Abstract
Objective
The predictive value of diabetic retinopathy on end-stage kidney disease (ESKD) has not been fully addressed in patients with type 2 diabetes and diabetic kidney disease.
Research design and methods
We studied 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease who were screened for diabetic retinopathy during the 1 month of kidney biopsy. We examined the association between retinopathy progression and renal lesions. We used Cox regression analyses to explore the risk of ESKD adjusting for known risk demographic and clinical variables. We assessed the incremental prognostic value of ESKD by adding diabetic retinopathy to the clinical variables.
Results
The diabetic retinopathy progression positively correlated with all scores of renal lesions, especially with the glomerular-based classification (r=0.41), scores of interstitial fibrosis (r=0.41) and diffuse lesion (r=0.48). During a median follow-up of 5.7 years, 114 patients developed ESKD. Adjusting for known risk factors of ESKD, the HR for ESKD (patients with no apparent retinopathy as a reference) were 1.96 (95% CI 0.62 to 6.17) for patients with mild non-proliferative diabetic retinopathy (NPDR), 3.10 (95% CI 1.45 to 6.65) for patients with moderate NPDR, 3.03 (95% CI 1.44 to 6.37) for patients with severe NPDR, and 3.43 (95% CI 1.68 to 7.03) for patients with proliferative diabetic retinopathy, respectively. Addition of the retinopathy grading to the clinical model alone improved the prognostic value (the global χ2 statistic increased from 155.2 to 164.5; p<0.001), which is an improvement equivalent to the addition of the renal lesion grading to the clinical model.
Conclusions
Retinopathy progression appeared to be associated with renal lesions and the development of ESKD. Our findings suggest that diabetic retinopathy and kidney disease share the same magnitude of disease progression, and therefore diabetic retinopathy may be useful for prognosticating the clinical course for diabetic kidney disease.
Keywords: Diabetic Retinopathy, Diabetic Kidney Disease, Risk, End-stage Kidney Disease, Cohort, Renal Pathology
Significance of this study.
What is already known about this subject?
Epidemiological studies in diabetes revealed that the concordance prevalence is high between diabetic retinopathy and renal dysfunction.
What are the new findings?
Diabetic retinopathy progression positively correlated with all scores of renal lesions.
The risk for end-stage kidney disease (ESKD) increased in a stepwise fashion, from mild non-proliferative diabetic retinopathy (NPDR) to moderate NPDR, to severe NPDR, to proliferative diabetic retinopathy, even after adjusting for known risk factors for ESKD.
Addition of the retinopathy grading to the clinical model alone improved the prognostic value, which is an improvement equivalent to the addition of the renal lesion grading to the clinical model.
How might these results change the focus of research or clinical practice?
Extensive screening for diabetic retinopathy may be a powerful tool in prognosticating the clinical course for diabetic kidney disease in patients with type 2 diabetes.
Introduction
Diabetes is now a global epidemic with multiple complications.1 Diabetic kidney disease, one of the major complications of diabetes, affects about 25%–40% of patients with diabetes,2 3 and is now the leading cause of end-stage kidney disease (ESKD) worldwide.3–5 It is also associated with increased rates of cardiovascular disease (CVD) and all-cause death.6 This predicament highlights the need for prognostic tools for these outcomes, helping physicians to decide on the intensity of multifactorial therapies in patients with diabetic kidney disease so as to ultimately alter their prognosis. However, methods to predict these adverse outcomes currently rely exclusively on urine albumin and estimated glomerular filtration rate (eGFR).7 Some pathological markers of diabetic kidney disease have shown to be of incremental prognostic value in patients with diabetic kidney disease.8 9 Renal biopsy is a valuable research tool; however, it is too laborious for routine use. In addition, traditionally, diabetic kidney disease has been clinically diagnosed without kidney biopsy unless patients were suspected to have another kidney disease.
Likewise, diabetic retinopathy is one of the microvascular complications in diabetes and affects about 30% of patients with diabetes.10–12 Clinically, the diagnosis of diabetic retinopathy is screened with a funduscopy, which is a relatively handy, non-invasive method, compared with kidney biopsy. Diabetic retinopathy can be classified by severity, according to the International Clinical Diabetic Retinopathy Disease Severity Scale.13 This classification addresses disease stages and provides patients with an opportunity for early interventions to prevent blindness. Previous epidemiological studies have shown the coexistence of diabetic retinopathy and diabetic kidney disease in both type 1 and type 2 diabetes.14–17 In addition, glycemic and blood pressure control reduces the incidence and progression of both retinopathy and kidney disease in patients with diabetes, suggesting a common pathogenesis of these two complications.18–24
We postulate that diabetic retinopathy and kidney disease share the common pathogenic mechanisms, resulting in the simultaneous initiation and concurrent progression of both complications, and thus the International Clinical Diabetic Retinopathy Disease Severity Scale13 could be useful for prognosticating the clinical course for diabetic kidney disease. The objectives of our study were to (1) evaluate the association between clinical findings in the retina and pathological lesions in kidney biopsy specimens; and (2) quantify the risk for ESKD, according to the severity of diabetic retinopathy, in patients with type 2 diabetes and biopsy-proven diabetic kidney disease.
Methods
Study design and population
This is a longitudinal, retrospective study of patients with type 2 diabetes aged 32–76 years who underwent clinical kidney biopsy between 1985 and 2015 at the Toranomon Hospital and the Toranomon Hospital Kajigaya and had a pathological diagnosis with diabetic kidney disease as the only glomerular disease diagnosis and were screened for diabetic retinopathy with a funduscopic examination during the 1 month of kidney biopsy. The majority of study patients, mainly inhabiting in Tokyo Metropolis and suburban areas (Kanagawa, Saitama, and Chiba Prefectures), were under the care of the two hospitals or satellite clinics, and they were followed up until December 2017. We excluded patients with a diagnosis of concomitant kidney diseases with diabetic kidney disease, patients who underwent a protocol transplant kidney biopsy, or patients who were lost to follow-up within 3 months.
Measurements
Clinical data were collected from the medical records at the time of kidney biopsy. Clinical data included age, gender, body mass index (BMI), known duration of diabetes, medical history of CVD, ever having smoked, use of renin-angiotensin-aldosterone system (RAAS) blockade, use of glucose-lowering agents, use of statin, use of erythropoietin stimulating agents, systolic blood pressure, diastolic blood pressure, hemoglobin, hemoglobin A1c, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, uric acid, serum creatinine, eGFR (calculated by the Modification of Diet in Renal Disease study equation for Japanese25), and urine albumin to creatinine ratio (UACR). Type 2 diabetes was defined as having diabetes onset after the age of 30 years and not taking insulin at the baseline visit to our hospitals. Diabetes duration was defined as the time from the date of diabetes onset to the date of kidney biopsy.
All kidney biopsy specimens were evaluated by (1) light microscopy with H&E, periodic acid-Schiff, and Masson trichrome stain; (2) immunofluorescence microscopy with IgG, IgA, IgM, direct fast scarlet, λ light chains, κ light chains, C3, and C1q; and (3) electron microscopy. Patients with at least 5 years’ duration of diabetes and proven glomerular basement membrane thickening on electron microscopy (glomerular basement membrane >430 nm in men or >395 nm in women26) were classified as having diabetic kidney disease, and pathological findings were evaluated according to the Renal Pathology Society (RPS) Diabetic Nephropathy Classification.27 Briefly, diabetic kidney disease was classified based on glomerular changes as follows: (1) class I: glomerular basement thickening and only mild, non-specific changes on light microscopy; (2) class II: mild (IIa) or severe (IIb) mesangial expansion without either nodular lesions or global sclerosis in >50% of the glomeruli; (3) class III: nodular lesions without global sclerosis in >50% of the glomeruli; and (4) class IV: global sclerosis in >50% of the glomeruli. Pathological findings other than glomerulus were also evaluated as follows: interstitial lesions (interstitial fibrosis and tubular atrophy (IFTA) (grades 0–3) and interstitial inflammation (grades 0–2)) and vascular lesions (arteriolar hyalinosis (grades 0–2) and arteriosclerosis (grades 0–2)). We also evaluated diffuse lesion (grades 0–3), subendothelial space widening (grades 0–1), exudative lesion (grades 0–1), mesangiolysis/microaneurysm (grades 0–1), and perihilar neovascularization (grades 0–1). Patients were excluded if immunofluorescence and electron microscopy confirmed concomitant kidney diseases, such as IgA nephropathy, membranous nephropathy, or light chain deposition diseases. These procedures were conducted by three pathologists.
Diabetic retinopathy was defined as having any of the following retinal microvascular lesions: microaneurysm, retinal dot, blot hemorrhage, or neovascularization.12 With the review of medical records on retinal examination or funduscopic examination, diabetic retinopathy was graded on the International Clinical Diabetic Retinopathy Disease Severity Scale13 as follows: (1) no apparent retinopathy; (2) mild non-proliferative diabetic retinopathy (NPDR): microaneurysms only; (3) moderate NPDR: any of microaneurysms, retinal dot and blot, hemorrhages, hard exudates or cotton wool spots, but no signs of severe NPDR; (4) severe NPDR: any of intraretinal hemorrhages (≥20 in each of four quadrants), definite venous beading (in two quadrants) or intraretinal microvascular abnormalities (in one quadrant), but no signs of proliferative diabetic retinopathy (PDR); and (5) PDR: one or more of neovascularization, vitreous or preretinal hemorrhages. Patients were also evaluated whether they had diabetic macular edema (DME), defined as having retinal thickening or hard exudates in the posterior pole. These evaluations were conducted by several ophthalmologists and reviewed by a chief ophthalmologist.
The outcomes of interest were ESKD, CVD, and all-cause death events, until the end of 2017. The primary outcome, ESKD, was defined as initiation of any hemodialysis, peritoneal dialysis, or renal transplantation, or death from uremia, and occurrence of ESKD was ascertained by reviewing the database of the Japanese Society for Dialysis Therapy (JSDT). Since 1968, the JSDT has kept a complete annual renal data registry (JSDT Renal Data Registry) that covers patients on dialysis.28 The secondary outcomes of this study were CVD and all-cause mortality. The event of CVD and death was ascertained from the medical records. Patients who did not reach the outcome of interest or who were lost to follow-up were censored at their last follow-up visit.
Statistical analyses
Baseline (at the time of kidney biopsy) clinical and pathological characteristics were quantified using median and IQR for continuous variables and percentages for categorical variables. Then, these characteristics were compared across the International Clinical Diabetic Retinopathy Disease Severity Scale, with p values for trend calculated by Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. Kaplan-Meier curves for time-to-event endpoints (ESKD, CVD and all-cause mortality) for each group in the International Clinical Diabetic Retinopathy Disease Severity Scale, taking time 0 as the date of kidney biopsy, were constructed. We used log-rank test to determine whether there was a difference in survival rate. The risks of ESKD, CVD and all-cause death for each group in the International Clinical Diabetic Retinopathy Disease Severity Scale, compared with the group with no apparent diabetic retinopathy as a reference, were estimated as the adjusted HRs with 95% CIs using multivariable Cox proportional hazards model adjusted for age, gender, known duration of diabetes, baseline eGFR and UACR. Variables with more than 20% missing values were not included in the analysis.
Global χ2 (likelihood ratio), Akaike’s information criterion (AIC), and Bayesian information criterion (BIC) were used to assess the incremental prognostic value of the International Clinical Diabetic Retinopathy Disease Severity Scale and the RPS Diabetic Nephropathy Classification over standard clinical assessment of established risk factors for ESKD (age, gender, BMI, diabetic duration, systolic blood pressure, hemoglobin A1c, triglycerides, eGFR, and UACR29).
Statistical tests were considered significant at p<0.05 (two-sided). All statistical analyses were conducted using Stata V.14.1.
Results
Baseline clinical and pathological characteristics stratified by retinopathy severity scale
Baseline (at the time of kidney biopsy) clinical and pathological characteristics among different grades of diabetic retinopathy in this study are listed in table 1. The median age of the total 232 patients was 59 years old, 78% were male, with BMI of 24, known duration of diabetes of 14 years, hemoglobin A1c of 7.3% (56 mmol/mol), baseline eGFR of 39 mL/min/1.73 m2, and UACR of 1.4 g/g.
Table 1.
Baseline clinical and pathological characteristics stratified by the International Clinical Diabetic Retinopathy Disease Severity Scale
| Total | No DR | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | ||
| (n=232) | (n=55) | (n=23) | (n=42) | (n=41) | (n=71) | P value | |
| Clinical characteristics | |||||||
| Age (years) | 59 (50, 67) | 60 (43, 69) | 64 (55, 72) | 65 (58, 69) | 59 (53, 66) | 52 (42, 62) | <0.001 |
| Male (%) | 78 | 85 | 70 | 90 | 68 | 72 | 0.037 |
| BMI (kg/m2) | 24 (22, 27) | 25 (22, 28) | 24 (22, 28) | 25 (22, 27) | 23 (21, 24) | 24 (22, 26) | 0.11 |
| Diabetes duration (years) | 14 (10, 21) | 11 (4, 19) | 17 (11, 25) | 14 (8, 23) | 16 (10, 25) | 15 (11, 20) | 0.052 |
| History of CVD (%) | 24 | 24 | 39 | 29 | 24 | 15 | 0.18 |
| Ever having smoked (%) | 61 | 67 | 57 | 69 | 59 | 56 | 0.53 |
| RAAS use (%) | 66 | 49 | 87 | 81 | 49 | 72 | <0.001 |
| Glucose-lowering agents use (%) | 81 | 60 | 87 | 83 | 88 | 89 | <0.001 |
| Statin use (%) | 29 | 24 | 40 | 29 | 14 | 38 | 0.23 |
| Systolic blood pressure (mm Hg) | 145 (132, 160) | 140 (126, 148) | 141 (131, 150) | 150 (140, 167) | 152 (140, 170) | 148 (135, 163) | 0.0053 |
| Diastolic blood pressure (mm Hg) | 80 (72, 90) | 80 (73, 90) | 80 (69, 87) | 80 (71, 87) | 80 (74, 90) | 81 (72, 90) | 0.68 |
| Hemoglobin A1c (%) | 7.3 (6.4, 8.9) | 7.2 (6.4, 8.9) | 7.0 (6.3, 8.5) | 7.1 (6.3, 8.2) | 8.1 (6.8, 10.2) | 7.1 (6.1, 9.1) | 0.20 |
| (mmol/mol) | 56 (46, 74) | 55 (46, 74) | 53 (45, 69) | 54 (45, 66) | 65 (51, 88) | 54 (43, 76) | 0.20 |
| Total cholesterol (mmol/L) | 5.4 (4.5, 6.6) | 5.5 (4.6, 6.3) | 5.3 (4.6, 5.9) | 5.2 (4.3, 5.6) | 5.5 (4.4, 7.0) | 5.7 (4.5, 7.2) | 0.27 |
| Triglycerides (mmol/L) | 1.8 (1.3, 2.4) | 2.2 (1.4, 3.4) | 1.8 (1.3, 2.6) | 1.7 (1.4, 2.2) | 1.5 (1.2, 2.0) | 1.9 (1.1, 2.6) | 0.032 |
| LDL-C (mmol/L) | 3.2 (2.6, 4.4) | 3.1 (2.6, 4.2) | 3.0 (2.8, 3.8) | 3.2 (2.5, 3.6) | 3.9 (2.6, 4.7) | 3.5 (2.9, 4.9) | 0.11 |
| HDL-C (mmol/L) | 1.0 (0.9, 1.3) | 1.0 (0.8, 1.2) | 1.0 (0.7, 1.3) | 1.0 (0.9, 1.4) | 1.2 (1.0, 1.4) | 1.1 (0.9, 1.4) | 0.38 |
| Uric acid (µmol/L) | 393 (333, 446) | 387 (309, 422) | 387 (315, 488) | 399 (357, 422) | 399 (351, 470) | 405 (321, 458) | 0.33 |
| eGFR (mL/min/1.73 m2) | 39 (25, 61) | 53 (37, 75) | 46 (36, 67) | 36 (29, 57) | 29 (16, 47) | 36 (23, 55) | <0.001 |
| UACR (g/g) | 1.4 (0.5, 3.2) | 0.7 (0.2, 1.5) | 1.2 (0.7, 3.3) | 1.2 (0.3, 2.3) | 1.8 (1.0, 3.2) | 2.1 (1.0, 4.4) | <0.001 |
| Pathological characteristics | |||||||
| RPS Diabetic Nephropathy Classification | <0.001 | ||||||
| Class I | 6 | 22 | 0 | 0 | 2 | 0 | |
| Class IIa | 20 | 40 | 35 | 19 | 5 | 8 | |
| Class IIb | 28 | 20 | 17 | 33 | 20 | 41 | |
| Class III | 32 | 11 | 39 | 34 | 44 | 38 | |
| Class IV | 14 | 7 | 9 | 14 | 29 | 13 | |
| IFTA | <0.001 | ||||||
| 0 | 6 | 22 | 4 | 0 | 0 | 1 | |
| 1 | 28 | 44 | 48 | 31 | 17 | 14 | |
| 2 | 33 | 20 | 22 | 31 | 41 | 42 | |
| 3 | 33 | 14 | 26 | 38 | 42 | 43 | |
| Interstitial inflammation | |||||||
| 0 | 10 | 25 | 18 | 5 | 0 | 4 | <0.001 |
| 1 | 77 | 71 | 78 | 79 | 83 | 76 | |
| 2 | 13 | 4 | 4 | 16 | 17 | 20 | |
| Arteriolar hyalinosis | <0.001 | ||||||
| 0 | 6 | 20 | 0 | 0 | 2 | 1 | |
| 1 | 13 | 22 | 22 | 10 | 7 | 10 | |
| 2 | 81 | 58 | 78 | 90 | 91 | 89 | |
| Arteriosclerosis | 0.009 | ||||||
| 0 | 7 | 14 | 13 | 7 | 0 | 3 | |
| 1 | 47 | 53 | 26 | 45 | 39 | 56 | |
| 2 | 46 | 33 | 61 | 48 | 61 | 41 | |
| Diffuse | <0.001 | ||||||
| 0 | 6 | 23 | 0 | 0 | 5 | 0 | |
| 1 | 15 | 41 | 11 | 14 | 5 | 7 | |
| 2 | 33 | 27 | 33 | 48 | 19 | 36 | |
| 3 | 46 | 9 | 56 | 38 | 71 | 57 | |
| Exudative | 66 | 32 | 78 | 62 | 81 | 80 | 0.002 |
| Subendothelial space widening | 67 | 27 | 88 | 81 | 81 | 78 | <0.001 |
| Mesangiolysis/microaneurysm | 46 | 14 | 56 | 38 | 62 | 60 | 0.005 |
| Perihilar neovascularization | 81 | 59 | 89 | 81 | 86 | 90 | 0.062 |
Data are expressed as mean (SD), median (25th, 75th percentiles), or percentage.
RPS Diabetic Nephropathy Classification: I, mild or non-specific light microscopy changes and electron microscopy-proven glomerular membrane thickening; IIa, mild mesangial expansion; IIb, severe mesangial expansion; III, nodular sclerosis (Kimmelstiel-Wilson lesion); IV, advanced diabetic glomerulosclerosis.
BMI, body mass index; CVD, cardiovascular disease; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IFTA, interstitial fibrosis and tubular atrophy; LDL-C, low-density lipoprotein cholesterol; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; RAAS, renin-angiotensin-aldosterone system blockade; RPS, Renal Pathology Society; UACR, urine albumin to creatinine ratio.
According to the International Clinical Diabetic Retinopathy Disease Severity Scale, 55 patients were classified as no diabetic retinopathy, 23 patients as mild NPDR, 42 patients as moderate NPDR, 41 patients as severe NPDR, and 71 patients as PDR. There were significant differences in age, gender, use of RAAS blockade, use of glucose-lowering agents, systolic blood pressure, triglycerides, eGFR, UACR, and scores of renal lesions across the severity scale of diabetic retinopathy.
Baseline clinical and pathological characteristics among patients with and without DME
There were significant differences in age, gender, systolic blood pressure, total cholesterol, eGFR, and UACR; however, all renal lesions except exudative lesions were not different between those with and those without DME (shown in online supplementary table S1).
bmjdrc-2019-000726supp001.pdf (61.5KB, pdf)
Correlation between severity scale of diabetic retinopathy and renal lesions
Table 2 shows the correlation between the severity scales of diabetic retinopathy with renal lesions. The severity scale of diabetic retinopathy positively correlated with the class of RPS Diabetic Nephropathy Classification (r=0.40) and with all scores of renal lesions, especially with scores of IFTA (r=0.41) and diffuse lesion (r=0.48). For further details about the association between each of retinal finding and renal finding, the results are available in online supplementary table S2.
Table 2.
Correlation between diabetic retinopathy grades and renal lesions
| RPS Diabetic Nephropathy Classification | IFTA | Interstitial inflammation | Arteriolar hyalinosis | Arteriosclerosis | Diffuse lesion | Exudative lesion | Subendothelial space widening | Mesangiolysis/microaneurysm | Perihilar neovascularization | |
| r | 0.40*** | 0.41*** | 0.30*** | 0.32*** | 0.13* | 0.48*** | 0.35*** | 0.27** | 0.33*** | 0.25** |
Spearman’s correlation coefficients (r) between the International Clinical Diabetic Retinopathy Disease Severity Scale and renal lesions were obtained using Spearman’s correlation rank test.
*P<0.05, **P<0.01, ***P<0.001.
IFTA, interstitial fibrosis and tubular atrophy; RPS, Renal Pathology Society.
Event-free survival of ESKD, CVD, and all-cause death according to the International Clinical Diabetic Retinopathy Disease Severity Scale
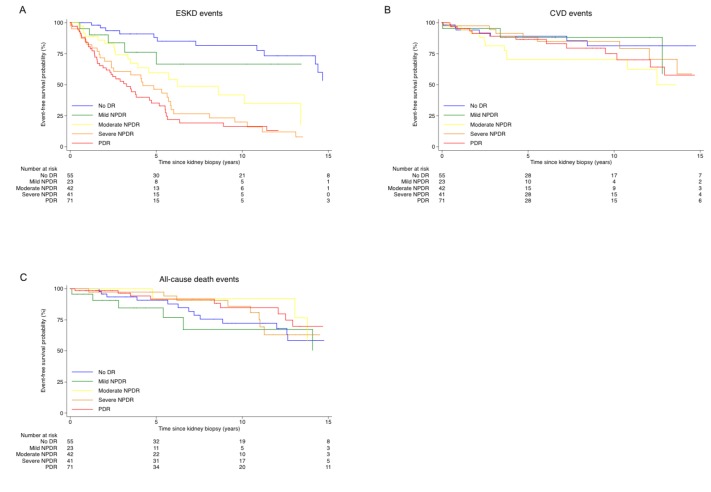
During a median follow-up of 5.7 years, 114 patients developed ESKD, 45 patients developed CVD, and 42 patients died. Figure 1 shows the Kaplan-Meier curves for time-to-event endpoints, according to the severity scale of diabetic retinopathy, taking time 0 as the date of kidney biopsy. The higher the severity scale of diabetic retinopathy went up, the poorer the ESKD prognosis became (log-rank test p<0.001). However, the severity scale of diabetic retinopathy was not shown to be a significant risk for CVD and all-cause mortality.
Figure 1.
Event-free survival for the 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. (A) Kaplan-Meier curves of ESKD event-free survival. (B) Kaplan-Meier curves of CVD event-free survival. (C) Kaplan-Meier curves of all-cause death event-free survival. CVD, cardiovascular disease; DR, diabetic retinopathy; ESKD, end-stage kidney disease; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Event-free survival of ESKD, CVD, and all-cause death among patients with and without DME
Online supplementary figure S1 shows the Kaplan-Meier curves for time-to-event endpoints among patients with and without DME, taking time 0 as the date of kidney biopsy. DME was associated with ESKD, but not with CVD or all-cause death.
bmjdrc-2019-000726supp002.pdf (133KB, pdf)
HR of ESKD, CVD, and all-cause death, according to the International Clinical Diabetic Retinopathy Disease Severity Scale
Table 3 shows the crude and adjusted HRs for the outcomes, according to the severity scale of diabetic retinopathy, among patients with type 2 diabetes and diabetic kidney disease. As compared with no diabetic retinopathy, the HR for ESKD increased in a stepwise fashion, from mild NPDR to moderate NPDR, to severe NPDR, to PDR, both in univariable and multivariable analyses: crude analyses: HR 1.35, 95% CI 0.49 to 3.76 for patients with mild NPDR; HR 2.89, 95% CI 1.42 to 5.86 for patients with moderate NPDR; HR 5.00, 95% CI 2.63 to 9.52 for patients with severe NPDR; HR 5.32, 95% CI 2.89 to 9.78 for patients with PDR; fully adjusted analyses: HR 1.96, 95% CI 0.62 to 6.17 for patients with mild NPDR; HR 3.10, 95% CI 1.45 to 6.65 for patients with moderate NPDR; HR 3.03, 95% CI 1.44 to 6.37 for patients with severe NPDR; HR 3.43, 95% CI 1.68 to 7.03 for patients with PDR, respectively. However, the univariable and multivariable Cox proportional hazard analyses showed no association between the severity of diabetic retinopathy and CVD as well as all-cause death.
Table 3.
Crude and adjusted risk for ESKD, CVD, and all-cause death, according to the severity scale of diabetic retinopathy, among patients with diabetic retinopathy, as compared with patients with no diabetic retinopathy
| ESKD | CVD | All-cause death | ||||||||||
| Model | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | Mild NPDR | Moderate NPDR | Severe NPDR | PDR |
| 1 | 1.35 (0.49 to 3.76) p=0.57 |
2.89 (1.42 to 5.86) p=0.003 |
5.00 (2.63 to 9.52) p<0.001 |
5.32 (2.89 to 9.78) p<0.001 |
1.17 (0.31 to 4.39) p=0.81 |
2.26 (0.93 to 5.52) p=0.072 |
1.22 (0.47 to 3.19) p=0.69 |
1.55 (0.67 to 3.60) p=0.31 |
1.44 (0.55 to 3.77) p=0.46 |
0.50 (0.16 to 1.53) p=0.22 |
0.84 (0.36 to 1.96) p=0.69 |
0.60 (0.26 to 1.39) p=0.23 |
| 2 | 1.53 (0.53 to 4.37) p=0.43 |
3.12 (1.49 to 6.58) p=0.003 |
6.15 (3.11 to 12.18) p<0.001 |
5.97 (3.20 to 11.14) p<0.001 |
1.40 (0.36 to 5.40) p=0.63 |
2.03 (0.81 to 5.13) p=0.13 |
1.55 (0.57 to 4.17) p=0.39 |
1.99 (0.84 to 4.69) p=0.12 |
0.67 (0.24 to 1.87) p=0.44 |
0.27 (0.09 to 0.85) p=0.025 |
0.74 (0.31 to 1.78) p=0.50 |
1.03 (0.42 to 2.53) p=0.96 |
| 3 | 1.96 (0.62 to 6.17) p=0.25 |
3.10 (1.45 to 6.65) p=0.004 |
3.03 (1.44 to 6.37) p=0.003 |
3.43 (1.68 to 7.03) p=0.001 |
1.58 (0.39 to 6.32) p=0.52 |
2.36 (0.89 to 6.26) p=0.085 |
2.01 (0.65 to 6.23) p=0.23 |
2.43 (0.92 to 6.43) p=0.073 |
0.57 (0.18 to 1.79) p=0.34 |
0.23 (0.07 to 0.74) p=0.014 |
0.50 (0.18 to 1.42) p=0.20 |
0.76 (0.28 to 2.04) p=0.59 |
HR (95% CI) and p values were determined for demographic and laboratory characteristics by univariable and multivariable Cox proportional hazard models.
Model 1: univariable (no diabetic retinopathy as reference).
Model 2: adjusted for demographic characteristics (age, gender, BMI, diabetic duration, ever having smoked, and systolic blood pressure).
Model 3: model 2 + adjusted for laboratory characteristics (hemoglobin A1c, triglycerides, eGFR, and UACR).
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; UACR, urine albumin to creatinine ratio.
HR of ESKD, CVD and all-cause death, among patients with DME
HRs for the outcomes among patients with DME as compared with patients with no DME are presented in online supplementary table S3. Similar to the findings with the severity of diabetic retinopathy, there was a higher risk of ESKD in those with DME in crude and demographic characteristics adjusted model. However, there was no association between DME and ESKD in the fully adjusted model. Likewise, the results showed no association between DME and CVD as well as all-cause death.
Incremental prognostic value of the International Clinical Diabetic Retinopathy Disease Severity Scale, RPS Diabetic Nephropathy Classification, and DME results over clinical information by global χ2, AIC, and BIC
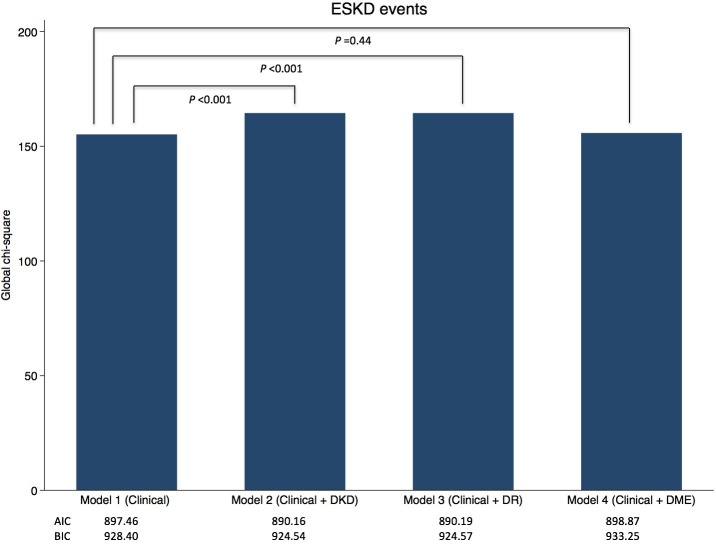
Figure 2 shows the statistics showing the incremental prognostic value of the International Clinical Diabetic Retinopathy Disease Severity Scale and the RPS Diabetic Nephropathy Classification over standard established clinical risk factors. The global χ2 statistic (likelihood ratio) increased from 155.21 to 164.48 (p<0.001) with the addition of the International Clinical Diabetic Retinopathy Disease Severity Scale to the clinical model alone, which showed an equivalent value of adding the RPS Diabetic Nephropathy Classification to the clinical model alone (global χ2 statistic increased from 155.21 to 164.51; p<0.001). Likewise, addition of the International Clinical Diabetic Retinopathy Disease Severity Scale to the clinical model alone improved the AIC value from 897.46 to 890.19, which is also an improvement equivalent to the addition of the RPS Diabetic Nephropathy Classification to the clinical model alone (AIC improved from 897.46 to 890.16). Conversely, adding DME to the clinical model did not show the incremental values (global χ2 statistic went from 155.21 to 155.80 (p=0.44), and AIC went from 897.46 to 898.87). Similar to the AIC statistics, the BIC statistics also improved when the International Clinical Diabetic Retinopathy Disease Severity Scale was added to the clinical model alone.
Figure 2.
Incremental prognostic value of the International Clinical Diabetic Retinopathy Disease Severity Scale, the Renal Pathology Society Diabetic Nephropathy Classification, and diabetic macular edema results over clinical information by global χ2, AIC, and BIC. Model 1: standard clinical assessment of established risk factors for ESKD model (age, gender, BMI, diabetic duration, systolic blood pressure, hemoglobin A1c, triglycerides, eGFR, and UACR). Model 2: model 1 + DKD. Model 3: model 1 + DR. Model 4: model 1 + DME. AIC, Akaike’s information criterion; BIC, Bayesian information criterion; BMI, body mass index; DME, diabetic macular edema; DKD, diabetic kidney disease; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; UACR, urine albumin to creatinine ratio.
Conclusions
This study demonstrated that diabetic retinopathy was correlated with changes in renal pathology, and a powerful predictor of ESKD, independent of established risk factors for ESKD, in patients with type 2 diabetes and biopsy-proven diabetic kidney disease. The study indicates that both microvascular complications—diabetic retinopathy and diabetic kidney disease—may simultaneously initiate and concurrently progress, and hence the International Clinical Diabetic Retinopathy Disease Severity Scale could be useful for prognosticating the clinical course for diabetic kidney disease. However, the degree of diabetic retinopathy was not associated with the excess risk of CVD (macrovascular complications) and all-cause death in our analysis.
The concordance prevalence between diabetic retinopathy and diabetic kidney disease has been investigated in previous cross-sectional studies both in type 1 and type 2 diabetes.14–17 However, studies implementing concrete analysis based on the correlation of anatomic measures of diabetic retinopathy and kidney disease are scarce. Only a couple of studies in type 1 diabetes investigated this and reported that anatomic changes of retina are correlated with structural changes of glomerulus.30 31 Our study showed, in accordance with previous anatomic studies, even though our study did not investigate structural changes of glomerulus, that there was a significant association between the severity of diabetic retinopathy and changes of renal pathology in patients with type 2 diabetes. The severity scale of diabetic retinopathy positively correlated with all scores of renal lesions, especially with the class of RPS Diabetic Nephropathy Classification based on glomerular damage, and scores of IFTA and diffuse lesion, all of which are major predictors of progression of diabetic kidney disease.32–35 Although the majority of the study population had both diabetic retinopathy and kidney disease to the same degree, a small number of the study population showed the discrepancy of the severities between diabetic retinopathy and kidney disease. For example, there were nine patients who had severe NPDR or PDR but had no kidney disease (class I) or mild kidney disease (class IIa). The percentage of men was higher in those nine patients than in the rest of the population (100% vs 77%, p=0.10), and also the prevalence of smoker was higher in those nine patients compared with the rest of the population (89% vs 60%, p=0.08), suggesting that male gender and smoking may have a detrimental effect on retina but less effect on kidney in patients with type 2 diabetes, although there were marginal significant differences between those nine patients and the remaining population. Conversely, there were 21 patients who had no retinopathy or mild NPDR but had advanced kidney disease (class III or IV). The prevalence of medical history of CVD was 57% in those 21 patients and 20% in the rest of the study population (p<0.001), indicating that medical history of CVD had a negative impact on kidney but had less impact on retina in patients with type 2 diabetes.
Beyond the association of diabetic retinopathy with diabetic kidney disease, there are only a couple of longitudinal studies investigating the impact of diabetic retinopathy on the prognosis of ESKD, CVD, and mortality in patients with diabetes.36–40 However, most of these studies did not take the severity of diabetic retinopathy into account. Further, the study population may have other kidney diseases other than diabetic kidney disease or concomitant kidney diseases because they were not biopsy-proven diabetic kidney diseases, which may distort the facts on each outcome. Our study provided a more indepth analysis of both the severity of diabetic retinopathy and diabetic kidney disease. We found that the risk for ESKD increased in a stepwise fashion, from mild NPDR to moderate NPDR, to severe NPDR, to PDR, even after adjusting for known risk factors for ESKD. Furthermore, we found that adding diabetic retinopathy to known risk factors for ESKD improved the prognostic value of ESKD, and also this incremental value was equivalent to the value of adding diabetic kidney scores to known risk factors. We believe that our finding may provide a profound impact on clinical practice because funduscopy examination is more convenient, less invasive, and less time-consuming, contrary to kidney biopsy. Our findings provide evidence that extensive screening of diabetic retinopathy may provide a powerful tool in predicting the risk of ESKD in patients with type 2 diabetes and diabetic kidney disease. Regarding the impact of diabetic retinopathy on CVD and death events, however, our analysis did not show a significant impact of diabetic retinopathy on CVD and all-cause mortality, which goes against previous studies that reported that diabetic retinopathy is a risk of CVD death in patients with diabetic kidney disease.38–40 These studies included a fairly large population of patients with a relatively long follow-up period, which can detect the statistical differences in CVD mortality in patients with diabetes. On the contrary, in our study, the number of outcomes may be too small to detect differences in CVD and all-cause death events across the retinopathy grading study.
Similar to the findings with the severity of diabetic retinopathy, there was a higher risk of ESKD in those with DME, although there was no association between DME and ESKD in the fully adjusted model. DME can occur at any stage of diabetic retinopathy, and if untreated it may cause visual loss.12 Our findings have clinical implications that screening for DME even in patients with mild diabetic retinopathy is a useful tool that provides an opportunity to prevent visual loss and predicts renal prognosis in patients with type 2 diabetes and diabetic kidney disease.
The strengths of our study are the use of longitudinal design rather than cross-sectional design, well validation of the grading of diabetic retinopathy and diabetic kidney disease, and the precise ascertainment of the outcomes, all of which enabled robust analysis of the risk of ESKD, CVD, and all-cause mortality in patients with type 2 diabetes and diabetic kidney disease. Several limitations of this study, however, should be mentioned. First, because of the nature of retrospective studies, there is inherent selection bias. For example, there is a possibility that study population was biopsied because they were suspected to have any kidney diseases other than diabetic kidney disease. On the contrary, however, we believe that the use of biopsy-proven rather than inaccurate clinical diagnosis of diabetic kidney disease provides a clear picture of the clinical course of diabetic kidney disease. Second, we did not consider changes in clinical variables and interventions as well during the follow-up period. Although considering them may have some advantages, we believe that the approach we adopted minimizes the risk of reverse causation in the interpretation of the results. Furthermore, we believe that the approach we used can rather see the clinical course of diabetic kidney disease as this is a daily practice cohort study. Finally, the study population was all Japanese, hence we cannot assure its generalizability to other populations.
In conclusion, the severity of diabetic retinopathy was highly correlated with changes in renal pathology, and a handy-yet-powerful predictor of ESKD, independent of known risk factors for ESKD, in patients with type 2 diabetes and biopsy-proven diabetic kidney disease. These findings imply that extensive screening for diabetic retinopathy may be a powerful tool in prognosticating the clinical course for diabetic kidney disease in patients with type 2 diabetes.
Footnotes
Contributors: MY, MM, JH and YU designed the study protocol, researched the data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. KK, TF, KO, KF and TW contributed to discussion, and reviewed and edited the manuscript. YU is the guarantor of this work, and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was supported in part by a grant for medical research from the Okinaka Memorial Institute for Medical Research, Tokyo, Japan. The funding source had no role in study design or execution, data analysis, manuscript writing, or manuscript submission.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: An institutional review board at each hospital approved the study protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. World Health Organization Global report on diabetes, 2016. Available: https://www.who.int/diabetes/global-report/en/
- 2. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 2016;316:602–10. 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. United States Renal Data System. 2016 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of health, National Institute of diabetes and digestive and kidney diseases, Bethesda, MD, 2016. [Google Scholar]
- 4. ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2015 Academic medical center, department of medical informatics, Amsterdam, the Netherlands, 2017. [Google Scholar]
- 5. Japanese Society for Dialysis Therapy Renal Data Registry. Annual Report JSDT renal data registry (JRDR). Renal Replacement Therapy 2014;3. [Google Scholar]
- 6. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. JASN 2013;24:302–8. 10.1681/ASN.2012070718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berhane AM, Weil EJ, Knowler WC, et al. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. CJASN 2011;6:2444–51. 10.2215/CJN.00580111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fufaa GD, Weil EJ, Lemley KV, et al. Brosius Fc 3rd, Yee B, Mauer M, Nelson Rg. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 2016;11:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mottl AK, Gasim A, Schober FP, et al. Segmental sclerosis and Extracapillary hypercellularity predict diabetic ESRD. JASN 2018;29:694–703. 10.1681/ASN.2017020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong TY, Klein R, Islam FMA, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141:446–55. 10.1016/j.ajo.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010;304:649–56. 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong TY, Cheung CMG, Larsen M, et al. Diabetic retinopathy. Nat Rev Dis Primers 2016;2 10.1038/nrdp.2016.12 [DOI] [PubMed] [Google Scholar]
- 13. Wilkinson CP, Ferris FL, Klein RE. Global diabetic retinopathy project group. proposed International clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. [DOI] [PubMed] [Google Scholar]
- 14. Parving HH, Hommel E, Mathiesen E, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J 1988;296:156–60. 10.1136/bmj.296.6616.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration: Pittsburgh epidemiology of diabetes complications study II. Diabetes 1990;39:1116–24. 10.2337/diab.39.9.1116 [DOI] [PubMed] [Google Scholar]
- 16. Parving H-H, Gall M-A, Skøtt P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992;41:758–62. 10.1038/ki.1992.118 [DOI] [PubMed] [Google Scholar]
- 17. Christensen PK, Larsen S, Horn T, et al. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 2000;58:1719–31. 10.1046/j.1523-1755.2000.00333.x [DOI] [PubMed] [Google Scholar]
- 18. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007;298:902–16. Review. PubMed PMID. [DOI] [PubMed] [Google Scholar]
- 19. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 20. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Uk prospective diabetes study (UKPDS) group. Lancet 1998;354:602. [PubMed] [Google Scholar]
- 21. Shichiri M, Kishikawa H, Ohkubo Y, et al. Long-Term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23:B21–9. [PubMed] [Google Scholar]
- 22. Adler AI, Stratton IM, Neil HA. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–9. 10.1136/bmj.321.7258.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berl T, Hunsicker LG, Lewis JB, et al. Impact of achieved blood pressure on cardiovascular outcomes in the irbesartan diabetic nephropathy trial. JASN 2005;16:2170–9. 10.1681/ASN.2004090763 [DOI] [PubMed] [Google Scholar]
- 24. Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005;16:3027–37. 10.1681/ASN.2004110919 [DOI] [PubMed] [Google Scholar]
- 25. Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol 2007;11:41–50. 10.1007/s10157-006-0453-4 [DOI] [PubMed] [Google Scholar]
- 26. Olson JL, Laszik ZG. Diabetic Nephropathy In: Jenette JC, Olson JL, Schwartz MM, et al., eds Heptinstall’s pathology of the kidney. 7th ed., 2015: 897–949. [Google Scholar]
- 27. Tervaert TWC, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. JASN 2010;21:556–63. 10.1681/ASN.2010010010 [DOI] [PubMed] [Google Scholar]
- 28. Japanese Society for Dialysis Therapy Renal Data Registry Annual report 2014, JSDT renal data registry (JRDR). Renal Replacement Therapy;3. [Google Scholar]
- 29. Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 2014;63:S39–62. 10.1053/j.ajkd.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 30. Klein R, Knudtson MD, Klein BEK, et al. The relationship of retinal vessel diameter to changes in diabetic nephropathy structural variables in patients with type 1 diabetes. Diabetologia 2010;53:1638–46. 10.1007/s00125-010-1763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein R, Zinman B, Gardiner R, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the renin-angiotensin system study. Diabetes 2005;54:527–33. 10.2337/diabetes.54.2.527 [DOI] [PubMed] [Google Scholar]
- 32. Furuichi K, Shimizu M, Yuzawa Y, et al. Research group of diabetic nephropathy, Ministry of health, labour and welfare of Japan, and Japan agency for medical research and development. clinicopathological analysis of biopsy-proven diabetic nephropathy based on the Japanese classification of diabetic nephropathy. Clin Exp Nephrol 2018;22:570–82. [DOI] [PubMed] [Google Scholar]
- 33. Furuichi K, Yuzawa Y, Shimizu M, et al. Nationwide multicentre kidney biopsy study of Japanese patients with type 2 diabetes. Nephrol Dial Transplant 2018;33:138–48. 10.1093/ndt/gfw417 [DOI] [PubMed] [Google Scholar]
- 34. Mise K, Hoshino J, Ueno T, et al. Prognostic Value of Tubulointerstitial Lesions, Urinary N -Acetyl- β -d-Glucosaminidase, and Urinary β 2-Microglobulin in Patients with Type 2 Diabetes and Biopsy–Proven Diabetic Nephropathy. CJASN 2016;11:593–601. 10.2215/CJN.04980515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mise K, Hoshino J, Ubara Y, et al. Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transplant 2014;29:109–18. 10.1093/ndt/gft349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moriya T, Tanaka S, Kawasaki R, et al. Japan diabetes complications Study Group. diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan diabetes complications study. Diabetes Care 2013;36:2803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang J, Wang Y, Li L, et al. Diabetic retinopathy may predict the renal outcomes of patients with diabetic nephropathy. Ren Fail 2018;40:243–51. 10.1080/0886022X.2018.1456453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Hecke MV, Dekker JM, Stehouwer CDA, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–9. 10.2337/diacare.28.6.1383 [DOI] [PubMed] [Google Scholar]
- 39. Juutilainen A, Lehto S, Rönnemaa T, et al. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007;30:292–9. 10.2337/dc06-1747 [DOI] [PubMed] [Google Scholar]
- 40. Kramer CK, Rodrigues TC, Canani LH, et al. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care 2011;34:1238–44. 10.2337/dc11-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000726supp001.pdf (61.5KB, pdf)
bmjdrc-2019-000726supp002.pdf (133KB, pdf)