Abstract
In developing countries nutritional deficit during prenatal and continuing in post‐natal life is very common. This condition leads to stunting and important metabolic changes. Over 30% of children in the world are stunted. The metabolic resultants of nutritional deficit during growth are classically known to aim at energy conservation. This review summarizes data from Brazil, a developing country undergoing the double burden of obesity and undernutrition, especially among the poor, and suggests that stunting or chronic undernutrition increases the risk of obesity and hypertension later in life. Around 60 million people are under the poverty line in Brazil. In São Paulo, the richest city of the country, 20% of the population live in slums and in Maceió, the capital of one of the poorest states, this percentage reaches 50%. Undernutrition in this population is around 20% among children, with high frequency of infections, anemia, and parasitic infestations, associated with poor sanitation. Among stunted adolescents, we found a high prevalence of hypertension (21%) that is a considerably higher estimate compared to non‐stunted adolescents (less than 10%). The prevalence of hypertension in undernourished pre‐school children, or in those who recovered from undernutrition, was higher than that in controls (29%, 20% and 2%, respectively, P < 0.001). Among stunted adults eating no more than 66% of the requirements (adjusted for stature), overweight/obesity was 35% in women and 25% in men. The prevalence of hypertension was 44% among stunted women and 18% among stunted men. Fifty per cent of stunted and obese women had hypertension. These data reinforce the important association between undernutrition and hypertension from childhood through adulthood. Health policies for preventing and combating childhood undernutrition should have an impact on the morbidity and mortality related to hypertension during adulthood.
Keywords: stunting, chronic malnutrition, undernutrition, obesity, hypertension, food intake
Poverty and undernutrition
Undernutrition is still considered one of the major problems in public health in many countries (Sedgh et al., 2000) affecting 30% or more of children under 5 years of age (Kabir et al., 1998); being the most important mortality cause (55%) in this age group in developing countries (UNICEF, 1998).
The socioeconomic condition of a country determines the quality of life of its citizens. In the last decades, the great difficulties in living conditions found in rural areas in most developing countries has led to large migration to urban areas, promoting the increase of slums. In Brazil, in 2000, 82% of the population lived in urban areas (IBGE, 2000). The increase of the urban population was not followed by an improvement at the same speed in infrastructure, creating a deficit of urban facilities, such as water, electricity and sewage supply. In addition, this demographic explosion brought to the cities a great number of people with a low level of education and therefore unsuitable for the growing complexity of the working market.
Worldwide, the most frequent type of undernutrition is stunting caused by chronic undernutrition, most frequently starting prenatally (Allen & Gillespie, 2001). Nutritional stunting is caused by insufficient maternal nutrition, intrauterine undernutrition, lack of breastfeeding until 6 months of age, later introduction of complementary feeding, inadequate (quantity and quality) complementary feeding, and impaired absorption of nutrients owing to infectious diseases (Allen & Gillespie, 2001).
In recent years a growing body of evidence has shown that prenatal undernutrition or stunting is related to non‐communicable diseases later (Barker, 1994; Velasquez‐Melendez et al., 1999; Sichieri et al., 2000). Studies linking early nutrition with adulthood disease have focused on fetal nutrition. However, childhood undernutrition remains extremely common in many developing countries and associations with later disease need to be established. The present review describes data from slum populations in Brazil showing an association between stunting/short stature and hypertension and obesity, without an apparent excess of food or fat intake.
Stunting and hypertension among adolescents living in slums in the city of São Paulo
Twenty‐two per cent of the Brazilian population live in the state of São Paulo, and 28% of these are concentrated in the city of São Paulo (IBGE, 2000). It has been estimated that 20% of the population of the city of São Paulo live in slums (SEHAB, 1994). Sanitation is very precarious in these environments. The majority of the constructions are in unhealthy and inappropriate land near rivers and subjected to constant floods.
We investigated the nutritional and health conditions of 53 adolescents (10–16 years of age, 27 boys and 26 girls, Tanner stage 2, 3, 4 and 5) living in slums. There was a large number of dwellings made of wood planks (49%), as well as reduced availability of city sewage (21%), and frequent use of untreated water (68%). Almost all families were under the poverty line for income (less than a dollar per capita per day). Mean height for girls was 149.8 cm, 5 cm below the mean National Center for Health Statistics (NCHS) value, and mean height for boys was 149.3 cm, 7 cm below the NCHS value (−0.8 ± 1.1 and −1.0 ± 1.4 z‐score respectively). The mean body mass index (BMI) for girls was 18.8 kg m−2 and for boys 17.6 kg m−2, values within the normal range for this age group (mean age = 13 years) (Fernandes et al., 2003).
Table 1 shows the percentage of children with blood pressure (BP) above the 90th and 95th percentiles adjusted for height percentiles according to the Update Second Task Force from 1996 (National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents, 1996). BP was measured using a mercury column sphygmomanometer, following the guidelines of this Task Force. More detailed description of the procedure was described elsewhere (Fernandes et al., 2003). In the literature, BP above the 90th percentile and below or equal to the 95th percentile represents a risk of hypertension. There was a high percentage of adolescents with diastolic hypertension (values above the 95th percentile). For systolic BP, no patient was above the 95th percentile. Considering the group of patients as a whole, the overall prevalence of diastolic arterial hypertension was 21% with a 95% confidence interval of 10% to 32%. No significant difference was observed between sexes. The prevalence of cases with systolic or diastolic BP above the 90th percentile was 51% (n = 27), with a 95% confidence interval of 37% to 65%. Six percent (n = 3) of these individuals had simultaneously systolic and diastolic arterial hypertension. Figure 1 illustrates the levels of diastolic BP found in this group of adolescents. Almost 100% of both boys and girls showed values of diastolic BP above the 50th percentile, being distributed around the 90th percentile.
Table 1.
Blood pressure values in relation to the 90th and 95th percentiles for boys and girls according to the stature percentile (National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents, 1996)
| Variable | Boys (Total n = 27) n (%) | Girls (Total n = 26) n (%) | Total n (%) | P‐value* |
|---|---|---|---|---|
| P90 < SBP < P95 (mmHg) | 0 | 3 | 3 (6) | 0.11 |
| SBP > P95 (mmHg) | 0 | 0 | 0 | |
| P90 < DBP < P95 (mmHg) | 9 (33) | 7 (27) | 16 (30) | 0.83 |
| DBP > P95 (mmHg) | 5 (19) | 6 (23) | 11 (21) | 0.94 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; P90, 90th percentile; P95, 95th percentile; *P value for the comparison between boys and girls.
Taken from Fernandes et al. (2003).
Figure 1.
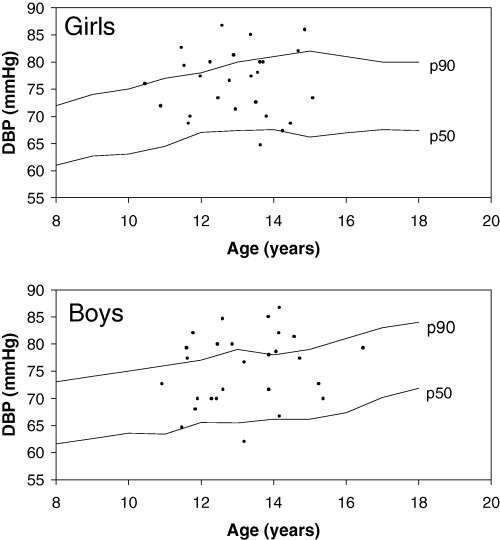
Diastolic blood pressure of 53 boys and girls recruited from population surveys of three slums in the city of São Paulo, Brazil. Mean ± SD of weight, stature and Body Mass Index (Z score), respectively: boys = −0.9 ± 1.0, −1.0 ± 1.4, −0.5 ± 0.5; and girls = −0.5 ± 0.9, −0.8 ± 1.1, −0.3 ± 0.7 (for more details see Fernandes et al., 2003). DBP, diastolic blood pressure.
Short stature, insufficient food intake, obesity and hypertension among adults living in slums in the city of Maceió
Alagoas, in the north‐eastern region of Brazil, is one of the poorest states of the country and has the highest income concentration (social exclusion index = 72.4%) and the worst human development index. This means that only 27.6% of the population is provided with basic public services. One‐third of its population is below the line of poverty (less than 1 US$ per capita per day), infant mortality stands at 68.4 per 1000 live births (IBGE, 2000). It was estimated that 50% of the people living in its capital, Maceió, live in slums.
We performed a census of the adult population of a slum community (age > 18 and < 60 years) in the city of Maceió. All the 315 shacks and 532 adults were investigated (after excluding pregnant women, the physically handicapped and those with prostheses, the final number was 416 individuals, 43% men and 57% women). The individuals who did not take part in the study (refusal, closed home, etc.) accounted for 11.8% of the population. Short stature was found in 22.6% of the adults, and overweight or obesity in 32% of women and 18% of men (Florêncio et al., 2001).
Assessment of food intake in this slum population revealed some intriguing results (2, 3). The methodology for this evaluation was recall inquiry applied to the entire adult population, on any given day, to provide information on the respondent's food intake during a single 24‐h period. The use of the Manual of Photographic Record for Dietary Inquiries by Zabotto (1996) was made, which illustrates the different servings, shown in grams and household measures. Detailed description of the methodology is given elsewhere (Florêncio et al., 2003). The average energy intake was very much below the requirements (66% for men and 60% for women). After adjusting for stature, the adequacy in energy intake for non‐stunted individuals reached only 78%, and 70% for stunted individuals. Stunted overweight and obese women consumed only 65% of the energy requirement adjusted to stature (Table 2).
Table 2.
Adequacy of energy intake in an adult population residing in a slum according to sex and anthropometric characteristics. Maceió, Alagoas – Brazil
| Sex | Energy (kJ) | Stunted | Non‐stunted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U | A | O | Total | U | A | O | Total | ||
| Male | Intake | 5882 | 6812 | 7226 | 6640 | 6501 | 7815 | 8373 | 7563 |
| % adequacy* | 54 | 63 | 66 | 61 | 60 | 72 | 77 | 70 | |
| Requirement adjusted to stature | 8987 | 8987 | 8987 | 8987 | 9614 | 9614 | 9614 | 9614 | |
| % adequacy | 65 | 76 | 80 | 74 | 68 | 81 | 87 | 79 | |
| Female | Intake | 4527 | 5029 | 4686 | 4747 | 5560 | 5497 | 6556 | 5871 |
| % adequacy* | 53 | 58 | 54 | 55 | 65 | 64 | 76 | 68 | |
| Requirement adjusted to stature | 7234 | 7234 | 7234 | 7234 | 7640 | 7640 | 7640 | 7640 | |
| % adequacy | 62 | 69 | 65 | 66 | 73 | 72 | 86 | 77 | |
U, underweight (BMI < 20); A, average weight (BMI ≥ 20 < 25); O, overweight + obesity (BMI ≥ 25); *Compared to the numbers established for the recommended dietary allowances (RDAs), taking 10878 and 8619 kJ as the mean values for men and women respectively.
Taken from Florêncio et al. (2003).
Table 3.
Macronutrient intake and respective percentage contribution in relation to total energy consumption, compared with gender and nutritional classification. Adult population residing in a slum, Maceió, Alagoas – Brazil
| Gender | Nutrient | Stunted | Non‐stunted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U | A | O | Total | U | A | O | Total | ||
| Male | Protein (g) | 52 (14.8) | 81 (19.9) | 76 (17.6) | 70 (17.5) | 73 (18.6) | 88 (18.8) | 96 (19.2) | 86 (18.8) |
| Carbohydrate (g) | 212 (60.4) | 218 (53.6) | 268 (62.1) | 233 (58.7) | 225 (57.9) | 262 (56.1) | 269 (53.8) | 252 (56.0) | |
| Fat (g) | 39 (24.8) | 48 (26.5) | 39 (20.3) | 42 (23.8) | 41 (23.5) | 52 (25.1) | 60 (27.0) | 51 (25.2) | |
| Female | Protein (g) | 46 (17.0) | 51 (17.0) | 52 (18.6) | 50 (17.5) | 65 (19.6) | 65 (19.8) | 77 (19.7) | 69 (19.7) |
| Carbohydrate (g) | 157 (58.0) | 173 (57.6) | 165 (58.9) | 165 (58.2) | 184 (55.4) | 180 (54.8) | 209 (53.4) | 191 (54.5) | |
| Fat (g) | 30 (25.0) | 34 (25.5) | 28 (22.5) | 31 (24.3) | 37 (25.0) | 37 (25.4) | 47 (27.0) | 40 (25.8) | |
U, underweight (BMI < 20); A, average weight (BMI ≥ 20 < 25); O, overweight + obesity (BMI ≥ 25).
The number in parentheses indicate the percentage of the respective macronutrient in relation to energy consumption.
Taken from Florêncio et al. (2003).
Table 3 shows the distribution of macronutrients according to their contribution to the total energy intake. The figures for all subgroups were similar. The total energy intake of the population was as follows: 18% protein, 58% carbohydrates and 24% lipids. The percentage of energy intake from fat was less than 30%, even for obese and overweight men or women.
The results obtained pointed to the non‐existence of differences in fat intake for stunted and obese men and women, when compared to their normal or undernourished counterparts, although they all ingested fewer calories than their respective current requirements. Therefore, it appears that the higher risk of obesity observed among stunted individuals cannot be explained by a higher fat or energy intake. On the other hand, the type of carbohydrate ingested has been considered as one important factor that could elevate the risk of obesity (Bray et al., 2004).
Obesity is acknowledged to be one of the main risk factors for non‐communicable diseases and is associated with an increased incidence of arterial hypertension (Stamler et al., 1978; Hsuch & Buchanan, 1994; Whelton, 1994). Particularly, the association between abdominal obesity and hypertension has been well documented (Bjorntorp & Rosmond, 2000; Bray & Champagne, 2004).
We have explored the association of short stature with hypertension and obesity in this adult population. The prevalence of hypertension was 28.5%, although the prevalence was strikingly different in men (18.4%) and women (38.5%). After stratification by stature quartiles, hypertension remained statistically significantly different, in both sexes, in the first two quartiles in comparison with the last two: 21.1% versus 14.6%, respectively (P = 0.01) in men; and 42.4% versus 34.6%, respectively (P = 0.013) in women (Table 4). Logistic regression was used to assess the odds ratio of arterial hypertension through the stature quartiles. Women in the 1st quartile showed an odds ratio of hypertension significantly greater than in the 4th quartile (2.23, 95% confidence interval: 1.67–2.90), although this result has not been observed in men. Table 5 shows the analysis of BP based on stature and BMI. Short‐stature obese women showed a higher prevalence of hypertension than the obese women in the other stature quartiles. Again, this pattern was not observed in men (Florêncio et al., 2004).
Table 4.
Prevalence (%) and odds ratio for arterial hypertension, based on stature quartiles in men and women living in a slum, Maceió, Alagoas–Brazil
| Stature quartiles | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Median height (cm) | n | Prevalence of hypertension (%) | Odds ratio (95% CI) | Median height (cm) | n | Prevalence of hypertension (%) | Odds ratio (95% CI) | |
| 1st | 158.2 | 42 | 18.0 | 1.12 (0.77–1.63) | 147.1 | 59 | 44.2 | 2.23 (1.67–2.90) |
| 2nd | 164.3 | 38 | 26.3 | 1.21 (0.97–1.51) | 152.4 | 56 | 40.7 | 2.16 (1.66–2.85) |
| 3rd | 168.5 | 52 | 12.2 | 1.03 (0.80–1.31) | 156.4 | 54 | 35.3 | 1.93 (1.53–2.45) |
| 4th | 174.2 | 47 | 17.0 | 1.0 | 161.6 | 68 | 33.9 | 1.0 |
| Total | 166.3 | 179 | 18.4 | 154.3 | 237 | 38.5 | ||
Taken from Florêncio et al. (2004).
Table 5.
Prevalence of arterial hypertension in overweight or obese a men and women, living in a slum, Maceió, Alagoas –Brazil
| Stature quartiles | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Median height (cm) | n | Prevalence of hypertension (%) | Odds ratio (95% CI) | Median height (cm) | n | Prevalence of hypertension (%) | Odds ratio (95% CI) | |
| 1st | 157.8 | 10 | 10.0 | 0.92 (0.50–1.78) | 146.4 | 20 | 50.0 | 1.98 (1.32–2.96) |
| 2nd | 164.0 | 8 | 25.0 | 1.16 (0.87–1.48) | 152.6 | 20 | 35.0 | 1.63 (1.06–2.49) |
| 3rd | 168.0 | 7 | 14.3 | 1.03 (0.56–1.68) | 156.3 | 17 | 35.3 | 1.65 (1.08–2.52) |
| 4th | 176.0 | 6 | 16.7 | 1.0 | 160.9 | 21 | 37.6 | 1.0 |
| Total | 166.3 | 31 | 16.5 | 154.3 | 78 | 39.5 | ||
Body Mass Index ≥ 25Kg/m2.
Taken from Florêncio et al. (2004).
Hypertension in undernourished children and in those with nutritional recovery
There is scant literature regarding BP in pre‐school children with growth retardation (Gaskin et al., 2000). We investigated the association between increased BP and undernutrition in pre‐school children of low socioeconomic level. Additionally, the BP of a group of children who had recovered from undernutrition was assessed.
In a cross‐sectional study we assessed the BP of 172 children older than 2 years living in slums in the city of São Paulo. Ninety‐one children were undernourished (height‐for‐age (H/A) or weight‐for‐age (W/A) Z‐score below −1 of the NCHS references). Among these, 26 (29%) had a W/A Z‐score < −1; 12 (13%) had a H/A Z‐score < −1, and 53 (58%) had both indices < −1. Twenty children had recovered from undernutrition (at the time of reevaluation both H/A and W/A scores were greater than −1 Z score) after an average time of 6.4 years. A control group of 61 non‐undernourished children was randomly selected among children living in the same neighbourhood as the cases and matched for age to the currently undernourished group (Sesso et al., 2004).
Mean systolic and diastolic BP, adjusted for several factors, were higher in the undernourished and recovered groups compared to the controls; however, the values were statistically significant only for diastolic BP (Table 6). No difference was detected between the two former groups.
Table 6.
Blood pressure (BP) of undernourished, recovered and control children. Mean values for systolic and diastolic BP, and numbers referring to the 90th and 95th percentiles a
| Blood pressure | Undernourished n = 91 | Recovered n = 20 | Controls n = 61 |
|---|---|---|---|
| Systolic BP b (mmHg) | 98.7 ± 1.0 | 99.4 ± 2.2 | 96.4 ± 1.2 |
| Diastolic BP b (mmHg) | 65.2 ± 0.6† | 66.5 ± 1.5† | 61.8 ± 0.8 |
| Systolic BP | |||
| >P90 and ≤ P95 | 11(12)† | 3 (15)* | 0 (0) |
| >P95 | 6 (7) | 1 (5) | 0 (0) |
| Diastolic BP | |||
| >P90 and ≤ P95 | 29 (32)†, § | 1 (5) | 12 (20) |
| >P95 | 22 (24)† | 3 (15)* | 1 (2) |
| Systolic or diastolic BP | |||
| >P90 and ≤ P95 | 38 (42)†, ‡ , c | 3 (15) d | 12 (20) |
| >P95 | 26 (29)† , c | 4 (20)* | 1 (2) |
P90, 90th percentile; P95, 95th percentile; aValues are expressed as mean ± SE, or number (%); bAdjusted for age, sex, race, birthweight, current weight and height; cTwo individuals had increased values of both systolic and diastolic BP; dOne individual had increased values of both systolic and diastolic BP.
P < 0.05,
P < 0.01 versus control;
P < 0.05,
P < 0.01 versus recovered.
Taken from Sesso et al. (2004).
A greater percentage of children in the undernourished and recovered groups had increased BP, particularly diastolic, compared to the controls. Considering alterations in systolic and/or diastolic BP (above the 95th percentile of the Update of the 2nd Task Force references) after adjusting for age, sex, race, height, birthweight, current weight and height, a significantly greater percentage of undernourished and recovered children had high BP compared to controls (29%, 20%, and 2%, respectively, P < 0.001). No significant difference was detected between undernourished and recovered children. The undernourished group had a greater proportion of children at a higher risk of hypertension (BP value above the 90th percentile and below or equal to the 95th percentile) than the other two groups (Table 6).
In univariate analysis there was a significantly positive correlation between systolic BP and age (r = 0.29), weight (r = 0.30) and height (r = 0.23), but not with birthweight (r = −0.04). The corresponding coefficients for diastolic BP were r = 0.46, r = 0.38, r = 0.41 and r = −0.02 respectively.
Multivariate regression analysis was performed to predict systolic and diastolic BP and all available variables were tested as independent ones. In these analyses, current weight was the only independent predictor variable affecting systolic BP. Nutritional group (undernourished or recovered compared to controls) significantly influenced diastolic BP controlling for several factors.
In a multivariate logistic regression analysis considering as dependent variable systolic or diastolic BP above the 95th percentile, nutritional group was the only significantly independent variable in the model. The odds ratio (95% confidence interval) of high BP in the undernourished and recovered groups compared to the controls was 29.17 (2.70–315) and 42.48 (2.37–763), respectively, after adjusting for age, gender, race, height, weight and birthweight.
Overall, a strikingly high percentage of the undernourished children in our study were found to have high BP, particularly diastolic. The prevalence rates of undernourished children with BP above the 90th percentile and below or equal to the 95th percentile (42%), and above the 95th percentile (29%) were remarkably greater than those in controls [20% and 2%, respectively (Table 6)], and considerably higher than the hypertension rates reported in other large national studies on children without nutritional alterations, which ranged from 0.8% to 7.2% (Bastos et al., 1993; Oliveira et al., 1999). Several authors have reported the association between prenatal undernutrition, as indicated by low birthweight, and hypertension in adult life (1989a, 1989b; Law & Shiell, 1996; Whincup et al., 1999; Eriksson et al., 2000). A fewer number of studies did not confirm these findings (Seidman et al., 1991; Lucas & Morley, 1994; Rahiala et al., 2002). In our study, birthweight was not a significant predictor of BP. In addition, we found that nutritional deficit during childhood was associated with high BP independently of birthweight (Sesso et al., 2004). Our data lend support to the hypothesis that undernutrition, not only prenatal as indicated in other studies, but also occurring during childhood, may be a risk factor for the development or aggravation of hypertension.
More interestingly, the findings that recovered (after 6.4 years) children tend to have higher BP levels than controls and values not significantly different from those currently undernourished suggest that the effects of undernutrition on BP may be persistent. The strength of this inference may be lessened by the small sample size of patients studied in this group, the cross‐sectional nature of this study design and the possibility that other factors related to undernutrition, such as the low socio‐economic status, may have contributed for this association.
Final remarks
The findings described in this review emphasize the need for monitoring BP in undernourished children. Undernutrition should be more often recognized as a factor associated with increased BP in childhood. It is suggested that not only intrauterine undernutrition but also its occurrence during childhood may influence the incidence of hypertension in adulthood.
There are a number of pathogenic mechanisms reported in both experimental and human studies, by which undernutrition intrautero or early in life could initiate or further increase higher BP: (1) activation of the renin‐angiotensin system (Godard et al., 1986; Kingdom et al., 1993); (2) alterations in vascular structure, function and compliance (Yoshida et al., 1995; Zureik et al., 1996); (3) increased sympathetic nervous activity (Phillips & Barker, 1997; Leon‐Quinto et al., 1998); (4) increased insulin sensitivity (Somova & Moodley, 2000); (5) plasma insulin‐like growth factor‐1 levels (Fall et al., 1995); (6) high plasma glucocorticoid concentrations (Jacobson et al., 1997; Langley‐Evans, 1997); and (7) reduction in nephron number (renal mass) associated with retarded fetal growth (Brenner & Chertow, 1994).
Physicians and other health care professionals practicing in developing countries and in large urban centers with low income populations should be aware of the association between early in life undernutrition and hypertension for a timely detection and treatment of hypertension, and to keep monitoring these individuals throughout their life. We also found a marked association between short stature, obesity and hypertension, mainly among adult women, living in poor conditions (Florêncio et al., 2001). Therefore, this group of people should deserve particular attention. Health policies for preventing and combating childhood undernutrition may have an impact on the morbidity and mortality related to hypertension during adulthood.
Acknowledgements
FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), FAPEAL (Fundação de Amparo à Pesquisa do Estado de Alagoas), IAEA (International Atomic Energy Agency).
References
- Allen L.H. & Gillespie S.R. (2001) What Works? A Review of the Efficacy and Effectiveness of Nutrition Interventions. United Nations Administrative Committee on Coordination/Sub‐Committee on Nutrition. ACC/SCN: Geneva. [Google Scholar]
- Barker D.J.P., Osmond C., Golding J., Kuh D. & Wadsworth M.E.J. (1989a) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. British Medical Journal, 298, 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P., Winter P.D., Osmond C., Margetts B. & Simmonds S.J. (1989b) Weight in infancy and death from ischemic heart disease. Lancet, 2, 577–580. [DOI] [PubMed] [Google Scholar]
- Barker D.J. (1994) Outcome of low birthweight. Hormone Research, 42, 223–230. [DOI] [PubMed] [Google Scholar]
- Bastos H.D., Macedo C.S. & Riyuzo M.C. (1993) Pressão arterial na infância. Journal of Pediatrics, 69, 107–115. [Google Scholar]
- Bjorntorp P. & Rosmond R. (2000) Neuroendocrine abnormalities in visceral obesity. International Journal of Obesity and Related Metabolic Disorders, 24 (Suppl. 2), 80–85. [DOI] [PubMed] [Google Scholar]
- Bray G.A. & Champagne C.M. (2004) Obesity and Metabolic Syndrome: implications for dietetics practitioners. Journal of the American Dietetic Association, 1, 86–89. [DOI] [PubMed] [Google Scholar]
- Bray G.A., Nielsen S.J. & Popkin B.M. (2004) Consumption of high‐fructose corn syrup in beverages may play a role in the epidemic of obesity. American Journal of Clinical Nutrition, 4, 537–543. [DOI] [PubMed] [Google Scholar]
- Brenner B. & Chertow G.M. (1994) Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. American Journal of Kidney Diseases, 23, 171–175. [PubMed] [Google Scholar]
- Eriksson J., Forsen T., Tuomilehto J., Osmond C. & Barker D. (2000) Fetal and childhood growth and hypertension in adult life. Hypertension, 36, 790–794. [DOI] [PubMed] [Google Scholar]
- Fall C.H., Pandit N.A. & Law C.M. (1995) Size at birth and plasma insulin‐like growth factor‐1 concentrations. Archives of Disease in Childhood, 73, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M.T., Sesso R., Martins P.A. & Sawaya A.L. (2003) Increased blood pressure in adolescents of low socioeconomic status with short stature. Pediatric Nephrology, 5, 435–439. [DOI] [PubMed] [Google Scholar]
- Florêncio T.T., Ferreira H.S., Cavalcante J.C., Luciano S.M. & Sawaya A.L. (2003) Food consumed does not account for the higher prevalence of obesity among stunted adults in a very Low income population in the Northeast of Brazil (Maceió, Alagoas). European Journal of Clinical Nutrition, 57, 437–446. [DOI] [PubMed] [Google Scholar]
- Florêncio T.T., Ferreira H.S., Cavalcante J.C. & Sawaya A.L. (2004) Short stature, obesity and arterial hypertension in a very low income population in North‐Eastern Brazil. Nutrition, Metabolism and Caridiovascular Diseases, 14, 26–33. [DOI] [PubMed] [Google Scholar]
- Florêncio T.T., Ferreira H.S., França A.P.T., Cavalcanti J.C. & Sawaya A.L. (2001) Obesity and undernutrition in a very low income population in the city of Maceió, Northeastern Brazil. British Journal of Nutrition, 86, 277–284. [DOI] [PubMed] [Google Scholar]
- Gaskin P.S., Walker S.P., Forrester T.E. & McGregor S.M.G. (2000) Early linear growth retardation and later blood pressure. European Journal of Clinical Nutrition, 54, 563–567. [DOI] [PubMed] [Google Scholar]
- Godard C.M., Munoz M., Sanchez M.A., Vallotton M.B. & Riondel A. (1986) A study of the renin‐angiotensin‐aldosterone system in severe infantile malnutrition. International Journal of Pediatric Nephrology, 7, 39–44. [PubMed] [Google Scholar]
- Hsuch W.A. & Buchanan T.A. (1994) Obesity hypertension. Endocrinology and Metabolism Clinics of North America, 23, 405–427. [PubMed] [Google Scholar]
- Instituo Brasileiro de Geografia e estatística (IBGE ). (2000) Censo 2000. Available at: http://www.ibge.gov.br
- Jacobson L., Zurakowski D. & Majzoub J.Á. (1997) Protein malnutrition increases plasma adrenocorticotropin and anterior pituitary propiomelanocortin messenger ribonucleic acid in the rat. Endocrinology, 138, 1048–1057. [DOI] [PubMed] [Google Scholar]
- Kabir I., Rahman M.M., Haider R., Mazumder R.N., Khaled M.A. & Mahalanabis D. (1998) Increased height gain of children fed a high‐protein diet during convalescence from shigellosis: a six‐month follow‐up study. Journal of Nutrition, 128, 1688–1691. [DOI] [PubMed] [Google Scholar]
- Kingdom J.C.P., McQueen J., Connell J.M.C. & Whittle M.J. (1993) Fetal angiotensin II levels and vascular (type1) angiotensin receptors in pregnancies complicated by uterine growth retardation. British Journal of Obstetrics and Gynecology, 100, 293–476. [DOI] [PubMed] [Google Scholar]
- Langley‐Evans S.C. (1997) Intrauterine programming of hypertension by glucocorticoids. Life Science, 60, 1213–1221. [DOI] [PubMed] [Google Scholar]
- Law C.M. & Shiell A.W. (1996) Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. Journal of Hypertension, 14, 935–941. [PubMed] [Google Scholar]
- Leon‐Quinto T., Magnan C. & Portha B. (1998) Altered activity of the autonomous nervous system as a determinant of the impaired beta‐cell secretory response after protein‐energy restriction in the rat. Endocrinology, 139, 3382–3389. [DOI] [PubMed] [Google Scholar]
- Lucas A. & Morley R. (1994) Does early nutrition in infants born before term programs later blood pressure? British Medical Journal, 309, 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents . (1996) Update on the 1987 Task Force Report on high blood pressure in children and adolescents: a Working Group report from the national high blood pressure education program. Pediatrics, 98, 649–658. [PubMed] [Google Scholar]
- Oliveira R.G., Lamounier J.A., Oliveira A.D.B., Castro M.D.R. & Oliveira J.S. (1999) Pressão arterial em escolares e adolescentes – O estudo de Belo Horizonte. Journal of Pediatrics, 75, 256–266. [DOI] [PubMed] [Google Scholar]
- Phillips D.I.W. & Barker D.J.P. (1997) Association between low birth weight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabetes Medicine, 14, 673–677. [DOI] [PubMed] [Google Scholar]
- Rahiala E., Tenhola S, Vanninen E., Herrgard E., Tikanoja T. & Martikainen A. (2002) Ambulatory blood pressure in 12‐year‐old children born small for gestational age. Hypertension, 39, 909–913. [DOI] [PubMed] [Google Scholar]
- Sedgh G., Herrera M.G., Nestel P., El Amin A. & Fawzi W.W. (2000) Dietary vitamin A intake and non‐dietary factors are associated with reversal of stunting in children. Journal of Nutrition, 130, 2520–2526. [DOI] [PubMed] [Google Scholar]
- SEHAB – São Paulo (cidade). Secretaria da Habitação e Desenvolvimento Urbano. (1994) Favelas na cidade de São Paulo. SEHAB: São Paulo. [Google Scholar]
- Seidman D.O., Lao A., Gale R., Stevenson O.K., Mashiach S. & Carron Y.L. (1991) Birth weight and current body weight and blood pressure in late adolescence. British Medical Journal, 302, 1235–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso R., Barreto G.P., Neves J. & Sawaya A.L. (2004) Malnutrition is associated with increased blood pressure in childhood. Nephron Clinical Practice, 97, 61–66. [DOI] [PubMed] [Google Scholar]
- Sichieri R., Siqueira K.S. & Moura A.S. (2000) Obesity and abdominal fatness associated with undernutrition early in life in a survey in Rio de Janeiro. International Journal of Obesity and Related Metabolic Disorders, 24, 614–618. [DOI] [PubMed] [Google Scholar]
- Somova L.I. & Moodley K. (2000) The effect of protein‐energy malnutrition on development of hypertension, glucose metabolism and insulin sensitivity in genetically hypertensive rats. Nutrition, Metabolism and Cardiovascular Disease, 10, 188–194. [PubMed] [Google Scholar]
- Stamler R., Stamler J., Algera G. & Roberts R. (1978) Weight and blood pressure. Findings in hypertension screening of 1 million Americans. Journal of the American Medical Association, 240, 1607–1609. [DOI] [PubMed] [Google Scholar]
- UNICEF . (1998) Situação mundial da infância. A nutrição em foco. Fundo das Nações Unidas para a Infância: Brasília. [Google Scholar]
- Velasquez‐Melendez G., Martins I.S., Cervato A.M., Fornés N.S., Marucci M. de F.N. & Coelho L.T. (1999) Relationship between stature, overweight and central obesity in the adult population in São Paulo, Brazil. International Journal of Obesity and Related Metabolic Disorders, 23, 639–644. [DOI] [PubMed] [Google Scholar]
- Whelton P.K. (1994) Epidemiology of hypertension. Lancet, 344, 101–106. [DOI] [PubMed] [Google Scholar]
- Whincup P.H., Bredow M., Payne F., Sadler S. & Golding J. (1999) Size at birth and blood pressure at 3 years of age. The Avon Longitudinal Study of Pregnancy and Childhood (ALSAPC). American Journal of Epidemiology, 149, 730–739. [DOI] [PubMed] [Google Scholar]
- Yoshida W.B., Muller S.S., Carvalho I., Fabris V.E., Naresse L.E. & Maffei F.H. (1995) Tensile strength and histological changes of abdominal aorta of malnourished rats. Cardiovascular Surgery, 3, 437–439. [DOI] [PubMed] [Google Scholar]
- Zabotto C.B. (1996) Registro Fotográfico para Inquéritos Dieteticos (photographic Record for Dietary Inquiries). Editora da UFG: Goiânia (in Portuguese). [Google Scholar]
- Zureik M., Bonithon‐Kopp C., Lecomte E., Siest G. & Ducimetiere P. (1996) Weights at birth and in early infancy, systolic pressure, and left ventricular structure in subjects aged 8–24 years. Hypertension, 27, 339–345. [DOI] [PubMed] [Google Scholar]


