Abstract
After decades of having the developed world’s highest rates of unintended pregnancy, the United States finally shows signs of improvement. This progress is likely due in large part to increased use of highly effective long-acting reversible methods of contraception. These methods can be placed and do not require any maintenance to provide years of contraception as effective as sterilization. Upon removal, fertility returns to baseline rates. This article addresses advances in both software—improved use and elimination of barriers to provide these methods; and hardware—novel delivery systems and devices.
Keywords: Contraceptive implant, IUD, long-acting reversible contraception
The United States has the highest rates of unintended pregnancy and abortion in the developed world, resulting in an imperative to improve these public health measures. Fortunately we are beginning to see declining rates at urban region, state, and national levels associated with removal of access barriers to long-acting reversible contraception (LARC), including intrauterine devices (IUDs) and contraceptive implants. Methods of LARC share the following attributes: they function for an extended period of time (at least 1 year), do not require any user action to maintain efficacy, and are as effective as sterilization (pregnancy rates are less than 1 per 100 women years), but unlike sterilization, they are completely reversible.
Significant advances in LARC service delivery over the past decade and new and future LARC methods will continue to favorably change the contraceptive landscape. Paul Blumenthal, M.D., M.P.H., at Stanford University refers to these two different forms of contraceptive developments as improvements in software (programmatic approaches and service delivery) and hardware (new methods) (1). This article will follow this approach in addressing both forms of LARC improvements.
LARC SOFTWARE ADVANCES
The central theme in recent and future LARC software developments is increasing access by breaking down initiation barriers. Barriers may include cost, clinical care access restrictions, unwarranted safety concerns, or expansion to previously restricted populations. Ongoing, multi-pronged software development will set the stage for future hardware successes.
LARC Promotion Reduces Unintended Pregnancies
Several studies promoting LARC devices documented declines in unintended pregnancy and abortion rates, fueling an intense interest in broadening LARC access and use. These studies featuring LARC methods have two important issues in common: [1] removing cost barriers to IUDs and implants; and [2] counseling patients on the superior efficacy and satisfaction associated with these devices. However, to avoid possible coercion while providing easy access to LARC devices, care must include the full range of contraceptive options (2, 3).
At the urban region level, the CHOICE project in St. Louis enrolled 9,256 women, offering no-cost access to their desired contraception for 3 years, and 75% chose LARC methods. Contraceptive pill, patch, or ring users were more than 20 times more likely to have an unintended pregnancy than LARC users (4). Moreover, teenage study participants had pregnancy and abortion rates less than half the rates St. Louis residents and the nation (5). At the state level in Iowa and Colorado, similar experiments showed remarkable decreases in teen pregnancies, unintended pregnancies, abortions, and preterm births (6–8).
Nationally, among women using contraception, a significant rise in the use of LARC devices from 2.4% in 2002 to 11.6% in 2012 occurred (9). Over this period, rates of unintended pregnancies and abortions declined (10). These successes may have contributed to the foundation for evidenced-based, professional organization guidelines to support increasing availability of LARC methods to all women (11, 12). More recently, counseling and clinic service provision focused on LARC methods demonstrated reductions in unintended pregnancy rates (13).
LARC Expansion in Clinical Care
In all settings where same-day LARC is provided, women are more likely to initiate the device than if a return visit is required. This increased uptake was demonstrated in post-abortion (14, 15) as well as postpartum settings (16–18). In a clinical setting requiring an initial visit to complete an order form and second visit IUD placement, only half (54%) actually returned for the device (19). More importantly, women who receive same-day IUD or implant insertions have lower short-term pregnancy rates than those with delayed insertions (14, 20, 21).
The most rigorous evidence supporting immediate first-trimester post-abortion IUD placement comes from a randomized, controlled trial (RCT) of 575 women (14). All of the women randomized to immediate postprocedure insertion received their IUD, compared with 71% randomized to delayed insertion. Within 6 months there were no pregnancies in the immediate insertion group, compared with five in the delayed insertion participants, none of whom received their IUD (14).
Evidence documenting the benefits of LARC use in the postpartum period is abundant. An assessment of 2006–2010 National Survey of Family Growth data found that 13% of postpartum women using short-acting hormonal contraception had a pregnancy within 18 months, compared with 0.5% of LARC users (adjusted hazard ratio 21.2, 95% confidence interval [CI] 6.2–72.8) (22). During the in-hospital postpartum period, the IUD expulsion rate is lowest with an immediate post-placental insertion (within 10 minutes) (23, 24). Expulsion rates among women obtaining immediate post-placental hormonal IUDs seem to be higher than for those receiving copper IUDs (25). Future research initiatives investigating optimal insertion approaches will address postpartum LARC delivery software adjustments to decrease expulsion rates.
Another area where LARC methods can greatly benefit women at high risk of unintended pregnancy is among emergency contraception (EC) users. The copper T380 IUD is the most effective method of EC (0.1% risk of pregnancy) (26), while providing ongoing contraception, regardless of insertion timing in the menstrual cycle or days since unprotected intercourse (27). One barrier to IUD insertion for EC is the preference by many women for a hormonal IUD, such as the 20-μg levonorgestrel (LNG20) IUD, over the copper T380 IUD (28). A recent study found a low risk of pregnancy when the LNG20 IUD was placed with use of oral LNG for EC (28). To further break down obstacles to the LNG20 IUD, data are needed to assess the risk of pregnancy when LNG20 IUDs are inserted alone for EC.
Data are amassing supporting extending the use of IUDs and implants beyond their US Food and Drug Administration (FDA) approval. Prospective monitoring of CHOICE study participants identified one pregnancy among 108 LNG IUD users beyond 5 years of use and no pregnancies in 123 contraceptive implant users reaching 4 years of use (29, 30). These data add to a large LNG 52 mg IUD study reporting no pregnancies among women followed from 5 to 7 years after insertion (30). Additional data are forthcoming on use of an LNG 52 mg IUD through 7 years from the ACCESS intrauterine system (IUS) phase 3 FDA study (31).
Dispelling Concerns over IUD Infection Risk
Concerns for upper genital tract infection and subsequent infertility have limited the widespread use of IUDs in the United States. Historically, the inappropriate selection of a comparison group, the overdiagnosis of pelvic inflammatory disease in IUD users, and the failure to control for confounding sexual behavior effects contributed to the misunderstanding of evidence and overestimation of infection risk (32). This misperception of increased risk is outdated, not well-supported by evidence, and should not be a barrier to utilization. The American College of Obstetricians and Gynecologists supports IUD placement and supports routine screening for sexually transmitted infections (STIs) but does not require screening before IUD insertion (11, 33, 34).
The safety of a single visit IUD insertion with simultaneous STI testing is demonstrated by an evaluation of 57,728 Kaiser Permanente Northern California patients (35). The overall rate of pelvic inflammatory disease in this study was 0.5%. There were no differences in infection rates between those receiving same-day STI testing, those screened within 3 months, or women without screening.
Other recent large, prospective studies also demonstrate pelvic infection rates of 0.5% with IUD use (36) and low infection rates for all women, including those at a high risk for STIs (37). An evidence-based approach to STI screening at the time of IUD insertion should follow current guidelines (38, 39) and should not delay or interfere with LARC initiation (40). Wide application of this evidence-based guideline is an important software update.
IUD Safety in Adolescents and Nulliparous Women
Provider concerns regarding the safety and acceptability of LARC devices limited uptake by both US adolescents and nulliparous women for decades. Fortunately, recent studies, including CHOICE (41) and ACCESS IUS (31), consciously included these women in their study designs, leading to evidence turning the tide on these outdated concerns. The recent, unprecedented decline in the US teen birth rate is a direct result of improved access to LARC methods (41, 42). The American Academy of Pediatrics (12) and American College of Obstetricians and Gynecologists (11, 34) now support LARC methods as first-line choices for adolescents and recommend LARC without restriction for nulliparous women.
Improving Reproductive Planning in Medically Complex Women
There are few populations in which improved LARC access could drastically decrease the risk of costly, adverse pregnancy outcomes more than in women with complex medical conditions. An unintended pregnancy in the setting of a poorly controlled disease increases risk of preterm delivery, congenital malformations, exposure to teratogens, and other maternal and fetal risks, which could be mitigated by disease control before conception (43–46). As the prevalence of chronic medical conditions rises in US reproductive-age women, removing LARC access barriers could have an important public health impact.
Women with chronic diseases are more likely to experience an unintended pregnancy and less likely to use LARC than healthy women (47, 48). Areas for improvement are evident in literature on disease-specific populations. Solid organ transplant patients and women with inflammatory bowel diseases are most likely to avoid any contraceptive method or rely only on condoms, despite desire to avoid pregnancy (49, 50). Women undergoing bariatric surgery are rarely referred for contraceptive counseling (51). Breast cancer patients experienced unintended pregnancies within 1 year of diagnosis owing to low contraceptive uptake (52). Collaborative care models to meet the needs of reproductive-age women managing complex conditions tend to be institutional endeavors, but epileptologists and rheumatologists are leading the way to broader improvements (53, 54). These initiatives are key: women with chronic conditions desire contraceptive counseling from their medical subspecialist and often see them more frequently than reproductive health providers. Unfortunately, many providers do not have the contraceptive knowledge to meet these needs, leading to misinformation and unfounded safety concerns (49,55–57).
To address knowledge gaps regarding contraceptive method safety for chronic conditions in reproductive-age women, the Centers for Disease Control and Prevention published expert guidance in the US Medical Eligibility Criteria for Contraceptive Use 2010 (US MEC) (58). Nearly all of the chronic diseases listed in the US MEC have safety rankings for LARC methods as category 1 (“no restriction”) or category 2 (“advantages outweigh theoretical or proven risks”). This guidance supports LARC methods as not only the most effective but also the safest options for these high-risk populations.
A companion guide to the US MEC, the US Selective Practice Recommendations, was published in 2013 to reduce barriers to contraceptive initiation and effective use (40). The US Selective Practice Recommendations provides evidence-based guidance to support same-day contraceptive provision and avoidance of unnecessary tests or examinations before initiation. Integration of these evidence-based resources into practice will allow women with a chronic disease to avoid barriers to care. Although contraceptive method selection is multifaceted for all women, recommendations based on safety and efficacy can be algorithmic. Development of evidence-based provider-facing decision support tools or patient-facing decision aids to provide risk assessment and contraceptive recommendations may be the next step to address barriers to LARC provision for all women, but especially for high-risk populations.
LARC HARDWARE ADVANCES
Development of LARC contraceptive methods, or “hardware,” includes new IUDs and subdermal implants to expand access to women with contraindications to estrogen-containing hormonal methods and potentially improve nonhormonal LARC method side effects. A definitive source of contraceptive methods under development is Calliope, the Contraceptive Pipeline Database (59). The website maintains a complete list and describes the stage of progress for each method.
Copper IUDs
Currently the only nonhormonal, FDA-approved LARC method available in the United States is the copper T380A IUD, Paragard (Teva Women’s Health). The copper IUD is being used by more than 200 million women globally (60, 61) and is produced for approximately $1 in many parts of the world. In addition to its long-acting efficacy, it is also the most effective emergency contraceptive, reducing the risk of pregnancy to 1 in 1,000 when placed within 5–7 days after unprotected intercourse (40). Despite its benefits, the increased bleeding and cramping associated with this device offer potential for improvement (62).
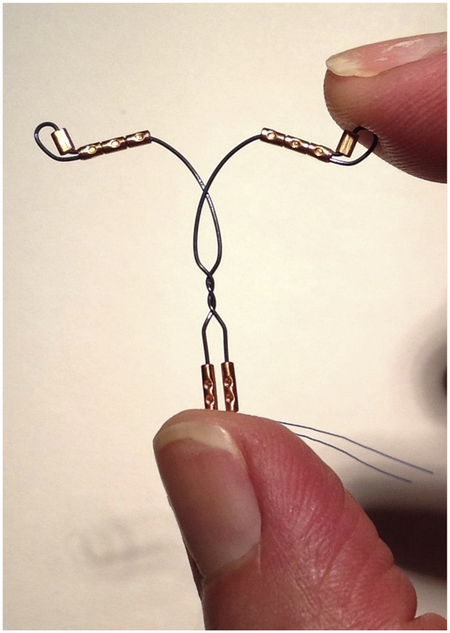
The VeraCept IUD (ContraMed) is a novel copper IUD on a flexible frame, accommodating the uterine contour with a lower total copper load to increase comfort and decrease bleeding (Fig. 1). The frame is made of nitinol, a nickel and titanium alloy, which is flexible and without memory. The insertion tube diameter is narrower than a copper T380, likely easing insertion. The 175 mm2 of copper surface area (less than half of the 380 mm2 in T380A) is strategically placed just inside the internal cervical os and bilaterally at the tubal ostea. Preliminary VeraCept studies enrolled 564 women in the Dominican Republic and reported only a single pregnancy, found to be ectopic, in more than 8,000 woman-months of use. In one of these studies, VeraCept IUD users reported less insertion discomfort, fewer expulsions, and fewer pain or bleeding removals than copper T380 users (63). The US enrollment is complete for a phase 2 FDA study, and planning is underway for the phase 3 study.
Figure 1.
Veracept IUD.
Turok. Recent and future IUD and implant advances. Fertil Steril 2016.
The intrauterine ball (IUB, OCON Medical) is another copper device using a nitinol frame loaded with several copper beads (Fig. 2). Published data are limited to small sample size reports. The most recent publication of the SCu380A IUB with a 12-mm diameter in 51 women showed 1 pregnancy, 14 expulsions (27%), and 8 removals (16%) at 12 months (64). The company website reports an unpublished trial comparing 245 SCu300A IUB users with 122 copper T380 IUD users, which found four pregnancies in the IUB group (1.63%, 95% CI 0.45–4.13) with similar cramping and bleeding reports (65). Additional data are required to determine the efficacy and utility of the IUB.
Figure 2.
Intrauterine ball.
Turok. Recent and future IUD and implant advances. Fertil Steril 2016.
Hormonal IUDs
The Mirena 52-mg LNG IUD with a 5-year FDA approval for use was the only US hormonal IUD available from 2001 to 2015. Medicines360, a nonprofit pharmaceutical company, developed a similar 52-mg LNG device, Liletta, with similar dimensions and performance characteristics as Mirena, and received FDA approval for 3 years of use in 2015. The 1-year Liletta pregnancy rate is 0.14 (95% CI 0.04–0.57) (31). With data to support up to 7 years of use for 52-mg LNG IUDs (29, 30), the phase 3 FDA Liletta study plans to continue follow-up to obtain a 7-year FDA contraceptive indication. Although this device has been safely and effectively used by nulliparous women (66), including more than 1,000 nulliparous women in the phase 3 Liletta study (31), Bayer Pharmaceuticals introduced a 13.5-mg LNG device (Skyla) in 2013. The Skyla inserter’s narrower profile makes for less painful and easier placement (67). However, the tradeoff is less frequent amenorrhea due to lower LNG volume. Bayer Pharmaceuticals completed a phase 3 study for an intermediary device intended for 5 years of use. This 19.5-mg LNG IUD maintains the narrow inserter diameter of Skyla and has bleeding characteristics intermediate to the lower and higher-volume LNG IUDs (68).
Frameless IUDs
A uterine size smaller than the dimensions of most IUDs is theorized to cause cramping and pain resulting in IUD removals (69, 70). This is plausible, because nearly half of women assessed in one study had a maximal uterine fundal width of <24 mm, smaller than the LNG 52 mg and copper T380A cross arms (both 32 mm), as well as the Skyla (28 mm) (69). A smaller or more flexible device may be better tolerated in younger and nulliparous women.
Frameless devices, available in nonhormonal and hormonal versions, have a single polypropylene suture core instead of a plastic “T” frame. Lacking cross arms, they minimally distort the uterine cavity. The frameless, copper bead containing GyneFix 200 (Fig. 3) and 330 IUDs are implanted in the endometrium at the uterine fundus (71). Studies on these devices began in 1985, and they are approved for use in the European Union, China, and Indonesia. Research demonstrated they are highly effective and comparable to the TCu380A IUD (72). A multicenter, randomized, comparative trial found some benefits to the frameless IUD, including fewer pregnancies in the second through eighth year of use, fewer ectopic pregnancies, and fewer removals for pain. The IUD removals for bleeding and other reasons were not significantly different. Overall, the pregnancy rate for the frameless IUD was 1.3 (95% CI 0.9–2.0) during the first year and 1.2 (95% CI 07–1.9) in years 2 through 8. However, the frameless IUD had more insertion failures, expulsions, and pregnancies within the first year of insertion when compared with the TCu380A (73).
Figure 3.
Gynefix 200 IUD.
Turok. Recent and future IUD and implant advances. Fertil Steril 2016.
The FibroPlant LNG-IUS (Fig. 4) is a frameless device, similar to the GyneFix IUD, with a thread and knot for uterine myometrium implantation with insertion (71). Like other LNG IUSs, the FibroPlant reduces menstrual bleeding, with 80% of 40 FibroPlant users experiencing amenorrhea after 2 years (74). In addition to the benefits of 5 years of highly effective contraception and improvements in bleeding offered by other LNG IUDs, insertion may be more tolerable for FibroPlant users.
Figure 4.
Fibroplant.
Turok. Recent and future IUD and implant advances. Fertil Steril 2016.
Unique IUD Inserters
Other innovative IUD devices available in Europe include the 5-year Femilis 60-mg LNG-IUS (Fig. 5) with a cross arm width of 28 mm; and the 3-year Femilis 40-mg Slim with a width of 24 mm (71). The advantage of the Femilis and Femilis Slim is the unique “push-in technique” of the inserter, which potentially easies insertion, lowers expulsion rates, and causes less pain with insertion and over use time (75). One prospective study of the Femilis and Femilis Slim included 143 parous and 92 nulliparous women and found no insertion failures and only one expulsion of each device, and 92.4% of women rated their pain as “none” or “slight” at the time of insertion (76). These findings associated with the unique inserter contribute to the potential desirability of this very effective method of contraception.
Figure 5.
Femilis IUD.
Turok. Recent and future IUD and implant advances. Fertil Steril 2016.
Novel IUD Approaches
A novel approach to address the increased menstrual pain and bleeding frequently occurring with the TCu380A IUD is the addition of indomethacin in five different Chinese copper IUDs (71, 77). Models of note include the Medicated Gamma Cu380, the Medicated Gamma Cu200, and the Active-γ-IUD. There is limited research available in the English literature regarding the many different devices; however, Chinese data demonstrate the benefits and efficacy of these devices when compared with other copper IUDs (71).
The Active-γ-IUD is a copper and steel T-shaped device composed of three layers: a y-shaped stainless steel wire, 200–300 mm2 of wound copper wire, and a stainless steel outer layer. A total of 25 mg of indomethacin is in silicon beads attached to the cross arms and to a silicon ring in the IUD body. Unpublished data report efficacy and side effect profiles for the Active-γ-IUD comparable to those of the TCu380A IUD.
The Medicated Gamma Cu380 is a device with a T-shaped stainless steel frame containing 380 mm2 of copper and 25 mg of indomethacin (71, 78). A study of 600 women using this device found no pregnancies over the course of 2 years, with an 8-year duration of action (77, 79). The Medicated Gamma Cu200 is a stainless steel device resembling the outline of the uterus. This device contains 200 mm2 of copper and 18 mg of indomethacin lining the interior IUD frame (77). Duration of action evidence for this device is unavailable (71).
Implants
For women who desire a LARC method and wish to avoid an IUD insertion, subdermal implants are the obvious choice. Nexplanon (Merck & Co.) is the only contraceptive implant currently FDA approved and marketed in the United States. It is approved for 3 years of use, though data suggest efficacy through 5 years (29). Although a 5-year, two-capsule levorgestrel implant (Norplant II or Jadelle) is FDA approved and available outside the United States (80, 81), it is not marketed or sold in the United States. To achieve lower cost, a generic version is manufactured in China, called the Sino-implant II. Several products currently being developed show future promise. The gestodene 36-mg single-rod implant, targeted for 3 years of use, is under development at the Shanghai Institute of Planned Parenthood Research (Shanghai Dahua Pharmaceuticals) (82) but will not meet the more stringent FDA requirements for US approval.
Development of biodegradable implants, an idea originally presented in 1979, would avoid removal requirements but comes at the cost of irreversibility (83). Although this concept is not new, no product has moved beyond the preclinical development phase. A simplistic analogy of one approach is biodegradable synthetic suture, in which a polymer of short-chain hydroxy acids is broken down in vivo by hydrolysis and enzymatic degradation. Impregnating a polymer with a progestin would allow for a contraceptive application. Progress for this contraceptive hardware advance is currently underway in several laboratories. Per the Calliope database, these include at least three promising options. One is a biodegradable contraceptive implant using poly(ω-pentadecalactone-co-p-dioxanone) co-polyesters (84), supported by FHI 360 under subcontracts from the US Agency for International Development and the Gates Foundation. A second is under investigation at the University of Washington and involves a biodegradable hydrogel 18-month rod containing contraceptive hormone microspheres. A third example under development by GeSea Biosciences with support by FHI 360 is an 18-month etonogestrel and cholesterol-fused pellet. An alternative approach to long-term contraception is an in situ depot/implant system in which a solution containing LNG and biodegradable polymers is injected and then forms a solid or semisolid reservoir. The drug is then slowly released over an extended period of time. This approach recently produced sustained LNG release from 2 weeks to 4 months in an ex vivo model (85).
Although implants offer benefits over IUDs, such as insertion without a pelvic examination, users report important side effects, namely weight gain and irregular bleeding. Recent studies offer important insights into these issues and may have implications for method continuation. The issue of weight gain for women using progestin-only contraception is addressed in a 2016 Cochrane Review on the subject that included five studies on implants and concludes that the overall quality of the evidence is low and that existing studies show limited evidence of weight change for progestin-only contraceptors compared with women using nonhormonal methods (86). A recent Jamaican RCT compared perceived and actual weight gain among 208 two-rod LNG implant initiators and 206 women who did not receive implant insertion until 3 months later. At 3 months after study enrollment more women randomized to the early insertion group reported perceived weight gain than those who had not received the implant (15% vs. 4%). Median weight gain at 3 months was 0.5 kg vs. 0.0 kg (P=.27), and the proportion of women gaining >2 kg was 23% vs. 22%, respectively (odds ratio 0.9, 95% CI 0.6–1.3) (87).
The most common reason for early implant removal is bothersome unscheduled bleeding (88), and RCTs have demonstrated methods to temporarily stop this. Guiahi et al. (89) randomized women with 7 or more consecutive days of bleeding to combined hormonal oral contraceptives or placebo. Among women receiving oral contraceptives, 88% stopped bleeding during the 14 days of treatment, compared with 38% of those receiving the placebo. The majority of oral contraceptive users (86%) resumed bleeding within 10 days of stopping the pills. Similar results were reported in an RCT of tamoxifen 10 mg twice daily vs. placebo for 7 days initiated at the onset of bothersome, unscheduled bleeding (90). Thirty days after treatment women in the tamoxifen group reported fewer days of bleeding (6 vs. 12 days, P=.05) and longer median duration of amenorrhea (30 vs. 8 days, P=.03). These studies create a platform on which to test the role of bleeding cessation in implant users and the effect on discontinuation.
In conclusion, the most rapid opportunities we have for further improvements in family planning care are broader application of the existing software advances discussed in this article. In the future we will see additional hardware advances that will expand the range of contraceptive opportunities for women. Now is the time for motivated and thoughtful clinicians to apply these proven technological updates in their daily practice and anticipate future advances. The combination of LARC software and hardware changes holds enormous potential to continue the positive trend we have seen in reducing US unintended pregnancies.
Acknowledgments
D.K.T. receives speaking honoraria from Allergan and Medicines360, is a consultant for Bioceptive, and serves on advisory boards for Allergan, Bayer, Pharmanest, and Teva. The Department of Obstetrics and Gynecology, University of Utah, receives contraceptive research funding from Bayer, Bioceptive, Medicines360, Merck, Teva, and Contramed. L.M.G. has nothing to disclose. S.L. has nothing to disclose.
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/12159-22748
REFERENCES
- 1.Blumenthal PD. Update in family planning: hardware and software improvements. Curr Opin Obstet Gynecol 2015;27:449–50. [DOI] [PubMed] [Google Scholar]
- 2.Gubrium AC, Mann ES, Borrero S, Dehlendorf C, Fields J, Geronimus AT, et al. Realizing reproductive health equity needs more than long-acting reversible contraception (LARC). Am J Public Health 2016;106:18–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins JA. Celebration meets caution: LARC’s boons, potential busts, and the benefits of a reproductive justice approach. Contraception 2014;89:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366: 1998–2007. [DOI] [PubMed] [Google Scholar]
- 5.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol 2012;120: 1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggs MA, Rocca CH, Brindis CD, Hirsch H, Grossman D. Did increasing use of highly effective contraception contribute to declining abortions in Iowa? Contraception 2015;91:167–73. [DOI] [PubMed] [Google Scholar]
- 7.Ricketts S, Klingler G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline in births among young, low-income women. Perspect Sex Reprod Health 2014;46: 125–32. [DOI] [PubMed] [Google Scholar]
- 8.Goldthwaite LM, Duca L, Johnson RK, Ostendorf D, Sheeder J. Adverse birth outcomes in Colorado: assessing the impact of a statewide initiative to prevent unintended pregnancy. Am J Public Health 2015;105:e60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol 2015;126:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016;374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee on Gynecologic Practice Long-Acting Reversible Contraception Working Group. Committee opinion no. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol 2015;126:e44–8. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Adolescence. Contraception for adolescents. Pediatrics 2014;134:e1244–56. [DOI] [PubMed] [Google Scholar]
- 13.Harper CC, Rocca CH, Thompson KM, Morfesis J, Goodman S, Darney PD, et al. Reductions in pregnancy rates in the USA with long-acting reversible contraception: a cluster randomised trial. Lancet 2015;386:562–8. [DOI] [PubMed] [Google Scholar]
- 14.Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med 2011;364:2208–17. [DOI] [PubMed] [Google Scholar]
- 15.Cremer M, Bullard KA, Mosley RM, Weiselberg C, Molaei M, Lerner V, et al. Immediate vs. delayed post-abortal copper T 380A IUD insertion in cases over 12 weeks of gestation. Contraception 2011;83:522–7. [DOI] [PubMed] [Google Scholar]
- 16.Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol 2010;116:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol 2011;117:1114–21. [DOI] [PubMed] [Google Scholar]
- 18.Ogburn JA, Espey E, Stonehocker J. Barriers to intrauterine device insertion in postpartum women. Contraception 2005;72:426–9. [DOI] [PubMed] [Google Scholar]
- 19.Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception 2012;86:694–7. [DOI] [PubMed] [Google Scholar]
- 20.Langston AM, Joslin-Roher SL, Westhoff CL. Immediate postabortion access to IUDs, implants and DMPA reduces repeat pregnancy within 1 year in a New York City practice. Contraception 2014;89:103–8. [DOI] [PubMed] [Google Scholar]
- 21.Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol 2012;206:481, e1–7. [DOI] [PubMed] [Google Scholar]
- 22.White K, Teal SB, Potter JE. Contraception after delivery and short interpregnancy intervals among women in the United States. Obstet Gynecol 2015; 125:1471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi IC, Farr G. Postpartum IUD contraception–a review of an international experience. Adv Contracept 1989;5:127–46. [DOI] [PubMed] [Google Scholar]
- 24.Lopez LM, Bernholc A, Hubacher D, Stuart G, Van Vliet HA. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database Syst Rev 2015:CD003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen BA, Reeves MF, Creinin MD, Schwarz EB. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception 2011;84:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012;27:1994–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turok DK, Godfrey EM, Wojdyla D, Dermish A, Torres L, Wu SC. Copper T380 intrauterine device for emergency contraception: highly effective at any time in the menstrual cycle. Hum Reprod 2013;28:2672–6. [DOI] [PubMed] [Google Scholar]
- 28.Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception 2016;93:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol 2015;125: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe P, Farley T, Peregoudov A, Piaggio G, Boccard S, Landoulsi S, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception 2016;93:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception 2015;92:10–6. [DOI] [PubMed] [Google Scholar]
- 32.Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet 2000;356:1013–9. [DOI] [PubMed] [Google Scholar]
- 33.American College of Obstetricians and Gynecologists. ACOG committee opinion no. 392. Intrauterine device and adolescents. Obstet Gynecol 2007;110:1493–5. [DOI] [PubMed] [Google Scholar]
- 34.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 121: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011;118:184–96. [DOI] [PubMed] [Google Scholar]
- 35.Sufrin CB, Postlethwaite D, Armstrong MA, Merchant M, Wendt JM, Steinauer JE. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol 2012;120:1314–21. [DOI] [PubMed] [Google Scholar]
- 36.Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement. Am J Obstet Gynecol 2016. May 12 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Positive testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the risk of pelvic inflammatory disease in IUD users. J Womens Health (Larchmt) 2015;24: 354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeFevre ML. US Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:902–10. [DOI] [PubMed] [Google Scholar]
- 39.Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 40.Curtis KM, Jatlaoui TC, Tepper NK, Zapata LB, Horton LG, Jamieson DJ, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep 2016;65:1–66. [DOI] [PubMed] [Google Scholar]
- 41.Secura GM, Madden T, McNicholas C, Mullersman J, Buckel CM, Zhao Q, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014;371:1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kost K, Maddow-Zimet I. U.S. teenage pregnancies, births and abortions, 2011: national trends by age, race and ethnicity. New York: Guttmacher Institute; 2016. [Google Scholar]
- 43.Mahadevan U, Sandborn WJ, Li DK, Hakimian S, Kane S, Corley DA. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology 2007; 133:1106–12. [DOI] [PubMed] [Google Scholar]
- 44.Hellerstedt WL, Pirie PL, Lando HA, Curry SJ, McBride CM, Grothaus LC, et al. Differences in preconceptional and prenatal behaviors in women with intended and unintended pregnancies. Am J Public Health 1998;88:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarz EB, Parisi SM, Handler SM, Koren G, Shevchik G, Fischer GS. Counseling about medication-induced birth defects with clinical decision support in primary care. J Womens Health (Larchmt) 2013;22:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia 2012. February 8, 10.1007/s00125-012-2455-y [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 47.Chor J, Rankin K, Harwood B, Handler A. Unintended pregnancy and postpartum contraceptive use in women with and without chronic medical disease who experienced a live birth. Contraception 2011;84:57–63. [DOI] [PubMed] [Google Scholar]
- 48.Champaloux SW, Tepper NK, Curtis KM, Zapata LB, Whiteman MK, Marchbanks PA, et al. Contraceptive use among women with medical conditions in a nationwide privately insured population. Obstet Gynecol 2015; 126:1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014;24:344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gawron LM, Gawron AJ, Kasper A, Hammond C, Keefer L. Contraceptive method selection by women with inflammatory bowel diseases: a cross-sectional survey. Contraception 2014;89:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mody SK, Hacker MR, Dodge LE, Thornton K, Schneider B, Haider S. Contraceptive counseling for women who undergo bariatric surgery. J Womens Health (Larchmt) 2011;20:1785–8. [DOI] [PubMed] [Google Scholar]
- 52.Guth U, Huang DJ, Bitzer J, Moffat R. Unintended pregnancy during the first year after breast cancer diagnosis. Eur J Contracept Reprod Health Care 2016;21:1–5. [DOI] [PubMed] [Google Scholar]
- 53.Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA, Davis AR. Contraceptive practices of women with epilepsy: findings of the epilepsy birth control registry. Epilepsia 2016;57:630–7. [DOI] [PubMed] [Google Scholar]
- 54.Guettrot-Imbert G, Morel N, Le Guern V, Plu-Bureau G, Frances C, Costedoat-Chalumeau N. Pregnancy and contraception in systemic and cutaneous lupus erythematosus. Ann Dermatol Venereol 2016. April 26 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 55.Toomey D, Waldron B. Family planning and inflammatory bowel disease: the patient and the practitioner. Fam Pract 2013;30:64–8. [DOI] [PubMed] [Google Scholar]
- 56.Jatlaoui TC, Cordes S, Goedken P, Jamieson DJ, Cwiak C. Family planning knowledge, attitudes and practices among bariatric healthcare providers. Contraception 2016;93:455–62. [DOI] [PubMed] [Google Scholar]
- 57.Dirksen RR, Shulman B, Teal SB, Huebschmann AG. Contraceptive counseling by general internal medicine faculty and residents. J Womens Health (Larchmt) 2014;23:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S medical eligibility criteria for contracepive use. MMWR Recomm Rep 2016;65:1–103. [DOI] [PubMed] [Google Scholar]
- 59.Calliope, the contraceptive pipeline database. Available at: https://pipeline.ctiexchange.org. Accessed September 9, 2016.
- 60.United Nations, Department of Economic and Social Affairs, Population Division. Trends in contraceptive use worldwide 2015. Available at: www.un.org/en/development/desa/population/publications/pdf/family/trendsContraceptiveUse2015Report.pdf. Accessed September 9, 2016.
- 61.United Nations, Department of Economic and Social Affairs, Population Division. Number of women who are married or in a union, 2015 revision. Available at: www.un.org/en/development/desa/population/theme/marriage-unions/marriage_estimates.shtml. Accessed September 9, 2016.
- 62.Hubacher D, Chen PL, Park S. Side effects from the copper IUD: do they decrease over time? Contraception 2009;79:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeves MF, Hathaway MJ, Canela Oleaga JM, Katz BH, Tal MG. A randomized single-blinded trial of VeraCept, a novel nitinol lowdose copper intrauterine contraceptive compared with a copper T380S intrauterine contraceptive. Obstet Gynecol 2015;125:5s. [Google Scholar]
- 64.Wiebe E, Trussell J. Discontinuation rates and acceptability during 1year of using the intrauterine ball (the SCu380A). Contraception 2016;93:364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.OCON Medical Ltd . Interim first year performance of the IUB SCU300A in comparison with the IUD TCU380A intrauterine device. Available at: www.oconmed.com/fileadmin/hormonfrei/user_upload/OCON_12M_IUB_SCu300A_vs_TCu380A_Report.pdf. Accessed September 9, 2016.
- 66.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol 2010;203:115, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol 2013;122:1205–13. [DOI] [PubMed] [Google Scholar]
- 68.Gemzell-Danielsson K, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two low-dose levonorgestrel intrauterine contraceptive systems: subgroup analyses of data from a phase III trial. PLoS One 2015;10:e0135309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wildemeersch D, Hasskamp T, Goldstuck N. Side effects of intrauterine devices are often related to disproportion with the endometrial cavity-is there a role for pre-insertion ultrasound? Eur J Obstet Gynecol Reprod Biol 2016; 201:215–7. [DOI] [PubMed] [Google Scholar]
- 70.Wildemeersch D. Not all IUDs fit in young nulliparous and adolescent women. J Fam Plann Reprod Health Care 2014;40:74–5. [DOI] [PubMed] [Google Scholar]
- 71.Hsia JK, Creinin MD. Intrauterine contraception. Semin Reprod Med 2016; 34:175–82. [DOI] [PubMed] [Google Scholar]
- 72.O’Brien PA, Marfleet C. Frameless versus classical intrauterine device for contraception. Cochrane Database Syst Rev 2005:CD003282. [DOI] [PubMed] [Google Scholar]
- 73.Meirik O, Rowe PJ, Peregoudov A, Piaggio G, Petzold M, IUD Research Group at the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction. The frameless copper IUD (GyneFix) and the TCu380A IUD: results of an 8-year multicenter randomized comparative trial. Contraception 2009;80: 133–41. [DOI] [PubMed] [Google Scholar]
- 74.Andrade A, Wildemeersch D. Menstrual blood loss in women using the frameless FibroPlant LNG-IUS. Contraception 2009;79:134–8. [DOI] [PubMed] [Google Scholar]
- 75.Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin 2007;34:137–66. [DOI] [PubMed] [Google Scholar]
- 76.Wildemeersch D, Janssens D, Vrijens M, Weyers S. Ease of insertion, contraceptive efficacy and safety of new T-shaped levonorgestrel-releasing intrauterine systems. Contraception 2005;71:465–9. [DOI] [PubMed] [Google Scholar]
- 77.Zhang S, Li Y, Yu P, Chen T, Zhou W, Zhang W, et al. In vitro release of cupric ion from intrauterine devices: influence of frame, shape, copper surface area and indomethacin. Biomed Microdevices 2015;17:19. [DOI] [PubMed] [Google Scholar]
- 78.Hu X, Li L, Zou Y, Wu S. A multicenter comparative study of UCu200, TCu380A, and medicated gamma-IUD devices inserted immediately after vacuum aspiration. Int J Gynaecol Obstet 2013;122:65–9. [DOI] [PubMed] [Google Scholar]
- 79.Bilian X. Chinese experience with intrauterine devices. Contraception 2007; 75:S31–4. [DOI] [PubMed] [Google Scholar]
- 80.Sivin I, Mishell DR Jr, Darney P, Wan L, Christ M. Levonorgestrel capsule implants in the United States: a 5-year study. Obstet Gynecol 1998;92:337–44. [DOI] [PubMed] [Google Scholar]
- 81.Sivin I, Alvarez F, Mishell DR Jr, Darney P, Wan L, Brache V, et al. Contraception with two levonorgestrel rod implants. A 5-year study in the United States and Dominican Republic. Contraception 1998;58:275–82. [DOI] [PubMed] [Google Scholar]
- 82.Chen H, Chen J. Research on the new one rod contraceptive implant containing gestodene. J Reprod Contracept 2007;18:86–8. [Google Scholar]
- 83.Benagiano G, Gabelnick HL. Biodegradable systems for the sustained release of fertility-regulating agents. J Steroid Biochem 1979;11:449–55. [DOI] [PubMed] [Google Scholar]
- 84.Liu J, Jiang Z, Zhang S, Liu C, Gross RA, Kyriakides TR, et al. Biodegradation, biocompatibility, and drug delivery in poly(ω-pentadecalactone-co-p-dioxanone) copolyesters. Biomaterials 2011;32:6646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janagam DR, Wang L, Ananthula S, Johnson JR, Lowe TL. An accelerated release study to evaluate long-acting contraceptive levonorgestrel-containing in situ forming depot systems. Pharmaceutics 2016;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, et al. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev 2016:CD008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gallo MF, Legardy-Williams J, Hylton-Kong T, Rattray C, Kourtis AP, Jamieson DJ, et al. Association of progestin contraceptive implant and weight gain. Obstet Gynecol 2016;127:573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S, et al. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod 2015;30:2527–38. [DOI] [PubMed] [Google Scholar]
- 89.Guiahi M, McBride M, Sheeder J, Teal S. Short-term treatment of bothersome bleeding for etonogestrel implant users using a 14-day oral contraceptive pill regimen: a randomized controlled trial. Obstet Gynecol 2015;126:508–13. [DOI] [PubMed] [Google Scholar]
- 90.Simmons K, Edelman A, Fu R, Jensen J. A short course of tamoxifen reduces unscheduled bleeding in etonogestrel contraceptive implant users. Obstet Gynecol 2016;127(Suppl 1):9S. [Google Scholar]