Abstract
Acute kidney injury (AKI) following cardiac surgery significantly increases morbidity and mortality risks. Improving existing clinical methods of identifying patients at risk of perioperative AKI may advance management and treatment options. This study investigated whether a combination of biomarkers and clinical factors pre and post cardiac surgery could stratify patients at risk of developing AKI. Patients (n = 401) consecutively scheduled for elective cardiac surgery were prospectively studied. Clinical data was recorded and blood samples were tested for 31 biomarkers. Areas under receiver operating characteristic (AUROCs) were generated for biomarkers pre and postoperatively to stratify patients at risk of AKI. Preoperatively sTNFR1 had the highest predictive ability to identify risk of developing AKI postoperatively (AUROC 0.748). Postoperatively a combination of H-FABP, midkine and sTNFR2 had the highest predictive ability to identify AKI risk (AUROC 0.836). Preoperative clinical risk factors included patient age, body mass index and diabetes. Perioperative factors included cardio pulmonary bypass, cross-clamp and operation times, intra-aortic balloon pump, blood products and resternotomy. Combining biomarker risk score (BRS) with clinical risk score (CRS) enabled pre and postoperative assignment of patients to AKI risk categories. Combining BRS with CRS will allow better management of cardiac patients at risk of developing AKI.
Subject terms: Cardiology, Predictive markers
Introduction
Acute kidney injury (AKI) is a major complication following cardiac surgery that can affect the function of multiple organs including the brain, lungs and gut. Acute kidney injury increases the risk of death resulting in major use of hospital resources and elevated costs1. Acute kidney injury occurs in almost 30% of patients following cardiac surgery2 and 50% in high risk patients i.e. diabetics3,4. In the UK, approximately 100,000 deaths per year are linked to AKI. Acute kidney injury costs the NHS between £434 and £620 million per year5.
Several diagnostic criteria have been used for diagnosis of AKI including the risk, injury, failure, loss, end-stage renal disease (RIFLE) criteria in 20046; AKI Network (AKIN) modified RIFLE criteria in 20077; 2012 Kidney Disease: Improving Global Outcomes (KDIGO) criteria8, which combines RIFLE and AKIN criteria. The KIDGO criteria rely on changes in serum creatinine (SCr) levels and urine output, which is not ideal. Oliguria frequently occurs following cardiac surgery. Sometimes it precedes increased SCr due to renal injury, but often is a physiological response to hypovolemia or hypotension. Thus, the specificity of urine output as a criterion for AKI is low, and if used alone could misclassify non AKI as AKI patients9. Moreover, at cardiac surgery, increases in SCr above preoperative baseline due to AKI may take several hours to develop due to the inevitable haemodilution effect of cardiopulmonary bypass. Accordingly, increases in SCr if used as a sole criterion for AKI could delay the time to AKI diagnosis and its potential management.
Existing strategies to prevent or reduce risk of AKI at cardiac surgery include (among others) maintaining a higher haematocrit perioperatively10 and supra normal blood pressure throughout the operation using vasopressors. However, the use of blood transfusion is costly and not risk free. Furthermore, maintaining a supra normal blood pressure perioperatively during cardiac surgery may heighten the risk of postoperative bleeding at the operative site with its attending complications. Accordingly, the anaesthesiologist must evaluate the risk/benefit balance of such reno-protective interventions for each patient. An ability to accurately stratify patients into AKI risk categories perioperatively would greatly assist in this decision and allow consideration of preventative strategies. However, in addition to predicting risk of AKI, earlier diagnosis might allow interventions such as earlier renal replacement therapy. In this context, some biomarkers have been evaluated to consider their utility in allowing preoperative prediction or perioperative diagnosis of AKI at cardiac surgery. Some of these studies were limited by small sample numbers and/or reduced areas under receiver operating characteristic (AUROC)11–14. McBride et al.15 have shown in elective cardiac surgery patients that IL-10 in postoperative plasma collected 2 hours after surgery and urinary transforming growth factor beta (TGFβ-1), collected 24 hours after surgery, were significantly higher in patients who developed early renal dysfunction. Furthermore, urinary IL-1Ra and sTNFR2 were significantly lower 24 hours postoperatively in late renal dysfunction patients.
Due to the different processes involved and the dynamic nature of AKI, it is unlikely that one biomarker will predict or diagnose AKI across a wide range of clinical conditions and quantify its severity. Therefore, the aim of this study was to investigate whether a combination of biomarkers and clinical factors could be used to stratify the risk of a patient developing AKI pre and post cardiac surgery earlier than routinely used clinical methods. The biomarkers selected for this study were likely to represent key pathways in AKI pathogenesis namely, inflammation, hypoperfusion and reperfusion (see Table 1).
Table 1.
Biomarkers and their functional status and pathophysiology
| Marker | Functional Status | Pathophysiology |
|---|---|---|
| IL-1α | Inflammation | Pro inflammatory cytokine involved in the malfunction, injury, and local inflammation of renal cells41,42 |
| IL-1β | Inflammation/Ischemia |
Pro inflammatory cytokine involved in the malfunction, injury, and local inflammation of renal cells25,41 IL-1β is generated by the injured epithelial proximal tubular cell and is an important mediator of endothelial ischemic injury43 |
| IL-2 | Inflammation | Pro inflammatory cytokine IL-2 is elevated in haemodialysis patients with uremic pruritus44 |
| IL-4 | Inflammation | Anti-inflammatory cytokine elevated in end stage renal disease45 |
| IL-6 | Inflammation/Ischemia |
Pro inflammatory cytokine involved in orchestration of the inflammatory response following acute renal insult46. Renal IL-6 expression in renal tubular epithelial cells is significantly increased in the pathogenesis of AKI47 IL-6 is generated by injured epithelial proximal tubular cells and is an important mediator of endothelial ischemic injury43 |
| IL-8 | Inflammation | IL-8 is generated by injured epithelial proximal tubular cells and is an important mediator of endothelial ischemic injury43 |
| IL-10 | Inflammation | IL-10 is an anti-inflammatory cytokine involved in the regulation and maintenance of normal renal function48 |
| VEGF | Inflammation | Pro inflammatory growth factor involved in angiogenesis42 |
| EGF | Mitogen | Intrarenal EGF expression is decreased in tubular injury; decreased urine EGF excretion is a marker for CKD progression49 |
| TNFα | Inflammation | Pro inflammatory cytokine associated with renal disease42 |
| IFNγ | Activator of macrophages | Cytokine involved in the pathophysiology of CKD50 |
| MCP-1 | Inflammation | Pro inflammatory cytokine involved in the pathogenesis of CKD42 |
| IGF-1 | Growth factor | Serum IGF-1 levels are positively associated with CKD51 |
| Eotaxin | Inflammation | Inflammatory marker, the chemokine eotaxin, is a predictor of the incidence of renal failure52. |
| IL-1Ra | Inflammation/Ischemia | Anti-inflammatory cytokine involved in renal ischemic reperfusion injury53 |
| PDGF-BB | Growth factor | Growth factor involved in driving renal fibrosis; independent of underlying kidney disease54 |
| IP-10 | Chemokine | Serum IP-10 is a marker for underlying renal disease55 |
| IL-12p40 | Inflammation | IL-12p40 is a key pro inflammatory cytokine involved in crescentic glomerulonephritis56 |
| sIL2Ra | Inflammation | Inflammatory modulator involved in the progression of interstitial fibrosis in CKD57 |
| sIL6R | Inflammation | Pro inflammatory cytokine which is elevated in patients with CKD58 |
| sTNFR1 | Inflammation | sTNFR1 is associated with kidney disease progression59 |
| sTNFR2 | Inflammation | sTNFR2 is a marker for kidney tissue damage60 |
| MMP9 | Inflammation | MMP9 increases the expression of TGF-β1 and promotes the occurrence of renal interstitial fibrosis61 |
| NGAL | Ischemia | NGAL is a non-invasive urinary biomarker for renal ischemia14 |
| CRP | Inflammation | Marker of inflammation in AKI62,63 |
| D-Dimer | Inflammation | D-Dimer levels are elevated in renal insufficiency64. |
| NSE | Enzyme | NSE is elevated in patients who present with kidney disease65 |
| H-FABP | Ischemia | H-FABP is a marker for detection of ischaemic injury33 |
| MK | Ischemia | After ischaemic reperfusion, MK is up-regulated in the proximal tubules. The absence of MK protects against renal ischaemic reperfusion injury by reducing the infiltration of leukocytes38 |
IL, interleukin; AKI, acute kidney disease; CKD, chronic kidney disease; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; TNFα, tumour necrosis factor alpha; IFNγ, interferon gamma; MCP, monocyte chemoattractant protein; IGF, insulin-like growth factor; IL-1Ra, interleukin-1 receptor antagonist; PDGF-BB, platelet-derived growth factor beta homodimer; IP-10, interferon gamma-induced protein 10; IL-12p40, interleukin-12 subunit p40; sIL2Ra, soluble interleukin-2 receptor alpha; sIL6R, soluble interleukin 6 receptor; sTNFR1, soluble tumour necrosis factor receptor-1; sTNFR2, soluble tumour necrosis factor receptor-2; MMP9, matrix metallopeptidase 9; TGFβ1, transforming growth factor beta 1; NGAL, neutrophil gelatinase-associated lipocalin; CRP, C-reactive protein; NSE, neuron-specific enolase; H-FABP, heart-type fatty acid-binding protein; MK, midkine.
Materials and Methods
Study population
Cardiac patients who were consecutively scheduled for elective cardiac surgery within the Cardiac Surgical Unit of the Royal Victoria Hospital, Belfast, UK between May 2012 and August 2013, were recruited into the study. Patients were excluded if they were <18 years of age, had preoperative or pretrauma dialysis-dependent renal failure or known significant renal disease. In addition, emergency surgery patients and patients with active malignancy, active endocarditis, sepsis, septic or cardiogenic shock, or had pre-operative haemodynamic instability prior to entrance into the study (known estimated glomerular filtration rate (eGFR < 30)) were excluded. The study complied with the Declaration of Helsinki, was approved by the Office for Research Ethics Committee Northern Ireland, the Royal Victoria Hospital Research Office Research Governance Committee and written informed consent was obtained from all participating patients. The study complied with Standards for Reporting Diagnostic Accuracy (STARD) guidelines16. Of the n = 401 patients recruited to the study, pre and postoperative samples were available from 344/401 (85.8%). Patient samples were not available from 57/401 (14.2%) and these patients were excluded from the study (Fig. 1).
Figure 1.
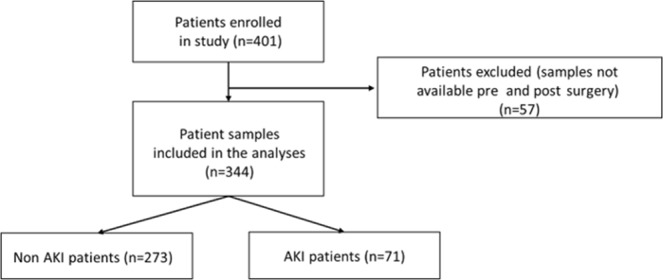
Trial flow diagram.
Clinical data collection
Clinical data was recorded for each patient from medical records that included baseline demographic characteristics, comorbidity data and current medications. Creatinine levels in patients pre and post surgery were measured by the hospital laboratory and were used to calculate eGFR through the Modification of Diet in Renal Disease (MDRD) study equation formula17. Surgical interventions included: administration of inotropes (e.g. dopamine, adrenaline, milrinone), surgical methods (e.g. valve surgery), cardio pulmonary bypass (CPB) and aortic clamping. Moreover, the length of patient admission (days) in the intensive care unit (ICU) and high dependency unit (HDU), as well as administration of interventions during and postoperatively (e.g. platelets), were also recorded. Clinical and demographic data for each patient were recorded on a case report form and stored on a database.
Sampling and laboratory methods
Patient blood samples (10 ml) were collected preoperatively and on day 1 postoperatively. The preoperative blood sample was collected following routine arterial line insertion prior to induction of anesthesia. Day 1 (within 24 hours) postoperative blood sample (10 ml) was collected from the same line. Patient blood samples were centrifuged, serum/plasma was aliquoted within 30 minutes of collection and stored at −80 °C.
Patient blood samples were analysed by Randox Clinical Laboratory Services (RCLS) (Antrim, UK) on cytokine arrays (Randox Laboratories Ltd, Crumlin, UK) for the following proteins: Cytokine I Array: Interleukin(IL)-1α, -1β, -2, -4, -6, -8, -10, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), tumour necrosis factor alpha (TNFα), interferon gamma (IFNγ), and monocyte chemoattractant protein 1 (MCP-1); Cytokine II Array: insulin growth like factor 1 (IGF-1), Eotaxin, interleukin-1 receptor antagonist (IL-1Ra), platelet-derived growth factor beta homodimer (PDGF-BB), interferon gamma induced protein 10 (IP-10), interleukin 12 subunit p40 (IL-12p40); and Cytokine IV Array: soluble interleukin 2 receptor alpha (sIL2Rα), soluble interleukin 6 receptor (sIL6R), soluble tumour necrosis factor receptor 1 (sTNFR1), soluble tumour necrosis factor receptor 2 (sTNFR2) and matrix metallopeptidase 9 (MMP9); using an Evidence Investigator analyser according to manufacturer’s instructions (Randox, Crumlin, UK). Neutrophil gelatinase-associated lipocalin (NGAL), C-reactive protein (CRP), D-Dimer, neuron-specific enolase (NSE), sTNFR1 were measured using Cerebral II Array (Randox, Crumlin, UK). Heart-type fatty-acid binding protein (H-FABP) was measured using an H-FABP IT assay (Randox, Crumlin, UK) on the RX Imola analyser (Randox, Crumlin, UK). Midkine (MK) was measured using a commercial ELISA according to manufacturer’s instructions (CellMid, Sydney, Australia). Serum creatinine was measured in the Kelvin Laboratory (Royal Victoria Hospital, Belfast) on the Cobas 8000c701 (Roche Diagnostics, Basel, Switzerland).
The limits of sensitivity for the biomarkers under investigation were as follows: Cytokine I - IL-2 4.9 pg/ml; IL-4 3.5 pg/ml; IL-6 0.4 pg/ml, IL-8 2.3 pg/ml; VEGF 10.8 pg/ml; IFNγ 2.1 pg/ml; TNFα 3.7 pg/ml; IL1α 0.9 pg/ml; MCP1 25.5 pg/ml; EGF 2.5 pg/ml; IL-10 1.1 pg/ml; IL-1β 1.3 pg/ml; Cytokine II - IL-1Ra 16.83 pg/ml, PDGF-BB 16.16 pg/ml, IP-10 7.81 pg/ml, IL-12p40 7.81 pg/ml; Cytokine IV - sIL2A 0.12 ng/ml; sIL6R 0.62 ng/ml; sTNFR1 0.09 ng/ml; sTNFR2 0.2 ng/ml; MMP9 3.03 ng/ml; CRP 0.67 mg/l; D-Dimer 2.1 ng/ml; NSE 0.26, NGAL 17.8 ng/ml; sTNFR1 0.24 ng/ml; MK 8.0 pg/ml; H-FABP 2.94 ng/ml and SCr 5 μmol/L. Biomarker values below the limit of detection (LOD) were recorded as 90% of LOD.
Outcome definitions
The development of AKI was defined as an eGFR drop of ≥25% from preoperative baseline on any of the recorded postoperative sampling days (days 1, 2 or 5) or at any time postoperatively.
Statistical analyses
Statistical analyses were performed using SPSS v2518. A Mann-Whitney U Test was used to identify significant biomarkers. Biomarkers with a p < 0.05 were considered significant. The ability of these biomarkers to predict AKI was further investigated using logistic regression (Backward Wald and Forced Entry). Areas Under the Receiver Operator Curve were generated pre and postoperatively for biomarker-based algorithms to provide a measure of how well the biomarker models distinguished between the two diagnostic groups (AKI vs. non AKI). The best combinations of biomarkers (with the greatest AUROC, sensitivity and specificity) were chosen to stratify patients at potential risk of developing AKI.
Results
A summary of baseline and clinical characteristics of the patients involved in the study are described in Table 2.
Table 2.
Summary of baseline and clinical characteristics of the study patients.
| non AKI (n = 273) | AKI (n = 71) | p value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 65.4 ± 11.6 | 68.6 ± 10.7 | 0.020 |
| Gender (male) | 192/273 (70.3%) | 50/71 (70.4%) | 0.988 |
| Weight (kg) | 80.9 ± 17.5 | 84.8 ± 16.6 | 0.061 |
| Height (cm) | 167.8 ± 11.4 | 165.1 ± 14.0 | 0.082 |
| BMI (kg/m2) | 28.9 ± 10.2 | 31.0 ± 6.0 | 0.001 |
| Comorbidities | |||
| Myocardial Infarction | 73/268 (27.2%) | 13/68 (19.1%) | 0.171 |
| Ischemic Heart Disease | 218/268 (81.3%) | 65/68 (95.6%) | 0.760 |
| Hypertension | 35/268 (13.1%) | 10/68 (14.7%) | 0.722 |
| Diabetes | 29/268 (10.8%) | 16/68 (23.5%) | 0.006 |
| Chronic Obstructive Pulmonary Disease | 9/268 (3.4%) | 3/68 (4.0 %) | 0.676 |
| Diverticulitis | 8/268 (3.0%) | 3/68 (4.4%) | 0.555 |
| Asthma | 6/268 (2.2%) | 2/68 (2.9%) | 0.735 |
| Transient Ischemic Attack | 6/268 (2.2%) | 1/68 (1.5%) | 0.692 |
| Peripheral Vascular Disease | 4/268 (1.5%) | 2/68 (2.9%) | 0.421 |
| Cerebrovascular Accident | 4/268 (1.5%) | 1/68 (1.5%) | 0.989 |
| Endocarditis | 1/268 (0.4%) | 1/68 (1.5%) | 0.294 |
| Pre surgery medications | |||
| Beta blockers | 186/266 (70.0%) | 47/68 (69.1%) | 0.897 |
| Calcium antagonists | 49/265 (18.5%) | 18/68 (26.5%) | 0.144 |
| Nitrates | 61/265 (23.0%) | 13/68 (19.1%) | 0.491 |
| Potassium channel blockers | 39/265 (14.7%) | 8/68 (11.8%) | 0.533 |
| ACE inhibitors | 131/265 (49.4%) | 36/68 (52.9%) | 0.606 |
| Angiotensin II blocker | 9/265 (3.4%) | 2/68 (2.9%) | 0.852 |
| Intraoperative conditions | |||
| Dopamine | 136/267 (50.9%) | 40/67 (59.7%) | 0.200 |
| Noradrenaline | 158/267 (59.2%) | 42/67 (62.7%) | 0.601 |
| Adrenaline | 10/266 (3.8%) | 4/67 (6.0 %) | 0.421 |
| Milrinone | 33/267 (12.4%) | 15/67 (22.4%) | 0.037 |
| CPB time (min) | 132.8 ± 50.7 | 152.1 ± 61.3 | 0.018 |
| Cross clamp time (min) | 91.7 ± 40.2 | 105.3 ± 47.6 | 0.018 |
| Operation time (min) | 296.3 ± 125.4 | 319.7 ± 108.5 | 0.029 |
| Intra-aortic balloon pump | 9/266 (3.4%) | 7/67 (10.4%) | 0.016 |
| Packed red blood cells | 126/266 (47.4%) | 41/67 (61.2%) | 0.043 |
| Fresh frozen plasma | 20/266 (7.5%) | 7/67 (10.4%) | 0.433 |
| Platelet bags | 25/266 (9.4%) | 8/67 (11.9%) | 0.534 |
| Operative method | |||
| Valve Surgery | 118/267 (44.2%) | 47/68 (69.1%) | <0.001 |
| CABG | 178/267 (66.7%) | 44/68 (64.7%) | 1.000 |
| Valve Surgery + CABG | 40/267 (15%) | 21/68 (30.9%) | 0.002 |
| Postoperative conditions | |||
| Dopamine | 156/268 (58.2%) | 51/67 (76.1%) | 0.008 |
| Noradrenaline | 163/268 (60.8%) | 51/67 (76.1%) | 0.023 |
| Adrenaline | 12/267 (4.5%) | 12/67 (17.9%) | <0.001 |
| Milrinone | 39/267 (14.6%) | 21/67 (31.3%) | 0.001 |
| Packed red blood cells | 110/267 (41.2%) | 36/67 (53.7%) | 0.065 |
| Fresh frozen plasma | 39/266 (14.7%) | 13/67 (19.4%) | 0.340 |
| Platelet bags | 39/266 (14.7%) | 18/67 (26.9%) | 0.018 |
| Resternotomy | 11/267 (4.1%) | 10/67 (14.9%) | 0.001 |
| Readmitted to intensive care | 1/267 (0.4%) | 0/68 (0.00%) | 0.614 |
| Length of admission (days) | 11.0 ± 8.0 | 13.1 ± 7.2 | <0.001 |
| Length of ICU admission (days) | 2.4 ± 3.0 | 4.1 ± 3.5 | <0.001 |
| Length of stay HDU (days) | 1.3 ± 1.0 | 1.6 ± 1.0 | 0.001 |
Data are presented as mean ± standard deviation or number/total (percentages). Note that patients presented with multiple comorbidities. BMI, body mass index; ACE, angiotensin-converting-enzyme; CPB, cardio pulmonary bypass; CABG, coronary artery bypass graft; ICU, intensive care unit; HDU, high dependency unit.
Estimated GFR was recorded on days 1, 2 and 5 following surgery. To increase the number of patients in each cohort, the analyses are based upon an ‘AKI any-day’ definition (i.e. development of AKI on days 1, 2 and 5). Patients were included in this category if their eGFR dropped ≥25% from baseline, following cardiac surgery.
Preoperative biomarkers
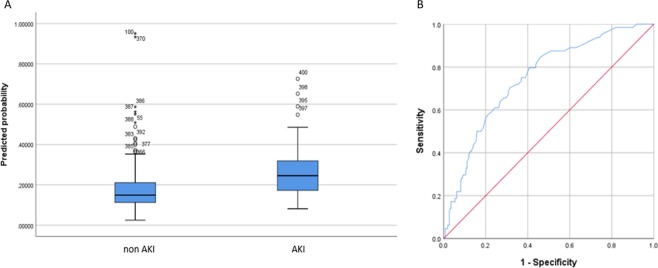
Preoperatively sTNFR1 or sTNFR2 had the highest predictive ability to identify patients at risk of developing AKI (Table 3) (sTNFR1 sensitivity 70.3%; specificity 68.5%; AUROC 0.748 (CI 0.684–0.812)) (Fig. 2a,b).
Table 3.
Serum biomarkers for predicting AKI pre and post cardiac surgery.
| Biomarkers | AUROC | CI | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Anytime | |||||
| Preoperative | sTNFR2 | 0.713 | 0.647–0.778 | 65.6% (42/64) | 65.9% (170/258) |
| sTNFR1 | 0.748 | 0.684–0.812 | 70.3% (45/64) | 68.5% (178/260) | |
| Postoperative | MK | 0.704 | 0.633–0.775 | 70.7% (41/58) | 61.3% (130/212) |
| H-FABP | 0.729 | 0.663–0.794 | 63.1% (41/65) | 68.1% (175/257) | |
| sTNFR2 | 0.762 | 0.699–0.825 | 69.2% (45/65) | 69.2% (175/253) | |
| sTNFR1 | 0.774 | 0.708–840.0 | 72.3% (47/65) | 74.0% (188/254) | |
| H-FABP + MK + sTNFR1 | 0.817 | 0.761–0.872 | 81.0% (47/58) | 67.8% (141/208) | |
| H-FABP + MK + sTNFR2 | 0.836 | 0.785–0.888 | 75.9% (44/58) | 69.1% (143/207) | |
AUROC, Sensitivity and specificity for serum biomarkers for predicting AKI pre and post cardiac surgery.
AKI, acute kidney injury; AUROC, area under receiver operating characteristic; CI, confidence interval; sTNFR2, soluble tumour necrosis factor receptor 2; sTNFR1, soluble tumour necrosis factor receptor 1; MK, midkine; H-FABP, heart-type fatty acid-binding protein.
Figure 2.
Preoperative biomarker for detecting AKI risk. (A) Serum sTNFR1 pre cardiac surgery was significantly higher in patients who developed AKI. (B) Soluble TNFR1 had the highest predictive ability to identify patients at risk of developing AKI (AUROC 0.748). AKI, acute kidney injury; sTNFR1, soluble tumour necrosis factor receptor 1; AUROC, area under receiver operating characteristic
Postoperative biomarkers
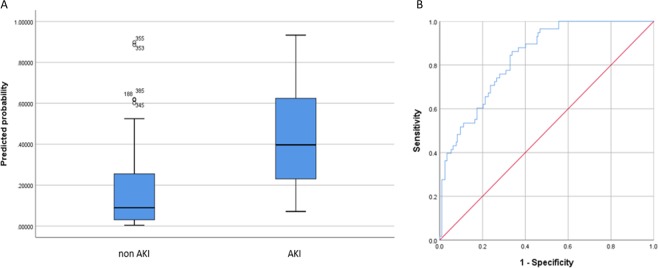
Postoperatively a combination of H-FABP, MK and sTNFR1 or sTNFR2 had the highest predictive ability to identify patients at risk of developing AKI (Table 3) (H-FABP + MK + sTNFR2 sensitivity 75.9%; specificity 69.1%; AUROC 0.836 (CI 0.785–0.888)) (Fig. 3a,b).
Figure 3.
Postoperative biomarker-based algorithm for detecting AKI risk. (A) Serum H-FABP, MK and sTNFR2 any time post surgery were significantly higher in patients who developed AKI. (B) H-FABP, MK and sTNFR2 had the highest predictive ability to identify patients at risk of developing AKI (AUROC 0.836). AKI, acute kidney injury; H-FABP, heart-type fatty acid-binding protein; MK, midkine; sTNFR2, soluble tumour necrosis factor receptor 2; AUROC, area under receiver operating characteristic
Clinical risk score (CRS)
The main clinical factors identified for patients at potential risk for the development of AKI pre and postoperatively are described in Tables 4 and 5, respectively. Patients who have a cumulative score of 0, no risk. Patients who score >1 e.g. a 65-year-old patient with a BMI of 26 and diabetes would have a cumulative score of 2.5 (highest risk).
Table 4.
Clinical factors identified for patients at risk of developing AKI pre cardiac surgery and CRS (result).
| Clinical Factors | Parameter | Result |
|---|---|---|
| Age |
<65 ≥65 |
0 1 |
| BMI |
<25 ≥25 <30 ≥30 |
0 0.5 1 |
| Diabetes |
No Yes |
0 1 |
AKI, acute kidney injury; CRS, clinical risk score; BMI, body mass index.
Table 5.
Clinical factors identified for patients at risk of developing AKI 24 hours post cardiac surgery and CRS (result).
| Clinical Factors | Parameter | Result |
|---|---|---|
| Age |
<65 ≥65 |
0 1 |
| BMI |
<25 ≥25 <30 ≥30 |
0 0.5 1 |
| Diabetes |
No Yes |
0 1 |
| CPB time (min) |
<130 ≥130 |
0 1 |
| Cross clamp time (min) |
<90 ≥90 |
0 1 |
| Operation time (min) |
<296 ≥296 |
0 1 |
| Intra-aortic balloon pump |
No Yes |
0 1 |
| Packed red blood cells |
No Yes |
0 1 |
| Platelet bags |
No Yes |
0 1 |
| Resternotomy |
No Yes |
0 1 |
AKI, acute kidney injury; CRS, clinical risk score; BMI, body mass index; CPB, cardio pulmonary bypass.
Biomarker risk score (BRS)
Biomarker combination algorithm(s) can be applied clinically to provide a patient risk score for developing AKI. Patients with a score equal to/or greater than the value of the set point (cut-off) would be categorised positive (AKI); whereas patients below the cut-off would be categorised negative (non AKI) (Table 6).
Table 6.
Post surgery patient score calculation and BRS.
| BRS | Patient score* |
|---|---|
| Negative | <0.200 |
| Positive | ≥0.200 |
*Patient Score = 7.322 + 1.773 *log(H-FABP) + 1.120 *log(MK) + 3.510 *log(sTNFR2).
The patient score equation was derived from logistic regression. The cut-off (0.200) was manually determined to optimise sensitivity while maintaining specificity. If the patient score was < 0.200 then BRS is negative for AKI, if the patient score ≥ 0.200 then BRS is positive for AKI.
BRS, biomarker risk score; H-FABP, heart-type fatty acid-binding protein; MK, midkine; sTNFR2, soluble tumour necrosis factor receptor 2; AKI, acute kidney injury.
Positive BRS is associated with higher risk for development of AKI, e.g. patients with negative BRS and high CRS are at lower risk of developing AKI (category 2) while patients with a positive BRS and low CRS are assigned to category 3 (high risk for the development of AKI) (Table 7) (See Supplementary Notes 1–3 and Supplementary Tables 1–8 for worked examples).
Table 7.
Proactive AKI clinical tool for management of patients pre- and post-cardiac surgery.
| Category | BRS | CRS | Clinical Management |
|---|---|---|---|
| 1 | Negative | Low | Routine pre or postoperative management |
| 2 | Negative | High | Assign to low risk management |
| 3 | Positive | Low | Assign to higher risk management |
| 4 | Positive | High | Assign to highest risk management |
BRS biomarker risk score: negative = non AKI, positive = AKI.
CRS clinical risk score pre cardiac surgery: low 0–1, high 1.5–3.
CRS clinical risk score post cardiac surgery: low 0–4, high 4.5–10.
AKI, acute kidney injury; BRS, biomarker risk score; CRS, clinical risk score.
Clinical utility; combining BRS with CRS: pre and postoperative management of patients at potential risk for the development of AKI
Combining BRS with CRS could assist with pre and postoperative management of patients at potential risk for the development of AKI. Four categories of risk were identified (Table 7); Categories 1 and 2 = low risk; Categories 3 and 4 = high risk. Combining the biomarkers with the clinical risk factors, preoperative and postoperative, improved the AUROC (See Supplementary Note 4 and Supplementary Table 9 for distribution of non AKI and AKI patients within the risk categories and Supplementary Note 5 and Supplementary Table 10 for further statistical analysis of biomarkers and clinical factors).
Discussion
The aim of this study was to investigate whether a combination of biomarkers and clinical characteristics/risk score could predict AKI earlier than SCr and oliguria in patients undergoing cardiac surgery. Although a large range of biomarkers were studied, the mediators identified in our model interestingly represented three important pathways for the pathogenesis of renal dysfunction, namely hypoperfusion (H-FABP), ischaemia reperfusion injury (MK) and proinflammatory insult (sTNFR1 or sTNFR2).
Of the n = 30 biomarkers investigated in the patient samples undergoing cardiac surgery, only serum sTNFR1 or sTNFR2 on their own proved to be the best predictive biomarkers pre surgery, whereas serum TNFα was not significant. Soluble TNFR1 and sTNFR2 are the soluble forms of their membrane-bound counterparts (mTNFR1 and mTNFR2) through which TNFα acts19. When sTNFR1 and sTNFR2 are released from the membrane, they bind free TNFα thus limiting its biological proinflammatory effects. Soluble TNFR1 and sTNFR2 are thus anti-inflammatory agents19. Similarly, postoperative serum sTNFR1 and sTNFR2 had biopredictive utility in combination with MK and H-FABP for AKI whereas TNFα did not.
There are several reasons why this may occur. Firstly, perioperative serum TNFα exhibits different kinetics to serum sTNFR1 and sTNFR2 responses. Serum TNFα has a transient and small increase prior to CPB followed by a second transient and small increase at the end of CPB20. These small transient increases may be caused in part, by surgically-induced coagulation disturbances, interaction of blood with the foreign surface of the CPB machine, and retransfusion of unwashed shed mediastinal blood perioperatively21. The un-sustained transient nature of the TNFα response reflects efficient mechanisms to clear blood TNFα from the circulation22.
Kinetically, unlike TNFα, the serum sTNFR1 and sTNFR2 anti-inflammatory response is larger and more sustained lasting over 24 hours20. Moreover, soluble sTNFR2 in blood increases progressively following cardiac surgery over at least a 2-day follow-up period21. In this regard, serum sTNFR1 and sTNFR2 responses differ from the blood response of other important anti-inflammatory cytokines such as IL-10 and IL-1Ra which rise and fall to baseline 24 hours perioperative20. Furthermore, it may be argued that because the blood IL-10 and IL-1Ra responses at cardiac surgery have been shown to be transient20, this may explain why these anti-inflammatory mediators lack biopredictive utility in our model.
The second reason may lie in the underlying pathogenesis of perioperative inflammatory-mediated renal failure. It has been suggested that perioperative increases in filtered TNFα, if unsuccessfully handled by the kidney, could directly injure renal tubules23.
Due to the transient nature of TNFα, it is not clinically practicable to measure its exact peak in serum or TNFα recovery from urine24. Moreover, serum sTNFR1 and sTNFR2 are >20 kDa and thus not as readily filtered by the tubules as monomeric TNFα. Therefore, increases in blood sTNFR1 and sTNFR2 are not likely to have a direct protective effect against tubular damage mediated by filtered TNFα. This may explain in part why blood increases in sTNFR1 and sTNFR2 were not linked with reduced AKI risk in our model. However, increased blood sTNFR1 and sTNFR2 were linked with AKI risk. This could be because the sustained increases in blood sTNFR1 and sTNFR2 are a proportionate and compensatory response to transient increases in blood TNFα22.
As already discussed, serum TNFα is barely detectable in preoperative blood in healthy individuals, whereas baseline preoperative serum sTNFR1 and sTNFR2 concentrations are constitutively expressed25. This should be understood in the context of other conditions known to modulate serum sTNFR1 and sTNFR2 levels. For example, both sTNFR1 and sTNFR2 were demonstrated as potential biomarkers for the identification of patients presenting with chronic kidney disease (CKD) by predicting outcome in either those with diabetic nephropathy26–28, or early or moderate CKD29, or underlying malignancy30.
However, in this study we show for the first time that higher baseline sTNFR1 and sTNFR2 in patients who have normal preoperative renal function may predict postoperative AKI risk. Elevated baseline serum sTNFR1 and sTNFR2 preoperatively is driven by a heightened proinflammatory response due to underlying cardiovascular disease processes e.g. atheroma31 which would constitute a perioperative AKI risk. Alternatively, a reduced preoperative renal ability to clear preoperative episodic TNFα pulses could lead to a requirement for higher compensatory sustained increased levels in baseline sTNFR1 and sTNFR2.
When the biomarkers (n = 30) were measured in patient serum samples post cardiac surgery at any time the combination of H-FABP, MK and sTNFR1 or sTNFR2 had the highest predictive ability for detecting patients at risk of developing AKI (AUROC 0.817 for H-FABP, MK and sTNFR1 and AUROC 0.836 for H-FABP, MK and sTNFR2 (Table 3)).
While serum sTNFR1 and sTNFR2 in our model may be an indirect reflection of the relative contribution of proinflammatory factors in pathogenesis of AKI, H-FABP in our model may reflect under perfusion of the kidney. Firstly, this increase in serum H-FABP could be secondary to the peri and postoperative myocardial dysfunction which commonly accompanies cardiac surgery32. Schaub et al.32, reported a 6-fold increase in H-FABP measured in blood from patients who experienced AKI at any time point (day 1–day 5 post cardiac operation). Moreover, H-FABP is released into the blood 30 minutes after an ischaemic event from myocytes33,34. The resulting suboptimal cardiac output could lead to renal hypoperfusion and AKI. Secondly, H-FABP is also produced by kidney distal tubular cells35. However, H-FABP expression in the myocardium is 20 times higher than renal tissue so H-FABP measured in the serum is more likely of myocardial origin36. Thirdly, because serum H-FABP is renally cleared, patients with diminished renal function, whether acute or chronic, have compromised H-FABP renal clearance which may further contribute to the elevated H-FABP levels.
Our model also identified MK as a significant factor in the postoperative biomarker combination to detect AKI. Midkine is a pleiotropic, heparin-binding growth factor involved in the pathogenesis of ischemia reperfusion injury. Necrosis and autophagy occur after ischaemic reperfusion injury resulting in vascular endothelial dysfunction and vascular congestion and oedema, reduced blood flow and migration of inflammatory cells to the kidney37. Infiltrating inflammatory cells release cytokines, reactive oxygen species (ROS) and other chemokines adding further insult to the already compromised kidney. Midkine promotes this process and is normally expressed at low levels in proximal tubules. However, it is up-regulated in proximal tubules after ischaemic reperfusion38. Of note, the absence of MK in MK-deficient mice protects against experimentally induced renal ischaemic reperfusion injury39.
The existing method of measuring SCr (eGFR) evaluates the result of AKI. In contrast, our biomarker combination of serum H-FABP, MK and sTNFR1 or sTNFR2, is based largely on the processes initiating and underlying the pathogenesis of AKI. Thus, the information provided by the biomarker combination has the potential to assist with earlier diagnosis and prediction of AKI.
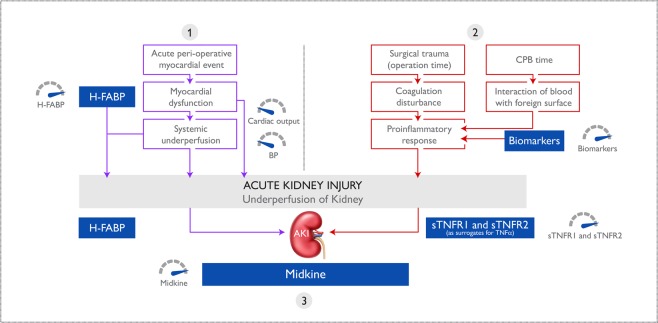
In summary, three main factors in perioperative AKI at cardiac surgery, namely proinflammatory-mediated tubular injury, renal under perfusion and ischemia reperfusion injury are utilised in our model. Soluble TNFR1 and sTNFR2 indicate perioperative proinflammatory load, H-FABP indicates the risk of renal under perfusion secondary to myocardial dysfunction, and MK suggests renal ischemia reperfusion injury. A potential mechanism of action for the biomarker combination is described in Fig. 4.
Figure 4.
Potential pathways involved in the pathogenesis of AKI. Three important pathways in the pathogenesis of AKI are represented by biomarkers in the model: (1) hypoperfusion (H-FABP), (2) proinflammation (sTNFR1 and sTNFR2 as surrogates for the transient TNFα response) and (3) ischaemia reperfusion injury (MK). Together with clinically measured variables, such as (among others) cardiac output and blood pressure (hypoperfusion and ischaemia reperfusion), cross clamp time and bypass time (proinflammation) biomarkers enable AKI patient risk categorisation. AKI, acute kidney injury; H-FABP, heart-type fatty acid-binding protein; sTNFR1, soluble tumour necrosis factor receptor 1; sTNFR2, soluble tumour necrosis factor receptor 2; TNFα, tumour necrosis factor alpha; MK, midkine.
The onset of AKI is multifactorial so in addition to biomarkers, clinical characteristics including age, BMI and diabetes were identified as risk factors for patients at potential risk for the development of AKI preoperatively (Table 4). These three clinical factors were also identified together with surgery-related factors, which included CPB time, cross-clamp time, operation time, whether the patient needed intra-aortic balloon pump, transfusion of blood or platelets and resternotomy, for identifying AKI in patients postoperatively (Table 5). To translate both the biomarker data and clinical characteristics into a proactive AKI clinical tool, the information was converted into a BRS and CRS, respectively (Tables 4, 5, 6 and 7). The results from the BRS and CRS combination will allow the clinician to identify patients at risk of AKI, administer appropriate treatments and monitor treatment efficacy. Thus, if a patient was identified as risk category 2, the current author (WMcB) would monitor the patient’s expected increases in creatinine and urea concentrations over several days retaining an expectation that dialysis requirement would be unlikely. A diuretic, if necessary, would be considered. If the patient was identified as category 3, the dialysis machine would be made available but not primed for potential use post surgery. The present author would be more hesitant to give a diuretic to this patient. However, if the patient was identified as category 4, the author would request that the dialysis machine was ready for use once the operation was completed.
The patients who developed AKI in the current study stayed an extra 2 days in hospital and 2 days longer in ICU (p = 0.000 and p = 0.000, respectively). Similarly, AKI patients had significant increased length of stay in the HDU (p = 0.001). Additional hospital stay is associated with increased costs. However, the low risk patients could potentially have been moved out of the HDU to a ward which would improve patient flow and free up beds and staff to accept new patients, with associated savings (2018/2019 costs per day in HDU is £1400 (excluding medication))40. Earlier diagnosis of AKI benefits the patient, clinician and improves use of hospital resources.
Limitations of the study
Patients undergoing cardiac surgery were included in the study and, therefore, AKI resulting from other serious diseases such as sepsis or drug-induced AKI, were not represented. This was an observational study, where biomarker analysis was completed post event. This limits conclusions since patient interventions were not influenced by our results. Strengths of the study include; the patients were considered not renally impaired preoperatively which enabled measurement of baseline biomarkers. This assisted with determination of biomarker levels post surgery and an understanding of the role of biomarkers in AKI development.
Conclusion
Measurement of sTNFR1 or sTNFR2 preoperatively predicted risk of a patient developing AKI following cardiac surgery. Measurement of a combination of biomarkers, namely H-FABP, MK, sTNFR1 or sTNFR2, at any time postoperatively identified patients with increased risk of developing AKI. Furthermore, deployment of a BRS in combination with CRS in routine practice could assist the clinician with appropriate patient management. This would allow identification of patients at higher risk of developing AKI pre and immediately postoperatively. Adoption of this novel proactive AKI clinical tool will (1) facilitate early identification of patients at risk of AKI, (2) allow timelier clinical decision-making, (3) alter current patient pathways, resulting in more efficient hospital resources utilisation and reduced hospital/ICU/HDU stay.
Supplementary information
Acknowledgements
We would like to thank Dr Moyna Bill (Consultant Cardiac Anaesthetist, Department of Cardiac Anaesthesia, Royal Victoria Hospital, Belfast) for her support throughout the project.
Author contributions
W.Mc.B., M.J.K., G.Mc.L., M.W.R. and J.V.L. made substantial contributions to conception and design, analysis and interpretation of data, revising the manuscript and given final approval of the version to be published. Furthermore, G.Mc.L. and, to a lesser extent, J.J. was responsible for data acquisition. A.D., D.M. and J.W. have made substantial contributions to analysis and interpretation of data and manuscript revision. P.F. and I.Y. provided conceptual support and contributed to manuscript revision.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
M.J.K., A.D., D.M., J.W., J.V.L. and M.W.R. are employees of Randox Laboratories Ltd but hold no shares in the Company. P.F. is the Managing Director and owner of Randox, a privately-owned Company. A patent has been submitted by Randox to protect the biomarkers identified from this work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: William T. McBride and Mary Jo Kurth.
Supplementary information
is available for this paper at 10.1038/s41598-019-53349-1.
References
- 1.Parolari A, et al. Risk Factors for Perioperative Acute Kidney Injury After Adult Cardiac Surgery: Role of Perioperative Management. Ann. Thorac. Surg. 2012;93:584–591. doi: 10.1016/j.athoracsur.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 2.Lagny M-G, et al. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol. 2015;16:76. doi: 10.1186/s12882-015-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dardashti A, Ederoth P, Algotsson L, Brondén B, Bjursten H. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014;147:800–807. doi: 10.1016/j.jtcvs.2013.07.073. [DOI] [PubMed] [Google Scholar]
- 4.Zarbock A, et al. Effect of Remote Ischemic Preconditioning on Kidney Injury Among High-Risk Patients Undergoing Cardiac Surgery. JAMA. 2015;313:2133. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 5.Facts and Stats|Kidney Care UK. Available at, https://www.kidneycareuk.org/news-and-campaigns/facts-and-stats/, (Accessed: 15th July 2019).
- 6.Bellomo R, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care. 2004;8:R204. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellum JA, Lameire N, Kdigo AKI. Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit. Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Md Ralib A, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Crit. Care. 2013;17:R112. doi: 10.1186/cc12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranucci M, et al. Acute Kidney Injury and Hemodilution During Cardiopulmonary Bypass: A Changing Scenario. Ann. Thorac. Surg. 2015;100:95–100. doi: 10.1016/j.athoracsur.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Bennett M, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin. J. Am. Soc. Nephrol. 2008;3:665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liangos O, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14:423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh CR, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J. Am. Soc. Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 15.McBride WT, et al. Cytokine phenotype, genotype, and renal outcomes at cardiac surgery. Cytokine. 2013;61:275–284. doi: 10.1016/j.cyto.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Bossuyt PM, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 18.IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
- 19.Fischer R, et al. Targeting sTNF/TNFR1 Signaling as a New Therapeutic Strategy. Antibodies. 2015;4:48–70. doi: 10.3390/antib4010048. [DOI] [Google Scholar]
- 20.McBride WT, Armstrong MA, Crockard AD, McMurray TJ, Rea JM. Cytokine balance and immunosuppressive changes at cardiac surgery: contrasting response between patients and isolated CPB circuits. Br. J. Anaesth. 1995;75:724–733. doi: 10.1093/bja/75.6.724. [DOI] [PubMed] [Google Scholar]
- 21.Allen SJ, et al. Cell Salvage Alters the Systemic Inflammatory Response After Off-Pump Coronary Artery Bypass Grafting Surgery. Ann. Thorac. Surg. 2007;83:578–585. doi: 10.1016/j.athoracsur.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Baker RC, Armstrong MA, Allen SJ, McBride WT. Role of the kidney in perioperative inflammatory responses. Br. J. Anaesth. 2002;88:330–4. doi: 10.1093/bja/88.3.330. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee PK, Hawksworth GM, McLay JS. Cytokine-stimulated nitric oxide production in the human renal proximal tubule and its modulation by natriuretic peptides: A novel immunomodulatory mechanism? Exp. Nephrol. 1999;7:438–48. doi: 10.1159/000020623. [DOI] [PubMed] [Google Scholar]
- 24.Bocci V, Pacini A, Pessina GP, Maioli E, Naldini A. Studies on tumor necrosis factor (TNF)–I. Pharmacokinetics of human recombinant TNF in rabbits and monkeys after intravenous administration. Gen. Pharmacol. 1987;18:343–6. doi: 10.1016/0306-3623(87)90088-7. [DOI] [PubMed] [Google Scholar]
- 25.Gormley SMC, et al. Plasma and Urinary Cytokine Homeostasis and Renal Dysfunction during Cardiac Surgery. Anesthesiology. 2000;93:1210–1216. doi: 10.1097/00000542-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Gohda T, et al. Circulating TNF Receptors 1 and 2 Predict Stage 3 CKD in Type 1 Diabetes. J. Am. Soc. Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niewczas MA, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 2012;23:507–15. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saulnier P-J, et al. Association of Serum Concentration of TNFR1 With All-Cause Mortality in Patients With Type 2 Diabetes and Chronic Kidney Disease: Follow-up of the SURDIAGENE Cohort. Diabetes Care. 2014;37:1425–1431. doi: 10.2337/dc13-2580. [DOI] [PubMed] [Google Scholar]
- 29.Tonelli M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 30.Atwell DM, Grichnik KP, Newman MF, Reves JG, McBride WT. Balance of proinflammatory and antiinflammatory cytokines at thoracic cancer operation. Ann. Thorac. Surg. 1998;66:1145–50. doi: 10.1016/S0003-4975(98)00592-X. [DOI] [PubMed] [Google Scholar]
- 31.Ma, G. & Bi, S. Effect of rosuvastatin on vascular endothelial functions and inflammatory factors of patients with type 2 diabetes mellitus and coronary heart disease. Exp. Ther. Med., 10.3892/etm.2018.6923 (2018). [DOI] [PMC free article] [PubMed]
- 32.Schaub JA, et al. Perioperative heart-type fatty acid binding protein is associated with acute kidney injury after cardiac surgery. Kidney Int. 2015;88:576–583. doi: 10.1038/ki.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelsers MMAL, Hermens WT, Glatz JFC. Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Kleine AH, et al. Immunohistochemical detection of very recent myocardial infarctions in humans with antibodies against heart-type fatty acid-binding protein. Cardiovasc. Pathol. 1993;2:63–69. doi: 10.1016/1054-8807(93)90014-S. [DOI] [PubMed] [Google Scholar]
- 35.Maatman RG, Van Kuppevelt TH, Veerkamp JH. Two types of fatty acid-binding protein in human kidney. Isolation, characterization and localization. Biochem. J. 1991;273(Pt 3):759–66. doi: 10.1042/bj2730759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heuckeroth RO, Birkenmeier EH, Levin MS, Gordon JI. Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J. Biol. Chem. 1987;262:9709–17. [PubMed] [Google Scholar]
- 37.Lauriat S, Linas SL. The role of neutrophils in acute renal failure. Semin. Nephrol. 1998;18:498–504. [PubMed] [Google Scholar]
- 38.Sato W, Sato Y. Midkine in nephrogenesis, hypertension and kidney diseases. Br. J. Pharmacol. 2014;171:879–887. doi: 10.1111/bph.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato W, et al. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J. Immunol. 2001;167:3463–9. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- 40.Dorset County Hospital Private Patient Tariff. Available at, https://www.dchft.nhs.uk/patients/private-patient-service/Documents/20190429-Private Patient Tariff SF 19-20.pdf.
- 41.Anders H-J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016;27:2564–75. doi: 10.1681/ASN.2016020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perlman, A. S. et al. Serum Inflammatory and Immune Mediators Are Elevated in Early Stage Diabetic Nephropathy Ann. Clin. Lab. Sci.45, 256-263 (2015). [PubMed]
- 43.Sharfuddin, A. A. & Molitoris, B. A. Pathophysiology of ischemic Acute Kidney Injury Nat. Rev. Nephrol.7, 189-200 (2011). [DOI] [PubMed]
- 44.Mihai S, et al. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018;2018:1–16. doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen SD, Phillips TM, Khetpal P, Kimmel PL. Cytokine patterns and survival in haemodialysis patients. Nephrol. Dial. Transplant. 2010;25:1239–1243. doi: 10.1093/ndt/gfp625. [DOI] [PubMed] [Google Scholar]
- 46.Zhang WR, et al. Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J. Am. Soc. Nephrol. 2015;26:3123–32. doi: 10.1681/ASN.2014080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nechemia-Arbely Y, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J. Am. Soc. Nephrol. 2008;19:1106–15. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinuani I, Beberashvili I, Averbukh Z, Sandbank J. Role of IL-10 in the progression of kidney disease. World J. Transplant. 2013;3:91–8. doi: 10.5500/wjt.v3.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isaka Y. Epidermal growth factor as a prognostic biomarker in chronic kidney diseases. Ann. Transl. Med. 2016;4:S62. doi: 10.21037/atm.2016.10.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.García-Sánchez O, López-Novoa JM, López-Hernández FJ. Interferon-γ Reduces the Proliferation of Primed Human Renal Tubular Cells. Nephron Extra. 2014;4:1–7. doi: 10.1159/000353587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teppala S, Shankar A, Sabanayagam C. Association between IGF-1 and chronic kidney disease among US adults. Clin. Exp. Nephrol. 2010;14:440–444. doi: 10.1007/s10157-010-0307-y. [DOI] [PubMed] [Google Scholar]
- 52.Roy MS, Janal MN, Crosby J, Donnelly R. Markers of endothelial dysfunction and inflammation predict progression of diabetic nephropathy in African Americans with type 1 diabetes. Kidney Int. 2015;87:427–33. doi: 10.1038/ki.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haq, M., Norman, J., Saba, S. R., Ramirez, G. & Rabb, H. Role of IL-1 in renal ischemic reperfusion injury. J. Am. Soc. Nephrol. 9 (1998). [DOI] [PubMed]
- 54.Ostendorf T, Boor P, van Roeyen CRC, Floege J. Platelet-derived growth factors (PDGFs) in glomerular and tubulointerstitial fibrosis. Kidney Int. Suppl. 2014;4:65–69. doi: 10.1038/kisup.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, et al. Serum IP-10 is useful for identifying renal and overall disease activity in pediatric systemic lupus erythematosus. Pediatr. Nephrol. 2018;33:837–845. doi: 10.1007/s00467-017-3867-1. [DOI] [PubMed] [Google Scholar]
- 56.Kitching AR, et al. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J. Am. Soc. Nephrol. 1999;10:752–9. doi: 10.1681/ASN.V104752. [DOI] [PubMed] [Google Scholar]
- 57.Lundberg S, Lundahl J, Gunnarsson I, Sundelin B, Jacobson SH. Soluble interleukin-2 receptor alfa predicts renal outcome in IgA nephropathy. Nephrol. Dial. Transplant. 2012;27:1916–1923. doi: 10.1093/ndt/gfr554. [DOI] [PubMed] [Google Scholar]
- 58.Lemesch S, et al. Mode of renal replacement therapy determines endotoxemia and neutrophil dysfunction in chronic kidney disease. Sci. Rep. 2016;6:34534. doi: 10.1038/srep34534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barr ELM, et al. High Baseline Levels of Tumor Necrosis Factor Receptor 1 Are Associated With Progression of Kidney Disease in Indigenous Australians With Diabetes: The eGFR Follow-up Study. Diabetes Care. 2018;41:739–747. doi: 10.2337/dc17-1919. [DOI] [PubMed] [Google Scholar]
- 60.Parodis I, et al. Serum soluble tumour necrosis factor receptor-2 (sTNFR2) as a biomarker of kidney tissue damage and long-term renal outcome in lupus nephritis. Scand. J. Rheumatol. 2017;46:263–272. doi: 10.1080/03009742.2016.1231339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H, et al. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013;2:84. doi: 10.5527/wjn.v2.i3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wannamethee SG, Whincup PH, Lennon L, Papacosta O, Lowe GD. Associations between fibrin D-dimer, markers of inflammation, incident self-reported mobility limitation, and all-cause mortality in older men. J. Am. Geriatr. Soc. 2014;62:2357–62. doi: 10.1111/jgs.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang Y, et al. C-reactive protein promotes acute kidney injury by impairing G 1/S-dependent tubular epithelium cell regeneration. Clin. Sci. 2014;126:645–659. doi: 10.1042/CS20130471. [DOI] [PubMed] [Google Scholar]
- 64.Lindner G, et al. D-dimer to Rule Out Pulmonary Embolism in Renal Insufficiency. Am. J. Med. 2014;127:343–347. doi: 10.1016/j.amjmed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Sharain, K., Hoppensteadt, D., Bansal, V., Singh, A. & Fareed, J. Progressive Increase of Inflammatory Biomarkers in Chronic Kidney Disease and End-Stage Renal Disease, Clin. Appl. Thromb. Hemost.19, 303-308 (2013). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.