Abstract
Infant and childhood growth result from and reflect a range of influences in pre‐ and postnatal life. These include nutrition, burden of infection and the psycho‐social environment. Nutrition in young children is dependent on individual level factors such as fetal experience, infant feeding and weaning practices, and on societal factors such as education of women and economic conditions. The relationship of early postnatal growth to adult disease may be indicative or causal, and may reveal both biological and sociological processes. Although non‐insulin‐dependent diabetes mellitus (NIDDM) and obesity are risk factors for ischaemic heart disease, the relationships of these three conditions to infant growth differ. Poor infant growth has been associated with higher levels of NIDDM and ischaemic heart disease, but lower levels of adult obesity. Most research has been of populations living in developed countries at different stages of nutritional transition. However, differences in context are not simply limited to the stage of the nutritional transition. They also need to consider the nature of that transition and its social correlates, which may result in the clustering of aetiological influences such as increased body mass and poverty. The size of effect of the relationship of infant growth to adult disease is important not only to determine its relative aetiological importance but also for its potential for public health policy. Such policy also needs to consider the relationships of infant growth to a range of outcomes, both health and human capital, which are not the subject of this workshop.
Keywords: infancy, growth, chronic disease, public health
Introduction
This paper will give a public health overview of the relationship of early postnatal growth to some common degenerative diseases of adult life – cardiovascular disease, non‐insulin‐dependent diabetes mellitus (NIDDM) and obesity. It will highlight challenges in interpreting the current state of evidence and give an overview of what is known at present. It will illustrate some of these general issues with reference to specific studies. It will also discuss the application of this knowledge to public health in countries that are going through a nutritional transition.
The term ‘early growth’ covers a range of possibilities from conception to later childhood. This paper will concentrate on the first 2 years of postnatal life, which will be referred to as infancy. Although any cut‐off point in the continuum of human growth is arbitrary, infancy is a concept with utility for public health. Firstly, it is clearly recognizable, beginning at birth and ending after a fixed time period. Secondly, infant growth has particular properties that distinguish it from growth in childhood – the growth curve is non‐linear and crossing of the centiles, particularly in early infancy, is the norm (Karlberg et al., 1994; Cole, 2004). Thirdly, some of the prime influences on postnatal growth in developing countries, such as feeding practice and the burden of infection, show substantial differences between infancy and childhood.
The World Health Organization (WHO) describes public health as a social and political concept. It is based in ‘a comprehensive understanding of the ways in which lifestyles and living conditions determine health status, and a recognition of the need to mobilise resources and make sound investments in policies, programmes and services which create, maintain and protect health by supporting healthy lifestyles and creating supportive environments for health’ (World Health Organization, 1998). From this, it follows that a public health perspective on evidence linking early growth and adult disease is focused on effecting change rather than dissection of the aetiology. Nonetheless, understanding of the mechanisms underlying relationships between early growth and adult disease would be helpful, though arguably not essential in developing policies or programmes to improve public health.
Unfortunately, detailed knowledge of these underlying mechanisms is not yet developed. Infant (and childhood) growth result from and reflect a range of influences in pre‐ and postnatal life. At a very broad level, these include nutrition, burden of infection and the psycho‐social environment. Just examining one of these influences in more detail is revealing. Nutrition in young children is dependent on individual level factors such as fetal experience, choice of infant feeding and weaning practices, and on societal factors such as the education of women and economic conditions. An obvious example of the complex nature of nutritional influences on infant growth is breastfeeding. The growth of breast‐fed infants is different to that of infants fed on other forms of milk, with some evidence that breastfeeding may protect against later obesity (Dietz, 2001; World Health Organization, 2004) [cross reference to Hediger paper]. One hypothesis to explain this is that the process of breastfeeding incorporates a balanced ‘supply and demand’ system, such that when a baby has received sufficient breast milk, both feeding behaviour of the baby and the nature of the breast milk change in a way that prevents ‘overfeeding’ (Daly & Hartmann, 1995). However, the mother may also exhibit changes in her behaviour in relation to different signals. Though breastfeeding evolved to provide sufficient nourishment to most young babies, the belief that they do not have enough milk to nourish their baby is a commonly cited reason for abandoning breastfeeding (Van Esterik & Butler, 2004). In addition, mothers may curtail breastfeeding (or choose alternative methods) because of family or societal pressures, for example, to return to work or to fulfil other domestic responsibilities. Thus, breastfeeding may reflect biological, behavioural or social processes, each or all of which may influence infant growth.
Interpretation of the current research evidence on infant growth and adult disease for public health action needs to address three challenges. The first is whether infant growth is on the causal pathway or acts only as an indicator. The example above illustrates the extent of this challenge. Until there is better information on both the determinants and consequences of infant growth, the causal nature of the relationships described in this paper and elsewhere will continue to be debated hotly (Kramer, 2000). Should this gap in knowledge be filled before public health action is justified? One of the classical methods for inferring causality is by observing the effect of experimental manipulation of the exposure variable (in this case, infant growth) on an outcome (adult disease). Animal models might be used, but these will always be limited in their application to humans. Experimental intervention in infants would have to be justified by a systematic collation of relevant evidence, including explicit consideration of the possibility for harm (Campbell et al., 2000). Thus, bringing the observational evidence together is an essential prerequisite for public health action. To some extent, arguments that require inference of causality before testing of public health action is undertaken are circular, because they cannot be resolved without recourse to intervention studies.
The next challenge relates to the assessment of infant size. Firstly, there is debate as to whether infant size or growth is the critical variable in predicting adult disease – in other words, is it the accumulation of size that is associated with adult health or the growth ‘journey’ by which this size is accrued at different times and at different speeds. Although birthweight and infant weight are closely correlated, crossing of centiles is common during infancy, and both the description and meaning of ‘catch‐up growth’ are matters of debate (Cole, 2004). Secondly, growth or size is often measured in infancy by weight or weight gain. These are summary measures of an infant's experience, yet in many studies, are the most complete or the only variables available to describe infant growth.
Finally, there are challenges that arise from the nature of the epidemiological evidence in this field. With rare exceptions, many of the studies in this field have been conducted in developed countries. Some studies of birthweight in relation to adult disease have been published from developing countries (Stein et al., 1996; Mi et al., 2000), but few describe associations with infant growth. Assessing the relationship between a putative exposure (infant growth) and a disease with a long latent period (cardiovascular disease or NIDDM) is challenging logistically. Most of the literature relates to historical cohort studies and were conducted on people whose infancy was experienced decades ago. Their lives would have been different in many ways from today's infants’. With relevance to growth, they are likely to have had a different experience of breastfeeding and weaning, and if bottle‐fed, the composition of their milk would have been different. They would have had a higher burden of infection than those born now in developed countries and a different burden to those born in developing countries. They would have been born into larger families and the educational attainment and relative income of their mothers is likely to have been lower than in developed countries now. Prospective cohort studies are rare and none yet exists which can address all adult outcomes of interest although some studies will soon have that capacity (Ferri et al., 2003). Though they will have the advantages of prospective data collection they will still be based on individuals whose infancies were unlike those of babies born today. Intervention studies in this field are extremely unusual.
Whilst the challenges described are daunting, scientists must try and address them both in assessing the current state of knowledge and in researching the knowledge gaps. The next section of this paper gives an overview of the studies relating infant growth to adult disease, and illustrates their interpretation with specific examples.
Although the focus of the workshop from which this paper derives was cardiovascular disease, obesity and NIDDM, a public health perspective must take a holistic view of human health. So in considering the relation of infant growth to adult health, the full range of adult disease must be addressed. One systematic public health approach to this would be to consider those diseases which result in the highest burden to the population. The Global Burden of Disease Study has estimated these by calculating, for different groups of countries, the disability adjusted life years (DALYs) lost as a consequence of various diseases or risks (Murray & Lopez, 1997). Table 1 sets out the 12 conditions that, between them, constitute the top 10 causes of DALYs lost for men and women. The number besides each condition gives the number of papers which describe the association of each condition with infant growth. These numbers are based on a rigorous search strategy of a number of literature databases as part of a systematic review and result from screening over 60 000 abstracts (J. Baird and D. Fisher, personal communication, August 2004). Only ischaemic heart disease and NIDDM have sufficient numbers of studies to be able to make general (rather than study‐specific) comments on the associations observed. Furthermore, this literature is dependent on three cohort studies, two British (MRC National Survey of Health & Development 1946 and Hertfordshire cohorts) and one Finnish (Helsinki), with few studies from developing countries. Thus, a first conclusion of an overview is that the literature is limited in size and geographical setting and is unlikely to expand much in the foreseeable future, because of the difficulty of conducting cohort studies. This makes judicious interpretation essential.
Table 1.
Papers reporting the association of infant growth or size with disease or conditions of adulthood*
| Ischaemic heart disease | 5 |
| Cerebrovascular disease | 1 |
| Major depression | 1 |
| Lung cancer | 2 |
| Road traffic accidents | 0 |
| Alcohol use | 0 |
| Dementia | 0 |
| Osteoarthritis | 1 |
| Chronic obstructive pulmonary disease | 0 |
| Self‐injury | 1 |
| Breast cancer | 1 |
| NIDDM | 3 |
NIDDM, non‐insulin‐dependent diabetes mellitus; Total number of papers is 13; *The top 10 causes of disability adjusted life years lost for men and women based on Global Burden of Disease Study (Murray & Lopez, 1997).
The available research shows consistent associations between low size in infancy (weight in the Hertfordshire cohorts and weight, body mass index (BMI) and height in the Helsinki study) and increased risk of ischaemic heart disease in men (Barker et al., 1989; Fall et al., 1995; Eriksson et al., 2001). The two studies in women did not demonstrate statistically significant associations, though results were in the same direction as in men (Osmond et al., 1993; Forsen et al., 2004). Some of these analyses also either demonstrated or implied an inverse relation between infant growth and ischaemic heart disease. Figure 1 illustrates data from the Helsinki cohort. The average growth of the cohort is set as a standard deviation score of zero, and so is expressed as a horizontal (dashed) line. The average growth (height, weight and BMI) of boys who went on to suffer from ischaemic heart disease in later life is also expressed as a standard deviation score. Compared to the cohort, these boys are small at birth and grow poorly in the first 1 to 2 years of life. Thereafter their growth is somewhat faster than the cohort overall, so that by the time they are 12 years old, they are of similar BMI to the cohort overall, though still shorter. Importantly, in this study and others, the relation between low infant size/growth and increased risk of ischaemic heart disease was independent of birthweight. Whilst it has been argued that fetal life is a critical period for the early origins of cardiovascular disease, this suggests that early postnatal life may also be critical. This is important for the design of public health interventions as well as for the understanding of aetiology. Interventions which can be applied postnatally to an infant will be of a different nature and may be more feasible than those which are designed to act in pregnancy, and therefore must involve the mother as well as the fetus.
Figure 1.
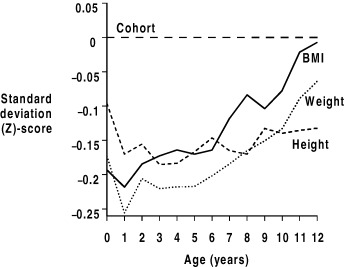
Growth of 357 boys who later developed ischaemic heart disease in a cohort of 4630 boys born in Helsinki, Finland. (Amended and reproduced based on Eriksson et al., 2001, with permission from the BMJ Publishing Group.)
As noted above, boys born in Helsinki in the early part of the 20th century are likely to be different in many ways compared with boys growing up in, say, rural India today. But comparison of their patterns of growth may, at least, indicate the potential risks of later disease to which they are being exposed.
Although diabetes and obesity are risk factors for ischaemic heart disease, the relationships of these three conditions to infant growth differ. The Helsinki studies, which are discussed in detail in the paper by Eriksson (cross reference) allow a direct comparison between the three outcomes. As noted above, ischaemic heart disease is associated with small size at birth and during infancy, followed by greater than average growth during childhood. The men and women in the Helsinki cohort who went on to develop NIDDM were smaller than average at birth and their growth in infancy was somewhat less than average, though this was not statistically significant (Eriksson et al., 2003a). Thereafter they had accelerated growth during childhood. Men and women who developed adult obesity were above average size at birth and had higher BMI during infancy (Eriksson et al., 2003b).
There are few studies of infant growth and diabetes with which to compare the Helsinki results. The Hertfordshire cohort study showed that low weight at 1 year was associated with increased risk of NIDDM in men (Hales et al., 1991). In a rare prospective cohort study from a developing country, India, impaired glucose tolerance and NIDDM in early adulthood was associated with rapid gain in body mass during childhood (Fig. 2). Though there was some suggestion that glucose intolerance was also associated with poor growth and low BMI in infancy, this was not statistically significant (Bhargava et al., 2004).
Figure 2.
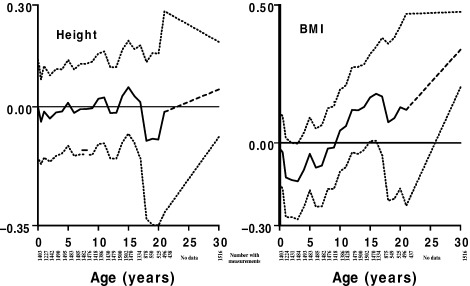
Childhood growth of men and women who later developed impaired glucose tolerance or diabetes New Delhi, age 26–32 years (N = 1518). (Bhargava et al., 2004, copyright © 2004 Massachusetts Medical Society. All rights reserved.)
By contrast, many studies have shown a positive relationship between size in infancy, particularly weight, and increased rates of teenage and adult obesity. In addition, this relationship is not dependent on the Helsinki or Hertfordshire cohorts, but derives from a variety of cohort studies around the world. For example, in a study of over 1000 teenagers in Brazil, the odds of having a BMI above 85th percentile was 3.5 times greater in those who had been more than one standard deviation score above the mean for weight‐for‐height at 2 years (Monteiro et al., 2003).
In summary, small size or poor growth during infancy is associated with increased risk of ischaemic heart disease and decreased risk of obesity, and the association with NIDDM is inconclusive. Thus, the known relationships between ischaemic heart disease, NIDDM and obesity seem unlikely to result from common aetiological origins in infant growth. However, the opposing time trends of ischaemic heart disease and obesity in developed countries may be explained in part by trends towards greater size in infancy.
The possibility that trends in infant size may be associated with disbenefits in adult life also needs to be examined in relation to non‐cardiovascular outcomes. The Hertfordshire cohort demonstrated an increase in mortality from ovarian cancer in women who were heaviest at 1 year (Barker et al., 1995). Recently, the 1946 cohort study has demonstrated a link between greater size in early childhood and breast cancer, though there was no association with infant size (De Stavola et al., 2004). This gives further evidence that relationships between early size and adult disease may be specific both to the period of early life and to the disease outcome. This is challenging for public health action, because it suggests that there will be a balance of benefits and disbenefits associated with particular patterns of growth. Attempts to alter patterns of early growth must assess that balance with care.
Whether or not to act on the limited evidence available is a matter of judgement. Such judgement might include the strength of the evidence and the likelihood of the associations being causal. A further important consideration is the potential size of effect. Data on which to calculate this is severely limited. The only published information in relation to early growth and ischaemic heart disease comes from the Helsinki cohort of men, and is illustrated in Fig. 3 (Barker et al., 2002). The cumulative incidence (as a percentage) of ischaemic heart disease is shown on the vertical axis and thirds of the distribution of BMI at 1 year on the horizontal axis. The open bars represent men whose BMI decreased between 3 and 11 years and the filled (grey) bars those whose body mass increased from 3 to 11 years. These increases were expressed as standard deviation scores, so are relative measures. If each man had been in the highest third of BMI at 1 years and had lowered his standard deviation score for BMI between 3 and 11 years the incidence of ischaemic heart disease would have been lowered by 40%. If causal, this is a considerable size of effect. However, the required changes in the population distribution of both body mass in infancy and change in body mass between 3 and 11 years are also considerable. Furthermore, it is not clear how changes of this magnitude could be effected, particularly as they imply opposite growth patterns at different ages (increase in average body mass in infancy and decrease in childhood). So, while changes in early growth have the potential to create reductions in adult disease (assuming the two are causally related), the means to achieve this potential are unclear.
Figure 3.
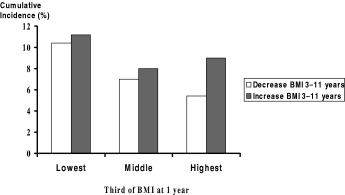
Cumulative incidence of ischaemic heart disease, by BMI at one year, and change in BMI at 3–11 years (3387 men born 1934–44, Helsinki, Finland).
Finally, infancy should not just be viewed as a training ground for adult life, with the sole aim of freedom from chronic degenerative disease. Policy makers as well as parents will want to assess the implications of changing patterns of infant growth for health in childhood, as well as later adult life. They will want to consider all aspects of health across the life course – from infancy to old age and from death and serious morbidity to aspects of human capital such as cognitive function and muscle strength. The United Nations Convention on the Rights of the Child states that children have the right to be consulted on matters of concern to them (UN Convention, 1999). As infants cannot express these views, those of their parents are salient. A recent review of lay perspectives of infant growth found that notions of what constituted healthy size and growth were dominated by the concept of ‘normality.’ Where growth differed from the constructed norm and a plausible explanation could not be found, growth became an important concern for parents. None of the studies reviewed sought opinions from parents about the acceptability or advisability of intervening to effect rate of growth (P. Lucas and H. Roberts, personal communication, 2004). This is a further gap in knowledge which needs to be addressed before intervention studies can be considered.
The research linking early growth to ischaemic heart disease, NIDDM and obesity offers the possibility of preventing some of the huge burden of disease that is associated with these disorders. However, it needs to be placed within a wider public health context which considers infants living in current societies around the world, and with their health across the whole of the life course at stake.
Acknowledgements
I am grateful to Janis Baird, David Fisher, Helen Roberts, Patricia Lucas and Jos Kleijan and my other colleagues at the Institute of Child Health, University College London, and the Medical Research Council Environmental Epidemiology Unit, Southampton, UK for their insights and discussions on this topic. Part of the work described in this paper was funded by the Medical Research Council, UK and the Department of Health for England. The views expressed in this paper are those of the author and not necessarily those of the Medical Research Council or the Department of Health.
References
- Barker D.J.P., Winter P.D., Osmond C., Margetts B. & Simmonds S.J. (1989) Weight in infancy and death from ischaemic heart disease. The Lancet, ii, 577–580. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P., Winter P.D., Osmond C., Phillips D.I.W. & Sultan H.Y. (1995) Weight gain in infancy and cancer of the ovary. The Lancet, 345, 1087–1088. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P., Eriksson J.G., Forsen T. & Osmond C. (2002) Fetal origins of adult disease, strength of effects and biological basis. International Journal of Epidemiology, 31, 1235–1239. [DOI] [PubMed] [Google Scholar]
- Bhargava S.K., Sachdev H.S., Fall C.H., Osmond C., Lakshmy R., Barker D.J. et al. (2004) Relation of serial changes in childhood body‐mass index to impaired glucose tolerance in young adulthood. The New England Journal of Medicine, 350, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M., Fitzpatrick R., Haines A., Kinmouth A.‐L., Sandercock P., Spiegelhalter D. & Tyrer P. (2000) Framework for design and evaluation of complex interventions to improve health. British Medical Journal, 321, 694–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T.J. (2004) Modeling postnatal exposures and their interactions with birth size. The Journal of Nutrition, 134, 201–204. [DOI] [PubMed] [Google Scholar]
- Daly S.E. & Hartmann P.E. (1995) Infant demand and milk supply. Part 1. Infant demand and milk production in lactating women. Journal of Human Lactation, 11, 21–26. [DOI] [PubMed] [Google Scholar]
- De Stavola B.L., Dos S.S.I., McCormack V., Hardy R.J., Kuh D.J. & Wadsworth M.E. (2004) Childhood growth and breast cancer. American Journal of Epidemiology, 159, 671–682. [DOI] [PubMed] [Google Scholar]
- Dietz W.H. (2001) Breastfeeding may help prevent childhood overweight. The Journal of the American Medicine Association, 285, 2506–2507. [DOI] [PubMed] [Google Scholar]
- Eriksson J.G., Forsen T., Tuomilehto J., Osmond C. & Barker D.J.P. (2001) Early growth and coronary heart disease in later life: longitudinal study, British Medical Journal, 322, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Forsen T., Osmond C. & Barker D. (2003a) Obesity from cradle to grave. International Journal of Obesity and Related Metabolic Disorders, 27, 722–727. [DOI] [PubMed] [Google Scholar]
- Eriksson J.G., Forsen T., Tuomilehto J., Osmond C. & Barker D.J. (2003b) Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia, 46, 190–194. [DOI] [PubMed] [Google Scholar]
- Fall C.H.D., Vijayakumar M., Barker D.J.P., Osmond C. & Duggleby S. (1995) Weight in infancy and prevalence of coronary heart disease in adult life. British Medical Journal, 310, 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri E., Bynner J. & Wadsworth M. (2003) Changing Britain, Changing Lives Three Generations at the Turn of the Century. Institute of Education: London. [Google Scholar]
- Forsen T., Osmond C., Eriksson J.G. & Barker D.J. (2004) Growth of girls who later develop coronary heart disease. Heart, 90, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C.N., Barker D.J.P., Clark P.M.S., Cox L.J., Fall C., Osmond C. et al. (1991) Fetal and infant growth and impaired glucose tolerance at age 64. British Medical Journal, 303, 1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg J., Jalil F., Lam B., Low L. & Yeung C.Y. (1994) Linear growth retardation in relation to the three phases of growth. European Journal of Clinical Nutrition, 48 (Suppl. 1), S25–S44. [PubMed] [Google Scholar]
- Kramer M.S. (2000) Invited commentary: association between restricted fetal growth and adult chronic disease: Is it causal? Is it important? American Journal of Epidemiology, 152, 605–608. [DOI] [PubMed] [Google Scholar]
- Mi J., Law C.M., Zhang K.‐L., Osmond C., Stein C. & Barker D. (2000) Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Annals of International Medicine, 132, 253–260. [DOI] [PubMed] [Google Scholar]
- Monteiro P.O., Victora C.G., Barros F.C. & Monteiro L.M. (2003) Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. International Journal of Obesity and Related Metabolic Disorders, 27, 1274–1282. [DOI] [PubMed] [Google Scholar]
- Murray C.J.L. & Lopez A.D. (1997) Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. The Lancet, 349, 1498–1504. [DOI] [PubMed] [Google Scholar]
- Osmond C., Barker D.J.P., Winter P.D., Fall C.H.D. & Simmonds S.J. (1993) Early growth and death from cardiovascular disease in women. British Medical Journal, 307, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C.E., Fall C.H.D., Kumaran K., Osmond C., Cox V. & Barker D.J.P. (1996) Fetal growth and coronary heart disease in South India, The Lancet, 348, 1269–1273. [DOI] [PubMed] [Google Scholar]
- UN Convention. (1999) United Nation Convention on the Rights of the Child. United Nations: Geneva. [Google Scholar]
- Van Esterik, P. & Butler, S. (2004) Breastfeeding and the well‐being of Families. Available at: http://www.waba.org.my/activitysheet/acsh5.htm
- World Health Organization. (1998) Health Promotion Glossary. World Health Organisation: Geneva. [Google Scholar]
- World Health Organization. (2004) Nutrition: Infant and Young Child. Exclusive Breastfeeding. Available at: http://www.who.int/child-adolescent-health/nutrition/infant_exclusive.htm


