Abstract
In the direct-acting antiviral (DAA) era for hepatitis C virus (HCV) infection, sustained virological response (SVR) is very high, but close attention must be paid to the possible occurrence of hepatocellular carcinoma (HCC) and reactivation of hepatitis B virus (HBV) in patients with co-infection who achieved SVR in short term. HCC occurrence was more often observed in patients with previous HCC history. We found occurrence of HCC in 178 (29.6%) of 602 patients with previous HCC history (15.4 months mean follow-up post-DAA initiation) but, in contrast, in only 604 (1.3%) of 45,870 patients without previous HCC history (18.2 months mean follow-up). Thus, in these guidelines, we recommend the following: in patients with previous HCC history, surveillance at 4-month intervals for HCC by ultrasonography (US) and tumor markers should be performed. In patients without previous HCC history, surveillance at 6- to 12-month intervals for HCC including US is recommended until the long-term DAA treatment effects, especially for the resolution of liver fibrosis, are confirmed. This guideline also includes recommendations on how to follow-up patients who have been infected with both HCV and HBV. When HCV was eradicated in these HBsAg-positive patients or patients with previous HBV infection (anti-HBc and/or anti-HBs-positive), it was shown that HBV reactivation or HBV DNA reappearance was observed in 67 (41.4%) of 162 or 12 (0.9%) of 1317, respectively. For these co-infected patients, careful attention should be paid to HBV reactivation for 24 weeks post-treatment.
Electronic supplementary material
The online version of this article (10.1007/s12072-019-09988-7) contains supplementary material, which is available to authorized users.
Keywords: HCV, HCC, DAA, SVR, Follow-up, Guideline, HBV
Introduction
Hepatocellular carcinoma (HCC) due to hepatitis C virus (HCV) infection is one of the major causes of liver-related death [1, 2]. Eradication of HCV could reduce the occurrence of HCC, as demonstrated by the long-term follow-up of patients who achieved sustained virological response (SVR) in the interferon era [3–5]. Thus, SVR could be the goal of antiviral therapy for HCV.
In the interferon era, as the duration of interferon-based therapy was longer than that of DAA therapy, the occurrence of HCC has occasionally been observed during the treatment. But these cases were omitted from studies or ignored by regarding them as pre-existing, and they were therefore unrelated to the interferon treatment [5, 6].
Now, in the age of DAAs, extremely high SVR rates, sometimes even 100%, have been reported [7–17]. However, there have been several reports on the unexpectedly high rate of early HCC occurrence despite the virus eradication [18–35]. In real-life settings, if these are actual cases, this incidence could be a shocking event to patients as well to attending physicians. This prompted us to collect data and provide a compact APASL practice guidelines.
In addition, in Asian countries, co-infections of HBV and HCV are more frequently observed. We are likely the first to elucidate the effects of DAA on the replication of HBV by such a high SVR for HCV. Therefore, we have also proposed a compact recommendation on how to follow co-infected patients.
Part I
Risk factors for the occurrence of HCC
These days, most patients seen at outpatient clinics are those whose HCV has been eradicated by the use of DAAs. Although more recent clinical studies and real-world studies have reported that DAA therapy decreased the risk of both de novo HCC and recurrent HCC in both cirrhotic and non-cirrhotic patients with HCV infection, several preliminary studies have dealt with the risk factors for the occurrence of HCC [29, 32, 34, 35]. These studies revealed that male gender, older age, alcohol abuse, diabetes mellitus, and the existence of cirrhosis are associated with the occurrence of HCC (Table 1) [29, 32, 34, 35]. Most of these studies were conducted 1–2 years after DAA treatment [11, 33]. Similar factors were also shown to be associated with increased HCC risk during the interferon era [36, 37].
Table 1.
Risk factors and odds ratio for HCC in direct-acting antiviral (DAA) combination-treated patients [29, 32, 34, 35]
| Risk factors for HCC | Odds ratio (95% CI), n, p value [Refs.] |
|---|---|
| Cirrhosis | 4.73 (3.34–6.68), total n = 19,581, HCC (n, cirrhosis, yes/no = 139/44), < 0.0001 [29] |
| Previous HCC history | 2.64 (0.90–7.74), total n = 864, HCC (n, previous HCC history, yes/no = 24/17), 0.075 [32] |
| Male gender |
2.63 (0.65–10), total n = 19,581, HCC (n, male, yes/no = 181/2), 0.17 [29] 2.09 (0.73–5.98), total n = 864, HCC (n, male, yes/no = 26/15), 0.167 [32] 1.49 (0.91–2.44), total n = 2249, HCC (n, male, yes/no = 55/23), 0.11 [35] |
| Alcohol abuse | 1.56 (1.11–2.18), total n = 19,581, HCC (n, alcohol, yes/no = 124/59), 0.01 [29] |
| Older age | 1.30 (0.96–1.76), total n = 19,581, HCC (n, >=65, yes/no = 71/112), 0.08 [29] |
| Diabetes mellitus | 1.28 (0.92–1.78), total n = 19,581, HCC (n, diabetes, yes/no = 96/87), 0.13 [29] |
| Drug use | 1.27 (0.91–1.75), total n = 19,581, HCC (n, drug, yes/no = 91/92), 0.15 [29] |
| Bilirubin | 1.25 (0.97–1.62), total n = 2249, HCC (n = 78), 0.08 [35] |
| Low albumin | 1.92 (1.16–3.22), total n = 2249, HCC (n = 78), 0.010 [35] |
| EOT-AFP (=>9 ng/mL) | 1.19 (1.07–1.34), total n = 1523, HCC (n = 20), 0.0027 [34] |
| Low platelet count | 1.01 (1.01–1.02), total n = 2249, HCC (n = 78), 0.011 [35] |
AFP α-fetoprotein, EOT end of treatment, n number
However, in addition to the abovementioned parameters, there is some dispute regarding the difference in occurrence of HCC between patients with and without previous HCC history [19–21, 38]. Thus, we conducted a literature search, investigating the occurrence of HCC in DAA-treated patients with and without previous HCC history (Table 2) [18–35]. A summary of the collected data is described in the following sections.
Table 2.
Occurrence of hepatocellular carcinoma (HCC) in patients with direct-acting antiviral (DAA) treatment and sustained virological response (SVR) [18–35]
| Authors (year) [references] | Total SVR patients (n) | Observation periods (mean months post-DAA initiation) | Patients with HCC occurrence [n (%)] | Annual incidence of HCC (%/year) |
|---|---|---|---|---|
| Minami et al. (2016) [18] | 22 | 5.8 | 4 (18) | 37.2 |
| Reig et al. (2016) [19] | 58 | 5.7 | 16 (27.6) | 58.1 |
| Torres et al. (2016) [20] | 84 | 12 | 0 (0) | 0 |
| Conti et al. (2016) [21] | 403 | 9 | 26 (6.5) | 8.7 |
| Kolly et al. (2017) [22] | 47 | 12 | 27 (57.4) | 57.4 |
| Cardoso et al. (2017) [23] | 54 | 18 | 4 (7.4) | 4.9 |
| Calleja et al. (2017) [24] | 70 | 12 | 21 (30) | 30 |
| Ikeda et al. (2017) [25] | 155 | 12 | 47 (30.2) | 30.2 |
| Mettke et al. (2017) [26] | 158 | 17.5 | 6 (3.8) | 2.61 |
| Nakao et al. (2017) [27] | 242 | 6 | 6 (2.5) | 5.0 |
| Nagata et al. (2017) [28] | 729 | 24.6 | 29 (4.0) | 1.95 |
| Kanwal et al. (2017) [29] | 19,518 | 15.8 | 183 (0.9) | 0.68 |
| Ioannou et al. (2017) [30] | 19,909 | 18 | 280 (1.4) | 0.93 |
| Cabibbo et al. (2018) [31] | 143 | 12 | 24 (16.8) | 16.8 |
| Ooka et al. (2018) [32] | 864 | 15 | 41 (4.7) | 3.76 |
| Reddy et al. (2018) [33] | 893 | 36 | 16 (1.8) | 0.60 |
| Ogawa et al. (2018) [34] | 1675 | 17 | 46 (2.7) | 1.91 |
| Calvaruso et al. (2018) [35] | 2140 | 14 | 64 (3.0) | 2.57 |
| Total | 47,164 | 14.6 (5.7–36) | 840 (1.8) | 14.6 (0–58.1) |
n number
Occurrence of HCC in patients without previous HCC history
The occurrence of HCC after SVR in patients without previous HCC history was reported in ten studies (Table 3). In those 10 studies, the total number of SVR patients ranged from 54 to 19,909 patients (mean: 4587 patients). The mean follow-up period of those studies was 18.2 months (range 9–36 months) post-DAA initiation. The overall occurrence rate of HCC after SVR in 45,870 patients without previous HCC history was 604 (1.3%) (range 0.9–7.4%) (Table 3) [21, 23, 26, 28–30, 32–35]. Thus, the annual occurrence rate of SVR patients by DAA without previous HCC history is no different from that of the interferon era [3–5, 39–41].
Table 3.
Occurrence of hepatocellular carcinoma (HCC) after direct-acting antiviral (DAA) treatment and sustained virological response (SVR) in patients without previous HCC history [21, 23, 26, 28–30, 32–35]
| Authors (year) [references] | Total SVR patients (n) | Observation periods (months post-DAA initiation) | Patients with HCC occurrence [n (%)] | Annual incidence of HCC (%/year) |
|---|---|---|---|---|
| Conti et al. (2016) [21] | 254 | 9 | 7 (2.7) | 3.60 |
| Cardoso et al. (2017) [23] | 54 | 18 | 4 (7.4) | 4.93 |
| Mettke et al. (2017) [26] | 158 | 17.5 | 6 (3.8) | 2.61 |
| Nagata et al. (2017) [28] | 652 | 21.6 | 7 (1.1) | 0.61 |
| Kanwal et al. (2017) [29] | 19,518 | 15.8 | 183 (0.9) | 0.68 |
| Ioannou et al. (2017) [30] | 19,909 | 18 | 280 (1.4) | 0.93 |
| Ooka et al. (2018) [32] | 769 | 15 | 17 (2.2) | 1.76 |
| Reddy et al. (2018) [33] | 893 | 36 | 16 (1.8) | 0.60 |
| Ogawa et al. (2018) [34] | 1523 | 17 | 20 (1.3) | 0.92 |
| Calvaruso et al. (2018) [35] | 2140 | 14 | 64 (3.0) | 2.57 |
| Total | 45,870 | 18.2 (9–36) | 604 (1.3) | 1.92 (0.60–4.93) |
n number
Therefore, the same guidelines and recommendations as were present in the time of interferon may apply to patients treated by DAAs, if there is no previous experience of HCC. Of course, regular follow-ups are necessary, according to the routinely set rules of the interferon era, especially among HCV patients with advanced liver fibrosis or cirrhosis (Table 3) [42, 43].
Occurrence of HCC in patients with previous HCC history
The occurrence of HCC after DAA treatment and SVR in patients with previous HCC history was reported in six studies (Table 4) [21, 24, 25, 28, 32, 34]. The total number of SVR patients ranged from 53 to 155 patients (mean: 100 patients). The mean follow-up period of these studies was 15.4 months (range 9–28 months) post-DAA initiation. The overall occurrence rate of HCC after DAA treatment and SVR in patients with previous HCC history was 29.6% (178/602) (range 17.1–71.6%) (Table 4) [21, 24, 25, 28, 32, 34].
Table 4.
Occurrence of hepatocellular carcinoma (HCC) after direct-acting antiviral (DAA) treatment and sustained virological response (SVR) in patients with previous HCC history [21, 24, 25, 28, 32, 34]
| Authors (year) [references] | Total SVR patients (n) | Observation periods (months post-DAA initiation) | Patients with HCC occurrence [n (%)] | Annual incidence of HCC (%/year) |
|---|---|---|---|---|
| Conti et al. (2016) [21] | 53 | 9 | 38 (71.6) | 95.5 |
| Calleja et al. (2017) [24] | 70 | 12 | 21 (30) | 30.0 |
| Nagata et al. (2017) [28] | 77 | 27.6 | 22 (28.6) | 12.4 |
| Ikeda et al. (2017) [25] | 155 | 12 | 47 (30.3) | 30.3 |
| Ooka et al. (2018) [32] | 95 | 15 | 24 (25.3) | 20.2 |
| Ogawa et al. (2018) [34] | 152 | 17 | 26 (17.1) | 12.1 |
| Total | 602 | 15.4 (9–27.6) | 178 (29.6) | 33.4 (12.1–95.5) |
n number
The very high incidence of HCC occurrence during and right after DAA treatment suggests that very careful attention should be paid to the possible occurrence of HCC in patients with previous HCC history.
Discussion
Risk of HCC occurrence among patients post-DAA treatment
In the interferon era, male gender, older age, and the existence of cirrhosis and other factors were shown to be associated with risk factors of HCC occurrence [42, 44, 45]. Also in the age of DAAs, similar factors are shown to be associated with this risk. In other words, the existence of cirrhosis, no SVR, male gender, alcohol abuse, older age, and diabetes mellitus are risk factors for HCC occurrence (Table 1) [29, 32, 34, 35]. Surveillance is recommended for SVR patients with any histologic stage of HCV with comorbidities, such as alcohol abuse and diabetes mellitus [1].
Of note, most importantly, the current survey revealed that the existence of previous HCC history is an independent, very high-risk factor for HCC occurrence post-DAA treatment.
In the interferon era, because the treatment duration was longer than that of DAA, several studies seemed to exclude HCC occurrence during and right after interferon treatment when analyzing their data. In fact, during the interferon era, many patients with HCC or cirrhosis could not receive interferon treatment. To some extent, this might explain the lower occurrence of HCC during or right after antiviral treatment.
It has been reported that several mechanisms may exist during and after DAA treatment, such as rapid immunological changes, that could lead to HCC occurrence [46–50]. Changes in cytokines and chemokines have been observed in HCC occurrence post-DAA treatment and it is possible that they may have affected tumor immunity [46–50].
DAA treatment increased the serum vascular endothelial growth factor (VEGF) level which is significantly related to the serum angiopoietin-2 level. These are risk factors for HCC occurrence post-DAA treatment [51, 52]. Rapid immunological changes, including in NKG2D systems, are also observed during and after DAA treatment [53, 54].
With such drastic “environmental changes” occurring in the liver due to the very powerful DAAs, pre-existing “occult neoplastic” or “dysplastic” cells may develop into classical tumors in a short time period. Ooka et al. reported that “dysplastic” nodules detected by ultrasonography (US) might turn into hyper-vascular “classical” HCC by rapid decrease of the immune surveillance system with rapid elimination of HCV [32, 46, 55]. Studies have proposed that the presence of “dysplastic” nodules by US has a much higher odds ratio (26 times) than previous HCC history [32, 56].
In fact, approximately 50% of HCC occurrence and recurrence cases are observed during and 1–2 years after DAA treatment [21, 33]. Although it is well known that patients with mild/no fibrosis and SVR have a lower risk of developing HCC, population-based studies were different from clinical practice guidelines. So, we recommend that, for patients with SVR and risk factors of HCC, surveillance for HCC should be conducted at shorter intervals, and especially within 2 years post-DAA treatment.
How to follow these patients? Among imaging modalities (US, CT, and MRI), US might be the most cost-effective and easily available modality.
The prognosis of HCC depends on earlier-stage detection and earlier treatment [1]. In addition to US, measurement of tumor markers may play a more important role in HCC screening. Among tumor markers for the diagnosis of HCC, measurement of AFP has been performed for decades [57, 58], although with some dispute regarding its significance. However, there have been numerous studies regarding multiple tests including lens culinaris agglutinin (LCA)-reactive AFP isoform (AFP-L3), which can differentiate an increase in AFP due to HCC from that in patients with benign liver disease. In addition, des-γ-carboxy prothrombin (DCP) is a very powerful measure for detecting early and small tumors [59–65]. These studies are mostly from Japan, and these two tests, AFP-L3 and DCP, could not be validated as they have not been available in many countries. However, AFP, AFP-L3, and DCP tests have now become increasingly available in many Asian countries. Thus, we recommended the measurements of these markers.
Once a blood test result is abnormal, further imaging modalities [gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI and/or dynamic CT] should be performed for the potential diagnosis of HCC occurrence [1, 32].
Thus, in patients with previous HCC history, surveillance of shorter 4-month intervals for HCC, including US with AFP, AFP-L3, and/or DCP, should be performed [1].
After successful eradication of HCV, regular follow-up of HCC, esophageal varices, and other complications of advanced liver fibrosis will be necessary if they existed at pre-treatment [66–68].
#1 Consensus statements and recommendations on follow-up of DAA-treated virus-eradicated HCV-infected patients
-
In patients without advanced liver fibrosis, or cirrhosis and without previous HCC history
- Before, during, and approximately 2 years after the end of treatment (EOT) with DAA, surveillance at 6-month intervals for HCC, including ultrasonography (US) with or without tumor markers, should be performed (C-2).
- After 2 years, surveillance at 12-month intervals for HCC, including US with or without AFP, could be performed (C-2).
-
In patients with advanced liver fibrosis or cirrhosis and without previous HCC history
Surveillance at 6-month intervals for HCC, including by US with AFP, lens culinaris agglutinin (LCA)-reactive AFP isoform (AFP-L3) and/or des-γ-carboxy prothrombin (DCP) should be performed (A-1).
-
In patients with previous HCC history
Surveillance at 4-month intervals for HCC, including by US with AFP, AFP-L3 and/or DCP, should be performed. In these cases, contrast-enhanced US (CEUS), dynamic CT, dynamic MRI or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI could be added (A-2).
SVR patients with alcohol abuse and/or diabetes mellitus should undergo surveillance for HCC regularly (A-1).
In patients with advanced liver fibrosis or cirrhosis, screening for esophageal and gastric varices by endoscopy should be performed especially if present at pre-treatment (A-1).
Grading of evidence and recommendations are shown in Supplementary Table 1.
Part II
HBV reactivation in patients with HCV and HBV co-infection
HBV infection is one of the major health problems in the world, with the highest rates being in Africa and the Asia–Pacific region [69]. Evaluation for HBV infection was also recommended for all persons with active HCV infection by the US Food and Drug Administration in 2004. However, the exact prevalence and characteristics of HBV DNA reappearance and clinical “reactivation” among patients treated by DAAs are not known in detail.
Therefore, we collected data from 14 studies on HBV DNA reappearance and clinical reactivation in HBV and HCV co-infected patients treated by DAAs (Table 5) [70–78].
Table 5.
Hepatitis B virus (HBV) reactivation or HBV DNA reappearance in patients with HBV and hepatitis C virus (HCV) co-infection after direct-acting antiviral (DAA) treatment [70–78]
| Authors (year) [references] | Total patients (n) | Observation periods (months post-EOT) | Patients with increases of HBV DNA greater than 1 log10 IU/mL or HBV DNA reappearance [n (%)] | Monthly incidence of HBV reactivation or HBV DNA reappearance (%/month) |
|---|---|---|---|---|
| HBsAg-positive patients | ||||
| Gane et al. (2016) [70] | 8 | 3 | 7 (87.5) | 29.2 |
| Doi et al. (2017) [71] | 4 | 3 | 2 (50) | 16.7 |
| Kawagishi et al. (2017) [72] | 4 | 3 | 2 (50) | 16.7 |
| Yeh et al. (2017) [73] | 7 | 3 | 7 (100)e | 33.3 |
| Mucke et al. (2017) [74] | 8 | 3 | 4 (50)b | 16.7 |
| Wang et al. (2017) [75] | 10 | 3 | 3 (33.3)d | 11.1 |
| Tamori et al. (2018) [76] | 12 | 3 | 3 (25)c | 8.3 |
| Liu et al. (2018) [77] | 109 | 3 | 39 (35.8)a | 13 |
| Total | 162 | 3 | 67 (41.4) | 18.1 (8.33–33.3) |
| HBsAg-negative patients positive for anti-HBc antibody and/or anti-HBs antibody | ||||
| Yeh et al. (2017) [73] | 57 | 3 | 0 (0) | 0 |
| Wang et al. (2017) [75] | 124 | 3 | 0 (0) | 0 |
| Doi et al. (2017) [71] | 155 | 3 | 3 (1.9) | 0.63 |
| Kawagishi et al. (2017) [72] | 153 | 3 | 4 (2.6) | 0.87 |
| Ogawa et al. (2018) [78] | 63 | 3 | 4 (6.3) | 2.1 |
| Tamori et al. (2018) [76] | 765 | 3 | 1 (0.1) | 0.33 |
| Total | 1317 | 3 | 12 (0.91) | 0.61 (0–2.1) |
HBsAg hepatitis B surface antigen, anti-HBc anti-hepatitis B core antibody, anti-HBs anti-hepatitis B surface antibody, EOT end of treatment, n number
aThree patients (one with cirrhosis and two without cirrhosis) began anti-HBV treatment: one entecavir (ETV) and two tenofovir disoproxil fumarate (TDF)
bThree patients (one with cirrhosis and two without cirrhosis) began TDF
cOne cirrhotic patient began TDF
dTwo patients, one had hepatic failure and one had icteric hepatitis
eOne icteric patient began ETV
HBsAg-positive group
Of these 14 studies, 8 reported the results of HBV DNA reappearance and clinical reactivation in HBsAg-positive patients treated by DAAs (Table 5) [70–77]. The number of patients enrolled in those 8 studies ranged from 4 to 109 patients (mean 20) and the mean observation period was 3 months post-EOT. The overall occurrence rate of HBV DNA reappearance and clinical reactivation among 162 patients treated by DAA was 41.4% (67/162) (range 25–100%). Thus, among HBsAg-positive patients, HBV DNA reappearance and reactivation are the frequent events through at least 12 weeks after EOT (Table 5).
HBsAg-negative group (anti-HBc- and/or anti-HBs-positive group)
Of the 14 studies (Table 5), 6 studies reported results on HBV DNA reappearance and clinical reactivation in HBsAg-negative, but positivity for anti-hepatitis B core (anti-HBc) antibody and/or anti-hepatitis B surface (anti-HBs) antibody at baseline [71–73, 75, 76, 78].
The number of patients enrolled in those 6 studies ranged from 57 to 765 patients (mean 219.5) and the mean observation period was 3 months post-EOT. The overall occurrence rate of HBV DNA reappearance and clinical reactivation among 1317 patients treated by DAAs was 0.91% (12/1317) (range 0–6.3%) (Table 5). Thus, in HBsAg-negative patients but positive for anti-HBc antibody and/or anti-HBs antibody at baseline; HBV reactivation and/or HBV DNA reappearance are rare events through 12 weeks after EOT (Table 5).
Discussion
Clinical pictures of HBV reactivation
HBsAg-positive group
Before the rituximab (humanized anti-CD20 monoclonal antibody) era, Lau et al. reported that among 15 HBsAg-positive patients with lymphoma treated with chemotherapy but deferred prophylactic lamivudine therapy, 8 (53%) had HBV reactivation defined as an increase of serum HBV DNA to more than 10 times of baseline [79]. Of these eight patients, seven (87.5%) had ‘hepatitis’, defined as a more than threefold increase of serum ALT on two consecutive determinations at least 5 days apart. Of these seven patients, anicteric and icteric hepatitis and hepatic failure were 5, 1, and 1, respectively [79]. Thus, of HBsAg-positive patients with lymphoma treated by chemotherapy without nucleos(t)ide analogs, 6.7% (1/15) had hepatic failure [79]. They also observed that, among 15 patients with lymphoma who received lamivudine 1 week before chemotherapy, none had HBV reactivation after chemotherapy [79]. Lok et al. also observed 18 HBV reactivations (67%) [6 icteric hepatitis (22%); 1 non-fatal hepatic failure (3.7%); and 1 death (3.7%)] among 27 Chinese patients who underwent induction cytotoxic therapy without prophylaxis for HBsAg-positive malignant lymphoma [80].
After rituximab was introduced as a potent drug for patients with malignant lymphoma, reactivation of HBV has been repeatedly shown in HBsAg-positive patients [81]. Wang et al. reported that rituximab/chemotherapy induced hepatic dysfunction in 13 (33%) of 40 HBsAg-positive patients with diffuse large B cell lymphoma [82].
HBV reactivation has also been reported in HBsAg-positive solid cancer patients who underwent chemotherapy or other molecular target therapies [69]. Among HBsAg-positive breast cancer patients receiving chemotherapy, the rates of HBV reactivation in patients without or with prophylactic lamivudine were 28.6% and 0%, respectively [83]. HBV reactivation during chemotherapy occurred independently of lymphoma (odds ratio: 5.0), breast cancer (odds ratio: 4.2), steroid use (odds ratio: 2.7), and HBV DNA positive at baseline (odds ratio: 8.4) [84].
Thus, APASL HBV guidelines have recommended that prophylactic nucleos(t)ide therapy should be given to HBsAg-positive cancer patients who receive cytotoxic and immunosuppressive therapy, regardless of HBV DNA levels for 12 months after cessation [69].
Regarding the treatment by DAAs for those co-infected with HBV and HCV, a variety of events, ranging from asymptomatic HBV reactivation/HBV DNA reappearance to clinically symptomatic reactivation characterized by elevation in HBV DNA and ALT were reported [70–77].
Collected results of eight studies of HBsAg-positive and co-infected patients treated with DAAs for HCV infection indicated that the rates of HBV reactivation were similar to HBsAg-positive patients with malignant lymphoma and cancer patients treated with cytotoxic drugs (~ 40%) [85, 86] (also see Table 5).
Regarding the severity of liver disease induced by this HBV reactivation in HBsAg-positive patients treated with DAAs, only limited data are available [75, 77, 87–89]. Bersoff-Matcha et al. reported that two cases with liver failure resulted in one death and one case of liver transplantation [87]. Wang et al. also reported one HBsAg-positive patient with liver failure due to HBV reactivation although most reported cases were asymptomatic increases of HBV DNA and/or ALT in the absence of concomitant liver injury [75].
Of note, Liu et al. reported that two patients had concomitant elevation of HBV DNA level with ALT elevation > 2 times the upper limit of normal at post-treatment week 48, of whom one commenced treatment with entecavir at post-treatment week 53 following the onset of malaise, anorexia, and nausea associated with sclera jaundice [77]. Holmes et al. reviewed two HBsAg-positive, co-infected patients who were treated by DAAs for HCV infection, and had fulminant hepatic failure and death: one was a 57-year-old female who was treated with daclatasvir plus asunaprevir, had HBV reactivation at week 8 from the start of DAAs, and she was treated with entecavir; the other was a 73-year-old female who was treated with daclatasvir plus asunaprevir, had HBV reactivation at week 7 from the start of DAAs, and she had stopped entecavir prior to the commencement of DAA therapy [88].
At present, we do not know the risk factors of HBV reactivation and associated liver failure, although several factors such as HBsAg levels and HBV DNA levels have been reported [76, 90].
For the safety of HBsAg-positive patients treated by DAAs, we recommend that prophylactic nucleos(t)ide therapy should be given before starting DAA therapy; nonetheless, further studies may also be needed to determine the duration of prophylactic nucleos(t)ide therapy.
HBsAg-negative, but positive for anti-HBc and/or anti-HBs group
Although the elimination of HBsAg is one of the goals in the treatment of HBV infection, HBV DNA reappeared in 15–33% patients after HBsAg seroclearance in the natural history of HBV infection and in post-anti-HBV treatment [91]. So, it is possible that HBV reactivation and/or HBV DNA reappearance may occur in patients of this group treated by DAAs, as well as patients who receive immunosuppressants or anti-cancer drugs (Table 6).
Table 6.
HBV reactivation in HBsAg-negative patients treated for lymphoma and solid tumors
| Types | Prophylactic nucleos(t)ide analogs | Total patients (n) | Incidence [n, (%)] | Authors (year) [references] |
|---|---|---|---|---|
| Lymphoma (without rituximab-based regimens) | NA | 72 | 10 (14%) | Lok et al. (1991) [80] |
| Lymphoma (with rituximab-based regimens) | NA | 39 | 7 (17.9%) | Huang et al. (2013) [93] |
| Hematologic malignancy (with rituximab-based regimens) | NA | 28 | 3 (10.7%) | Buti et al. (2014) [94] |
| Lymphoma (with rituximab-based regimens) | NA | 578 | 36 (6.3%) | Mozessohn et al. (2015) [92] |
| Solid cancer | NA | 27 | 2 (7.4%) | Hagiwara et al. (2012) [98]. |
| Solid cancer | NA | 321 | 1 (0.3%) | Kim et al. (2014) [97] |
NA not applicable
With the administration of rituximab without antiviral treatment, clinical HBV reactivation was estimated at 6.3% in HBsAg-negative/anti-HBc-positive patients with lymphoma [92]. Prior to use of rituximab, Lok et al. also observed 10 HBV reactivations (14%) [1 icteric hepatitis (2%); 1 non-fatal hepatic failure (2%); and no death (0%)] of 72 HBsAg-negative patients with malignant lymphoma treated by chemotherapy without prophylactic treatment of nucleos(t)ide analogs (Table 6) [80].
Thus, in the rituximab era, once HBsAg-negative patients who received rituximab including chemotherapy for malignant lymphoma had HBV reactivation (6.3–17.9%) (Table 6) [92–94], higher mortality rates (12.5–50%) were seen [95].
In breast cancer, HBV fetal reactivation was occasionally observed in HBsAg-negative patients who underwent chemotherapy [96]. Kim et al. reported that HBV reactivation occurred in 1 (0.3%) of 321 HBsAg-negative and anti-HBc-positive patients with solid cancers during anti-cancer chemotherapy [97]. Hagiwara et al. reported that HBV reactivation occurred in 2 (7.4%) of 27 HBsAg-negative and anti-HBc/anti-HBs-positive patients with solid cancers during anti-cancer chemotherapy [98].
Jun et al. reported that 2 (10%), 8 (5.3%), 4 (5.5%), and 2 (0.9%) HBV reactivations were observed in 20 HBsAg(−)/anti-HBc(+)/anti-HBs(−), 151 HBsAg(−)/anti-HBc(+)/anti-HBs(+), 73 HBsAg(−)/anti-HBc(−)/anti-HBs(−), and 227 HBsAg(−)/anti-HBc(−)/anti-HBs(+) patients undergoing hematopoietic stem cell transplantation, respectively [99]. Of note, the incidence of HBV reactivation in these HBsAg-negative patients was not low (5.9%) [99, 100], although most patients with solid cancers remained unscreened for HBV-resolved infection [101, 102].
A summary of six studies of HBsAg-negative cases indicates that the overall occurrence rate of HBV reactivation and/or HBV DNA reappearance is lower (0.91%) (Table 5).
The prevalence rates of HBV reactivation and/or HBV DNA reappearance in patients of the HBsAg-negative/anti-HBc-positive group by DAAs seem equal to or less than those with chemotherapy for breast cancer, one of the non-hematologic malignancies.
Regarding the severity of liver disease induced by this HBV reactivation in HBsAg-negative patients treated by DAA, only limited data are available [103, 104]. Two HBsAg-negative patients who developed hepatic failure after DAA treatment have been reported (Table 7) [103, 104]. We do not know the exact risk factors of HBV reactivation in HBsAg-negative patients treated with DAAs although several factors have been reported [71–73, 75, 76, 78].
Table 7.
Cases with HBV reactivation-related hepatic failure among co-infected patients treated by DAAs
| # | Age (years)/gender | Treatment for HCV (GT) | Severity, ALT levels | Treatment for HBV (GT/HBeAg)/outcome | Authors (year) [references] |
|---|---|---|---|---|---|
| HBsAg-positive patients treated by DAAs | |||||
| 1 | 57/Female | Daclatasvir/Asunaprevir (unknown) | Hepatic failure, ALT 2114 IU/L | Entecavir (unknown/unknown)/death | Holmes et al. (2017) [88] |
| 2 | 73/Female | Daclatasvir/Asunaprevir (unknown) | Hepatic failure, ALT 462 IU/L | Entecavir (unknown/unknown)/death | Holmes et al. (2017) [88] |
| 3 | 53/Female | Sofosbuvir/Ribavirin (GT1) | ALT 1417 IU/L | No description (unknown/HBeAg-)/no description | Holmes et al. (2017) [88] |
| 4 | 53/Male | Ledipasvir/Sofosbuvir (GT1) [co-infection with HIV] | ALT 1026 IU/L | Tenofovir (GTD/HBeAg-)/alive | De Monte et al. (2016) [89] |
| HBsAg-negative patients treated by DAAs | |||||
| 5 | 59/Female | Sofosbuvir/Simeprevir (GT1b) | Hepatic failure, ALT 2263 IU/L | Tenofovir (unknown/unknown)/liver transplantation | Ende et al. (2015) [103] |
| 6 | 83/Female | Daclatasvir/Asunaprevir (GT1b) | Hepatic failure, ALT 1066 IU/L | Entecavir (GTB1/unknown)/death | Hayashi et al. (2016) [104] |
GT genotype, ALT alanine aminotransferase, HBeAg hepatitis B e antigen
There are no standard management regimens for HBV reactivation among HBsAg-negative patients, even for those treated with rituximab including chemotherapy. It has been reported that monthly monitoring of HBV DNA is useful for preventing HBV reactivation-related hepatitis among B cell non-Hodgkin lymphoma patients with resolved HBV infection following rituximab plus corticosteroid including chemotherapy [105].
Physicians should be aware of the risk of HBV reactivation in HBsAg-negative patients. Although further studies are needed to compare the efficacy and cost effectiveness of different preventive strategies, we should at least perform careful monitoring of these patients, and if needed, we should administer nucleos(t)ide analogs against HBV DNA reactivation/reappearance. Regarding nucleos(t)ide analogs, as lamivudine and telbivudine are limited, entecavir or tenofovir would be preferred.
#2 Consensus statements and recommendations on follow-up of HBV and HCV co-infected patients treated with DAA in Asia–Pacific region
Before starting DAA treatment, HBsAg should be examined in high endemic areas of HBV infection (A-1).
In HBsAg-positive patients with advanced fibrosis, cirrhosis or previous HCC, pre-emptive nucleos(t)ide analog treatment should be started to prevent HBV reactivation (A-1).
In HBsAg-positive patients without advanced fibrosis, cirrhosis or previous HCC history, pre-emptive nucleos(t)ide analog treatment is effective for HBV infection (A-1), or close monitoring should be recommended during DAA treatment and through 24 weeks after EOT (B-1). Stopping should follow APASL HBV guidelines.
In HBsAg-negative patients who are positive for anti-HBc antibody and/or anti-HBs antibody when abnormal liver function tests are observed during DAA treatment and after EOT, HCV RNA, HBsAg and HBV DNA should be examined. Nucleos(t)ide analogs should be used to treat HBV reactivation (B-1).
Grading of evidence and recommendations are shown in Supplementary Table 1.
Conclusion
During DAA treatment, host immunological changes may occur although DAA treatment can lead to higher SVR rates with shorter treatment duration and less serious adverse events in most patients infected with HCV [10]. First, we have created guidelines for the monitoring of HCC occurrence based on its accumulated data for it (Fig. 1). Second, we have constructed compact guidelines for patients with HBsAg and anti-HBc and/or anti-HBs antibody (Fig. 2).
Fig. 1.

Surveillance/monitoring algorithm for patients with hepatitis C virus and sustained virological response by direct-acting antivirals (DAAs). HCC Hx history of hepatocellular carcinoma, Adv Fibrosis advanced liver fibrosis, US ultrasonography, T Markers: α-fetoprotein (AFP), lens culinaris agglutinin (LCA)-reactive AFP isoform (AFP-L3) and/or des-γ-carboxy prothrombin (DCP)
Fig. 2.
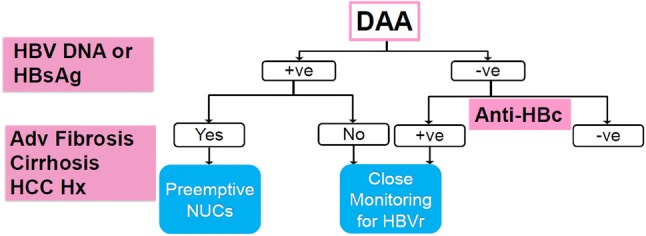
Surveillance/monitoring algorithm for patients co-infected with hepatitis C virus and hepatitis B virus (HBV) and treated with direct-acting antivirals (DAAs). HCC Hx history of hepatocellular carcinoma, Adv Fibrosis advanced liver fibrosis, NUCs nucleos(t)ides, HBsAg hepatitis B virus surface antigen, anti-HBc ant-hepatitis B virus core antibody, HBVr HBV reactivation and/or HBV DNA reappearance, +ve positive, -ve negative
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- GT
Genotype
- HCC
Hepatocellular carcinoma
- DAAs
Direct-acting antivirals
- SVR
Sustained virological response
- EOT
End of treatment
- US
Ultrasonography
- AFP
α-Fetoprotein
- AFP-L3
Lens culinaris agglutinin (LCA)-reactive AFP isoform
- DCP
Des-γ-carboxy prothrombin
Funding
None.
Compliance with ethical standards
Conflict of interest
Dr. Tatsuo Kanda received research grants from Merck Sharp and Dohme (MSD), Chugai Pharm, and AbbVie. Prof. Masao Omata received research grants from Gilead, Eisai, and Ono Pharma. The founding sponsors played no role in the study design, data collection, analyses, interpretation, writing of the manuscript, or in the decision to publish the results. The other authors have declared that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not necessary, see above.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650–655. [PubMed] [Google Scholar]
- 3.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Yu ML, Huang CF, Dai CY, Huang JF, Chuang WL. Long-term effects of interferon-based therapy for chronic hepatitis C. Oncology. 2007;72(Suppl 1):16–23. doi: 10.1159/000111703. [DOI] [PubMed] [Google Scholar]
- 5.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702–726. doi: 10.1007/s12072-016-9717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762–768. doi: 10.1111/jvh.12312. [DOI] [PubMed] [Google Scholar]
- 8.Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645–653. doi: 10.1016/S1473-3099(15)70099-X. [DOI] [PubMed] [Google Scholar]
- 9.Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 10.Lau G, Benhamou Y, Chen G, Li J, Shao Q, Ji D, et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: a phase 2, open-label, proof-of-concept study. Lancet Gastroenterol Hepatol. 2016;1:97–104. doi: 10.1016/S2468-1253(16)30015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson IM, Lawitz E, Gane EJ, Willems BE, Ruane PJ, Nahass RG, et al. Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology. 2017;153:113–122. doi: 10.1053/j.gastro.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 12.Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. Sofosbuvir, Velpatasvir, and Voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376:2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 13.Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 14.Wei L, Xie Q, Hou JL, Tang H, Ning Q, Cheng J, et al. Ledipasvir/sofosbuvir for treatment-naive and treatment-experienced Chinese patients with genotype 1 HCV: an open-label, phase 3b study. Hepatol Int. 2018;12:126–132. doi: 10.1007/s12072-018-9856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CH, Chen YS, Wang SS, Liu CJ, Su TH, Yang HC, et al. Sofosbuvir-based interferon-free direct acting antiviral regimens for heart transplant recipients with chronic hepatitis C virus infection. Clin Infect Dis. 2018;66:289–292. doi: 10.1093/cid/cix787. [DOI] [PubMed] [Google Scholar]
- 16.Reau N, Kwo PY, Rhee S, Brown RS, Jr, Agarwal K, Angus P, et al. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection. Hepatology. 2018;68:1298–1307. doi: 10.1002/hep.30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoj K, Nayak SL, Gupta E, Kataria A, Sarin SK. Generic sofosbuvir-based direct-acting antivirals in hepatitis C virus-infected patients with chronic kidney disease. Liver Int. 2018;38:2137–2148. doi: 10.1111/liv.13863. [DOI] [PubMed] [Google Scholar]
- 18.Minami T, Tateishi R, Nakagomi R, Fujiwara N, Sato M, Enooku K, et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol. 2016;65:1272–1273. doi: 10.1016/j.jhep.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: first, do no harm by withdrawing treatment. J Hepatol. 2016;65:862–864. doi: 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Kolly P, Waidmann O, Vermehren J, Moreno C, Vögeli I, Berg T, et al. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: a European multicentre study. J Hepatol. 2017;67:876–878. doi: 10.1016/j.jhep.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P, et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;65:1070–1071. doi: 10.1016/j.jhep.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J Hepatol. 2017;66:1138–1148. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda K, Kawamura Y, Kobayashi M, Kominami Y, Fujiyama S, Sezaki H, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci. 2017;62:2932–2942. doi: 10.1007/s10620-017-4739-z. [DOI] [PubMed] [Google Scholar]
- 26.Mettke F, Schlevogt B, Deterding K, Wranke A, Smith A, Port K, et al. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther. 2018;47:516–525. doi: 10.1111/apt.14427. [DOI] [PubMed] [Google Scholar]
- 27.Nakao Y, Hashimoto S, Abiru S, Komori A, Yamasaki K, Nagaoka S, et al. Rapidly growing, moderately differentiated HCC: a clinicopathological characteristic of HCC occurrence after IFN-free DAA therapy? J Hepatol. 2018;68:854–855. doi: 10.1016/j.jhep.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933–939. doi: 10.1016/j.jhep.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(996–1005):e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018;68:25–32. doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther. 2017;46:688–695. doi: 10.1111/apt.14256. [DOI] [PubMed] [Google Scholar]
- 32.Ooka Y, Kanda M, Obi S, Nakamura M, Ogasawara S, Suzuki E, et al. Prediction of the very early occurrence of HCC right after DAA therapy for HCV infection. Hepatol Int. 2018;12:523–530. doi: 10.1007/s12072-018-9895-5. [DOI] [PubMed] [Google Scholar]
- 33.Reddy KR, Pol S, Thuluvath PJ, Kumada H, Toyota J, Chayama K, et al. Long-term follow-up of clinical trial patients treated for chronic HCV infection with daclatasvir-based regimens. Liver Int. 2018;38:821–833. doi: 10.1111/liv.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, et al. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther. 2018;47:104–113. doi: 10.1111/apt.14380. [DOI] [PubMed] [Google Scholar]
- 35.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155(411–421):e4. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Yu ML, Huang CF, Yeh ML, Tsai PC, Huang CI, Hsieh MH, et al. Time-degenerative factors and the risk of hepatocellular carcinoma after antiviral therapy among hepatitis C virus patients: a model for prioritization of treatment. Clin Cancer Res. 2017;23:1690–1697. doi: 10.1158/1078-0432.CCR-16-0921. [DOI] [PubMed] [Google Scholar]
- 37.Janjua NZ, Chong M, Kuo M, Woods R, Wong J, Yoshida EM, et al. Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada. J Hepatol. 2017;66:504–513. doi: 10.1016/j.jhep.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Zavaglia C, Okolicsanyi S, Cesarini L, Mazzarelli C, Pontecorvi V, Ciaccio A, et al. Is the risk of neoplastic recurrence increased after prescribing direct-acting antivirals for HCV patients whose HCC was previously cured? J Hepatol. 2017;66:236–237. doi: 10.1016/j.jhep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–1055. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Gao F, Yuan G, Shi K, Huang Y, Chen Y, et al. Ten-year follow-up analysis of chronic hepatitis C patients after getting sustained virological response to pegylated interferon-α and ribavirin therapy. J Viral Hepat. 2016;23:971–976. doi: 10.1111/jvh.12574. [DOI] [PubMed] [Google Scholar]
- 41.Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013;57:230–236. doi: 10.1093/cid/cit234. [DOI] [PubMed] [Google Scholar]
- 42.Shiratori Y, Omata M. Predictors of the efficacy of interferon therapy for patients with chronic hepatitis C before and during therapy: how does this modify the treatment course? J Gastroenterol Hepatol. 2000;15(Suppl):E141–E151. doi: 10.1046/j.1440-1746.2000.02116.x. [DOI] [PubMed] [Google Scholar]
- 43.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motoyama H, Tamori A, Kubo S, Uchida-Kobayashi S, Takemura S, Tanaka S, et al. Stagnation of histopathological improvement is a predictor of hepatocellular carcinoma development after hepatitis C virus eradication. PLoS One. 2018;13:e0194163. doi: 10.1371/journal.pone.0194163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki R, Meyer K, Moriyama M, Kato N, Yokosuka O, Ray RB, et al. Rapid hepatitis C virus clearance by antivirals correlates with immune status of infected patients. J Med Virol. 2019;91:411–518. doi: 10.1002/jmv.25310. [DOI] [PubMed] [Google Scholar]
- 47.Carlin AF, Aristizabal P, Song Q, Wang H, Paulson MS, Stamm LM, et al. Temporal dynamics of inflammatory cytokines/chemokines during sofosbuvir and ribavirin therapy for genotype 2 and 3 hepatitis C infection. Hepatology. 2015;62:1047–1058. doi: 10.1002/hep.27971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hengst J, Falk CS, Schlaphoff, Deterding K, Manns MP, Cornberg M, et al. Direct-acting antiviral-induced hepatitis C virus clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. J Infect Dis. 2016;214:1965–1974. doi: 10.1093/infdis/jiw457. [DOI] [PubMed] [Google Scholar]
- 49.Sung PS, Lee EB, Park DJ, Lozada A, Jang JW, Bae SH, et al. Interferon-free treatment for hepatitis C virus infection induces normalization of extrahepatic type I interferon signaling. Clin Mol Hepatol. 2018;24:302–310. doi: 10.3350/cmh.2017.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlton-Smith C, Holmes JA, Naggie S, Lidofsky A, Lauer GM, Kim AY, et al. IFN-free therapy is associated with restoration of type I IFN response in HIV-1 patients with acute HCV infection who achieve SVR. J Viral Hepat. 2018;25:465–472. doi: 10.1111/jvh.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villani R, Facciorusso A, Bellanti F, Tamborra R, Piscazzi A, Landriscina M, et al. DAAs rapidly reduce inflammation but increase serum VEGF level: a rationale for tumor risk during Anti-HCV treatment. PLoS One. 2016;11:e0167934. doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faillaci F, Marzi L, Critelli R, Milosa F, Schepis F, Turola E, et al. Liver angiopoietin-2 is a key predictor of de novo or recurrent hepatocellular cancer after hepatitis C virus direct-acting antivirals. Hepatology. 2018;68:1010–1024. doi: 10.1002/hep.29911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu PS, Nakamoto N, Taniki N, Ojiro K, Amiya T, Makita Y, et al. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One. 2017;12:e0179096. doi: 10.1371/journal.pone.0179096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmes JA, Carlton-Smith C, Kim AY, Dumas EO, Brown J, Gustafson JL, et al. Dynamic changes in innate immune responses during direct-acting antiviral therapy for HCV infection. J Viral Hepat. 2019;26:362–372. doi: 10.1111/jvh.13041. [DOI] [PubMed] [Google Scholar]
- 55.Toyoda H, Kumada T, Tada T, Mizuno K, Sone Y, Kaneoka Y, et al. Impact of previously cured hepatocellular carcinoma (HCC) on new development of HCC after eradication of hepatitis C infection with non-interferon-based treatments. Aliment Pharmacol Ther. 2018;48:664–670. doi: 10.1111/apt.14914. [DOI] [PubMed] [Google Scholar]
- 56.Sato T, Kondo F, Ebara M, Sugiura N, Okabe S, Sunaga M, et al. Natural history of large regenerative nodules and dysplastic nodules in liver cirrhosis: 28-year follow-up study. Hepatol Int. 2015;9:330–336. doi: 10.1007/s12072-015-9620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minami T, Tateishi R, Kondo M, Nakagomi R, Fujiwara N, Sato M, et al. Serum alpha-fetoprotein has high specificity for the early detection of hepatocellular carcinoma after hepatitis C virus eradication in patients. Medicine (Baltimore) 2015;94:e901. doi: 10.1097/MD.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, et al. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–1262. doi: 10.1002/hep.26442. [DOI] [PubMed] [Google Scholar]
- 59.Aoyagi Y, Isemura M, Suzuki Y, Sekine C, Soga K, Ozaki T, et al. Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet. 1985;2:1353–1354. doi: 10.1016/s0140-6736(85)92643-1. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita F, Tanaka M, Satomura S, Tanikawa K. Prognostic significance of Lens culinaris agglutinin A-reactive alpha-fetoprotein in small hepatocellular carcinomas. Gastroenterology. 1996;111:996–1001. doi: 10.1016/s0016-5085(96)70067-7. [DOI] [PubMed] [Google Scholar]
- 61.Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol. 1999;94:650–654. doi: 10.1111/j.1572-0241.1999.00930.x. [DOI] [PubMed] [Google Scholar]
- 62.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol. 2014;49:555–563. doi: 10.1007/s00535-013-0883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aoyagi Y, Oguro M, Yanagi M, Mita Y, Suda T, Suzuki Y, et al. Clinical significance of simultaneous determinations of alpha-fetoprotein and des-gamma-carboxy prothrombin in monitoring recurrence in patients with hepatocellular carcinoma. Cancer. 1996;77:1781–1786. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1781::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 64.Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17–30. doi: 10.1007/s12072-007-9038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu T, Takami Y, Wada Y, Tateishi M, Matsushima H, Mikagi K, et al. Double- and triple-positive tumor markers predict early recurrence and poor survival in patients with hepatocellular carcinoma within the milan criteria and child-pugh class A. J Gastrointest Surg. 2017;21:957–966. doi: 10.1007/s11605-017-3394-1. [DOI] [PubMed] [Google Scholar]
- 66.D’Ambrosio R, Aghemo A, Rumi MG, Primignani M, Dell’Era A, Lampertico P, et al. The course of esophageal varices in patients with hepatitis C cirrhosis responding to interferon/ribavirin therapy. Antivir Ther. 2011;16:677–684. doi: 10.3851/IMP1807. [DOI] [PubMed] [Google Scholar]
- 67.Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, Conte E, et al. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016;151(130–139):e2. doi: 10.1053/j.gastro.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 68.Lens Sabela, Alvarado-Tapias Edilmar, Mariño Zoe, Londoño María-Carlota, LLop Elba, Martinez Javier, Fortea Jose Ignacio, Ibañez Luís, Ariza Xavier, Baiges Anna, Gallego Adolfo, Bañares Rafael, Puente Angela, Albillos Agustín, Calleja Jose Luís, Torras Xavier, Hernández-Gea Virginia, Bosch Jaume, Villanueva Cándid, Forns Xavier, García-Pagán Juan Carlos. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153(5):1273-1283.e1. doi: 10.1053/j.gastro.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 69.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gane EJ, Hyland RH, An D, Svarovskaia ES, Brainard D, McHutchison JG. Ledipasvir and sofosbuvir for HCV infection in patients coinfected with HBV. Antivir Ther. 2016;21:605–609. doi: 10.3851/IMP3066. [DOI] [PubMed] [Google Scholar]
- 71.Doi A, Sakamori R, Tahata Y, Urabe A, Morishita N, Yamada R, et al. Frequency of, and factors associated with, hepatitis B virus reactivation in hepatitis C patients treated with all-oral direct-acting antivirals: analysis of a Japanese prospective cohort. Hepatol Res. 2017;47:1438–1444. doi: 10.1111/hepr.12919. [DOI] [PubMed] [Google Scholar]
- 72.Kawagishi N, Suda G, Onozawa M, Kimura M, Maehara O, Ohara M, et al. Comparing the risk of hepatitis B virus reactivation between direct-acting antiviral therapies and interferon-based therapies for hepatitis C. J Viral Hepat. 2017;24:1098–1106. doi: 10.1111/jvh.12737. [DOI] [PubMed] [Google Scholar]
- 73.Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, et al. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol. 2017;32:1754–1762. doi: 10.1111/jgh.13771. [DOI] [PubMed] [Google Scholar]
- 74.Mücke VT, Mücke MM, Peiffer KH, Weiler N, Welzel TM, Sarrazin C, et al. No evidence of hepatitis B virus reactivation in patients with resolved infection treated with direct-acting antivirals for hepatitis C in a large real-world cohort. Aliment Pharmacol Ther. 2017;46:432–439. doi: 10.1111/apt.14177. [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, et al. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2017;15:132–136. doi: 10.1016/j.cgh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 76.Tamori A, Abiru S, Enomoto H, Kioka K, Korenaga M, Tani J, et al. Low incidence of hepatitis B virus reactivation and subsequent hepatitis in patients with chronic hepatitis C receiving direct-acting antiviral therapy. J Viral Hepat. 2018;25:608–611. doi: 10.1111/jvh.12840. [DOI] [PubMed] [Google Scholar]
- 77.Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, et al. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology. 2018;154:989–997. doi: 10.1053/j.gastro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 78.Ogawa E, Furusyo N, Murata M, Toyoda K, Hayashi T, Ura K. Potential risk of HBV reactivation in patients with resolved HBV infection undergoing direct-acting antiviral treatment for HCV. Liver Int. 2018;38:76–83. doi: 10.1111/liv.13496. [DOI] [PubMed] [Google Scholar]
- 79.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 80.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 81.Langman L, Cornell LD. Mechanism of Action of Immunosuppressive Drugs. In: Chang A, editor. Diagnostic Pathology. Transplant Pathology. 1. Altona: Amirsys Publishing Inc; 2014. [Google Scholar]
- 82.Wang F, Xu RH, Luo HY, Zhang DS, Jiang WQ, Huang HQ, et al. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B-cell lymphoma. BMC Cancer. 2008;8:115. doi: 10.1186/1471-2407-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Long M, Jia W, Li S, Jin L, Wu J, Rao N, et al. A single-center, prospective and randomized controlled study: can the prophylactic use of lamivudine prevent hepatitis B virus reactivation in hepatitis B s-antigen seropositive breast cancer patients during chemotherapy? Breast Cancer Res Treat. 2011;127:705–712. doi: 10.1007/s10549-011-1455-9. [DOI] [PubMed] [Google Scholar]
- 84.Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, et al. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–1311. doi: 10.1038/sj.bjc.6601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Z, Jiang L, Liang G, Song E, Jiang W, Zheng Y, et al. Hepatitis B virus reactivation in breast cancer patients undergoing chemotherapy: a review and meta-analysis of prophylaxis management. J Viral Hepat. 2017;24:561–572. doi: 10.1111/jvh.12672. [DOI] [PubMed] [Google Scholar]
- 86.Kumagai K, Takagi T, Nakamura S, Sawada U, Kura Y, Kodama F, et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol. 1997;8(Suppl 1):107–109. [PubMed] [Google Scholar]
- 87.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the US food and drug administration adverse event reporting system. Ann Intern Med. 2017;166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 88.Holmes JA, Yu ML, Chung RT. Hepatitis B reactivation during or after direct acting antiviral therapy—implication for susceptible individuals. Expert Opin Drug Saf. 2017;16:651–672. doi: 10.1080/14740338.2017.1325869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Monte A, Courjon J, Anty R, Cua E, Naqvi A, Mondain V, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27–30. doi: 10.1016/j.jcv.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 90.Liu CH, Liu CJ, Su TH, Fang YJ, Yang HC, Chen PJ, et al. Hepatitis B virus reactivation in patients receiving interferon-free direct-acting antiviral agents for chronic hepatitis C virus infection. Open Forum Infect Dis. 2017;4:028. doi: 10.1093/ofid/ofx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura M, Kanda T, Nakamoto S, Haga Y, Sasaki R, Jiang X, et al. Reappearance of serum hepatitis B viral DNA in patients with hepatitis B surface antigen seroclearance. Hepatology. 2015;62:1329. doi: 10.1002/hep.27693. [DOI] [PubMed] [Google Scholar]
- 92.Mozessohn L, Chan KK, Feld JJ, Hicks LK. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat. 2015;22:842–849. doi: 10.1111/jvh.12402. [DOI] [PubMed] [Google Scholar]
- 93.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 94.Buti M, Manzano ML, Morillas RM, García-Retortillo M, Martín L, Prieto M, et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: the Preblin study. PLoS One. 2017;12:e0184550. doi: 10.1371/journal.pone.0184550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol. 2009;90:13–23. doi: 10.1007/s12185-009-0359-5. [DOI] [PubMed] [Google Scholar]
- 96.Ide Y, Ito Y, Takahashi S, Tokudome N, Kobayashi K, Sugihara T, et al. Hepatitis B virus reactivation in adjuvant chemotherapy for breast cancer. Breast Cancer. 2013;20:367–370. doi: 10.1007/s12282-010-0213-x. [DOI] [PubMed] [Google Scholar]
- 97.Kim E, Yune S, Ha JM, Lee WJ, Hwang JW, Paik YH, et al. Hepatitis B virus reactivation during anti-cancer chemotherapy in patients with past hepatitis B virus infection. Hepatogastroenterology. 2014;61:1704–1711. [PubMed] [Google Scholar]
- 98.Hagiwara S, Sakurai T, Nishina S, Tanaka K, Ikeda M, Ueshima K, et al. Characteristic pattern of reactivation of hepatitis B virus during chemotherapy for solid cancers. Dig Dis. 2012;30:541–546. doi: 10.1159/000343056. [DOI] [PubMed] [Google Scholar]
- 99.Jun CH, Kim BS, Oak CY, Lee DH, Cho E, Cho SB, et al. HBV reactivation risk factors in patients with chronic HBV infection with low replicative state and resolved HBV infection undergoing hematopoietic stem cell transplantation in Korea. Hepatol Int. 2017;11:87–95. doi: 10.1007/s12072-016-9747-0. [DOI] [PubMed] [Google Scholar]
- 100.Lin CL, Kao JH. Hepatitis B reactivation in patients receiving immunosuppressive therapy: a hidden menace. Hepatol Int. 2017;11:31–33. doi: 10.1007/s12072-016-9782-x. [DOI] [PubMed] [Google Scholar]
- 101.Hwang JP, Fisch MJ, Lok AS, Zhang H, Vierling JM, Suarez-Almazor ME. Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center. BMC Cancer. 2013;13:534. doi: 10.1186/1471-2407-13-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung KS, Seto WK, Lai CL, Yuen MF. Prevention and management of hepatitis B virus reactivation in cancer patients. Hepatol Int. 2016;10:407–414. doi: 10.1007/s12072-015-9692-3. [DOI] [PubMed] [Google Scholar]
- 103.Ende AR, Kim NH, Yeh MM, Harper J, Landis CS. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep. 2015;9:164. doi: 10.1186/s13256-015-0630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayashi K, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Nishimura D, et al. A case of acute hepatitis B in a chronic hepatitis C patient after daclatasvir and asunaprevir combination therapy: hepatitis B virus reactivation or acute self-limited hepatitis? Clin J Gastroenterol. 2016;9:252–256. doi: 10.1007/s12328-016-0657-4. [DOI] [PubMed] [Google Scholar]
- 105.Kusumoto S, Tanaka Y, Suzuki R, Watanabe T, Nakata M, Takasaki H, et al. Monitoring of hepatitis B virus (HBV) DNA and risk of HBV reactivation in B-Cell lymphoma: a prospective observational study. Clin Infect Dis. 2015;61:719–729. doi: 10.1093/cid/civ344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


