Abstract
It is important to support women to exclusively breastfeed for 6 months and continue breastfeeding for 24 months and beyond. It is also necessary to provide the poor with access to affordable ways to improve the quality of complementary foods. Currently, many countries do not have the legal and policy environment necessary to support exclusive and continued breastfeeding. Legislative and policy changes are also necessary for introducing complementary food supplements, allowing them to be marketed to those who need them, and ensuring that marketing remains appropriate and in full compliance with the International Code of Marketing of Breastmilk Substitutes. This paper aims to illustrate the above with examples from Indonesia and to identify legislative requirements for supporting breastfeeding and enabling appropriate access to high‐quality complementary food supplements for children 6–24 months of age. Requirements include improved information, training, monitoring and enforcement systems for the International Code of Marketing of Breastmilk Substitutes; implementation and monitoring of the Baby‐Friendly Hospital Initiative; establishment of a registration category for complementary food supplements to enhance availability of high‐quality, low‐cost fortified products to help improve young child feeding; clear identification and marketing of these products as complementary food supplements for 6–24‐month‐olds so as to promote proper use and not interfere with breastfeeding.
Keywords: complementary food supplements, lipid nutrient supplements, micronutrient powders, breastfeeding, Code of Marketing, baby‐friendly hospitals, infant and young child nutrition
Introduction
Exclusive breastfeeding for the first 6 months of life and continued breastfeeding up to 24 months and beyond promotes optimal infant and young child growth and development (World Health Organization & UNICEF 2003). While young infants thrive best when exclusively breastfed, by the time they reach 6 months of age, timely, adequate, safe and properly fed complementary foods need to be introduced in addition to continued breastfeeding in order to fully meet their nutrient needs (World Health Organization & UNICEF 2003). The period around the introduction of complementary foods, unfortunately, is also the time that growth faltering starts to manifest itself (Victora et al. 2010). A balanced diet based on locally available foods including animal products can provide optimal nutrition to the young child. However, large proportions of the population in developing countries cannot afford a balanced diet (Kothari & Abderrahim 2010; de Pee et al. 2010). Because deficient dietary intake during the so‐called ‘window of opportunity’ (the 1000 days between conception and the second birthday) can lead to irreversible impairment of physical and mental growth, development, immunity and future economic potential (Victora et al. 2008), it is important to support women to exclusively breastfeed for 6 months and continue breastfeeding for 24 months and beyond. Additionally, it is also necessary to provide the poor with access to affordable ways to improve the quality of the foods fed to children to complement breast milk after 6 months of age.
Often, the habitual diets of poor children 6–24 months of age in developing countries lack certain micronutrients and essential fatty acids, all essential for growth and development. Rather than replacing the local foods or requiring mothers to cook a different meal for their young children, fortifying the child's portion of the family meal with these nutrients can be a feasible approach to addressing dietary deficiencies.
Complementary food supplements (CFS) are fortified food‐based products to be added to other foods (as ‘point of use’ or ‘home’ fortificants) or eaten alone to improve both macronutrient and micronutrient intake of children 6–24 months of age. Multiple micronutrient powders (MNP) are also used for home fortification, and they can be comprised of micronutrients alone or contain other factors/ingredients, such as essential fatty acids, amylase, and flavouring. Their inclusion as an addition to the traditional foods supports local feeding practices rather than competing with them. Additionally, because of their low water content, they are resistant to microbial proliferation, resistant to spoilage, and the micronutrients in these products cannot interact with each other chemically because there is no water to do so. Studies have documented improved child growth with consumption of the CFS, Nutributter® (Nutriset SAS, Malaunay, France), a fortified peanut‐based paste (2007, 2008) and fortified soy flour (Wang et al. 2007; Chen et al. 2010).
Currently, however, many countries do not have the legal and policy environment to support exclusive and continued breastfeeding. Legislative and policy changes are also necessary for introducing CFS, allowing them to be marketed to those who need them, and at the same time ensuring that marketing remains appropriate and in full compliance with the International Code of Marketing of Breastmilk Substitutes where it applies, and within the spirit of the Code where products are not addressed by the Code. This paper aims to illustrate the above with examples from Indonesia and to identify legislative and policy requirements for supporting breastfeeding and enabling appropriate access to high‐quality CFS for children 6–24 months of age.
Key messages
-
•
Improved information, training, monitoring and enforcement systems for the International Code of Marketing of Breastmilk Substitutes are needed to prevent marketing practices that are harmful to breastfeeding.
-
•
Monitoring of Baby‐Friendly hospitals to ensure compliance is essential to improve breastfeeding practices.
-
•
A registration category needs to be established for complementary food supplements to enhance availability of high‐quality, low‐cost fortified products that do not interfere with breastfeeding and help improve young child feeding.
-
•
Products need to be clearly identified and marketed as complementary food supplements for 6–24‐month‐olds so as to promote proper use and not interfere with breastfeeding.
Methods
We reviewed infant and young child feeding (IYCF) practices, legislation, food regulations and national policies related to IYCF in Indonesia. We propose changes to strengthen support for improved IYCF practices.
Results
The Republic of Indonesia is the fourth most populous country in the world, with an estimated population size of around 240 million. An estimated 4.4 million infants are born annually [Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International 2008]. Although the past decades have seen some major improvements in the health of the Indonesian people (UNICEF 2009), the poorer segments of the population still suffer from lack of access to adequate hygiene, sanitation, food and health care. Twenty‐one per cent of the population lives below the international poverty line of less than USD1.25 per person per day, which means that approximately 50 million Indonesians are living in poverty (The World Bank Group 2010). The poorer 60% of the population spends between USD 3.70–11 on food per month per person (World Bank 2010).
Despite declines in recent years, Indonesia still has the highest maternal mortality rate in Southeast Asia, estimated at 228/100 000 live births [Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International 2008], an infant mortality rate of 31/1000 live births, and an under five mortality rate of 41/1000 (UNICEF 2008). The national prevalence of stunting among children under five is 36.8% (Agency for Health Research and Development. Republic of Indonesia 2008). Among infants 6–11 months, 35% were already stunted, and among young children 12–23 months, this figure increases to 41% (WHO 2009). In addition, micronutrient deficiencies are common, with anaemia prevalence among children under five as high as 50–60% in some areas, while zinc and vitamin A deficiency are also common (Church World Service et al. 2008) (Dijkhuizen et al. 2001). Alarmingly, anaemia is most prevalent among 12‐ to 23‐month‐old children, and has been found to be as high as 60–75% (de Pee et al. 2004).
IYCF practices and the nutrient gap
Indonesian infants are generally breastfed until well into their second year or beyond, and the median duration of any breastfeeding is 21 months [Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International 2008]. However, only 32% of infants are exclusively breastfed in the first 6 months of life, as against the goal of 100%. Ironically, breastfeeding practices (early initiation, no prelacteal feeds) are noticeably better among poorer, less educated, rural women whose delivery was not attended by a health professional [Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International 2008]. Despite the fact that this practice is prohibited by law, distribution of breast milk substitutes within hospitals has been observed (Besar et al. 2004).
The use of infant formula is widespread in Indonesia, even among breastfeeding infants (Fig. 1). Increasing rates of exclusive breastfeeding and discouraging the use of breast milk substitutes would help improve health of Indonesian infants and their mothers.
Figure 1.
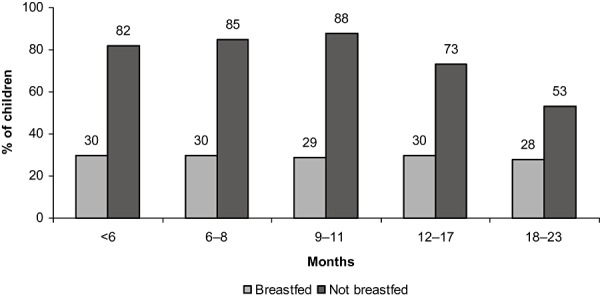
Per cent of children receiving infant formula in the preceding day and night (Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International 2008).
According to the 2007 Demographic and Health Survey (DHS) [Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International 2008], half the children at 6–8 months did not consume carotene‐rich fruit and vegetables the day and night before the survey, while only about 30% of breastfeeding and 40% of non‐breastfeeding infants consumed meat, fish, poultry and eggs. Only 42% of breastfed and 51% of non‐breastfed children 6–23 months of age consumed foods made with fat or oil. It can be assumed that if these children's intake of animal‐source foods is also low, they are likely to consume low levels of essential fatty acids, especially if they are not breastfed.
Continued breastfeeding ensures children receive the nutrients in breast milk that they cannot attain in adequate amounts from food (such as calcium and high‐quality protein) unless they are fed with other animal and milk products. But of non‐breastfed children aged 12‐17 months, 24% received no milk products on the preceding day; and at the age of 18–23 months, this increased to 36%. Even though 75% of children aged 6–23 months received animal products on the preceding day, the amount consumed is likely to be small given the high cost and, so, is unlikely to significantly contribute to overall micronutrient intake.
World Health Organization (WHO) and UNICEF recommend the use of complementary products in addition to breast milk after 6 months of age when there is a gap in critical nutrients (World Health Organization & UNICEF 2008). The use of linear programming models for rural and peri‐urban Indonesian poor demonstrated that even with adequate intake of breast milk and use of the currently available fortified infant cereals, soy‐based foods and animal‐source foods in amounts that are affordable to the poor, it is difficult to provide children aged 9–11 months with adequate iron, zinc, calcium and B vitamins because of the cost constraints faced by the average family (Ferguson et al. 2006; Santika et al. 2009). A recent survey in West Timor reported consumption of vitamin A‐rich foods to be only 113 RE/day among children aged 12–35 months, while the Recommended Daily Allowance is 400 RE/day (Church World Service et al. 2008). Given the current dietary pattern, there is clearly a need for affordable CFS containing sufficient levels of fortification with the critical micronutrients and essential fatty acids to improve the local diet of infants and young children.
Until recently, the Ministry of Health (MOH) provided, free of charge, fortified blended food to poor children aged 6–11 months and fortified biscuits to poor children aged 12–23 months but has now started distributing a MNP ‘Taburia’ during child health sessions (posyandu) in selected areas instead of these products. The MOH, however, has insufficient funds to distribute MNP to all poor children and, thus, distributes them to selected regions. The product is made locally in East Java and has the potential to be marketed for wider availability. It is registered as a food supplement. A similar product registered in the same category is also being marketed by a subsidiary of a company that also markets infant formula and cereals. As both these products fall in a general category not specifically created for products for older infants and young children, there is no language to ensure that these products are marketed in line with the Code of Marketing of Breastmilk Substitutes or in the spirit of the Code, supporting breastfeeding.
Policy and regulatory environment with regards to IYCF
Breastfeeding
The Indonesian government gives much attention to IYCF, in particular, to supporting breastfeeding (Minister of Health Republic of Indonesia 2004). A 2009 Health Bill (UU 36/2009) made exclusive breastfeeding until 6 months mandatory and any person ‘intentionally obstructing breastfeeding’ is punishable with imprisonment for up to 1 year and a fine of up to USD 10 000. If a corporation commits this unlawful act, the fine can be tripled. In addition, the business license and/or legal entity status of the corporation can be revoked (Republic of Indonesia 2009).
Marketing of breast milk substitutes
Indonesia ratified part of the International Code for the Marketing of Breastmilk Substitutes (the Code) (World Health Organization 1981) in 1997 (Minister of Health, Republic of Indonesia 1997). This decree (which is currently being revised) only addresses infants 0–11 months, thus providing no guidance for marketing of products targeted at young children aged 12 months or older. Furthermore, the language surrounding complementary foods has not lent itself to easy interpretation.
The decree defines breast milk substitutes as ‘food products that are marketed or otherwise meant as infant food and used to substitute breast milk partially or completely’, while complementary foods are defined as ‘food products that are marketed or otherwise stated as foods for infants over 4 months old to meet their nutritional needs in addition to breast milk’ 1 . While the definitions are complex, the distinction is critical. Foods marketed or intended as a substitute for breast milk are covered by Code limitations. However, foods marketed as a complement to fulfil the nutrient gaps from 4 months and older are not breast milk substitutes and, thus, may be promoted. Unfortunately, early in the document the text does not clarify the distinction between infant formula and complementary foods, which may add to confusion. Further in the document, there is specific mention of ‘complementary food given using a bottle and nipple’ (and not other complementary foods) as being a ‘commercial breast milk substitute’. While complementary food given using a bottle and a nipple would clearly be covered under the Code, it is not the only manner in which to identify when a complementary food is serving as a breast milk substitute (and as such is subject to the Code). The complexity of defining when a complementary food becomes a breast milk substitute, as well as the language used in the Indonesian decree, might explain some parties' reluctance to accept complementary foods as an indispensable part of older infants' and young children's diets and the need to ethically promote them. The outdated use of 4 months rather than 6 months for age of introduction of solids within the Indonesian Code may have further contributed to a less than wholehearted universal endorsement of the need to actively encourage the use of high‐quality complementary foods and supplements. (This will be changed to 6 months in the forthcoming revised decree.)
As for all food products, a registration permit from the National Agency for Food and Drug Control (BPOM) is required to distribute breast milk substitutes, including imported products. Although quite specific on what exactly is prohibited in terms of marketing of breast milk substitutes, no legal penalties are detailed in the Ministerial Decree of 1997. It only mentions ‘administrative penalties, starting from a verbal reprimand to withdrawal of permits as per the governing laws’ 2 . In spite of these existing laws, and certainly in part because of the lack of legal penalties, and difficulties with setting up a monitoring system and systematic enforcement, there are multiple reports of retail level promotion of infant formula, follow‐up formula and complementary foods for infants under 6 months, with little or no public sector action to address these Code violations. (Besar et al. 2004).
Baby‐Friendly Hospital policies
Since the early 1990s, the Indonesian government has made progress in its support of appropriate IYCF practices, including adoption of the Baby‐Friendly Hospital Initiative (BFHI) (UNICEF 2009), launched in 1991 by WHO and UNICEF. A maternity facility can be designated ‘baby‐friendly’ when it does not accept free or low‐cost breast milk substitutes, feeding bottles or teats, and has implemented the Ten Steps Towards Effective Breastfeeding, which include ‘Give newborn infants no food or drink other than breast milk, unless medically indicated’. In 2004, a decree from the Minister of Health officially promoted exclusive breastfeeding until 6 months, supported by the Ten Steps Towards Effective Breastfeeding (World Health Organization & UNICEF 1989) together with continued breastfeeding until 2 years along with appropriate complementary feeding (Ministry of Health Republic Indonesia 2004). This was, however, only legislated in October 2009 (Republic of Indonesia 2009).
Despite the fact that legislation supporting breastfeeding references the Ten Steps, it does not require implementation of the BFHI. Reports indicate that implementation of the Baby‐Friendly Hospital Initiative has long been neglected, although efforts are being made to revitalize it (Laksono et al. 2010).
There is a need to monitor Baby‐Friendly hospitals on an annual basis and require annual recertification so that those not meeting the requirements can be informed and encouraged to make appropriate changes. The DHS illustrates that 70% of infants born in health facilities were given prelacteal feeds, and 57% were not breastfed in the first hour after delivery, illustrating that these facilities were not Baby Friendly. There is also a need to include Baby‐Friendly Hospital practices within accreditation criteria for hospitals so that these become an established practice just as standard operational procedures and hospital by‐laws are compulsory for hospital accreditation.
In addition to Baby‐Friendly policies for hospitals, maternity leave policies and employer policies providing time, space and support for breastfeeding are also critical for exclusive and continued breastfeeding. However, these are beyond the scope of this paper, which is focused on policies directly relating to availability, marketing, use and misuse of infant food products.
National Codex
The Indonesian National Standard for Complementary Foods (SNI MP‐ASI) (Head of the National Bureau of Standards 2005) consists of four parts: instant cereal powders, biscuits, ready‐to‐cook products and ready‐to‐eat products. The standard defines complementary foods as ‘nutritious foods given in addition to breastmilk to infants aged 6 months and older or on medical indication, until the age of 24 months'. The formulation of this standard took 4 years and involved all stakeholders including the associations of food and beverages producing companies and baby‐food producers, universities, professional organizations, non‐governmental organizations (NGOs) and related government institutions.
Unfortunately, there is no category within this national standard for CFS. Therefore, the only commercially available food products falling under this category for infants and young children are instant cereals, biscuits and a few ready‐to‐cook products. All these products are currently fortified to some degree. The contents of these products comply with the national Codex standards as this is a prerequisite for the registration and marketing of these products. However, there is a concern that they could potentially interfere with continued breastfeeding because of the caloric content, recommended portion size and the recommended frequency of feeding, which are often not in line with best practices (Ten Year Strategy to Reduce Vitamin and Mineral Deficiencies MIYCN Working Group: Formulations Subgroup 2009). For example, there are products that are suggested for use from 4 months of age that are in line with existing national Codex regulations; however, relevant World Health Assembly resolutions subsequent to the International Code and other international guidelines identify 6 months as the appropriate age for the introduction of complementary foods. Examples such as this lead to understandable concern from the nutrition and child health community, and indeed all those who support optimal infant feeding and should be rectified.
The fortified infant cereals sold in Indonesia typically contain 200–210 kcal per portion of 40‐50 g per serving and suggest multiple servings per day. This already exceeds the recommended energy intake from complementary foods for infants 6–8 months old who are breastfeeding and is almost half the requirement for children 12–23 months of age who are breastfeeding (Pan American Health Organization/World Health Organization 2003). Biscuits contain 78–90 kcal per serving and typically 6 g sugar (30%E) in approximately 20 g per biscuit. This is in line with the National Codex that requires infant cereals to contain at least 0.8 kcal per gram and biscuits at least 4 kcal per gram (Head of the National Bureau of Standards 2005). National guidelines, however, should consider a lower limit for sugar (such as less than 10%) (Ten Year Strategy to Reduce Vitamin and Mineral Deficiencies MIYCN Working Group: Formulations Subgroup 2009) and lower the suggested serving sizes of complementary foods (e.g. 25 g) so as to ensure that these foods do not interfere with breastfeeding. Current pending revision of National Codex guidelines also suggests exclusion of products containing trans‐fatty acids. Because it is currently not required that infant food labels contain information on trans‐fatty acid content, it is likely that many products sold in Indonesia do contain trans‐fatty acids.
Support and promotion of optimal IYCF practices
Both the MOH and NGOs/International organizations have extensively promoted breastfeeding, in particular exclusive breastfeeding for the first 6 months, and many policies and guidelines are in place to support optimal practices. However, there are still many hurdles to overcome as evidenced by the unsatisfactory exclusive breastfeeding rates. The rate of exclusive breastfeeding for children less than 6 months of age is just 32.4%, and continued breastfeeding at 2 years is 50.3% [Statistics Indonesia (Badan Pusat Statistic – BPS) and Macro International)].
In contrast to breastfeeding interventions, complementary feeding interventions have been relatively neglected – perhaps because the messages are more complicated and more context specific than for exclusive breastfeeding. It is telling that the MOH has set behaviour change communications for improved complementary feeding as a goal in its National Action Plan for Food & Nutrition 2006–2010. It might be useful to set targets for complementary feeding practices such as increasing the per cent of children 6–24 months of age who receive iron rich or iron fortified food (World Health Organization 2008).
Promoting CFS and protecting breastfeeding: what needs to be done?
CFS such as lipid‐based nutrient supplements (e.g. Nutributter®) should be part of a larger strategy to combat childhood malnutrition, which also includes exclusive breastfeeding for 6 months, continued breastfeeding for up to 2 years and beyond, improved dietary diversity, appropriate frequency of feeding, and the inclusion of animal products in young children's diets. As part of a broader nutrition strategy, CFS provide a powerful tool with which to address the poor dietary quality of the complementary diet (Adu‐Afarwuah et al. 2007; Wang et al. 2007; Dewey et al. 2009; de Pee & Bloem 2009). In order to provide access to these products, barriers to use and promotion need to be addressed, and controls ensuring their proper use and promotion need to be in place.
Category in Codex
Many countries follow Codex Alimentarius when setting their national legislation, but Codex does not yet have a category under which CFS fall. Codex Alimentarius, therefore, needs to revise their texts to include a category for ‘complementary food supplements’: products that can supplement the nutritional content of locally accepted complementary foods. This category should at least include lipid‐based supplements, and it could serve as a model for individual country standards.
As the revision of the Codex Alimentarius standards and guidelines is a lengthy process, it is possible for individual countries to initiate a process to revise their own national Codex to allow appropriate use of CFS. This has already been done in some countries. For example, there is a standard for fortified soy flour and for supplements for complementary foods in China.
Product registration
Currently in Indonesia, as in most countries, there is no special category for CFS such as MNPs or lipid nutrient supplements. Complementary foods can only be registered if they fall in one of four very distinct categories: cereal‐based, biscuits, ready‐to‐eat and ready‐to‐cook. This greatly limits companies' opportunity to produce and market innovative products to reduce malnutrition as part of the local diet. Any food that is marketed for infants and young children has to fall under the current categories, and no other category allows for foods targeted at this group or any other particular age group.
However, there are two other categories under which CFS could fall: food supplements (as is currently the case with MNPs) or ‘foods for special uses’. As these are loosely defined and non‐age specific, it is easier to fit a product in one of these categories. This has the advantage of providing wider access to products that help fill the nutrient gap of young children. Unfortunately, it also opens the way to uncontrolled and misleading promotion and marketing.
In order to be able to combine wide access to high‐quality products designed to prevent malnutrition among vulnerable 6–24 months old children, and strict regulation as to the labelling and marketing of these products to prevent inappropriate use, it is essential that a new, specific category be defined for these products.
The establishment of the existing categories for complementary foods in Indonesia took 4 years. In order for the registration rules to be altered or for a new subcategory to be added, a long process needs to take place, including numerous meetings with experts in the field and all companies in the industry. It is, therefore, expedient for the public sector to initiate and manage the process of developing a new category for CFS.
Clearly identify products as complementary foods rather than breast milk substitutes
Once a category is created for CFS, products should be labelled and marketed in a manner such that they will be used solely as a complementary food and are not misused as a substitute for breastfeeding. In order to avoid misrepresentation of the product, certain guidelines emanating from the Code need to be followed:
-
•
The age of introduction should not precede 6 months and should be clearly stated on all packaging. If pictures are used, children should appear older than 6 months and show achievement of a physical or developmental milestone clearly reached after 6 months.
-
•
Instruction should be given to serve the product with a daily ration of food that is less than the recommended daily energy intake from complementary foods for a breastfed child. Large servings (which are often now recommended for many infant cereals) would interfere with continued breastfeeding and, thus, act as a breast milk substitute. (See Table 1.)
-
•
To further ensure that the product is not misused, the importance of exclusive breastfeeding for the first 6 months and continued breastfeeding to 2 years and beyond should be clearly stated in a conspicuous way on product packaging and in marketing messages.
-
•
All marketing messages should make clear that the product is for children 6–24 months as well as make clear the points listed above.
Table 1.
Energy needs from complementary foods (kcal/day) by feeding status (Pan American Health Organization/World Health Organization 2003).
| Age of child (months) | Energy needs from complementary foods (kcal/day) | |
|---|---|---|
| Breastfed | Not breastfed | |
| 6–8 | 200 | 600 |
| 9–11 | 300 | 700 |
| 12–23 | 550 | 900 |
These requirements are in keeping with the current wording in the International Code and subsequent World Health Assembly (WHA) resolutions. (World Health Organization 1981) (Quinn et al. 2010). Explicit national guidelines stating these requirements would help clarify which product is a complementary food to be promoted and which one serves as a breast milk substitute and is, therefore, subject to all marketing restrictions outlined in the Code. The national guidelines should also give guidance on which nutrition and health claims are acceptable for such products, if any.
Enforce the Code of Marketing of Breastmilk Substitutes
A landscape analysis of the health system was conducted in 2010, coordinated by the Ministry of Health and Bappenas (the National Planning Board) with technical and financial support of UNICEF, WHO and WFP Indonesia. One of the recommendations of this analysis was that a government regulation (in contrast to a decree which is less enforceable) should be approved to control the marketing of breast milk substitutes and develop a mechanism for monitoring and enforcement (Ministry of Health/Bappenas/UNICEF/WHO 2010). Without effective monitoring and enforcement systems to ensure compliance, legislation implementing the Code is of little value. Aguayo et al. (2003) found that comparable levels of Code violations were observed in Burkina Faso, where there is regulating legislation, and in Togo, where there is no legislation, and concluded that legislation must be accompanied by effective information, training and monitoring systems to ensure that healthcare providers and manufacturers comply with evidence‐based practice and the Code. Improved education about, and enforcement of, the Code is critical both to protect breastfeeding as well as to responsibly allow for the necessary opening of a clearly defined space to promote appropriate complementary feeding in addition to breastfeeding for 6–24 months.
Discussion
Breastfeeding advocates have done an enormous job in getting breastfeeding on the Indonesian health agenda to the extent that exclusive breastfeeding for the first 6 months of life is now mandatory in Indonesia. In addition to protecting breastfeeding, the importance of timely, adequate and safe complementary feeding needs to be emphasized.
The Indonesian Code itself is not easy to interpret on the topic of complementary foods leaving it open to multiple interpretations that can, and do, result in irresponsible marketing of these products. Furthermore, it was not designed to address CFS, which did not exist at the time that the Code was drafted but have now been shown to be beneficial.
While previously the Indonesian government received funding for special health department activities from infant formula manufacturers, this policy has now been changed and existing contracts will be discontinued (Supriyono 2010). In line with WHA Resolution 58.32 (World Health Assembly 2005), the government will no longer use sponsor funds for its health activities from companies that produce breast milk substitutes but instead will work together with non‐profit organizations such as WHO, UNICEF and others. These changes were necessary and should be applauded. Further government and multistakeholder action needs to be taken to fully implement and enforce the International Code of Marketing of Breastmilk Substitutes and ensure Baby‐Friendly Hospitals are certified and follow the practices outlined in the 10 Steps to Successful Breastfeeding (World Health Organization & UNICEF 1989), including not distributing infant formula. Along with this, the government now needs to open the door for the private sector groups that comply with the Code of Marketing of Breastmilk Substitutes to develop and appropriately promote CFS for children aged 6–24 months in order to promote optimal development during the crucial 1000‐day window of opportunity from birth to 2 years.
Source of funding
Authors (Soekarjo, Zehner) received support from the Global Alliance for Improved Nutrition.
Conflict of interest statement
No conflicts of interest have been declared.
Footnotes
This was in 1997, when it was generally assumed complementary feeding (CF) should start at 4 months of age.
Punishment is mentioned in UU 36/2009.
References
- Adu‐Afarwuah S., Lartey A., Brown K.H., Zlotkin S., Briend A. & Dewey K.G. (2007) Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition 86, 412–420. [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S., Lartey A., Brown K.H., Zlotkin S., Briend A. & Dewey K.G. (2008) Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. American Journal of Clinical Nutrition 87, 929–938. [DOI] [PubMed] [Google Scholar]
- Agency for Health Research and Development. Republic of Indonesia (December 2008) Basic Health Research (Riskesdas) 2007 National Report (Badan Penelitian dan Pengembangan Kesehatan Departemen Kesehatan, Republik Indonesia Riset Kesehatan Dasar (RISKESDAS) 2007, Laporan Nasional 2007. Desember 2008. Available at: http://www.gizi.net/download/statgizi-nas-riskesdas%202007.pdf (Accessed 31 January 2011).
- Aguayo V.M., Ross J.S., Kanon S. & Ouedraogo A.N. (2003) Monitoring compliance with the International Code of Marketing of Breastmilk Substitutes in west Africa: multisite cross sectional survey in Togo and Burkina Faso. British Medical Journal 326, 127–132. Available at: http://www.bmj.com/content/326/7381/127.1.full (Accessed 20 December 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besar D.S. et al (2004). Indonesia code violations. Available at: http://aimi-asi.org/wp/wp-content/files/ibfan%20report%20indo.pdf (Accessed 11 January 2011).
- Chen C.M., Wang Y.Y. & Chang S.Y. (2010) Effect of in‐home fortification of complementary feeding on intellectual development of Chinese children. Biomedical and Environmental Sciences 23, 83–91. [DOI] [PubMed] [Google Scholar]
- Church World Service, Helen Keller International, CARE (2008) Nutrition Survey in East Nusa Tenggara (NTT). Final Report.
- Dewey K.G., Yang Z. & Boy E. (2009) Systematic review and meta‐analysis of home fortification of complementary foods. Maternal & Child Nutrition 5, 283–232. [Google Scholar]
- Dijkhuizen M.A., Wieringa F.T., West C.E. & Muherdiyantiningsih, Muhilal (2001) Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia. American Journal of Clinical Nutrition 73, 786–791. [DOI] [PubMed] [Google Scholar]
- Ferguson E.L., Darmon N., Fahmida U., Fitriyanti S., Harper T.B. & Premachandra I.M. (2006) Design of optimal food‐based complementary feeding recommendations and identification of key ‘problem nutrients’ using goal programming. Journal of Nutrition. 136, 2399–2404. [DOI] [PubMed] [Google Scholar]
- Head of the National Bureau of Standards (2005) Decision of the Head of the National Bureau of Standards (SK Kepala BSN Nomor 52/KEP/BSN/05/2005) dated 23 Mei 2005 regarding the formulation of four Indonesian National Standards Available at: http://websisni.bsn.go.id/index.php?/sk_main/surat_keputusan/sksni/1/91 (Accessed 5 November 2010).
- Kothari, M & Abderrahim, N (2010) Nutrition Update 2010. ICF Macro: Calverton, MD. [Google Scholar]
- Laksono T., Soeharsono S., Budihardja S., Kirana P., Erna M., Handy A.F. et al (2010) Reducing child mortality in Indonesia. Bulletin of the World Health Organization [serial on the Internet] 88, 642–642. Available at: http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0042-96862010000900002&lng=en (Accessed 11 January 2011). doi: 10.1590/S0042‐96862010000900002. 20865063 [Google Scholar]
- Ministry of Health/Bappenas/UNICEF/WHO (2010) The Landscape Analysis Indonesia Country assessment. September Jakarta, Indonesia.
- Minister of Health, Republic of Indonesia (1997) Ministerial Decree Number 237, 1997 Available at: http://www.selasi.net/keputusan-menteri/kemmenkes-237-tahun-1997 (Accessed 5 November 2010).
- Ministry of Health Republic of Indonesia (2004) Keputusan Menteri Kesehatan Republik Indonesia No. 450/Menkes/IV/2004 tentang Pemberian Air Susu Ibu (ASI) secara Eksklusif pada Bayi di Indonesia, 10 April 2004. Available at: http://www.gizi.net/kebijakan-gizi/download/SK-ASI-Eksklusif.pdf (Accessed 5 November 2010).
- Pan American Health Organization/World Health Organization (2003) Guiding Principles for Complementary Feeding of the Breastfed Child Available at: http://www.who.int/child_adolescent_health/documents/a85622/en/index.html (Accessed 21 January 2011).
- de Pee S., Martini E., Moench‐Pfanner R., Firdaus M.A., Stormer A., Halati S. et al (2004) Nutrition and Health Trends in Indonesia 1999‐2003. Nutrition & Health Surveillance System Annual Report 2003. Jakarta, Indonesia: Helen Keller International.
- de Pee S., Moench‐Pfanner R., Martini E., Zlotkin S., Darnton‐Hill I. & Bloem M.W. (2007) Home fortification in emergency response and transition programming: experiences in Aceh and Nias, Indonesia. Food and Nutrition Bulletin 28, 189–197. [DOI] [PubMed] [Google Scholar]
- de Pee S. & Bloem M.W. (2009) Current and potential role of specially formulated foods and food supplements for preventing malnutrition amung 6‐ to 23‐month‐old children and for treating moderate malnutrition amoung 6‐ to 59‐month‐old children. Food and Nutrition Bulletin 30 (Suppl.), S434–S463. [DOI] [PubMed] [Google Scholar]
- de Pee S., Brinkman H.J., Webb P., Godfrey S., Darnton‐Hill I., Alderman H., Semba R.D., Piwoz E. & Bloem M.W. (2010) How to ensure nutrition security in the global economic crisis to protect and enhance development of young children and our common future. The Journal of Nutrition 140, 138S–142S. Epub 2009 Nov 25. [DOI] [PubMed] [Google Scholar]
- Quinn V., Zehner E., Schoefield D., Guyon A. & Huffman S.L. (2010) Using the code of marketing of breast‐milk substitutes to guide the marketing of complementary foods to protect optimal infant feeding practices. Global Alliance for Improved Nutrition (GAIN). Geneva, Switzerland.
- Republic of Indonesia (2009) Health Bill (Law no 36, 2009) Available at: http://www.depdagri.go.id/produk-hukum/2009/10/13/undang-undang-no-36-tahun-2009 (Accessed 5 November 2010).
- Santika O., Fahmida U. & Ferguson E.L. (2009) Development of food based complementary feeding recommendations for 9‐ to 11‐month‐old peri‐urban Indonesian infants using linear programming. Journal of Nutrition 139, 135–141. [DOI] [PubMed] [Google Scholar]
- Statistics Indonesia (Badan Pusat Statistik – BPS) and Macro International (2008) Indonesia Demographic and Health Survey (2007) Calverton, Maryland, USA: BPS and Macro International Available at: http://www.measuredhs.com/pubs/pub_details.cfm?id=897&srchTp=home (Accessed 15 November 2010).
- Supriyono A. (2010) Government Health Activities will no longer Use Sponsor Funds [Kegiatan Kesehatan Pemerintah tak Lagi Pakai Dana Sponsor] Republika – Thursday, August 12, 2010. Available at: http://www.repulika.co.id (Accessed 5 November 2010).
- Ten Year Strategy to Reduce Vitamin and Mineral Deficiencies, Maternal, Infant and Young Child Nutrition Working Group: Formulations Subgroup (2009) Formulations for fortified complementary foods and supplements: review of successful products for improving the nutritional status of infants and young children. Food and Nutrition Bulletin 30 (Suppl.), S239–S255. [PubMed] [Google Scholar]
- The World Bank Group (2010) PovcalNet Available at: http://iresearch.worldbank.org/PovcalNet/povcalSvy.html (Accessed 5 November 2010).
- UNICEF (December 2008) The State of the World's Children 2009: Maternal and Newborn Health UNICEF. New York, USA. Available at: http://www.unicef.org/publications/index_47127.html (Accessed 5 November 2010).
- UNICEF (12 August, 2009) The Baby Friendly Hospital Initiative Available at: http://www.unicef.org/nutrition/index_24806.html (Accessed 5 November 2010).
- Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Richter L., Sachdev H.S. & Maternal and Child Undernutrition Study Group (2008) Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., de Onis M., Hallal P.C., Blössner M. & Shrimpton R. (2010) Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–e480. Epub 2010 Feb 15. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen C., Wang F. & Wang K. (2007) Effects of nutrient fortified complementary food supplements on growth of infants and young children in poor rural area in Gansu Province. Journal of Hygiene Research 36, 78–81. (in Chinese). [PubMed] [Google Scholar]
- World Health Assembly (25 May 2005) WHA 58.32 Infant and Young Child Nutrition Available at: http://www.who.int/gb/ebwha/pdf_files/WHA58/WHA58_32-en.pdf (Accessed 5 November 2010).
- World Health Organization (1981) International Code of Marketing of Beastmilk Substitutes. World Health Organization: Geneva. Available at: http://www.infactcanada.ca/crackcode.htm (Accessed 21 February 2011). [Google Scholar]
- World Health Organization (2008) Indicators for Assessing Infant and Young Child Feeding Practices: Conclusions of a Consensus Meeting Held 6‐8 November 2007 in Washington D.C., USA. WHO: Geneva. [Google Scholar]
- World Health Organization (18 July 2009) Global Database on Child Growth and Malnutrition Available at: http://www.who.int/nutgrowthdb/database/countries/who_standards/idn.pdf (Accessed 5 November 2010).
- World Health Organization & UNICEF (1989) Protecting Promoting and Supporting Breastfeeding: The Special Role of Maternity Services, a joint WHO/UNICEF statement. Geneva, 1989 Available at: http://www.unicef.org/newsline/tenstps.htm (Accessed 19 December 2010).
- World Health Organization & UNICEF (2003) Global Strategy for Infant and Young Child Feeding. WHO: Geneva. Available at: http://www.who.int/nutrition/publications/infantfeeding/9241562218/en/index.html (Accessed 6 April 2010). [Google Scholar]
- World Health Organization & UNICEF (2008) Strengthening Actions to Improve Feeding of Infants and Young Children 6‐23 Months of Age in Nutrition and Child Health: Report of Proceedings, Geneva, 6‐9 October 2008. WHO: Geneva. [Google Scholar]


