Abstract
Objective
The aim of this study was to assess the cost effectiveness of semaglutide versus dulaglutide, as an add-on to metformin monotherapy, for the treatment of type 2 diabetes (T2D), from a Canadian societal perspective.
Methods
The Swedish Institute for Health Economics Cohort Model of T2D was used to assess the cost effectiveness of once-weekly semaglutide (0.5 or 1.0 mg) versus once-weekly dulaglutide (0.75 or 1.5 mg) over a 40-year time horizon. Using data from the SUSTAIN 7 trial, which demonstrated comparatively greater reductions in glycated hemoglobin (HbA1c), body mass index and systolic blood pressure with semaglutide, compared with dulaglutide, a deterministic base-case and scenario simulation were conducted. The robustness of the results was evaluated with probabilistic sensitivity analyses and 15 deterministic sensitivity analyses.
Results
The base-case analysis indicated that semaglutide is a dominant treatment option, compared with dulaglutide. Semaglutide was associated with lower total costs (Canadian dollars [CAN$]) versus dulaglutide for both low-dose (CAN$113,287 vs. CAN$113,690; cost-saving: CAN$403) and high-dose (CAN$112,983 vs. CAN$113,695; cost-saving: CAN$711) comparisons. Semaglutide resulted in increased quality-adjusted life-years (QALYs) and QALY gains, compared with dulaglutide, for both low-dose (11.10 vs. 11.07 QALYs; + 0.04 QALYs) and high-dose (11.12 vs. 11.07 QALYs; + 0.05 QALYs) comparisons. The probabilistic sensitivity analysis showed that for 66–73% of iterations, semaglutide was either dominant or was considered cost effective at a willingness-to-pay threshold of CAN$50,000.
Conclusions
From a Canadian societal perspective, semaglutide may be a cost-effective treatment option versus dulaglutide in patients with T2D who are inadequately controlled on metformin monotherapy.
Electronic supplementary material
The online version of this article (10.1007/s41669-019-0131-6) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| This is the first application of the Swedish Institute for Health Economics Cohort Model of T2D, a validated and transparent model, in assessing the cost effectiveness of semaglutide versus dulaglutide from a Canadian societal perspective, incorporating both a simplified base-case and an alternative scenario analysis. |
| Semaglutide is demonstrably a cost-effective treatment option versus dulaglutide in patients with T2D inadequately controlled on metformin monotherapy, providing evidence to support decision makers in the Canadian healthcare system in recommending semaglutide as a glucagon-like peptide-1 receptor agonist for reimbursement. |
Introduction
In Canada, the number of people with diabetes was 2.6 million in 2017 and is expected to rise to 3.2 million in 2045 [1]. Type 2 diabetes (T2D) accounts for over 90% of diabetes cases [2]. The disease is chronic, progressive, and complex, and is associated with numerous complications, such as cardiovascular (CV) disease, which is the most common cause of death for people with diabetes [3]. In 2010, the predicted economic burden of both direct and indirect costs of T2D in Canada was 12.2 billion Canadian dollars (CAN$) per annum [4], and this cost is projected to increase to CAN$16.9 billion by 2020 [5]. Diabetic complications and their management contribute significantly to the health and economic burden [6].
Several new treatments for T2D have recently become available and, given the burden of the disease, it is important to assess and compare the value for money of these interventions. Economic modeling techniques have become recommended [7] and widely used tools that consider the long-term clinical outcomes and economic value of such new interventions, thereby aiding decision making in the selection of treatments for reimbursement. Cost-effectiveness analyses go beyond the efficacy results of clinical trials and individual drug costs, and reflect country-specific differences in local guidelines, healthcare systems and their willingness to pay, and costs of complications, among other factors, all of which may impact the evaluation of economic viability.
Given the complex, multifactorial and chronic nature of T2D, designing an economic model that can effectively assess the impact of interventions is challenging [8]. There are a range of long-term economic models for diabetes, including CORE [9, 10], Economic and Health Outcomes Model of Type 2 Diabetes Mellitus (ECHO-T2DM) [11, 12], United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Models 1 [13] and 2 [14] and Cardiff [15, 16]. However, despite being used in health technology assessment (HTA) submissions around the world, the optimal design of these models is still under discussion by expert groups [8], and being considered by the Canadian Agency for Drugs and Technology in Health (CADTH) [17]. For the purpose of this analysis, we sought to use a model that was easy to use, open source, transparent, able to address uncertainty, and applicable to more than one compound as these qualities were deemed to be the most appropriate and desired by Canadian stakeholders. An existing economic model that met these criteria, developed by the Swedish Institute for Health Economics (IHE), is the IHE Cohort Model of T2D. This Microsoft® Excel-based model, with Visual Basic for Applications (VBA) coding, utilizes Markov health states. These aspects deliver the requirements of simplified and accessible use; verifiable methodology, assumptions and calculations; generation of probabilistic analyses to address uncertainty; and being applicable for any treatment for diabetes. This externally validated model [18] is expected to satisfactorily approximate the characteristics, treatment options and treatment patterns of patients with diabetes in clinical practice in Canada. From an economic perspective, the model addresses all relevant factors and is an appropriate choice for assessing the cost effectiveness of interventions for T2D from a Canadian societal perspective.
For these reasons, the IHE Cohort Model of T2D was selected for the current study to assess the cost effectiveness of once-weekly semaglutide (Novo Nordisk, Denmark) versus once-weekly dulaglutide (Eli Lilly and Company, USA), two glucagon-like peptide-1 receptor agonists (GLP-1RAs) recently approved in Canada (2018 and 2016, respectively) for the treatment of T2D [19, 20].
In the global phase III clinical trial program SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes), semaglutide was compared with a range of other treatments for T2D [21–27]. The SUSTAIN 7 trial compared once-weekly semaglutide with once-weekly dulaglutide, as an add-on to metformin [23]. After 40 weeks of treatment, reductions from baseline in glycated hemoglobin (HbA1c) and body weight were superior with semaglutide for both the high-dose comparison (1.0 mg semaglutide vs. 1.5 mg dulaglutide) and low-dose comparison (0.5 mg semaglutide vs. 0.75 mg dulaglutide) [p < 0.0001] [23]. Furthermore, a significantly greater proportion of subjects with T2D achieved clinically meaningful glycemic targets (HbA1c < 7.0%; p < 0.0021 for the high-dose comparison, p < 0.0001 for the low-dose comparison) and weight-loss responses (≥ 5% weight loss; p < 0.0001 for both dose comparisons) with semaglutide compared with dulaglutide, while exhibiting similar safety profiles [23].
The aim of this study was to assess the cost effectiveness of once-weekly semaglutide versus once-weekly dulaglutide, as an add-on to metformin, for the treatment of T2D, from a Canadian societal perspective.
Methods
Model Overview
The IHE Cohort Model of T2D was constructed in Microsoft® Excel 2013, with the aid of built-in VBA, and uses Markov health states to capture important micro- and macrovascular complications and premature mortality associated with T2D. A simplified overview is presented in Fig. 1. The model compares two treatment strategies, which are defined by the user in terms of treatments used and the associated expected effect on glycemic control, weight change and risk of hypoglycemia. The cycle length is 1 year, with a maximum time horizon of 40 years. Uncertainty in the model parameters (second-order uncertainty) is captured by means of probabilistic sensitivity analysis (PSA). Time-varying transition probabilities are calculated on the basis of characteristics of the cohort (e.g. diabetes duration, demographics, biomarkers) and user-defined selection of risk equations. Mortality risk equations are sourced from UKPDS 68 [13] or UKPDS 82 [14]; macrovascular risk equations are sourced from the Swedish National Diabetes Register (NDR) [28], UKPDS 68 [13] or UKPDS 82 [14]; and microvascular risk equations are sourced from other diabetes models [29–31]. Further details on the model structure are presented in Online Resource 1, and in the model validation study [18]. Previously, the model has been applied to assess the cost effectiveness of several new antihyperglycemic treatment alternatives [32–35] and has been used for reimbursement decisions by the Dental and Pharmaceutical Benefits Agency (TLV) in Sweden [36, 37]. The National Board of Health and Welfare in Sweden has also used the model in the development of the 2015 National Guidelines for Diabetes Care [38].
Fig. 1.
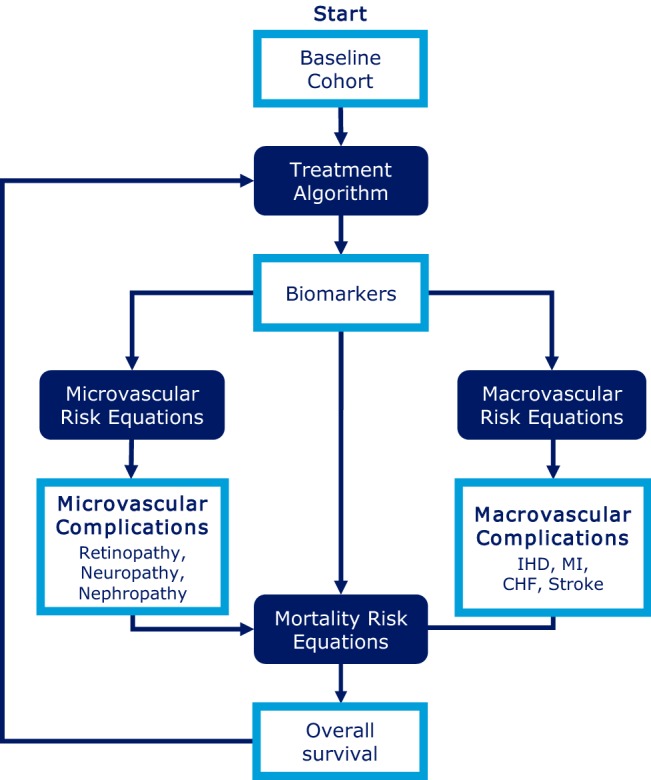
The IHE cohort model of T2D. CHF congestive heart failure, IHD ischemic heart disease, IHE Swedish Institute of Health Economics, MI myocardial infarction, T2D type 2 diabetes
Base-Case Analysis
A deterministic base-case simulation (using a 40-year time horizon) and a PSA using 1000 iterations were conducted. In line with modeling conducted in the CADTH 2017 report [39], the UKPDS 82 risk equations for macrovascular complications and mortality were chosen [14]. Furthermore, these equations also include separate risk equations for secondary events for some complications. Additional key assumptions are presented in Table 1. In addition, a scenario analysis, in which modeled treatment patterns were changed to reflect alternative, clinically relevant circumstances, and 15 deterministic sensitivity analyses (DSAs) were conducted.
Table 1.
Key assumptions
| Setting | Value |
|---|---|
| Perspective | Societal |
| Time horizon | 40 years |
| Discounting of health and costsa | 1.50% |
| Baseline cohort characteristics | Head-to-head clinical trial (SUSTAIN 7) [23] |
| Treatment effects | Head-to-head clinical trial (SUSTAIN 7) [23] |
| Treatment algorithm | Assume 3-year treatment duration then discontinue initial treatments and assume treatment with basal insulin |
| Macrovascular and mortality risk equations | UKPDS 82 [14] |
| Microvascular risk equations | WESDR, REP [29, 31] |
| Micro- and macrovascular complications cost | CADTH therapeutic review (2017) [39], Ward et al. (2014) [52] |
| Disutility weights | CADTH therapeutic review (2017) [39], Bagust and Beale (2005) [51] |
CADTH Canadian Agency for Drugs and Technologies in Health, REP Rochester Epidemiology Project, UKPDS United Kingdom Prospective Diabetes Study, WESDR Wisconsin Epidemiological Study of Diabetic Retinopathy
aThe discounting rate was chosen based on the Canadian Guidelines for the Economic Evaluation of Health Technologies [67]
To ensure transparency and reproducibility, the Diabetes Modeling Input Checklist (Eighth Mount Hood Challenge) and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist were completed during the set-up and reporting of this study [40, 41] and are included in the electronic supplementary material (Online Resources 11 and 12).
Treatment Algorithm
In the simulation, patients initiate treatment with either semaglutide (0.5 or 1.0 mg) or dulaglutide (0.75 or 1.5 mg). For a 3-year period, GLP-1RA treatment is assumed to be maintained, after which semaglutide and dulaglutide are discontinued and basal insulin initiated. Although no data were available specifically showing the benefits of GLP-1RAs up to 3 years, a quantitative investigation into physician treatment practices in five major European countries showed that the mean treatment duration for GLP-1RAs was 29.35 months [42]. For the modeling analysis, this was rounded up to 3 years (36 months) because treatment switching can only occur at the end of the model’s annual cycles. The SUSTAIN 6 trial provides additional support for long-term benefits of GLP-1RAs; in this trial, semaglutide provided significant and sustained improvements in clinical parameters, especially body weight, over 104 weeks [24]. The 3-year timeframe is also consistent with previously published cost-effectiveness studies [43–45].
The following assumptions were made for the simulation period: HbA1c (%) and body mass index (BMI) remain at the level observed after the first-year treatment effect has been applied; systolic blood pressure (SBP) and lipid levels ‘drift’ upwards following application of the treatment effect; the efficacy of basal insulin treatment maintains HbA1c at 8.0%; and upon discontinuation of GLP-1RA treatment, BMI reverts to baseline values [13]. These assumptions, in regard to treatment with basal insulin, were later tested in a scenario analysis.
Scenario Analysis
The scenario analysis simulates the impact of an alternative disease progression that more accurately represents the management of diabetes in clinical practice. Specifically, HbA1c is assumed to ‘drift’ upward at a rate of 0.14% per year according to data in the study by Kahn et al. [46], which has also been applied in other modeling studies [47–49]. When this drifting results in patients’ HbA1c levels reaching 8.0%, GLP-1RA treatment is discontinued and insulin treatment initiated. In contrast to the base-case, the scenario analysis models the effects of insulin treatment on HbA1c, BMI and hypoglycemia. Treatment effects were derived from the study by Willis et al. that estimated parsimonious prediction equations for changes in HbA1c and weight, as well as hypoglycemic events [50], and estimated using the CORE specification equation for insulin-naïve patients, while rates for non-severe and severe hypoglycemia were based on insulin-naïve patients with an average HbA1c of 7.5–8.0% (Online Resource 9). Insulin treatment results in HbA1c reductions, but once HbA1c levels again reach 8.0%, the assumption is the same as in the base-case analysis, in that HbA1c levels are fixed at 8.0% for the remainder of the simulation, i.e. for the remainder of the model’s time horizon. A PSA using 1000 iterations was also performed for this analysis.
Deterministic Sensitivity Analyses
Fifteen DSAs were conducted to assess the robustness of the base-case result, encompassing changes in clinical parameters (DSA 1–3, 6–10, 15), time horizons (DSA 4–5), and cost parameters (DSA 11–14).
Cohort Data
Data on patient demographics and clinical characteristics were sourced from SUSTAIN 7 results (Table 2).
Table 2.
Baseline characteristics
| Total cohort (both arms) | |
|---|---|
| Demographics | |
| Age, years | 55.67 |
| Female, % | 44.79 |
| Ethnicity, %a | |
| Caucasian | 82.82 |
| Black | 5.67 |
| Hispanic | 11.51 |
| Risk factors and biomarkers | |
| Diabetes duration, years | 7.42 |
| Smoker, % | 14.01 |
| HbA1c, % | 8.22 |
| SBP, mmHg | 132.96 |
| TC, mmol/Lb | 4.69 |
| LDL, mmol/Lb | 2.65 |
| HDL, mmol/Lb | 1.16 |
| TG, mmol/Lb | 2.05 |
| BMI, kg/m2 | 33.50 |
| HR, bpm | 75.19 |
| WBC, 1 × 106 | 7.39 |
| eGFR, mL/min/1.73 m2 | 97.20 |
| History of complications, % | |
| Retinopathy | |
| Background diabetic retinopathy | 2.9 |
| Severe visual loss | 0.3 |
| PDR | 0.0 |
| ME | 0.0 |
| PDR and ME | 0.0 |
| Neuropathy | |
| Symptomatic | 9.1 |
| Peripheral vascular disease | 0.7 |
| Lower-extremity amputation | 0.4 |
| Nephropathy | |
| Microalbuminuria | 1.6 |
| Macroalbuminuria | 0.5 |
| End-stage renal disease | 0.0 |
| Macrovascular complications | |
| Ischemic heart disease | 14.5 |
| Congestive heart failure | 5.4 |
| Myocardial infarction | 4.3 |
| Stroke | 2.2 |
Values are based on the SUSTAIN 7 trial [23] and are reported as means
BMI body mass index, bpm beats per minute, eGFR estimated glomerular filtration rate, HDL high-density lipoprotein, HbA1c glycated hemoglobin, HR heart rate, LDL low-density lipoprotein, ME macular edema, PDR proliferative diabetic retinopathy, SBP systolic blood pressure, TC total cholesterol, TG triglycerides, WBC white blood cell count
aThe SUSTAIN trial also included the ethnic groups Asian/Pacific Islander and Australian, which were included in the Caucasian category
bValues have been converted from mg/dL to mmol/L by multiplying the mg/dL values by 0.0259 (TC, HDL, LDL) and 0.0113 (TG)
Treatment Effects and Adverse Events
Treatment effects on HbA1c, SBP, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, BMI, estimated glomerular filtration rate (eGFR), and non-severe and severe hypoglycemic events for patients treated with semaglutide and dulaglutide were sourced from SUSTAIN 7 (Online Resource 2), where possible [23]. Data on treatment effects from SUSTAIN 7 that had not previously been published were derived from supplementary post hoc analyses.
Health-Related Quality of Life
Baseline data and patient characteristics for health-related quality of life (HRQoL) were estimated from the CODE-2 study, which used multivariate regression techniques and a data set of 4461 patients with T2D to attribute HRQoL decrements to specific individual patient characteristics, treatments and health complications [51]. Decrements for each episode of micro- and macrovascular complications were subtracted from baseline values. Disutilities for HRQoL used in the CADTH 2017 report were applied wherever possible [39], and supplemented with data from cross-sectional studies (Online Resource 3).
Costs
Canada-specific costs for micro- and macrovascular complications and hypoglycemic events were derived from the CADTH 2017 report wherever possible. Costs that were unavailable from this report were sourced from a US study that used direct data analysis and microcosting to estimate costs, converted to Canadian dollars and adjusted for inflation (Online Resources 4 and 5) [52, 53]. All costs are reported in 2017 Canadian dollars.
The cost for both semaglutide and dulaglutide was CAN$195.06 for a package supplying 4 weeks of treatment, equivalent to CAN$6.97 per day or CAN$2544 per year. Annual costs of CAN$1259 were associated with basal insulin treatment, and comprised CAN$757 for neutral protamine Hagedorn (NPH) insulin, CAN$177 for needles and CAN$325 for test strips (Online Resource 6).
Indirect costs (e.g. loss of work productivity) are an important consideration in cost-effectiveness analyses because they account for the wider societal impact of diseases and treatment interventions. Data on the association between specific diabetes complications and loss of productivity are scarce, and a conservative assumption was made that diabetes-related loss of productivity was mainly attributable to severe stages of micro- and macrovascular complications. Because of a lack of data suitable for economic modeling of productivity losses in Canada, assumptions were based on a Danish register study from 2013, in which an estimation of absences from work (days per year) associated with diabetes-related complications was determined from 34,882 individuals (age 18–70 years) with hospital-diagnosed diabetes [53]. Data for productivity loss and employment-to-population ratio used to estimate indirect costs are shown in Online Resources 7 and 8.
Results
Base-Case and Probabilistic Sensitivity Analysis
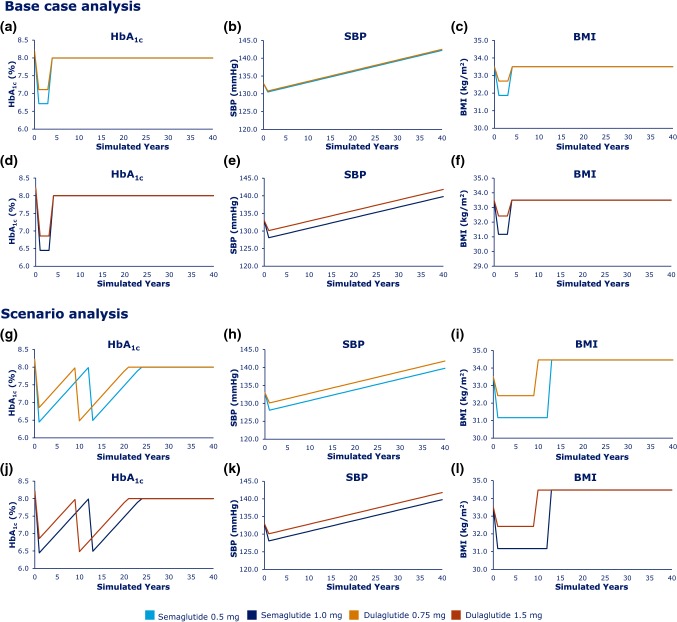
The simulated progression in risk factors over time for semaglutide and dulaglutide are shown in Fig. 2, comparing semaglutide 0.5 mg with dulaglutide 0.75 mg (Fig. 2a–c), and semaglutide 1.0 mg with dulaglutide 1.5 mg (Fig. 2d–f). The progression in HbA1c (Fig. 2a, d), SBP (Fig. 2b, e), and BMI (Fig. 2c, f) over time is in line with the findings for semaglutide compared with dulaglutide, as observed over 40 weeks in the SUSTAIN 7 trial [23].
Fig. 2.
Base-case progression over time in a HbA1c, b SBP, and c BMI for the low-dose comparison, and in d HbA1c, e SBP, and f BMI for the high-dose comparison. Scenario progression over time in g HbA1c, h SBP, and i BMI for the low-dose comparison, and in j HbA1c, k SBP, and l BMI for the high-dose comparison. BMI body mass index, HbA1c glycated hemoglobin, SBP systolic blood pressure
These results led to differences in modeled cost and health outcomes over time. Overall, while the daily acquisition cost of semaglutide and dulaglutide is identical, the total cost of treatment is predicted to be slightly higher with semaglutide versus dulaglutide for both the low-dose (CAN$25,501 vs. CAN$25,481) and high-dose (CAN$25,534 vs. CAN$25,476) comparisons, which may be related to the slightly higher simulated survival associated with semaglutide. For micro- and macrovascular complications, semaglutide was associated with lower cumulative incidences of retinopathy, nephropathy, myocardial infarction and stroke (Online Resource 10). Differences were smallest for neuropathy and no benefit was evident with semaglutide versus dulaglutide for peripheral vascular disease. Differences were greatest for CV events; semaglutide was associated with a relative risk reduction (RRR) of 3.10% and 2.70% for the occurrence of first stroke and second or subsequent stroke, respectively, for the high-dose comparison. However, these differences are effectively minimized as the base-case assumes a fixed 3-year treatment duration, after which differences in HbA1c and BMI are eliminated. As these are important risk factors for predicting micro- and macrovascular complications, the reductions in HbA1c and BMI achieved during this limited timeframe are likely to lead to even small increases in survival. The impact of these assumptions was tested in the scenario analysis described below.
Semaglutide was associated with lower overall total costs for both the low-dose (CAN$113,287 vs. CAN$113,690) and high-dose (CAN$112,983 vs. CAN$113,695) comparisons, representing total cost savings with semaglutide of CAN$403 and CAN$711, respectively (Table 3). Furthermore, semaglutide generated quality-adjusted life-year (QALY) gains of 0.04 for the low-dose comparison and 0.05 for the high-dose comparison, i.e. semaglutide was dominant (Table 3).
Table 3.
Deterministic base-case analysis for semaglutide versus dulaglutide as an add-on to metformin
| Deterministic base-case | ||||||
|---|---|---|---|---|---|---|
| Semaglutide 0.5 mg |
Dulaglutide 0.75 mg |
Difference | Semaglutide 1.0 mg |
Dulaglutide 1.5 mg |
Difference | |
| Health gain | ||||||
| Survival after 40 years, % | 4.6 | 4.6 | 0 | 4.7 | 4.6 | 0.1 |
| Life-years | 17.79 | 17.77 | 0.02 | 17.81 | 17.77 | 0.05 |
| QALYs | 11.10 | 11.07 | 0.04 | 11.12 | 11.07 | 0.05 |
| Direct costs, CAN$ | ||||||
| Antihyperglycemic treatment | 25,501 | 25,481 | 20 | 25,534 | 25,476 | 58 |
| Hypoglycemia | 181 | 231 | − 50 | 212 | 236 | − 24 |
| Macrovascular complications | ||||||
| IHD | 13,755 | 13,628 | 127 | 13,717 | 13,765 | − 48 |
| CHF | 7664 | 7692 | − 27 | 7687 | 7719 | − 33 |
| MI | 8655 | 8627 | 28 | 8573 | 8674 | − 101 |
| Stroke | 5482 | 5510 | − 29 | 5356 | 5518 | − 161 |
| Microvascular complications | ||||||
| Retinopathy | 1306 | 1354 | − 48 | 1288 | 1319 | − 31 |
| Neuropathy | 20,564 | 20,703 | − 139 | 20,510 | 20,579 | − 69 |
| Nephropathy | 2983 | 3075 | − 93 | 2935 | 3007 | − 72 |
| Total | 86,090 | 86,302 | − 212 | 85,812 | 86,292 | − 480 |
| Production loss costs, CAN$ | 27,197 | 27,388 | − 191 | 27,172 | 27,403 | − 231 |
| Total costs, CAN$ | 113,287 | 113,690 | − 403 | 112,983 | 113,695 | − 711 |
| Incremental cost-effectiveness ratio | ||||||
| Per life-year gained | Dominant | Dominant | ||||
| Per QALY gained | Dominant | Dominant | ||||
CAN$ Canadian dollars, CHF congestive heart failure, IHD ischemic heart disease, MI myocardial infarction, QALY quality-adjusted life-year
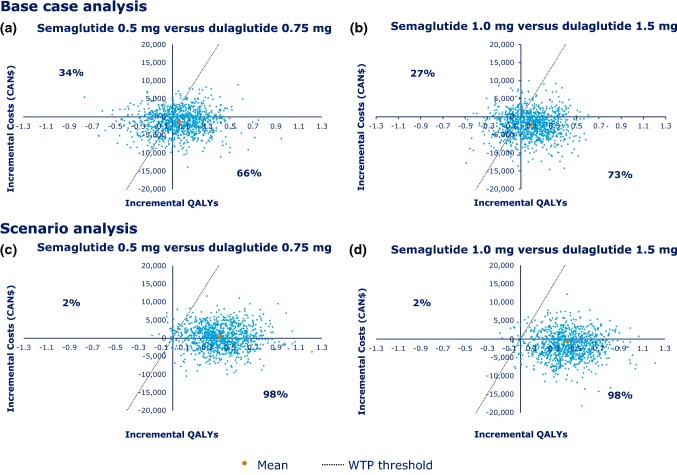
The PSA of the base-case is presented in Fig. 3, which shows the cost-effectiveness plane (CEP) for 1000 iterations in the PSA. With the majority (66–73%) of the iterations below the willingness-to-pay (WTP) threshold of CAN$50,000, semaglutide was considered to be cost effective. In the base-case PSA analysis for semaglutide 0.5 mg versus dulaglutide 0.75 mg, the mean incremental QALYs and costs were 0.06 and CAN$ − 1288, respectively, and, at a WTP threshold of CAN$50,000, 66% of the incremental cost-effectiveness ratios (ICERs) were cost effective (Fig. 3a). For semaglutide 1.0 mg versus dulaglutide 1.5 mg, the mean incremental QALYs and costs were 0.09 and CAN$ − 2191, respectively, and, at a WTP threshold of CAN$50,000, 73% of ICERs were cost effective (Fig. 3b).
Fig. 3.
CEP based on incremental costs and QALYs in the base-case analysis for the a low-dose and b high-dose comparison, and in the scenario analysis for the c low-dose and d high-dose comparison. a In the semaglutide 0.5 mg vs. dulaglutide 0.75 mg comparison, at a WTP threshold of CAN$50,000, 66% of ICERs were cost effective. b In the semaglutide 1.0 mg vs. dulaglutide 1.5 mg comparison, at a WTP threshold of CAN$50,000, 73% of ICERs were cost effective. c In the semaglutide 0.5 mg vs. dulaglutide 0.75 mg comparison, at a WTP threshold of CAN$50,000, 98% of ICERs were cost effective. d In the semaglutide 1.0 mg vs. dulaglutide 1.5 mg comparison, at a WTP threshold of CAN$50,000, 98% of ICERs were cost effective. CAN$ Canadian dollars, CEP cost-effectiveness plane, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year, WTP willingness to pay
Scenario Analysis
In the more clinically relevant scenario analysis, there was greater differentiation between semaglutide and dulaglutide in the progression of the risk factors HbA1c (Fig. 2g, j), SBP (Fig. 2h, k), and BMI (Fig. 2i, l) over time.
Semaglutide was associated with higher total costs than dulaglutide for both dose comparisons, with total increased expenditure of CAN$1305 and CAN$1977, respectively (Table 4). Comparisons of semaglutide versus dulaglutide generated QALY gains of 0.35 for the low-dose comparison and 0.40 for the high-dose comparison, giving ICERs of CAN$5585 and CAN$3296, respectively, per QALY.
Table 4.
Deterministic sensitivity analyses and scenario analysis results
| Label | Low-dose comparison | High-dose comparison | |||||
|---|---|---|---|---|---|---|---|
| Semaglutide 0.5 mg vs. dulaglutide 0.75 mg | Semaglutide 1.0 mg vs. dulaglutide 1.5 mg | ||||||
| Incremental total cost, CAN$ | Incremental total QALYs | ICER | Incremental total cost, CAN$ | Incremental total QALYs | ICER | ||
| Base-case | Assuming 3-year treatment duration, then discontinuation of initial treatments; HbA1c and BMI remain at the level attained | − 403 | 0.04 | Dominant | − 711 | 0.05 | Dominant |
| Scenario analysis | Assuming HbA1c ‘drifts’ upward at a rate of 0.14% per year, with GLP-1RA treatment discontinued and insulin treatment initiated when patients drift up to the HbA1c threshold of 8% | 1977 | 0.35 | 5585 | 1305 | 0.40 | 3296 |
| DSA1 | Change in HbA1c is the only difference in efficacy between treatments: all other effect parameters are set to zero | − 432 | 0.02 | Dominant | − 427 | 0.02 | Dominant |
| DSA2 | Only statistically significantly different treatment effects from the trials are included in the simulation | − 501 | 0.03 | Dominant | − 386 | 0.04 | Dominant |
| DSA3 | The UKPDS 68 risk equations are used instead of UKPDS 82 | − 603 | 0.04 | Dominant | − 733 | 0.06 | Dominant |
| DSA4 | 20-year time horizon | − 413 | 0.03 | Dominant | − 666 | 0.04 | Dominant |
| DSA5 | 10-year time horizon | − 303 | 0.02 | Dominant | − 415 | 0.02 | Dominant |
| DSA6 | The differences in BMI between treatments are assumed to be maintained throughout the patient’s lifetime | − 533 | 0.13 | Dominant | − 913 | 0.16 | Dominant |
| DSA7 | The upper 95% CI for change in HbA1c is used in the semaglutide arm | − 514 | 0.04 | Dominant | − 830 | 0.06 | Dominant |
| DSA8 | The lower 95% CI for change in HbA1c is used in the semaglutide arm | − 289 | 0.03 | Dominant | − 581 | 0.05 | Dominant |
| DSA9 | The upper 95% CI for change in BMI is used in the semaglutide arm | − 410 | 0.04 | Dominant | − 718 | 0.07 | Dominant |
| DSA10 | The lower 95% CI for change in BMI is used in the semaglutide arm | − 400 | 0.02 | Dominant | − 705 | 0.05 | Dominant |
| DSA11 | Cost of complications assumed to be 10% higher | − 427 | 0.04 | Dominant | − 770 | 0.05 | Dominant |
| DSA12 | Cost of complications assumed to be 10% lower | − 379 | 0.04 | Dominant | − 667 | 0.05 | Dominant |
| DSA13 | Cost of drugs + 10% | − 401 | 0.04 | Dominant | − 710 | 0.05 | Dominant |
| DSA14 | Cost of drugs − 10% | − 405 | 0.04 | Dominant | − 722 | 0.05 | Dominant |
| DSA15 | No explicit insulin-treatment effects assumed; patients’ HbA1c levels remain at 8.0% following upward ‘drift’ to this threshold | 1561 | 0.36 | 4397 | 905 | 0.38 | 2397 |
BMI body mass index, CI confidence interval, CAN$ Canadian dollars, DSA deterministic sensitivity analysis, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year, UKPDS United Kingdom Prospective Diabetes Study
The higher total cost of semaglutide is explained by the assumed drift in HbA1c, whereby patients were only switched to basal insulin once HbA1c levels had drifted up to the threshold of 8.0%. For both low- and high-dose comparisons, patients taking semaglutide experienced a greater initial drop in HbA1c at the beginning of the simulation, compared with dulaglutide. Consequently, patients in both the low- and high-dose semaglutide arms remained on GLP-1RA treatment for 3 years longer than those receiving dulaglutide treatment, before switching to the less costly insulin treatment.
Figure 3 shows the CEP for 1000 iterations in the PSA for the scenario analysis; in the majority of iterations (98%), semaglutide was cost effective (i.e. below the WTP threshold of CAN$50,000). For semaglutide 0.5 mg versus dulaglutide 0.75 mg, the mean incremental QALY gains were 0.41, and the mean incremental costs were CAN$530; at a WTP threshold of CAN$50,000, 98% of the iterations were cost effective (Fig. 3c). For semaglutide 1.0 mg versus dulaglutide 1.5 mg, the mean incremental QALY gains were 0.42 and the mean incremental costs were CAN$ − 613; at a WTP threshold of CAN$50,000, 98% of the iterations were cost effective (Fig. 3d).
Deterministic Sensitivity Analyses
Findings from the DSAs support the results of the base-case. Semaglutide was dominant in nearly all DSAs, regardless of changes in clinical parameters, time horizons, or cost parameters. Details of the sensitivity analyses for low- and high-dose comparisons of semaglutide and dulaglutide are shown in Table 4.
When changing clinical parameters, and even using the more extreme assumptions of DSA1 (change in HbA1c was the only difference in efficacy between treatments, with differences in all other effect parameters set to zero), semaglutide still dominated dulaglutide. While the remaining DSAs demonstrated QALY gains of 0.02–0.07, in DSA6, where treatment-related differences in BMI were maintained throughout the patient’s lifetime, QALY gains of 0.13 for the low-dose comparison and 0.16 for the high-dose comparison were observed, with costs decreased by CAN$533 and CAN$913, respectively. Alternatively, in DSA15, an assumption is made regarding an upwards drift in HbA1c (0.14% per year) following initial treatment effects until 8.0%, at which point GLP-1RA treatment is discontinued. However, in contrast to the scenario analysis, in which the effects of insulin on HbA1c and BMI are taken into consideration, the insulin treatment maintains HbA1c levels at 8.0%, as in the base-case. With no insulin treatment effects assumed, semaglutide was cost effective, compared with dulaglutide, for both the low-dose (QALY gains of 0.36; cost increase CAN$1561; ICER 4397) and high-dose (QALY gains of 0.38; cost increase CAN$905; ICER 2397) comparisons. As with the scenario analysis, the increase in total cost is related to patients having semaglutide treatment for longer, compared with dulaglutide, with associated costs offset by reduced complications (results not shown). QALYs increased due to a larger separation in HbA1c level and BMI over time between treatment arms and because patients taking dulaglutide started insulin therapy (which is associated with more hypoglycemic events) earlier than semaglutide. These results yielded a low ICER of CAN$1900 per QALY gained.
When the time horizon was shortened to 20 (DSA4) or 10 years (DSA5), semaglutide dominated dulaglutide. However, as would be expected for the shorter timeframes, the QALY gains and total cost differences decreased. Semaglutide also dominated dulaglutide when the cost of complications or the costs of drugs were adjusted by 10%.
Discussion
This analysis from a Canadian perspective demonstrated that treatment with semaglutide results in reduced micro- and macrovascular complications and QALY gains, compared with dulaglutide treatment. Total costs were also lower with semaglutide than with dulaglutide, although the difference was modest. The reduced cumulative incidences of micro- and macrovascular complications observed at the end of the simulation, which are the result of the superior treatment effects associated with semaglutide, mean that patients’ life expectancy increases. Because the time spent on semaglutide and dulaglutide treatment was equivalent, with the exception of the scenario analysis and DSA15, the intervention costs should be comparable. However, it is likely that increased treatment costs for semaglutide are underpinned by the small survival difference favoring semaglutide during the first 3 years of the simulation. Micro- and macrovascular complications impose a significant cost burden to the Canadian economy, and treatments such as semaglutide that have the potential to reduce such complications may limit these costs (direct and indirect), in addition to reducing CV morbidity and mortality among patients [3]. The outcomes from the base-case analysis, which allowed for a straightforward comparison of the treatments, resulted in QALY gains and lower total costs, so that semaglutide dominated dulaglutide. Although the reported gains might be considered marginal, the base-case does not permit marked separation in the risk factors, uses a short 3-year treatment duration, and assumes that changes in HbA1c and BMI progress in the same way, irrespective of the treatment arm. These factors effectively minimize any differences between treatment arms, and can be considered to be conservative assumptions, so that efficiencies in a real-life setting could be even greater.
In contrast, while the more complex scenario analysis may better represent clinical practice, it has the disadvantage of introducing more uncertainty (e.g. through integration of the treatment effects of insulin). Nevertheless, additional QALY gains were observed for both the low-dose and high-dose comparisons in the scenario analysis (0.35 and 0.40 QALYs), compared with the base-case (0.04 and 0.05 QALYs). Since, at the start of the simulation, patients taking semaglutide experience a more robust HbA1c reduction, irrespective of dose comparison, they remain on therapy longer than patients treated with dulaglutide, before switching to insulin. Consequently, despite increased direct drug costs from living longer, there are also cost offsets. Not only is the spending on insulin lower in the semaglutide arm, because patients taking dulaglutide start insulin treatment earlier, but there are also lower costs over a longer time period due to reduced micro- and macrovascular complications. Indeed, the ICERs for semaglutide are favorable, generating ICERs of CAN$5585 (low-dose comparison) and CAN$3296 (high-dose comparison), respectively, per QALY gained. The majority of ICERs for semaglutide versus dulaglutide fell below the WTP threshold of CAN$50,000, indicating that semaglutide is likely to be cost effective in the Canadian setting.
As with any modeling study, results should be interpreted with caution. One potential limitation is generalizing from international trial data to a Canadian population. However, as in this study, initial evaluations of cost effectiveness are frequently based on trial data for both patient characteristics and treatment effects, thus maintaining the high internal validity, with the effects of the drugs observed in the trial directly applicable to those patients participating in the trial. The estimation of the HRQoL decrements was based on a European population, which may also limit the applicability to the Canadian population. To the authors’ knowledge, there are no Canadian-specific HRQoL equations or any validation of the CODE-2 or UKPDS HRQoL equations, therefore values previously accepted by Canadian HTA authorities (including CADTH) were applied. Furthermore, two US-based sets of risk equations in patients with T2D—RECODe [60] and BRAVO [61]—have been published, of which the BRAVO equations [61] could be of interest for cost-effectiveness modeling in a Canadian population, supplementing the UKPDS equations. However, as these sets of equations are not currently applicable in the economic model chosen for this study, the well-established risk equations from the UKPDS were used. Additionally, only one study was used to extrapolate outcomes over a time horizon of 40 years, and the study was restricted to patients receiving metformin monotherapy. In clinical practice, many patients will have received additional drugs prior to semaglutide or dulaglutide [54]. While the results are unlikely to be substantially different for patients receiving prior oral antihyperglycemic therapy, further modeling would be required to explore this question.
Finally, because the most common cause of death in adults with T2D is CV disease [3], there is a need for further modeling that incorporates data from CV outcomes trials (CVOTs) [8, 17, 55]. The CADTH has recently highlighted the need to identify a model that encompasses the CV benefits of T2D treatments [17]. In UKPDS 80, there were benefits in some macrovascular complications observed after many years, confirming the importance of HbA1c in these effects [56]. However, recent CVOTs have demonstrated CV benefits after only a few years, indicating that although HbA1c control is beneficial for macrovascular complications, CV complications in diabetes may be influenced by aspects beyond the traditional risk factors such as HbA1c [57]. As such, current risk equations are likely to underestimate the CV benefits that are associated with the treatments studied in these CVOTs, and discussions are ongoing on how to incorporate findings from CVOTs into standard long-term modeling [55, 57]. A recent cost-effectiveness analysis of canagliflozin versus sitagliptin incorporated findings from the CANVAS CVOT trial [47]. Although the data could not be included in this study, semaglutide has been investigated in SUSTAIN 6, a phase III CVOT that demonstrated a 26% reduction in the risk of major adverse CV events [24], and dulaglutide has been investigated in the REWIND CVOT [58], with preliminary results reporting significantly reduced major adverse CV events [59]. Consequently, the CV benefits seen in these trials could not be accounted for, such that this study might underestimate the value of both semaglutide and dulaglutide in reducing macrovascular complications and their associated costs.
The strengths of this study include the use of a validated and transparent economic model to assess both the base-case (simplified approach) and the more clinically relevant scenario analysis in the same study, as well as the extensive use of deterministic and probabilistic sensitivity analyses to evaluate the robustness of the results.
This study is the first comparing the cost effectiveness of semaglutide versus dulaglutide in a Canadian setting, and builds on the current evidence that exists on the economic value of GLP-1RAs. Although relevance across borders is limited, other cost-effectiveness studies of semaglutide versus dulaglutide have validated the cost effectiveness of semaglutide [62, 63], and the reimbursement of semaglutide has been approved in Denmark [64], Ireland [65], and Switzerland [66].
Conclusions
Our findings suggest that semaglutide may be a cost-effective treatment option, compared with dulaglutide, in patients inadequately controlled on metformin monotherapy, and, ultimately, may offer a preferred treatment for the Canadian healthcare system.
Data Availability Statement
All data used in the analyses are available in the article tables and electronic supplementary material. The health economic diabetes model described in this study is proprietary of the Swedish IHE, Lund, Sweden. Access to the model is at the discretion of the IHE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Adam Fridhammar of the Swedish IHE for reviewing the simulations and assumptions made in the analyses and contributing with feedback and technical support regarding the model. The authors also thank Stacy Carl-McGrath (AXON Communications) and Jeshika Singh (PHMR) for medical writing and editorial assistance, funded by Novo Nordisk A/S.
Author contributions
PJ, JHB, SP, AL and PB provided the study concept and design, and contributed to the analysis and interpretation of data. LAL contributed to the analysis and interpretation of data. All authors contributed to the drafting of the paper, critically revised the paper for intellectual content, were involved in the final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Compliance with Ethical Standards
Funding
This study was supported and open access publication was sponsored by Novo Nordisk A/S.
Conflict of interest
Pierre Johansen, Aiden R. Liu and Peter Bech are employees of Novo Nordisk. Pierre Johansen was employed by the Swedish IHE prior to the time of this study. Jonas Håkan-Bloch was employed by Novo Nordisk at the time this study was conducted. Sofie Persson is employed by IHE, which received grant funding from Novo Nordisk. Lawrence A. Leiter received no payment or services related to this study, but has received research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GSK, Janssen, Merck, Novo Nordisk and Sanofi, and has been on advisory panels and provided continuing medical education for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Servier.
References
- 1.International Diabetes Federation. Canada Country Report 2017 & 2045. IDF Diabetes Atlas, 8th ed. Brussels: International Diabetes Federation; 2017. https://reports.instantatlas.com/report/view/704ee0e6475b4af885051bcec15f0e2c/CAN. Accessed Oct 2018.
- 2.International Diabetes Federation. IDF Diabetes Atlas, 7th ed. Brussels. International Diabetes Federation; 2017. http://www.diabetesatlas.org. Accessed June 2018.
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2013;37:S1–S212. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Somerville R. An economic tsunami: the cost of diabetes in Canada. Toronto: Canadian Diabetes Association; 2009. https://www.diabetes.ca/CDA/media/documents/publications-and-newsletters/advocacy-reports/economic-tsunami-cost-of-diabetes-in-canada-english.pdf. Accessed Oct 2018.
- 6.O’Brien JA, Patrick AR, Caro JJ. Cost of managing complications resulting from type 2 diabetes mellitus in Canada. BMC Health Serv Res. 2003;3(1):7. doi: 10.1186/1472-6963-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 8.Govan L, Wu O, Lindsay R, Briggs A. How do diabetes models measure up? A review of diabetes economic models and ADA guidelines. J Health Econ Outcomes Res. 2015;3(2):132–152. doi: 10.36469/9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 11.Willis M, Johansen P, Nilsson A, Asseburg C. Validation of the economic and health outcomes model of type 2 diabetes mellitus (ECHO-T2DM) Pharmacoeconomics. 2017;35(3):375–396. doi: 10.1007/s40273-016-0471-3. [DOI] [PubMed] [Google Scholar]
- 12.Willis M, Asseburg C, He J. Validation of economic and health outcomes simulation model of type 2 diabetes mellitus (ECHO-T2DM) J Med Econ. 2013;16(8):1007–1021. doi: 10.3111/13696998.2013.809352. [DOI] [PubMed] [Google Scholar]
- 13.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47(10):1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 14.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–1933. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 15.McEwan P, Ward T, Bennett H, Bergenheim K. Validation of the UKPDS 82 risk equations within the Cardiff Diabetes Model. Cost Eff Resour Alloc. 2015;13:12. doi: 10.1186/s12962-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwan P, Peters JR, Bergenheim K, Currie CJ. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster) Curr Med Res Opin. 2006;22(1):121–129. doi: 10.1185/030079906X80350. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Agency for Drugs and Technologies in Health (CADTH). Therapeutic review of third-line drugs for type 2 diabetes—an update. 2018. https://www.cadth.ca/news/therapeutic-review-third-line-drugs-type-2-diabetes-update. Accessed Oct 2018.
- 18.Lundqvist A, Steen Carlsson K, Johansen P, Andersson E, Willis M. Validation of the IHE cohort model of type 2 diabetes and the impact of choice of macrovascular risk equations. PloS One. 2014;9(10):e110235. doi: 10.1371/journal.pone.0110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novo Nordisk Canada Inc. Ozempic® approved in Canada for the treatment of adults with type 2 diabetes. 2018. https://www.newswire.ca/news-releases/ozempic-approved-in-canada-for-the-treatment-of-adults-with-type-2-diabetes-668432133.html. Accessed 5 Mar 2018.
- 20.Canadian Agency for Drugs and Technologies in Health (CADTH). Dulaglutide. 2015. https://www.cadth.ca/dulaglutide. Accessed Sept 2018.
- 21.Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 22.Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratley RE, Aroda VR, Lingvay I, Ludemann J, Andreassen C, Navarria A, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 24.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 25.Aroda VR, Bain SC, Cariou B, Piletic M, Rose L, Axelsen M, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 26.Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbol JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 27.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 28.Kiadaliri AA, Gerdtham UG, Nilsson P, Eliasson B, Gudbjornsdottir S, Carlsson KS. Towards renewed health economic simulation of type 2 diabetes: risk equations for first and second cardiovascular events from Swedish register data. PloS One. 2013;8(5):e62650. doi: 10.1371/journal.pone.0062650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagust A, Hopkinson PK, Maier W, Currie CJ. An economic model of the long-term health care burden of Type II diabetes. Diabetologia. 2001;44(12):2140–2155. doi: 10.1007/s001250100023. [DOI] [PubMed] [Google Scholar]
- 30.Brown JB, Russell A, Chan W, Pedula K, Aickin M. The global diabetes model: user friendly version 3.0. Diabetes Res Clin Pract. 2000;50(Suppl 3):S15–S46. doi: 10.1016/s0168-8227(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 31.Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Zbrozek AS, Dong F, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–734. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- 32.Ericsson Å, Lundqvist A. Cost effectiveness of insulin degludec plus liraglutide (IDegLira) in a fixed combination for uncontrolled type 2 diabetes mellitus in Sweden. Appl Health Econ Health Policy. 2017;15(2):237–248. doi: 10.1007/s40258-016-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ericsson Å, Glah D, Lorenzi M, Jansen JP, Fridhammar A. Cost-effectiveness of liraglutide versus lixisenatide as add-on therapies to basal insulin in type 2 diabetes. PloS One. 2018;13(2):e0191953. doi: 10.1371/journal.pone.0191953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steen Carlsson K, Persson U. Cost-effectiveness of add-on treatments to metformin in a Swedish setting: liraglutide vs sulphonylurea or sitagplitin. J Med Econ. 2014;17(9):658–669. doi: 10.3111/13696998.2014.933110. [DOI] [PubMed] [Google Scholar]
- 35.Kiadaliri AA, Gerdtham UG, Eliasson B, Carlsson KS. Cost-utility analysis of glucagon-like peptide-1 agonists compared with dipeptidyl peptidase-4 inhibitors or neutral protamine Hagedorn basal insulin as add-on to metformin in type 2 diabetes in Sweden. Diabetes Ther. 2014;5(2):591–607. doi: 10.1007/s13300-014-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Dental and Pharmaceutical Benefits Agency (TLV). Lyxumia is included in the reimbursement system with limitation (Lyxumia ingår i högkostnadsskyddet med begränsning). 2015. https://www.tlv.se/beslut/beslut-lakemedel/begransad-subvention/arkiv/2015-02-26-lyxumia-ingar-i-hogkostnadsskyddet-med-begransning.html. Accessed Jan 2018.
- 37.The Dental and Pharmaceutical Benefits Agency (TLV). Xultophy is included in the reimbursement system with limitation (Xultophy ingår i högkostnadsskyddet med begränsning). 2015. https://www.tlv.se/beslut/beslut-lakemedel/begransad-subvention/arkiv/2015-06-15-xultophy-ingar-i-hogkostnadsskyddet-med-begransning.html. Accessed Oct 2018.
- 38.Socialstyrelsen. National guidelines for diabetes care—support for governance and management. 2015. http://www.socialstyrelsen.se/publikationer2015/2015-4-12. Accessed Oct 2018.
- 39.Canadian Agency for Drugs and Technologies in Health (CADTH). Therapeutic review. New drugs for type 2 diabetes: second-line therapy—science report. 2018. https://www.cadth.ca/sites/default/files/pdf/TR0012_T2D_Science_Report.pdf. Accessed Jun 2018. [PubMed]
- 40.Palmer AJ, Si L, Tew M, Hua X, Willis MS, Asseburg C, et al. Computer modeling of diabetes and its transparency: a report on the Eighth Mount Hood challenge. Value Health. 2018;21(6):724–731. doi: 10.1016/j.jval.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Heap G. Type 2 diabetes current treatment detailed, expanded analysis: physician insights (EU5). Decision Resources Group. 2017. https://decisionresourcesgroup.com/report/716654-biopharma-type-2-diabetes-current-treatment-detailed/. Accessed Jan 2019.
- 43.Hunt B, Vega-Hernandez G, Valentine WJ, Kragh N. Evaluation of the long-term cost-effectiveness of liraglutide vs lixisenatide for treatment of type 2 diabetes mellitus in the UK setting. Diabetes Obes Metab. 2017;19(6):842–849. doi: 10.1111/dom.12890. [DOI] [PubMed] [Google Scholar]
- 44.Mezquita-Raya P, Ramirez de Arellano A, Kragh N, Vega-Hernandez G, Pohlmann J, Valentine WJ, et al. Liraglutide versus lixisenatide: long-term cost-effectiveness of GLP-1 receptor agonist therapy for the treatment of type 2 diabetes in Spain. Diabetes Ther. 2017;8(2):401–415. doi: 10.1007/s13300-017-0239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt B, Kragh N, McConnachie CC, Valentine WJ, Rossi MC, Montagnoli R. Long-term cost-effectiveness of two GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus in the Italian setting: liraglutide versus lixisenatide. Clin Ther. 2017;39(7):1347–1359. doi: 10.1016/j.clinthera.2017.05.354. [DOI] [PubMed] [Google Scholar]
- 46.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 47.Willis M, Neslusan C, Nilsson A, Asseburg C. Assessing the value of canagliflozin (CANA) vs. sitagliptin (SITA) as second-line therapy in the U.S.—the importance of considering evidence from the CANVAS program. Diabetes. 2018;67(Suppl 1):1272-P. [Google Scholar]
- 48.National Institute for Health and Care Excellence. Multiple technology appraisal: canagliflozin, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes. 2016. https://www.nice.org.uk/guidance/ta390/documents/committee-papers. Accessed Oct 2018.
- 49.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management [NG28]. Appendix F: full health economics report. 2015. https://www.nice.org.uk/guidance/ng28/evidence/appendix-f-full-health-economics-report-pdf-2185320355. Accessed Jan 2019.
- 50.Willis M, Asseburg C, Nilsson A, Johnsson K, Kartman B. Multivariate prediction equations for HbA1c lowering, weight change, and hypoglycemic events associated with insulin rescue medication in type 2 diabetes mellitus: informing economic modeling. Value Health. 2017;20(3):357–371. doi: 10.1016/j.jval.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–230. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 52.Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012) J Med Econ. 2014;17(3):176–183. doi: 10.3111/13696998.2014.882843. [DOI] [PubMed] [Google Scholar]
- 53.Eckert KA, Carter MJ, Lansingh VC, Wilson DA, Furtado JM, Frick KD, et al. A simple method for estimating the economic cost of productivity loss due to blindness and moderate to severe visual impairment. Ophthalmic Epidemiol. 2015;22(5):349–355. doi: 10.3109/09286586.2015.1066394. [DOI] [PubMed] [Google Scholar]
- 54.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42:S1–S325. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Willis M, Neslusan C, Johansen P, Nilsson A. The importance of considering the evolving evidence base on cardiovascular (CV) effects of antihyperglycemic agents (AHAs) on estimates of “value for money”. Presented at American Diabetes Association (ADA) 77th scientific sessions, San Diego, CA, USA, 9–13 June 2017.
- 56.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 57.Evans M, Johansen P, Vraziv H. Incorporating cardioprotective effects of once-weekly semaglutide in estimates of health benefits for patients with type 2 diabetes. Diabetes. 2018;67(Suppl 1):1273-P. [Google Scholar]
- 58.ClinicalTrials.gov. Researching cardiovascular events with a weekly incretin in diabetes (REWIND). 2017. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed July 2017.
- 59.Eli Lilly. Trulicity® (dulaglutide) demonstrates superiority in reduction of cardiovascular events for broad range of people with type 2 diabetes. 2018. https://www.prnewswire.com/news-releases/trulicity-dulaglutide-demonstrates-superiority-in-reduction-of-cardiovascular-events-for-broad-range-of-people-with-type-2-diabetes-300743485.html. Accessed Nov 2018.
- 60.Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017;5(10):788–798. doi: 10.1016/S2213-8587(17)30221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shao H, Fonseca V, Stoecker C, Liu S, Shi L. Novel risk engine for diabetes progression and mortality in USA: building, relating, assessing, and validating outcomes (BRAVO) Pharmacoeconomics. 2018;36(9):1125–1134. doi: 10.1007/s40273-018-0662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for the treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–621. doi: 10.1111/dom.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tikkanen C, Johansen P, Hunt B, Malkin S, Pollock R. Once-weekly semaglutide provides better health outcomes compared to dulaglutide as dual therapy in the treatment of type 2 diabetes: a cost-effectiveness analysis. Presented at the EASD 54th annual meeting, Berlin, Germany, 1–5 October 2018.
- 64.Laegemiddelstyrelsen (Danish Medicines Agency). Ozempic® får generelt klausuleret tilskud. 2018. https://laegemiddelstyrelsen.dk/da/nyheder/2018/ozempic-faar-generelt-klausuleret-tilskud/. Accessed Aug 2018.
- 65.Health Service Executive Ireland. Ozempic. Health Service Executive Ireland. 2018. https://www.sspcrs.ie/druglist/search/category/1?description=ozempic. Accessed Oct 2018.
- 66.Bundesamt für Gesundheit BAG. BAG-Bulletin: Informationsmagazin für medizinische Fachpersonen und Medienschaffende. Bundesamt für Gesundheit BAG. 2018. https://www.bag.admin.ch/dam/bag/de/dokumente/cc/Kampagnen/Bulletin/2018/BU_38_18.pdf.download.pdf/BU_38_18_DE.pdf. Accessed Oct 2018.
- 67.Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 2017. https://cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed Aug 2018.
- 68.Fenwick EK, Xie J, Ratcliffe J, Pesudovs K, Finger RP, Wong TY, et al. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Investig Ophthalmol Vis Sci. 2012;53(2):677–684. doi: 10.1167/iovs.11-8992. [DOI] [PubMed] [Google Scholar]
- 69.Canadian Agency for Drugs and Technologies in Health (CADTH). Optimal use recommendations for second- and third-line therapy for patients with type 2 diabetes. CADTH optimal use report. Ottawa, CADTH; 2013. https://www.cadth.ca/sites/default/files/pdf/OP0512_Diabetes_RecsReport_2nd_3rd-line_e.pdf. Accessed June 2018. [PubMed]
- 70.Currie CJ, McEwan P, Poole C, Valentine WJ, Palmer AJ, Lammert M, et al. Comments on: long-term clinical and cost outcomes of treatment with biphasic insulin aspart 30/70 versus insulin glargine in insulin-naive type 2 diabetes patients: cost-effectiveness analysis in the UK setting. Curr Med Res Opin. 2006;22(5):967–969. doi: 10.1185/030079906X00000. [DOI] [PubMed] [Google Scholar]
- 71.Sørensen J, Ploug U. The cost of diabetes-related complications: registry-based analysis of days absent from work. Econ Res Int. 2013;8:article ID 618039.
- 72.Working Days. Working days in Canada. 2017. http://www.workingdays.ca/workingdays_holidays_2017.htm. Accessed Aug 2018.
- 73.Johansen P, Håkan-Bloch J, Liu AD, Persson S, Toresson Grip E. Cost-effectiveness of once-weekly semaglutide 1.0 mg vs. dulaglutide 1.5 mg as add-on to metformin in the treatment of type 2 diabetes in Canada. Diabetes. 2018;67(Suppl 1):136-LB. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the analyses are available in the article tables and electronic supplementary material. The health economic diabetes model described in this study is proprietary of the Swedish IHE, Lund, Sweden. Access to the model is at the discretion of the IHE.