Abstract
Maternal folic acid deficiency is an underlying risk for neural tube defects (NTDs). China has one of the highest prevalences of NTDs, and the prevalence rates of NTDs vary by region. We characterized plasma folate level and dietary folate intake among Chinese women of childbearing age by region (North and South, East and West, urban and rural) to provide evidence for establishing policy to prevent NTDs. A total of 1003 women of childbearing age from five provinces in China were interviewed. Fasting blood samples were collected. Plasma folate concentrations were determined by a microbiological assay. Dietary intake data were collected using a 24‐h recall. Both the plasma folate concentrations and dietary folate intake of women in the South (25.9 nmol L−1 and 211.0 µg day−1) were higher than those of women in the North (13.3 nmol L−1 and 189.2 µg day−1). In the North, plasma folate concentrations and dietary folate intake of women in rural areas were lower than those of women in urban areas, whereas, in the South, an opposite pattern was observed. No difference was found between women in the East and West, in either the North or South regions. Plasma folate and dietary folate intake among Chinese women of childbearing age were suboptimal and varied by region. Different folic acid supplementation approaches and dosage should be undertaken to improve folate status of women in different areas. Particular attention should be paid to women in the North, especially in northern rural areas.
Keywords: folate, folic acid, plasma folate status, dietary folate intake, women of childbearing age, neural tube defects
Introduction
Maternal folic acid deficiency was established as an underlying risk for neural tube defects (NTDs), and folic acid supplementation during the periconceptional period could reduce the risk of NTDs (MRC Vitamin Study Research Group 1991; Czeizel 1993; Berry et al. 1999). Many governments and organizations recommend that women of childbearing age take folic acid supplements daily (Van Allen et al. 1993; Centers for Disease Control and Prevention 1995; Institute of Medicine 1998; Rasmussen et al. 1998) and/or implement mandatory or voluntary food folic acid fortification to prevent NTDs (Food & Drug Administration 1996; Freire et al. 2000; Metz et al. 2002; Ray et al. 2002a).
China is among the high NTD‐prevalent countries, and the birth prevalence rates of NTDs vary by region. Rates in southern China (about 5 per 10 000) were similar to those in developed countries, while rates in northern China (about 20 per 10 000), especially in Shanxi province (higher than 60 per 10 000), were the highest in the world (Dai et al. 2002; International Clearinghouse for Birth Defects Monitoring Systems 2004). Rates in rural areas were higher than those in urban areas (Xiao et al. 1990; Dai et al. 2002), and rates tended to decline from East to West (Xiao et al. 1990; Chinese Birth Defects Monitoring Collaborative Group 1992). The Chinese government has been considering undertaking strategies to reduce the occurrence of NTDs. In 1993, the Ministry of Health recommended women of childbearing age take 400 µg of folic acid daily (Department of Science, Technology and Education, Ministry of Health 1996). The government conducted a pilot study on fortifying flour with eight kinds of nutrients, including 200 µg folic acid per 100 g flour in some areas since 2002 (Chai 2006).
However, data on blood folate status and dietary folate intake of Chinese women of childbearing age are lacking. The few studies that reported the blood folate or dietary folate status of women in China were limited regionally to Beijing (Gao et al. 2003) and Shanxi (Li et al. 1996; Zhang et al. 2006; Ren et al. 2007), with very high prevalence of NTDs, or Shanghai (Shrubsole et al. 2001), Anqing (Ronnenberg et al. 2000) and Jiangsu (Li et al. 1996; Ren et al. 2007), with very low prevalence of NTDs, or focused on middle‐aged and older women (Dyer et al. 2003; Hao et al. 2003). Existing evidence was insufficient to assess folate status among women of childbearing age countrywide. It would be a challenge for the Chinese government to make an appropriate policy to improve folate status among women of childbearing age and reduce NTDs nationwide without information on how folate status varies within the country.
The purpose of this paper was to characterize the plasma folate level and dietary folate intake among Chinese women of childbearing age by region (North and South, East and West, urban and rural), to provide evidence for establishing national policy to improve folate status among women of childbearing age and prevent NTD‐affected pregnancies, as well as to provide baseline data for evaluating the impact of the intervention policy in the future.
Materials and methods
Subjects
As a part of the Study on Nutrition Status among Chinese Women of Childbearing Age, the present study was conducted in three northern provinces – Liaoning, Shandong and Gansu – and two southern provinces – Guangdong and Sichuan. In terms of approximate geographical location (Fig. 1), and for the purpose of analysis, we combined Liaoning and Shandong to represent northeast, Gansu to represent northwest, Guangdong to represent southeast, and Sichuan to represent southwest. In each province, we selected one city and one county, and from each, one community or village was selected as project sites. The socioeconomic status of the project site was representative of the provinces. Recruitment was conducted between April and May 2005. Using residential registration data, we identified eligible women in the communities or villages. The inclusion criteria of subjects were: (1) ages 18–30 years; (2) not pregnant or breastfeeding; and (3) free from hypertension, diabetes, cancer, and heart, liver, renal and gastrointestinal diseases, as well as other serious diseases. Each community or village had about 150 to 200 eligible women. We distributed leaflets about the study to eligible women, informed and invited them to participate in the study, and took the first 100 women who consented. The Institutional Review Board of Peking University Health Science Center reviewed and approved the study protocol.
Figure 1.
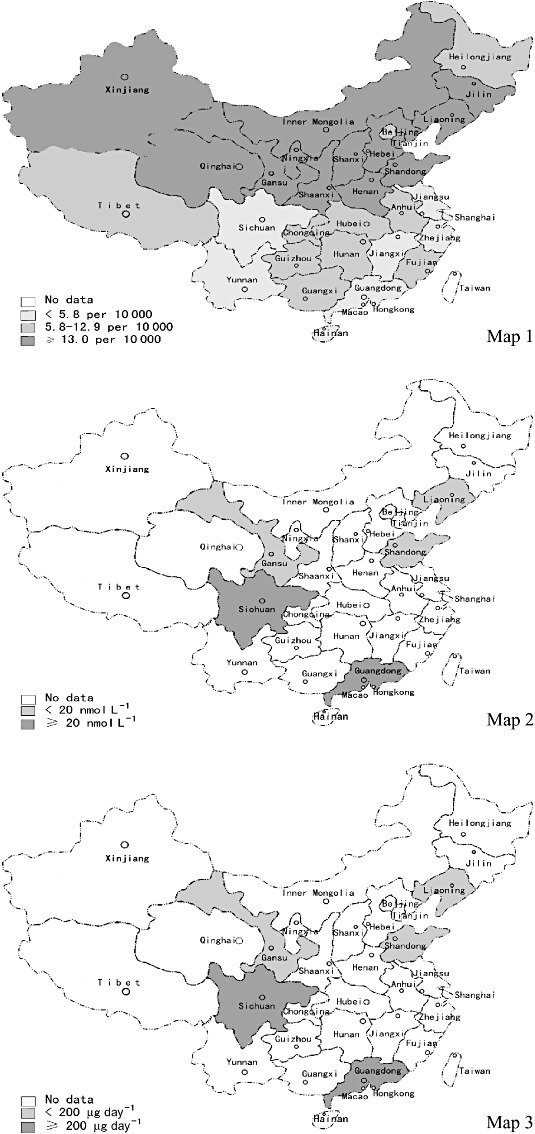
Distributions of prevalence of neural tube defects (NTDs), plasma folate concentration and dietary folate intake in China. Map 1: Data of prevalences of NTDs are from the study of Dai and co‐authors (Dai et al., 2002). The cut‐off values, 5.8 per 10 000 and 13.0 per 10 000, are the average prevalence rate of NTDs in the South and that in the whole of China. Maps 2 and 3: Data of plasma folate concentration and dietary folate intake are from our study. The cut‐off values are established by us.
In order to achieve stable estimates of means of the studied nutrients, including folate and Vitamins A, B6, and B12, and to characterize the difference in means and deficient rates by region, based on estimates of means and deficient rates of the studied nutrients from previous studies in China, we calculated the sample size at the 0.05 significance level [95% confidence interval (CI)] and the statistical power of 90% to detect a minimum detectable difference as 15% of the estimates of studied nutrients. Finally, we determined to recruit 100 women from each site to meet the appropriate sample size for most studied nutrients (Zhang 2006).
Measurements
At the community health centre, a trained interviewer administered a structured questionnaire to collect subject information on sociodemographic and personal characteristics, comprising age, ethnicity, educational level, current cigarette and alcohol drinking, use of oral contraception, and use of folic acid supplements or multivitamins containing folic acid. Women's dietary data were collected using a 24‐h recall. Folate values of foods were based on Chinese Food Composition (Yang 2005). Body weight in light clothing and height without shoes were measured to the nearest 0.1 kg and 0.1 cm with a beam weighing scale and a height scale, respectively. Body mass index (BMI) was calculated as weight (kg)/height (m)2. A total of 9 mL overnight fasting blood was collected from each subject using venipuncture by a qualified nurse.
Blood samples were drawn into K3EDTA‐containing Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged within 1 h of collection. Plasma and red blood cells were separated and frozen at −20°C. All specimens were transported on dry ice to the central laboratory of the Institute of Reproductive and Child Health, Peking University, and stored at −70°C before nutritional analyses. The plasma folate concentrations were determined by a microbiological assay (O'Broin & Kelleher 1992). The intra‐ and inter‐assay coefficients of variation were less than 9% across the full range of folate concentrations.
Statistical analysis
All data summarization and analysis were performed using SPSS package for Windows, version 11.5 (SPPS, Chicago, IL, USA). As the distributions of plasma folate concentrations and dietary folate intake were positively skewed, natural logarithmic transformations were used to normalize the distributions, and inverse transformations were used to provide geometric means and their 95% CI. Folate deficiency was defined as plasma folate less than 6.8 nmol L−1[from radioassay, i.e. 9.2 nmol L−1 converted to result of microbiological assay (see Note)] (Herbert & Das 1994). Chi‐square test was used to compare deficient rates and linear regression analyses (using SPSS general linear models procedure) were conducted to compare means of plasma folate concentration and dietary folate intake (as the dependent variable, respectively) in the North vs. South, East vs. West and urban vs. rural (regions as the fixed factors), adjusted for potentially confounding variables (as the covariates), i.e. age, BMI, educational level, multivitamin and/or folic acid use. All P values were two‐sided and statistical significance was set at P ≤ 0.05.
Results
A total of 1008 women took part in the study. Three women whose blood samples were hemolytic and two women who did not complete dietary recall were excluded, leaving 1003 women (99.5%) for analysis. Demographic characteristics are shown in Table 1. The mean age of participants was 25.7 (±SD 3.3) years and mean BMI was 21.3 (±SD 2.8). The mean age and mean BMI differed between some regions. Most women (97.3%) were ethnic Han Chinese, and there were no marked regional differences. Most women (96.4%) finished middle school education or above; the distributions of level of education differed between some regions, but most notably between urban and rural. Use of cigarettes, alcohol and oral contraception were not prevalent among Chinese women, being 2.2%, 2.9% and 1.2%, respectively, and did not vary between regions. Only 3.0% of women reported that they consumed folic acid supplement or multivitamins containing folic acid more than once per week in the past 3 months. The rates were no substantial differences between most regions.
Table 1.
Characteristics of Chinese women of childbearing age in the study
| Characteristic | South | North | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| East | West | East | West | ||||||
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | ||
| Number of women | 100 | 100 | 100 | 100 | 201 | 202 | 100 | 100 | 1003 |
| Age (years, mean ± SD) | 25.1 ± 3.3 | 25.0 ± 3.2 | 23.2 ± 2.9 | 26.4 ± 2.6 | 26.0 ± 3.4 | 26.9 ± 2.9 | 25.3 ± 3.0 | 26.3 ± 3.5 | 25.7 ± 3.3 |
| BMI (mean ± SD) | 19.9 ± 2.4 | 21.4 ± 3.5 | 20.1 ± 2.3 | 21.3 ± 2.4 | 21.2 ± 2.4 | 22.9 ± 3.0 | 21.0 ± 2.6 | 20.9 ± 2.3 | 21.3 ± 2.8 |
| Han ethnic group (%) | 100.0 | 100.0 | 97.0 | 95.0 | 95.5 | 95.5 | 99.0 | 100.0 | 97.3 |
| Level of education (%) | |||||||||
| Elementary | 1.0 | 3.0 | 0.0 | 11.0 | 0.0 | 6.9 | 1.0 | 6.0 | 3.6 |
| Middle School | 6.0 | 66.0 | 11.1 | 79.0 | 2.0 | 73.8 | 4.0 | 48.0 | 36.7 |
| High School | 41.0 | 28.0 | 10.1 | 9.0 | 47.0 | 18.3 | 38.0 | 28.0 | 28.5 |
| College and above | 52.0 | 3.0 | 78.8 | 1.0 | 51.0 | 1.0 | 57.0 | 18.0 | 31.3 |
| Current cigarette smoking (%) | 2.0 | 0.0 | 3.0 | 2.0 | 1.0 | 5.4 | 1.0 | 1.0 | 2.2 |
| Current alcohol drinking (%) | 4.0 | 2.0 | 3.0 | 4.0 | 4.5 | 1.5 | 4.0 | 0.0 | 2.9 |
| Oral contraception use (%) | 3.0 | 0.0 | 1.0 | 0.0 | 1.5 | 2.5 | 0.0 | 0.0 | 1.2 |
| Multivitamin and/or folic acid use (%) | 6.0 | 1.0 | 8.0 | 0.0 | 3.0 | 0.5 | 4.0 | 4.0 | 3.0 |
Table 2 shows the mean plasma folate concentrations by region, and 1, 2 are the corresponding graphical representation. The geometric mean of women living in the South was 25.9 nmol L−1 (95% CI: 24.7–27.1) and that of women living in the North was 13.3 nmol L−1 (95% CI: 12.8–13.7). In both urban and rural areas, and the East and West, women living in the South had higher plasma folate concentrations than those living in the North. After controlling for age, BMI, education level, and multivitamins and/or folic acid supplement use, the significant difference between southerners and northerners was unchanged. In the North, urban women had higher plasma folate concentrations than rural women, whereas in the South, urban women had lower plasma folate concentrations than rural women. Both in the North and South, there was no significant difference in plasma folate concentrations between the East and West.
Table 2.
Plasma folate concentrations and folate deficient rates among Chinese women of childbearing age by region
| Region | Geometric mean (95% CI, nmol L−1) | Deficient rate (%) † | |
|---|---|---|---|
| Crude | Adjusted* | ||
| South, overall | 25.9 (24.7–27.1) | 26.2 (25.0–27.4) ‡ | 1.5 ‡ |
| East | |||
| Overall | 26.2 (24.7–27.8) | 26.5 (24.9–28.2) ‡ | 1.0 ‡ |
| Urban | 24.4 (22.2–26.9) | 24.3 (22.1–26.6) ‡ , § | 2.0 ‡ |
| Rural | 28.1 (26.3–30.0) | 28.8 (26.3–31.5) ‡ | 0.0 ‡ |
| West | |||
| Overall | 25.6 (23.9–27.4) | 26.0 (24.4–27.7) ‡ | 2.0 ‡ |
| Urban | 22.4 (20.4–24.6) | 23.2 (21.0–25.6) ‡ , § | 4.0 § |
| Rural | 29.3 (26.7–32.2) | 29.1 (26.4–32.0) ‡ | 0.0 ‡ |
| North, overall | 13.3 (12.8–13.7) | 13.6 (13.1–14.1) | 20.0 |
| East | |||
| Overall | 12.6 (12.0–13.1) | 12.9 (12.4–13.4) | 22.8 ¶ |
| Urban | 13.1 (12.3–13.9) | 14.1 (13.1–14.7) § | 19.4 ¶ |
| Rural | 12.1 (11.4–12.9) | 11.5 (10.7–12.3) | 26.1 |
| West | |||
| Overall | 14.7 (13.8–15.7) | 14.6 (13.7–15.6) | 14.5 |
| Urban | 16.4 (15.0–18.0) | 16.5 (15.1–18.1) § | 9.0 § |
| Rural | 13.2 (12.1–14.4) | 12.9 (11.8–14.1) | 20.0 |
Geometric means of plasma folate concentration were controlled for age, body mass index, education level and multivitamins and/or folic acid supplement use.
Folate deficiency rates using 6.8 nmol L−1 (from radioassay, i.e. 9.2 nmol L−1 converted to result of microbiological assay) as cut‐off value.
P < 0.01 for the comparison with the North.
P < 0.01 for the comparison with rural areas in the same region.
P < 0.01 for the comparison with the west in the South/North.
Figure 2.
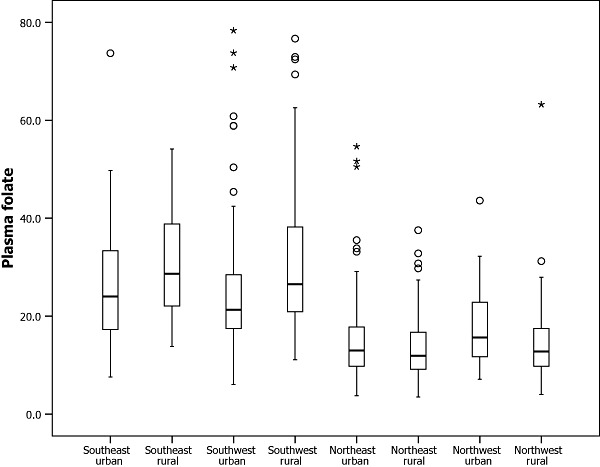
Distribution of raw plasma folate concentrations among Chinese women of childbearing age by regions.
Using 6.8 nmol L−1 as the cut‐off value, 1.5% and 20.0% of the participants in the South and North were plasma folate deficient, respectively. The deficiency rates amongst women in the North, especially in the northeast (22.8%), were higher than those of women in the South. There was no significant difference in folate deficiency rates between urban and rural women in the southeast and northeast. In the southwest, the folate deficiency rate in the urban area (4.0%) was higher than that in the rural area (0.0%). But in the northwest, the deficiency rate in the rural area (20.0%) was higher than that in the urban area (9.0%).
Table 3 shows the mean dietary folate concentrations by region, and 1, 3 are the corresponding graphical representations. The geometric mean of dietary folate intake of the subjects was 211.0 µg day−1 (95% CI: 201.3–221.1) in the South and 189.2 µg day−1 (95% CI: 181.8–196.9) in the North. Dietary folate intake of the southerners was higher than that of the northerners. After adjusting for women' s age, BMI, education level, and multivitamins and/or folic acid supplement use, the significant difference between southerners and northerners was unchanged. In the northwest, dietary intake of urban women was significantly higher than that of rural women. However, in the South, dietary intake of urban women was less than that of rural women but not to a statistically different degree. With the exception of lower dietary intake of rural women in the northwest compared with rural women in the northeast, there was no significant difference in dietary folate intake between women living in the East and those living in the West.
Table 3.
Geometric mean of dietary folate intake among Chinese women of childbearing age by region (95% CI, µg day–1)
| Region | Crude | Adjusted* |
|---|---|---|
| South, overall | 211.0 (201.3–221.1) | 212.9 (202.8–223.6) † |
| East | ||
| Overall | 213.7 (200.8–227.5) | 215.1 (201.0–230.3) † |
| Urban | 200.1 (182.2–219.7) | 205.0 (185.5–226.5) |
| Rural | 228.2 (210.3–247.5) | 225.9 (204.7–249.3) † |
| West | ||
| Overall | 208.2 (194.1–223.4) | 210.4 (196.4–225.5) † |
| Urban | 205.5 (184.5–229.0) | 201.4 (181.3–223.7) |
| Rural | 211.0 (193.0–230.8) | 220.8 (198.4–245.8) † |
| North, overall | 189.2 (181.8–196.9) | 188.0 (180.8–195.6) |
| East | ||
| Overall | 192.3 (183.5–201.4) | 190.9 (181.8–200.4) |
| Urban | 194.5 (180.7–209.5) | 199.2 (185.3–214.2) |
| Rural | 190.0 (179.5–201.2) | 181.5 (167.9–196.2) ‡ |
| West | ||
| Overall | 183.2 (169.9–197.4) | 182.7 (170.8–195.5) |
| Urban | 203.9 (185.2–224.6) | 211.2 (191.3–233.0) § |
| Rural | 164.3 (147.0–183.7) | 159.1 (144.5–175.3) |
Geometric means of dietary folate intake were controlled for age, body mass index, education level and multivitamins and/or folic acid supplement use.
P < 0.01 for the comparison with the North.
P < 0.01 for the comparison with the west in the South/North.
P < 0.01 for the comparison with rural areas in the same region.
Figure 3.
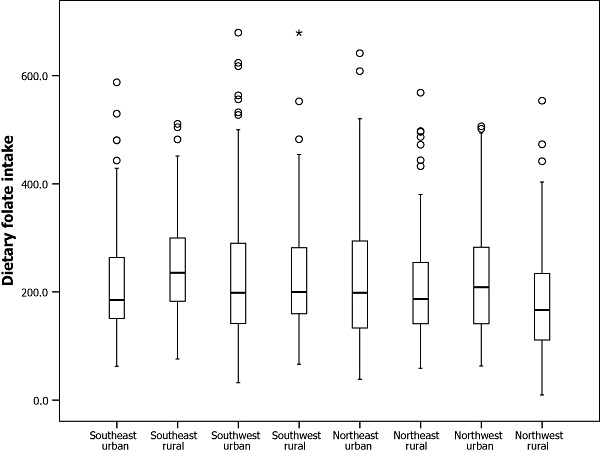
Distribution of raw dietary folate intake among Chinese women of childbearing age by regions.
Discussion
This is the first large sample and broad coverage study to report plasma folate status and dietary folate intake among Chinese women of childbearing age. Also, for the first time in China, folate status, especially dietary folate intake among women of childbearing age, was characterized by region. Results of our study showed that the plasma folate status and dietary folate intake among Chinese women of childbearing age was far from optimal, especially among women living in northern China.
Results of our study showed that the plasma folate status among women of childbearing age in northern China was lower than that of women in southern China. The geometric mean concentration [13.3 nmol L−1 (95% CI: 12.8–13.7)] among women in northern China was about the mean concentration [9.7 ± 4.3 nmol L−1 from radioassay, i.e. 13.5 ± 5.6 nmol L−1 converted to result of microbiological assay] among Chilean women of childbearing age before flour fortification with 220 µg/100 g folic acid, but far lower than the mean (37.2 ± 9.5 nmol L−1 from radioassay, i.e. 59.1 ± 13.2 nmol L−1 converted to result of microbiological assay) among Chilean women after fortification (Hertrampf et al. 2003); lower than the median [4.8 ng mL−1 (95% CI: 4.5–3.4) from radioassay, i.e. 15.3 nmol L−1 (95% CI: 14.3–16.8) converted to result of microbiological assay] among women of childbearing age in the United States before cereal‐grain fortification with 140 µg/100 g folic acid, lower than the 10th per centile [6.4 ng mL−1 (95% CI: 5.8–7.0) from radioassay, i.e. 21.0 nmol L−1 (95% CI: 18.9–23.2) converted to result of microbiological assay] among women in the United States after fortification (Centers for Disease Control and Prevention 2002). The geometric mean [25.9 nmol L−1 (95% CI: 24.7–27.1)] among women in southern China was higher than the mean concentration among Chilean women before flour fortification but lower than the mean among Chilean women after fortification. And it was about the 75th per centile [7.8 ng mL−1 (95% CI: 7.3–8.3) from radioassay, i.e. 26.1 nmol L−1 (95% CI: 24.3–28.0) converted to result of microbiological assay] among women in the United States before fortification, and was between the 10th and the 25th per centile [9.1 ng mL–1 (95% CI: 8.7–9.5) from radioassay, i.e. 30.9 nmol L−1 (95% CI: 29.4–32.4) converted to result of microbiological assay] of women in the United States after fortification (Centers for Disease Control and Prevention 2002). The deficient rates of plasma folate of the northerners were also higher than those of the southerners. Using 6.8 nmol L−1 (from radioassay, i.e. 9.2 nmol L−1 converted to result of microbiological assay) as the cut‐off value, 20.0% and 1.5% of the participants in the North and South, respectively, were plasma folate deficient. Moreover, 65.9% and 12.0% of Chinese women of childbearing age in the North and South, respectively, did not achieve the serum folate levels (≥15.9 nmol L−1) associated with very low risk for NTDs (Daly et al. 1995)
To our knowledge, no large sample dietary folate intake data amongst Chinese women of childbearing age have been reported. Our study showed that dietary folate intake amongst Chinese women of childbearing age was low. The geometric mean of total dietary folate intake amongst women of childbearing age in southern China was 211.0 µg day–1 (95% CI: 201.3–221.1). It was about the mean (217 ± 5.2 µg day–1) of women at 20–39 years old in the United States before fortification, but less than the mean (294 ± 12.6 µg day–1) of women at 20–39 years old in the United States after fortification (Dietrich et al. 2005). The geometric mean of total dietary folate intake among women of childbearing age in northern China was 189.2 µg day–1 (95% CI: 181.8–196.9). It was less than the mean of women at 20–39 years old in the United States before fortification and after fortification. The distribution of dietary folate intake of the subjects was similar to the distribution of the plasma folate concentration: dietary folate intake among women in the North was less than in the South, with urban northerners higher than rural northerners, urban southerners lower than rural southerners, and in either the North or South regions, there was no significant difference between east and west. However, we found that the difference in dietary folate intake was not as significant as plasma folate concentrations between women in different areas. For example, plasma folate concentration of women in the South was 1.9 times that of women in the North, but the dietary folate intake of women in the South was only 1.1 times that of women in the North. Several factors may be contributory. First, some kinds of green vegetables consumed frequently by the women in the South are special local products. We could not determine their folate content from the Chinese Food Composition Table, and so we estimated them using the average folate content of vegetables. This might be an underestimation, resulting in the dietary folate intake of women in the South being underestimated on the whole. Second, analysis of food sources of dietary intake showed that women in the South obtained folate mainly from vegetables and fruits, while women in the North obtained folate mainly from grains. Cooking, processing and storage can destroy some folate in food (Chinese Nutrition Society 2000). Grains are often stored, cooked and processed more extensively than vegetables and fruits, thus the lower actual folate intake for women in the North than in the South. Third, although most (97.3%) women in the study were of Han ethnicity, genetic backgrounds might still play a role. Studies shown that Han populations in the North had a higher frequency of a mutation in the gene encoding methylenetetrahydrofolate reductase enzyme (MTHFR) than those in the South (Wilcken et al. 2003; Zhu et al. 2006), and the mutated form of MTHFR is associated with decreased plasma concentrations (Molloy et al. 1997). The low plasma folate concentration in women in the North may be partly attributable to their higher frequency of MTHFR mutation.
Results of our study showed that the geographical distributions of plasma folate and dietary folate intake among Chinese women of childbearing age were inversely associated with the distributions of birth prevalence rates of NTDs between North and South China (Xiao et al. 1990; Berry et al. 1999; Dai et al. 2002) (Fig. 1). It confirmed the negative association between maternal folate status and occurrence of NTDs. Moreover, Xiao et al. suggested that the prevalence of NTDs tended to decline from east to west in China (Xiao et al. 1990; Chinese Birth Defects Monitoring Collaborative Group 1992). For the first time, we compared the plasma folate concentrations and dietary folate intake between women in the east and west. Results showed neither plasma folate concentration nor dietary folate intake among women in the east and west, in either the North or South regions, had significant difference. We reviewed articles on the prevalence of NTDs in China. No other article compared prevalence of NTDs in the East with that in the West. Using data on NTD prevalence of each province in China reported by Dai et al. (2002), we did not find the trend Xiao et al. (1990) reported. Further studies are needed to clarify the distribution of NTD prevalence and folate status among people in the East and West of China.
As mentioned earlier, our studies showed that folate status of Chinese women of childbearing age was far from optimal for preventing NTDs. Hence, many women, especially those living in the North, would enter pregnancy with severely compromised folate status. Increasing dietary folate intake and improving blood folate status among Chinese women of childbearing age are urgent. Studies suggested a substantial increase in women's blood folate status (Lawrence et al. 1999; Ray et al. 2002b; Hertrampf et al. 2003) and a significant decrease in the risk of NTDs (Honein et al. 2001; Gucciardi et al. 2002; Persad et al. 2002; Ray et al. 2002a; Williams et al. 2002; De Wals et al. 2003; Hertrampf & Cortes 2004; Lopez‐Camelo et al. 2005) after folic acid fortification in the United States, Canada and Chile. The effectiveness of folic acid fortification was striking. Fortifying food with folic acid may also be an effective approach to improving the suboptimal folate status of women in China. The Chinese government has conducted pilot studies on food fortification since 2002. However, according to the result of our study, two problems should be noted before popularizing food fortification with folic acid in China. One issue is the different baseline levels of folate status between women in South and North China. Our study showed the geometric mean concentration of plasma folate among women of childbearing age in Northern China was near the median among women of childbearing age in the United States before fortification, and that among women in southern China was near two times that of women in the North, higher than the 75th per centile among women in the United States before fortification and near the 25th per centile after fortification. It indicated that fortifying food with the 200 µg/100 g dosage, which is higher than the 140 µg/100 g dosage used in the United States, would be appropriate for northerners, whereas it might be excessive for southerners. Although folic acid is generally regarded as safe, with some studies suggesting that folic acid intake may also reduce the risk of other kinds of birth defects, such as oral–facial clefts (Goh et al. 2006; Badovinac et al. 2007) and congenital heart diseases (Goh et al. 2006), stroke (Wang et al. 2007), breast cancer (Ericson et al. 2007), colorectal cancer (Sanjoaquin et al. 2005) and neuroblastoma (Olshan et al. 2002; French et al. 2003), other studies suggested that consuming supplements containing folic acid or food fortified with folic acid may increase the risk of certain kinds of cancer (Cole et al. 2007; Mason et al. 2007). Morris et al. (2007) suggested that in seniors with low vitamin B12 status, high serum folate was associated with anaemia and cognitive impairment (Morris et al. 2007). Hao et al. reported that 5.5% of Chinese adults were deficient in vitamin B12 (Hao et al. 2004). Vitamin B12 was not included in the fortification formula in China (Chai 2006). Although the role that folate plays in cancer development and its association with anaemia and cognitive impairment in seniors need to be proven, we should be mindful that more folate is not better in all circumstances and folic acid fortification may have adverse effects in subpopulation groups not originally targeted for fortification, and use care in developing public health policy (Kim 2004; Ulrich 2007; Ulrich & Potter 2007). Much attention and further study are needed to establish the appropriate folic acid dosages for food fortification in different areas of China to maximize effective prevention while avoiding potential adverse effects. The other problem is the inherent difficulty with implementing food fortification in China. Presently, only a few large‐ or medium‐sized mills participate in the national flour fortification programme, producing 1 million tons of fortified flour yearly, which accounts for less than 1% of total flour output in China and covers only 7.7‰ of the Chinese population (Chai 2006). In rural areas, most people bring their own grain to mills, preferring to produce and eat their own flour (Zhang et al. 2006). It is difficult to spread fortified flour throughout China in a short period. Before fortified food is widely available and acceptable all over the country, it is necessary to undertake some measures to promote periconceptional folic acid supplements for NTD prevention among Chinese women of childbearing age, especially those living in the northern rural.
A limitation of this study was the sample size was too small for some purpose. One hundred women from each project site met the recommended minimum sample size needed to achieve stable estimates of means of the studied nutrients and to characterize the difference in means and deficient rates by regions. However, 100 participants did not meet the appropriate sample size to estimate plasma folate deficient rates in the South (≥496), to compare deficient rates between urban and rural in the North (≥153) and to compare plasma folate deficient rates between rural and urban in the South (≥286). In addition, a 24‐h recall was used to obtain dietary folate intake data in this study. Although the 24‐h recall method is the most often used dietary assessment tool in large clinical studies, such as the National Health and Nutrition Examination Surveys and Nationwide Food Consumption Survey in the United States, a 24‐h recall is not an ideal methodology to assess individuals' dietary nutrients intake but is more suitable to assess dietary intake of a population with a large sample size (Buzzard 1998). By and large, the method was appropriate to the purpose of our study, i.e. assessing the folate intake among women in different regions. However, it was insufficient to assess each individual's dietary intake or to assess whether an individual reaches the recommended nutrient intake. More studies using 24 h recall and other dietary intake assessment methods are needed to evaluate the results of our study and to estimate dietary folate intake among Chinese people.
In conclusion, the present study suggests that plasma folate and dietary folate intake among Chinese women of childbearing age were suboptimal and varied by region. Different folic acid supplementation approaches and different folic acid dosage for food fortification should be undertaken to improve folate status of women in different areas to prevent NTDs and avoid potential adverse effects. Particular attention should be paid to women in the North, especially rural women. To ensure preventive effects before food fortification is implemented all over the country, the Chinese government and health care staff should undertake measures to encourage women of childbearing age to consume 400 µg of folic acid daily.
Note
The Bio‐Rad QuantaPhase II radioassay (BR) and microbiological assay (MA) are the two principal methods used to measure plasma and red blood cell folate. Studies showed that the radioassay produces lower results than microbiological assay. To compare the results of our study from MA with that from radioassay in the United States, we converted results from BR to MA using the equation established by Fazili et al.[log10 MA = 0.0504 + (1.0958 × log10 BR) + (0.6358 × IND) − (0.4105 × IND × log10 BR), IND = 0 for BR results ≤ 45 nmol L−1 and IND = 1 for BR results ≥ 45 nmol L−1 and IND‐1 for BR results ≥ 45 nmol L−1] (Fazili et al. 2007).
Key messages
-
•
Maternal folic acid deficiency is an underlying risk for neural tube defects (NTDs). China is among the high NTD‐prevalent countries, and the prevalence rates of NTDs vary by region.
-
•
This study showed plasma folate concentrations and dietary folate intake among Chinese women of childbearing age were suboptimal and varied by region.
-
•
Plasma folate concentrations and dietary folate intake of women in the South were higher than those of women in the North.
-
•
In the North, plasma folate concentrations and dietary folate intake of women in rural areas was lower than those of women in urban areas, whereas, in the South, an opposite pattern was observed.
-
•
In China, different folic acid supplementation approaches and dosage should be undertaken to improve folate status of women in different areas.
Conflicts of interest
None declared.
Acknowledgements
We would like to thank the official support from the Department of Maternal and Child Health Care and Community Health of the Chinese Ministry of Health. We thank the health care staff at project sites in Gansu, Liaoning, Guangdong, Sichuan and Shandong Province for their effort in the coordination of the fieldwork. We are grateful to all the women who participated in the study. We thank the reviewers of the manuscript for their constructive suggestions. This work was supported by the Study on Nutrition Status among Chinese Women of Childbearing Age (grant number WP/2004/CHN/RPH/3.4/001), a programme sponsored by the World Health Organization. No conflicts of interest are involved in this work.
References
- Badovinac R.L., Werler M.M., Williams P.L., Kelsey K.T. & Hayes C. (2007) Folic acid‐containing supplement consumption during pregnancy and risk for oral clefts: a meta‐analysis. Birth Defects Research Part A: Clinical and Molecular Teratology 79, 8–15. [DOI] [PubMed] [Google Scholar]
- Berry R.J., Li Z., Erickson J.D., Li S., Moore C.A., Wang H. et al. (1999) Prevention of neural‐tube defects with folic acid in China. The New England Journal of Medicine 341, 1485–1490. [DOI] [PubMed] [Google Scholar]
- Buzzard M. (1998) 24‐hour dietary recall and food record methods In: Nutritional Epidemiology (ed. Willett W.), 2nd edn, pp. 50–73. Oxford University Press: New York. [Google Scholar]
- Centers for Disease Control and Prevention (1995) Knowledge and use of folic acid by women of childbearing age – United States, 1995. Morbidity and Mortality Weekly Report 44, 716–718. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2002) Folate status in women of childbearing age, by race/ethnicity – United States, 1999–2000. Morbidity and Mortality Weekly Report 51, 808–810. [PubMed] [Google Scholar]
- Chai W. (2006) China flour fortification today and tomorrow – challenges and our suggestion. In: Proceedings of improving health and nutrition in China: fortification of wheat flour (eds Chinese Cereals and Oils Association & Center for Public Nutrition and Development of China), pp. 87–97. 10–11 May, Beijing.
- Chinese Birth Defects Monitoring Collaborative Group (1992) Altas of Birth Defects in China. Chengdu Map Publishing House: Chengdu, China. [Google Scholar]
- Chinese Nutrition Society (2000) Chinese Dietary Reference Intakes. China Light Industry Press: Beijing. [Google Scholar]
- Cole B.F., Baron J.A., Sandler R.S., Haile R.W., Ahnen D.J., Bresalier R.S. et al. (2007) Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Journal of the American Medical Association 297, 2351–2359. [DOI] [PubMed] [Google Scholar]
- Czeizel A.E. (1993) Prevention of congenital abnormalities by periconceptional multivitamin supplementation. British Medical Journal 306, 1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Zhu J., Zhou G., Wang Y., Wu Y., Miao L. et al. (2002) Dynamic monitoring of neural tube defects in China during 1996 to 2000. Zhonghua Yu Fang Yi Xue Za Zhi 36, 402–405. [PubMed] [Google Scholar]
- Daly L.E., Kirke P.N., Molloy A., Weir D.G. & Scott J.M. (1995) Folate levels and neural tube defects. Implications for prevention. Jama 274, 1698–1702. [DOI] [PubMed] [Google Scholar]
- De Wals P., Rusen I.D., Lee N.S., Morin P. & Niyonsenga T. (2003) Trend in prevalence of neural tube defects in Quebec. Birth Defects Research Part A: Clinical and Molecular Teratology 67, 919–923. [DOI] [PubMed] [Google Scholar]
- Department of Science, Technology and Education, Ministry of Health (1996) Collection of 10‐year 100 Marketable Scientific Research Findings. Hongqi Press: Beijing. [Google Scholar]
- Dietrich M., Brown C.J. & Block G. (2005) The effect of folate fortification of cereal‐grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non‐supplement users in the United States. Journal of the American College of Nutrition 24, 266–274. [DOI] [PubMed] [Google Scholar]
- Dyer A.R., Elliott P., Stamler J., Chan Q., Ueshima H. & Zhou B.F. (2003) Dietary intake in male and female smokers, ex‐smokers, and never smokers: the INTERMAP Study. Journal of Human Hypertension 17, 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson U., Sonestedt E., Gullberg B., Olsson H. & Wirfalt E. (2007) High folate intake is associated with lower breast cancer incidence in postmenopausal women in the Malmo Diet and Cancer Cohort. The American Journal of Clinical Nutrition 86, 434–443. [DOI] [PubMed] [Google Scholar]
- Fazili Z., Pfeiffer C.M. & Zhang M. (2007) Comparison of serum folate species analyzed by LC‐MS/MS with total folate measured by microbiologic assay and Bio‐Rad radioassay. Clinical Chemistry 53, 781–784. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (1996) Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register 61, 8781–8797. [Google Scholar]
- Freire W.B., Hertrampf E. & Cortes F. (2000) Effect of folic acid fortification in Chile: preliminary results. European Journal of Pediatric Surgery 10(Suppl. 1), 42–43. [PubMed] [Google Scholar]
- French A.E., Grant R., Weitzman S., Ray J.G., Vermeulen M.J., Sung L. et al. (2003) Folic acid food fortification is associated with a decline in neuroblastoma. Clinical Pharmacology and Therapeutics 74, 288–294. [DOI] [PubMed] [Google Scholar]
- Gao X., Yao M., McCrory M.A., Ma G., Li Y., Roberts S.B. et al. (2003) Dietary pattern is associated with homocysteine and B vitamin status in an urban Chinese population. The Journal of Nutrition 133, 3636–3642. [DOI] [PubMed] [Google Scholar]
- Goh Y.I., Bollano E., Einarson T.R. & Koren G. (2006) Prenatal multivitamin supplementation and rates of congenital anomalies: a meta‐analysis. Journal of Obstetrics and Gynaecology Canada 28, 680–689. [DOI] [PubMed] [Google Scholar]
- Gucciardi E., Pietrusiak M.A., Reynolds D.L. & Rouleau J. (2002) Incidence of neural tube defects in Ontario, 1986–1999. Canadian Medical Association Journal 167, 237–240. [PMC free article] [PubMed] [Google Scholar]
- Hao L., Ma J., Stampfer M.J., Ren A., Tian Y., Tang Y. et al. (2003) Geographical, seasonal and gender differences in folate status among Chinese adults. The Journal of Nutrition 133, 3630–3635. [DOI] [PubMed] [Google Scholar]
- Hao L., Tian Y., Tang Y. & Li Z. (2004) Comparative study of plasma vitamin B12 concentration among Chinese adults. Yingyang Xuebao 26, 19–22. [Google Scholar]
- Herbert V. & Das K.C. (1994) Folic acid and vitamin B12 In: Modern Nutrition in Health and Disease (eds Shils M.E., Olson J.A. & Shike M.), 8th edn, pp. 402–425. Lea & Febiger: Philadelphia, PA. [Google Scholar]
- Hertrampf E. & Cortes F. (2004) Folic acid fortification of wheat flour: Chile. Nutrition Reviews 62, S44–S48; discussion S49. [DOI] [PubMed] [Google Scholar]
- Hertrampf E., Cortes F., Erickson J.D., Cayazzo M., Freire W., Bailey L.B. et al. (2003) Consumption of folic acid‐fortified bread improves folate status in women of reproductive age in Chile. The Journal of Nutrition 133, 3166–3169. [DOI] [PubMed] [Google Scholar]
- Honein M.A., Paulozzi L.J., Mathews T.J., Erickson J.D. & Wong L.Y. (2001) Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. Journal of the American Medical Association 285, 2981–2986. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (1998) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline, pp. 196–305. National Academy Press: Washington, DC. [PubMed] [Google Scholar]
- International Clearinghouse for Birth Defects Monitoring Systems (2004) Annual Report 2004. International Centre for Birth Defects: Rome. [Google Scholar]
- Kim Y.I. (2004) Will mandatory folic acid fortification prevent or promote cancer? American Journal of Clinical Nutrition 80, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Lawrence J.M., Petitti D.B., Watkins M. & Umekubo M.A. (1999) Trends in serum folate after food fortification. Lancet 354, 915–916. [DOI] [PubMed] [Google Scholar]
- Li Z., Liu X., Chen Y., Chang H., Chen L., Tang Y. et al. (1996) Difference in prevalence and seasonal variations of folate deficiencies among pre‐marital women from high and low risk areas of neural tube defects. Zhongguo Yousheng Youyu Zazhi 7, 1–4. [Google Scholar]
- Lopez‐Camelo J.S., Orioli I.M., Da Graca Dutra M., Nazer‐Herrera J., Rivera N., Ojeda M.E. et al. (2005) Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. American Journal of Medical Genetics 135, 120–125. [DOI] [PubMed] [Google Scholar]
- Mason J.B., Dickstein A., Jacques P.F., Haggarty P., Selhub J., Dallal G. et al. (2007) A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiology Biomarkers & Prevention 16, 1325–1329. [DOI] [PubMed] [Google Scholar]
- Metz J., Sikaris K.A., Maxwell E.L. & Levin M.D. (2002) Changes in serum folate concentrations following voluntary food fortification in Australia. The Medical journal of Australia 176, 90–91. [DOI] [PubMed] [Google Scholar]
- Molloy A.M., Daly S., Mills J.L., Kirke P.N., Whitehead A.S., Ramsbottom D. et al. (1997) Thermolabile variant of 5,10‐methylenetetrahydrofolate reductase associated with low red‐cell folates: implications for folate intake recommendations. Lancet 349, 1591–1593. [DOI] [PubMed] [Google Scholar]
- Morris M.S., Jacques P.F., Rosenberg I.H. & Selhub J. (2007) Folate and vitamin B‐12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. American Journal of Clinical Nutrition 85, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338, 131–137. [PubMed] [Google Scholar]
- O'Broin S. & Kelleher B. (1992) Microbiological assay on microtitre plates of folate in serum and red cells. Journal of Clinical Pathology 45, 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshan A.F., Smith J.C., Bondy M.L., Neglia J.P. & Pollock B.H. (2002) Maternal vitamin use and reduced risk of neuroblastoma. Epidemiology 13, 575–580. [DOI] [PubMed] [Google Scholar]
- Persad V.L., Van den Hof M.C., Dube J.M. & Zimmer P. (2002) Incidence of open neural tube defects in Nova Scotia after folic acid fortification. Canadian Medical Association Journal 167, 241–245. [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L.B., Andersen N.L., Andersson G., Lange A.P., Rasmussen K., Skak‐Iversen L. et al. (1998) Folate and neural tube defects. Recommendations from a Danish working group. Danish Medical Bulletin 45, 213–217. [PubMed] [Google Scholar]
- Ray J.G., Meier C., Vermeulen M.J., Boss S., Wyatt P.R. & Cole D.E. (2002a) Association of neural tube defects and folic acid food fortification in Canada. Lancet 360, 2047–2048. [DOI] [PubMed] [Google Scholar]
- Ray J.G., Vermeulen M.J., Boss S.C. & Cole D.E. (2002b) Increased red cell folate concentrations in women of reproductive age after Canadian folic acid food fortification. Epidemiology 13, 238–240. [DOI] [PubMed] [Google Scholar]
- Ren A., Zhang L., Hao L., Li Z., Tian Y. & Li Z. (2007) Comparison of blood folate levels among pregnant Chinese women in areas with high and low prevalence of neural tube defects. Public Health Nutrition 10, 762–768. [DOI] [PubMed] [Google Scholar]
- Ronnenberg A.G., Goldman M.B., Aitken I.W. & Xu X. (2000) Anemia and deficiencies of folate and vitamin B6 are common and vary with season in Chinese women of childbearing age. The Journal of Nutrition 130, 2703–2710. [DOI] [PubMed] [Google Scholar]
- Sanjoaquin M.A., Allen N., Couto E., Roddam A.W. & Key T.J. (2005) Folate intake and colorectal cancer risk: a meta‐analytical approach. International Journal of Cancer 113, 825–828. [DOI] [PubMed] [Google Scholar]
- Shrubsole M.J., Jin F., Dai Q., Shu X.O., Potter J.D., Hebert J.R. et al. (2001) Dietary folate intake and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Research 61, 7136–7141. [PubMed] [Google Scholar]
- Ulrich C.M. (2007) Folate and cancer prevention: a closer look at a complex picture. The American Journal of Clinical Nutrition 86, 271–273. [DOI] [PubMed] [Google Scholar]
- Ulrich C.M. & Potter J.D. (2007) Folate and cancer – timing is everything. Journal of the American Medical Association 297, 2408–2409. [DOI] [PubMed] [Google Scholar]
- Van Allen M.I., Fraser F.C., Dallaire L., Allanson J., McLeod D.R., Andermann E. et al. (1993) Recommendations on the use of folic acid supplementation to prevent the recurrence of neural tube defects. Clinical Teratology Committee, Canadian College of Medical Geneticists. Canadian Medical Association Journal 149, 1239–1243. [PMC free article] [PubMed] [Google Scholar]
- Wang X., Qin X., Demirtas H., Li J., Mao G., Huo Y. et al. (2007) Efficacy of folic acid supplementation in stroke prevention: a meta‐analysis. Lancet 369, 1876–1882. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Bamforth F., Li Z., Zhu H., Ritvanen A., Renlund M. et al. (2003) Geographical and ethnic variation of the 677C > T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. Journal of Medical Genetics 40, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.J., Mai C.T., Edmonds L.D., Shaw G.M., Kirby R.S., Hobbs C.A. et al. (2002) Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology 66, 33–39. [DOI] [PubMed] [Google Scholar]
- Xiao K.Z., Zhang Z.Y., Su Y.M., Liu F.Q., Yan Z.Z., Jiang Z.Q. et al. (1990) Central nervous system congenital malformations, especially neural tube defects in 29 provinces, metropolitan cities and autonomous regions of China: Chinese Birth Defects Monitoring Program. International Journal of Epidemiology 19, 978–982. [DOI] [PubMed] [Google Scholar]
- Yang Y.X. (2005) Chinese Food Composition, 2004. Peking University Medical Press: Beijing. [Google Scholar]
- Zhang L. (2006) Study on Micronutrients Status in Women Aged 18–30 and Establishment of Models for Prediction of Population Micronutrients Level. Institute of Reproductive and Child Health, Peking University: Beijing. [Google Scholar]
- Zhang L., Ren A., Li Z., Hao L., Tian Y. & Li Z. (2006) Folate concentrations and folic acid supplementation among women in their first trimester of pregnancy in a rural area with a high prevalence of neural tube defects in Shanxi, China. Birth Defects Research Part A: Clinical and Molecular Teratology 76, 461–466. [DOI] [PubMed] [Google Scholar]
- Zhu J., Ren A., Hao L., Pei L., Liu J., Zhu H. et al. (2006) Variable contribution of the MTHFR C677T polymorphism to non‐syndromic cleft lip and palate risk in China. American Journal of Medical Genetics 140, 551–557. [DOI] [PubMed] [Google Scholar]


