Abstract
The structural modification of natural pyrethrins has led to a number of synthetic pyrethroid insecticides, and each compound has its own characteristics. At present, pyrethroid insecticides are applied not only for household use, the original use for pyrethrins, but also for a wide range of uses such as crop protection, pharmaceuticals, and veterinary applications. Quoting primary sources, this review describes the historical view of structural modifications of pyrethroids, with a focus on structural similarities, and their use.
Keywords: pyrethroids, structural modification, pyrethrins, insecticide, lead natural products
Introduction
Tanacetum cinerariifolium, commonly called an “insecticidal flower,” is one of three pyrethrum species and has been used as insect powder in the Dalmatian region since the Middle Ages due to its insecticidal activity in the ovule. Active ingredients extracted from dried pyrethrum, named pyrethrins, were among the few insect control agents available in the world before the invention of synthetic insecticides, but they were inconvenient for practical use.1) However, with the invention of a mosquito stick in 1890 (a mosquito coil in 1895) by Eiichiro Ueyama, a founder of the Japanese company Dainippon Jochugiku (Kincho), the use of pyrethrins gradually spread throughout the world due to its advantages in controlling flying insects and its ease of use. The mosquito coil is still one of the most popular materials for preventing mosquito bites without changing the coil shape. Even now, there is a demand for pyrethrins as natural insecticides, and 10,000 tons of dried petals are produced annually.2)
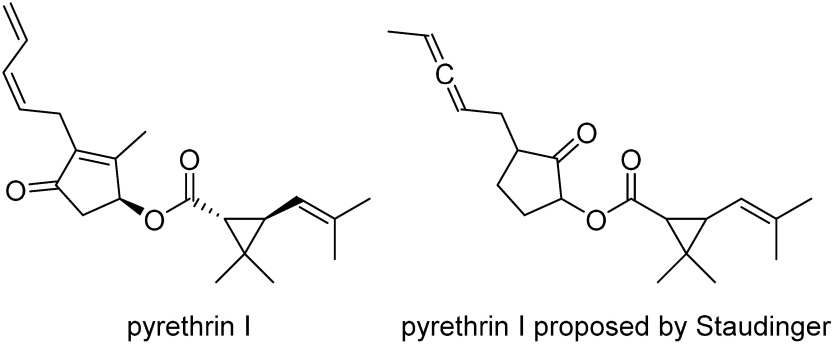
Six pyrethrins have been identified as insecticidal compounds in nature, and pyrethrin I (Fig. 1) is the most important component because of its overall insecticidal efficacy and abundance. Pyrethrin I has a unique ester structure constructed from substituted cyclopentenolone (called pyrethrolone) and a substituted cyclopropanecarboxylic acid (called chrysanthemic acid). Pyrethrins have fast action (i.e., a fast-acting effect), called a knockdown effect, which paralyzes insect pests by modifying the kinetics of the voltage-sensitive sodium channel.3) Additionally, they are active ingredients in mosquito coils, which are safe to mammals and have excellent heat transpiration. However, since pyrethrin I is constructed from many unstable partial structures, such as a trialkyl-substituted double bond, cyclopentenolone, and conjugated diene, the use of pyrethrins as agricultural insecticides has been very limited. Furthermore, since the supply of natural pyrethrins was sometimes unreliable, the industrial production of pyrethrins was expected but proved difficult because of structural complications. In order to solve these problems, extensive structural modifications of pyrethrins have been conducted for over half a century, and a number of derivatives, the so-called synthetic pyrethroids, have been born.
Fig. 1. Structure of pyrethrin I and the structure proposed by Stäudinger.6).
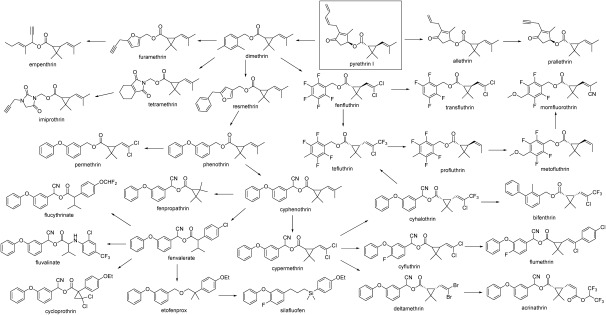
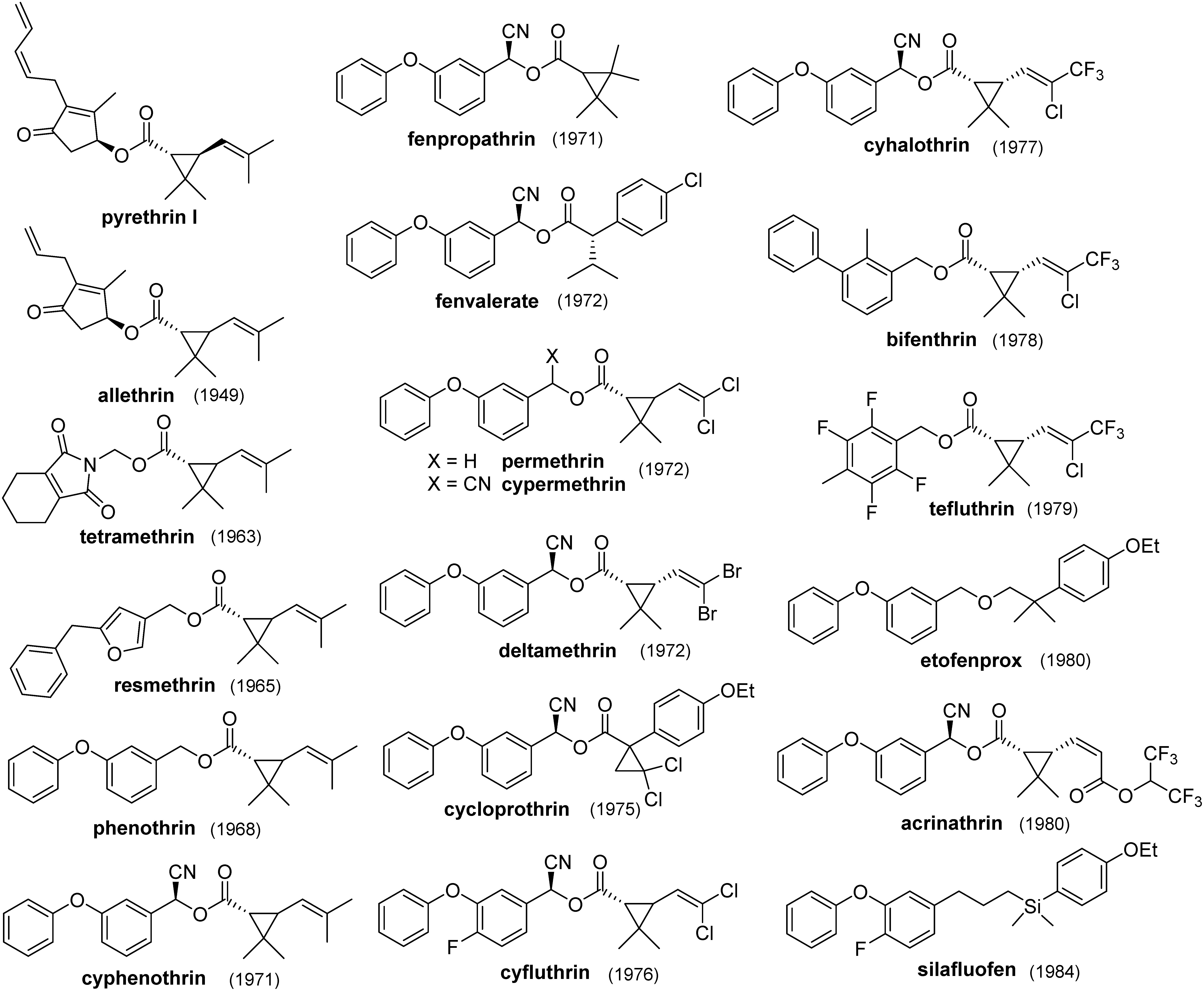
Figure 2 shows the brief history of the structural modifications of pyrethroids. The structural modifications of both alcohol and acid moieties in natural pyrethrins have led to a number of synthetic pyrethroids with diverse structural features. Many researchers have participated in the competition to develop superior new pyrethroids. As a result, a variety of structural modifications have been achieved, and more than thirty compounds are on the market. In this review, the historical flow of the structural modifications of pyrethroids is summarized, focusing on structural similarities. This article quotes as many primary sources (i.e., patents and literature) as possible.
Fig. 2. Structural modification tree of pyrethroids. Most synthetic pyrethroids contain asymmetric carbons in their structures, and their insecticidal actions are very stereospecific. Commercialized products are either isomer mixtures, a single isomer, or various product specifications. In many cases, they have been replaced by a resolved form (chiral switch). Only the most important isomers are shown here. Generally, both cis- and trans-isomers on the cyclopropane ring are insecticidal, but cis-isomers are preferred in agricultural use and trans-isomers are preferred in household use; however, there are some exceptions.
1. Early Studies of Natural Pyrethrin Structures
As Japan had a big share in the pyrethrum market in the early 1900s, many of the early pyrethrum studies were conducted in Japan. The first isolation of pyrethrins was reported by Fujitani.4) He claimed in the report that pyrethrins are ester compounds. In 1923, Yamamoto reported that the acid part of pyrethrins is 3-butenyl-2,2-dimethylcyclopropanecarboxylic acid.5) The following year, two prestigious chemists, Stäudinger and Ružička at Eidgenössische Technische Hochschule Zürich (ETH), published a paper of several hundred pages reporting detailed structural studies. The pyrethrin I structure they proposed is shown in Fig. 1.6) Their works were great achievements in natural product chemistry in light of the scientific level at that time (there were no spectroscopic analytical instruments), although we now know that the proposed structure contained some mistakes. The correct planar structure was elucidated in 1945,7) and a complete structure, including stereochemistry, was determined in 19588); this became the basis for subsequent developments of pyrethroid chemistry. The first synthetic pyrethroid, allethrin, was invented at the United States Department of Agriculture (USDA) in 1949,9) and its patent10) was opened to the public the following year.11) Several companies, including Sumitomo Chemical, successfully commercialized allethrin and got into the pyrethroid business in 1953.12) It is notable that a complete stereochemistry of pyrethrin I was determined five years after the launching of allethrin. This means that the researchers did not know which isomer was active when allethrin was launched.
2. Structural Modification of the Alcohol Part of Pyrethrin I
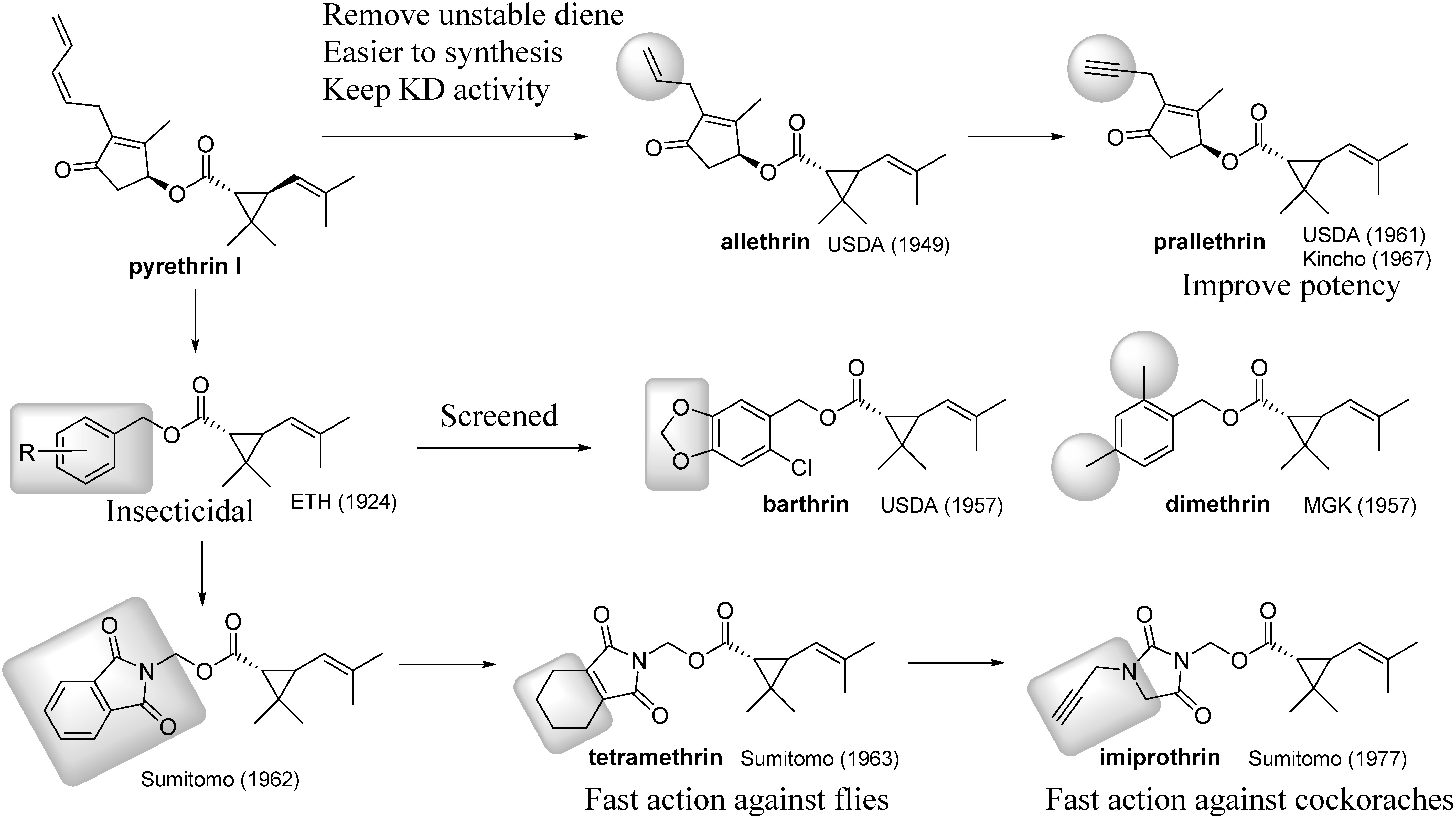
Most chemists wanted to convert the complicated pyrethrolone, the alcohol part of pyrethrin I, to a simpler one. Several attempts are shown in Fig. 3.† The first successful modification achieved was allethrin synthesis, in which one vinyl group was removed from the unstable diene structure. Allethrin exhibited knockdown activity comparable to that of pyrethrin I (i.e., fast action). Even now allethrin is widely used as an active ingredient in mosquito killers such as mosquito coils and aerosol spray. Replacement of the allyl group of allethrin with a propargyl group was previously evaluated by the USDA in 1961, but the efficacy was only 60% that of allethrin.13) In contrast, Dainippon Jochugiku reported that the propargyl derivative was 1.2 times more potent than allethrin.14) They proposed that the difference might be due to the difference in sample purity and/or the different bioassay methods. Subsequently, Sumitomo Chemical established a chiral synthesis of this moiety15) and succeeded in commercializing it as prallethrin.16) Prallethrin was the best cyclopentenolone-type pyrethroid, especially in terms of the knockdown effect on various house pests, such as mosquitoes, flies, and cockroaches.
Fig. 3. Structural modifications of the alcohol part with the aim of improving fast action.
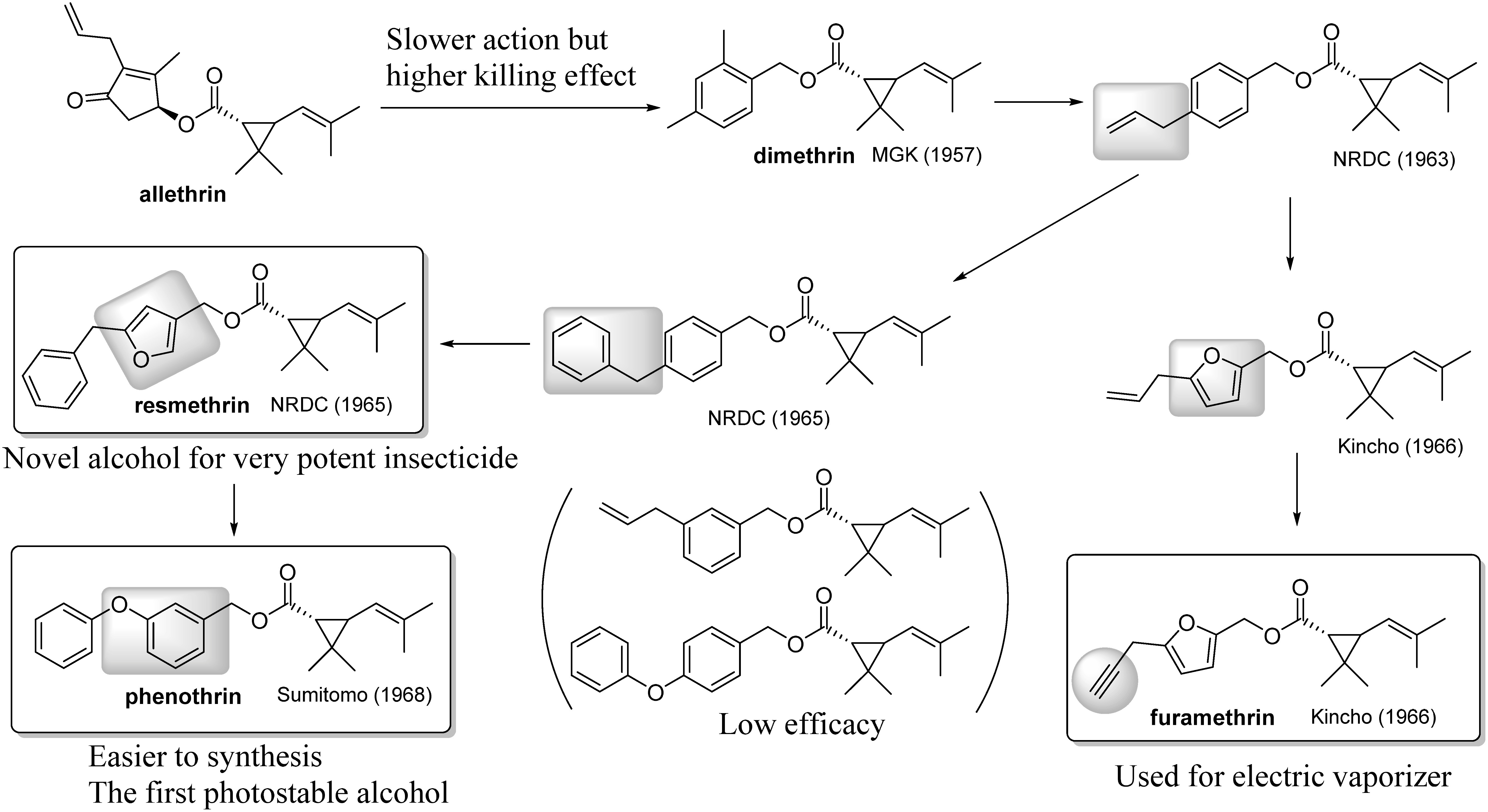
The synthetic variations of chrysanthemate have already been reported by Stäudinger and Ružička in a paper on the structural elucidation of natural pyrethrins.6) They found that substituted benzyl ester and some unsaturated aliphatic alcohol esters have insecticidal activity.17) Barthel et al. of the USDA18) and McLaughlin Gormley King Co. (MGK)19) randomly screened various benzyl esters to discover barthrin and dimethrin (Fig. 3). These compounds have a potent killing effect against houseflies and are more easily synthesized than pyrethrin I,20) but their knockdown activities against houseflies were only one-tenth that of pyrethrin I’s.21) This work encouraged many researchers to perform further modifications. The first breakthrough was realized as a carbodiimide-N-hydroxymethylol ester, tetramethrin,22) via a phthalimide-N-hydroxymethylol ester.23) Tetramethrin showed faster knockdown action against houseflies than did allethrin and pyrethrins, with lower oral acute toxicity on mice.24) This finding led to the invention of imiprothrin, which was registered in 1996 as a very fast knockdown agent against crawling insects, especially cockroaches.25)
The paragraph above focused on the fast action of pyrethroids. Although all of these compounds do not necessarily have an excellent killing effect on insects, barthrin and dimethrin have some killing effect, but it is inferior to that of pyrethrins. These studies were followed by that of Elliott et al. of the National Research Development Corp. (NRDC) (Fig. 4). As a result, a 5-benzyl-3-furfuryl alcohol ester, resmethrin, was invented via a para-allyl- or para-benzyl-benzyl alcohol ester. However, it is very difficult to synthesize as compared with the substituted benzyl esters invented at that time.26) As resmethrin has an outstanding lethal effect against various insects, it was commercialized as a household insecticide and is still one of the most important mosquito-killing agents. Independently, Dainippon Jochugiku found that 2-furfuryl alcohol esters also have significant insecticidal activity.27) In particular, furamethrin, in which the allyl group was replaced by the propargyl group, exhibited superior performance as an active ingredient in an electric vaporizer.28) Another important development was the Sumitomo Chemical discovery of phenothrin, in which the furan ring of resmethrin was replaced by a benzene ring. This replacement made synthesis easier and improved photostability. Because of these properties, 3-phenoxybenzyl alcohol would be used in many pyrethroids that were invented subsequently. The NRDC had already evaluated para-benzyl-substituted benzyl alcohol ester in 1965. Since they did not evaluate meta-substituted ones at that time, they lost the patent right.29) Why did they lag behind Sumitomo in the discovery of phenothrin? They already had data indicating that meta-allylbenzyl and para-phenoxybenzyl esters exhibit only weak activity compared to para-allyl and para-benzylbenzyl esters, respectively.30) Accordingly, they might have concluded that para-substitution and the methylene bridge between the benzene and benzyl groups are very important in eliciting insecticidal activity.
Fig. 4. Structural modifications of the alcohol part with the aim of improving lethal action.
Around the same time, BASF introduced an ethynyl group at the α-position of various benzyl-type pyrethroids such as dimethrin and found enhanced potency as compared to that of unsubstituted derivatives (Fig. 5).31) This finding was immediately applied to various known pyrethroids, as in the case of S-2684 derived from phenothrin.32) However, this conversion had not been applied in the case of resmethrin33) and was highly limited in the case of phenothrin.34) On the other hand, replacing the ethynyl group in S-2684 with a cyano group improved insecticidal efficacy. The derivative of S2684 was commercialized as cyphenothrin, which was mainly used as an active ingredient in cockroach killers.35) Cyphenothrin is also recognized as the first Type II pyrethroid, which is generally characterized by the presence of an α-cyano group, as compared to the former pyrethroids, called Type I pyrethroids. Most pyrethroids that have been developed for agricultural use to date contain a cyano group, as described later. Type II pyrethroids not only improve killing efficacy but also have a different effect on sodium channels. Type I pyrethroids tend to activate sodium channels, while Type II pyrethroids prolong the activated state of channels.36)
Fig. 5. Introduction of an ethynyl/cyano group into the α-position of alcohol.
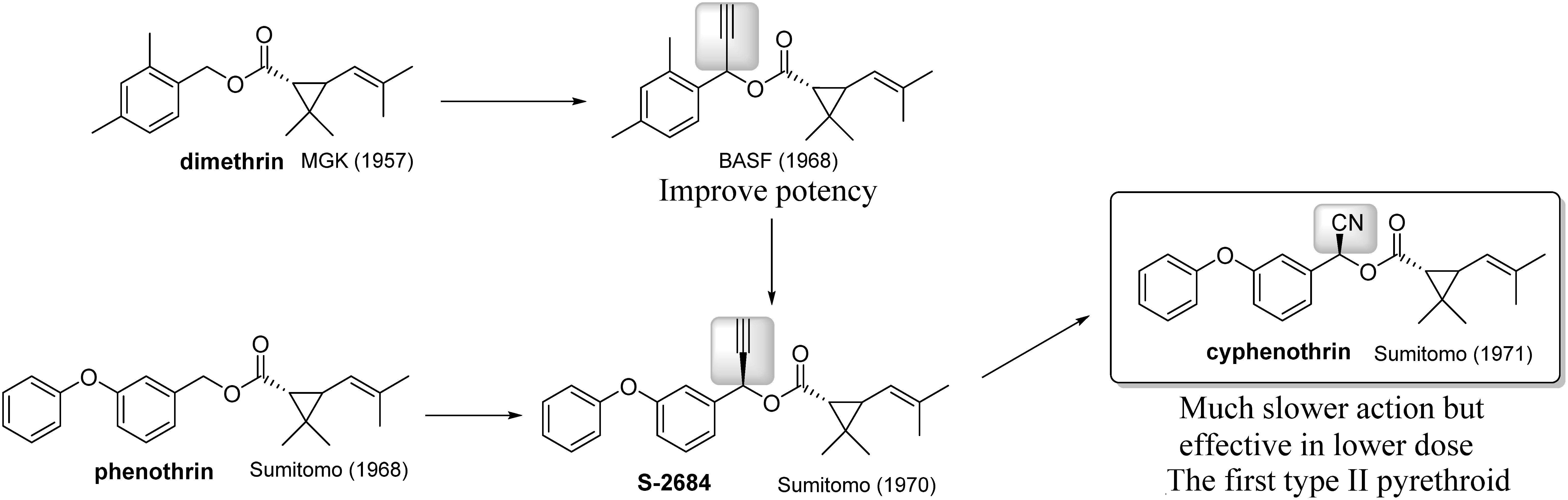
3. Challenge to Develop an Active Ingredient for Crop Protection
Cyphenothrin’s lethal action is superior to that of the former pyrethroids, although the agricultural use of this reagent has been very limited. What are the drawbacks of cyphenothrin for agricultural use? First, the manufacturing cost of the acid part, especially construction of the cyclopropane ring, is too expensive as compared with the improvement in crop yield. Second, trisubstituted double bonds in the acid part are not stable against oxygen and sunlight. Third, the insecticidal spectrum is not good against insects living in the field. It was expected that these problems might be solved by replacing the acid part with some inexpensive and stable substructures.
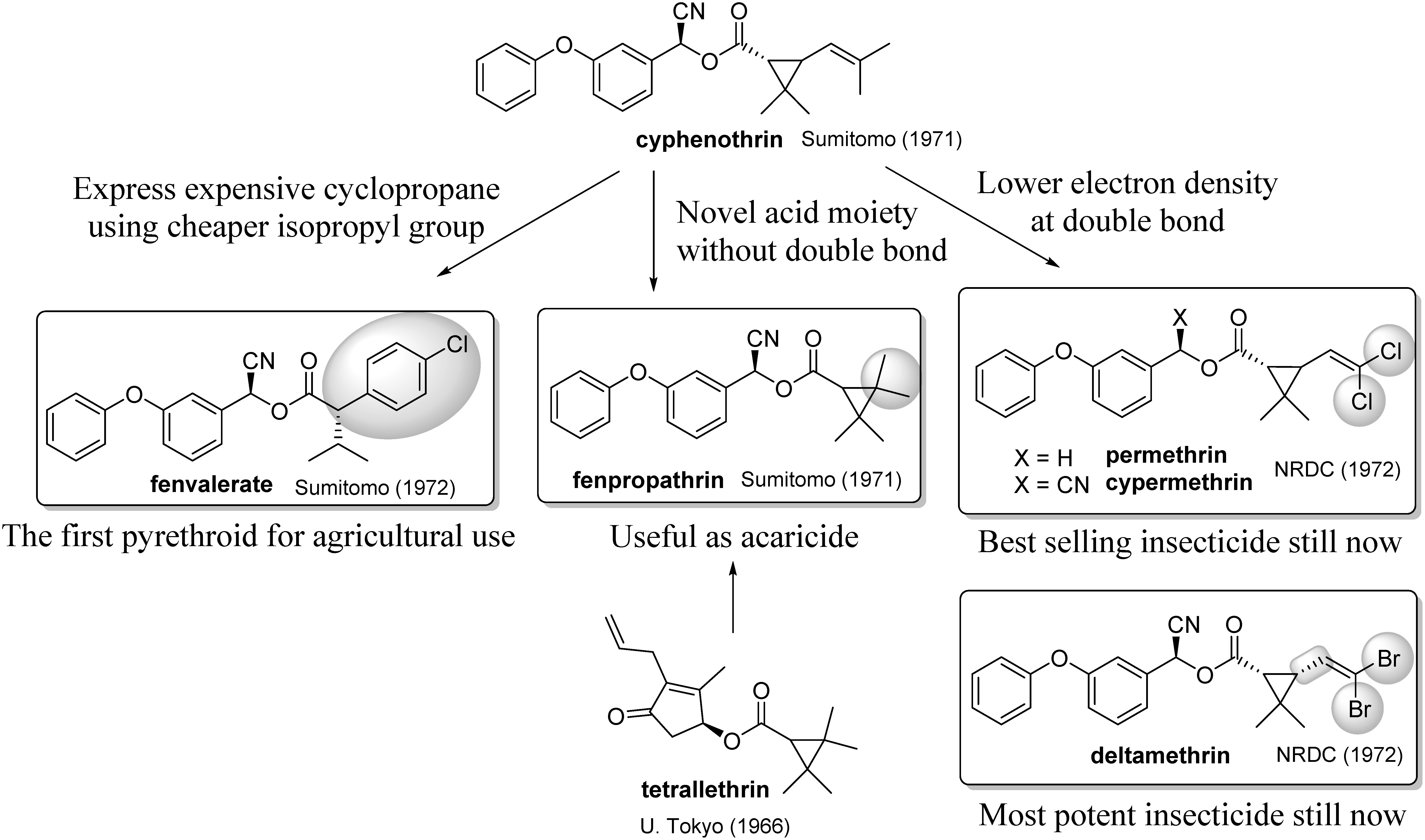
Figure 6 shows three successful approaches to overcoming the issues above. The first invented compound was fenpropathrin, the design of which was based on the finding at the University of Tokyo that the tetramethylcyclopropanecarboxylic acid used in tetrallethrin37) could be used as a pyrethroid acid to increase the stability against oxygen and sunlight. Fenpropathrin was effective against several agricultural pests,38) especially mite pests in fruit.39) Fenpropathrin was later launched in a niche market. The second approach is to replace the cyclopropane ring with something inexpensive to reduce the synthetic cost. This was accomplished by opening the cyclopropane ring and replacing the remaining part with a substituted benzene ring, resulting in fenvalerate.40) This compound became the first pyrethroid for agricultural use (especially for the control of cotton pests). The third approach is to substitute the methyl groups connecting the double bond to halogen atoms to reduce photooxidative instability. This acid part was first reported by Farkaš et al. in 1958.41) They synthesized the allethrin analog and achieved the enhancement of insecticidal activity. However, their research was not well known and was discontinued because the photostable alcohol part having high lethal activity was not known when they did their research. However, a combination of this acid with the alcohol part of phenothrin and cyphenothrin made permethrin, cypermethrin, and deltamethrin, which exhibited a new order of insecticidal activity42) with photostability.43) These analogues were highly useful, not only in the field of household insecticides but also as agricultural insecticides.44) The remaining task in the industry was to find economical methods of synthesizing this unique acid, permethric acid. To that end, many industrial laboratories have joined the race to look for a cheap process of synthesizing permethric acid.
Fig. 6. Structural modifications of cyphenothrin for use as a crop protection agent.
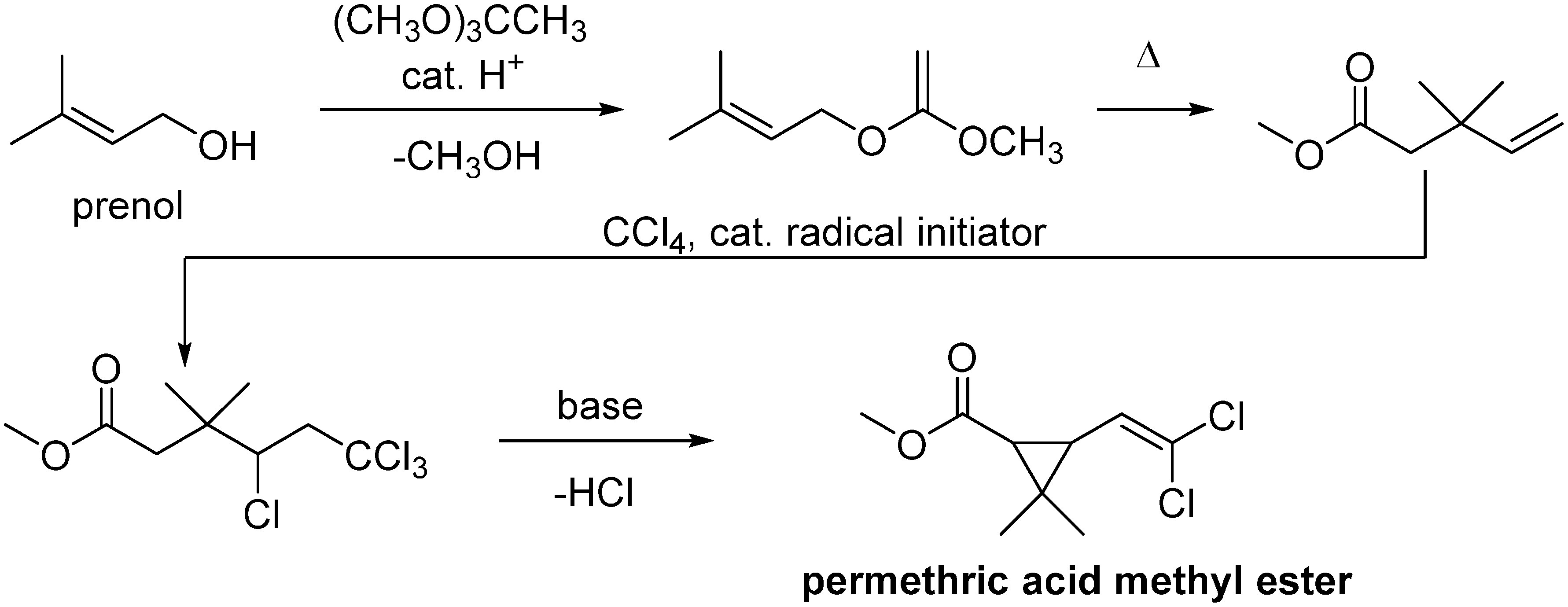
The method of synthesis described in Elliott’s patent is summarized in Fig. 7. This process is superior on a laboratory level, but there are several problems to be overcome: i) several steps are required to manufacture the starting material, which is unfavorable to making cheap agricultural insecticides; ii) it contains a risky reaction, such as ozone oxidation; and iii) it contains low atom economy reactions, such as that of carbon tetrachloride with triphenylphosphine. Among the various synthetic methods that were attempted in an effort to overcome these issues in many companies and institutes, the process (shown in Fig. 8) used by the Sagami Chemical Research Center was the winner of this synthetic race.45) The condensation of prenyl alcohol and orthoacetate ester caused the Claisen rearrangement to make 3,3-dimethyl-4-pentenoate. The obtained product was condensed with carbon tetrachloride and cyclized by treating with a base to give a molecular target. This process is one of the superior industrial synthesis processes because starting materials are cheap except for prenol and the process is constructed by short steps, redox-neutral reaction, and high atom economy (byproducts: methanol and the salt of hydrogen chloride). Later, the problem of industrializing prenol synthesis was successfully solved by Kuraray.46)
Fig. 7. Synthetic scheme of permethric acid as described in Elliott’s patent.
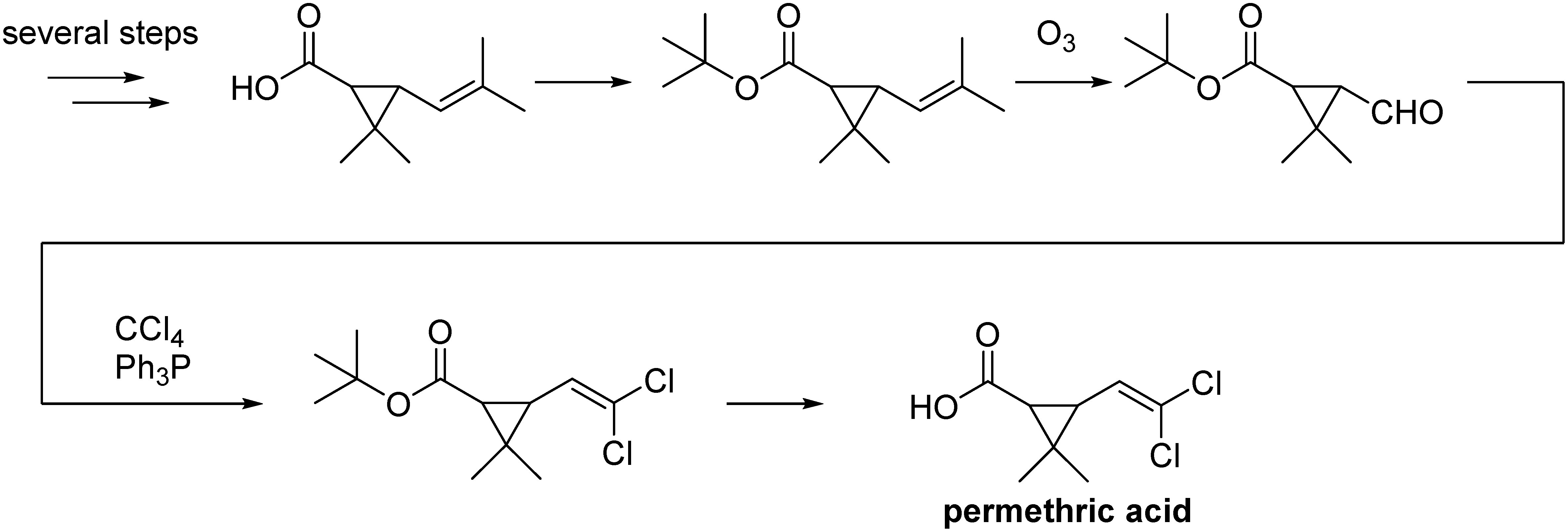
Fig. 8. Synthetic scheme of permethric acid by Sagami.
4. Fluorine Chemistry in Structural Modifications of Pyrethroids
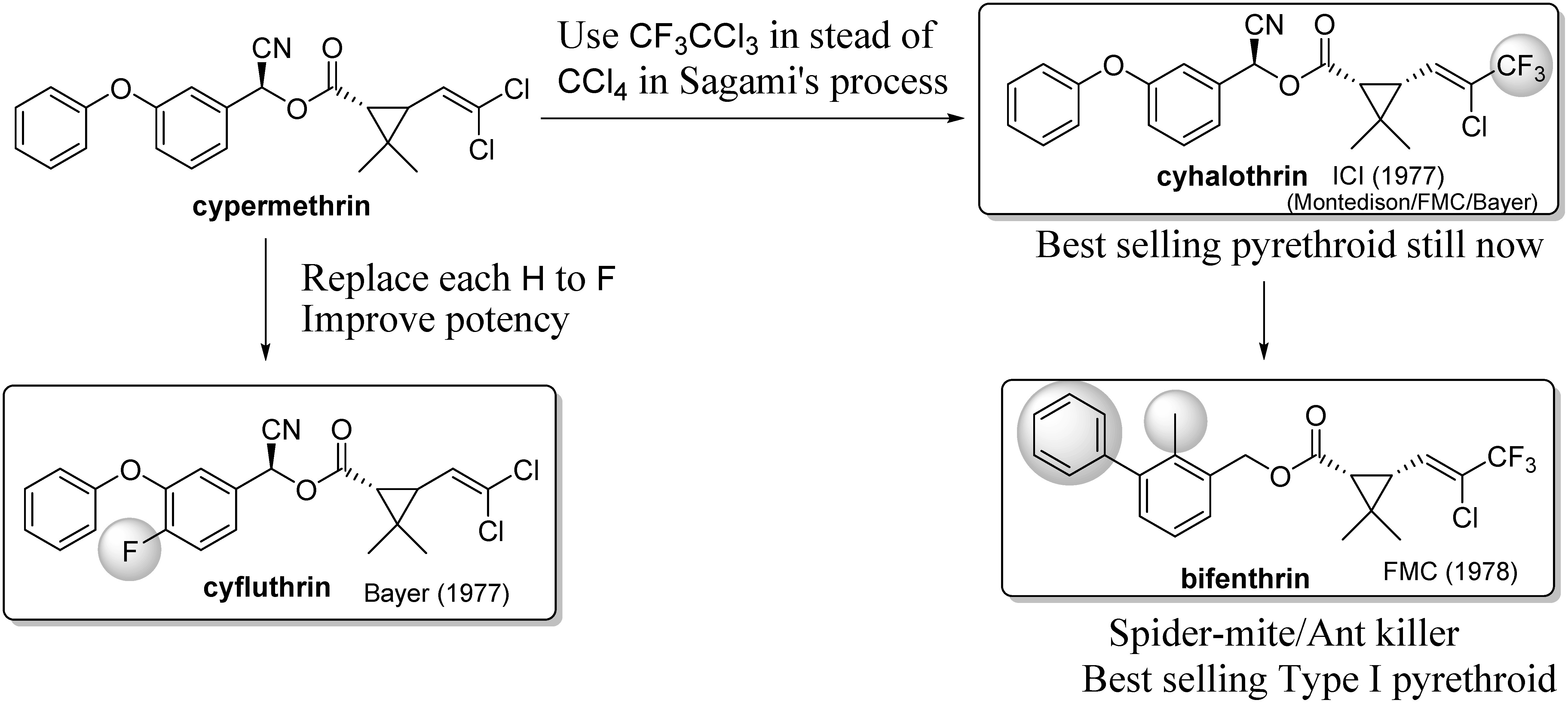
The application of fluorine chemistry in studies for the discovery of pharmaceuticals and agrochemicals had become popular in the 1970s.47) Fluorine chemistry also successfully applied the structural modifications of pyrethroid chemistry (Fig. 9). The first example of success was cyfluthrin.48) Bayer revealed that only the 4-position of the benzyl group in cypermethrin needed to be fluorinated to improve insecticidal efficacy.49) Another approach was the replacement of one chlorine of cypermethrin with a trifluoromethyl group. This acid part can be prepared according to the process shown in Fig. 8, except that carbon tetrachloride was replaced with CFC-113a (CF3CCl3). This method was invented by four research groups independently, including Imperial Chemical Industries (ICI),50) Montedison,51) FMC,52) and Bayer.53) Although the first applicant, ICI, was a newcomer to pyrethroid chemistry, they commercialized cyhalothrin, which is now the best-selling pyrethroid in the agricultural insecticide market. This new acid part was also used in bifenthrin. Bifenthrin carries a different type of substituted benzyls as the alcohol part that is characterized by the biphenyl substructure and the methyl group at the ortho-position.54) Bifenthrin exhibits a wide spectrum of pesticidal activity, particularly against mites, and is now the best-selling Type I pyrethroid. Note that bifenthrin is usually considered to be a Type I pyrethroid because the α-cyano group is missing. However, several studies have suggested that it acts as a mixture of Type I and Type II pyrethroids.55)
Fig. 9. Introduction of fluorine into pyrethroids.
Another remarkable application of fluorine chemistry to pyrethroids involved polyfluorobenzyl esters (Fig. 10). The first polyfluorobenzyl ester pyrethroid may have been reported by Elliott. He described the synthesis of pentafluorobenzyl chrysanthemate in his patent document.56) Although the results of insecticidal tests of several alcohol esters were described in the patent, no result for a pentafluorobenzyl ester was described in it.56) It is likely that his research focused on the 4-allylbenzyl alcohol ester and that the ester was only used as a standard in the gas chromatography analysis. Sumitomo Chemical reported that pentachlorobenzyl tetramethylcyclopropanecarboxylate exhibits activity comparable to that of existing pyrethroids, but their studies appeared to be focused on the tetramethylcyclopropanecarboxylic acid part.57) Ten years later, Bayer found that permethric acid pentafluorobenzyl ester exhibits superior activity against various insects. trans-Isomers such as fenfluthrin exhibited especially fast action against dipterous insects. On the other hand, cis-isomers, such as NAK1901, were useful for controlling soil insects due to their higher volatility, higher hydrophilicity, and higher stability in soil bacteria relative to other pyrethroids.58) Fenfluthrin and NAK1901 were abandoned in the late 1970s for economic reasons59) and/or due to their high susceptibility to nucleophilic attack at the 4-fluorine in the benzyl group.60) However, ICI invented tefluthrin by replacing the 4-fluorine with a methyl group as a soil insect control agent in combination with the acid part of cyhalothrin.61) On the other hand, Bayer replaced the 4-fluorine in fenfluthrin with a hydrogen atom to develop transfluthrin as a household insecticide.62)
Fig. 10. Polyfluorobenzyl ester pyrethroid.
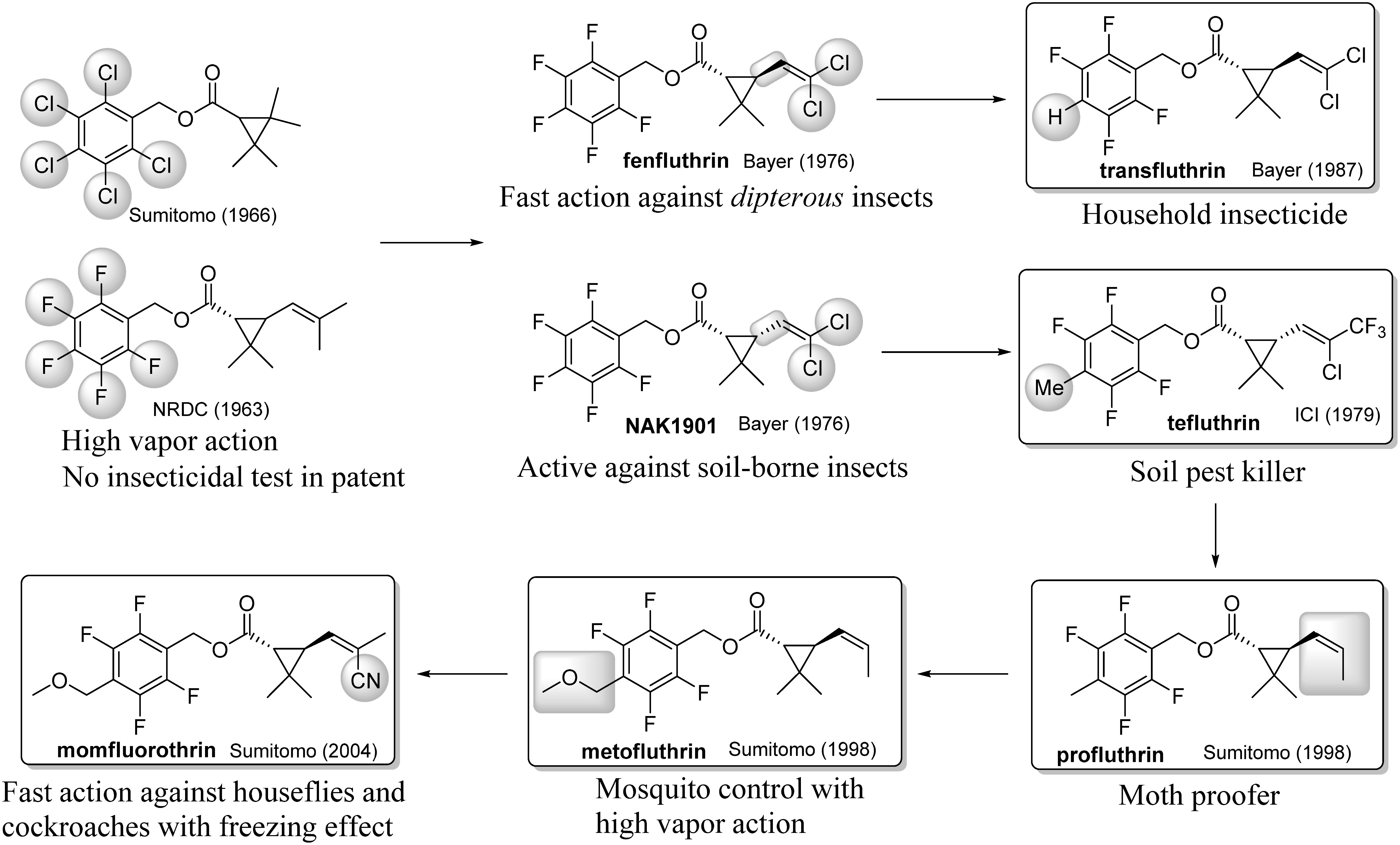
The author’s group removed one methyl group from the side chain, called norchrysantemic acid, as a smaller molecular structure while maintaining insecticidal activity.63) A 4-methoxymethyl derivative, metofluthrin, exhibited extremely high knockdown activity in vapor action against various insects, especially mosquitoes. A 4-methyl derivative, profluthrin, had an insecticidal effect against various fabric insects that was superior to that of traditional moth proofers.64) Momfluorothrin was invented by the introduction of a cyano group into the acid part of the metofluthrin, which exhibited excellent knockdown efficacy against not only houseflies but also German cockroaches. One potential reason for these activities may be that the cyano group forms a hydrogen bond to the channel protein, facilitating noncovalent interactions with the biological target.65)
5. Beyond Cyclopropane and Ester Frameworks
The invention of fenvalerate made a breakthrough in pyrethroid chemistry by escaping 2,2-dimethylcyclopropanecarboxylic acid ester dogma, as mentioned above. Further, drastic structural modifications were inspired by this finding (Fig. 11). The first example was inspired by the similarity of the acid part of fenvalerate to valine. Zoecon replaced the acid part of fenvalerate with N-aryl valine, which is now known as fluvalinate.66) Fluvalinate has very low toxicity to honeybees. Another attempt was made to form a hybrid of DDT and pyrethroid structures. The Commonwealth Scientific and Industrial Research Organisation (CSIRO) had originally discovered DDT analogues such as GH7467) and CP5154368) during a structure–activity relationship study of DDTs intended to overcome their environmental problem. However, these compounds were abandoned because of the 1974 energy crisis.69) Cycloprothrin that is a hybrid compound CP51543 (acid part) and pyrethroid (alcohol part) exhibited high insecticidal activity with low fish and daphnia toxicity.70) The low aquatic organism toxicity is an important property of insecticides for paddy rice fields. In this sense, cycloprothrin was the first pyrethroid suitable for use in paddy rice fields.
Fig. 11. Development of non-dimethylcyclopropanecarboxylic acid esters.
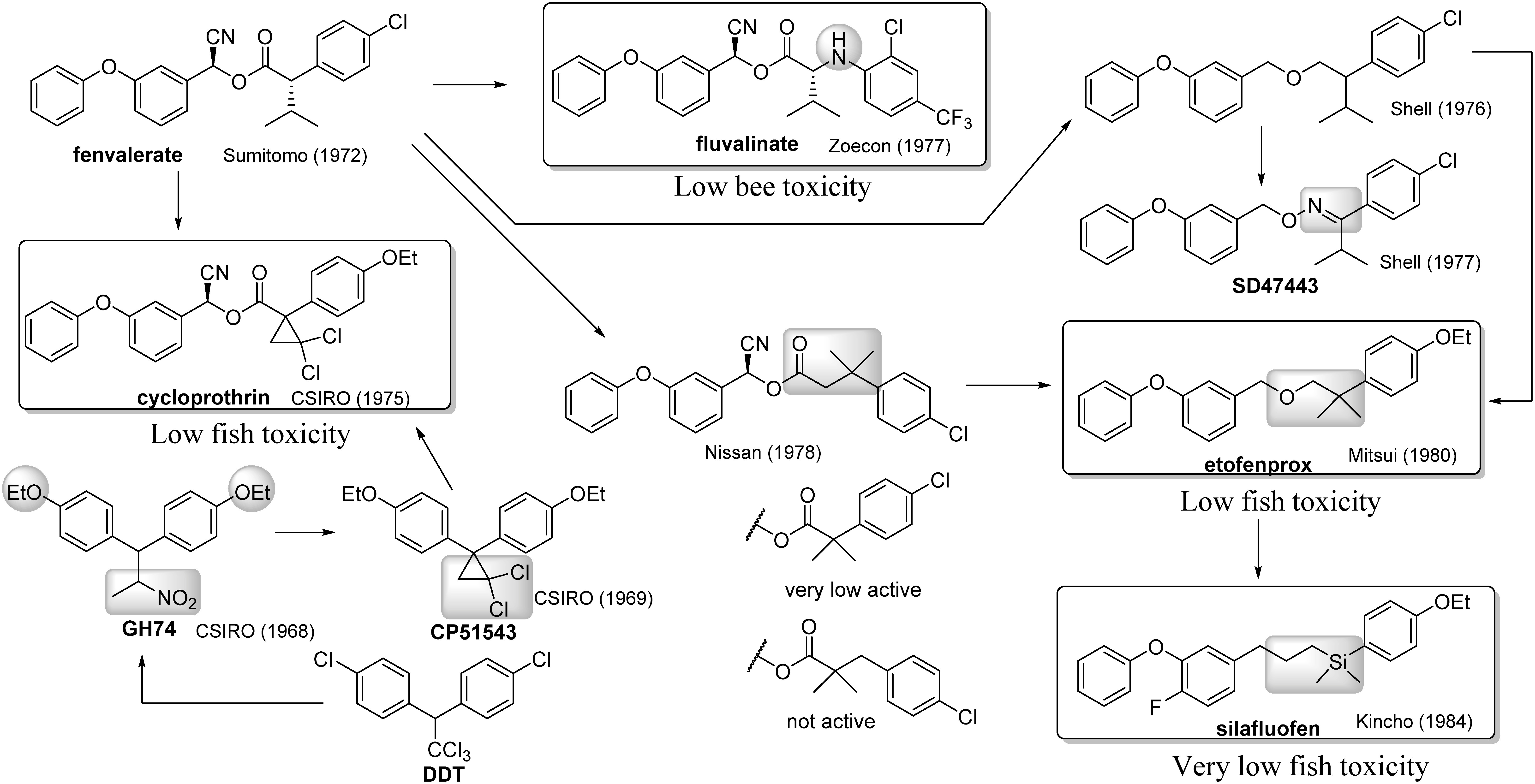
While drastic structural modifications had been attempted to overcome the ester framework, the derivatives elicited only weak activities.71) Shell reported that ether compounds inspired from the fenvalerate structure exhibit some insecticidal activities.72) However, their study was withdrawn because the potencies of their compounds were nearly 1/100 that of fenvalerate.73) The first breakthrough from this approach was SD47443, in which oxime ether was considered as a replacement for the ester framework.74) SD47443 exhibited insecticidal activity comparable to that of typical pyrethroids.75) Meanwhile, Nissan Chemical found β-gem-dimethyl-β-phenylpropionic acid to be applicable as a pyrethroid acid,76) even though similar structures such as α-gem-dimethylphenylacetates and α-gem-dimethyl-β-phenylpropionates were nearly inactive.77) Mitsui Toatsu found that gem-dimethyl derivatives lacking the carbonyl group of the ester linkage (namely, converting the ester linkage to the ether linkage) exhibited excellent insecticidal activity.78) This finding led to the development of etofenprox, which had not only broad-spectrum insecticidal activity but also low fish toxicity.79) Silafluofen, invented by Dainippon Jochugiku, was the first commercialized organosilicon insecticide that was driven by changing three substructures of etofenprox: i) replacing quarterly carbon with a silicon atom, ii) replacing ether oxygen with a methylene group, and iii) introducing a fluorine atom at the 4-position of the benzyl group that was inspired from the cyfluthrin structure. Substitution with a silicon atom slightly reduced its insecticidal potency but was very effective at reducing fish toxicity.80) Silafluofen is the only pyrethroid compound with a rank A classification in the Japanese fish toxicity classification system.
6. Application of Pyrethroids for Specialized Uses
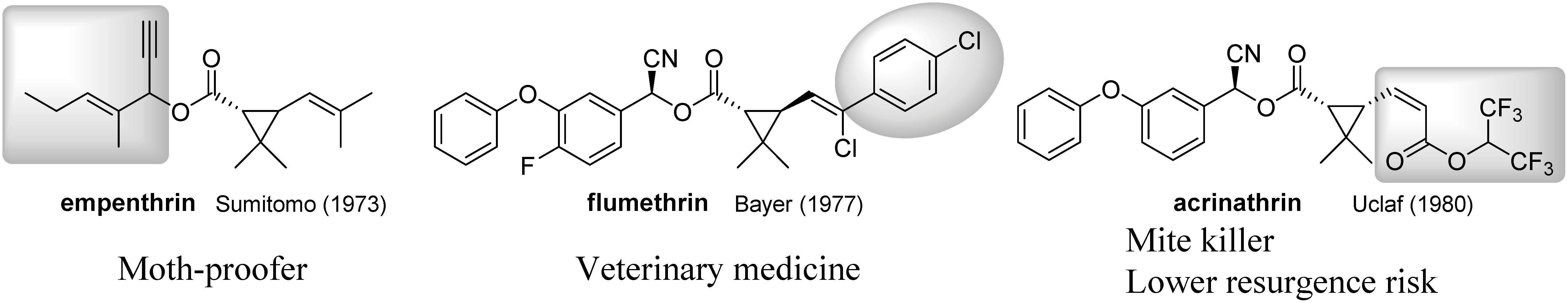
The structural diversity of pyrethroids is very great, and each compound exhibits characteristic biological properties, some of which have been commercialized for specific applications (Fig. 12). Empenthrin, which is currently the most volatile commercialized pyrethroid,81) has been widely used as an active ingredient in moth proofers.82) Empenthrin was inspired by the α-ethynyl moiety of furamethrin obtained in the course of studies on the synthesis of furamethrin, even though α-ethynyl-benzyl esters had already been reported by BASF, as mentioned above.83) Flumethrin is structurally characterized as having the (E)-chlorine in cyfluthrin replaced by a 4-chlorophenyl group.84) Since flumethrin exhibits specific activity against parasitic insects and ticks on various cattle, it is used as active ingredient in veterinary medicine.85) Acrinathrin, in which structurally unique (Z)-acrylic acid exists in the acid side chain, exhibits high miticidal activity.86) Pyrethroids have a broad spectrum of insecticidal activity and sometimes tend to cause resurgence, but this is less likely with acrinathrin because the overall insecticidal activity of acrinathrin is lower than that of typical pyrethroids.87)
Fig. 12. Pyrethroids containing unique structures.
Conclusion
A synthetic pyrethroid, allethrin, was invented by the USDA and commercialized by Sumitomo Chemical in 1953. Since then, a large number of researchers have joined the race to discover superior pyrethroid insecticides. Each structural transformation described in this article is a historically inevitable flow of ideas rather than the inventions of geniuses. Pyrethroids developed mainly for agricultural use are shown in Fig. 13 in order of their year of invention. As seen in this figure, many pyrethroids for agricultural use have entered the market since the commercialization of fenvalerate. All of them were invented between 1971 and 1984. This means that even great and structurally convertible compounds such as pyrethrin I were effective as lead compounds only for around 10 years.88) A similar situation is observed in the case of strobilurin fungicides, which are structurally convertible and numerous commercialized products. I believe that these examples show that finding new lead compounds is always important for chemists. It is highly desirable to discover further structural modifications while maintaining the properties of natural pyrethrins and develop new pyrethroids that can be used in environments where humans and pets live so as to be prepared for new future threats.89) Another review paper entitled “Discovery and development of pyrethroid insecticides” has been prepared by Dr. Matsuo and recently published.90)
Fig. 13. Leading pyrethroids ordered by year of invention (for agricultural use after cyphenothrin).
Acknowledgments
I would like to thank Professor Hideto Miyoshi, Kyoto University, for encouraging me to write this review. I am grateful to the colleagues of Sumitomo Chemical Co. Ltd. for supporting my research on new pyrethroids.
†Four digits enclosed in parentheses indicate the year of the priority date of the patent or the publication date of the journal in which each compound was originally described.
References
- 1).S. Machida: “Kayaributa no Nazo,” Shinchosha, pp. 13–40, Japan, 2001. (in Japanese).
- 2).N. Ueyama: Kagaku to Kogyo 70, 590–592 (2017) (in Japanese). [Google Scholar]
- 3).D. M. Soderlund, J. M. Clark, L. P. Sheets, L. S. Mullin, V. J. Piccirillo, D. Sargent, J. T. Stevens and M. L. Weiner: Toxicology 171, 3–59 (2002). [DOI] [PubMed] [Google Scholar]
- 4).J. Fujitani: Arch. Exp. Pathol. Pharmakol. 61, 41–75 (1909) (in German). [Google Scholar]
- 5).R. Yamamoto: J. Chem. Soc. Jpn. 44, 311–330 (1923) (in Japanese). [Google Scholar]
- 6).H. Stäudinger and L. Ružička: Helv. Chim. Acta 7, 177–448 (1924) (in German). [Google Scholar]
- 7).F. B. LaForge and W. F. Barthel: J. Org. Chem. 10, 114–120 (1945). [Google Scholar]
- 8).Y. Katsuda, H. Chikamoto and Y. Inoue: Bull. Agric. Chem. Soc. Jpn. 22, 427–428 (1958). [Google Scholar]
- 9).M. S. Schechter, N. Green and F. B. LaForge: J. Am. Chem. Soc. 71, 3165–3173 (1949). [Google Scholar]
- 10).M. S. Schechter and F. B. LaForge: U. S. Pat. US 2574500 (1951).
- 11).Chem. Eng. News 28, 941–942 (1950). [Google Scholar]
- 12).H. J. Sanders and A. W. Taff: Ind. Eng. Chem. 46, 414–426 (1954). [Google Scholar]
- 13).W. A. Gersdorff and P. G. Piquett: J. Econ. Entomol. 54, 1250–1252 (1961). [Google Scholar]
- 14).Y. Katsuda, T. Chikamoto, H. Ogami, H. Hirobe and T. Kunishige: Agric. Biol. Chem. 33, 1361–1362 (1969). [Google Scholar]
- 15).S. Mitsuda, T. Umemura and H. Hirohara: Appl. Microbiol. Biotechnol. 29, 310–315 (1988). [Google Scholar]
- 16).T. Matsuo, T. Nishioka, M. Hirano, Y. Suzuki, K. Tsushima, N. Itaya and H. Yoshioka: Pestic. Sci. 11, 202–218 (1980). [Google Scholar]
- 17).H. Stäudinger and L. Ružička: Helv. Chim. Acta 7, 448–458 (1924) (in German). [Google Scholar]
- 18).W. F. Barthel, B. H. Alexander, J. B. Gahan and P. G. Piquett (U.S. Government): U. S. Pat. US 2886485 (1959).
- 19).Benzol Products Co. and Mclaughlin Gormley King Co.: Brit. Pat. Appl. GB 848379 (1960).
- 20).W. F. Barthel and B. H. Alexander: J. Org. Chem. 24, 1012–1014 (1958). [Google Scholar]
- 21).W. A. Gersdorff, S. K. Freeman and P. G. Piquett: J. Agric. Food Chem. 7, 548–550 (1959). [Google Scholar]
- 22).T. Kato, K. Ueda, S. Horie, T. Mizutani K. Fujimoto and Y. Okuno (Sumitomo Chemical Co., Ltd.): Jpn. Tokkyo Kokoku Koho JP 40008535 (1965) (in Japanese).
- 23).T. Kato, K. Ueda, S. Kuramoto, Y. Okuno and K. Fujimoto (Sumitomo Chemical Co., Ltd.): Jpn. Tokkyo Kokoku Koho JP 39018142 (1964) (in Japanese).
- 24).T. Kato, K. Ueda and K. Fujimoto: Agric. Biol. Chem. 28, 914–915 (1964). [Google Scholar]
- 25).M. Hirano, N. Itaya, I. Ohno, Y. Fujita and H. Yoshioka: Pestic. Sci. 10, 291–294 (1979). [Google Scholar]
- 26).W. F. Barthel: Bull. World Health Organ. 44, 349–352 (1970). [Google Scholar]
- 27).Y. Katsuda (Dainippon Jochugiku Co., Ltd.): Brit. Pat. Appl. GB 1133554 (1968).
- 28).Y. Katsuda (Dainippon Jochugiku Co., Ltd.): Brit. Pat. Appl. GB 1215310 (1970).
- 29).J. E. Casida: Environ. Health Perspect. 34, 189–202 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).M. Elliott: Bull. World Health Organ. 44, 315–324 (1970). [PMC free article] [PubMed] [Google Scholar]
- 31).R. Vollrath, A. Nuerrenbach and H. Adolphi (Badische Anilin- & Soda-Fabrik A.-G.): U. S. Pat. US 3673215 (1972).
- 32).T. Fujimoto, Y. Okuno, H. Matsuo, N. Itaya, T. Mizutani and S. Kitamura (Sumitomo Chemical Co., Ltd.): Jpn. Tokkyo Kokoku Koho JP 49027331 (1974) (in Japanese).
- 33).M. Elliott, N. F. Janes, B. P. S. Khambay and D. A. Pulman: Pestic. Sci. 14, 182–190 (1983). [Google Scholar]
- 34).T. Matsuo, N. Itaya, T. Mizutani, N. Ohno, K. Fujimoto, Y. Okuno and H. Yoshioka: Agric. Biol. Chem. 40, 247–249 (1976). [Google Scholar]
- 35).T. Matsuo, N. Itaya, Y. Okuno, T. Mizutani and N. Ohno (Sumitomo Chemical Co., Ltd.): Jpn. Tokkyo Kokoku Koho JP 51005450 (1976) (in Japanese).
- 36).A. Lund and T. Narahashi: Pestic. Biochem. Physiol. 20, 203–216 (1983). [Google Scholar]
- 37).M. Matsui and T. Kitahara: Agric. Biol. Chem. 31, 1143–1150 (1967). [Google Scholar]
- 38).C. R. Harris and H. J. Svec: J. Econ. Entomol. 69, 625–629 (1976). [Google Scholar]
- 39).F. R. Hall: J. Econ. Entomol. 72, 441–446 (1979). [Google Scholar]
- 40).K. Fujimoto, N. Ohno, Y. Okuno, T. Mizutani, N. Ohno, M. Hirano, N. Itaya and T. Matsuo (Sumitomo Chemical Co., Ltd., Japan): Jpn. Pat. Appl. JP 49026425 (1974) (in Japanese).
- 41).J. Farkaš, P. Kouřím and F. Šorm: Chem. Listy 52, 688–694 (1958) (in Czech). [Google Scholar]
- 42).M. Elliott, A. W. Farnham, N. F. Janes, P. H. Needham and D. A. Pulman: Nature 248, 710–711 (1974). [DOI] [PubMed] [Google Scholar]
- 43).M. Elliott, A. W. Farnham, N. F. Janes, P. H. Needham, D. A. Pulman and J. H. Stevenson: Nature 246, 169–170 (1973). [DOI] [PubMed] [Google Scholar]
- 44).M. Elliott, N. F. Janes and D. A. Pulman (National Research Development Corp.): Brit. Pat. Appl. GB 1413491 (1975).
- 45).K. Kondo, K. Matsui, A. Negishi and Y. Takahatake (Sagami Chemical Research Center): Jpn. Pat. Appl. JP 51032543 (1976) (in Japanese).
- 46).Y. Fujita and T. Nishida: J. Oleo Sci. 29, 814–821 (1980) (in Japanese). [Google Scholar]
- 47).P. Maienfisch and R. G. Hall: Chimia (Aarau) 58, 93–99 (2004). [Google Scholar]
- 48).R. A. Fuchs, I. Hammann, W. Behrenz, B. Homeyer and W. Stendel (Bayer A.-G.): Ger. Offen. DE 2709264 (1978) (in German).
- 49).R. A. Fuchs, I. Hammann, W. Behrenz, B. Homeyer and W. Stendel (Bayer A.-G.): Ger. Offen. DE 2615435 (1977) (in German).
- 50).R. K. Huff (Imperial Chemical Industries Ltd., UK): U. S. Pat. US 4183948 (1978).
- 51).P. Piccardi, F. Corda, F. Gozzo, A. Menconi and A. Longoni (Montedison S.p.A.): Brit. Pat. Appl. GB 2015519 (1979).
- 52).J. F. Engel (FMC Corp., USA): Eur. Pat. Appl. EP 3336 (1979).
- 53).R. Lantzsch, H. Hagemann, I. Hammann, W. Behrenz and B. Homeyer (Bayer A.-G.): Ger. Offen. DE 2831193 (1980).
- 54).J. F. Engel (FMC Corp.): U. S. Pat. US 4235927 (1980).
- 55).D. W. Gammon, Z. Liu, A. Chandrasekaran, S. F. El-Naggar, Y. A. Kuryshev and S. Jackson: Pest Manag. Sci. 75, 1190–1197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).M. Elliott (National Research Development Corp.): Brit. Pat. Appl. GB 1078511 (1967).
- 57).M. Matsui, K. Ueda, T. Mizutani, N. Itaya, S. Kitamura, A. Fujinami, K. Fujimoto and Y. Okuno (Sumitomo Chemical Co., Ltd.): Brit. Pat. Appl. GB 1178857 (1970).
- 58).K. Naumann, E. Klauke, A. Marhold and B. Homeyer (Bayer A.-G.): Ger. Offen. DE 2840992 (1980) (in German).
- 59).K. Naumann: Pestic. Sci. 52, 3–20 (1998). [Google Scholar]
- 60).H. Yoshioka: “Rational Approaches to Structure, Activity, and Ecotoxicology of Agrochemicals,” eds. by W. Draber and T. Fujita, CRC Press, United States, pp. 186–213, 1992.
- 61).P. D. Bentley and N. Punja (Imperial Chemical Industries Ltd.): Brit. Pat. Appl. GB 2046732 (1980).
- 62).K. Naumann and W. Behrenz (Bayer A.-G.): U. S. Pat. US 4889872 (1989).
- 63).K. Ujihara, T. Mori, T. Iwasaki, M. Sugano, Y. Shono and N. Matsuo: Biosci. Biotechnol. Biochem. 68, 170–174 (2004). [DOI] [PubMed] [Google Scholar]
- 64).K. Ujihara and T. Iwasaki (Sumitomo Chemical Co., Ltd.): Jpn. Pat. Appl. JP 2000063329 (2000) (in Japanese).
- 65).T. Mori, Y. Tanaka, T. Uekawa, J. Oshita, M. Yamada, Y. Shono and H. Okamoto: Kandokon 28, 87–90 (2017). [Google Scholar]
- 66).C. A. Henrick and B. A. Garcia (Zoecon Corp.): U. S. Pat. US 4243819 (1981).
- 67).G. Holan: Bull. World Health Organ. 44, 353–362 (1971). [PMC free article] [PubMed] [Google Scholar]
- 68).G. Holan: Nature 232, 644–647 (1971). [DOI] [PubMed] [Google Scholar]
- 69).G. Holan (Commonwealth Scientific and Industrial Research Organization): Brit. Pat. Appl. GB 1218495 (1971).
- 70).G. Holan: Nature 221, 1025–1029 (1969). [DOI] [PubMed] [Google Scholar]
- 71).https://csiropedia.csiro.au/cycloprothrin-the-first-designer-insecticide/ (Accessed 12 Jun., 2019)
- 72).G. Holan, D. F. O’Keefe, C. Virgona and R. Walser: Nature 272, 734–736 (1978). [Google Scholar]
- 73).M. Elliott and N. F. Janes: Chem. Soc. Rev. 7, 473–505 (1978). [Google Scholar]
- 74).M. J. Bull and R. J. G. Searle (Shell Internationale Research Maatschappij B. V., Netherland): Brit. Pat. Appl. GB 1570982 (1980).
- 75).M. Elliott, A. W. Farnham, N. F. Janes and B. P. S. Khambay: Pestic. Sci. 23, 215–230 (1988). [Google Scholar]
- 76).A. C. Henry (Shell Oil Co., USA): U. S. Pat. US 4079149 (1978).
- 77).K. Nanjyo, N. Katsuyama, A. Kariya, T. Yamamura, S.-B. Hyeon, A. Suzuki and S. Tamura: Agric. Biol. Chem. 44, 217 (1980). [Google Scholar]
- 78).K. Ozawa, S Ishii, M. Hayashi, M. Hirose and R. Nonaka (Nissan Chemical Industries, Ltd.): Jpn. Pat. Appl. JP 55009044 (1980) (in Japanese).
- 79).A. Baydar, M. Elliott, A. Farnham, N. Janes and B. Khambay: Pestic. Sci. 23, 231–246 (1988). [Google Scholar]
- 80).K. Nakatani, S. Numata, T. Inoue, K. Kodaka, T. Ishii, T. Toyama, H. Tachibana, T. Udagawa and M. Gohbara (Mitsui Toatsu Chemicals, Inc., Japan): Jpn. Pat. Appl. JP 56154427 (1981).
- 81).K. Nakatani, S. Numata, T. Inoue, K. Kokaka, T. Ishii, T. Toyama, H. Tachibana, T. Udagawa and M. Gohbara (Mitsui Toatsu Chemicals, Inc.): U. S. Pat. US 4397864 (1983).
- 82).S. McN. Sieburth, C. J. Manly and D. W. Gammon: Pestic. Sci. 28, 289–307 (1990). [Google Scholar]
- 83).S. Kitamura, N. Itaya, Y. Okuno, N. Ohno, T. Matsuo, M. Hirano, T. Mizutani and H. Takeda (Sumitomo Chemical Co., Ltd.): Brit. Pat. Appl. GB 1424170 (1976).
- 84).S. Aoki, K. Kunita, I. Nitta and A. Nishimura (Earth Chemical Co., Ltd.): Jpn. Kokai Tokkyo Koho JP 5690004 (1981) (in Japanese).
- 85).K. Yoshida, S. Tsuda and Y. Okuno: Seni Gakkaishi 40, T254–T262 (1984). [Google Scholar]
- 86).N. Matuso: Pestic. Sci. 52, 21–28 (1998). [Google Scholar]
- 87).R. Fuchs, I. Hammann, W. Stendel (Bayer A.-G.): U. S. Pat. US 4611009 (1986).
- 88).H. J. Schnitzerling, J. Nolan and S. Hughes: Exp. Appl. Acarol. 6, 47–54 (1989). [DOI] [PubMed] [Google Scholar]
- 89).J. Martel, J. Tessier and A. Teche (Roussel-UCLAF): U. S. Pat. US 4542142 (1985).
- 90).https://cropscience.bayer.jp/ja/home/product_detail/?id=18953&kind=3 (Accessed 12 Jun., 2019) (in Japanese).
- 91).K. Naumann: “Synthetic Pyrethroid Insecticides: Structures and Properties,” eds. by G. Haug and H. Hoffmann, Springer-Verlag, Germany, pp. 183–211, 1990.
- 92).Y. Katsuda: Top. Curr. Chem. 314, 1–30 (2012). [DOI] [PubMed] [Google Scholar]
- 93).N. Matsuo: Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 95, 378–400 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]