Abstract
Objective
Diabetes in pregnancy and consequently the need for treatment with antidiabetic medication (ADM) has become increasingly prevalent. The prevalence and patterns of use of ADM in pregnancy from 2006 onward in seven different countries was assessed.
Research design and methods
Data sources included individually linked data from the nationwide health registers in Denmark (2006–2016), Finland (2006–2016), Iceland (2006–2012), Norway (2006–2015), Sweden (2006–2015), state-wide administrative and claims data for New South Wales, Australia (2006–2012) and two US insurance databases: Medicaid Analytic eXtract (MAX; 2006–2012, public) and IBM MarketScan (2012–2015, private). The prevalence of ADM use was calculated as the proportion of pregnancies with at least one filled prescription of an ADM in the 90 days before pregnancy or within the three trimesters of pregnancy.
Results
Prevalence of any ADM use in 5 279 231 pregnancies was 3% (n=147 999) and varied from under 2% (Denmark, Norway, and Sweden) to above 5% (Australia and US). Insulin was the most used ADM, and metformin was the most used oral hypoglycemic agent with increasing use over time in all countries. In 11.4%–62.5% of pregnancies with prepregnancy use, ADM (primarily metformin) was discontinued. When ADM treatment was initiated in late pregnancy for treatment of gestational diabetes mellitus, insulin was most often dispensed, except in the US, where glibenclamide was most often used.
Conclusions
Prevalence and patterns of use of ADM classes varied between countries and over time. While insulin remained the most common ADM used in pregnancy, metformin use increased significantly over the study period.
Keywords: drug utilization, gestational diabetes mellitus, insulin, oral antidiabetics, pharmacoepidemiology, population-based studies, pregestational diabetes, pregnancy
Significance of this study.
What is already known about this subject?
Rates of pregestational and gestational diabetes are increasing.
Traditionally, insulin has been the first-line drug in treatment of diabetes during pregnancy, however in the last decade, guidelines have started to promote oral hypoglycemic agent use in pregnancy.
What are the new findings?
Prevalence of any antidiabetic medication use in pregnancy varied from under 2% (Denmark, Norway, and Sweden) to above 5% (Australia and US).
Insulin remained the most common antidiabetic medication used in pregnancy and metformin use increased significantly over the study period.
How might these results change the focus of research or clinical practice?
The increasing number of women using different classes of antidiabetic medication in pregnancy highlights the need for better evidence on the short-term and long-term safety of oral hypoglycemic agents relative to insulin for mothers and infants
Introduction
Diabetes mellitus is an increasingly common disease worldwide, and a growing number of women of reproductive age have pregestational diabetes.1 Likewise, gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy, affecting over 15% of pregnancies worldwide.2 Obesity is related to the development of diabetes, and as the burden of the obesity epidemic grows, both pregestational diabetes and GDM are increasing in prevalence. Consequently, so too is the need for antidiabetic medication (ADM) use in pregnancy.3
Until recently, insulin was the only medication recommended for use in pregnancy for women with pregestational diabetes. Women treated with oral hypoglycemic agents (OHA) were advised to switch to insulin when they became pregnant4 (G1; see online supplementary table S1 for guideline references). A handful of small clinical studies have found metformin to be as effective as insulin for treatment of diabetes during pregnancy without increasing the risk of neonatal or maternal morbidity.5–7 Hence, some Nordic guidelines now promote the continuation of metformin from prepregnancy into the gestational period, with the addition of insulin if glycemic control is not reached (G4, G7, and G9). Additionally, metformin is recommended for the management of metabolic and menstrual irregularities in women with polycystic ovary syndrome (PCOS) and in treatment of infertility.8 9
bmjdrc-2019-000759supp001.pdf (75KB, pdf)
Insulin has also been the first-line drug treatment for GDM where management by lifestyle modification is not sufficient to control blood glucose levels. Randomized control trials (RCTs) have demonstrated the efficacy of metformin for treatment of GDM compared with insulin,10 11 and its use is supported in GDM treatment guidelines (G7 and G15). Glibenclamide (glyburide), another OHA, has been included in some US guidelines for treatment of GDM (G11 and G13).
Evidence is lacking on the short-term and long-term safety of OHA relative to insulin for mothers and infants.3 12–15 The first step towards addressing this critical issue is to assess to what extent and how OHA are used in pregnancy. A multinational study assessed ADM use in pregnancy from 2004 to 2009 in seven European countries.16 Our study extends their study period by an additional 6 years resulting in five times the number of pregnancies.
This study aimed to assess ADM utilization during pregnancy from 2006 to 2016 in seven countries on three continents, including trends in use of insulin and OHA as well as the characteristics of women and their pregnancies according to ADM use patterns in early and late pregnancy.
Methods
Study setting and data sources
This observational drug utilization study included all pregnancies recorded from 2006 to the end of the available data in the respective databases. The national medical birth registers in each Nordic country include data on all pregnancies resulting in the delivery of live born and stillborn infants from week 12 (Norway) or week 22 (Denmark, Finland, Iceland, and Sweden). For Sweden, all pregnanices of mothers who delivered at least one liveborn child were included. Unique personal identity numbers allow for linkage of data from the medical birth registers to prescribed drug registers in which dispensations of prescribed medications are recorded.
In Australia, New South Wales (NSW) is the most populous state, and the NSW Perinatal Data Collection is a statutory collection about pregnancy and outcomes for all live and stillbirths of at least 20 weeks of gestation or at least 400 g birth weight. Birth records were linked to the Pharmaceutical Benefits Scheme database and restricted to pregnancies among concessional beneficiaries for whom the database has complete capture of dispensed prescription medications.
From the US, nationwide cohorts of pregnant women linked to their live-born infants from two healthcare utilization databases were included: the Medicaid Analytic eXtract (US MAX) covering publicly insured women, and the IBM MarketScan Commerical Claims and Encounters Database containing commercially insured women.
In the Nordic countries and Australia, gestational age was determined by ultrasound examination in the first or second trimester, from which the date of last menstrual period (LMP) was confirmed. In the US, gestational age was assigned using previously validated algorithms based on international statistical classification of disease (ICD-9) codes. Additional information on the data sources is presented in online supplementary table S2.
Exposure definition and prevalence of use
ADM was identified according to the WHO Anatomical Therapeutic Chemical classification code A10 ‘Drugs used in diabetes’ (online supplementary table S3). Any ADM use in pregnancy was defined as at least one recorded dispensation from 90 days before LMP to the end of pregnancy. Prevalence of use in each population was calculated as the number of pregnancies with any ADM use divided by the total number of deliveries per year. The prevalence of use of specific classes of ADM was similarly calculated based on the number of pregnancies with at least one dispensation of insulins, biguanides, sulfonylureas or other ADM.
Four exposure periods were defined: prepregnancy (PRE: from 90 days before LMP to the day before LMP), first trimester (T1; 0–97 days of gestation), second trimester (T2; 98–202 days of gestation) and third trimester (T3; 203 days of gestation to delivery). The trimester definitions used in the Finnish data were: T1=0–84, T2=85–182, and T3=183 days of gestation to delivery.
ADM use patterns and related pregnancy characteristics
Pregnancies with ADM use were categorized into three mutually exclusive, predefined ADM use patterns based on the dispensation patterns before and during pregnancy: continuous ADM use was defined as having a dispensation in both early (PRE or T1) and late (T2 or T3) pregnancy; discontinued ADM use in prepregnancy or early pregnancy was defined as having at least one dispensation in PRE or in T1, with no further dispensations; late pregnancy ADM initiation was defined as having the first dispensation of an ADM in T2 or T3.
Characteristics of pregnancies within these three ADM use patterns were described including information, as available from each data source, on maternal age at delivery (categorized as ≤24, 25–29, 30–34, 35–39, and ≥40 years), parity (categorized as 1, 2, 3, and ≥4), body mass index (categorized as <18, 18–24, 25–29, 30–34, 35–39, and ≥40 kg/m2), smoking in the first trimester of pregnancy (yes/no), and cohabitation with a partner (yes/no). ADM class per use pattern was categorized as insulin only, biguanides only, other ADM only (all other ADM excluding insulin and biguanides), or mixed ADM (more than one class of ADM).
When available, information about diagnoses of pregestational diabetes, GDM, infertility, and PCOS was collected from the birth registers, by ICD-10 codes in the Nordic patient registers and the Australian data,17 or based on ICD-9 codes in the US healthcare utilization databases. However, because of the variability in quality and coverage within and between data sources, these diagnoses were not used to identify indications of ADM use but only for descriptive purposes.
Late pregnancy ADM initiation: assessing ADM class use
To examine which medications were used as first-line pharmacological treatment of GDM, the ADM class of the first dispensation in pregnancies with late-pregnancy ADM initiation (T2 or T3) was categorized into ‘insulin’, ‘biguanide’, or ‘other ADM’. Prevalence of each ADM category was calculated, as well as the proportion of these pregnancies with a second class of ADM dispensed.
Types of insulin use over time
Prevalence of different types of insulin use was calculated for all pregnancies with at least one dispensation of insulin and categorized into ’human insulin only’, ’insulin analogue only’, and ‘both human and analogue insulin’.
Results
Any ADM use in pregnancy
A total of 5 279 231 pregnancies from the seven countries were included, of which 3% (n=1 47 999) had at least one recorded ADM dispensation in pregnancy.
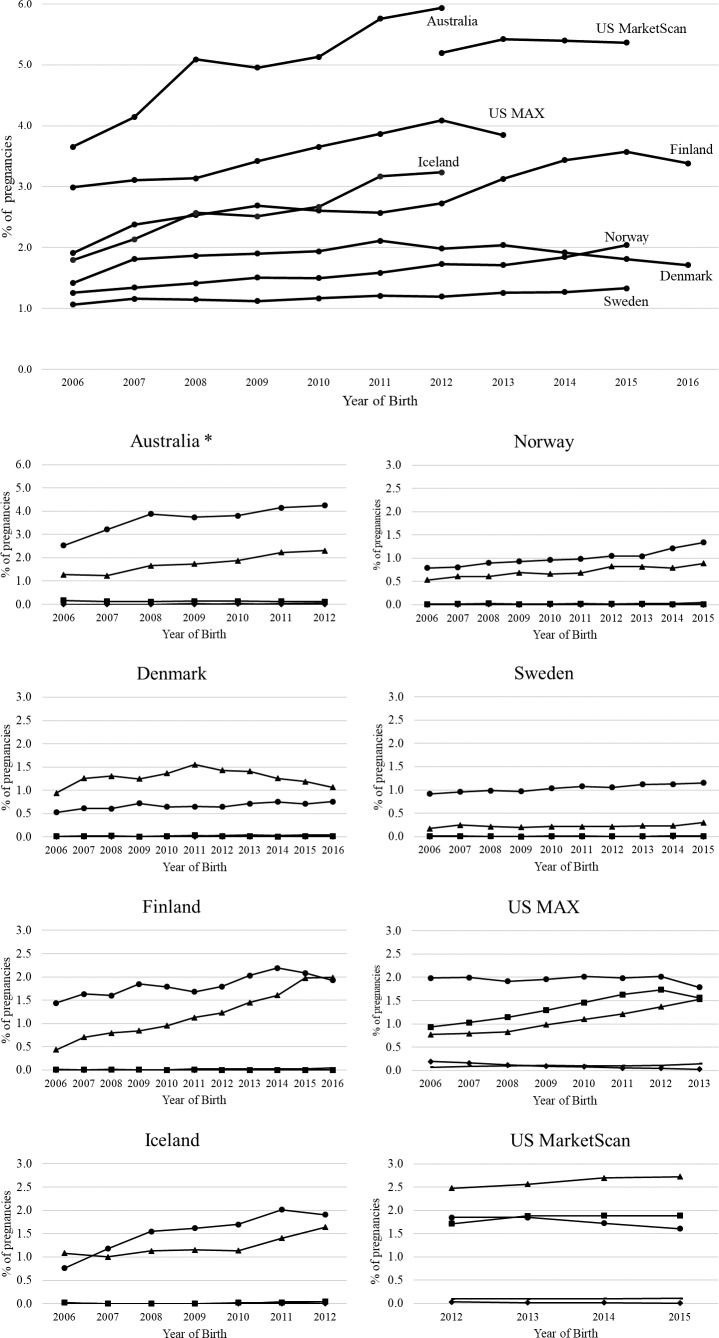
Figure 1 shows the trends of any ADM use in pregnancy over time for each country. Overall, with fewer than 2% of pregnancies, Denmark, Norway, and Sweden had the lowest use of ADM consistently over time. Highest overall use was in Australia, which increased from 3.7% in 2006 to 5.9% in 2012, followed by US MarketScan, from 5.2% in 2012 to 5.4% in 2015. ADM use increased in Iceland (from 1.8% to 3.2%), Finland (from 1.9% to 3.4%), Norway (from 1.3% to 2.0%), and US MAX (from 3.0% to 3.9%) from the first to last year of their respective study periods. Of the OHA, within the biguanide and sulfonylurea classes, respectively, metformin and glibenclamide were used almost exclusively. Insulin was the most used ADM in all countries except in Denmark, where twice as many women used metformin compared with insulin and in the US MarketScan database where metformin was most commonly used. Metformin use increased over time in Finland, Iceland, and US MAX so that by the end of their respective study periods, it was as frequently used as insulin. There were few dispensations of thiazolidinediones (0%–0.2% of all pregnancies) or other ADM classes (0.0%–0.15% of all pregnancies) including alpha glucosidase inhibitors, dipeptidyl peptidase 4 inhibitors, glucagon-like peptide-1 analogs, sodium-glucose cotransporter 2a inhibitors, and other blood glucose-lowering drugs, and when dispensed, they were dispensed only once in the pregnancy period.
Figure 1.
Prevalence of use of antidiabetic medication (ADM) in pregnancy presented by country. Black circle=insulin; black triangle=biguanides; black square=sulfonylureas; black Diamonds=thiazolidinediones, no marker=other ADM. *The y-axis scale for Australia is double that of the other countries, and the prevalence of ADM should be interpreted accordingly. Note: within the biguanide and sulfonylurea classes, respectively, metformin and glibenclamide were almost exclusively dispensed. MAX, Medicaid Analytic eXtract.
ADM use patterns and related pregnancy characteristics
Table 1 shows the characteristics of pregnancies within the three identified ADM use patterns.
Table 1.
Characteristics of women and pregnancies in three ADM use patterns presented by country
| Country | Australia | Denmark | Finland | Iceland | Norway | Sweden | US MAX | US MarketScan | ||||||||||||||||
| Years | 2006–2012 | 2006–2016 | 2006–2016 | 2006–2012 | 2006–2015 | 2006–2015 | 2006–2012 | 2012–2015 | ||||||||||||||||
| Total pregnancies, n | 114 671 | 653 659 | 652 263 | 32 267 | 590 640 | 1 081 535 | 1 253 241 | 900 955 | ||||||||||||||||
| Any ADM use, n (%) | 5665 (4.9) | 12 146 (1.9) | 17 735 (2.7) | 834 (2.6) | 9789 (1.7) | 12 905 (1.2) | 43 559 (3.5) | 45 366 (5.0) | ||||||||||||||||
| Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | |
| % of total pregnancies | 0.8 | 1.1 | 3.0 | 0.5 | 1.2 | 0.2 | 0.7 | 0.4 | 1.6 | 0.5 | 0.9 | 1.1 | 0.6 | 0.5 | 0.5 | 0.6 | 0.1 | 0.5 | 1.1 | 0.4 | 1.9 | 1.3 | 1.3 | 2.4 |
| % of any ADM users | 16.0 | 22.5 | 61.1 | 26.2 | 62.5 | 11.3 | 25.6 | 15.5 | 59.0 | 20.5 | 35.5 | 44.0 | 35.7 | 32.4 | 31.9 | 49.2 | 12.5 | 38.3 | 32.8 | 11.4 | 55.8 | 26.7 | 26.6 | 46.7 |
| Age, years | ||||||||||||||||||||||||
| ≤24 | 14.0 | 23.1 | 10.3 | 8.5 | 7.0 | 4.4 | 15.1 | 6.8 | 7.7 | 9.9 | 13.9 | 6.8 | 13.0 | 9.1 | 7.5 | 11.6 | 8.8 | 7.8 | 23.7 | 34.5 | 22.6 | 6.7 | 7.1 | 5.6 |
| 25–29 | 21.2 | 31.9 | 18.1 | 27.9 | 37.2 | 19.5 | 30.2 | 34.4 | 21.2 | 29.2 | 43.6 | 25.6 | 28.8 | 33.8 | 21.6 | 26.8 | 29.4 | 19.8 | 27.6 | 33.4 | 28.6 | 21.6 | 28.8 | 17.9 |
| 30–34 | 30.0 | 24.3 | 32.2 | 36.1 | 39.0 | 33.6 | 31.3 | 39.8 | 33.3 | 34.5 | 28.7 | 28.6 | 32.8 | 36.5 | 33.1 | 34.0 | 39.6 | 32.9 | 27.1 | 21.4 | 26.7 | 38.5 | 41.8 | 37.1 |
| 35–39 | 22.7 | 16.7 | 28.4 | 21.4 | 14.7 | 31.5 | 17.7 | 16.4 | 27.6 | 18.1 | 12.8 | 32.2 | 20.4 | 17.3 | 27.7 | 21.5 | 18.0 | 27.8 | 16.3 | 8.5 | 16.4 | 26.4 | 18.5 | 29.8 |
| ≥40 | 9.8 | 4.0 | 11.0 | 6.0 | 2.0 | 10.9 | 5.7 | 2.6 | 10.1 | 8.2 | 1.0 | 6.8 | 5.0 | 3.3 | 10.0 | 6.2 | 4.2 | 11.7 | 5.4 | 2.2 | 5.7 | 8.8 | 3.7 | 9.6 |
| Missing | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Parity | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||
| 1 | 20.3 | 26.2 | 15.9 | 45.6 | 60.8 | 32.5 | 43.5 | 61.3 | 28.9 | 33.9 | 52.0 | 28.6 | 41.9 | 54.4 | 30.5 | 41.9 | 54.0 | 31.8 | ||||||
| 2 | 33.7 | 38.3 | 30.8 | 35.9 | 31.5 | 32.7 | 33.5 | 28.8 | 33.9 | 39.2 | 32.4 | 36.5 | 35.6 | 31.6 | 36.3 | 35.3 | 34.4 | 32.5 | ||||||
| 3 | 18.7 | 21.0 | 22.1 | 12.0 | 6.5 | 20.4 | 13.3 | 7.4 | 19.3 | 19.3 | 13.2 | 24.5 | 15.1 | 10.4 | 19.3 | 14.1 | 7.9 | 17.9 | ||||||
| ≥4 | 27.2 | 14.4 | 31.0 | 6.4 | 1.3 | 14.4 | 9.6 | 2.4 | 17.9 | 7.6 | 2.4 | 10.4 | 7.4 | 3.6 | 13.9 | 8.7 | 3.6 | 17.8 | ||||||
| Missing | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| BMI, early pregnancy | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||
| <18.5 | 0.9 | 2.3 | 1.46 | 1.4 | 1.6 | 0.5 | 0.5 | 0.72 | 0.6 | 0.1 | 0.1 | 0.1 | ||||||||||||
| 18.5–24 | 41.2 | 41.8 | 20.5 | 41.7 | 37.0 | 18.9 | 15.9 | 15.2 | 11.4 | 33.8 | 23.1 | 18.0 | ||||||||||||
| 25–29 | 26.9 | 23.7 | 26.4 | 25.7 | 25.9 | 28.6 | 13.7 | 12.6 | 15.4 | 32.4 | 27.5 | 28.4 | ||||||||||||
| 30–34 | 14.6 | 17.4 | 24.0 | 15.0 | 20.1 | 25.0 | 8.7 | 11.13 | 12.4 | 16.0 | 23.0 | 24.4 | ||||||||||||
| 35–39 | 8.0 | 7.8 | 13.5 | 9.2 | 10.2 | 15.2 | 4.1 | 5.67 | 6.1 | 7.7 | 12.7 | 14.2 | ||||||||||||
| ≥40 | 4.8 | 3.8 | 11.6 | 5.7 | 4.1 | 10.4 | 1.5 | 2.62 | 4.1 | 3.7 | 6.1 | 8.3 | ||||||||||||
| Missing | 3.4 | 3.2 | 2.55 | 1.3 | 1.5 | 1.5 | 55.6 | 52.1 | 50.0 | 6.3 | 7.6 | 6.6 | ||||||||||||
| Smoking, early pregnancy* | N/A | N/A | N/A | |||||||||||||||||||||
| No | 75.2 | 80.0 | 78.6 | 82.2 | 89.7 | 79.4 | 81.0 | 89.6 | 79.3 | 76.6 | 80.8 | 74.1 | 88.6 | 91.0 | 89.2 | 97.0 | 97.4 | 97.7 | 98.0 | 98.6 | 98.0 | |||
| Yes | 24.5 | 19.5 | 20.8 | 15.0 | 8.3 | 16.7 | 16.3 | 8.4 | 18.0 | 9.2 | 8.1 | 11.0 | 7.5 | 4.5 | 7.6 | 3.0 | 2.6 | 2.3 | 2.0 | 1.4 | 2.0 | |||
| Missing | 0.4 | 0.7 | 0.6 | 2.7 | 2.1 | 3.9 | 2.7 | 2.0 | 2.7 | 14.2 | 11.1 | 14.9 | 3.9 | 4.5 | 3.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| Relationship status† | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||
| Cohabiting | 70.6 | 75.4 | 73.6 | 53.0 | 57.1 | 59.9 | 89.9 | 95.5 | 91.4 | 84.8 | 93.6 | 87.5 | 91.7 | 94.7 | 90.4 | 89.0 | 91.0 | 86.7 | ||||||
| Not cohabiting | 29.4 | 24.6 | 26.4 | 47.0 | 42.9 | 40.0 | 9.8 | 4.9 | 8.7 | 14.0 | 5.1 | 12.3 | 7.3 | 4.7 | 8.9 | 2.3 | 1.4 | 3.7 | ||||||
| Missing | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.1 | 1.2 | 1.4 | 0.3 | 1.0 | 0.6 | 0.7 | 8.7 | 7.5 | 9.6 | ||||||
Continuous ADM use was defined as at least one ADM dispensation in the 90 days before pregnancy or in trimester 1 and at least one dispensation in trimester 2 or 3.
Discontinued ADM use in prepregnancy and early pregnancy was defined as having at least one dispensation in the 90 days before pregnancy or in trimester 1 with no further dispensations.
Late pregnancy ADM initiation was defined as a first ADM dispensation in trimester 2 or 3.
*For Australia, smoking anytime during pregnancy.
†For Denmark, this variable reflects married and registered partnerships.
ADM, antidiabetic medication; BMI, body mass index;MAX, Medicaid Analytic eXtract; N/A, data not available.
Continuous ADM use
Of all pregnancies with ADM use, 16% (Australia), 26% (Denmark and Finland), 21% (Iceland), 36% (Norway), 49% (Sweden), 33% (US MAX), and 27% (US MarketScan) of pregnancies used ADM continuously through pregnancy. Continuous ADM users were generally older and more often smokers than women with discontinued ADM use. Table 2 shows the prevalence of pregestational diabetes diagnoses within each ADM use pattern. Specifically, the prevalence of type 1 diabetes diagnoses varied greatly between countries for women with continuous ADM use in pregnancy: 17.5% in the US datasets, 45.6% in Iceland, and from 60% to 80% in the remaining Nordic countries (Australia did not have data separately for type 1 diabetes). This is also reflected in the pattern of ADM class dispensing within this group of pregnancies: Finland, Denmark, Norway and Sweden had the highest rates of pregnancies with only insulin use when compared with Iceland, Australia, and the US where more pregnancies used oral ADM.
Table 2.
Antidiabetic medication class and diagnoses in women and pregnancies in three ADM use patterns presented by country
| Australia | Denmark | Finland | Iceland | Norway | Sweden | US MAX | US MarketScan | |||||||||||||||||
| Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | Continuous (%) | Discontinued (%) | Late pregnancy (%) | |
| ADM class | ||||||||||||||||||||||||
| Insulin only | 46.8 | 10.8 | 90.7 | 75.9 | 1.1 | 97.0 | 72.5 | 3.5 | 60.1 | 54.4 | 1.7 | 95.3 | 71.2 | 4.7 | 80.6 | 86.6 | 10.0 | 96.6 | 70.2 | 6.7 | 46.7 | 30.2 | 1.8 | 33.6 |
| Biguanides only * | 2.0 | 81.3 | 7.0 | 8.1 | 98.1 | 1.9 | 7.9 | 93.9 | 26.6 | 19.6 | 96.0 | 2.7 | 10.6 | 93.1 | 15.0 | 1.4 | 86.8 | 2.1 | 11.0 | 50.3 | 5.5 | 28.5 | 93.0 | 6.1 |
| Other ADM only † | 1.4 | 0.7 | 0.3 | 0.0 | 0.3 | 0.8 | 0.2 | 0.6 | 4.2 | 0.0 | 0.6 | 0.0 | 0.3 | 0.7 | 0.1 | 0.0 | 1.1 | 0.1 | 6.1 | 12.2 | 38.2 | 2.4 | 1.6 | 52.1 |
| Mixed ADM ‡ | 49.8 | 6.5 | 2.0 | 16.0 | 0.6 | 0.4 | 19.5 | 2.0 | 9.1 | 26.0 | 1.7 | 1.9 | 17.8 | 1.5 | 4.3 | 12.0 | 2.0 | 1.2 | 12.7 | 30.8 | 9.5 | 39.0 | 3.7 | 8.2 |
| Recorded diagnoses§ | ||||||||||||||||||||||||
| Pregestational diabetes | 56.8 | 11.1 | 4.6 | 80.8 | 4.0 | 18.7 | 82.9 | 4.1 | 1.4 | 57.9 | 0.7 | 4.6 | 81.7 | 6.8 | 14.6 | 89.8 | 10.5 | 8.9 | 63.8 | 18.8 | 3.0 | 41.8 | 3.9 | 1.9 |
| GDM | 23.8 | 11.9 | 77.7 | 1.7 | 6.2 | 51.2 | 0.0 | 0.0 | 84.0 | 28.7 | 9.1 | 91.6 | 8.2 | 8.1 | 78.5 | 22.4 | 7.1 | 91.9 | 1.8 | 0.4 | 86.7 | 45.4 | 24.0 | 96.2 |
| Infertility | N/A | N/A | N/A | 0.9 | 1.9 | 0.2 | 0.1 | 0.1 | 0.0 | N/A | N/A | N/A | 0.2 | 1.4 | 0.2 | 15.1 | 56.2 | 15.7 | 1.1 | 5.7 | 0.6 | 22.5 | 40.5 | 12.3 |
| PCOS | N/A | N/A | N/A | 8.3 | 36.3 | 6.1 | 0.2 | 0.4 | 0.1 | 13.5 | 8.8 | 2.5 | 7.8 | 30.7 | 3.3 | 3.0 | 21.7 | 3.2 | 3.3 | 21.6 | 0.5 | 35.6 | 62.3 | 7.4 |
Continuous ADM use was defined as at least one ADM dispensation in the 90 days before pregnancy or in trimester 1 and at least one dispensation in trimester 2 or 3.
Discontinued ADM use in prepregnancy and early pregnancy was defined as having at least one dispensation in the 90 days before pregnancy or in trimester 1 with no further dispensations.
Late pregnancy ADM initiation was defined as a first ADM dispensation in trimester 2 or 3.
*Within the biguanide ADM class, metformin was almost exclusively dispensed.
†Within the other ADM category, glibenclamide was almost exclusively dispensed.
‡Mixed ADM was defined as dispensations of more than one class of ADM in the same pregnancy period.
§Pre-existing diabetes category combines type 1 and type 2 diabetes diagnoses. The row percentages may add to more than 100% due to the individual receiving more than one type of diabetes diagnosis. Diagnoses should be interpreted with caution due to the likelihood of misclassification of recorded diagnosis.
ADM, antidiabetic medication; GDM, gestational diabetes mellitus;MAX, Medicaid Analytic eXtract; N/A, data not available; PCOS, polycystic ovary syndrome.
Discontinued ADM use in prepregnancy or early pregnancy
ADM use was discontinued in 11%–35% of pregnancies with ADM use in all countries, except Denmark, where 62.5% had discontinued use. Compared with all other pregnancies with ADM use, women with discontinued use of ADM had lower parity. The majority of the pregnancies in this group (82%–98%) had dispensations of only metformin, with the exception of pregnancies in US MAX where 50% had dispensations of only metformin and 31% had dispensations of more than one class of ADM. Women with discontinued ADM use had the highest rates of infertility and PCOS diagnoses of all ADM use patterns.
Late pregnancy ADM initiation
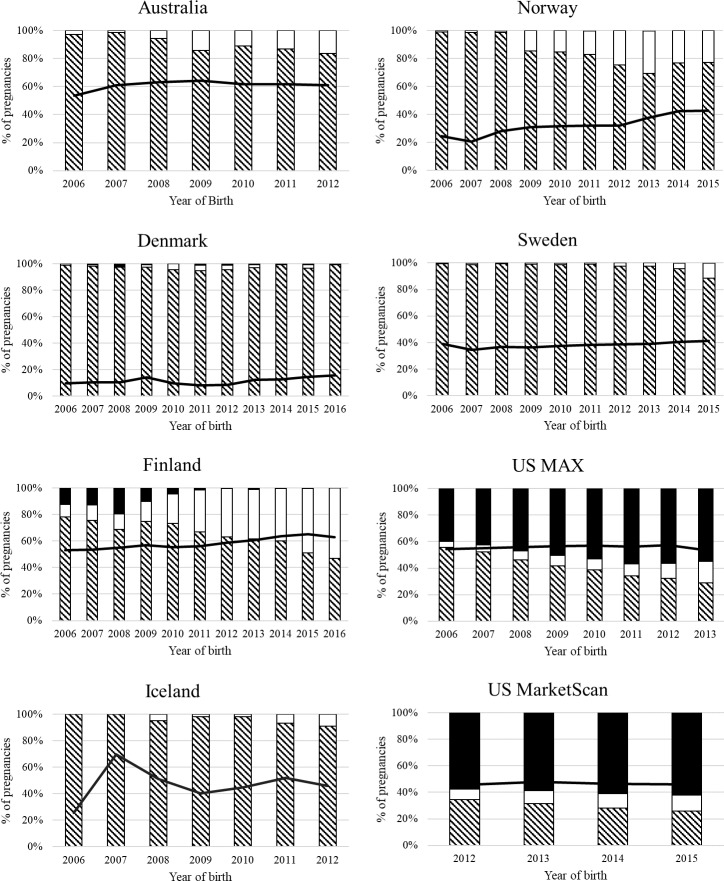
Over the entire time period for each country, the percentage of all pregnancies with late pregnancy initiation of ADM ranged from 0.2% (Denmark) to 3.0% (Australia), representing from 11% (Denmark) to 59% (Finland) of pregnancies with ADM use. This percentage increased over time in all countries except in the US databases, which remained steady as shown in figure 2. Women with late pregnancy initiation had, compared with all other ADM use patterns, a higher BMI in all countries where data were available and were older in all countries except in the US MAX database, where the age distribution was similar in continuous and late pregnancy ADM users. The majority of pregnancies with late pregnancy ADM initiation (52%–96%) had a recorded diagnosis of GDM.
Figure 2.
ADM class dispensing patterns in late pregnancy in 2006, 2012, 2015/2016 presented by country. Striped bar=insulin, white bar=biguanides, black bar=other ADM, black line=% of all pregnancies with late pregnancy initiation of ADM defined as first ADM dispensation in trimester 2 or 3. ADM, antidiabetic medication; MAX, Medicaid Analytic eXtract.
Among the pregnancies with late pregnancy ADM initiation, insulin was most often the first dispensed ADM in all countries, with the exception of the US where it was most often glibenclamide. Towards the end of the study period in Australia, Norway, Finland, Iceland, and Sweden, metformin increasingly became the first ADM dispensed (figure 2). In Finland from 2006 to 2010, up to 20% of pregnancies had a dispensation of guar gum, an add-on medication for diabetes treatment. A second class of ADM was dispensed after the first in 0.4% (Denmark) to 9.5% (US MAX) of pregnancies with late pregnancy ADM initiation (table 2). The majority of these pregnancies began with a metformin dispensation followed by an insulin dispensation, except in Norway, where an insulin dispensation was followed by metformin in all cases, and in the US, where 54% and 56% (MarketScan and MAX, respectively) of these pregnancies began with glibenclamide and then were dispensed insulin.
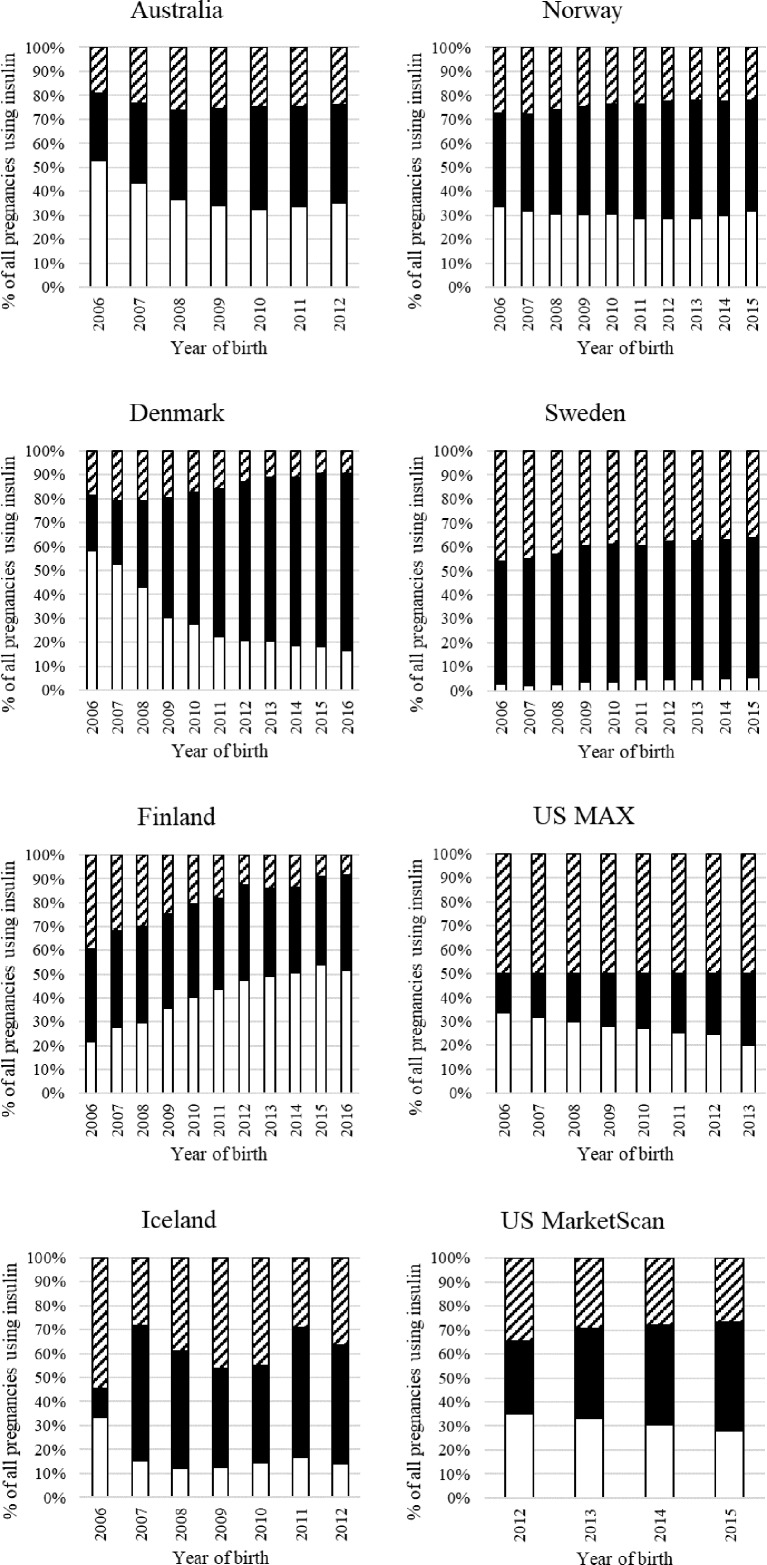
Type of insulin
Insulin analogs were the most commonly dispensed insulins in all countries, frequently combined with human insulin (figure 3). Finland was the only country where human insulin use increased over time. Denmark had a marked decrease in the number of pregnancies that were dispensed both types of insulin. Sweden had the lowest use of human insulin, which accounted for less than 5% of pregnancies with insulin use.
Figure 3.
Type of insulin dispensed in pregnancy over time by country. White bar=human insulin; black bar=analog insulins; striped bar=both human and analog insulin. MAX, Medicaid Analytic eXtract.
Discussion
Summary of our findings
In this study of over 5 million pregnancies from seven countries from 2006 onward, 1%–6% of pregnant women used ADM in the 3 months before or during pregnancy. ADM use generally increased over time, and insulin remained the most commonly used drug. Metformin use increased, most markedly in Finland and Iceland. Glibenclamide was rarely used outside of the US, where its use predominated the choice of pharmacologic treatment for GDM.
These results are in agreement with the ADM utilization in pregnancy study by Charlton et al (2016),16 which covered ADM use data from 2004 to 2009 in seven European countries, including Denmark and Norway.
ADM use patterns
Pregnancies were classified into three distinct predefined ADM use patterns based on ADM dispensation patterns in the 3 months before and during pregnancy (continuous use, discontinued use, and late pregnancy initiation). Maternal characteristics were consistent with expectations for each use pattern. As an example, women with continuous use of ADM throughout pregnancy were assumed to be treated for pregestational diabetes. The majority of continuous ADM users were dispensed only insulin, and in Denmark, Finland, Norway, and Sweden, the majority of these pregnancies had a recorded diagnosis of type 1 diabetes. This is in-line with the knowledge that the Nordic countries have the highest prevalence of type 1 diabetes globally and relatively low prevalence of type 2 diabetes in young women, except for Iceland, where prevalence rates are more similar to those of Australia and the US, which is also reflected in our data.18–20
Pregnancies with late pregnancy ADM initiation were assumed to be in women diagnosed with and pharmacologically treated for GDM. Characteristics of this group, compared with women who did not use ADM and those who had continuous ADM use during pregnancy, include higher age and BMI, which are known risk factors for GDM.21 This late pregnancy ADM initiation group may also include women who were managing pregestational type 2 diabetes with lifestyle modification who required the addition of ADM medication for glycemic control in pregnancy. However, without precise information about indication we are unable to specifically identify this subgroup of women.
Lastly, women with discontinued ADM use were assumed to be predominantly women being treated with metformin for PCOS and infertility before their conception of the recorded pregnancy. This is supported by the lower parity among women in this group and the higher rates of PCOS and infertility diagnoses compared with the other ADM use patterns. Similarly, Charlton et al 16 found a high level of discontinuation of metformin during pregnancy and furthermore, a US study from 2001 to 2007 reported that most women (67%) using metformin before pregnancy had a diagnosis for polycystic ovaries or infertility and that two-thirds did not continue ADM use during pregnancy.22 However, women with PCOS also have a higher risk for developing GDM,23 and a possible scenario is that a woman is using metformin prepregnancy, discontinues before or soon after conception, and then in late pregnancy begins ADM use for GDM treatment. This treatment pattern would unavoidably result in them being classified into the continuous ADM use pattern. Furthermore, women with chronic conditions where medication is necessary or strongly recommended, including diabetes, have been reported to discontinue their medication use due to the perception of harm,24 and therefore, it cannot be ruled out that some women in this category discontinued their ADM treatment for diabetes. Notably, Denmark had the highest percentage of pregnancies with discontinued use of ADM (62.5%) and 95%–99% of them used metformin.
Patterns in ADM class use over time
Charlton et al 16 reported a slight increase in overall insulin prescribing from 2004 to 2009 and by 2005, insulin analogs were the most common type of insulin prescribed to pregnant women. We found that insulin analogs continued to be the most dispensed insulin type from 2006 to 2015, alone or most often in combination with human insulin. Drug reimbursement schemes may affect the prevalence of use of the different types of insulin in some countries. For example, in Norway, insulin analogs are not eligible for reimbursement for type 2 diabetes or GDM until after human insulin has been tried.25
Two notable and interconnected trends continued over the years in both the Charlton et al study (ending in 2009) and our study data (ending in 2016). First, there was an increase in overall ADM use over time, primarily attributable to an increase in OHA use in pregnancy. Second, there was an increase in the number of pregnancies pharmacologically treated for GDM overall, however with great variability in the prevalence between countries and databases.
The observed trend of increasing oral ADM use in pregnancy over time is likely due to two main factors: the increase in both pregestational diabetes and GDM,26–28 as well as the issuing of guidelines recommending the use of OHA during pregnancy for treatment of diabetes. In 2008, the results of an RCT showing no difference in perinatal outcomes between metformin and insulin treatment for GDM were published,11 and a number of studies with similar conclusions followed.29 The UK’s National Institute for Health and Care Excellence 2008 guidelines for diabetes treatment in pregnancy swiftly incorporated these results (G16), beginning a trend of other European guidelines advising on the suitability of metformin for treatment of diabetes in pregnancy. This likely explains the increase in the number of pregnancies with metformin use over time in all countries in this study, and even in Sweden, where clinical guidelines only started recommending the use of metformin in pregnancy in 2010 for continued type 2 diabetes treatment and in 2015 for GDM. In Denmark, metformin use peaked in 2011 and then started to decline, mimicking the metformin use pattern in the general population, after which an increase in newer oral ADM was observed but which was not reflected in our pregnancy data.30
We report an almost doubling in the use of metformin during pregnancy in NSW, Australia. The 2016 guidelines of the Royal Australian College of General Practitioners and Diabetes Australia state that metformin can be used in pregnancy for the treatment of type 2 diabetes; however, the patient should be informed of the lack of long-term safety information. For GDM, the guidelines state that metformin has not been approved (G1). Adherence to these guidelines is reflected in our results.
US guidelines still maintain that insulin is the only safe choice for pharmaceutical treatment of diabetes in pregnancy, but the American Diabetes Association recommendations state that metformin and glibenclamide can be used with the caveat of there being no longer term follow-up on the children exposed to these medications in pregnancy (G12 and G14). A previous study in the US MarketScan database showed that glibenclamide use increased from 2000 to 2011 to replace insulin as the most common GDM treatment from 2007 onward.31 Our study includes US MarketScan data from 2012 to 2015 and shows that glibenclamide continues to be the most commonly used ADM for GDM treatment, and overall, metformin is the most commonly used ADM in this population of privately insured pregnant women. In the US MAX database, which is a population of pregnant women publicly insured by Medicaid, insulin remained the most commonly dispensed ADM; however, glibenclamide use increased specifically as first-line treatment for GDM.
The variability in the number of pregnancies being treated for GDM is likely a result of a number of factors, including the distribution of lifestyle risk factors among pregnant women such as an older age and higher BMI at pregnancy within each population,28 as well as an ongoing debate on the screening guidelines and diagnostic criteria for GDM. In 2013, in an attempt to have a single global guideline, the WHO published diagnostic criteria for the diagnosis of hyperglycemia in pregnancy (G17). Many countries have been slow or reluctant to adopt these recommendations resulting in continued variability in practice between countries and even within countries.32 However, Australia in 2015 (G1) and Sweden in 2018 (G10) implemented the WHO guidelines resulting in a lower threshold for identifying hyperglycemia in pregnancy and now a doubling to tripling of the number of women diagnosed with GDM in both countries is anticipated.33 34 Hence, the use of ADM in pregnancy is expected to continue to rise overall and specifically in countries adopting these WHO guidelines.
Future research
The increasing rates of metformin use in pregnancy can be expected to continue as previously unknown benefits of its use outside diabetes are explored. For example, two recently published RCTs have reported that metformin taken during pregnancy reduces maternal weight gain in obese mothers without diabetes and reduces the risk of late miscarriage and preterm birth in women with PCOS.35 36 However, there is insufficient high-quality evidence confirming the safety and efficacy of metformin and other OHA on key maternal and infant health outcomes.3 12–15 For example, prenatal exposure to metformin compared with insulin or placebo in RCTs has been reported to be associated with increased offspring weight.37 38 Furthermore, there is concern about the long-term metabolic health of children exposed to metformin and glibenclamide in utero.3 Hence, large-scale, long-term safety studies are needed.
Strengths and limitations
The strengths of this drug utilization study are based on the use of a common protocol to collect similar data about ADM use in pregnancy from seven different countries. The Nordic data were population based and included all pregnancies and dispensations within the countries within the study period. The Australian NSW region data included pregnancies from all social security beneficiaries, and the privately and publicly insured health utilization data from the US ensured representativeness of the total US population.
As data were collected from drug dispensations and healthcare utilization data, there is no participant recall bias on medication use during pregnancy. However, dispensation data do not capture actual consumption and adherence to the medication. Lastly, in our data, the indication was not recorded as part of the medication dispensation or reimbursement, and diagnoses of diabetes, PCOS and infertility had varying quality and completeness in the databases and registers resulting in under-ascertainment of diabetes diagnoses. Consequently, ADM use patterns were created based on ADM dispensation patterns; however, it was reassuring that data on maternal characteristics were consistent with expectations for each use pattern.
Conclusions
In conclusion, from 2006 to 2016, ADM use in pregnancy increased and specifically OHA, including metformin in all countries and glibenclamide in the US. This increase can be attributed to the increase in the number of women with pregestational diabetes and GDM in pregnancy, the adoption of OHA in treatment guidelines for diabetes in pregnancy and expanding indications for the use of metformin in women of childbearing age. The number of women treated with ADM in pregnancy is expected to continue to increase and calls for additional large studies on the safety of OHA compared with insulin with regards to both short-term and long-term consequences for mothers and their children.
Acknowledgments
The authors would like to thank Pär Karlsson (Centre for Pharmacoepidmiology, Karolinska Institutet), Professor Mette Nørgaard (Aarhus University), and Professor Anders Engeland (University of Bergen). The authors would like to thank the NSW Ministry of Health, the Australian Government Department of Health and Ageing and the Department of Human Services for providing data. The authors would also like to thank the Centre for Health Record Linkage and the Australian Institute for Health and Welfare for conducting the linkage of records.
Footnotes
Contributors: Concept and design: all authors. Analysis: CEC, AnH, MKL, DT, and YY. Interpretation of data: all authors. Drafting of article: CEC, JMC, LP, and IO. Critical revision for important intellectual content: all authors. CEC takes full responsibility for the work as a whole, including the study design, access to data and the decision to submit and publish the manuscript.
Funding: This study was funded by NordForsk as part of the Nordic Pregnancy Drug Safety Studies project (Project No: 83539) and the Research Council of Norway as part of the International Pregnancy Drug Safety Studies (InPreSS) (Project No: 273366) both awarded to KF at Norwegian Institute of Public Health (NIHP). Linkage of the Australian data was supported by an Australian National Health and Medical Research Council Project grant (No. 1028543). GB was supported by the Swedish Society of Medicine (InPreSS grant) and the Stockholm County Council (clinical postdoctoral appointment). HZ was funded by a Scientia Fellowship awarded by UNSW. BTB, SH-D and KFH were supported by the grant R01HD097778 from the Eunice Kennedy Shriver National Institute for Child Health & Human Development. YY’s salary is paid by unrestricted grants from the Lundbeck Foundation (R232-2016-2462 and R265-2017-4069), unrelated to this work. The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Competing interests: CEC, GB, HK, LP, and IO are employees at the Centre for Pharmacoepidemiology, which receives grants from several entities (pharmaceutical companies, regulatory authorities and contract research organizations) for the performance of drug safety and drug utilization studies, unrelated to this work. HLG reports fees and grants from pharmaceutical companies in her previous position at Oslo University Hospital, unrelated to this work. SH-D reports receiving research grants to her institution from Eli Lilly, GlaxoSmithKline, and the National Institutes of Health and consulting fees from Roche unrelated to this work and having served as an epidemiologist with the North America AED pregnancy registry, which is funded by multiple companies. KFH reports receiving research grants to her institution from Eli Lilly, Pfizer, GlaxoSmithKline, and Boehringer-Ingelheim, unrelated to this work. BTB reports receiving grants to his institution from Eli Lilly, GlaxoSmithKline, Pacira, Baxalta, Pfizer, and Aetion unrelated to this work and having served on an expert panel for a postpartum hemorrhage quality improvement project that was conducted by the Association of Women’s Health, Obstetric, and Neonatal Nurses and funded by a grant from Merck for Mothers. All other authors have no conflicts of interest to declare.
Patient consent for publication: Not required.
Ethics approval: Ethical committee/institutional review board approval has been granted for use of each registers/datasets from each participating country.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. This observational study is based on individual level data from the respective population-based health registers and healthcare utilization databases. The authors are not allowed, by law, to publicly share this data. Therefore, the authors are not able to make this data fully available to the public. However, aggregate-level data supporting the findings of the current study are available from the corresponding author upon reasonable request.
References
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 2. Guariguata L, Linnenkamp U, Beagley J, et al. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract 2014;103:176–85. 10.1016/j.diabres.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 3. Feghali MN, Scifres CM. Novel therapies for diabetes mellitus in pregnancy. BMJ 2018;362 10.1136/bmj.k2034 [DOI] [PubMed] [Google Scholar]
- 4. Ekpebegh CO, Coetzee EJ, van der Merwe L, et al. A 10-year retrospective analysis of pregnancy outcome in pregestational type 2 diabetes: comparison of insulin and oral glucose-lowering agents. Diabet Med 2007;24:253–8. 10.1111/j.1464-5491.2007.02053.x [DOI] [PubMed] [Google Scholar]
- 5. Ainuddin JA, Karim N, Zaheer S, et al. Metformin treatment in type 2 diabetes in pregnancy: an active controlled, parallel-group, randomized, open label study in patients with type 2 diabetes in pregnancy. J Diabetes Res 2015;2015:325851a:325851–11. 10.1155/2015/325851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Panchaud A, Rousson V, Vial T, et al. Pregnancy outcomes in women on metformin for diabetes or other indications among those seeking teratology information services. Br J Clin Pharmacol 2018;84:568–78. 10.1111/bcp.13481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polasek TM, Doogue MP, Thynne TRJ. Metformin treatment of type 2 diabetes mellitus in pregnancy: update on safety and efficacy. Ther Adv Drug Saf 2018;9:287–95. 10.1177/2042098618769831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–92. 10.1210/jc.2013-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the International evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–18. 10.1093/humrep/dey256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLoS One 2013;8:e64585 10.1371/journal.pone.0064585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (mig tofu): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care 2018;6:e000456 10.1136/bmjdrc-2017-000456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown J, Grzeskowiak L, Williamson K, et al. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev 2017;11 10.1002/14651858.CD012037.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbour LA, Scifres C, Valent AM, et al. A cautionary response to SMFM statement: pharmacological treatment of gestational diabetes. Am J Obstet Gynecol 2018;219:367.e1–367.e7. 10.1016/j.ajog.2018.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feig DS, Murphy K, Asztalos E, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multi-center randomized controlled trial. BMC Pregnancy Childbirth 2016;16:173 10.1186/s12884-016-0954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindsay RS, Loeken MR. Metformin use in pregnancy: promises and uncertainties. Diabetologia 2017;60:1612–9. 10.1007/s00125-017-4351-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charlton RA, Klungsøyr K, Neville AJ, et al. Prescribing of antidiabetic medicines before, during and after pregnancy: a study in seven European regions. PLoS One 2016;11:e0155737 10.1371/journal.pone.0155737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bell JC, Ford JB, Cameron CA, et al. The accuracy of population health data for monitoring trends and outcomes among women with diabetes in pregnancy. Diabetes Res Clin Pract 2008;81:105–9. 10.1016/j.diabres.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 18. Akerblom HK, Reunanen A. The epidemiology of insulin-dependent diabetes mellitus (IDDM) in Finland and in northern Europe. Diabetes Care 1985;8(Suppl 1):10–16. 10.2337/diacare.8.1.S10 [DOI] [PubMed] [Google Scholar]
- 19. Helgason T, Danielsen R, Thorsson AV. Incidence and prevalence of type 1 (insulin-dependent) diabetes mellitus in Icelandic children 1970-1989. Diabetologia 1992;35:880–3. 10.1007/BF00399936 [DOI] [PubMed] [Google Scholar]
- 20. Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine 2014;42:698–702. 10.1016/j.mpmed.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mirghani Dirar A, Doupis J. Gestational diabetes from a to Z. World J Diabetes 2017;8:489–511. 10.4239/wjd.v8.i12.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawrence JM, Andrade SE, Avalos LA, et al. Prevalence, trends, and patterns of use of antidiabetic medications among pregnant women, 2001-2007. Obstet Gynecol 2013;121:106–14. 10.1097/AOG.0b013e318278ce86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mustaniemi S, Vääräsmäki M, Eriksson JG, et al. Polycystic ovary syndrome and risk factors for gestational diabetes. Endocr Connect 2018;7:859–69. 10.1530/EC-18-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolgast E, Lindh-Åstrand L, Lilliecreutz C. Women's perceptions of medication use during pregnancy and breastfeeding-A Swedish cross-sectional questionnaire study. Acta Obstet Gynecol Scand 2019;98:856–64. 10.1111/aogs.13570 [DOI] [PubMed] [Google Scholar]
- 25. Statens Legemiddelverket (Norwegian Medicines Agency) Refusjonslisten (Refund list), 2018. Available: https://legemiddelverket.no/offentlig-finansiering/refusjonslisten-refusjonssok [Accessed 07 Jan 2019].
- 26. Bardenheier BH, Imperatore G, Devlin HM, et al. Trends in pre-pregnancy diabetes among deliveries in 19 U.S. states, 2000-2010. Am J Prev Med 2015;48:154–61. 10.1016/j.amepre.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bardenheier BH, Imperatore G, Gilboa SM, et al. Trends in gestational diabetes among hospital deliveries in 19 U.S. states, 2000-2010. Am J Prev Med 2015;49:12–19. 10.1016/j.amepre.2015.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fadl HE, Simmons D. Trends in diabetes in pregnancy in Sweden 1998-2012. BMJ Open Diabetes Res Care 2016;4:e000221 10.1136/bmjdrc-2016-000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015;350:h102 10.1136/bmj.h102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christensen DH, Rungby J, Thomsen RW. Nationwide trends in glucose-lowering drug use, Denmark, 1999-2014. Clin Epidemiol 2016;8:381–7. 10.2147/CLEP.S113211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camelo Castillo W, Boggess K, Stürmer T, et al. Trends in glyburide compared with insulin use for gestational diabetes treatment in the United States, 2000-2011. Obstet Gynecol 2014;123:1177–84. 10.1097/AOG.0000000000000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agarwal MM. Gestational diabetes mellitus: an update on the current international diagnostic criteria. World J Diabetes 2015;6:782–91. 10.4239/wjd.v6.i6.782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong VW, Lin A, Russell H. Adopting the new World Health organization diagnostic criteria for gestational diabetes: how the prevalence changes in a high-risk region in Australia. Diabetes Res Clin Pract 2017;129:148–53. 10.1016/j.diabres.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 34. Changing diagnostic criteria for gestational diabetes in Sweden (2018) welcome to the CDC4G study [Accessed 2019-04-30].
- 35. Syngelaki A, Nicolaides KH, Balani J, et al. Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med 2016;374:434–43. 10.1056/NEJMoa1509819 [DOI] [PubMed] [Google Scholar]
- 36. Løvvik TS, Carlsen SM, Salvesen Øyvind, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2019;7:256–66. 10.1016/S2213-8587(19)30002-6 [DOI] [PubMed] [Google Scholar]
- 37. van Weelden W, Wekker V, de Wit L, et al. Long-Term effects of oral antidiabetic drugs during pregnancy on offspring: a systematic review and meta-analysis of follow-up studies of RCTs. Diabetes Ther 2018;9:1811–29 https://doi.org/ 10.1007/s13300-018-0479-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanem LGE, Stridsklev S, Júlíusson PB, et al. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J Clin Endocrinol Metab 2018;103:1612–21 https://doi.org/ 10.1210/jc.2017-02419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000759supp001.pdf (75KB, pdf)