.6 ± 6.2 | 9.6 ± 4.6 | 11.6 ± 5.0 | <0.001 | <0.001 |
| Plasma 25(OH)D (nmol l−1) | 61 | 4 | 60.6 ± 22.4 | 62.7 ± 27.0 | 67.7 ± 28.2 | 77.0 ± 24.8 | 80.0 ± 19.7 | 0.003 | 0.001 |
| Dietary folate equivalents* | |||||||||
| Intake from FD (μg day−1) | 61 | 4 | 175 ± 75 | 247 ± 141 | 271 ± 128 | 303 ± 162 | 331 ± 204 | <0.001 | <0.001 |
| Serum folate (nmol l−1) | 62 | 11 | 19.2 ± 6.3 | 24.1 ± 11.4 | 24.1 ± 8.8 | 24.3 ± 8.8 | 24.9 ± 8.5 | 0.004 | 0.054 |
| Iodine* | |||||||||
| Intake from FD (μg day−1) | 67 | 2 | 97 ± 52 | 126 ± 55 | 131 ± 48 | 148 ± 62 | 183 ± 61 | <0.001 | <0.001 |
| Urinary iodine (μg per 24 h) | 63 | 4 | 91 ± 59 | 156 ± 91 | 175 ± 106 | 172 ± 106 | 208 ± 80 | <0.001 | <0.001 |
Correctly classified if classified into same or adjacent quintiles, grossly misclassified if classified into opposing quintiles. *Including dietary supplements. †Mann–Whitney U‐test. ‡Linear trend.
Correlations between the FFQ and reference measures were influenced by pregnancy‐related nausea. When participants who reported nausea were excluded, the correlation between FFQ and FD intakes increased from r = 0.27 to r = 0.49 for energy, and from r = 0.28 to r = 0.43 for protein. The correlation between FFQ protein intake and urinary nitrogen excretion increased from r = 0.27 to r = 0.58 (P < 0.01 for all).
Total energy expenditure based on the motion sensor registration ranged from 7.87 to 12.88 MJ day−1 (mean 10.02 MJ day−1). Energy intake calculated by the FD varied from 5.66 to 12.4 MJ day−1 and corresponded to on average 91% of the energy expenditure. Energy intake calculated by the FFQ varied from 5.00 to 16.67 MJ day−1 and corresponded to on average 96% of the energy expenditure.
Correcting for the attenuating effect of random within‐person error improved the correlations between the FFQ and the FD for energy, protein, fat, calcium and fatty acids. For vitamin D (food only), the day‐to‐day variation was so large that the corrected correlation was larger than 1 (Table 4).
Table 4.
Attenuation factors and their effect on the correlations between the food frequency questionnaire and the food diary (n = 119)
| Attenuation factor | Correlations, unadjusted r | Energy‐adjusted r | Attenuation and energy‐adjusted r | |
|---|---|---|---|---|
| Energy | 1.20 | 0.27 | 0.32 | |
| Protein | 1.27 | 0.28 | 0.44 | 0.56 |
| Fat | 1.38 | 0.23 | 0.39 | 0.54 |
| SAFA | 1.36 | 0.34 | 0.33 | 0.45 |
| MUFA* | 1.37 | 0.15 ns | 0.32 | 0.44 |
| PUFA* | 1.39 | 0.30 | 0.45 | 0.63 |
| Calcium* | 1.43 | 0.37 | 0.33 | 0.47 |
| Vitamin D* | 3.36 | 0.30 | 0.34 | Infeasible value >1 |
MUFA, mono‐unsaturated fatty acids; PUFA, polyunsaturated fatty acids; SAFA, saturated fatty acids. *Food only. ns P > 0.05, all other P < 0.05.
The intake of bread, meat, seafood and sweets was significantly larger calculated by the FFQ than by the FD. The correlation coefficients between the FFQ and the FD for intake of foods and food groups were stronger than those for nutrients (mean average 0.48 vs. 0.36), and tended to be stronger for the beverages coffee, tea and milk (Table 5) than for bread, fish and meat.
Table 5.
Calculated median intake of major food groups by the food frequency questionnaire (FFQ) and by the weighed food diary (FD) and Spearman correlations (n = 119)
| Food group | FFQ intake (g day−1) Median (P5, P95) | FD intake (g day−1) Median (P5, P95) | Spearman correlations | |
|---|---|---|---|---|
| Crude r | Energy‐adjusted r ‡‡ | |||
| Milk (drink) | 260 (0, 1200) | 210 (16, 660) | 0.66 | 0.67 |
| Dairy foods | 450 (60, 1300) | 360 (100, 790) | 0.58 | 0.59 |
| Brown bread | 170 †† (43, 320) | 110 (27, 210) | 0.41 | 0.37 |
| All breads | 190 †† (70, 350) | 160 (72, 260) | 0.36 | 0.30 |
| Eggs | 8 (2, 40) | 13 (0, 55) | 0.35 | 0.35 |
| Meat | 110 †† (57, 170) | 90 (20, 190) | 0.33 | 0.21 |
| Fish* and seafood † | 31 †† (7, 84) | 26 (0, 86) | 0.43 | 0.49 |
| Fruit | 250 (52, 510) | 180 (74, 460) | 0.39 | 0.32 |
| Juice | 150 (10, 500) | 150 (0, 530) | 0.50 | 0.50 |
| Raw vegetables ‡ | 84 (18, 240) | 86 (11, 200) | 0.42 | 0.48 |
| All vegetables § | 170 (59, 400) | 150 (48, 380) | 0.34 | 0.48 |
| Margarine/butter | 9 (0, 42) | 9 (0, 42) | 0.65 | 0.64 |
| Chocolates/sweets | 27 †† (9, 120) | 25 (0, 76) | 0.38 | 0.34 |
| Soft drinks ¶ | 110 (8, 1000) | 125 (0, 670) | 0.48 | 0.51 |
| Tea** | 120 (0, 710) | 93 (0, 500) | 0.53 | 0.54 |
| Coffee | 20 (0, 480) | 13 (0, 330) | 0.80 | 0.80 |
Fish in mixed dishes not included (fish soup, fish au gratin, fish wok, fish pudding).
† Roe, crabs, scallops, prawns.
‡ Leafy green vegetables, tomatoes, cucumber, peppers.
§ Sum of all vegetables except potatoes.
¶ Including both sugar sweetened and artificial sweetened.
**Black, green and herb tea.
Test of difference between the FFQ and the FD, P < 0.01 (Wilcoxon signed rank test).
‡‡ Energy adjustment: intake per 10 MJ (g per 10 MJ).
Classification into quintiles showed that on average, 68% of the women were classified into the same or adjacent quintile when ranked by the FFQ and the FD, whether absolute or energy‐adjusted intakes were examined, and on average, less than 10% were misclassified into opposite quintiles by the two methods (Table 6).
Table 6.
Cross classification of subjects by quintiles of calculated intake of nutrients and foods from the food frequency questionnaire (FFQ) and the food diary (FD)
| Absolute intake (g day−1) | Energy‐adjusted intake (g per 10 MJ) | |||
|---|---|---|---|---|
| Correctly classified (%) | Grossly misclassified (%) | Correctly classified (%) | Grossly misclassified (%) | |
| Nutrients* | ||||
| Energy | 66 | 13 | ||
| Protein | 62 | 13 | 71 | 6 |
| Fat | 61 | 17 | 61 | 4 |
| Carbohydrate | 67 | 13 | 62 | 8 |
| Added sugar | 65 | 6 | 57 | 9 |
| Dietary fibre | 67 | 13 | 76 | 9 |
| Beta‐carotene | 66 | 13 | 65 | 6 |
| Retinol | 68 | 13 | 63 | 13 |
| Vitamin D | 71 | 0 | 72 | 0 |
| Vitamin E | 73 | 6 | 74 | 4 |
| Thiamine | 76 | 4 | 77 | 8 |
| Riboflavine | 68 | 4 | 69 | 6 |
| Niacin equivalents | 70 | 4 | 69 | 4 |
| Vitamin B6 | 74 | 6 | 76 | 6 |
| Folate | 61 | 4 | 67 | 2 |
| Vitamin C | 59 | 17 | 62 | 11 |
| Calcium | 69 | 13 | 66 | 13 |
| Phosphorous | 71 | 8 | 71 | 6 |
| Potassium | 70 | 4 | 61 | 3 |
| Magnesium | 70 | 2 | 68 | 11 |
| Iron | 66 | 9 | 66 | 15 |
| Zinc | 71 | 11 | 74 | 13 |
| Copper | 68 | 9 | 66 | 6 |
| Iodine | 67 | 2 | 68 | 6 |
| Selenium | 64 | 6 | 63 | 15 |
| Food groups | ||||
| Dairy foods | 72 | 0 | 75 | 0 |
| All breads | 65 | 6 | 64 | 11 |
| Meat | 64 | 6 | 59 | 4 |
| Fish † and seafood ‡ | 66 | 6 | 73 | 4 |
| Fruit | 66 | 6 | 60 | 4 |
| Juice | 71 | 6 | 78 | 11 |
| Vegetables § | 61 | 4 | 67 | 4 |
| Chocolate/sweets | 63 | 8 | 70 | 6 |
| Soft drinks ¶ | 72 | 11 | 73 | 8 |
| Tea** | 77 | 11 | 76 | 9 |
| Coffee | 94 | 0 | 94 | 2 |
Correctly classified if classified into same or adjacent quintiles, grossly misclassified if classified into opposing quintiles (n = 119). *Food and supplements. †Fish in mixed dishes not included (fish soup, fish au gratin, fish wok, fish pudding). ‡Roe, crabs, scallops, prawns. §All vegetables except potatoes. ¶Including both sugar sweetened and artificial sweetened.**Black, green and herb tea.
Discussion
This is the first validation study of a FFQ conducted in pregnant women in Norway. It was undertaken because a new FFQ was developed for assessment of diet in MoBa. There was relatively good agreement between the FFQ and the weighed food record for energy, nutrients and foods. The associations between the two dietary methods were influenced by day‐to‐day variation in the FD and, apparently, dietary changes from nausea. The validity of the FFQ was supported by increasing biomarker concentrations across increasing quintiles of FFQ intakes for protein, vitamin D and iodine. Several statistical approaches have been applied in order to present the data and to express the validity of the FFQ relative to the reference measures.
The Bland–Altman method showed that the level of agreement between the FFQ and the FD was better at the group level than at the individual level, as the mean difference between the methods was small, whereas the confidence limits were wide (1, 2). The plots indicated that, for most nutrients and food groups, the difference between the FFQ and FD increased with increasing intake. This often occurs in situations where the measurement error is a relatively constant fraction of the reading. The Bland–Altman plot of fruit, juice and vegetable intake (Fig. 2) did not show a similar increase in the difference between the methods with increasing intake, indicating a more precise identification of high and low intakes. It may also indicate that under‐reporting may be less common with food items considered healthy, than with energy‐yielding food items. A study from Iceland regarding quality of dietary assessment methods showed that women specifically under‐reported foods high in sugar and fat (Olafsdottir et al. 2006). The validity of fruit and vegetable intake measured by the new MoBa FFQ relative to biological markers of fruit and vegetable intake has been described previously (Brantsæter et al. 2007).
The correlations between the MoBa FFQ and the FD are comparable to those reported in other validation studies in pregnant women (Robinson et al. 1996; De Vriese et al. 2001; Erkkola et al. 2001; Mikkelsen et al. 2006), and lower than those reported in most non‐pregnant populations (Andersen et al. 1995; Byers 2001; Subar et al. 2001). The average correlation of absolute intakes between the FFQ and FD in our study was 0.36 for nutrients and 0.48 for foods. In a validation study in Finnish pregnant women, the average correlation was 0.37 for nutrients and 0.47 for foods (Erkkola et al. 2001), while in a validation study in US pregnant women, the correlation was higher than 0.5 for most nutrients (Brown et al. 1996). There are few published validation studies in pregnant women, and the studies are difficult to compare because of differences in the FFQ instruments, reference methods and days of recording, and because the studies cover various periods of pregnancy. We found that correlations for energy and protein intakes between the FFQ and the FD were stronger when women who reported nausea were excluded. Nausea may have been less evident at the time of the FD than at the time of the questionnaire, and thus attenuate the correlations. Similar findings regarding nausea were reported in another validation study in pregnant women (Robinson et al. 1996).
The correlations between the independent reference measures (motion sensor and biological markers) and the dietary assessments were stronger for the FD than for the questionnaire. This is not surprising in view of the FD being conducted at the same time as the energy and biomarker assessments, which also has been reported in other validation studies in non‐pregnant (McKeown et al. 2001) and in pregnant women (Robinson et al. 1996; Mikkelsen et al. 2006).
Twenty‐hour urinary nitrogen has been frequently used for the validation of dietary protein intake, and strong correlations have been demonstrated when multiple urine samples have been collected (Bingham et al. 1995; Day et al. 2001). The use of 24‐h urinary nitrogen for validation of protein intake depends on the assumption that subjects are in nitrogen balance (Bingham 2003), a condition that is not present during pregnancy. In spite of this assumption, and in spite of having only one urine sample from each participant, Mikkelsen et al. (2006) found that urinary nitrogen in pregnant women correlated with protein intake by a 7‐day FD (r = 0.64, P < 0.001) but not with a FFQ, whereas, we found that urinary nitrogen correlated both with the FD (r = 0.65, P < 0.001) and, weakly, with the FFQ protein intake (r = 0.27, P = 0.004). In comparison with their study, we found weaker increase in serum folate across increasing folate intake than they did using erythrocyte folate. The two studies were conducted at different stages of pregnancy, which may partially explain some of these differences.
What constitutes a satisfactory level of correlation is totally dependent on the relation under investigation. In most validation studies, correlation coefficients between dietary methods are considered poor if <0.30, fair if 0.30–0.49, and good if >0.50 (Hankin et al. 1991). However, correlations alone are not sufficient to give credence to the FFQ, as illustrated by 3, 6. In spite of relatively modest correlations between the FFQ estimates and reference measures, the FFQ was able to distinguish between high and low consumers (Q1 vs. Q5) for all the FD estimates, as well as for urinary nitrogen, urinary iodine, plasma 25(OH)D and serum folate. The degree of misclassification was small, while around two‐thirds of the subjects were classified into the same or adjacent quintile by the FFQ and reference measures. Classification into the same or adjacent quintile by the two dietary methods was similar to that reported for a questionnaire used for assessment of diet in pregnant women in Finland (Erkkola et al. 2001), and for the questionnaire used in the Danish National Birth Cohort (Mikkelsen et al. 2006). In future studies of diet and disease, the ability of the FFQ to identify high and low consumers is important, and our results showed that the FFQ was capable of this. However, the fact that about one‐third of participants were not classified to the same or adjacent quintile by the two methods raises concerns. This lack of agreement may actually in large part be due to limitations of the reference measure. A major challenge in validation of FFQs is the selection of the appropriate reference measures. It is not possible to measure the ‘true’ habitual dietary intake. Dietary assessments aimed at determining current intake are likely to interfere with the subject's everyday habits and cause a distortion of intake (Cade et al. 2002). Furthermore, the repeatability of the FD in our pregnant subjects is not known, but we know that many change their eating patterns during pregnancy (Rifas‐Shiman et al. 2006). The FFQ covers a longer time span and may actually be a better reflection of habitual intake than a weighed FD covering intake over a few specific days. Therefore, several biological markers were included as additional reference measures in our study. However, biomarkers may be influenced by factors other than the dietary intake (Bates et al. 1997), and classification into the same or adjacent quintiles by the FFQ and biomarkers [urinary nitrogen excretion, plasma 25(OH)D, serum folate and urinary iodine excretion] did not result in higher agreement than the comparison of FFQ and FD estimates (Table 3).
Evaluating the validity of reported energy intakes provides a valuable check on the general quality of the dietary data in any study (Livingstone & Black 2003). A linear relationship between total energy expenditure and energy intake was not found (Table 3) and cannot be expected during pregnancy due to individual variation in energy balance. The energy expenditure measurement indicated, however, that underreporting was more extensive with the FD than with the questionnaire. This was confirmed by the Bland–Altman method, as the mean difference between energy intake and expenditure was twice as large for the FD (−930 kJ) as for the FFQ (−406 kJ) in spite of the FD being at the same time as the motion sensor measurement. Comparison of reported energy intake with energy expenditure measured by the doubly labelled water method (Schoeller 2002), has revealed that under‐reporting is a serious error in food recording in all population groups, including pregnant women (Forsum et al. 1992; Goldberg et al. 1993).
Women in the validation study were not, fraction wise, totally representative of the target population with regard to age, BMI, parity, marital status, smoking habits and education. However, there is little reason to believe the results would have been very different with a completely unbiased sample. Furthermore, the median daily intakes of energy, protein and fat (9.3 MJ, 86 g and 75 g) in the validation study sample are comparable to the intake of energy, protein and fat (9.4 MJ, 84 g and 77 g, respectively) in a larger sample of MoBa participants (Meltzer et al. 2008). It was important to obtain a broad range of intake within the sample, and this was achieved (Table 2). The sample size of 119 subjects is reasonable for a validation study, and the subjects came from the population for which the questionnaire was designed (Willett 1998).
In the present validation study, a 4‐day weighed food record was chosen as the dietary reference measure. Recording (i.e. weighing and measuring) of food, drink and dietary supplements does not rely on memory or the ability to estimate portion sizes. However, it is demanding and includes the possibility to under‐report intake or to eat differently during the recording period. Furthermore, few days of food recording may represent a limited estimate of the habitual intake. Upon collecting dietary data at the individual level, there is a trade‐off between the burden one can impose on the included subjects and the accuracy, and hence the usefulness of the data. Four days can be sufficient if the sample size is large, but foods eaten rarely will not be accurately assessed (Stram et al. 1995). Adjusting for the within‐person variation during the 4 days of food recording (Table 4) is an approach developed to compensate for the limited observation time of the FD compared with the questionnaire (Willett 1998). We included this approach to illustrate that the agreement between the FFQ and FD has been influenced by the short time frame of the FD.
Participants were asked not to alter their food habits during the recording days, but many admitted that snacks were omitted during the 4‐day recording period. This may explain the relatively modest correlation for added sugar (crude: 0.36, energy adjusted: 0.29) shown in Table 2.
Limitations of the reference measures and lack of standardized timing between the test and reference measures may have attenuated our results. Use of an external marker like para‐aminobenzoic acid to verify completeness of urine collection (Bingham 2003) was considered unacceptable in this population, and one woman did not want to wear the motion sensor due to fear of radiation on the fetus. Wearing the motion sensor was the reference measure with the lowest compliance.
Non‐fasting blood specimens were obtained so that participants did not have to take time off from work in the morning. The impact of using non‐fasting blood specimens is uncertain. Ahn et al. (2005) found no evidence of circadian variation in folate pharmacokinetics, while previous studies have reported this for lipids, cholesterol and zinc (Kanabrocki et al. 1983; Romon et al. 1997). Non‐fasting blood samples were previously used in a validation study of carotenoid intake by FFQ (McNaughton et al. 2005). The analysis of serum/plasma biomarkers were based on a single blood sample from each participant, but contrary to urine measurements, it has been shown that a single sample may accurately rank individuals (van Kappel et al. 2001; Dixon et al. 2006). Dixon et al. (2006) reported no advantage of two blood samples over one, suggesting reasonably stable ranking of individuals for carotenoids and tocopherol in women with only one blood sample.
In summary, the present validation study indicates that the MoBa FFQ produces a realistic and relatively precise estimate of the habitual intake of energy, nutrients and food groups among pregnant Norwegian women, and is a valid tool for categorizing pregnant women according to high and low intakes of energy, nutrients and foods.
Acknowledgements
The authors would like to thank all the women who participated in the validation study. The study was financially supported by the Research Council of Norway, and by the European Commission, 6th Framework Programme, Priority 5 on Food Quality and Safety (FOOD Contract No. 016320 Integrated Project), ‘Newborns and Genotoxic Exposure Risk: Development and application of biomarkers of dietary exposure to genotoxic chemicals and of biomarkers of early effects, using mother–child birth cohorts and biobanks (NewGeneris)’. None of the authors had a conflict of interest.
References
- Ahn E., Kapur B. & Koren G. (2005) Study on circadian variation in folate pharmacokinetics. Canadian Journal of Clinical Pharmacology 12, e4–e9. [PubMed] [Google Scholar]
- Aksnes L. (1994) Simultaneous determination of retinol, alpha‐tocopherol, and 25‐hydroxyvitamin D in human serum by high‐performance liquid chromatography. Journal of Pediatric Gastroenterology and Nutrition 18, 339–343. [DOI] [PubMed] [Google Scholar]
- Andersen L.F., Nes M., Lillegaard I.T., Sandstad B., Bjørneboe G.E. & Drevon C.A. (1995) Evaluation of a quantitative food frequency questionnaire used in a group of Norwegian adolescents. European Journal of Clinical Nutrition 49, 543–554. [PubMed] [Google Scholar]
- Bates J.C., Thurnham D.I., Bingham S.A., Margetts B.M. & Nelson M. (1997) Biochemical markers of nutrient intake In: Design Concepts in Nutritional Epidemiology (eds Margetts B.M. & Nelson M.), 2nd edn, pp 170–240. Oxford University Press: Oxford. [Google Scholar]
- Bingham S.A. (2003) Urine nitrogen as a biomarker for the validation of dietary protein intake. Journal of Nutrition 133(Suppl. 3), 921S–924S. [DOI] [PubMed] [Google Scholar]
- Bingham S.A., Cassidy A., Cole T.J., Welch A., Runswick S.A., Black A.E. et al. (1995) Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. British Journal of Nutrition 73, 531–550. [DOI] [PubMed] [Google Scholar]
- Blaker B. & Aarsland M. (1989) Mål og vekt for matvarer [Household Measures and Weights of Foods]. Landsforeningen for kosthold og helse [National Association for Nutrition and Health]: Oslo. [Google Scholar]
- Bland J.M. & Altman D.G. (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310. [PubMed] [Google Scholar]
- Brantsæter A.L., Haugen M., Rasmussen S.E., Alexander J., Samuelsen S.O. & Meltzer H.M. (2007) Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutrition 10, 274–283. [DOI] [PubMed] [Google Scholar]
- Brown J.E., Buzzard I.M., Jacobs D.R. Jr, Hannan P.J., Kushi L.H., Barosso G.M. et al. (1996) A food frequency questionnaire can detect pregnancy‐related changes in diet. Journal of the American Dietetic Association 96, 262–266. [DOI] [PubMed] [Google Scholar]
- Brussaard J.H., Brants H.A., Hulshof K.F., Kistemaker C. & Lowik M.R. (1997) Iodine intake and urinary excretion among adults in the Netherlands. European Journal of Clinical Nutrition 51(Suppl. 3), S59–S62. [PubMed] [Google Scholar]
- Byers T. (2001) Food frequency dietary assessment: how bad is good enough? American Journal of Epidemiology 154, 1087–1088. [DOI] [PubMed] [Google Scholar]
- Cade J., Thompson R., Burley V. & Warm D. (2002) Development, validation and utilisation of food‐frequency questionnaires: a review. Public Health Nutrition 5, 567–587. [DOI] [PubMed] [Google Scholar]
- Dahl L., Meltzer H.M., Opsahl J.A. & Julshamn K. (2003) Iodine intake and status in two groups of Norwegians. Scandinavian Journal of Nutrition 47, 170–178. [Google Scholar]
- Day N., McKeown N., Wong M., Welch A. & Bingham S. (2001) Epidemiological assessment of diet: a comparison of a 7‐day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. International Journal of Epidemiology 30, 309–317. [DOI] [PubMed] [Google Scholar]
- De Vriese S.R., De Henauw S., De Backer G., Dhont M. & Christophe A.B. (2001) Estimation of dietary fat intake of Belgian pregnant women. Comparison of two methods. Annals of Nutrition and Metabolism 45, 273–278. [DOI] [PubMed] [Google Scholar]
- Dixon L.B., Subar A.F., Wideroff L., Thompson F.E., Kahle L.L. & Potischman N. (2006) Carotenoid and tocopherol estimates from the NCI Diet History Questionnaire are valid compared with multiple recalls and serum biomarkers. Journal of Nutrition 136, 3054–3061. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. (2001) Diet image: a new perspective on the food‐frequency questionnaire. Nutrition Reviews 59, 370–372. [DOI] [PubMed] [Google Scholar]
- Erkkola M., Karppinen M., Javanainen J., Rasanen L., Knip M. & Virtanen S.M. (2001) Validity and reproducibility of a food frequency questionnaire for pregnant Finnish women. American Journal of Epidemiology 154, 466–476. [DOI] [PubMed] [Google Scholar]
- Forsum E., Kabir N., Sadurskis A. & Westerterp K. (1992) Total energy expenditure of healthy Swedish women during pregnancy and lactation. American Journal of Clinical Nutrition 56, 334–342. [DOI] [PubMed] [Google Scholar]
- Godfrey K.M. & Barker D.J. (2001) Fetal programming and adult health. Public Health Nutrition 4, 611–624. [DOI] [PubMed] [Google Scholar]
- Goldberg G.R., Prentice A.M., Coward W.A., Davies H.L., Murgatroyd P.R., Wensing C. et al. (1993) Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. American Journal of Clinical Nutrition 57, 494–505. [DOI] [PubMed] [Google Scholar]
- Greeley S., Storbakken L. & Magel R. (1992) Use of a modified food frequency questionnaire during pregnancy. Journal of the American College of Nutrition 11, 728–734. [DOI] [PubMed] [Google Scholar]
- Hankin J.H., Wilkens L.R., Kolonel L.N. & Yoshizawa C.N. (1991) Validation of a quantitative diet history method in Hawaii. American Journal of Epidemiology 133, 616–628. [DOI] [PubMed] [Google Scholar]
- Hunter D. (1998) Biochemical indicators of dietary intake In: Nutritional Epidemiology (ed. Willett W.C.), 2nd edn, pp 174–243. Oxford University Press: New York. [Google Scholar]
- Hustvedt B.E., Christophersen A., Johnsen L.R., Tomten H., McNeill G., Haggarty P. et al. (2004) Description and validation of the ActiReg®: a novel instrument to measure physical activity and energy expenditure. British Journal of Nutrition 92, 1001–1008. [DOI] [PubMed] [Google Scholar]
- Isaksson B. (1980) Urinary nitrogen output as a validity test in dietary surveys. American Journal of Clinical Nutrition 33, 4–5. [DOI] [PubMed] [Google Scholar]
- Jacques P.F., Sulsky S.I., Sadowski J.A., Phillips J.C., Rush D. & Willett W.C. (1993) Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. American Journal of Clinical Nutrition 57, 182–189. [DOI] [PubMed] [Google Scholar]
- Kanabrocki E.L., Scheving L.E., Olwin J.H., Marks G.E., McCormick J.B., Halberg F. et al. (1983) Circadian variation in the urinary excretion of electrolytes and trace elements in men. American Journal of Anatomy 166, 121–148. [DOI] [PubMed] [Google Scholar]
- Van Kappel A.L., Steghens J.P., Zeleniuch‐Jacquotte A., Chajes V., Toniolo P. & Riboli E. (2001) Serum carotenoids as biomarkers of fruit and vegetable consumption in the New York Women's Health Study. Public Health Nutrition 4, 829–835. [DOI] [PubMed] [Google Scholar]
- Lauritsen J. (2005) FoodCalc. Current Version. Available at: http://www.ibt.ku.dk/jesper/foodcalc (accessed 5 July 2005).
- Livingstone M.B. & Black A.E. (2003) Markers of the validity of reported energy intake. Journal of Nutrition 133(Suppl. 3), 895S–920S. [DOI] [PubMed] [Google Scholar]
- McKeown N.M., Day N.E., Welch A.A., Runswick S.A., Luben R.N., Mulligan A.A. et al. (2001) Use of biological markers to validate self‐reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. American Journal of Clinical Nutrition 74, 188–196. [DOI] [PubMed] [Google Scholar]
- McNaughton S.A., Marks G.C., Gaffney P., Williams G. & Green A. (2005) Validation of a food‐frequency questionnaire assessment of carotenoid and vitamin E intake using weighed food records and plasma biomarkers: the method of triads model. European Journal of Clinical Nutrition 59, 211–218. [DOI] [PubMed] [Google Scholar]
- Magnus P., Irgens L.M., Haug K., Nystad W., Skjaerven R. & Stoltenberg C. (2006) Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). International Journal of Epidemiology 35, 1146–1150. [DOI] [PubMed] [Google Scholar]
- Masson L.F., McNeill G., Tomany J.O., Simpson J.A., Peace H.S., Wei L. et al. (2003) Statistical approaches for assessing the relative validity of a food‐frequency questionnaire: use of correlation coefficients and the kappa statistic. Public Health Nutrition 6, 313–321. [DOI] [PubMed] [Google Scholar]
- Meltzer H.M., Brantsæter A.L., Alexander J. & Haugen M. & the MoBa Study Group (2008) Methodological challenges when monitoring the diet of pregnant women in a large study; experiences from the Norwegian Mother and Child Cohort Study (MoBa). Maternal and Child Nutrition 4, 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T.B., Osler M. & Olsen S.F. (2006) Validity of protein, retinol, folic acid and n‐3 fatty acid intakes estimated from the food‐frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutrition 9, 771–778. [DOI] [PubMed] [Google Scholar]
- Nelson M. (1997) The validation of dietary assessment In: Design Concepts in Nutritional Epidemiology (eds Margetts B.M. & Nelson M.), 2nd edn, pp 241–272. Oxford University Press: Oxford. [Google Scholar]
- Olafsdottir A.S., Thorsdottir I., Gunnarsdottir I., Thorgeirsdottir H. & Steingrimsdottir L. (2006) Comparison of women's diet assessed by FFQs and 24‐hour recalls with and without underreporters: associations with biomarkers. Annals of Nutrition and Metabolism 50, 450–460. [DOI] [PubMed] [Google Scholar]
- Rifas‐Shiman S.L., Rich‐Edwards J.W., Willett W.C., Kleinman K.P., Oken E. & Gillman M.W. (2006) Changes in dietary intake from the first to the second trimester of pregnancy. Paediatric and Perinatal Epidemiology 20, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimestad A.H., Borgejordet Å., Vesterhus K.N., Sygnestveit K., Løken E.B., Trygg K. et al. (2001) Den Store Matvaretabellen/The Norwegian Food Composistion Table. Statens råd for ernæring og fysisk aktivitet, Statens næringsmiddeltilsyn, Institutt for ernæringsforskning: Oslo. [Google Scholar]
- Robinson S., Godfrey K., Osmond C., Cox V. & Barker D. (1996) Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. European Journal of Clinical Nutrition 50, 302–308. [PubMed] [Google Scholar]
- Romon M., Le Fur C., Lebel P., Edme J.L., Fruchart J.C. & Dallongeville J. (1997) Circadian variation of postprandial lipemia. American Journal of Clinical Nutrition 65, 934–940. [DOI] [PubMed] [Google Scholar]
- Schoeller D.A. (2002) Validation of habitual energy intake. Public Health Nutrition 5, 883–888. [DOI] [PubMed] [Google Scholar]
- Stram D.O., Longnecker M.P., Shames L., Kolonel L.N., Wilkens L.R., Pike M.C. et al. (1995) Cost‐efficient design of a diet validation study. American Journal of Epidemiology 142, 353–362. [DOI] [PubMed] [Google Scholar]
- Subar A.F., Thompson F.E., Kipnis V., Midthune D., Hurwitz P., McNutt S. et al. (2001) Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. American Journal of Epidemiology 154, 1089–1099. [DOI] [PubMed] [Google Scholar]
- Willett W.C. (1998) Nutrition Epidemiology, 2nd edn Oxford University Press: New York. [Google Scholar]
- World Health Organization (1985) Energy and Protein Requirements. Report of a joint FAO/WHO/UNU expert consultation. WHO Technical Report No. 724. World Health Organization: Geneva. [PubMed] [Google Scholar]
- Yates A.A., Schlicker S.A. & Suitor C.W. (1998) Dietary reference intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. Journal of the American Dietetic Association 98, 699–706. [DOI] [PubMed] [Google Scholar]


