Abstract
Slow growth during fetal life and infancy is often followed by accelerated weight gain in childhood. These patterns of growth seem to precede the development of coronary heart disease (CHD) and type 2 diabetes in adult life. Patterns of growth associated with CHD and type 2 diabetes in adult life are described based upon findings from the Helsinki Birth Cohort Study. We are beginning to understand that adult degenerative diseases are associated with different patterns of early growth. Yet it is not clear what optimal growth is and how it can be achieved. Most data suggest that the development of many non‐communicable diseases involve a number of interactions including genetic ones. Therefore these diseases can best be focused upon from a life cycle perspective.
Keywords: type 2 diabetes, coronary heart disease, growth patterns
Many chronic diseases originate early in life and people who have been small at birth – as a result of reduced intrauterine growth – have increased rates of cardiovascular disease and type 2 diabetes in adult life (Barker et al., 1989; Hales et al., 1991; Osmond et al., 1993; Eriksson et al., 1999). According to the FOAD hypothesis, an undernourished fetus makes persisting metabolic adaptations predisposing to, e.g. coronary heart disease (CHD) and type 2 diabetes (Barker, 1998). The increased risk for CHD and type 2 diabetes associated with a small body size at birth is largely modified by both childhood growth and adult characteristics (Eriksson et al., 1999; Eriksson et al., 2001, , 2003a). Therefore the patterns of growth leading to chronic diseases in adulthood will best be understood from a life cycle perspective. The early origins and patterns of growth related to CHD and type 2 diabetes will be described based upon findings in Finland in the Helsinki Birth Cohort Study.
The Helsinki Birth Cohort Study
Two study cohorts consisting of 15 846 individuals born at Helsinki University Central Hospital and who grew up in Helsinki have been followed (Eriksson & Forsén, 2002). Data from the ‘older cohort’ consisting of 7086 individuals born 1924–33, include birth characteristics as well as growth data between 7 and 15 years of age. The ‘younger cohort’ born 1934–44 (n = 8760) has detailed data on childhood growth from birth up to 12 years of age. A third cohort born at the Midwives’ Hospital in Helsinki during 1934–44, including 2345 men has more recently been studied – it has similar growth data as the cohort born at Helsinki University Central Hospital (Forsén et al., 2004). Information on early growth has been collected from birth, child welfare and school records. These records include information on health and growth, but also information on socio‐economic factors during childhood. All cohorts have been followed up from 1971 onwards by register linkage to national Finnish registers providing information on both morbidity and mortality. A clinical examination of 2500 individuals will provide more detailed information on metabolic and genetic aspects and their associations with growth and adult health outcomes.
Coronary heart disease, fetal and infant growth
The first study reporting an association between low birthweight, low weight in infancy and CHD in adult life was based upon data from Hertfordshire, UK (Barker et al., 1989). By now there is a large number of studies showing that people who were small at birth have increased rates of CHD and its risk factors later in life (Barker, 1998).
In the Helsinki Birth Cohort Study, deaths from CHD were associated with low birthweight, but also with thinness at birth (Eriksson et al., 1999). Men with a birthweight <2500 g had hazard ratios for CHD of 3.63 (95% CI 2.02–6.51) compared with the group with a birthweight >4000 g. The growth of women who developed CHD differed slightly from that of the men (Forsén et al., 1999). Hazard ratios for CHD fell with increasing birthweight among the women, but the pattern of fetal growth that most strongly predicted CHD was shortness at birth. The hazard ratio for developing CHD in women increased by 10.2% for each centimetre decrease in length at birth – after adjustment for gestation.
Slow growth during infancy increases the risk of CHD in later life. Low weight at 1 year of age added to the CHD risk independently of size at birth (Eriksson et al., 2001). Body size at 1 year of age was a remarkably strong predictor of later CHD independently of birthweight (Forsén et al., 2004). Hazard ratios for CHD according the body size at 1 year of age are given in Table 1.
Table 1.
Hazard ratios for coronary heart disease in Finnish men born 1934–44 according to body weight 1 year of age
| Weight at 1 year (kg) | Hazard ratio (95% CI) |
|---|---|
| <8 | 3.39 (1.55–7.43) |
| −9 | 2.48 (1.30–4.75) |
| −10 | 2.04 (1.11–3.73) |
| −11 | 1.10 (0.58–2.06) |
| −12 | 0.98 (0.49–1.95) |
| >12 | 1.00 |
| P‐value for trend | <0.0001 |
Coronary heart disease and childhood growth
An interesting and important question is whether the increased risk for CHD associated with a small birth and infant size is modified by childhood growth. The Helsinki Birth Cohort Study has largely contributed to our present knowledge in this area.
Findings from the older Finnish birth cohort showed that deaths from CHD were associated with a small birth size followed by an above average body mass index (BMI) during later childhood (Forsén et al., 1997). These findings show that the consequences of becoming relatively overweight in childhood are largely conditioned and modified by growth in utero.
Data from the younger birth cohort have provided important information on growth between birth and 12 years of age. Infant and childhood growth of the men who developed CHD in adult life is shown in Fig. 1 (Eriksson et al., 2001). Growth is expressed as z‐scores with the z‐score for the whole cohort being set at zero. An individual maintaining a steady position as either large or small in relation to other individuals follows a horizontal path on the graph. Those men who later developed CHD had a small body size at birth and during infancy. Thereafter they experienced accelerated gain in weight and BMI; however, their heights remained below average.
Figure 1.
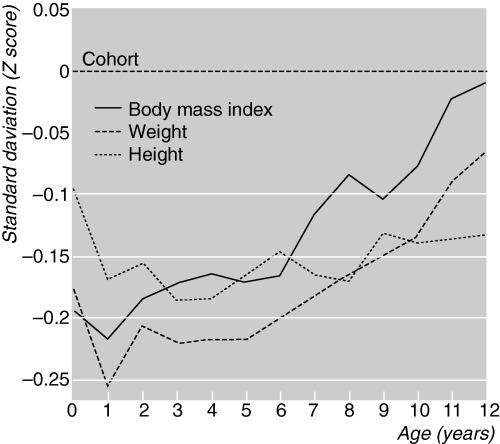
Growth of 357 boys who later developed coronary heart disease in a cohort of 4630 boys born in Helsinki. Reproduced with permission from British Medical Journal 2001; 322, 949–953.
These findings show that weight gain in infancy is associated with a reduced risk of CHD – irrespective of body size at birth. This piece of information is important as promoting weight gain in infancy is standard practice. A high weight gain during the first year is advantageous, contrary to accelerated growth past 1 year of age, which increases the risk of CHD – especially among those with a small birth size.
Socio‐economic factors, early growth and CHD
Non‐optimal socio‐economic circumstances are associated with increased rates of cardiovascular disease in adult life. Fetal and childhood growth and socio‐economic factors cannot be strictly separated from each other, and the risk for CHD is modified by the socio‐economic status in childhood (Barker et al., 2001). In the Helsinki Birth Cohort Study thinness at birth was related to an increased risk for CHD most strongly among men with lower socio‐economic status in childhood. Likewise low adult socio‐economic status and poor educational attainment were also associated with an increased risk for CHD.
Type 2 diabetes, fetal and infant growth
Small body size at birth has been associated with increased rates of impaired glucose tolerance and type 2 diabetes in adult life in several studies (Hales et al., 1991; Rich‐Edwards et al., 1999; Forsén et al., 2000). The Hertfordshire study showed that the disease was also associated with a low weight at 1 year of age. This has later been confirmed in the Helsinki Birth Cohort Study (Eriksson et al., 2003a). Fetal and infant growth of individuals who later develop type 2 diabetes resembles much the growth of those who develop CHD.
Type 2 diabetes and childhood growth
Childhood growth of those who later developed type 2 diabetes showed several similarities with the growth of those who later developed CHD. Children who later developed type 2 diabetes had small body size at birth and at 1 year, after which their weights and BMIs rose progressively to exceed the average. Their heights rose more slowly and reached the average (Eriksson et al., 2003a).
Obese individuals are more likely to develop type 2 diabetes but those who developed type 2 diabetes were not overweight at birth or during infancy. At what point does the increased risk for obesity and type 2 diabetes become evident in the growth pattern? After the age of 2 years the adiposity of young children as measured by BMI decreases to a minimum around 6 years of age before increasing again, i.e. the ‘adiposity rebound’ (Rolland‐Cachera et al., 1984). Figure 2 shows the cumulative incidence of type 2 diabetes in adult life in relation to age at adiposity rebound. Those children who had an adiposity rebound at the earliest ages had the highest prevalence of type 2 diabetes in adult life. The prevalence of type 2 diabetes decreased from 8.6% in those in whom the adiposity rebound occurred before the age of 5 years to 1.8% in those in whom it occurred after the age of 7 years (Eriksson et al., 2003a).
Figure 2.
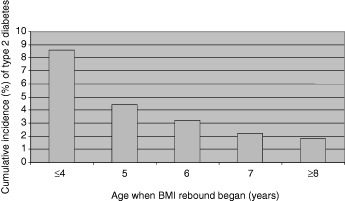
Cumulative incidence (%) of type 2 diabetes in adult life in relation to age at adiposity rebound.
Table 2 shows the relation between age at adiposity rebound, BMI/weight at 1 year and BMI at 12 years of age and the cumulative adult incidence of type 2 diabetes. Early adiposity rebound was preceded by thinness at birth and during infancy, yet associated with above average BMI at 12 years of age and high rates of type 2 diabetes in adult life. An early adiposity rebound is associated with the development of both obesity and type 2 diabetes (Eriksson et al., 2003b). However, the patterns of growth that lead to these two disorders differ. People who become obese tend to be big at birth, whereas people who develop type 2 diabetes tend to have low birthweight and to be small or thin at birth (Eriksson et al., 2003b). A recent unique population‐based longitudinal study of children born in Delhi, India about 30 years ago showed quite similar results as the Finnish study. Those who showed impaired glucose regulation had a low BMI up to 2 years of age, followed by an early adiposity rebound and accelerated increase in BMI until adult life (Barghava et al., 2004).
Table 2.
Age at adiposity rebound in relation to body mass index (BMI) and weight at 1 year of age, BMI at 12 years of age and prevalence of type 2 diabetes in adult life
| Age at adiposity rebound (years) | BMI at 1 year (kg/m2) | Weight at 1 year (kg) | BMI at 12 years (kg/m2) | Prevalence of type 2 diabetes (%) |
|---|---|---|---|---|
| ≤4 | 16.9 | 9.7 | 20.1 | 8.6 |
| 5 | 16.9 | 9.6 | 17.9 | 4.4 |
| 6 | 17.7 | 10.3 | 17.2 | 3.2 |
| 7 | 18.2 | 10.4 | 17.1 | 2.2 |
| ≥8 | 18.4 | 10.5 | 16.9 | 1.8 |
| P‐value for trend | <0.001 | <0.001 | <0.001 | <0.001 |
Underlying mechanisms
Numerous studies support the importance of events during critical periods of growth and development in the pathogenesis of CHD and type 2 diabetes. There are a number of possible mechanisms by which a non‐optimal early growth combined with accelerated weight gain in childhood could lead to CHD and type 2 diabetes. Babies who are thin at birth have less muscle tissue (Eriksson et al., 2002). If an individual born thin develops a relatively high BMI in childhood he might have a disproportionately high fat mass (Kahn et al., 2000; Eriksson et al., 2002). This again may be associated with insulin resistance as well as the metabolic syndrome, both well‐known risk factors for CHD and type 2 diabetes.
People born small with an impaired growth during infancy might have experienced poor liver growth leading to dyslipidemia and CHD in later life (Nathanielsz & Hanson, 2003). Support for this comes from the Hertfordshire study where low weight at 1 year has been associated with an atherogenic lipid profile in adult life (Fall et al., 1992; Barker et al., 1993). Similar associations between weight at one and adult lipid profiles have also been observed in Helsinki. Lipid metabolism is largely regulated by the liver, and one could speculate that poor infant growth is associated with persisting changes in the function of the liver.
The processes linking low weight gain in infancy with an early adiposity rebound are unknown. It could reflect a postweaning, infant diet low in fat but high in protein, followed by a childhood diet high in fat. Another explanation for the association between small birth size, early adiposity rebound and later type 2 diabetes is persisting alterations in body composition. An early adiposity rebound could be associated with life long setting of hormones and growth factors that facilitate the deposition of fat thereby predisposing to obesity and type 2 diabetes.
The patterns of growth that predispose to adult diseases are complex and the importance of distinguishing between early and late ‘catch‐up’ in growth needs to be stressed. ‘Catch‐up’ growth, during infancy, is beneficial while crossing of centiles in later childhood is associated with an increased disease risk. There is no evidence in the current literature supporting the view that accelerated growth during infancy in low birthweight babies would be harmful. On the contrary there is considerable evidence about the deleterious effects of childhood overweight and obesity. Therefore treatment and prevention of childhood obesity should be high health priorities especially in individuals with an increased disease risk because of early patterns of growth.
Acknowledgements
The study has been supported by Academy of Finland, British Heart Foundation, Finnish Heart Foundation, Finnish Diabetes Association, Finska Läkaresällskapet and Sohlberg Foundation.
References
- Barghava S.K., Sachdev H.S., Fall C.H.D., Osmond C., Lakshmy R., Barker D.J.P. et al. (2004) Relation of serial changes in childhood body‐mass index to impaired glucose tolerance in young adulthood. New England Journal of Medicine, 350, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J. (1998) Mothers, Babies and Health in Later Life, 2nd edn Churchill Livingstone: London. [Google Scholar]
- Barker D.J.P., Forsén T., Uutela A., Osmond C. & Eriksson J.G. (2001) Size at birth and resilience to effects of poor living conditions in adult life: longitudinal study. British Medical Journal, 323, 1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P., Martyn C.N., Osmond C., Hales C.N. & Fall C.H.D. (1993) Growth in utero and serum cholesterol concentrations in adult life. British Medical Journal, 307, 1524–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P., Osmond C., Winter P.D., Margetts B. & Simmonds S.J. (1989) Weight in infancy and death from ischaemic heart disease. Lancet, 2, 577–580. [DOI] [PubMed] [Google Scholar]
- Eriksson J.G. & Forsén T.J. (2002) Childhood growth and coronary heart disease in later life. Annals of Medicine, 34, 157–161. [PubMed] [Google Scholar]
- Eriksson J., Forsén T., Osmond C. & Barker D. (2003b) Obesity from cradle to grave. International Journal of Obesity and Related Metabolic Disorders, 27, 722–727. [DOI] [PubMed] [Google Scholar]
- Eriksson J.G., Forsén T., Tuomilehto J., Osmond C. & Barker D.J.P. (2001) Early growth and coronary heart disease in later life: longitudinal study. British Medical Journal, 322, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Forsén T., Tuomilehto J., Osmond C. & Barker D. (2002) Size at birth, fat free mass and resting metabolic rate in adult life. Hormone and Metabolic Research, 34, 72–76. [DOI] [PubMed] [Google Scholar]
- Eriksson J.G., Forsén T., Tuomilehto J., Osmond C. & Barker D.J.P. (2003a) Early adiposity rebound in childhood and risk of type 2 diabetes in adult life. Diabetologia, 46, 322–329. [DOI] [PubMed] [Google Scholar]
- Eriksson J.G., Forsén T., Tuomilehto J., Winter P.D., Osmond C. & Barker D.J.P. (1999) Catch‐up growth in childhood and death from coronary heart disease: longitudinal study. British Medical Journal, 318, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall C.H.D., Barker D.J.P., Osmond C., Winter P.D., Clark P.M.S. & Hales C.N. (1992) Relation of infant feeding to adult serum cholesterol concentration and death from ischaemic heart disease. British Medical Journal, 304, 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsén T., Eriksson J.G., Osmond C. & Barker D.J.P. (2004) The infant growth of boys who later develop coronary heart disease. Annals of Medicine, 36, 389–392. [DOI] [PubMed] [Google Scholar]
- Forsén T., Eriksson J.G., Tuomilehto J., Osmond C. & Barker D.J.P. (1999) Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. British Medical Journal, 319, 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsén T., Eriksson J., Tuomilehto J., Reunanen A., Osmond C. & Barker D. (2000) The fetal and childhood growth of persons who develop type 2 diabetes. Annals of Internal Medicine, 133, 176–182. [DOI] [PubMed] [Google Scholar]
- Forsén T., Eriksson J.G., Tuomilehto J., Teramo K., Osmond C. & Barker D.J.P. (1997) Mother's weight in pregnancy and coronary heart disease in Finnish men. British Medical Journal, 315, 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C.N., Barker D.J.P., Clark P.M.S., Cox L.J., Fall C., Osmond C. et al. (1991) Fetal and infant growth and impaired glucose tolerance at age 64. British Medical Journal, 303, 1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn H.S., Narayan K.M.V., Williamson D.F. & Valdez R. (2000) Relation of birth weight to lean and fat thigh tissue in young men. International Journal of Obesity and Related Metabolic Disorders, 24, 1–6. [DOI] [PubMed] [Google Scholar]
- Nathanielsz P.W. & Hanson M.A. (2003) The fetal dilemma: spare the brain and spoil the liver. Journal of Physiology, 548, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C., Barker D.J., Winter P.D., Fall C.H.D. & Simmonds S.J. (1993) Early growth and death from cardiovascular disease in women. British Medical Journal, 307, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich‐Edwards J.W., Colditz G.A., Stampfer M.J., Willett W.C., Gillman M.W., Hennekens C.H. et al. (1999) Birthweight and the risk for type 2 diabetes mellitus in adult women. Annals of Internal Medicine, 130, 278–284. [DOI] [PubMed] [Google Scholar]
- Rolland‐Cachera M.F., Deheeger M., Bellisle F., Sempe M., Guilloud‐Bataille M., Patois E. et al. (1984) Adiposity rebound in children: a simple indicator for predicting obesity. American Journal of Clinical Nutrition, 39, 129–135. [DOI] [PubMed] [Google Scholar]


