Abstract
In this study, efficient knock-in (KI) of human epidermal growth factor (hEGF) cDNA at the ovalbumin (OV) locus in cultured chicken cells was achieved using adenovirus as a delivery for CRISPR/Cas9 elements and optimizing donor vector construction. The strategy of recruiting donor DNA to the insertion site further improved the KI efficiency. The inserted hEGF cDNA can expressed in primary oviduct cells and secreted hEGF promoted proliferation of Hela cells. Moreover, we achieved efficient KI in blastoderm cells without altering their induction in vitro and obtained germline chimeric KI chicken embryos by transplanting KI blastoderm cells as well as injecting adenovirus directly, in vivo. Our results provided an efficient KI method for chicken cells and embryos, and lay the foundation for more convenient production of KI chicken at the OV locus, which will promote the development of oviduct-specific bioreactor.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1966-3) contains supplementary material, which is available to authorized users.
Keywords: Chicken ovalbumin locus, Knock-in, CRISPR/Cas9, Adenovirus, Bioreactor
Introduction
In recent decades, therapeutic recombinant proteins have found use in industrial, medical, and scientific applications. A transgenic chicken used as an in vivo bioreactor to synthesize therapeutic recombinant proteins, as a component of egg white, is referred to as oviduct-specific bioreactor (Woodfint et al. 2018). To produce a highly efficient oviduct-specific bioreactor, exogenous protein genes are expressed by truncated chicken ovalbumin (OV) promoter, which is considered to be the strongest oviduct-specific promoter, yielding more than 50% of the total egg white protein content, and exogenous protein genes are integrated at random sites within the chromosomal DNA (Petitte and Mozdziak 2007). However, the amount of exogenous protein produced in egg white is relatively low because of the truncation of the OV promoter. In contrast, site-specific insertion of exogenous DNA in the OV locus, a so-called knock-in (KI), should enable efficient exogenous DNA expression and be less detrimental to the health of the host chicken. This assumption was proven in a recent study: human interferon-β (hIFN-β) cDNA was inserted at the translation initiation site of the OV locus in primordial germ cells (PGCs) via CRISPR/Cas9-based KI and produced KI hens expressing high levels of hIFN-β in egg white (Oishi et al. 2018). It is worth noting that the PGC-mediated method is the only way to produce precision genome engineering (PGE and KI is a PGE type) chickens due to the establishment of PGC lines, which provide the ability to enrich PGE-positive cells from a large number of negative cells (Oishi et al. 2016; Park et al. 2014; Schusser et al. 2013; Taylor et al. 2017). However, the process of establishing PGC lines is technically demanding and resource intensive. Whether alternative methods can be used to produce PGE chicken, especially KI at OV locus, is still unknown.
A blastodermal cell (BC)-mediated method is a potential method of producing KI chicken. BCs are pluripotent cells derived from the zona pellucida of stage X (EG&K) chicken embryos, and germline chimeras were produced after freshly isolated BCs were transplanted into the subgerminal cavity of embryos (Carsience et al. 1993). Compared with PGCs, which are rare in the embryo, BCs can be easily obtained in large quantities from fertilized eggs (Petitte et al. 1990). A limitation of the BC-mediated method is that in vitro cultured BCs have extremely low germline transmission capacity (Lavial and Pain 2010; van de Lavoir et al. 2006), but this limitation may be resolved by germline induction in vitro by retinoic acid, which can promote germline-specific gene expression in BCs (Tang et al. 2017, 2019). Since BCs are not cell lines and cannot be cultured in vitro long-term or for large-scale amplification, another limitation of the BC-mediated method is the inability to enrich for KI-positive cells. Therefore, establishing an efficient KI method in BCs is essential, after which enrichment for KI-positive cells will be dispensable. To date, this has not been achieved.
Another potential non-PGC culture method for producing KI chickens is direct in vivo transfection of PGCs by injecting CRISPR/Cas9 plasmids complexed with lipofectamine intravenously into HH stage 14 embryos, although only chickens expressing GFP integrated at random sites are currently produced by this method (Cooper et al. 2018; Tyack et al. 2013; Wang et al. 2019). Obviously, a highly efficient KI method suitable for in vivo injection is essential; otherwise, this method may result in a low frequency of KI offspring being generated from germline chimeric roosters.
In this study, we used adenovirus as a delivery method for CRISPR/Cas9 elements and optimized donor vector and Cas9 protein construction, targeting to improve the KI efficiency of human epidermal growth factor (hEGF), a therapeutic recombinant protein used to promote the regeneration of wounded skin, at the OV locus. We demonstrate that this method works efficiently in induced BCs and obtained germline chimeric KI embryos. We further demonstrate the ability to obtain germline chimeric KI embryos via direct injection in vivo.
Materials and methods
Construction of plasmids and production of recombinant adenoviruses
The gRNA target site is CTCTAGCCATGGTATACCT, which was located in the second exon of OV and has been shown to have high CRISPR/Cas9 targeting efficiency in previous study (the gRNA was named OVATg3 in this study) (Oishi et al. 2016).
Codons of the hEGF gene were optimized for expression in the chicken according to previous study (Park et al. 2015). 2A peptide and chicken lysozyme signal peptide sequences were placed before the hEGF gene to allow expression and secretion of hEGF, and 6 × His-tag were placed after the hEGF gene to facilitate detection of the hEGF content. The hEGF insertion cassette containing the above sequences and homology arms was synthesized from Sangon Biotech (Shanghai, China). To express the Cas9–TALE fusion protein, Cas9 and TALE (transcription activator-like effector, targeting TGAACCGCATCGAGCTG) were connected by a (G4S)6 linker. The fragment containing TALE and (G4S)6 linker was synthesized from Sangon Biotech.
To construct the HDR donor, the hEGF insertion cassette was inserted into the KpnI–HindIII sites of adenovirus shuttle plasmid pShuttle (Addgene, catalog no. 16402), named pShuttle-HDR. The functional component of the HMEJ donor was PCR-amplified from pShuttle-HDR by adding gRNA target sites at 5ʹends of the primers, and inserted into the KpnI–HindIII sites of pShuttle, named pShuttle-HMEJ. The functional component of the MMEJ donor was PCR-amplified from pShuttle-HDR by reserving micro-arms and adding gRNA target sites at 5ʹends of the primers and inserted into the KpnI–HindIII sites of pShuttle, named pShuttle-MMEJ. To construct Bait-HMEJ donors, using pShuttle-HMEJ as template, a TALE target site (Bait) and a XhoI site were added to 5ʹends of the reverse primer, a XhoI site was added to 5ʹends of the forward primer, and after the entire pShuttle-HMEJ plasmid was PCR-amplified, the products were digested with XhoI endonuclease and then self-ligated to generate pShuttle-Bait-HMEJs. Recombinant adenoviruses as donors were produced using the above shuttle plasmids and adenoviral backbone vector pAdEasy-1 (Addgene, catalog no. 16400) according to standard procedures of the AdEasy system. High titter recombinant adenoviruses containing Cas9, Cas9–TALE, or Inside-Bait-HMEJ were customized in Vigene Biosciences (Jinan, China).
Cell KI efficiency test
Cell KI efficiency was tested by single-cell PCR. The cells to be detected were made into a single-cell suspension, washed two times in phosphate-buffered saline (PBS) buffer, and transferred to a PCR tube individually. Two microliters of lysis buffer (0.1% tween 20, 0.1% Triton X-100, and 4 mg/ml proteinase K) was previously added to each tube. The samples were incubated at 56 °C for 30 min and proteinase K heat-inactivated at 99 °C for 10 min. The products of the lysis program were used as templates in a nest PCR analysis. Simultaneous amplification of the 5ʹ and 3ʹ ends was considered KI-positive. Primer sequences are listed in Table 1.
Table 1.
Primers for nest PCR analysis
| Name | Primer sequences (5′ to 3′) |
|---|---|
| Nest-5′-1 |
CAGCACTCAGTACGCATA ACCTCTCACCGATATAACC |
| Nest-5′-2 |
CACACTGGCTATACAATAGTTG TCACCGCATGTTAGAAGAC |
| Nest-3′-1 |
GCAGAGGAAGTCTTCTAACA AACTTACTGGCAGGATTGG |
| Nest-3′-2 | GAACAGCGATAGCGAGTG |
| GGCAAGGCTGAACGAATA |
Cell isolation and culture
The animals used in this study were SPF Hyline chicken (Gallus gallus) purchased from Zhushun Biology Animal Centre (Nanjing, China).
For isolation and culture of BCs, the zona pellucida was isolated from stage X (EG&K) embryos as previously described (Johnson and Giles 2006), was washed twice with DMEM/F12 medium (Gibco, Carlsbad, CA, USA), and gently dispersed. Cells were resuspended in Essential 8™ medium (E8 medium, Gibco) containing 1% penicillin/streptomycin (Gibco) after centrifugation at 350×g. The cells were seeded at a density of 2 × 104 cells/cm2 in vitronectin (Gibco)-coated culture plates (Costar, Corning, NY, USA). Upon reaching 70–80% confluency, cells were passaged by washing and pipetting with Ca2+/Mg2+-free PBS (Gibco). For germline induction of BCs, BCs were cultured in E8 medium-containing 10 ng/ml human 1eukemia inhibitory factor (LIF, Sino Biological, China) and 10 ng/ml human stem cell factor (SCF, Sino Biological) for 5–7 days, and induced in E8 medium-containing 1 μM all-trans retinoic acid (RA, Selleck, Houston, TX, USA) and 10 ng/ml human bone morphogenetic protein 4 (BMP4, Sino Biological).
For isolation and culture of primary oviduct cells, chicken oviducts from 16 to 19 week old hens were isolated by opening the magnum segment of the oviduct and cutting it into small pieces. The tissues were digested with 0.2% Type I collagenase (Sigma-Aldrich, Steinheim, Germany) for 30 min and continued to digest for an additional 30 min after the addition of three volumes of 0.25% trypsin (Gibco). After pipetting gently multiple times, three volumes of DMEM/F12 medium were added to stop digestion and the remaining tissue was removed by a 70 μm cell strainer. Cells were resuspended in DMEM/F12 medium and centrifuged at 350×g. The cells were seeded in DMEM/F12 medium-containing 10% fetal bovine serum (FBS, Gibco), 1% nonessential amino acids (Gibco), and 1% penicillin/streptomycin in gelatin (Gibco)-coated culture plates (Costar) after PBS washing two times at a density of 5 × 104 cells/cm2.
For isolation and culture of primary chicken embryonic fibroblasts (CEFs), embryos at embryonic day 9 (E9) had their internal organs, heads, and limbs removed, and then cut into small pieces. The tissues were digested with 0.25% trypsin for 10 min, and then processed according to the above protocol of oviduct cells. CEFs were seeded at a density of 1 × 104 cells/cm2.
In vitro and in vivo transfection of adenoviruses
When the cells were cultured in vitro, the serum-free medium was used instead of the original medium, and the adenoviruses were added in accordance with the multiplicity of infection (MOI) of 200. After 4 h, replaced the new complete medium and continued to culture. To direct injection in vivo, the chicken eggs were hatched to HH stage 15–17 (about 2.5 days), and a window of about 8 mm in diameter was opened on the equatorial plane of each egg, and 2–5 μl of adenoviruses were injected into the peripheral blood vessels of each embryo. Sealed the windows with Parafilm™ membrane (Bemis, Neenah, WI, USA) and continued to hatch the eggs. In vitro and in vivo transfection of adenoviruses, the proportion of Cas9/Cas9–TALE adenovirus, and donor adenovirus was 1:1.
Western blot analysis
Adherent primary oviduct cells were lysed in the radioimmunoprecipitation assay (RIPA) buffer supplemented with Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA, USA). After blocking with 5% BSA at room temperature for 2 h, primary antibodies directed against 6 × His (1:1000, Abcam, Cambridge, UK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:5000, Abcam) were added and the blots were incubated overnight at 4 °C. Primary antibodies were detected using species-specific horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000, Cell Signaling Technology, Boston, MA, USA) at room temperature for 2 h.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of hEGF in supernatants of KI oviduct cells were determined using a 6 × His-tag ELISA Kit (Abcam) according to the manufacturer’s protocol. Briefly, oviduct cells were cultured for 24 h after being transfected with adenoviruses, replaced with fresh medium, and cultured for further 72 h. The supernatants were collected and add 50 µl to each ELISA well and incubate 2 h at room temperature. Aspirate and wash each well three times, add 50 µl prepared primary detector antibody to each well, incubate 1 h at room temperature. Aspirate and wash each well three times, add 50 µl prepared HRP labeled secondary detector antibody, incubate 1 h at room temperature. Aspirate and wash each well three times, add 100 µl HRP Development Solutionto each well. Add a stop solution after 10 min and read at 450 nm.
In vitro assay of cell proliferation by hEGF
Collected the supernatants of oviduct cells transfected with donor adenovirus and Cas9–TALE adenovirus. Based on the results of the ELISA assay, the supernatants were mixed with DMEM/F12 medium to give a secreted hEGF concentration of 1 ng/ml, and added 5% FBS, 1% nonessential amino acids, and 1% penicillin/streptomycin. The commercial recombinant hEGF (Sino Biologica) produced from E. coli was used as a positive control. Hela cells were cultured and grow to 50% confluence in 96-well plate; the above media were added for further culture. For cell proliferation testing, media were replaced with fresh DMEM/F12 medium-containing diluted CCK-8 reagent (1:10, Dojindo Laboratories, Japan), and incubated for 2 h at 37 °C. The CCK-8 reaction product was quantified by measuring absorbance at 450 nm.
Reverse transcription and quantitative PCR (qRT-PCR) analyses
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. PrimeScript RT Master Mix reverse transcription kit (TaKaRa, Japan) was used for cDNA synthesis. Reverse transcription products were amplified with SYBR Premix Ex Taq PCR kit (TaKaRa). PCR amplification was performed in an automated StepOne system (Applied Biosystems, Carlsbad, CA, USA). Primer sequences are listed in Table 2.
Table 2.
Primers for qRT-PCR analysis
| Genes | Accession no. | Primer sequences (5′ to 3′) |
|---|---|---|
| Prdm1 | XM_004940353.2 |
ACACAGCGGAGAGAGACCAT GCACAGCTTGCACTGGTAAG |
| Dazl | NM_204218 |
CTGGGGAGCAAAGAAACTACG CAAAGGTGTTCCTCAGACGGT |
| Cvh | NM_204708 |
GGGAAGATCAGTTTGGTGGA GACAAAGAAAGGCTGCAAGG |
| Tudor | 603107842F1 | GCCATCCAGGTCACTTCATTG CCAGCACATACACCGACAGA |
| PouV | NM_001110178 |
GCCAAGGACCTCAAGCACAA ATGTCACTGGGATGGGCAGA |
| Nanog | NM_001146142.1 |
CAGCAGACCTCTCCTTGACC AAGCCCTCATCCTCCACAGC |
| β-actin | NM_205518 |
GAACCCCAAAGCCAACAGA GGAGGGCGTAGCCTTCATAGA |
T7E1 enzyme digestion assay
Total DNA was extracted from CEFs, after transfecting with CRISPR/Cas9 adenovirus, using DNA extraction kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. Using the above DNA as templates, products of PCR amplification were gel purified. Then, a solution containing 5 μl PCR products, 1.1 μl NEBuffer 2.1, and 4.4 μl ultrapure water was incubated at 99 °C for 10 min, and then annealed at room temperature for at least 30 min. Hybridized PCR products were digested with 5 IU T7E1 enzyme (New England Biolabs, Ipswich, MA) for 30 min at 37 °C and finally subjected to 2% agarose gel electrophoresis. Bands were subjected to gray intensity analysis using the software Image J (National Institutes of Health, US).
Fluorescence-activated cell sorting
E12 gonads were minced into pieces and digested with 0.2% Type I collagenase (Sigma-Aldrich) for 20 min, and then, three volumes of 0.25% trypsin (Gibco) added and the mixture digested for an additional 20 min. The mixture was disrupted into a single-cell suspension by pipetting and filtrating through 70 μm nylon mesh. After centrifugation, cells were washed with PBS and fixed in 4% paraformaldehyde at room temperature for 20 min, followed by permeabilization with 0.5% Triton X-100 (Sigma-Aldrich) in PBS for 10 min. After incubation with blocking buffer containing 5% BSA for 20 min, the cells were stained with anti-Cvh antibodies (1:100, Bioss, Beijing, China) at 4 °C overnight and rewarmed at 37 °C for 45 min. Alexa Fluor 597-conjugated goat anti-rabbit IgG (1:1000, Abcam) was added and cells were incubated at room temperature for 1 h. Cells were centrifuged, resuspended in FACS buffer (PBS containing 2% FBS, 0.1% sodium azide, and 1 mM EDTA), and analyzed and sorted with a flow cytometer (FACSAria II, Becton–Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
SPSS v16.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze data sets. Data presented in Fig. 5c were analyzed by Chi-square test, whereas Student’s t test was used for the analysis of other data.
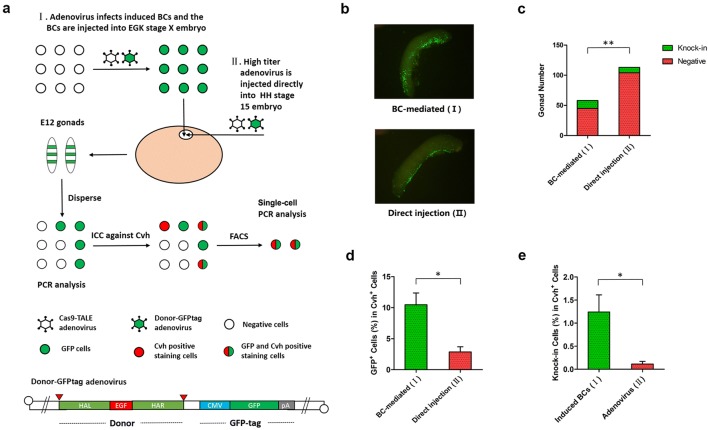
Fig. 5.
Germline chimeric KI chicken embryo production using BC-mediated and direct injection methods. a Diagram of the BC-mediated method, direct injection method, and germline chimerism rate detection. b Representative chimeric gonads. c Statistics of KI-positive gonads; PCR was used to determine whether KI was positive. d GFP-positive cell chimerism rate in germ cells and e germline chimerism rate were detected by single-cell PCR. Values are the mean ± SEM (n = 3), *p < 0.05; **p < 0.01
Results
Optimization of KI efficiency in primary chicken embryonic fibroblast (CEFs)
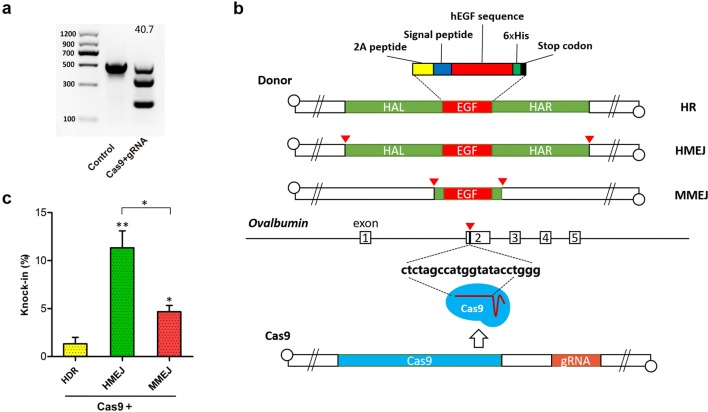
The insertion site which we chose is located in the second exon of OV, which is 125 bp behind the start codon and has been shown to have high targeting efficiency for CRISPR/Cas9 systems (Oishi et al. 2016). To allow expression and secretion of hEGF from cells, 2A peptide and chicken lysozyme signal peptide sequences were placed before the hEGF gene. To facilitate detection of the hEGF content, 6 × His-tag were placed after the hEGF gene. We chose adenovirus to deliver CRISPR/Cas9 elements due to its high transfection efficiency in primary cells. The T7E1 enzyme assay showed that the CRISPR/Cas9 targeting efficiency using adenovirus delivery was 40.7% in CEFs (Fig. 1a). As a linear double-stranded DNA virus, adenovirus can be used as a donor by the homology-directed repair (HDR) pathway. Previous studies have shown that in vivo cleavage of double-stranded donor DNA could promote CRISPR/Cas9-based targeted integration, and we chose two of these strategies, microhomology-mediated end joining (MMEJ) (Nakade et al. 2014; Yao et al. 2017a), and homology-mediated end joining (HMEJ) (Yao et al. 2017b; Zhang et al. 2017), to explore whether these strategies are efficient in an adenovirus delivery method. After CEFs were co-infected with CRISPR/Cas9 adenovirus and one of the three donor (HDR, HMEJ, and MMEJ) adenoviruses (Fig. 1b), single-cell PCR results showed that KI efficiency was significantly higher with the HMEJ donor (11.3%) than with MMEJ (4.7%) and HDR (1.3%) (Fig. 1c).
Fig. 1.
KI efficiency at OV locus for different donors in CEFs. a CRISPR/Cas9 targeting efficiency by T7E1 enzyme assay after adenovirus transfection. b Diagram of insertion site at OV locus and different adenovirus donor structures. EGF means the hEGF insertion cassette. c KI efficiency detected by single-cell PCR at OV locus for different donors. Values are the mean ± SEM (n = 3), *p < 0.05; **p < 0.01
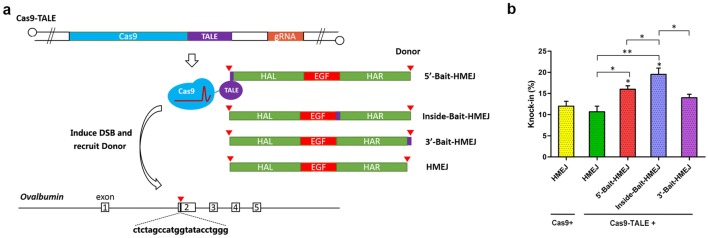
Next, we explored the method of recruitment of the donor to the insertion site to further improve KI efficiency (Gu et al. 2018; Ma et al. 2017). We constructed a fusion protein of Cas9 and transcription activator-like effector (TALE) and generated three different Bait-HMEJ donors by PCR amplification from an HMEJ donor with a TALE-targeting sequence (Bait) (Fig. 2a). After the above components were delivered into CEFs using adenovirus, the results of single-cell PCR showed that the Cas9–TALE fusion protein did not significantly affect KI efficiency compared to wild-type Cas9, while Bait-HMEJ donors effectively improved KI efficiency, the highest of which was Inside-Bait-HMEJ, which placed the TALE-targeting sequence between two homology arms, and was significantly more efficient than HMEJ donor co-infecting with wild-type Cas9 or Cas9–TALE (19.5% compared 12.0% and 10.7%, respectively) (Fig. 2b).
Fig. 2.
The effect of using donor recruitment to the insertion site on KI efficiency. a Diagram detailing recruiting the donors to the insertion site and different adenovirus donor structures. b KI efficiency detected by single-cell PCR for different donors. Values are the mean ± SEM (n = 3), *p < 0.05; **p < 0.01
KI in chicken primary oviduct cells (OCEs) and blastodermal cells (BCs)
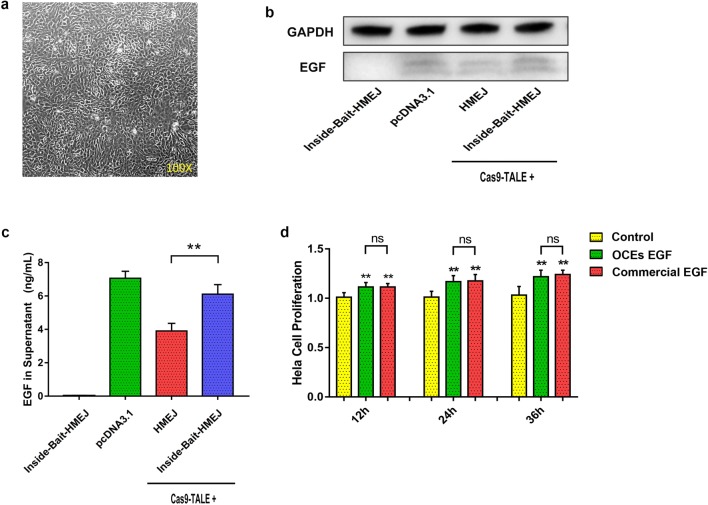
Endogenous OV promoter works only in primary oviduct cells. Therefore, to test whether hEGF cDNA can be expressed by knock-into the insertion site on the OV locus, we cultured primary oviduct cells in vitro (Fig. 3a) and assayed for the expression of hEGF protein after KI in oviduct cells. The results of western blot and ELISA analysis showed that the expression of hEGF protein was detected in primary oviduct cells when they were co-transfected with Cas9–TALE and donors, and the expression level when using Bait-HMEJ donor was obviously higher than that of the HMEJ donor (Fig. 3b, c). To evaluate the biological activity of secreted hEGF proteins, we tested the effect of hEGF protein on proliferation of Hela cells. The results showed that biological activity of secreted hEGF proteins was not significantly different from commercial hEGF (Fig. 3d).
Fig. 3.
Expression of hEGF protein after KI in primary oviduct cells. a Morphology of in vitro cultured primary oviduct cells. b Expression of hEGF protein after KI in primary oviduct cells lysates by western blotting. Group of pcDNA3.1 refers to electrotransferring pcDNA3.1 plasmid containing CMV promoter and hEGF CDS into primary oviduct cells, as positive control. c Content of hEGF protein in supernatants after KI in primary oviduct cells detected by ELISA. d In vitro proliferation assay of Hela cell treating by different supernatants with or without hEGF. Control means the supernatant of oviduct cells transfected with donor adenovirus only oviduct cells. EGF means the supernatant of oviduct cells transfected with donor adenovirus and Cas9–TALE adenovirus, which contains the hEGF secreted by oviduct cells. Commercial EGF means the control supernatant adding commercial hEGF. *p < 0.05; **p < 0.01, ns no significant
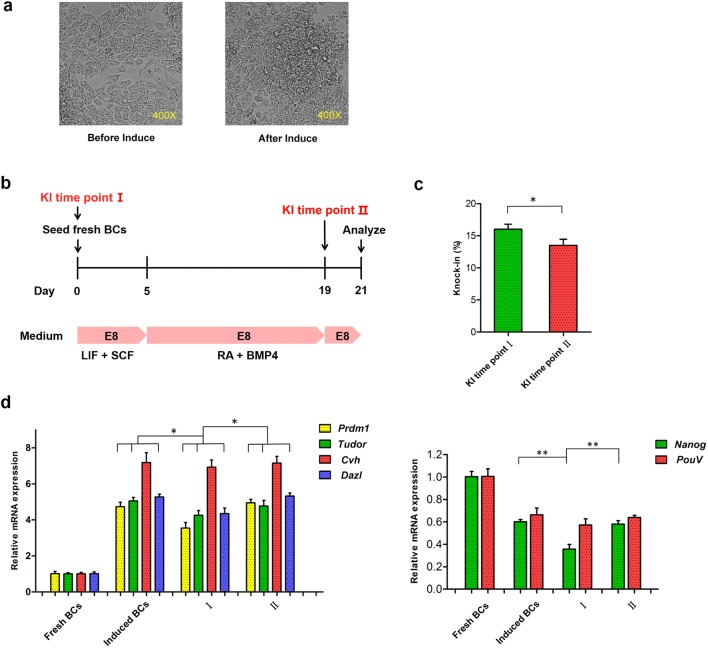
Next, we performed KI on BCs. In vitro cultured BCs require germline induction to increase germline chimeric efficiency, and BCs from before and after induction showed differing phenotypes, transforming from a single layer to multiple layers (Fig. 4a). We performed KI at different time points to explore whether we could achieve higher KI efficiency and lower impact induction efficiency in BCs (Fig. 4b). The results showed that the efficiency of KI before induction was significantly higher than that after induction (16.0% and 13.5%, respectively, Fig. 4c). However, qRT-PCR results showed that KI before induction resulted in decreased expression of germline-specific and pluripotency-related genes, while KI after induction did not alter the expression of these genes (Fig. 4d). Therefore, we concluded that KI after induction is a better approach.
Fig. 4.
KI efficiency in BCs at different time points and the effect of KI on BCs induction. a Different states of BCs before and after induction. b Timeline for induction and time points of KI. c KI efficiency detected by single-cell PCR in BCs at different time points. d The mRNA expression of germline-specific genes and pluripotency-related genes in BCs. Data are representative of results in three independent experiments, values are the mean ± SEM (n = 3), and each condition is normalized to β-actin abundance. *p < 0.05; **p < 0.01
Germline chimeric KI chicken embryo production using the BC-mediated method and the direct injection method
We produced germline chimeric KI chicken embryos via in vitro induction and KI of BCs and transplanted them into the subgerminal cavity of recipient embryos (BC-mediated method). In addition, we also used direct in vivo injection of high titre adenovirus (> 1 × 1010 PFU/ml) to produce germline chimeric KI chicken embryos (direct injection method) and compared the production efficiency of the two methods. To identify the cells infected with adenovirus, we used the adenovirus with GFP tag to deliver the Bait-HMEJ donor (donor-GFPtag adenovirus) (Fig. 5a). We demonstrated that BCs, which have undergone in vitro induction and KI, can migrate to the gonads, and that direct injection of adenovirus can also infect gonad cells; both methods can produce gonad chimeric KI chicken embryos (Fig. 5b), but the proportion of KI-positive gonads from the BC-mediated method was significantly higher than that from the direct injection method (13/58 and 9/113, respectively, Fig. 5c); moreover, the GFP-positive cell ratio and KI-positive cell ratio in germ cells (germline chimerism rate) obtained via the BC-mediated method were also significantly higher than that of the direct injection method (only three individuals with the highest values were counted, 10.46% and 2.87% in GFP-positive cell ratio, and 1.24% and 0.11% in germline chimerism rate, respectively) (Fig. 5d, e).
Discussion
In this study, we used adenovirus for delivery, optimized the donor structure, and improved donor recruitment to increase the efficiency of KI in various chicken cells with a goal to explore new methods of producing KI chickens. The efficiency of KI is one of the most decisive factors in determining the production methods of KI animals. For example, in the production of KI mice, the KI efficiency when using conventional homologous recombination is about 10−3–10−9 (Komor et al. 2017), so only mouse embryonic stem cell (mES)-mediated methods can be used. With the use of gene-editing tools such as CRIPSR/Cas9, which introduce a double-stranded break (DSB) into the genomic locus, the efficiency of KI is greatly increased to 0.1–20% (Komor et al. 2017); thus, KI mice can be produced by oocyte cytoplasmic injection, which greatly reduces the cost and time of the production cycle. Similarly, currently, KI chickens can only be produced by the PGC-mediated method, because this method is feasible even when KI efficiency is low (Oishi et al. 2018; Taylor et al. 2017; Tyack et al. 2013). If KI efficiency can be improved to a certain extent, establishing other KI chicken production methods will be possible.
DSB-based KI usually depends on HDR, which is a homology driven DNA incorporation stimulated by DSB, and therefore, increasing HDR can improve KI efficiency (Devkota 2018). However, approaches for increasing HDR, i.e., chemical/genetic activation or manipulation of the cell cycle, may be harmful to target cells. Of additional concern, these approaches are difficult to implement in vivo. Recent studies show that after DSB, there are other precise repair pathways besides HDR that can also drive DNA incorporation; MMEJ and HMEJ are two representatives of these repair pathways (Nakade et al. 2014; Yao et al. 2017a, b; Zhang et al. 2017). In this study, our results demonstrated that the KI efficiency of HMEJ donor was 8.7 times that of the HDR donor, whereas the KI efficiency of the MMEJ donor was 3.6 times that of HDR donor (Fig. 1c). The reason for the lower efficiency of HDR in this study may due to the large size of the adenoviral DNA backbone (~ 35 kb), for linear DNA, the longer the backbone at both ends of the homology arm, the lower the KI efficiency (Yao et al. 2018). It is noteworthy that the MMEJ donor contains a very short homology arm (5–25 bp), whereas the HDR or MHEJ donor usually contains long homology arms (from 500 bp to several kb). Therefore, the construction of the MMEJ donor saves labor and time. We believe that the MMEJ donor will be suitable for use in the production of KI chickens by the PGC-mediated method, because this method does not require extremely high KI efficiency. Physically recruiting donors to the insertion site is another approach of increasing KI efficiency which results in less intervention to targeted cells (Gu et al. 2018; Lee et al. 2017; Ma et al. 2017). In this study, we recruited donors for the first time by fusing TALE to the C-terminal of Cas9 protein; this method increased the KI efficiency of MHEJ donor by 0.6-fold (Fig. 2b). Compared with previous studies, this recruitment approach does not require chemical modification in the donor, leaving it suitable for adenovirus delivery.
In this study, we evaluated two potential KI chicken production methods, the BC-mediated method and the direct injection method, after increasing KI efficiency. Currently KI chicken can only be produced by PGC-mediated methods due to PGC lines ability to proliferate rapidly and maintain the potential for germline chimerism after long-term in vitro culture and cryopreservation (Oishi et al. 2016; Park et al. 2014; Schusser et al. 2013; Taylor et al. 2017). This was a breakthrough technology for PGE chicken production, but establishing PGC lines while preserving the ability for germline chimerism is very difficult. Currently, only a few research teams have managed, and this process takes massive time and resources. Compared with the PGC-mediated method, the advantages of the direct injection method are a simple production process, short time-cycle, and versatility among different chicken breeds (Cooper et al. 2018; Tyack et al. 2013; Wang et al. 2019). Unlike liposomes used in previous studies, in this study, we used adenovirus (titre = 5 × 1010 PFU/ml) for direct injection in vivo, and the GFP-positive cell chimerism rate was 2.87% (Fig. 5d) which is higher than in previous studies of injection using liposomes (~ 1%), but the embryonic germline chimerism rate (KI-positive cell rate) in this study was only 0.11% (Fig. 5e). Theoretically, the efficiency of this method depends on virus titres, and we believe that if the virus titre can be increased, the results may improve. Interestingly, although using relatively low titre adenovirus (~ 109 PFU/ml), the embryonic germline chimerism rate in the BC-mediated method is considerable (1.13%) (Fig. 5e). Therefore, the BC-mediated method may achieve a balance between the PGC-mediated method and the direct injection method: it can obtain a large number of available cells in a short period of time and is not limited by viral titres. However, the BC-mediated method is not as efficient as the PGC-mediated method, because it cannot enrich for KI-positive cells, and the production process is more complicated than direct injection method. Nevertheless, our study provides two potential alternatives to the production of KI chickens for researchers who lack the technology and resources to master the PGC-mediated method.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Special Fund for Technology Innovation Guidance of Guangxi Province [2017AD10038], Special Fund for Technology Innovation Guidance of Nanning [CG20180038], National Natural Science Foundation of Guangxi [2018JJA130061], and National Natural Science Foundation of China [31760648].
Author contributions
XT and KC designed the experiments. XQ, NX, and YX worked together to carry out the experiments. XT and XQ performed the data analysis and wrote the manuscript. FY, XW, HH, and QL participated in some experiments. All authors discussed the results and commented on the manuscript.
Data availability
All data generated or analyzed during this study are included in this article.
Compliance with ethical standards
Conflict of interest
We declare no competing financial interests.
Ethics standards
All experiments were performed in accordance with the guidelines of the regional Animal Ethics Committee, and the Institutional Animal Care and Use Committee of Guangxi University approved all experiments (Approval number: GXU2016-089).
Footnotes
Kuiqing Cui and Xiaochuan Tang are co-corresponding authors.
Xiaolian Qin, Ning Xiao, and Yu Xu have contributed equally to this work.
Contributor Information
Kuiqing Cui, Email: kqcui@126.com.
Xiaochuan Tang, Email: tangxiaochuan7@163.com.
References
- Carsience RS, Clark ME, Gibbins AMV, Etches RJ. Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development. 1993;117(2):669–675. doi: 10.1242/dev.117.2.669. [DOI] [PubMed] [Google Scholar]
- Cooper CA, Doran TJ, Challagulla A, Tizard MLV, Jenkins KA. Innovative approaches to genome editing in avian species. J Anim Sci Biotechnol. 2018;9:15. doi: 10.1186/s40104-018-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devkota S. The road less traveled: strategies to enhance the frequency of homology-directed repair (HDR) for increased efficiency of CRISPR/Cas-mediated transgenesis. BMB Rep. 2018;51(9):437–443. doi: 10.5483/bmbrep.2018.51.9.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Posfai E, Rossant J. Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat Biotechnol. 2018 doi: 10.1038/nbt.4166. [DOI] [PubMed] [Google Scholar]
- Johnson PA, Giles JR. Use of genetic strains of chickens in studies of ovarian cancer. Poult Sci. 2006;85(2):246–250. doi: 10.1093/ps/85.2.246. [DOI] [PubMed] [Google Scholar]
- Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;168(1–2):20–36. doi: 10.1016/j.cell.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavial F, Pain B. Chicken embryonic stem cells as a non-mammalian embryonic stem cell model. Dev Growth Differ. 2010;52(1):101–114. doi: 10.1111/j.1440-169X.2009.01152.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Mackley VA, Rao A, Chong AT, Dewitt MA, Corn JE, Murthy N. Synthetically modified guide RNA and donor DNA are a versatile platform for CRISPR–Cas9 engineering. Elife. 2017 doi: 10.7554/elife.25312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Zhuang F, Hu X, Wang B, Wen XZ, Ji JF, Xi JJ. Efficient generation of mice carrying homozygous double-floxp alleles using the Cas9-Avidin/Biotin-donor DNA system. Cell Res. 2017;27(4):578–581. doi: 10.1038/cr.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S, Tsubota T, Sakane Y, Kume S, Sakamoto N, Obara M, Suzuki KT. Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat Commun. 2014;5:5560. doi: 10.1038/ncomms6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep. 2016;6:23980. doi: 10.1038/srep23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Yoshii K, Miyahara D, Tagami T. Efficient production of human interferon beta in the white of eggs from ovalbumin gene-targeted hens. Sci Rep. 2018;8(1):10203. doi: 10.1038/s41598-018-28438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Lee HJ, Kim KH, Kim JS, Han JY. Targeted gene knockout in chickens mediated by TALENs. Proc Natl Acad Sci USA. 2014;111(35):12716–12721. doi: 10.1073/pnas.1410555111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Lee HG, Moon JK, Lee HJ, Yoon JW, Yun BN, Han BK. Deposition of bioactive human epidermal growth factor in the egg white of transgenic hens using an oviduct-specific minisynthetic promoter. FASEB J. 2015;29(6):2386–2396. doi: 10.1096/fj.14-264739. [DOI] [PubMed] [Google Scholar]
- Petitte JN, Mozdziak PE. The incredible, edible, and therapeutic egg. Proc Natl Acad Sci USA. 2007;104(6):1739–1740. doi: 10.1073/pnas.0611652104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitte JN, Clark ME, Liu G, Gibbins AMV, Etches RJ. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development. 1990;108(1):185–196. doi: 10.1242/dev.108.1.185. [DOI] [PubMed] [Google Scholar]
- Schusser B, Collarini EJ, Yi H, Izquierdo SM, Fesler J, Pedersen D, Leighton PA. Immunoglobulin knockout chickens via efficient homologous recombination in primordial germ cells. Proc Natl Acad Sci USA. 2013;110(50):20170–20175. doi: 10.1073/pnas.1317106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Xu S, Zhang H, Chen Q, Li R, Wu W, Liu H. Retinoic acid promotes expression of germline-specific genes in chicken blastoderm cells by stimulating Smad1/5 phosphorylation in a feeder-free culture system. BMC Biotechnol. 2017;17(1):17. doi: 10.1186/s12896-017-0332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Shi J, Qin X, Xiao N, Li R, Hu H, Wang X. Retinoic acid (RA) and bone morphogenetic protein 4 (BMP4) restore the germline competence of in vitro cultured chicken blastodermal cells. Vitro Cell Dev Biol Anim. 2019 doi: 10.1007/s11626-019-00324-9. [DOI] [PubMed] [Google Scholar]
- Taylor L, Carlson DF, Nandi S, Sherman A, Fahrenkrug SC, McGrew MJ. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development. 2017;144(5):928–934. doi: 10.1242/dev.145367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyack SG, Jenkins KA, O’Neil TE, Wise TG, Morris KR, Bruce MP, Doran TJ. A new method for producing transgenic birds via direct in vivo transfection of primordial germ cells. Transgenic Res. 2013;22(6):1257–1264. doi: 10.1007/s11248-013-9727-2. [DOI] [PubMed] [Google Scholar]
- van de Lavoir MC, Mather-Love C, Leighton P, Diamond JH, Heyer BS, Roberts R, Etches RJ. High-grade transgenic somatic chimeras from chicken embryonic stem cells. Mech Dev. 2006;123(1):31–41. doi: 10.1016/j.mod.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wang ZB, Du ZQ, Na W, Jing JH, Li YM, Leng L, Li H. Production of transgenic broilers by non-viral vectors via optimizing egg windowing and screening transgenic roosters. Poult Sci. 2019;98(1):430–439. doi: 10.3382/ps/pey321. [DOI] [PubMed] [Google Scholar]
- Woodfint RM, Hamlin E, Lee K. Avian bioreactor systems: a review. Mol Biotechnol. 2018;60(12):975–983. doi: 10.1007/s12033-018-0128-x. [DOI] [PubMed] [Google Scholar]
- Yao X, Wang X, Hu X, Liu Z, Liu J, Zhou H, Yang H. Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res. 2017;27(6):801–814. doi: 10.1038/cr.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Wang X, Liu J, Hu X, Shi L, Shen X, Yang H. CRISPR/Cas9-mediated precise targeted integration in vivo using a double cut donor with short homology arms. EBioMedicine. 2017;20:19–26. doi: 10.1016/j.ebiom.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Zhang M, Wang X, Ying W, Hu X, Dai P, Yang H. Tild-CRISPR allows for efficient and precise gene knockin in mouse and human cells. Dev Cell. 2018;45(4):526e525–536e525. doi: 10.1016/j.devcel.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Li XL, Li GH, Chen W, Arakaki C, Botimer GD, Zhang XB. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 2017;18(1):35. doi: 10.1186/s13059-017-1164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article.