Abstract
Epidemiological observations of associations between early life nutrition and long‐term disease risk have prompted detailed experimental investigation of the biological basis of programming. Studies using rodent or large animal models have clearly established the biological plausibility of nutritional programming and are now yielding important information on underlying mechanisms. Nutritional interventions in pregnancy, including global food restriction, protein restriction, micronutrient restriction and excess fat feeding, determine a consistent cluster of disorders in the resulting offspring. The common association of such diverse nutritional disturbances with hypertension, glucose intolerance and adiposity suggests that a small number of simple common mechanisms are active in response to fetal nutrient imbalance. Studies of rodent models indicate that fetal undernutrition determines adult adiposity. It is unclear whether the increase in central adiposity is related to increased food intake or reduced energy expenditure, although evidence exists to suggest that both may act together. Rats subject to intrauterine protein restriction exhibit increased preference for high fat foods. Feeding of energy dense foods to rats that were undernourished in utero promotes a greater degree of obesity than is noted in animals subject to adequate nutrition in fetal life. There is evidence to suggest that programming of appetite may stem from remodelling of hypothalamic structures that control feeding and programming of the expression of genes involved in responses to orexogenic hormones. The early life programming of appetite and obesity is a complex phenomenon and our understanding of how maternal nutrition determines later energy balance is at a very early stage.
Keywords: obesity, programming, appetite, rat, undernutrition
The biological plausibility of programming
Associations between growth in fetal life or infancy and later cardiovascular disease and type 2 diabetes have been recognized since the late 1980s (Barker, 2003). These epidemiological studies have been the subject of considerable controversy, but have withstood robust criticism (Huxley et al., 2002), finally emerging as the Developmental Origins of Health and Disease (DOHAD) hypothesis (Gluckman & Hanson, 2004). One of the main problems for epidemiologists working in this area has been to demonstrate the biological plausibility of programming as a phenomenon, as the ideal prospective cohort studies that would provide detailed nutritional information spanning the entire periconceptual and early postnatal periods with follow‐up of babies through to middle age and beyond, either do not exist or are too lengthy and time‐consuming to attempt. The development of relevant animal models of nutritional programming has therefore provided essential support for the DOHAD paradigm (Bertram & Hanson, 2001; Langley‐Evans, 2001).
A very broad range of animal models have successfully demonstrated that exposure of the developing fetus to undernutrition has long‐term effects on health and physiological functions (Langley‐Evans, 2004). Early attempts to model the DOHAD hypothesis focused on the epidemiological association between disease and birth weight and sought to induce intrauterine growth retardation in rats or guinea pigs by either uterine ligation or severe restriction of food intake (also termed global nutrient restriction) (Persson & Jansson, 1992; Woodall et al., 1996). Other models have considered the impact of variation in macronutrient intake, with low (Langley‐Evans, 2004) or high protein (Daenzer et al., 2002) diets and high fat (Khan et al., 2003) diets fed to pregnant rats or mice. A third group of models have investigated the effects of gestational restriction of micronutrient intake, with a focus on iron (Gambling et al., 2003), zinc (Beach et al., 1982) and calcium (Bergel & Belizan, 2002) deficiency in rodents. There are also a number of large animal models of fetal programming as the sheep has long been a preferred species for fetal physiologists (Symonds et al., 2004). Importantly many of these models show long‐term programming effects without any impact on fetal weight at birth, hence exposing one of the major limitations of epidemiology in this area.
Although the small and large animal models utilize a diverse set of nutritional insults in utero, the range of phenotypic endpoints is remarkably consistent and narrow. Hypertension, glucose intolerance, insulin resistance and obesity are reported effects for most of the models (Langley‐Evans, 2004). The occurrence of such a consistent cluster of effects following diverse insults suggests that undernutrition of any form may activate a relatively small number of simple common pathways. These are described in detail by other reviews (Langley‐Evans, 2001; Drake & Seckl, 2004; Gluckman & Hanson, 2004; Young et al., 2004), but the concept that undernutrition or, in particular an imbalance of nutrients in the diet, acts as a stressor resulting in disturbance of the normal materno‐fetal endocrine milieu is supported by a large and robust body of literature (Langley‐Evans et al., 1996a; Drake & Seckl, 2004).
Nutritional programming of obesity
Associations between fetal growth, maternal nutrition and later obesity in humans have not been clearly demonstrated. Whilst data from the Dutch Hunger Winter of 1944/45 indicate that exposure to famine in early gestation appeared to programme obesity in later life (Ravelli et al., 1999), data relating adult obesity to birth anthropometry generally show that there is a U‐shaped relationship, with both low and high birth weights predicting later adiposity (Martorell et al., 2001). The early postnatal diet is perhaps a more critical determinant of later obesity with many studies now suggesting that infants who are breastfed are less likely to become obese than those who are exclusively formula fed (von Kries et al., 1999). The study of Bergmann et al. (2003) for example, indicates that formula feeding results in an earlier adiposity rebound and a near trebling of the prevalence of obesity at age 6. A lot of the current emphasis of human research in this area is upon the interaction between prenatal and postnatal factors, as catch‐up growth following fetal growth retardation appears to be a strong risk factor for obesity (Eriksson et al., 2003).
Animal studies demonstrating prenatal programming of adiposity predate the Barker Hypothesis. Jones et al. (1983) modelled the Dutch Hunger Winter findings by feeding pregnant rats 50% of ad libitum intake for the first 2 weeks of pregnancy. This manipulation had no effect on adiposity in the offspring but when these rats were fed a high fat diet, those subject to prenatal undernutrition were found to have more body fat than control animals. Anguita et al. (1993) used the same protocol and showed increased carcass fat in the female offspring at 6 weeks of age, without any need for high fat feeding to induce obesity. Obesity in rodents can also be programmed by prenatal exposure to low protein diets (Bellinger et al., 2003), and to high protein diets (Daenzer et al., 2002), supporting the hypothesis that any intrauterine disturbance of nutrient balance may induce common metabolic responses.
Exploration of the interaction between prenatal and postnatal dietary factors is proving to be a fruitful approach for the elucidation of mechanisms through which obesity may be programmed in animals. There are a number of candidate mechanisms for which supporting evidence has been published. Daenzer et al. (2002) demonstrated that fetal exposure to high protein (40% by weight) diets reduced total energy expenditure, with thermogenesis or physical activity expenditure components being specifically affected. Variation in fibre type in skeletal muscle may also be important in determining energy metabolism, and this may be subject to intrauterine influences (Brameld et al., 2003). Most animal models, however, implicate changes in the central control of appetite and feeding behaviour as the key step towards obesity programmed by nutrition in early life.
Fetal programming of appetite, feeding behaviour and energy balance
Studies at the human population level strongly implicate nutrition in the early postnatal period as a factor determining lifetime risk of obesity. Animal models, as described above, generally only consider this period as a secondary challenge following prenatal insult. Plagemann et al. (1992), however, have developed a model of overfeeding in rats in which reduction of litter size in the postnatal period promotes increased suckling and energy intake. In the long term these offspring become hyperphagic, obese and develop insulin resistance and other endocrine disorders. In this model it is clear that there is remodelling of key hypothalamic structures and in particular an over‐expression of galanin, an orexogenic peptide known to stimulate intake of fats (Plagemann et al., 1999).
Hyperphagia is also a consequence of prenatal nutritional disturbance. Vickers et al. (2000) have shown that subjecting pregnant rats to severe food restriction (feeding only 30% of ad libitum intake) promotes profound intrauterine growth retardation in their offspring. These growth‐retarded pups grow up to be hyperphagic and when provided with a hypercaloric diet from weaning, develop pronounced central adiposity.
Recent work in our laboratory has also shown effects of the prenatal diet upon long‐term appetite and feeding behaviours. Exposure of the rat fetus to a low protein diet in utero has no gross effects on fetal growth (Langley‐Evans et al., 1996b), but from weaning such animals exhibit hypertension (Langley & Jackson, 1994) and altered glucose handling (Langley et al., 1994). As mature animals the low protein exposed animals begin to deposit significantly more abdominal fat, although the effect is not large and is gender‐specific (Bellinger et al., 2003). The lack of gross effect may be as a result the type of diet fed to laboratory rats. Standard rat and mouse chow diets have been formulated to prevent obesity and have a low fat content. Feeding a hypercaloric, high fat diet, as in the Vickers study may be necessary to elicit frank obesity. When fed this antiobesogenic chow diet, rats exposed to low protein diets in utero have reduced food intake (Bellinger et al., 2004). However, if offered a choice of high fat, high carbohydrate and high protein foodstuffs they have an increased appetite for fat, which promotes an elevation of energy intake in females, but not in males (Fig. 1) (Bellinger et al., 2004).
Figure 1.
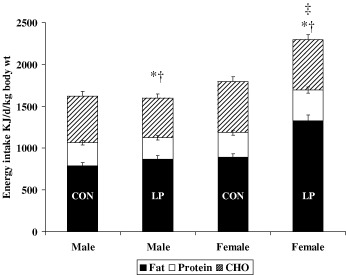
Energy intake in 12‐week‐old animals exposed to a low protein diet in utero. Rats were fed a control (18% casein) or a low protein (9% casein) diet throughout pregnancy. At delivery all rats were fed the same standard diet. At 12 weeks of age the offspring were allowed to self‐select their diet from a choice of high fat, high protein or high carbohydrate food. Data show mean energy intake from different macronutrient sources ± SEM. *indicates fat‐derived energy intake differs from control (P < 0.05), †indicates CHO‐derived energy intake differs from control (P < 0.05). ‡indicates overall energy intake differs from control animals (P < 0.05). CON, control; LP, low protein diet in utero.
The feeding trends elicited by offering rats a self‐selection diet depend on timing. Critical phases appear to lie in the period of fetal development and postnatally. From a postnatal perspective, we note that providing the self‐selection protocol to maternal low protein diet exposed rats at weaning enhances their fat preference, whereas if its introduction is delayed until 6 months postweaning the effect is absent (Bellinger et al., 2004). This suggests that a critical window for programming a fat preference, and presumably obesity, lies in the adolescent phase of life and just into young adulthood. Experiments in which maternal undernutrition is targeted at specific periods in early, mid‐ or late gestation in the rat indicate that these have different effects on appetite to undernutrition throughout pregnancy (Bellinger & Langley‐Evans, 2005a). Studies of this nature may be useful in determining how hyperphagia and a preference for fat become established.
Molecular basis of appetite programming
The common occurrence of a persistent hyperphagic state in animals exposed to either fetal undernutrition or early postnatal overfeeding suggests that less than optimal nutrition at critical phases of development may promote adaptive responses that modify the structures of key hypothalamic nuclei responsible for appetite control. Gluckman & Hanson (2004) suggest that the developing organism mounts a predictive adaptive response that anticipates the long‐term environment and provides a survival advantage. The balance of evidence however, is more consistent with the hypothesis that programming is a product of disrupted development. For example, Bennis‐Taleb et al. (1999) have shown that exposure of the rat fetus to a low protein diet modifies vascularization of the cerebral cortex. Plageman et al. (2000) have extended this work and demonstrated that the early nutritional environment essentially hard‐wires the brain to favour a hyperphagic state. The offspring of rats fed low protein diets throughout gestation and lactation showed differences in whole brain volume and volume of the paraventricular and ventromedial nuclei of the hypothalamus (Plagemann et al., 2000). These two regions also showed increases in neuronal density, but with fewer neurones staining for key peptides involved in appetite control, such as neuropeptides Y and cholecystokinin.
Structural adaptations within the brain following on from early insult may therefore permanently predispose the animal to increased appetite. We propose that this appetite may also be rendered selective for particular nutrients or sources of energy through changes in the profile of neuropeptides and their receptors that are expressed in hypothalamic centres. We have found evidence that expression of these proteins may be programmed in utero (Bellinger & Langley‐Evans, 2005b). Gene microarray studies indicate that the expression of a relatively narrow profile of genes in the hypothalamus is programmed by fetal exposure to a maternal low protein diet (Fig. 2). Prominent among those genes with altered expression in the hypothalamus of rats subject to a brief period of protein restriction in early gestation are those encoding peptides that control appetite, including the galanin receptor subtype 2. This provides an interesting parallel to the overfeeding model of 1999, 1992).
Figure 2.
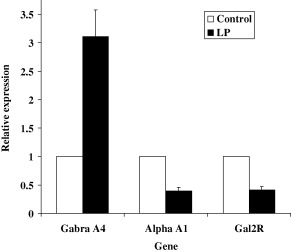
Hypothalamic expression of genes involved in appetite regulation. Expression of 10 000 genes and open reading frames was determined by microarray analysis using pooled RNA samples from n = 6 rats exposed to control diet (18% casein) in utero or n = 6 low protein diet (9% casein) for the first week of fetal life (0–7 days gestation). Data show relative expression in low protein diet exposed offspring at 12 weeks of age. Microarray analysis showed that only 19 genes were up‐regulated and 24 genes were down‐regulated by intrauterine undernutrition (taking a twofold change in expression as the chief criterion for identification as up‐ or down‐regulated). Gabra A4, gamma‐aminobutyric acid receptor subunit 4; Alpha‐A, alpha‐adrenergic receptor A1; Gal2R, galanin receptor subtype 2.
In addition to hard‐wiring of the brain in a manner that determines expression of orexogenic hormones and receptors, under‐ or overnutrition during early development may impact on other aspects of behaviour. Our microarray studies also suggest that there are changes in hypothalamic expression of taste and pheremone receptors. These may in part determine perception of palatability of foods in a manner that favours high fat feeding, but the latter are also important in determining social and sexual behaviour in rats. Vickers et al. (2003) have noted that prenatal undernutrition decreases adult levels of physical activity in rats. This will clearly impact upon energy expenditure levels and hence obesity, and indeed when prenatal undernutrition is followed by a hypercaloric diet from weaning the reduction in activity level is exacerbated. In rodents at least, behaviour is strongly determined by the central nervous system and in particular the hippocampus. If the hypothalamus is hard‐wired by the prevailing environment during development it is reasonable to expect that other brain regions could be similarly affected.
Rodents are of course not humans and the observations of altered appetite and behaviour following early nutritional insults in rats are not directly applicable to human health and disease. However, the general concept that the developing brain may be vulnerable to such insults is likely to be applicable to all mammalian species. Further work in this area of hypothalamic programming is likely to yield new approaches to our understanding of the aetiology of obesity in children.
Acknowledgements
The Biotechnology and Biological Research Council and the British Heart Foundation fund the Center for Reproduction and Early Life at the School of Biosciences.
References
- Anguita R.M., Sigulem D.M. & Sawaya A.L. (1993) Intrauterine food restriction is associated with obesity in young rats. Journal of Nutrition, 123, 1421–1428. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P. (2003) The developmental origins of adult disease. European Journal of Epidemiology, 18, 733–736. [DOI] [PubMed] [Google Scholar]
- Beach R.S., Gershwin M.E. & Hurley L.S. (1982) Gestational zinc deprivation in mice: persistence of immunodeficiency for three generations. Science, 218, 469–471. [DOI] [PubMed] [Google Scholar]
- Bellinger L. & Langley‐Evans S.C. (2005a) Programming of appetite and adiposity in male rats is dependent on timing of prenatal nutritional insult. Proceedings of the Nutrition Society (in press).
- Bellinger L. & Langley‐Evans S.C. (2005b) Prenatal exposure to a low protein diet and programming of fat metabolism. Proceedings of the Nutrition Society (in press).
- Bellinger L., Lilley C. & Langley‐Evans S.C. (2003) Prenatal exposure to a low protein diet programmes a preference for high fat foods in the rat. Pediatric Research, 53, 603. [DOI] [PubMed] [Google Scholar]
- Bellinger L., Lilley C. & Langley‐Evans S.C. (2004) Prenatal exposure to a maternal low protein diet programmes a preference for high fat foods in the young adult rat. British Journal of Nutrition, 92, 513–520. [DOI] [PubMed] [Google Scholar]
- Bennis‐Taleb N., Remacle C., Hoet J.J. & Reusens B. (1999) A low protein isocaloric diet during gestation affects brain development and alters permanently cerebral cortex blood vessels in rat offspring. Journal of Nutrition, 129, 1613–1619. [DOI] [PubMed] [Google Scholar]
- Bergel E. & Belizan J.M. (2002) A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. British Journal of Obstetrics and Gynaecology, 109, 540–545. [PubMed] [Google Scholar]
- Bergmann K.E., Bergmann R.L., Von Kries R., Bohm O., Richter R., Dudenhausen J.W. et al. (2003) Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast‐feeding. International Journal of Obesity and Related Metabolic Disorders, 27, 162–172. [DOI] [PubMed] [Google Scholar]
- Bertram C.E. & Hanson M.A. (2001) Animal models and programming of the metabolic syndrome. British Medical Bulletin, 60, 103–121. [DOI] [PubMed] [Google Scholar]
- Brameld J.M., Fahey A.J., Langley‐Evans S.C. & Buttery P.J. (2003) Nutritional and hormonal control of muscle growth and fat deposition. Archives of Animal Breed, 46, 143–156. [Google Scholar]
- Daenzer M., Ortmann S., Klaus S. & Metges C.C. (2002) Prenatal high protein exposure decreases energy expenditure and increases adiposity in young rats. Journal of Nutrition, 132, 142–144. [DOI] [PubMed] [Google Scholar]
- Drake A. & Seckl J.R. (2004) Impact of intrauterine exposure to glucocorticoids upon fetal development and adult pathophysiology In: Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition (ed. Langley‐Evans SC.), pp 381–418 . CABI: Wallingford, UK. [Google Scholar]
- Eriksson J.G., Forsen T.J., Osmond C. & Barker D.J. (2003) Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care, 26, 3006–3010. [DOI] [PubMed] [Google Scholar]
- Gambling L., Dunford S., Wallace D.I., Zuur G., Solanky N., Srai S.K. et al. (2003) Iron deficiency during pregnancy affects postnatal blood pressure in the rat. Journal of Physiology, 552, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D. & Hanson M.A. (2004) The developmental origins of the metabolic syndrome. Trends in Endocrinology and Metabolism, 15, 183–187. [DOI] [PubMed] [Google Scholar]
- Huxley R., Neil A. & Collins R. (2002) Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet, 360, 659–665. [DOI] [PubMed] [Google Scholar]
- Jones A.P., Simson E.L. & Friedman M.I. (1983) Gestational undernutrition and the development of obesity in rats. Journal of Nutrition, 114, 1482–1484. [DOI] [PubMed] [Google Scholar]
- Khan I.Y., Taylor P.D., Dekou V., Seed P.T., Lakasing L., Graham D. et al. (2003) Gender‐linked hypertension in offspring of lard‐fed pregnant rats. Hypertension, 41, 168–175. [DOI] [PubMed] [Google Scholar]
- Von Kries R., Koletzko B., Sauerwald T., Von Mutius E., Barnert D., Grunert V. et al. (1999) Breast feeding and obesity: cross sectional study. British Medical Journal, 319, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley S.C., Browne R.F. & Jackson A.A. (1994) Altered glucose tolerance in rats exposed to maternal low protein diets in utero . CBP, 109A, 223–229. [DOI] [PubMed] [Google Scholar]
- Langley S.C. & Jackson A.A. (1994) Increased systolic blood pressure in adult rats, induced by fetal exposure to maternal low protein diets. Clinical Science, 86, 217–222. [DOI] [PubMed] [Google Scholar]
- Langley‐Evans S.C. (2001) Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proceedings of the Nutrition Society, 60, 505–513. [DOI] [PubMed] [Google Scholar]
- Langley‐Evans S.C. (2004) Fetal programming of adult disease: an overview In: Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition (ed. Langley‐Evans SC.), pp 1–20 . CABI: Wallingford, UK . [Google Scholar]
- Langley‐Evans S.C., Gardner D.S. & Jackson A.A. (1996b) Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. Journal of Reproduction Fertile, 106, 307–312. [DOI] [PubMed] [Google Scholar]
- Langley‐Evans S.C., Phillips G.J., Benediktsson R., Gardner D.S., Edwards C.R.W., Jackson A.A. et al. (1996a) Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta, 17, 169–172. [DOI] [PubMed] [Google Scholar]
- Langley‐Evans S.C., Phillips G.J. & Jackson A.A. (1994) In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clinical Nutrition, 13, 319–324. [DOI] [PubMed] [Google Scholar]
- Martorell R., Stein A.D. & Schroeder D.G. (2001) Early nutrition and later adiposity. Journal of Nutrition, 131, 874S–880S. [DOI] [PubMed] [Google Scholar]
- Persson E. & Jansson T. (1992) Low birth weight is associated with elevated adult blood pressure in the chronically catheterized guinea‐pig. Acta Physiologica Scandinavica, 145, 195–196. [DOI] [PubMed] [Google Scholar]
- Plagemann A., Harder T., Rake A., Melchior K., Rohde W. & Dorner G. (1999) Increased number of galanin‐neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Brain Research, 818, 160–163. [DOI] [PubMed] [Google Scholar]
- Plagemann A., Harder T., Rake A., Melchior K., Rohde W. & Dorner G. (2000) Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. Journal of Nutrition, 130, 2582–2589. [DOI] [PubMed] [Google Scholar]
- Plagemann A., Heidrich I., Gotz F., Rohde W. & Dorner G. (1992) Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Experimental and Clinical Endocrinology, 99, 154–158. [DOI] [PubMed] [Google Scholar]
- Ravelli A.C., Van Der Meulen J.H., Osmond C., Barker D.J. & Bleker O.P. (1999) Obesity at the age of 50 y in men and women exposed to famine prenatally. American Journal of Clinical Nutrition, 70, 811–816. [DOI] [PubMed] [Google Scholar]
- Symonds M.E., Gardner D.S., Pearce S. & Stephenson T. (2004) Endocrine responses to fetal undernutrition: the growth hormone (GH): insulin‐like growth factor (IGF) axis In: Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition (ed.Langley‐Evans SC.), pp 353–380 . CABI: Wallingford, UK . [Google Scholar]
- Vickers M.H., Breier B.H., Cutfield W.S., Hofman P.L. & Gluckman P.D. (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. American Journal of Physiology. Endocrinology and Metabolism, 279, E83–E87. [DOI] [PubMed] [Google Scholar]
- Vickers M.H., Breier B.H., McCarthy D. & Gluckman P.D. (2003) Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 285, R271–R273. [DOI] [PubMed] [Google Scholar]
- Woodall S.M., Johnston B.M., Breier B.H. & Gluckman P.D. (1996) Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatrics Research, 40, 438–443. [DOI] [PubMed] [Google Scholar]
- Young L.E., Rees W.D. & Sinclair K.D. (2004) Programming in the pre‐implantation embryo In: Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition (ed. Langley‐Evans SC.), pp 333–352 . CABI: Wallingford, UK. [Google Scholar]


