Abstract
The Siglec family of cell surface receptors have emerged as attractive targets for cell directed therapies due to their restricted expression on immune cells, endocytic properties and ability to modulate receptor signaling. Human Siglec-8, for instance has been identified as a therapeutic target for the treatment of eosinophil and mast cell disorders. A promising strategy to target Siglecs involves the use of liposomal nanoparticles with multivalent display of Siglec ligands. A key challenge for this approach is the identification of a high affinity ligand for the target Siglec. Here we report the development of a ligand of Siglec-8 and its closest murine functional ortholog Siglec-F that is capable of targeting liposomes to cells expressing Siglec-8 or -F. A glycan microarray library of synthetic 9-N-sulfonyl sialoside analogs was screened to identify potential lead compounds. The best ligand, 9-N-(2-naphthyl-sulfonyl)-Neu5Acα2–3-[6-O-sulfo]-Galβ1–4GlcNAc (6′-O-sulfo NSANeu5Ac) combined the lead 2-naphthyl sulfonyl C-9 substituent with the preferred sulfated scaffold. The ligand 6′-O-sulfo NSANeu5Ac was conjugated to lipids for display on liposomes to evaluate targeted delivery to cells. Targeted liposomes showed strong in vitro binding/uptake and selectivity to cells expressing Siglec-8 or -F, and when administered to mice, exhibit in vivo targeting to Siglec-F+ eosinophils.
Graphical Abstract
The Sialic acid-binding immunoglobulin-type lectins (Siglecs) are a family of cell surface receptors with restricted expression on one or a few immune cell types.1–2 Due to their expression patterns, endocytic properties, and ability to modulate receptor signaling, Siglecs have emerged as attractive targets for cell directed therapies.3–16 Examples include targeting Siglec-1 on macrophages,6–7 Siglec-2 on B cells,8–10 Siglec-3 on mast cells,11–13 Siglec-9 on T cells,14 and Siglec-15 on osteoclasts.15, 17 Furthermore, Siglec-8 has been identified as a therapeutic target for the treatment of eosinophil and mast cell disorders, with antibodies targeting Siglec-8 in clinical trials.18–22 A promising alternative to antibodies to target Siglecs involves use of a multivalent display of Siglec ligands presented on nanoparticles or polymers.3 Indeed, ligand mediated targeting of Siglecs has been used to deliver diagnostic or therapeutic agents to a variety of immune cells.6, 23–28 A key challenge for this strategy is the identification of selective, high affinity ligands for the target Siglec.
Natural sialylated glycan ligands of Siglecs have variable selectivity and generally low monovalent affinity (0.1–3 mM). Glycan ligands gain avidity through multivalent interactions.29–31 These glycan scaffolds, however, can serve as starting points to develop more optimal synthetic ligands. It has been well established that modifying positions on sialic acid can modulate the selectivity and affinity of Siglec ligands.32 A productive strategy to identify ligands has involved synthesizing and screening libraries of glycan analogs.33–39 While high affinity ligands have been developed for many Siglecs,3, 40–41 suitable ligands are currently unavailable for Siglec-8 or its closest murine functional ortholog Siglec-F, the latter also being prominently expressed on eosinophils. In view of the therapeutic potential of targeting eosinophils and mast cells we set out to develop selective ligands for Siglec-8 and -F. We describe here a ligand of Siglec-8 and -F capable of targeting Siglec-8 and -F positive cells, and in vivo targeting of murine eosinophils.
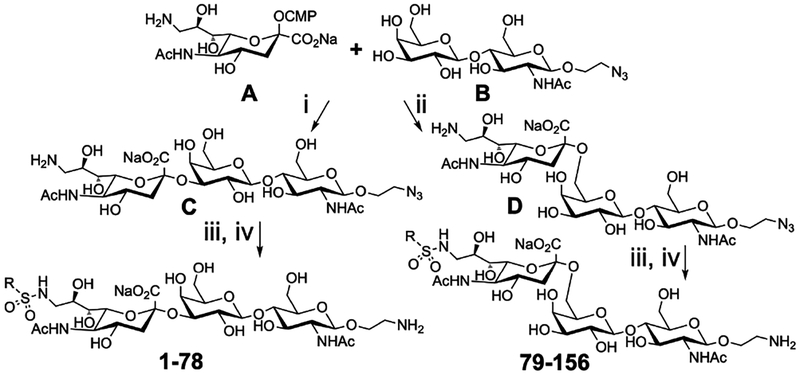
While initial attempts to identify ligands for Siglec-8 and -F by screening established sialoside analog libraries with amide-linked substituents at C-5/−9 yielded no ‘hits’, we obtained promising evidence of binding of Siglec-8 and -F to a focused panel of C-9 sulfonamide analogs (1-17 α2–3, Scheme 1, Figure 1 and S2). Based on these results we chemoenzymatically synthesized an expanded library of sulfonamide-based C-9 modified sialoside analogs (1-78 α2–3; 79-156 α2–6) (Scheme 1). Although, Siglec-8 and -F are known to bind 6′-O-sulfo Neu5Acα2–3Galβ1–4GlcNAc42 and 6′-O-sulfo Sialyl-Lewis-X43 we constructed the library based off C-9 modification of the synthetically more accessible non-sulfated Neu5Acα2–3- and Neu5Acα2–6-Galβ1–4GlcNAc scaffolds. Briefly, the 9-NH2 trisaccharides (C) and (D) were synthesized and purified on ~50 mg scales by reacting CMP-9-NH2-Neu5Ac44 (A) with Galβ1–4GlcNAc-ethyl azide45 (B) using either P. multocida α2,3-sialyltransferase46 or P. damsela α2,6-sialyltransferase47, respectively. The sulfonamide analogs were synthesized from C and D in parallel two-step one pot reactions (0.5 mg scale) by reacting the 9-NH2 groups with a panel of substituted sulfonyl chlorides (~2 eq. RSO2Cl, see Table S1). Completed reactions (TLC) were placed in a vacuum desiccator to remove the methanol. After quenching excess RSO2Cl with H2O (pH 9–10), the ethyl azide aglycones were reduced using trimethyl phosphine (PMe3, 2 eq.) to generate an ethyl amine linker. The reactions were concentrated under reduced pressure then the products were diluted to 0.1 mM (H2O) without further purification for printing on the glycan microarray. In total, the library consists of 156 sulfonamide sialoside analogs. The glycans were printed directly on amine-reactive N-hydroxy succinimide (NHS)-activated glass slides (Schott Nexterion® Slide-H).33 Neu5Acα2–3Galβ1–4GlcNAc (C1) which is the parent scaffold used to construct the analog library, and 6′-O-sulfo Neu5Acα2–3Galβ1–4GlcNAc (C2) which is the known Siglec-8 and -F ligand were included as controls. The printed slides were washed to remove excess synthetic reagents.
Scheme 1. Chemo-enzymatic synthesis of sulfonamide analogs.a.
aReagents and conditions: (i) Pasteurella multocida α2,3-sialyltransferase; (ii) Photobacterium damsela α2,6-sialyltransferase; (iii) RSO2Cl (1-156, see Table S1), DIEA (5 eq.), CH3OH; (iv) PMe3 (2 eq.), THF, H2O (pH 9).
Figure 1.
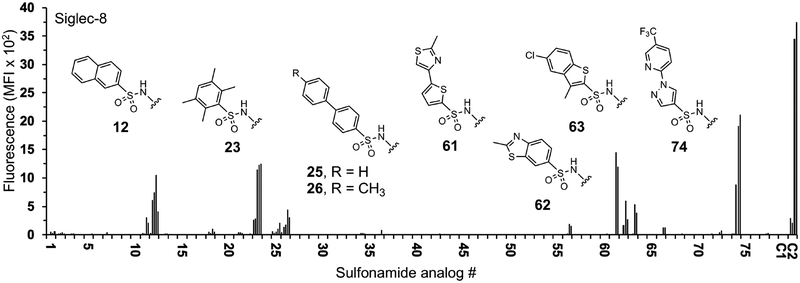
Binding of recombinant Siglec-8 to the α2,3-sialyl sulfonamide analog array. Arrays were screened to identify C-9 substituents of sialic acid that show increased binding compared to control glycan C1. Human Siglec-8 COMP (10 μg/ml) was pre-complexed with anti-penta-histidine IgG-Alexa Fluor 488 (5 μg/ml). The complexed proteins were overlaid onto the array. After incubation the slides were washed then scanned for fluorescence. Analogs 1-78 correspond to the groups listed in Table S1. Shown is mean fluorescence intensity of Siglec-8 binding. Each glycan was printed at 100, 20, 4, 0.8, and 0.16 μM in 4 replicates each (increasing concentration from left to right). The controls are Neu5Acα2–3Galβ1–4GlcNAc (C1) and 6′-O-sulfo Neu5Acα2–3Galβ1–4GlcNAc (C2).
The sulfonamide analog array (1-156) was screened against fluorescently labeled recombinant Siglec-8 COMP (Cartilage Oligomeric Matrix Protein)48 or Fc and Siglec-F Fc chimeras to identify substituents that show increased binding compared to C1 (Figures 1, S1, and S2). As expected, Siglec-8 and -F bound the 6′-O sulfated C2 (down to 4 μM) however neither bound C1. Binding of the Siglecs to the analogs was markedly different. Siglec-8 bound to only a few of the α2–3 analogs while Siglec-F bound strongly to many (1-78, Figure 1 and S2). Each Siglec bound to only a few of the α2,6 analogs (79-156, Figure S1 and S2). Siglec-8 showed robust binding to several ligands (12, 23, 61, 74). We selected 12 as a lead hit since it showed strong binding down to 0.8 μM. It comprises Neu5Ac modified at C-9 with a 2-naphthyl sulfonyl group (NSANeu5Ac, NSA = 2-naphthyl sulfonamide). To further optimize this hit we also synthesized 158 (NSANeu5Acα2–3[6SO4]Galβ1–4GlcNAc, 6′-O-sulfo NSANeu5Ac), which combines the NSANeu5Ac with the preferred scaffold C2 (Scheme S1).
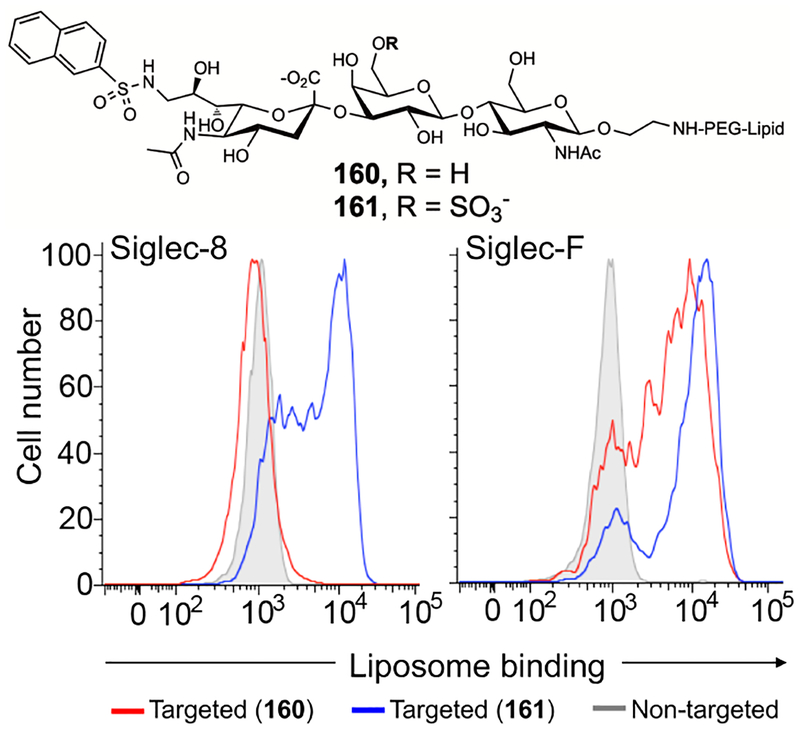
We next used a cell binding assay to evaluate if liposomes displaying ligands C2, 12, or 158 could bind Chinese hamster ovary (CHO) cells expressing Siglec-8 or -F (Figure 2 and S3).49 Accordingly, ligands C2, 12, and 158 were synthesized, purified then coupled to NHS activated PEGylated lipid to generate the conjugates 159–161 (Scheme S1–S3). ‘Targeted’ liposomes containing these glyco-PEG-lipid conjugates (2 mol%) and fluorescent (Alexa Fluor® 647) lipids were formulated as described in the supporting information. Fluorescent ‘non-targeted’ liposomes without Siglec ligand were used as a control. Siglec-8 and -F expressing CHO cells were incubated with targeted (159-161) or non-targeted liposomes for one hour, washed then analyzed for binding of fluorescent liposomes by flow cytometry (Figure 2 and S3). Liposomes formulated with either 160 or 161 strongly bound Siglec-F expressing cells with slight preference for 161. In contrast Siglec-8 expressing CHO cells only bound liposomes containing 161. In contrast, liposomes displaying the natural ligand C2 did not bind to either Siglec-8 or -F expressing CHO cell (Figure S3). This result demonstrates that both the C-9 2-naphthyl sulfonamide and 6′-O-sulfation of 161 are required to support binding of liposomes to Siglec-8 expressing cells. Accordingly, we proceeded to further evaluate liposomes decorated with 161.
Figure 2.
In vitro binding/uptake of fluorescent targeted liposomes displaying 160 or 161 to CHO cells expressing Siglec-8 and -F. Cells were treated with 20 μM fluorescent targeted liposomes (2 mol% of 160 or 161) at 37 °C for 1 hour. Liposome binding was assessed by flow cytometry. Low binding to some cells reflects loss of Siglec expression (see Figure S4).
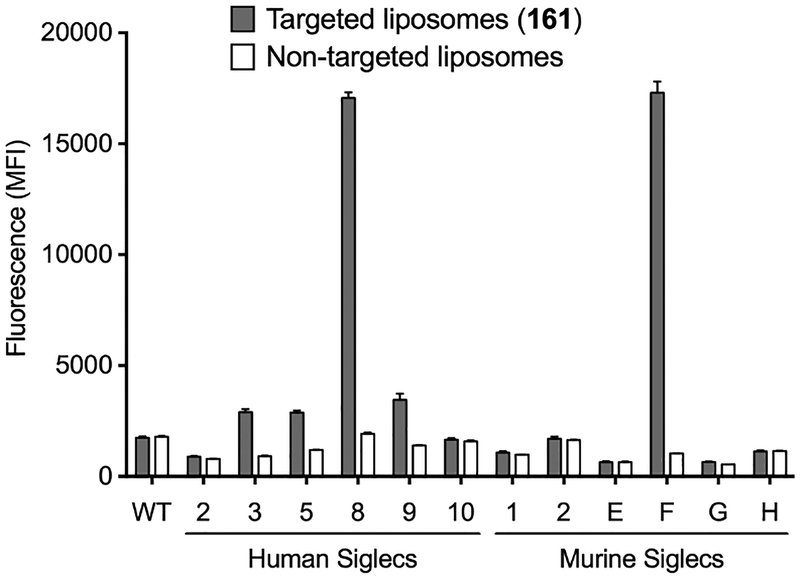
To assess the degree of specificity for Siglec-F/8, the targeted (161) liposomes were then assessed against a panel of cell lines expressing several human and murine Siglecs. Cells were incubated with fluorescent targeted (161, 2 mol%) or non-targeted liposomes and analyzed by flow cytometry as described above. For murine Siglecs, targeted liposomes (161) bound strongly to and were highly selective for Siglec-F (Figure 3). For the human Siglecs, the targeted liposomes showed strong binding to Siglec-8 and to a lesser extent to Siglec-3, −5, and −9. We further tested if targeted liposomes (161) bound bone marrow derived eosinophils (bmEos) generated from wild-type (Siglec F+/8−) and various transgenic (Siglec F+/8+, Siglec F−/8+, and Siglec F−/8−)50–51 mice (Figure S5). In all cases the targeted liposomes bound to cells expressing Siglec-F and/or −8, However, no appreciable binding was observed with bmEos from Siglec F−/8− mice. These results demonstrate that liposomes decorated with 161 selectively bind to Siglec-8 and -F expressing cells.
Figure 3.
Flow cytometry analysis of in vitro binding/uptake of targeted (161, 2 mol%) or non-targeted (no ligand) fluorescent liposomes to cells expressing human and murine Siglecs. Binding is shown as mean fluorescence intensity (MFI) ± SEM (n = 3).
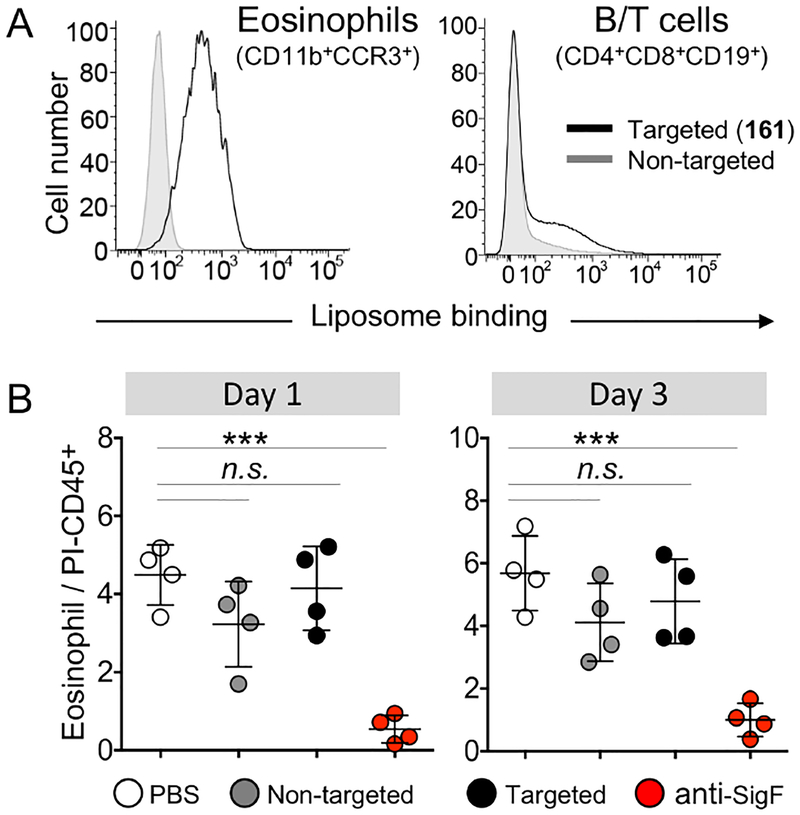
To evaluate targeting cells in vivo, we investigated whether Siglec-F expressing eosinophils in the spleen of normal mice could be labeled with fluorescent liposomes containing 161 (Figure 4A, S6, and S7). To this end, wild type (WT) mice were intravenously given fluorescent targeted (161) or non-targeted liposomes. After 1 and 24 hours, the spleens were harvested and the binding/uptake of liposomes by splenocytes was analyzed by flow cytometry. Populations of eosinophils (CD11b+CCR3+), B (CD19+) and T (CD4+/CD8+) cells were identified using antibodies to surface markers of each cell type (Figure 4A). Analysis of the gated cell populations revealed that the targeted liposomes strongly labeled Siglec-F+ eosinophils in vivo. There was no appreciable binding of targeted liposomes to any other cell type, however, a small amount of labeling of B cells was observed (Figure 4A and S6 and S7). Since Siglec-G is a B cell Siglec, and we had observed a slight but significant labeling of Siglec-G expressing CHO cells not evident in Figure 3, we compared in vitro labeling of splenocytes from WT and Siglec-G knockout (KO) mice (Figure S8), and found that the weak binding to B cells is Siglec-G mediated.
Figure 4.
(A) Targeted liposomes (161) bind eosinophils in vivo. WT mice were intravenously given either targeted (161, 2 mol%, n=3) or non-targeted (no ligand, n=3) fluorescent liposomes. After one hour, splenocytes were harvested, stained with antibodies and analyzed by flow cytometry. In vivo binding of targeted (black line) or non-targeted liposomes (grey) to eosinophils (CD11b+CCR3+, left) or B/T cells (CD4+CD8+CD19+, right) were overlaid. Representative overlays are shown. See also Figure S6. (B) Targeted liposomes (161) do not alter eosinophil frequency in vivo. WT mice (n=4) were intravenously given PBS (white), non-targeted liposomes (grey), targeted liposomes (161, 5 mol%) (black), or anti-Siglec-F (red). Eosinophil frequencies in the blood were analyzed 1- and 3-days post injection and determined by dividing cells that were CD11b+CCR3+ by live immune cells (PI−CD45+). *** P <0.001; N.S., not significant (P >0.05) determined by 1-way ANOVA followed by Tukey’s test.
Lastly, previous work demonstrated that anti-Siglec-F and −8 antibodies deplete eosinophils in vivo.52–55 Moreover, glycan ligand mediated ligation of Siglec-8 was also observed to increase death of human eosinophils in vitro.55–56 To determine if the targeted liposomes (161) impacted eosinophil survival in vivo, WT mice were intravenously injected with either anti-Siglec-F antibody57 or liposomes containing 5 mol% 161 (Figure 4B). Blood eosinophil frequency was then determined 1- and 3-days post injection by flow cytometry. Eosinophils were identified as CD11b+CCR3+ and their frequency was determined by comparison to total live peripheral blood leukocytes (CD45+PI−). As seen by others,52 anti-Siglec-F potently decreased eosinophil frequencies at both time points. In contrast, there was no significant change observed in eosinophil frequencies in mice injected with ligand targeted liposomes relative to control mice. The results suggest that anti-Siglec-F may deplete eosinophils in vivo by an immune dependent mechanism rather than ligation dependent signaling of Siglec-F. Furthermore, they suggest that targeted (161) liposomes could serve as a vehicle for delivery of agents to eosinophils without causing their depletion.
In summary, we have described the successful development of a sulfonamide-based glycan analog ligand, 6′-O-sulfo NSANeu5Ac that binds both Siglec-8 and -F. The sulfonamide substituent was identified by screening a glycan analog microarray against recombinant Siglec-8 and -F. The best ligand combined the C-9 sulfonamide with the preferred sulfated glycan scaffold (NSANeu5Acα2–3[6-SO4]Galβ1–4GlcNAc). Targeted liposomes showed strong binding to Siglec-F and −8 expressing cells. Evaluation of the targeted liposomes in mice showed effective in vivo targeting of eosinophils without significantly affecting eosinophil frequency. We envisage that incorporating this novel ligand of Siglec-8 and -F into nanoparticle delivery systems will allow targeting of eosinophils and modulation of immune cell responses.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Heart, Lung, and Blood Institute (P01HL107151 to J.C.P., B.S.B., Z.Z., and R.S.L.) and the National Institute of Allergy and Infectious Diseases (U19AI136443 to J.C.P., B.S.B., and R.S.L.) as well as NIH grants AI132790, AI136443, and AI051043 to J.C.P.. Funding support to E.K. from Austrian science fund (FWF W1241, DK-MOLIN and FWF P-26185-B19) as well as Austrian Marshall Plan Foundation. We thank Prof. Ajit Varki (UCSD) for the Siglec-F KO mice, Prof. Yang Liu (Univ. Michigan) for the Siglec-G KO mice.
Footnotes
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website. Experimental procedures, characterization data, and NMR spectra for representative compounds, including Schemes S1-S3, and Figures S1-S8 (PDF)
Dr. Bochner receives remuneration for serving on the scientific advisory board of Allakos, Inc.; and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and Up-ToDate®. He is a co-inventor on existing Siglec-8-related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during development and potential sales of such products. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
All other authors declare no competing financial interests.
REFERENCES
- (1).Crocker PR; Paulson JC; Varki A, Siglecs and their roles in the immune system. Nat Rev Immunol 2007, 7 (4), 255–266. [DOI] [PubMed] [Google Scholar]
- (2).Macauley MS; Crocker PR; Paulson JC, Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 2014, 14 (10), 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Angata T; Nycholat CM; Macauley MS, Therapeutic Targeting of Siglecs using Antioody- and Glycan-Based Approaches. Trends Pharmacol Sci 2015, 36 (10), 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Adams OJ; Stanczak MA; von Gunten S; Laubli H, Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology 2018, 28 (9), 640–647. [DOI] [PubMed] [Google Scholar]
- (5).Xiao H; Woods EC; Vukojicic P; Bertozzi CR, Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A 2016, 113 (37), 10304–10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Edgar LJ; Kawasaki N; Nycholat CM; Paulson JC, Targeted Delivery of Antigen to Activated CD169(+) Macrophages Induces Bias for Expansion of CD8(+) T Cells. Cell Chem Biol 2019, 26 (1), 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Delputte PL; Van Gorp H; Favoreel HW; Hoebeke I; Delrue I; Dewerchin H; Verdonck F; Verhasselt B; Cox E; Nauwynck HJ, Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PloS one 2011, 6 (2), e16827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fry TJ; Shah NN; Orentas RJ; Stetler-Stevenson M; Yuan CM; Ramakrishna S; Wolters P; Martin S; Delbrook C; Yates B; Shalabi H; Fountaine TJ; Shern JF; Majzner RG; Stroncek DF; Sabatino M; Feng Y; Dimitrov DS; Zhang L; Nguyen S; Qin H; Dropulic B; Lee DW; Mackall CL, CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018, 24 (1), 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ereno-Orbea J; Sicard T; Cui H; Mazhab-Jafari MT; Benlekbir S; Guarne A; Rubinstein JL; Julien JP, Molecular basis of human CD22 function and therapeutic targeting. Nat Commun 2017, 8 (1), 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sullivan-Chang L; O’Donnell RT; Tuscano JM, Targeting CD22 in B-cell malignancies: current status and clinical outlook. BioDrugs 2013, 27 (4), 293–304. [DOI] [PubMed] [Google Scholar]
- (11).Kim MY; Yu KR; Kenderian SS; Ruella M; Chen S; Shin TH; Aljanahi AA; Schreeder D; Klichinsky M; Shestova O; Kozlowski MS; Cummins KD; Shan X; Shestov M; Bagg A; Morrissette JJD; Sekhri P; Lazzarotto CR; Calvo KR; Kuhns DB; Donahue RE; Behbehani GK; Tsai SQ; Dunbar CE; Gill S, Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173 (6), 1439–1453 e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Herrmann M; Krupka C; Deiser K; Brauchle B; Marcinek A; Ogrinc Wagner A; Rataj F; Mocikat R; Metzeler KH; Spiekermann K; Kobold S; Fenn NC; Hopfner KP; Subklewe M, Bifunctional PD-1 x alphaCD3 x alphaCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood 2018, 132 (23), 2484–2494. [DOI] [PubMed] [Google Scholar]
- (13).Duan S; Koziol-White CJ; Jester WF Jr.; Nycholat CM; Macauley MS; Panettieri RA Jr.; Paulson JC, CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J Clin Invest 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stanczak MA; Siddiqui SS; Trefny MP; Thommen DS; Boligan KF; von Gunten S; Tzankov A; Tietze L; Lardinois D; Heinzelmann-Schwarz V; von Bergwelt-Baildon M; Zhang W; Lenz HJ; Han Y; Amos CI; Syedbasha M; Egli A; Stenner F; Speiser DE; Varki A; Zippelius A; Laubli H, Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest 2018, 128 (11), 4912–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kameda Y; Takahata M; Mikuni S; Shimizu T; Hamano H; Angata T; Hatakeyama S; Kinjo M; Iwasaki N, Siglec-15 is a potential therapeutic target for postmenopausal osteoporosis. Bone 2015, 71, 217–226. [DOI] [PubMed] [Google Scholar]
- (16).Wang J; Sun J; Liu LN; Flies DB; Nie X; Toki M; Zhang J; Song C; Zarr M; Zhou X; Han X; Archer KA; O’Neill T; Herbst RS; Boto AN; Sanmamed MF; Langermann S; Rimm DL; Chen L, Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 2019, 25 (4), 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sato D; Takahata M; Ota M; Fukuda C; Tsuda E; Shimizu T; Okada A; Hiruma Y; Hamano H; Hiratsuka S; Fujita R; Amizuka N; Hasegawa T; Iwasaki N, Siglec-15-targeting therapy increases bone mass in rats without impairing skeletal growth. Bone 2018, 116, 172–180. [DOI] [PubMed] [Google Scholar]
- (18).Kiwamoto T; Kawasaki N; Paulson JC; Bochner BS, Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther 2012, 135 (3), 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Farid S; Mirshafiey A; Razavi A, Siglec-8 and Siglec-F, the new therapeutic targets in asthma. Immunopharmacol Immunotoxicol 2012, 34 (5), 721–726. [DOI] [PubMed] [Google Scholar]
- (20).Legrand F; Cao Y; Wechsler JB; Zhu X; Zimmermann N; Rampertaap S; Monsale J; Romito K; Youngblood BA; Brock EC; Makiya MA; Tomasevic N; Bebbington C; Maric I; Metcalfe DD; Bochner BS; Klion AD, Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: Receptor expression and targeting using chimeric antibodies. J Allergy Clin Immunol 2018, 143 (6), 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Simon D; Simon HU, Therapeutic strategies for eosinophilic dermatoses. Curr Opin Pharmacol 2019, 46, 29–33. [DOI] [PubMed] [Google Scholar]
- (22).O’Sullivan JA; Carroll DJ; Cao Y; Salicru AN; Bochner BS, Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J Allergy Clin Immunol 2018, 141 (5), 1774–1785 e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Rhee JK; Baksh M; Nycholat C; Paulson JC; Kitagishi H; Finn MG, Glycan-targeted virus-like nanoparticles for photodynamic therapy. Biomacromolecules 2012, 13 (8), 2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Duong BH; Tian H; Ota T; Completo G; Han SF; Vela JL; Ota M; Kubitz M; Bovin N; Paulson J; Nemazee D, Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med 2010, 207 (1), 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Courtney AH; Bennett NR; Zwick DB; Hudon J; Kiessling LL, Synthetic antigens reveal dynamics of BCR endocytosis during inhibitory signaling. Acs Chem Biol 2014, 9 (1), 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Spence S; Greene MK; Fay F; Hams E; Saunders SP; Hamid U; Fitzgerald M; Beck J; Bains BK; Smyth P; Themistou E; Small DM; Schmid D; O’Kane CM; Fitzgerald DC; Abdelghany SM; Johnston JA; Fallon PG; Burrows JF; McAuley DF; Kissenpfennig A; Scott CJ, Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med 2015, 7 (303), 303ra140. [DOI] [PubMed] [Google Scholar]
- (27).Pang L; Macauley MS; Arlian BM; Nycholat CM; Paulson JC, Encapsulating an Immunosuppressant Enhances Tolerance Induction by Siglec-Engaging Tolerogenic Liposomes. Chembiochem 2017, 18 (13), 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sun Y; Hong S; Xie R; Huang R; Lei R; Cheng B; Sun DE; Du Y; Nycholat CM; Paulson JC; Chen X, Mechanistic Investigation and Multiplexing of Liposome-Assisted Metabolic Glycan Labeling. J Am Chem Soc 2018, 140 (10), 3592–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Lee RT; Lee YC, Affinity enhancement by multivalent lectincarbohydrate interaction. Glycoconjugate J 2000, 17 (7–9), 543–551. [DOI] [PubMed] [Google Scholar]
- (30).Dam TK; Gerken TA; Brewer CF, Thermodynamics of multivalent carbohydrate-lectin cross-linking interactions: importance of entropy in the bind and jump mechanism. Biochemistry 2009, 48 (18), 3822–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Blixt O; Collins BE; van den Nieuwenhof IM; Crocker PR; Paulson JC, Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem 2003, 278 (33), 31007–31019. [DOI] [PubMed] [Google Scholar]
- (32).Zaccai NR; Maenaka K; Maenaka T; Crocker PR; Brossmer R; Kelm S; Jones EY, Structure-guided design of sialic acid-based Siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure 2003, 11 (5), 557–567. [DOI] [PubMed] [Google Scholar]
- (33).Blixt O; Han SF; Liao L; Zeng Y; Hoffmann J; Futakawa S; Paulson JC, Sialoside analogue arrays for rapid identification of high affinity siglec ligands. J Am Chem Soc 2008, 130 (21), 6680–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Abdu-Allah HH; Watanabe K; Completo GC; Sadagopan M; Hayashizaki K; Takaku C; Tamanaka T; Takematsu H; Kozutsumi Y; Paulson JC; Tsubata T; Ando H; Ishida H; Kiso M, CD22-antagonists with nanomolar potency: the synergistic effect of hydrophobic groups at C-2 and C-9 of sialic acid scaffold. Bioorg Med Chem 2011, 19 (6), 1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rillahan CD; Schwartz E; McBride R; Fokin VV; Paulson JC, Click and Pick: Identification of Sialoside Analogues for Siglec-Based Cell Targeting. Angew Chem Int Edit 2012, 51 (44), 11014–11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mesch S; Lemme K; Wittwer M; Koliwer-Brandl H; Schwardt O; Kelm S; Ernst B, From a library of MAG antagonists to nanomolar CD22 ligands. ChemMedChem 2012, 7 (1), 134–143. [DOI] [PubMed] [Google Scholar]
- (37).Kelm S; Madge P; Islam T; Bennett R; Koliwer-Brandl H; Waespy M; von Itzstein M; Haselhorst T, C-4 modified sialosides enhance binding to Siglec-2 (CD22): towards potent Siglec inhibitors for immunoglycotherapy. Angew Chem Int Ed Engl 2013, 52 (13), 3616–3620. [DOI] [PubMed] [Google Scholar]
- (38).Briard JG; Jiang H; Moremen KW; Macauley MS; Wu P, Cell-based glycan arrays for probing glycan-glycan binding protein interactions. Nat Commun 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bull C; Heise T; van Hilten N; Pijnenborg JFA; Bloemendal VRLJ; Gerrits L; Kers-Rebel ED; Ritschel T; den Brok MH; Adema GJ; Boltje TJ, Steering Siglec-Sialic Acid Interactions on Living Cells using Bioorthogonal Chemistry. Angew Chem Int Edit 2017, 56 (12), 3309–3313. [DOI] [PubMed] [Google Scholar]
- (40).Magesh S; Ando H; Tsubata T; Ishida H; Kiso M, High-affinity ligands of Siglec receptors and their therapeutic potentials. Curr Med Chem 2011, 18 (23), 3537–3550. [DOI] [PubMed] [Google Scholar]
- (41).Bull C; Heise T; Adema GJ; Boltje TJ, Sialic Acid Mimetics to Target the Sialic Acid-Siglec Axis. Trends Biochem Sci 2016, 41 (6), 519–531. [DOI] [PubMed] [Google Scholar]
- (42).Yu HF; Gonzalez-Gil A; Wei YD; Fernandes SM; Porell RN; Vajn K; Paulson JC; Nycholat CM; Schnaar RL, Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 2017, 27 (7), 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Propster JM; Yang F; Rabbani S; Ernst B; Allain FH; Schubert M, Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci U S A 2016, 113 (29), E4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Han S; Collins BE; Bengtson P; Paulson JC, Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol 2005, 1 (2), 93–97. [DOI] [PubMed] [Google Scholar]
- (45).Blixt O; Brown J; Schur MJ; Wakarchuk W; Paulson JC, Efficient preparation of natural and synthetic galactosides with a recombinant beta-1,4-galactosyltransferase-/UDP-4 ‘-gal epimerase fusion protein. J Org Chem 2001, 66 (7), 2442–2448. [DOI] [PubMed] [Google Scholar]
- (46).Yu H; Chokhawala H; Karpel R; Yu H; Wu BY; Zhang JB; Zhang YX; Jia Q; Chen X, A multifunctional Pasteurella multocida sialyltransferase: A powerful tool for the synthesis of sialoside libraries. J Am Chem Soc 2005, 127 (50), 17618–17619. [DOI] [PubMed] [Google Scholar]
- (47).Yu H; Huang SS; Chokhawala H; Sun MC; Zheng HJ; Chen X, Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: A P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew Chem Int Edit 2006, 45 (24), 3938–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gonzalez-Gil A; Porell RN; Fernandes SM; Wei YD; Yu HF; Carroll DJ; McBride R; Paulson JC; Tiemeyer M; Aoki K; Bochner BS; Schnaar RL, Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology 2018, 28 (10), 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Nycholat CM; Rademacher C; Kawasaki N; Paulson JC, In Silico-Aided Design of a Glycan Ligand of Sialoadhesin for in Vivo Targeting of Macrophages. J Am Chem Soc 2012, 134 (38), 15696–15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).O’Sullivan JA; Wei YD; Carroll DJ; Moreno-Vinasco L; Cao Y; Zhang FR; Lee JJ; Zhu Z; Bochner BS, Frontline Science: Characterization of a novel mouse strain expressing human Siglec-8 only on eosinophils. J Leukocyte Biol 2018, 104 (1), 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Angata T; Hingorani R; Varki NM; Varki A, Cloning and characterization of a novel mouse Siglec, mSiglec-F - Differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. Journal of Biological Chemistry 2001, 276 (48), 45128–45136. [DOI] [PubMed] [Google Scholar]
- (52).Zimmermann N; McBride ML; Yamada Y; Hudson SA; Jones C; Cromie KD; Crocker PR; Rothenberg ME; Bochner BS, Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 2008, 63 (9), 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Song DJ; Cho JY; Lee SY; Miller M; Rosenthal P; Soroosh P; Croft M; Zhang M; Varki A; Broide DH, Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol 2009, 183 (8), 5333–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Mao H; Kano G; Hudson SA; Brummet M; Zimmermann N; Zhu Z; Bochner BS, Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PloS one 2013, 8 (6), e68143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Carroll DJ; O’Sullivan JA; Nix DB; Cao Y; Tiemeyer M; Bochner BS, Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol 2018, 141 (6), 2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Hudson SA; Bovin NV; Schnaar RL; Crocker PR; Bochner BS, Eosinophil-Selective Binding and Proapoptotic Effect in Vitro of a Synthetic Siglec-8 Ligand, Polymeric 6 ‘-Sulfated Sialyl Lewis X. J Pharmacol Exp Ther 2009, 330 (2), 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gicheva N; Macauley MS; Arlian BM; Paulson JC; Kawasaki N, Siglec-F is a novel intestinal M cell marker. Biochem Biophys Res Commun 2016, 479 (1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.