Abstract
We previously reported that the trans-18:2 fatty acid trans-10, cis-12 conjugated linoleic acid (t10,c12-CLA) stimulates mammary gland development independent of estrogen and its receptor. Given the negative consequences of dietary trans-fatty acids on various aspects of human health, we sought to establish whether other trans-fatty acids could similarly induce ovary-independent mammary gland growth in mice. Prepubertal BALB/cJ mice were ovariectomized at 21 days of age then were fed diets enriched with cis-9, trans-11 CLA (c9,t11-CLA), or mixtures of trans-18:1 fatty acids supplied by partially hydrogenated sunflower, safflower, or linseed oil. The resultant mammary phenotype was evaluated 3 weeks later and compared to the growth response elicited by t10,c12-CLA, or the defined control diet. Whereas partially hydrogenated safflower oil increased mammary gland weight, none of the partially hydrogenated vegetable oils promoted mammary ductal growth. Similarly, the c9,t11-CLA supplemented diet was without effect on mammary development. Taken together, our data emphasize a unique effect of t10,c12-CLA in stimulating estrogen-independent mammary gland growth manifest as increased mammary ductal area and elongation that was not recapitulated by c9,t11-CLA or the partially hydrogenated vegetable oil diets.
Keywords: Mammary gland, Trans-fatty acids, Conjugated linoleic acid, Partially hydrogenated vegetable oil
Introduction
The level of trans-fatty acids in the modern food supply has increased dramatically over the last century [1]. Partially hydrogenated plant and marine oils have served as an inexpensive substitute for butterfat while improving the texture of processed foods, and increasing product shelf life [2]. Despite these many advantages, epidemiologic evidence has revealed that trans-fatty acids significantly elevate the risk of atherosclerosis [3], where a mere 2% increase in energy intake from trans-fatty acids increased risk for developing cardiovascular disease by 23% [4]. Trans-fatty acids also negatively affect serum lipids by elevating low density lipoprotein and lowering high density lipoprotein cholesterol [5], while the isomeric profile of dietary trans-fatty acids can differentially impact blood lipoprotein profiles [6]. Moreover, the results from multiple studies indicate that trans-fatty acids have a negative effect on systemic inflammation leading to increased levels of C-reactive protein, interleukin-6, and tumor necrosis factor-α [7], while also exacerbating insulin resistance in populations with comorbidities such as obesity [8].
The majority of dietary trans-fatty acids are trans-18:1 isomers produced during the partial hydrogenation of plant oils [9]. In some cases, trans-fatty acids can account for 60% of total fatty acids in partially hydrogenated vegetable oils (PHVO) [10]. An alternative source of dietary trans-fatty acids is fat from ruminant animals, where the level of trans-fatty acids in typical ruminant-derived foods is approximately 2–4% of total fatty acids [10]. The pre-dominant trans-fatty acid in ruminant-derived fats is trans-18:1, with smaller amounts of trans-18:2 cis-9, trans-11 conjugated linoleic acid (c9,t11-CLA) [10]. Minor amounts of the trans-18:2 fatty acid trans-10, cis-12 CLA (t10,c12- CLA) are present in ruminant-derived foods, whereas this isomer accounts for approximately half of the CLA in mixed CLA supplements marketed for weight loss [11].
Dietary fats have long been implicated in modifying development of the normal mammary gland [12–14] and its susceptibility to tumorigenesis [15]. Generally speaking, unsaturated fatty acids stimulate the growth of normal and transformed mammary epithelial cells in vitro [16] and in vivo [17], whereas saturated fatty acids restrict their growth [16]. Moreover, we recently identified that a diet supplemented with t10,c12-CLA stimulated the mammary glands of ovariectomized (OVX) prepubertal mice to undergo allometric growth, independent of any requirement for estrogen (E) and its receptor (ER) [17]. Our experiments further indicated a role for altered insulin/IGF-1 signaling in conjunction with concomitant metabolic dysregulation in mediating this response [17]. In addition to these findings, we and others have shown that dietary t10,c12-CLA promotes hyperinsulinemia [18], hepatic steatosis [19], inflammation [20], and lipoatrophy [21] while also stimulating precocious lobuloalveolar development in the mammary glands [22], and accelerating tumorigenesis in mice overexpressing ErbB2 [23].
Policies to minimize the consumption of trans-fatty acids have only been implemented in recent times [24], leaving in place questions about the long-standing impacts of their intake on human health and disease. Given parallels between t10,c12-CLA and other dietary trans-fatty acids, we sought to compare the ability of various mixtures of trans-fatty acid isomers to promote E-independent mammary growth. Our findings reveal that this response is specific to t10,c12-CLA, and does not occur in response to dietary c9,t11-CLA or mixtures of different trans-18:1 isomers derived from PHVO. These data further highlight the varying effects of diet on the developing mammary gland.
Materials and Methods
Animals
All experiments were conducted with the approval of the University of California, Davis Institutional Animal Care and Use Committee. BALB/cJ mice (Jackson Laboratory, Bar Harbor, ME, USA) were used in all experiments. Mice were housed under a 14:10 h light-dark cycle, and allowed ad libitum feed and water. Mice were bilaterally ovariectomized (OVX) at 21 days of age, prior to the onset of puberty. Mice were anesthetized with ketamine/xylazine (IP, 60 and 10 mg/kg, respectively) and received post-operative buprenorphine (SC, 0.05 mg/kg) analgesia. Mice were fed a control diet after OVX then were assigned to the experimental diets the following day.
Diets
The control (CON) and t10,c12-CLA diets used in Experiment 1 were as described [17]. The c9,t11-CLA diet used in Experiment 1 was the CON diet with 1% fat by weight as c9,t11-CLA (Table 1). All PHVO diets (Experiment 2) were formulated from an AIN-93G base diet and contained 15% total fat by weight, where 10% of the fat was soybean oil. The PHVO diets contained 5% fat as either partially hydrogenated sunflower (PH-SUN), partially hydrogenated safflower (PH-SAF), or partially hydrogenated linseed (PH-LIN) oil by weight. The control diet (TFA-CON) contained 5% stearic acid by weight. The c9,t11/t10,c12-CLA diet in Experiment 2 contained 2.3% stearic acid and 2.7% Clarinol-G80 that supplied approximately 1% 9,11 and 1% t10,c12-CLA by weight. The level of t10,c12-CLA in this diet was approximately equivalent to that used in the diet that contained t10,c12-CLA in Experiment 1.
Table 1.
Fatty acid composition (g/100 g fatty acids) of experimental diets (Experiment 1)
| Fatty acid | Diet |
||
|---|---|---|---|
| Control | c9,t11-CLA | t10,c12-CLA | |
| 16:0 | 10.98 | 10.18 | 10.49 |
| 18:0 | 3.81 | 3.63 | 3.79 |
| 18:1 c9 | 20.62 | 20.42 | 19.25 |
| 18:2 n6 | 54.15 | 48.25 | 48.36 |
| 18:3 n3 | 6.60 | 5.95 | 5.92 |
| c9,t11-CLA | 0.00 | 6.39 | 1.19 |
| t10,c12-CLA | 0.00 | 0.99 | 6.81 |
| Σa SFA | 15.87 | 14.95 | 15.31 |
| Σ MUFA | 22.11 | 21.85 | 20.59 |
| Σ PUFA | 60.77 | 54.24 | 54.31 |
| Σ CLA | 0.00 | 7.80 | 8.47 |
| Σ TFA | 0.42 | 0.34 | 0.35 |
Indicates sum of fatty acid class
All PHVO diets were enriched for trans-18:1 fatty acids containing between 11.37 g and 13.58 g trans-18:1/100 g fatty acids (Table 2). Both the TFA-CON and c9,t11/t10,c12-CLA diet were low in total trans-18:1, containing 0.18 g and 0.10 g trans-18:1/100 g fatty acids respectively (Table 2). All PHVO diets contained similar amounts of total saturated fatty acids, cis-monounsaturated fatty acids, and cis-polyunsaturated fatty acids, while both the TFA-CON and c9,t11/t10,c12-CLA diets contained a slightly higher amount of total fatty acids in these classes (Table 2). The TFA-CON and PHVO diets contained no CLA (Table 2) [6].
Table 2.
Fatty acid profile (g/100 g fatty acid) of experimental diets (Experiment 2)
| Fatty acid | Diet |
||||
|---|---|---|---|---|---|
| TFA-CON | PH-LIN | PH-SAF | PH-SUN | c9,t11/t10,c12-CLA | |
| 14:0 | 0.46 | 0.19 | 0.20 | 0.18 | 0.32 |
| 16:0 | 12.75 | 9.33 | 9.46 | 8.43 | 10.98 |
| 18:0 | 16.51 | 14.46 | 13.95 | 14.35 | 9.76 |
| 18:1 t4 | 0.00 | 0.11 | 0.19 | 0.21 | 0.00 |
| 18:1 t5 | 0.00 | 0.26 | 0.45 | 0.52 | 0.00 |
| 18:1 t6-t8 | 0.03 | 2.87 | 3.60 | 4.79 | 0.01 |
| 18:1 t9 | 0.03 | 1.77 | 1.48 | 1.83 | 0.01 |
| 18:1 t10 | 0.03 | 1.98 | 1.66 | 2.05 | 0.02 |
| 18:1 t11 | 0.06 | 1.62 | 1.57 | 1.47 | 0.04 |
| 18:1 t12 | 0.02 | 1.72 | 1.81 | 1.70 | 0.01 |
| 18:1 13/t14 | 0.00 | 2.24 | 2.59 | 1.78 | 0.00 |
| 18:1 c9 | 24.74 | 18.19 | 17.68 | 18.13 | 23.35 |
| 18:1 t15 | 0.00 | 1.06 | 1.21 | 1.02 | 0.00 |
| 18:1 c11 | 1.05 | 1.45 | 1.45 | 1.41 | 1.15 |
| 18:1 c12 | 0.05 | 0.47 | 0.51 | 0.43 | 0.03 |
| 18:1 c13 | 0.04 | 0.37 | 0.42 | 0.32 | 0.03 |
| 18:1 c14 | 0.01 | 0.56 | 0.78 | 0.45 | 0.00 |
| 18:2 c9,c12 (n-6) | 37.49 | 35.23 | 34.87 | 34.71 | 36.81 |
| 20:0 | 0.31 | 0.25 | 0.29 | 0.30 | 0.30 |
| 18:3 9c,12c,15c (n-3) | 4.52 | 4.26 | 4.26 | 4.33 | 4.31 |
| c9,t11-CLA | 0.00 | 0.00 | 0.00 | 0.00 | 5.28 |
| t10,c12-CLA | 0.00 | 0.00 | 0.00 | 0.00 | 5.30 |
| 22:0 | 0.31 | 0.21 | 0.27 | 0.45 | 0.27 |
| Σa Others | 1.58 | 1.42 | 1.29 | 1.14 | 2.04 |
| Σ SFA | 30.35 | 24.44 | 24.17 | 23.72 | 21.63 |
| Σ MUFA cis | 25.89 | 23.27 | 23.44 | 22.51 | 24.54 |
| Σ PUFA cis | 42.01 | 39.49 | 39.14 | 39.05 | 41.12 |
| Σ C18:1 trans | 0.18 | 11.37 | 11.97 | 13.58 | 0.10 |
| Total FA content (%) | 13.55 | 13.10 | 12.89 | 13.91 | 12.76 |
Indicates sum of fatty acid class
While the total level of trans-18:1 fatty acids in each PHVO diet was similar, the isomeric profile of these fatty acids differed. The PH-LIN diet was lowest in trans-6–8 18:1, while levels were highest in the PH-SUN diet (Table 2). In parallel, the PH-LIN diet contained more trans-9–10 18:1 than the PH-SAF diet (Table 2). The PH- SUN diet had the highest content of trans-6–8 18:1 and was lower in trans-11–15 18:1 (Table 2). The PH-SAF diet contained higher amounts of trans-11–15 18:1 (Table 2).
Experiment 1: Effect of c9,t11-CLA on Ovary-Independent Postnatal Mammary Growth
Mice OVX at 21 days of age were assigned to the CON diet or that with 1% fat as either 9,11 or t10,c12-CLA. Mice were maintained on these diets for 21 days and then euthanized by CO2 inhalation followed by exsanguination. Experiments were repeated in three independent cohorts (n = 4–7/group/cohort).
Experiment 2: Effect of PHVO on Ovary-Independent Postnatal Mammary Growth
Mice OVX at 21 days of age were assigned to the TFA-CON diet or that with 5% fat as PH-SUN, PH-SAF, PH-LIN, or c9,t11/t10,c12-CLA. Mice were fed these diets for 21 days ad libitum then were euthanized by CO2 inhalation followed by exsanguination.
Mammary Gland Whole Mount Preparation and Analysis
Whole mounts were prepared from the 4th inguinal mammary glands [25]. Briefly, mammary glands were excised, spread on a glass slide, and air-dried prior to fixation in either Carnoy’s (Experiment 1, cohort 1) or Tellyesniczky’s (Experiment 1, cohort 2 and 3; Experiment 2) fixative. Samples were dehydrated in graded ethanol, cleared in xylene, and coverslipped with Cytoseal 280 (Fisher Scientific, Waltham, MA, USA). Digital images were captured (Qimaging QiCAM RGB Cooled CCD) using an Olympus SZX16 stereomicroscope.
Ductal elongation was measured as the distance from the teat to the furthest ductal terminus using either digital calipers (Experiment 1) or the line tool in the open-source software FIJI [26]. Ductal area was determined using the magic wand tool to select only the ductal network following background subtraction, color deconvolution, and image binarization using FIJI [26]. To quantify ductal branchpoints, images were first adjusted by performing background subtraction, color deconvolution, and local contrast enhancement [26]. Ductal branchpoints were then quantified as the number of ductal bifurcations that were manually tagged using FIJI [26] (Experiment 1), or as the number of junctions detected using Angiotool (Experiment 2) [27]. Branchpoint density was calculated by expressing branchpoint number per ductal area.
Fatty Acid Analysis
The fatty acid profile of the diets was analyzed using a modified method of the direct transesterification method of Sukhija and Palmquist [28] as we have described previously [29]. An aliquot of the solution, containing the fatty acid methyl esters (FAME), was taken for GLC analysis. Total lipids were extracted from epithelium-free mammary fat pad and omental fat according to the method of Bligh and Dyer [30] using a mixture of methanol:chloroform:water (2:2:1.8, by vol). The lipids were transesterified with methanolic sodium methoxide (0.5 M NaOCH3/methanol, 15 min at room temperature). The GLC analysis of all FAME extracts was performed on a GC-2010 gas chromatograph (Shimadzu) equipped with a flame ionization detector using a CP-Sil 88 capillary column (100 m × 0.25 mm I.D. × 0.25 μm film thickness, Varian). GLC conditions were described previously [29]. Fatty acid results were expressed as percentages (g/100 g fatty acids) of fatty acids detected with a chain length between 10 and 24 carbon atoms. The lower limit of detection was <0.001/100 g fatty acids.
Statistics
Data were analyzed using the Proc GLM procedure in SAS (Cary, NC, USA). Experiment 1 was a Random Complete Block Design and was analyzed by one-way ANOVA with blocking by experimental cohort. Experiment 2 was a completely randomized design and was analyzed by one-way ANOVA. Body weight data was analyzed using the Proc GLM procedure with repeated measures. Means were significant when P < 0.05. Data were transformed where appropriate.
Results
Experiment 1: Effect of c9,t11-CLA on Ovary-Independent Mammary Growth
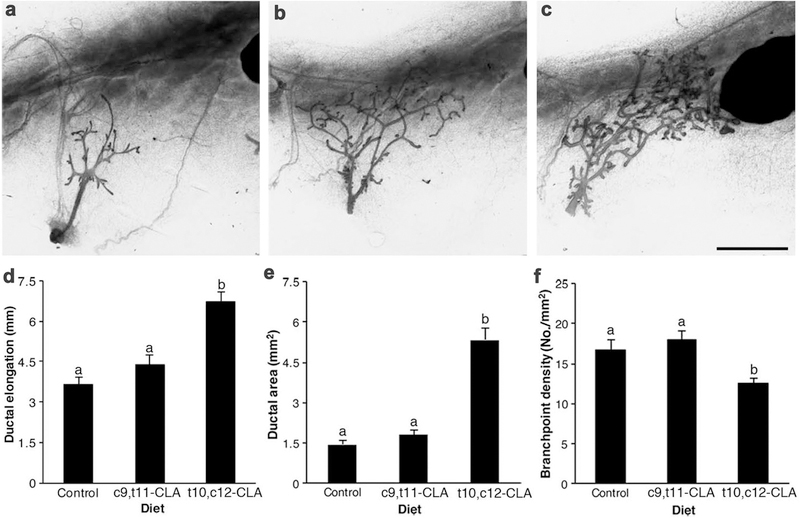
We previously showed that t10,c12-CLA stimulated mammary growth independent of E and the ER [17]. Given that both isomers of CLA (t10,c12-CLA and c9,t11-CLA) are present in CLA dietary supplements [31], as well as in ruminant-derived meat and dairy products [32], we sought to determine if the effect of 10,12 was recapitulated by dietary c9,t11-CLA. As anticipated, t10,c12-CLA increased ductal elongation in OVX mice, whereas dietary c9,t11-CLA had no effect (Fig. 1d). Furthermore, t10,c12-CLA increased ductal area, whereas the glands from mice fed c9,t11-CLA were unchanged (Fig. 1e). There was no increase in branchpoint density in response to c9,t11-CLA, whereas branchpoint density was reduced in mice fed t10,c12-CLA. These findings emphasize that the primary effect of t10,c12-CLA on the mammary glands is increased ductal elongation (Fig. 1f). From these data we conclude that despite similarities between these CLA isomers, only t10,c12-CLA evokes a proliferative response in the mammary gland.
Fig. 1.
Dietary trans-10, cis-12 conjugated linoleic acid (t10,c12 CLA), but not cis-9, trans-11 conjugated Linoleic acid (c9,t11 CLA), stimulates mammary gland growth in ovariectomized mice. Representative images of whole mounts from inguinal mammary glands of 42d old mice fed either the (a) control diet or that with 1% fat as (b) c9,t11 or (c) t10,c12 CLA. Mice were ovariectomized at 21 days, and fed the experimental diet from 22 days until euthanasia at 42 days of age. Scale bar 2 mm. d Mammary ductal elongation was the distance from the teat to the furthest-reaching ductal terminus. e Ductal area was measured using FIJI. f Branchpoint density was the number of ductal bifurcations counted using FIJI normalized for ductal area. Data are mean ± SEM from three experimental cohorts (n = 4–7/diet/cohort). a,bMeans with different letters are different (P < 0.05)
Effect of PHVO Diets on Ovary-Independent Mammary Growth (Experiment 2)
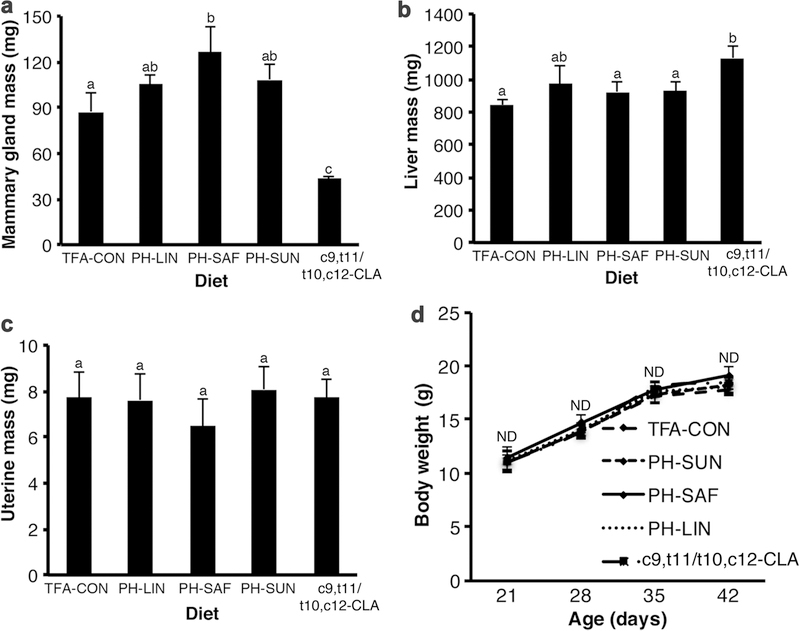
We next investigated the effects of different PHVO on ovary-independent mammary gland growth. Given that one hallmark of t10,c12-CLA supplementation is reduced adiposity [20] and increased liver mass [18], we measured the wet mass of both the liver and the lymph node-free 4th inguinal mammary gland. As anticipated, the diet containing the CLA isomer mixture (c9,t11/t10,c12-CLA) reduced the mass of the mammary glands, whereas the mammary glands of mice fed the PH-SAF diet were heavier than those from mice fed TFA-CON- and c9,t11/t10,c12-CLA (Fig. 2a). In parallel, the c9,t11/t10,c12-CLA diet, but not the PHVO diets, increased liver mass (Fig. 2b). Uterine mass was unchanged across the different diet groups (Fig. 2c). There was no effect of the various diets on body weight (Fig. 2d).
Fig. 2.
Ovariectomized mice fed a diet containing partially hydrogenated safflower oil have increased mammary gland mass. Mice were ovariectomized at 21 days and fed either the control diet (TFA-CON) or that with 5% fat as partially hydrogenated (PH) Linseed oil (PH-LIN), PH-SAF, partially hydrogenated sunflower oil (PH-SUN), or 2.7% conjugated linoleic acid (c9,t11/t10,c12 CLA) from 22 days of age until euthanasia at 42 days of age. Wet organ mass was measured for (a) lymph node-free mammary gland, (b) liver, and (c) uterus. d Body weight (g) was measured at 21, 28, 35, and 42 days of age. Data are mean ± SEM (n = 6–8/diet). a, b, cMeans with different letters are different (P < 0.05). ND no difference
We also determined the fatty acid profile of omental adipose tissue (Table 4) and the 4th inguinal epithelium-free mammary fat pad (distal to the teat and supramammary lymph node, Table 3). The fatty acid profile of both the omental and epithelium-free mammary fat pad depots generally reflected that of the diet. In parallel, the total level of cis-polyunsaturated fatty acids was greatest in fat depots from c9,t11/t10,c12-CLA-fed mice (Tables 3, 4).
Table 4.
Fatty acid profile (mol%) of omental fat samples collected from ovariectomized mice fed the control diet (TFA-CON), or the control diet with 5% fat as partially hydrogenated linseed oil (PH-LIN) partially hydrogenated safflower oil (PH-SAF), or partially hydrogenated sunflower oil (PH-SUN), or the control diet with 2.7% fat as conjugated linoleic acid (c9,t11/t10,c12-CLA)
| Fatty acid | Diet |
P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFA-CON |
PH-LIN |
PH-SAF |
PH-SUN |
c9,t11/t10,c12-CLA |
|||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| 14:0 | 1.76a | 0.22 | 1.85a | 0.33 | 1.90a | 0.19 | 1.51a | 0.25 | 0.73b | 0.08 | 0.0003 |
| 16:0 | 20.44a | 0.42 | 20.06a | 0.44 | 20.22a | 0.41 | 18.34b | 0.38 | 17.80b | 0.64 | 0.0014 |
| 18:0 | 5.44a | 0.40 | 4.22c | 0.18 | 4.34bc | 0.30 | 4.40bc | 0.18 | 5.10ab | 0.32 | 0.0260 |
| 18:1 4t | 0.00a | 0.00 | 0.02b | 0.00 | 0.04c | 0.00 | 0.05d | 0.00 | 0.00a | 0.00 | <0.0001 |
| 18:1 5t | 0.00a | 0.00 | 0.06b | 0.00 | 0.11c | 0.01 | 0.13d | 0.01 | 0.00a | 0.00 | <0.0001 |
| 18:1 6–8t | 0.01a | 0.00 | 1.20b | 0.06 | 1.53c | 0.10 | 2.24d | 0.13 | 0.01a | 0.00 | <0.0001 |
| 18:1 9t | 0.04a | 0.00 | 1.27b | 0.06 | 1.04c | 0.03 | 1.40b | 0.05 | 0.02d | 0.00 | <0.0001 |
| 18:1 10t | 0.01a | 0.00 | 1.01b | 0.04 | 0.87b | 0.03 | 1.06b | 0.04 | 0.03c | 0.00 | <0.0001 |
| 18:1 11t | 0.03a | 0.00 | 0.64b | 0.02 | 0.65b | 0.04 | 0.67b | 0.04 | 0.03c | 0.00 | <0.0001 |
| 18:1 12t | 0.02a | 0.00 | 0.99b | 0.04 | 1.13b | 0.06 | 1.23b | 0.05 | 0.01a | 0.00 | <0.0001 |
| 18:1 9c | 34.34a | 0.46 | 30.00bc | 0.47 | 29.38b | 0.47 | 29.78bc | 0.56 | 31.29c | 0.67 | <0.0001 |
| 18:1 11c | 1.22a | 0.04 | 1.64b | 0.10 | 1.58b | 0.07 | 1.63b | 0.09 | 1.45ab | 0.07 | 0.0051 |
| 18:1 12c | 0.03a | 0.00 | 0.27b | 0.01 | 0.30c | 0.01 | 0.26b | 0.01 | 0.06d | 0.00 | <0.0001 |
| 18:1 13c | 0.02a | 0.00 | 0.21b | 0.01 | 0.27c | 0.01 | 0.24d | 0.01 | 0.03a | 0.00 | <0.0001 |
| 18:1 14c/16t | 0.00a | 0.00 | 0.21b | 0.01 | 0.31c | 0.01 | 0.18d | 0.01 | 0.01a | 0.00 | <0.0001 |
| 18:2 9c,12c (n-6) | 27.49a | 0.64 | 26.60a | 0.82 | 26.53a | 0.63 | 27.99a | 0.51 | 32.29b | 0.71 | <0.0001 |
| 20:0 | 0.11a | 0.01 | 0.09bc | 0.01 | 0.10ac | 0.01 | 0.09bc | 0.01 | 0.08c | 0.01 | 0.0151 |
| 18:3 9c,12c,15c (n-3) | 2.43ab | 0.09 | 2.51a | 0.08 | 2.51a | 0.07 | 2.64a | 0.09 | 2.26b | 0.08 | 0.0381 |
| c9,t11-CLA | 0.03a | 0.00 | 0.40b | 0.03 | 0.35b | 0.03 | 0.35b | 0.03 | 3.68c | 0.15 | <0.0001 |
| t10,c12-CLA | 0.00a | 0.00 | 0.01a | 0.01 | 0.00a | 0.00 | 0.00a | 0.00 | 2.09b | 0.19 | <0.0001 |
| 22:0 | 0.16a | 0.01 | 0.15a | 0.01 | 0.15a | 0.01 | 0.16a | 0.01 | 0.09b | 0.00 | <0.0001 |
| ∑e SFA | 29.25a | 0.47 | 27.61a | 0.89 | 27.91a | 0.41 | 25.59b | 0.55 | 24.46b | 0.38 | <0.0001 |
| ∑ MUFA cis | 39.79a | 0.33 | 36.74b | 0.37 | 36.36b | 0.51 | 35.64bc | 0.64 | 34.16c | 0.85 | <0.0001 |
| ∑ PUFA cis | 30.79a | 0.67 | 29.98a | 0.86 | 29.95a | 0.65 | 31.57a | 0.55 | 35.20b | 0.78 | 0.0002 |
| ∑ CLA | 0.03a | 0.00 | 0.42b | 0.03 | 0.37b | 0.03 | 0.36b | 0.03 | 6.05c | 0.35 | <0.0001 |
| ∑ 18:1 trans | 0.11a | 0.00 | 5.40b | 0.23 | 5.66b | 0.28 | 6.97c | 0.29 | 0.12a | 0.00 | <0.0001 |
Data are mean and SE (n = 5/diet)
Least squares means are different P < 0.05
Indicates sum of fatty acid class
Table 3.
Fatty acid profile (mol%) of epithelium-free mammary fat pads collected from ovariectomized mice fed the control diet (TFA-CON), or the control diet with 5% fat as partially-hydrogenated linseed oil (PH-LIN), partially-hydrogenated safflower oil (PH-SAF), or partially hydrogenated sunflower oil (PH-SUN), or the control diet with 2.7% fat as conjugated linoleic acid (c9,t11/t10,c12-CLA)
| Fatty acid | Diet |
P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFA-CON |
PH-LIN |
PH-SAF |
PH-SUN |
c9,t11/t10,c12-CLA |
|||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| 14:0 | 2.54a | 0.34 | 2.54a | 0.57 | 2.61a | 0.47 | 2.21a | 0.44 | 0.96b | 0.13 | 0.050 |
| 16:0 | 18.56a | 0.33 | 18.27ab | 0.37 | 18.22abc | 0.59 | 16.84c | 0.24 | 17.12bc | 0.65 | 0.046 |
| 18:0 | 5.34a | 0.49 | 4.24a | 0.32 | 4.34a | 0.53 | 4.30a | 0.34 | 5.40a | 0.43 | 0.135 |
| 18:1 4t | 0.00a | 0.00 | 0.02b | 0.00 | 0.04c | 0.01 | 0.04c | 0.00 | 0.00a | 0.00 | <0.0001 |
| 18:1 5t | 0.00a | 0.00 | 0.06b | 0.00 | 0.10c | 0.01 | 0.12c | 0.01 | 0.00a | 0.00 | <0.0001 |
| 18:1 6–8t | 0.01a | 0.00 | 1.15b | 0.10 | 1.41b | 0.15 | 2.06c | 0.17 | 0.01a | 0.00 | <0.0001 |
| 18:1 9t | 0.04a | 0.00 | 1.22bc | 0.09 | 1.00b | 0.08 | 1.38c | 0.08 | 0.02d | 0.00 | <0.0001 |
| 18:1 10t | 0.02a | 0.00 | 0.89b | 0.07 | 0.77b | 0.05 | 0.99b | 0.07 | 0.03c | 0.00 | <0.0001 |
| 18:1 11t | 0.03a | 0.00 | 0.69b | 0.05 | 0.69b | 0.08 | 0.66b | 0.04 | 0.03a | 0.00 | <0.0001 |
| 18:1 12t | 0.02a | 0.01 | 1.00b | 0.07 | 1.09bc | 0.10 | 1.19c | 0.08 | 0.02a | 0.00 | <0.0001 |
| 18:1 9c | 35.81a | 0.24 | 31.96b | 0.51 | 31.39b | 0.98 | 31.58b | 0.54 | 30.92b | 1.00 | 0.0004 |
| 18:1 11c | 1.49a | 0.08 | 1.84b | 0.11 | 1.83b | 0.13 | 1.81b | 0.11 | 1.46a | 0.10 | 0.0279 |
| 18:1 12c | 0.04a | 0.01 | 0.28bc | 0.01 | 0.30b | 0.02 | 0.26c | 0.01 | 0.06a | 0.00 | <0.0001 |
| 18:1 13c | 0.03a | 0.00 | 0.20b | 0.01 | 0.25c | 0.01 | 0.22bc | 0.01 | 0.02a | 0.00 | <0.0001 |
| 18:1 14c/16t | 0.01a | 0.00 | 0.20b | 0.01 | 0.27c | 0.02 | 0.16d | 0.01 | 0.01a | 0.00 | <0.0001 |
| 18:2 9c,12c (n-6) | 27.10a | 0.81 | 26.13a | 1.14 | 26.46a | 1.36 | 27.49a | 0.92 | 31.89b | 1.09 | 0.0063 |
| 20:0 | 0.10ab | 0.01 | 0.08b | 0.01 | 0.08b | 0.01 | 0.08b | 0.01 | 0.11a | 0.01 | 0.0562 |
| 18:3 9c,12c,15c (n-3) | 2.16a | 0.06 | 2.20a | 0.11 | 2.23a | 0.11 | 2.28a | 0.09 | 2.42a | 0.09 | 0.3368 |
| CLA 9c,11t | 0.04a | 0.00 | 0.37b | 0.03 | 0.30b | 0.02 | 0.36b | 0.03 | 3.63c | 0.21 | <0.0001 |
| CLA 10t,12c | 0.00a | 0.00 | 0.00a | 0.00 | 0.00a | 0.00 | 0.00a | 0.00 | 2.23b | 0.23 | <0.0001 |
| 22:0 | 0.16a | 0.01 | 0.14a | 0.01 | 0.15a | 0.01 | 0.14a | 0.01 | 0.08b | 0.00 | 0.0003 |
| ∑e SFA | 28.65a | 0.47 | 27.00ab | 1.05 | 27.17ab | 0.87 | 25.11bc | 0.64 | 24.48c | 0.34 | 0.0031 |
| ∑ MUFA cis | 41.06a | 0.41 | 38.41b | 0.67 | 37.84b | 1.17 | 37.49b | 0.81 | 33.97c | 1.27 | 0.0005 |
| ∑ PUFA cis | 30.12a | 0.80 | 29.11a | 1.17 | 29.52a | 1.39 | 30.53a | 0.94 | 35.39b | 1.13 | 0.0035 |
| ∑ CLA | 0.04a | 0.00 | 0.39b | 0.03 | 0.31b | 0.02 | 0.37b | 0.03 | 6.12c | 0.45 | <0.0001 |
| ∑ 18:1 trans | 0.12a | 0.01 | 5.23b | 0.38 | 5.37bc | 0.48 | 6.60c | 0.46 | 0.12a | 0.01 | <0.0001 |
Data are mean and SE (n = 4–5/diet)
Least squares means are different P < 0.05
Indicates sum of fatty acid class
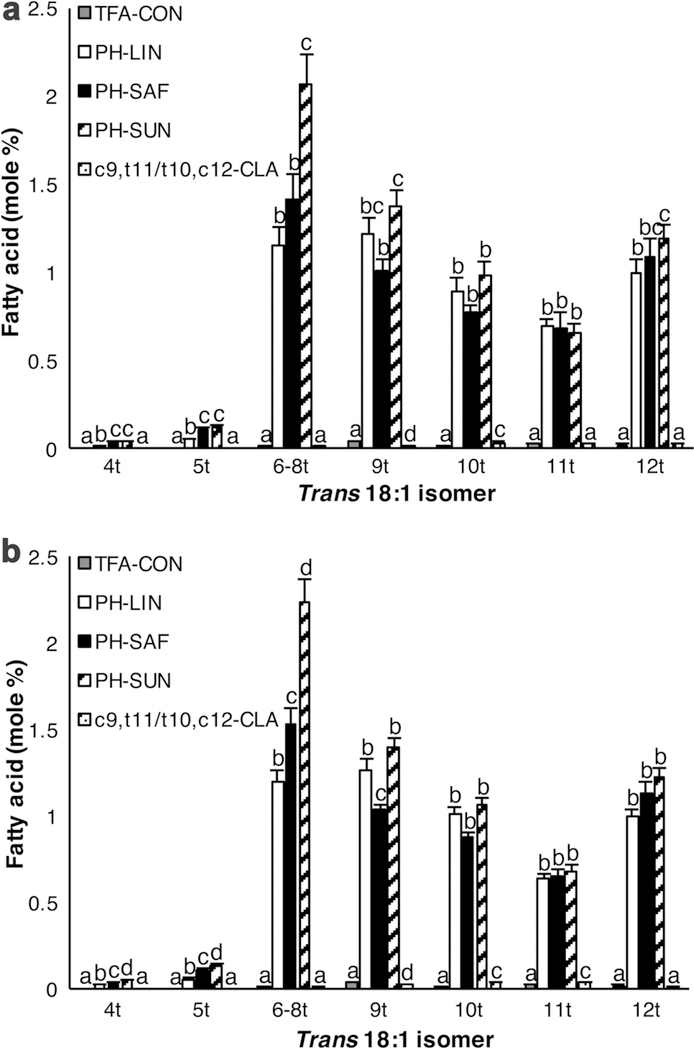
Fat depots from mice fed the various PHVO had higher levels of trans-18:1 than TFA-CON or c9,t11/t10,c12-CLA-fed mice, where mice fed PH-SUN had a significantly higher trans-18:1 content in the mammary fat pads than mice fed TFA-CON, c9,t11/t10,c12-CLA, or PH-LIN diet (Table 3). Consumption of the PH-SUN diet resulted in greater accumulation of trans-6–8 18:1 in the mammary fat pad compared to all other diets (Fig. 3a; Table 3).
Fig. 3.
Distribution of different trans-18:1 isomers in adipose tissue from ovariectomized mice fed the control diet (TFA-CON) or that with 5% fat as partially hydrogenated linseed oil (PH-LIN), partially hydrogenated safflower oil (PH-SAF), partially hydrogenated sunflower oil (PH-SUN), or 2.7% conjugated linoleic acids (c9,t11/t10,c12 CLA). Mice were ovariectomized at 21 days and fed the experimental diets from 22 days of age until euthanasia at 42 days of age. Fatty acid methyl esters from (a) epithelium-free mammary fat pad and (b) omental fat were analyzed by gas-liquid chromatography. X-axis indicates position of trans double bond of 18:1 fatty acids. Data are mean ± SEM (n = 4–5/diet). a b c dMeans with different letters are different (P < 0.05) for a given isomer
Mice fed the PHVO treatments had a higher level of c9,t11-CLA in both the omental (Table 4) and mammary fat pads (Table 3), despite the absence of c9,t11-CLA in the diet. The content of vaccenic acid (trans-11 18:1), the precursor to c9,t11-CLA, was greater in the mammary fat pads (Table 3; Fig. 3a) and omental fat (Table 4; Fig. 3b) of PHVO-fed mice compared to mice fed the control or c9,t11/t10,c12-CLA diets. This distribution closely reflected the diet composition, where the PHVO diets contained more trans-11 18:1 than the control and c9,t11/t10,c12-CLA diets (Table 2). As anticipated, the accumulation of CLA was greatest in tissues from mice fed the c9,t11/t10,c12-CLA diet (Tables 3, 4). However, the content of c9,t11-CLA was higher than t10,c12-CLA in the mammary fat pads and omental fat from mice fed the c9,t11/t10,c12-CLA diet (Tables 3, 4), despite there being approximately equal amounts of c9,t11 and t10,c12-CLA in the diet (Table 2).
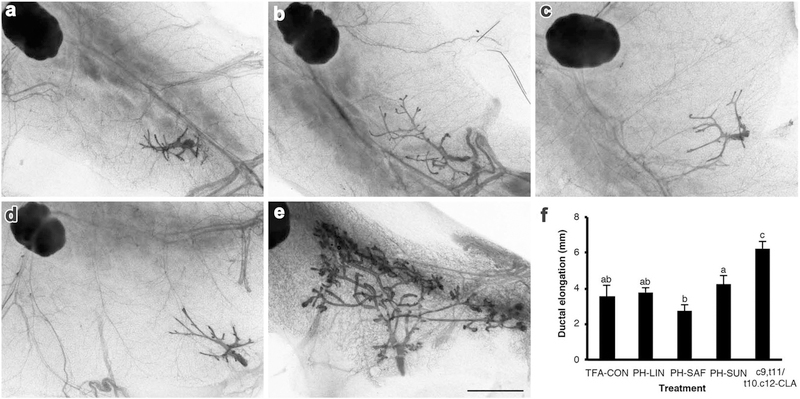
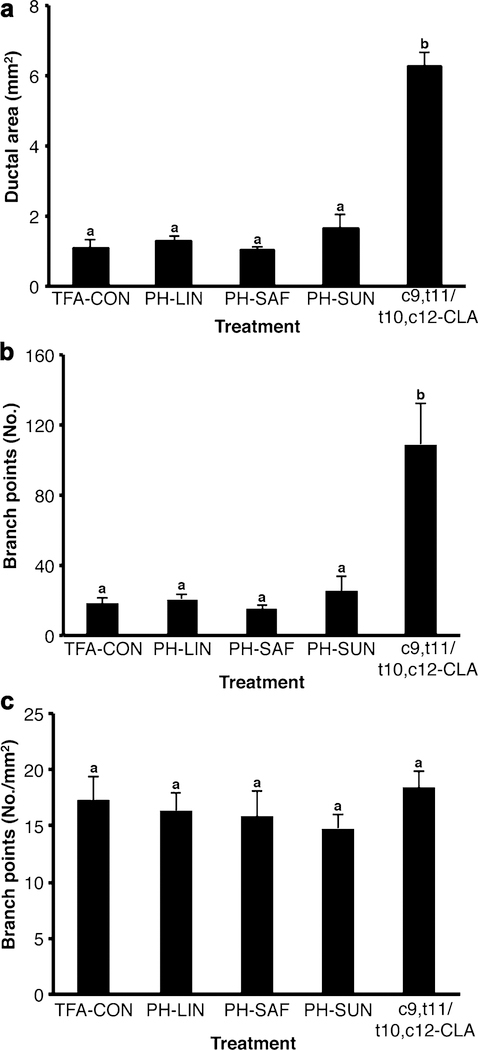
While the c9,t11/t10,c12-CLA mixture stimulated ovary-independent mammary growth, there was no effect of the various PHVO diets on ductal elongation compared to that in mice fed the TFA-CON diet (Fig. 4f). Similarly, mammary ductal area was increased by dietary c9,t11/t10,c12-CLA, but was unaffected by the PH-LIN, PH-SAF, or PH-SUN diets (Fig. 5a). Moreover, whereas dietary c9,t11/t10,c12-CLA increased total branchpoint number (Fig. 5b), there was no effect of diet on branchpoint density once branchpoint number was expressed relative to ductal area (Fig. 5c).
Fig. 4.
Dietary conjugated linoleic acid (c9,t11/t10,c12-CLA), but not various dietary trans-18:1 isomers, stimulates mammary gland growth in ovariectomized mice. Representative images of whole mounts from inguinal mammary glands of 42 days old mice fed either the (a) control diet (TFA-CON) or that with 5% fat as (b) partially hydrogenated linseed oil (PH-LIN), (c) partially hydrogenated safflower oil (PH-SAF), (d) partially hydrogenated sunflower oil (PH-SUN), or (e) 2.7% conjugated linoleic acids (c9,t11/t10,c12 CLA). Mice were ovariectomized at 21 days, then fed the experimental diets from 22 days until euthanasia at 42 days of age. Scale bar 2 mm. f Mammary ductal elongation was the distance from the teat to the furthest-reaching ductal terminus. Data are mean ± SEM (n = 6–8/diet). a, b, cMeans with different letters are different (P < 0.05)
Fig. 5.
Dietary conjugated linoleic acid (c9,t11/t10,c12-CLA), but not various dietary trans-18:1 fatty acids, increases mammary gland branch point number in ovariectomized mice. Mice were ovariectomized at 21 days and fed the either the control diet (TFA-CON) or that with 5% fat as partially hydrogenated linseed oil (PH-LIN), PH-SAF, partially hydrogenated sunflower oil (PH-SUN), or 2.7% conjugated linoleic acids (c9,t11/t10,c12-CLA) from 22 days of age until euthanasia at 42 days of age. a Mammary ductal area was measured using ImageJ. b Mammary gland branch point number was measured using AngioTool. c Branchpoint density was branchpoint number normalized to ductal area. Data are mean ± SEM (n = 6–8/diet). a, bMeans with different letters are different (P < 0.05)
Discussion
The Ability of Trans-Fatty Acids to Stimulate E-Independent Mammary Growth is Type-Specific and Isomer-Specific
Multiple lines of evidence have highlighted the deleterious effects of dietary trans-fatty acids on human health [3, 5, 9, 33]. While the contribution of individual fatty acids is often difficult to parse in epidemiological studies, considering the effects of different trans-fatty acid isomers in controlled experiments is crucial. We previously showed that dietary t10,c12-CLA, a trans-18:2 isomer, stimulates allometric mammary growth and tumorigenesis in OVX mice [17]. Those experiments supported that t10,c12-CLA-induced mammary growth independent of E and ER given that inhibition of E biosynthesis or antagonism of ER failed to block t10,c12-CLA-mediated mammary growth [17]. In parallel, we highlighted that metabolic dysregulation including well- documented insulin resistance could be implicated in t10,c12-CLA-stimulated mammary growth, and that administering the insulin-sensitizer rosiglitazone abrogated this response [17]. Here we establish that diet-stimulated mammary gland growth is specific to t10,c12-CLA and cannot be recapitulated by c9,t11-CLA or diets enriched with different mixtures of trans-18:1 isomers derived from PHVO. Although we cannot exclude the potential for an effect of different trans-fatty acid isomers on the mammary glands after a longer exposure period or when fed at different levels, we conclude that E-independent mammary gland growth is t10,c12-CLA-specific within the confines of this defined model system.
Dietary Trans-18:1 Isomers Are Metabolized to Trans-18:2 Fatty Acids In Vivo
The c9,t11-CLA content in the mammary fat pads and omental fat from mice fed PH-LIN, PH-SAF, and PH-SUN was approximately 10-fold greater than in control-fed mice. Given that the PH-LIN, PH-SAF, or PH-SUN diets were devoid of c9,t11-CLA, its accumulation in adipose tissue likely reflects conversion of trans-11 18:1 to c9,t11-CLA by delta-9-desaturase [34, 35]. Along these lines, Loor et al. found that the CLA content in the livers and whole carcasses of lactating mice was approximately equal when they were fed diets containing either a CLA mixture or trans-18:1 fatty acids [34]. Separately, CLA accumulated in the triacylglycerides but not the phospholipid fraction of adipose tissue in mice fed a diet containing trans-11 18:1, indicating that desaturation of trans-11 18:1 likely occurred in adipose tissue [36]. Moreover, inhibition of delta-9 desaturase with cyclopropenoic fatty acids prevented consversion of trans-11 18:1 to c9,t11-CLA in rats [37]. Given that the amount of trans-11 18:1 in the PH-LIN, PH-SAF, and PH-SUN diets was similar, it follows that the extent of c9,t11-CLA accumulation did not differ across the diet groups.
Our data also indicate that compared to t10,c12, c9,t11-CLA is preferentially incorporated into both the mammary fat pad and omental fat, given that the content of c9,t11- CLA in both fat depots was elevated despite approximately equal amounts of c9,t11 and t10,c12-CLA in the c9,t11/ t10,c12-CLA mixed isomer diet. Given the magnitude of the difference and the low content of trans-11 18:1 in the c9,t11/t10,c12-CLA diet preparation, conversion of trans-11 18:1 to c9,t11-CLA by delta-9 desaturase is likely only partially responsible for the difference. Given that mice are coprophagous, some CLA in the tissues may have come from the feces. Along these lines, the content of t10,c12-CLA in lipids extracted from the livers and hearts of pigs fed a mixed CLA diet was lower than that supplied by the diet, while incorporation of 9,11 into liver phospholipids was significantly higher than t10,c12-CLA [38]. In parallel, layer hens fed a diet containing mixed CLA had less t10,c12-CLA accumulate in tissues relative to the amount present in the diet [39]. Taken together, these data emphasize that c9,t11-CLA selectively incorporated into tissues across various monogastric species.
A preponderance of evidence points to the deleterious effects of dietary trans-fatty acids on human health that has led to broad policy initiatives limiting their presence in the human diet [33]. While our previous findings highlighted a novel effect of dietary t10,c12-CLA on estrogen-independent mammary gland growth [17], our data herein do not support a similar effect of diets high in trans-18:1 fatty acids supplied by various PHVO. At the same time, c9,t11-CLA, which is most common in dietary supplements, as well as is a minor constituent of ruminant-derived foodstuffs, also had no effect. Combined, these data indicate a specific effect of t10,c12-CLA on mammary gland development.
Acknowledgements
We thank the UC Davis Department of Animal Science Small Animal Colony manager Sandra Weisker and student staff. We thank Courtney Preseault for her assistance with fatty acid analysis. Research was funded in part by a Ruth L. Kirschstein National Research Service Award (F31 CA189421) from the National Institutes of Health National Cancer Institute (to GEB).
Abbreviations
- ANOVA
Analysis of variance
- c9,t11-CLA
Cis-9, trans-11 conjugated linoleic acid
- CLA
Conjugated linoleic acid
- CON
Control diet experiment 1
- TFA-CON
Control diet experiment 2
- E
Estrogen
- ER
Estrogen receptor
- IP
Intraperitoneal
- OVX
Ovariectomized
- PH-LIN
Partially hydrogenated linseed oil
- PH-SAF
Partially hydrogenated safflower oil
- PH-SUN
Partially hydrogenated sunflower oil
- PHVO
Partially hydrogenated vegetable oil
- SC
Subcutaneous
- t10,c12-CLA
Trans-10, cis-12 conjugated linoleic acid
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors have no conflicts of interest to disclose.
References
- 1.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J (2005) Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81:341–354 [DOI] [PubMed] [Google Scholar]
- 2.Micha R, Mozaffarian D (2008) Trans fatty acids: effects on cardiometabolic health and implications for policy. Prostaglandins Leukot Essent Fatty Acids 79:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascherio A, Hennekens CH, Buring JE, Master C, Stampfer MJ, Willett WC (1994) Trans-fatty acids intake and risk of myocardial infarction. Circulation 89:94–101 [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (2006) Trans fatty acids and cardiovascular disease. N Engl J Med 354:1601–1613 [DOI] [PubMed] [Google Scholar]
- 5.Mensink RP, Katan MB (1990) Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 323:439–445 [DOI] [PubMed] [Google Scholar]
- 6.Kraft J, Spiltoir JI, Salter AM, Lock AL (2011) Differential effects of the trans-18:1 isomer profile of partially hydrogenated vegetable oils on cholesterol and lipoprotein metabolism in male F1B hamsters. J Nutr 141:1819–1826 [DOI] [PubMed] [Google Scholar]
- 7.Micha R, Mozaffarian D (2009) Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 5:335–344 [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Willett WC (2007) Trans fatty acids and cardiovascular risk: a unique cardiometabolic imprint? Curr Atheroscler Rep 9:486–493 [DOI] [PubMed] [Google Scholar]
- 9.Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC (2010) Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc 110:585–592 [DOI] [PubMed] [Google Scholar]
- 10.Gebauer SK, Chardigny JM, Jakobsen MU, Lamarche B, Lock AL, Proctor SD, Baer DJ (2011) Effects of ruminant trans fatty acids on cardiovascular disease and cancer: a comprehensive review of epidemiological, clinical, and mechanistic studies. Adv Nutr 2:332–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ (2011) Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr 141:1286–1291 [DOI] [PubMed] [Google Scholar]
- 12.Olson LK, Tan Y, Zhao Y, Aupperlee MD, Haslam SZ (2010) Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int J Obes (Lond) 34:1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsch CW, DeHoog JV, O’Connor DH, Sheffield LG (1985) Influence of dietary fat levels on development and hormone responsiveness of the mouse mammary gland. Cancer Res 45:6147–6154 [PubMed] [Google Scholar]
- 14.Welsch CW, O’Connor DH (1989) Influence of the type of dietary fat on developmental growth of the mammary gland in immature and mature female BALB/c mice. Cancer Res 49:5999–6007 [PubMed] [Google Scholar]
- 15.Zhao Y, Tan YS, Aupperlee MD, Langohr IM, Kirk EL, Troester MA, Schwartz RC, Haslam SZ (2013) Pubertal high fat diet: effects on mammary cancer development. Breast Cancer Res 15:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrington CA, Hosick HL (1985) Effects of dietary fat on the growth of normal, preneoplastic and neoplastic mammary epithelial cells in vivo and in vitro. J Cell Sci 75:269–278 [DOI] [PubMed] [Google Scholar]
- 17.Berryhill GE, Gloviczki JM, Trott JF, Aimo L, Kraft J, Cardiff RD, Paul CT, Petrie WK, Lock AL, Hovey RC (2012) Diet-induced metabolic change induces estrogen-independent allometric mammary growth. Proc Natl Acad Sci USA 109:16294–16299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clement L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P (2002) Dietary trans-10, cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res 43:1400–1409 [DOI] [PubMed] [Google Scholar]
- 19.Stout MB, Liu LF, Belury MA (2011) Hepatic steatosis by dietary-conjugated linoleic acid is accompanied by accumulation of diacylglycerol and increased membrane-associated protein kinase C epsilon in mice. Mol Nutr Food Res 55:1010–1017 [DOI] [PubMed] [Google Scholar]
- 20.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME (2006) Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a micro-array and histological analysis. Physiol Genom 27:282–294 [DOI] [PubMed] [Google Scholar]
- 21.Nagao K, Inoue N, Ujino Y, Higa K, Shirouchi B, Wang YM, Yanagita T (2008) Effect of leptin infusion on insulin sensitivity and lipid metabolism in diet-induced lipodystrophy model mice. Lipids Health Dis 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foote MR, Giesy SL, Bernal-Santos G, Bauman DE, Boisclair YR (2010) t10, c12-CLA decreases adiposity in peripubertal mice without dose-related detrimental effects on mammary development, inflammation status, and metabolism. Am J Physiol Regul Integr Comp Physiol 299:R1521–R1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ip MM, McGee SO, Masso-Welch PA, Ip C, Meng X, Ou L, Shoemaker SF (2007) The t10, c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis 28:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unnevehr LJ, Jagmanaite E (2008) Getting rid of trans fats in the US diet: policies, incentives and progress. Food Policy 33:497–503 [Google Scholar]
- 25.Rasmussen SB, Young LJT, Smith GH (2000) Preparing mammary gland whole mounts from mice In: Ip MM, Asch BB (eds) Methods in mammary gland biology and breast cancer research. Springer, Boston [Google Scholar]
- 26.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Meth 9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zudaire E, Gambardella L, Kurcz C, Vermeren S (2011) A computational tool for quantitative analysis of vascular networks. PLoS One 6:e27385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sukhija PS, Palmquist DL (1988) Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J Agric Food Chem 36:1202–1206 [Google Scholar]
- 29.Lock AL, Preseault CL, Rico JE, DeLand KE, Allen MS (2013) Feeding a C16:0-enriched fat supplement increased the yield of milk fat and improved conversion of feed to milk. J Dairy Sci 96:6650–6659 [DOI] [PubMed] [Google Scholar]
- 30.Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- 31.Mougios V, Matsakas A, Petridou A, Ring S, Sagredos A, Melissopoulou A, Tsigilis N, Nikolaidis M (2001) Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J Nutr Biochem 12:585–594 [DOI] [PubMed] [Google Scholar]
- 32.Palmquist DL, Lock AL, Shingfield KJ, Bauman DE (2005) Biosynthesis of conjugated linoleic acid in ruminants and humans. Adv Food Nutr Res 50:179–217 [DOI] [PubMed] [Google Scholar]
- 33.Brownell KD, Pomeranz JL (2014) The trans-fat ban—food regulation and long-term health. N Engl J Med 370:1773–1775 [DOI] [PubMed] [Google Scholar]
- 34.Loor JJ, Lin X, Herbein JH (2002) Dietary trans-vaccenic acid (trans11–18:1) increases concentration of cis9, transll-conjugated linoleic acid (rumenic acid) in tissues of lactating mice and suckling pups. Reprod Nutr Dev 42:85–99 [DOI] [PubMed] [Google Scholar]
- 35.Kraft J, Hanske L, Mockel P, Zimmermann S, Hartl A, Kramer JK, Jahreis G (2006) The conversion efficiency of trans-11 and trans-12 18:1 by Delta9-desaturation differs in rats. J Nutr 136:1209–1214 [DOI] [PubMed] [Google Scholar]
- 36.Santora JE, Palmquist DL, Roehrig KL (2000) Trans-vaccenic acid is desaturated to conjugated linoleic acid in mice. J Nutr 130:208–215 [DOI] [PubMed] [Google Scholar]
- 37.Lock AL, Corl BA, Barbano DM, Bauman DE, Ip C (2004) The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by delta9-desaturase in rats. J Nutr 134:2698–2704 [DOI] [PubMed] [Google Scholar]
- 38.Kramer JK, Sehat N, Dugan ME, Mossoba MM, Yurawecz MP, Roach JA, Eulitz K, Aalhus JL, Schaefer AL, Ku Y (1998) Distributions of conjugated linoleic acid (CLA) isomers in tissue lipid classes of pigs fed a commercial CLA mixture determined by gas chromatography and silver ion-high-performance liquid chromatography. Lipids 33:549–558 [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Huang Y, Wang HQ, Chen ZY (2003) Isomeric distribution of conjugated linoleic acids (CLA) in the tissues of layer hens fed a CLA diet. J Agric Food Chem 51:5654–5660 [DOI] [PubMed] [Google Scholar]